Abstract
Zebrafish as a neurogenetic model system depends on the correct neuroanatomical understanding of its brain organization. Here, we address the unresolved question regarding a possible zebrafish homologue of the dorsal pallial division, the region that in mammals gives rise to the isocortex. Analyzing the distributions of nicotine adenine dinucleotide phosphate diphorase (NADPHd) activity and parvalbumin in the anterior zebrafish telencephalon, we show that against previous assumptions the central (Dc) zone possesses its own germinative region in the dorsal proliferative zone. We define the central (Dc) zone as topologically corresponding to the dorsal pallial division of other vertebrates (mammalian isocortex). In addition, we confirm through BrdU-labeling experiments that the posterior (Dp) zone is formed by radial migration and homologous to the mammalian piriform cortex. Based on our results, we propose a new developmental and organizational model of the zebrafish pallium—one which is the result of a complex outward-inward folding.
Keywords: amygdala, cortex, eversion, evagination, forebrain, migration, ray-finned fish, teleost
1. Introduction
The mammalian isocortex is considered the crowning achievement of evolution because it forms the neurological substrate for cognitive and emotive human mental processes (Rakic, 2009). It develops from what is called the dorsal pallial division. Searching for the evolutionary origin of this structure has been one of the most challenging questions in comparative neurology (Medina and Abellan, 2009). A dorsal pallial division homologous to the mammalian isocortex evolved with jawed vertebrates (gnathostomes) and is present in diverse anamniotes like sharks, lungfish, and frogs (Gonzalez and Northcutt, 2009; Northcutt, 1981; Northcutt, 2009; Pombal et al., 2009; Rodriguez-Moldes, 2009; Wicht and Northcutt, 1998). Ray-finned fish (actinopterygians) like zebrafish have been denied this privilege. Comparative studies have not established a distinct cortex homologue (Northcutt, 2008). We also lack specific markers that could help identify the cortex region. Molecular markers (pax6 and reelin) which label the mammalian cortex in a characteristic, stage dependent manner are not expressed in regions qualifying for a cortex homologue in zebrafish (Costagli et al., 2002; Wullimann and Rink, 2001). Also, none of the extensive molecular and gene expression studies on embryonic and larval stages of zebrafish have indicated a cortex homologue (Mueller and Wullimann, 2005; Mueller and Wullimann, 2009).
The main obstacle for identifying pallial divisions in zebrafish is the unusual development of the teleostean telencephalon (fig. 1). The telencephala of zebrafish and other ray-finned fish develop through a unique process of outward folding called eversion. The exact nature of this eversion process has been a subject of debate for the past 130 years. A number of eversion models have been proposed, ranging from very simple to highly elaborate (Braford, 1995; Braford, 2009; Butler, 2000; Gage, 1883; Nieuwenhuys, 2009; Northcutt and Davis, 1986; Northcutt, 2008; Studnička, 1894; Wullimann and Mueller, 2004; Yamamoto et al., 2007). However, little developmental evidence has validated any of these models. As a result, there is no consensus on the exact anatomical delineation of even well established pallial homologies such as the teleostean pallial amygdala, the hippocampus, and the piriform cortex (Nieuwenhuys, 2009; Northcutt, 2008). Yet, all of the participants in the current debate agree that the exact anatomical delineation of these homologies and the identification of the dorsal pallium depend on a complete topological analysis of the teleostean eversion (Nieuwenhuys, 1962; Nieuwenhuys, 2009).
Fig. 1.
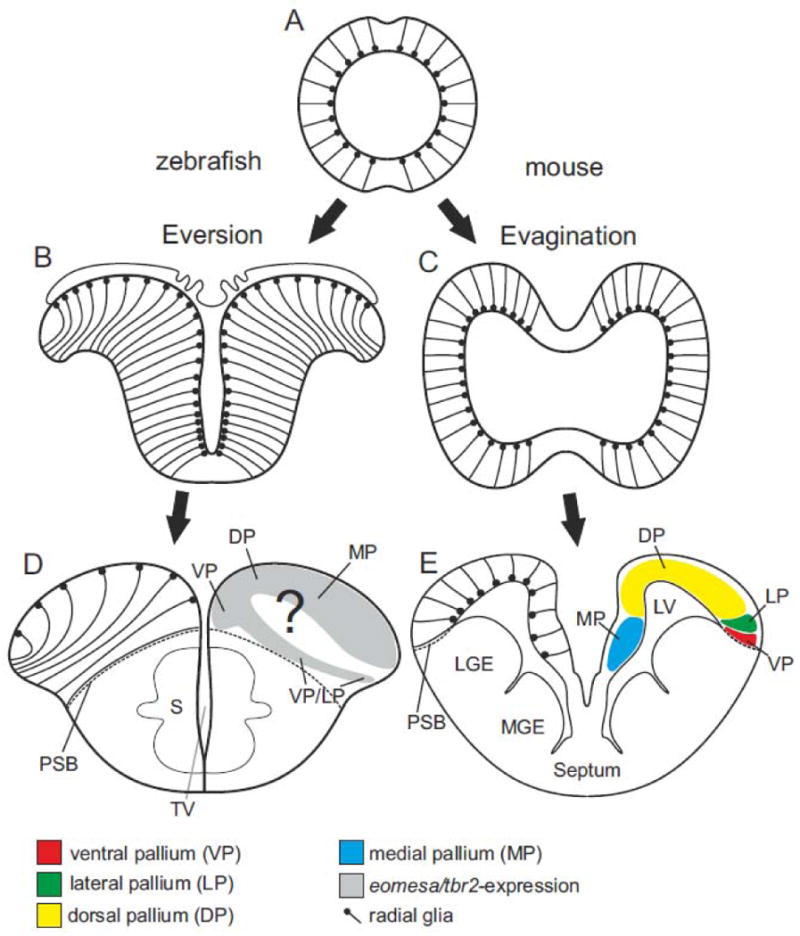
Development of the telencephalon in teleosts and mammals. (A) Coronal view of the vertebrate anterior neural tube giving rise to the telencephalon. (B and D) The teleostean telencephalic outward folding (eversion) leads to a dorsal telencephalon (pallium) where proliferative zone and ventricular surface are located on its dorsalmost site (indicated by location and orientation of radialglia). The development of the medial (MP), dorsal (DP), lateral (LP), and ventral (VP) pallial divisions which in mammals give rise to the hippocampus, cortex, piriform cortex and pallial amygdala is poorly understood. Eversion models simplified after (Nieuwenhuys, 1990) and (Mueller and Wullimann, 2009) (C and D) The mammalian (mouse) telencephalon develops through evagination. Proliferative zones are inwardly oriented towards the ventricle. Pallial divisions in mouse simplified (Puelles et al., 2000).
We chose a comparatively simple yet effective method for deciphering the zebrafish pallium. To determine and map true pallial histogenetic units, we studied consecutive sections of adult zebrafish that were stained against nicotine adenine dinucleotide phosphate diphorase (NADPHd) activity and parvalbumin. The differential staining patterns of both of these markers visualized pallial zones and their topological site of origin. For the first time, we show that the central (Dc) zone reaches the dorsal proliferative zone at the rostral pole of the telencephalon. Dc comprises its own germinative zone of origin and, thus, is a true pallial histogenetic unit. In a subsequent BrdU longterm labeling experiment, we provide additional evidence that the posterior (Dp)-zone, is the result of radial migration as proposed earlier (Wullimann and Mueller, 2004). We also defined the topological origin of both of these divisions. In sum, we here propose a modification of the partial eversion model (Wullimann and Mueller, 2004)—one that recognizes the central (Dc) zone as a true pallial division topologically corresponding to the dorsal pallium.
2. Results
NADPHd-Activiy as a Marker for Pallial Units
The contrasting coloration of the NADPHd-activity stain allowed us to distinguish the individual zones of the zebrafish pallium (fig. 2A). The lateral (Dl) zone located at the dorsolateral part was marked most distinctly in dark blue. In contrast, the central (Dc) zone at the core of the pallium only showed sparse NADPHd-positive cells. The medial (Dm) zone facing Dl as well as the posterior (Dp) zone ventral to Dl were free of NADPHd-activity (fig 2A). The locations of the pallial zones are the basis of the common topographical nomenclature (fig. 2A-C) (Nieuwenhuys, 1990). Our results, however, deliver the foundation for a topological terminology insofar as we define the four true histogenetic pallial units and their topological sites of origin.
Fig. 2.
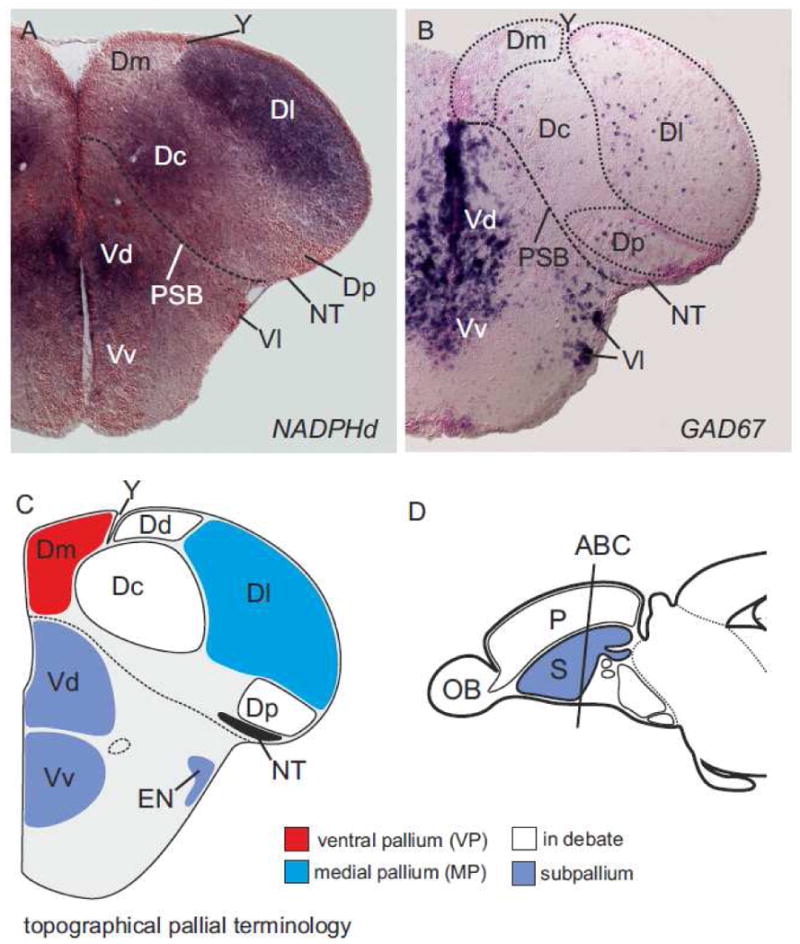
Coronal sections of a zebrafish telencephalon stained against NADPHd-activity. (A) The differential distribution of nicotine adenine dinucleotide phosphate diphorase- (NADPHd-) activity reveals distinct pallial divisions in the rostral pole of the zebrafish telencephalon. The lateral (Dl) zone of the pallium is strongly NADPHd-positive and can easily be discerned from the weakly stained central (Dc) zone and the unstained medial (Dm) and posterior (Dp) zones. (B) For comparison. GAD67 mRNA is a marker for GABAergic cells. The pallium is defined by scarse GAD67-expressing cells and, thus, can be distinguished from the subpallium, which is defined by dense GAD67-expressing cell populations in the subpallial parts of the telencephalon, namely the dorsal (Vd) and ventral (Vv) zones of the ventral telencephalon (Vd) and the entopeduncular nucleus (EN). (C) Schematic coronal sections with topographical nomenclature. Note that the dorsal (Dd) zone of the dorsal telencephalon is not found in figs. 1A+B.
NADPHd-Activity At The Rostral Pole of the Telencephalon
We traced these topological sites of origins analyzing consecutive sections of the rostral pole of adult zebrafish. Here we capitalized on the fact that, at its rostral pole, all major zones of the pallium are easily discernable with a stain against NADPHd-activity (figs. 3A-E). We found that the position of the pallial zones at the rostral pole of the telencephalon (described in figs. 2A-C) differed significantly from those seen at midlevels (figs. 3A-E). At most anterior sections (figs. 3A+B), the bluish NADPHd-positive Dl zone did not face the medial (Dm) zone but sat laterally to the sparsely NADPHd-positive central (Dc) zone. Dc was in these anterior sections vertically sandwiched between the medial (Dm) and the lateral (Dl) zones. Most importantly, the central (Dc) zone—our candidate for a dorsal pallial division—extended to the dorsal proliferative matrix (marked by arrows in figs. 3A+B). Dc, thus, comprises its own germinative field in the dorsal proliferation zone.
Fig. 3.
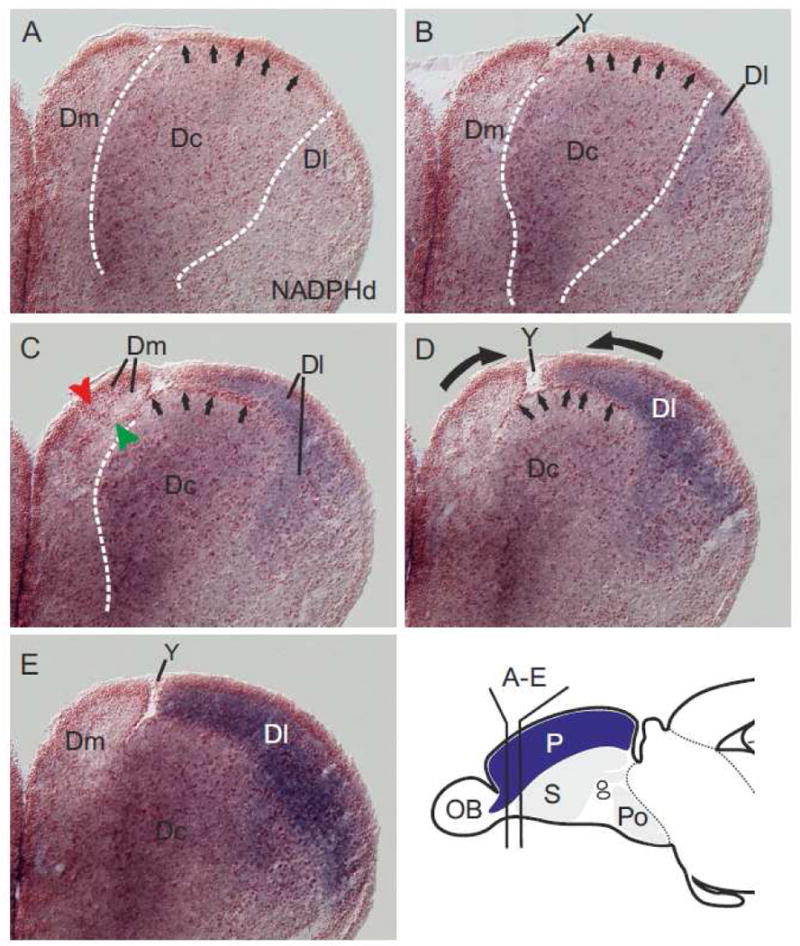
NADPHd activity in consecutive sections of the zebrafish pallium. (A and B) In these anteriormost sections, the central (Dc) zone is vertically sandwiched between the medial (Dm) and the lateral (Dl) zones. Here, the central (Dc) zone includes its germinative field in the proliferative matrix (arrows in figs. 1A and B). The sulcus ypsiloniformis (Y) appears as a small indentation (fig. 1B) between the medial (Dm) and central (Dc) zone. (C and D) The dark-blue-stained lateral (Dl) zone increasingly covers the central (Dc) zone on more posterior sections. At this level, the central (Dc) zone appears ventrally displaced and the sulcus ypsiloniformis is visible as a large gap (Y) between the medial (Dm) and lateral (Dl) zones. Note, Dc's germinative zone is visible on top of the ventrally displaced central (Dc) zone (arrows in figs. 2C, D). (E) The lateral (Dl) zone completely covers the central (Dc) zone at subsequent posterior sections. We hypothesize, that the sulcus ypsiloniformis (Y in figs. 3B-E) and the central position of Dc are results of a complex outward-inward folding (compare fig. 7 A). Thick black arrows indicate the protrusions of the medial (Dm) and lateral (Dl) zones during this hypothesized folding process.
At more posterior levels (fig. 3C) the lateral (Dl) zone began to cover the central (Dc) zone including its dorsalmost proliferative matrix (marked by arrows). Also a large indentation started to become visible between Dl and Dm. More caudally, this indentation enlarged (fig. 3D) and formed the sulcus ypsiloniformis (Y) (fig. 3E). On more caudal sections, the dorsal proliferation zone of Dc —the germinative sheet of origin— was visible as a distinctive line of red cells (arrows in figs. 3B+C) delineating the border to neighboring medial (Dm) and lateral (Dl) zones. At more caudal sections, the strongly NADPHd-positive lateral (Dl) zone fully covered this germinative sheet (arrows pointing on red cells in figs. 3C+D) and the entire sparsely labeled central (Dc) zone (figs. 3C-E). Together, these findings indicate that during development the central (Dc) zone sinks ventrally into the center of the pallium. We call this process invagination in our outward-inward folding hypothesis in fig. 7A. The major force of this process may be the differential growth processes of the medial (Dm) and lateral (Dl) zones during development. We hypothesize that during development the medial (Dm) and lateral (Dl) zone grow laterally and medially, respectively. This processes we call protrusions (fig. 7A).
Fig. 7.
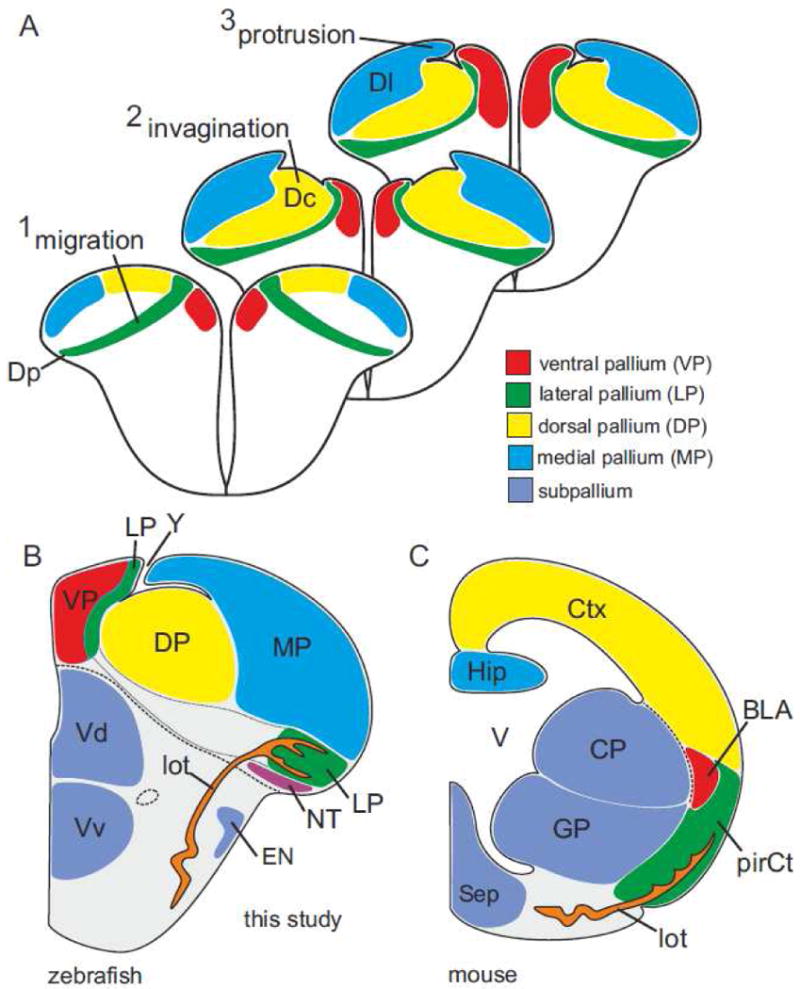
Complex eversion model and topological organization of the zebrafish pallium. (A) The teleostean pallium consists of the same four pallial divisions present in mammals. Adult location and topological origin are obscured by three major developmental events. First, the posterior (Dp) zone (the lateral pallium, LP) is the result of radial migration across the entire pallium as suggested earlier (Wullimann and Mueller, 2004). Second, the central (Dc) zone at the core of the pallium is the dorsal pallium (DP) that invaginates from a dorsalmost position. Third, the lateral (Dl) zone is the medial pallium (MP) that medially protrudes during development. Note, this model explains the origin of the sulcus ypsiloniformis as a corollary of the invagination. (B) Schematic section with topological nomenclature. The zebrafish pallium and its four pallial divisions compared to mouse. The zebrafish telencephalon possesses a ventral pallium (VP) that topologically corresponds to the mammalian basolateral amygdala, a lateral pallium (LP) corresponding to the mammalian piriform cortex, a dorsal pallium (DP) corresponding to the (iso-) cortex and, a medial pallium (MP) corresponding to the hippocampus. Note, the lateral olfactory tract (lot) of zebrafish is homologous to the tract with the same name in mouse. (C) Mouse telencephalon simplified after (Medina et al., 2004; Puelles et al., 2000).
This displacement of Dc by Dl alters our understanding of the sulcus ypsiloniformis (Y) as well (fig. 3B). The sulcus ypsiloniformis (Y) has been interpreted as an independent and stable anatomical landmark between Dm and Dl. Our results, however, indicated that this is true only for posterior levels of the telencephalon. At most anterior levels, the sulcus ypsiloniformis (Y) marked the border between the medial (Dm) zone and the central (Dc) zone (fig. 3B). This is because here the central (Dc) zone, including its germinative proliferative layer, reaches the dorsal top of the telencephalon. A dorsal (Dd) zone is absent in zebrafish.
Parvalbumin Immunoreactivity Confirms Four Pallial Units
To confirm that the zebrafish pallium comprises only four histogenetic units, we examined the distribution of parvalbumin. And, indeed, parvalbumin differentially marked the same four pallial zones in the adult zebrafish as NADPHd (figs. 4A-D). For example, the lateral (Dl) zone displayed many parvalbumin-positive cells ventrally adjacent to the dorsal proliferative layer, parvalbumin-positive cells in the periphery of Dl, as well as strongly labeled parvalbumin-positive fibers (nicely displayed in fig. 5E). The central (Dc) zone, in contrast, markedly differed from the overlying lateral (Dl) zone through the absence of parvalbumin-positive cells in both, its proliferative layer and in its periphery. The presence of many parvalbumin fibers in Dc sets this pallial unit apart from the adjacent medial (Dm) and the posterior (Dp) zones, which both lack expression of parvalbumin. Note, also the lateral olfactory tract (lot), which is known to send olfactory projections to the posterior (Dp) zone as well as to the nucleus taeniae (Levine and Dethier, 1985; Northcutt, 2008) is defined by the absence of parvalbumin-positive fibers (fig. 4A).
Fig. 4.
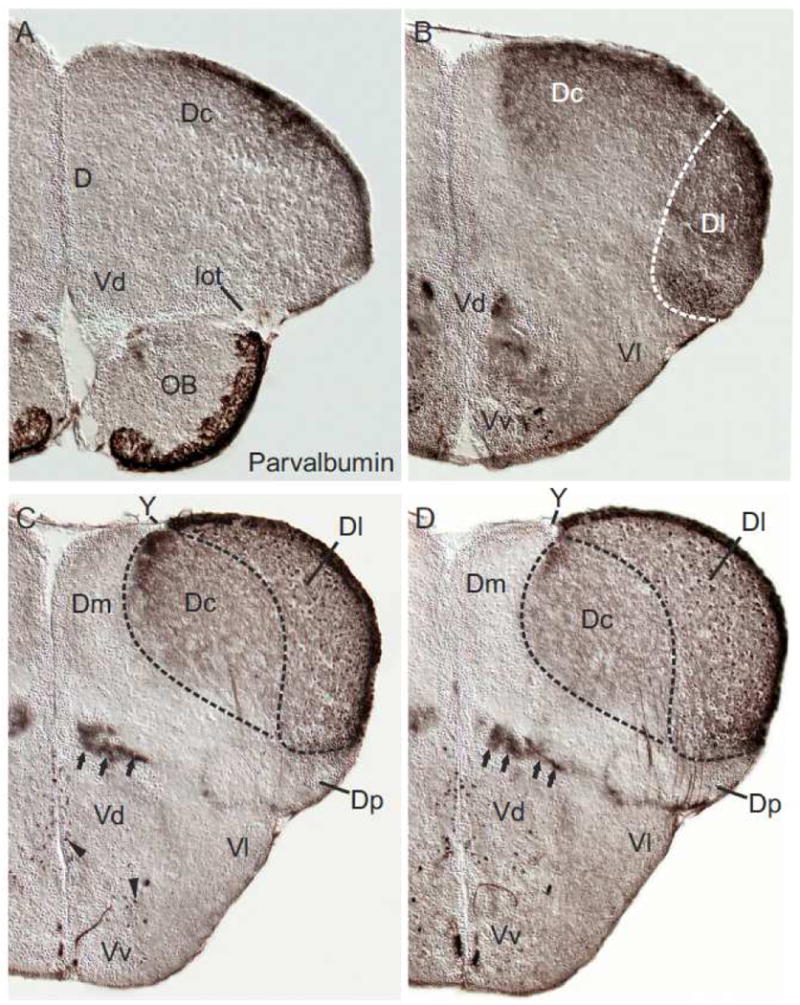
Coronal sections of a zebrafish telencephalon stained against parvalbumin. (A-D) The differential distribution of parvalbumin-positive cells and fibers reveals all four pallial divisions in the anterior zebrafish telencephalon. The lateral (Dl) zone of the pallium shows many parvalbumin-positive cells ventrally adjacent to the dorsal proliferation zone and its periphery (nicely seen in 4C+D) and displays many parvalbumin-positive fibres (A-D). In contrast, the central (Dc) zone shows also many parvalbumin-positive fibers but is defined by the absence of parvalbumin-positive cells. The medial (Dm) zone is largely free of parvalbumin-positive structures at all of these levels. The lateral olfactory tract (lot; fig. A) and its principal projection area, the posterior (Dp) zone (figs. C+D), are both free of parvalbumin. Sparse parvalbumin-positive neurons are found in the ventral (Vv) and dorsal (Vd) nuclei of the subpallium (figs. B-D). Parvalbumin-positive fibre bundles at the pallial-subpallial boundary can be followed from these anterior sections (arrow in B) that send projections to the central (Dc) and lateral (Dl) zones. Note, the sulcus ypsiloniformis (Y) is a large indentation at these anterior levels (C+D) and Dc reaches the ventricular surface formed by it. A dorsal (Dd) zone described in other teleosts is missing according to our results.
Fig. 5.
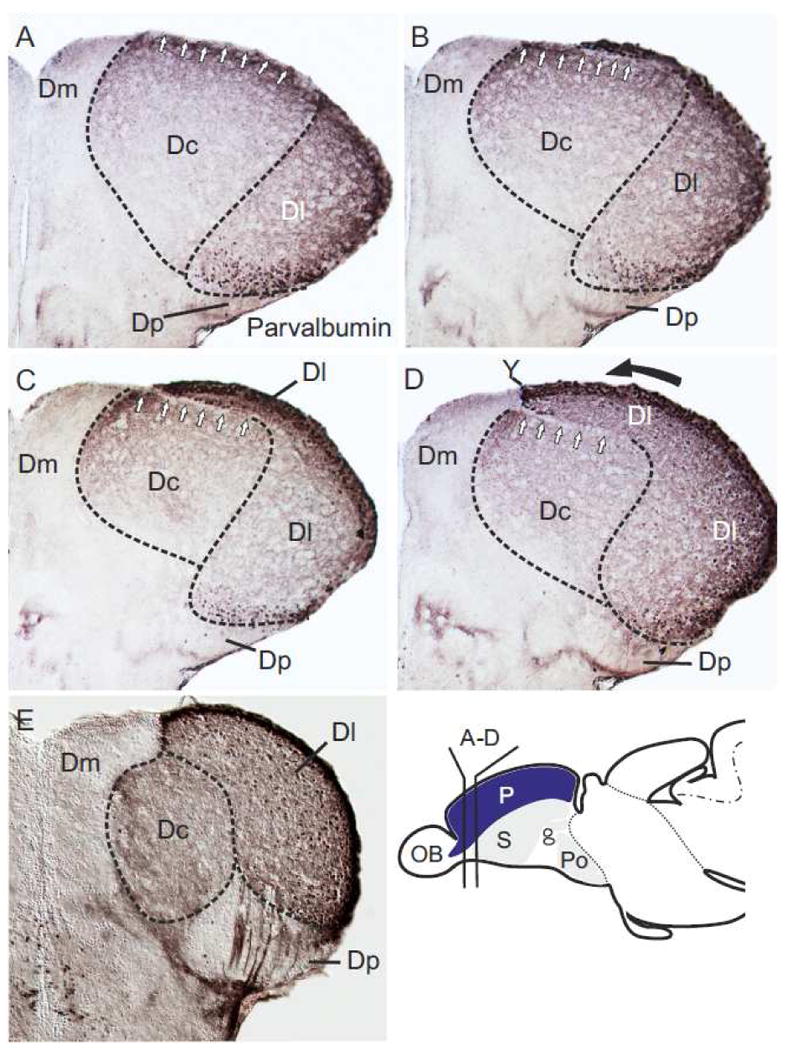
Distribution of Parvalbumin in consecutive sections of the zebrafish pallium. (A-E) Parvalbumin is the second marker that allows discerning all four true histogenetic units in the zebrafish pallium. The central (Dc) zone is defined by many parvalbumin-positive fibers, whereas the lateral (Dl) zone also exhibits many parvalbumin-positive cells ventrally adjacent to the proliferation zone and in its periphery. The medial (Dm) and posterior (Dp) zones are largeley free of parvalbumin. (A and B) Anteriorly, Dc extents to the dorsal proliferation and, thus, includes its germanitive field in the proliferative matrix (white arrows in figs. A and B). At this level, the central (Dc) zone is vertically sandwiched between the medial (Dm) and the lateral (Dl) zones confirming the results of the NADPHd-activity stains. Note, in this case, the sulcus ypsiloniformis (Y) does not appear as gap (fig. D) between the medial (Dm) and central (Dc) zone. (B-D) On subsequent more posterior sections, the strongly labeled lateral (Dl) zone increasingly covers the central (Dc) zone. (E) The lateral (Dl) zone completely covers the central (Dc) zone on midlevels of the telencephalon. Thick black arrows indicate the protrusions of the medial (Dm) and lateral (Dl) zones during the hypothesized outward-folding process (compare fig. 7A).
The majority of parvalbumin-positive fibers in the central (Dc) and lateral (Dl) zone likely arise from two main sources: an anterior and a posterior one. Anteriorly, parvalbumin-positive fibers form fiber bundles at the pallial-subpallial boundary (black arrows, figs. 4C+D) that can be traced towards the rostral pole of the telencephalon; these fiber bundles arise from the dorsal (Vd) and ventral (Vv) nuclei of the subpallium. Here, many parvalbumin-positive cells display the morphology of projecting neurons (black arrowheads, figs. 4C+D). At posterior levels, Dc and Dl appear to receive parvalbumin-positive projections via the partially labeled lateral forebrain bundle. These projections likely arise from nuclei of the preglomerular complex, which is known to projects to both, the lateral (Dl) as well as the central (Dc) zone in goldfish (Northcutt, 2006).
Parvalbumin-Immunoreactivity Confirms The Histogenetic Unit Dc
To confirm that the central (Dc) zone is a true histogenetic unit, we examined the distribution of parvalbumin-reactivity at the most rostral pole of the pallium. Indeed, the central (Dc) zone reached its own germinative zone within the periventricular site of proliferation (white arrows in fig. 5A) when sandwiched between the medial (Dm) and the lateral (Dl) zones. The lateral (Dl) zone, defined by strongly labeled parvalbumin-positive cells ventrally lining the dorsal proliferative zone, appeared to protrude medially. It increasingly covered the central (Dc) regions in subsequent sections (figs. 5B-D). Note, that the neuroepithelium anteriorly covering the central (Dc) zone (white fig. 5A), is horizontally sandwiched between Dc and Dl on more posterior sections (arrows in figs. 5B-D). At midlevels of the pallium, this neurepithelial sheet was no longer visible between the central (Dc) and lateral (Dl) zones (fig. 4D, 5E). Here, the lateral (Dl) zone marked by many parvalbumin-positive cells completely covered Dc and was attached to the medially facing medial (Dm) zone. Likewise, an open sulcus ypsiloniformis was only visible at more anterior sections (Y in figs. 4CD). Again, no dorsal (Dd) zone was found in these stainings, confirming the absence of this zone in zebrafish.
In sum, our results show that the zebrafish pallium, like the mammalian one, possesses four true histogenetic divisions. In zebrafish these four are the medial (Dm), the lateral (Dl), the posterior (Dp), and the central (Dc) zones. The central (Dc) zone is a distinct developmental entity (i.e. a histogenetic unit)—one that possesses its own field of origin in the dorsal proliferative matrix. We hypothesize that Dc is ventrally displaced during development where its initial dorsal position shifts to the center of the pallium. The main force of this invagination process might be due to the medially oriented protrusion of the lateral (Dl) zone. A dorsal (Dd) zone is not present in zebrafish in contrast to earlier reports (Wullimann et al., 1996). The overgrown part of Dl has been misinterpreted as a region corresponding to Dd.
The posterior zone (Dp) is the result of radial migration
To identify corresponding pallial divisions between teleosts and mammals—and, most importantly, to define Dc as topologically corresponding to the mammalian dorsal pallium—we need to understand the topological origin of each of these four domains. Apart from the central (Dc) zone, the posterior (Dp) zone of the teleostean telencephalon was the other key divisions with disputed place of origin (Nieuwenhuys, 2009). Yet, returning to our NADPHd-stained sections (in particular fig. 3C), we get a first glance at Dp's genesis. In fig. 3C, the medial (Dm) zone is vertically subdivided. We can differentiate between a vertical field formed by densely populated cells (red arrowhead in fig. 3C) and a parallel vertical field formed by scattered cells (green arrowhead in fig. 3C) running alongside the former. We interpret this second subdivision as an adult reminiscent trace of an extensive radial migration across the pallium. This migration exiles the posterior (Dp) zone to the pial surface below the lateral (Dl) zone as has been suggested in the partial eversion model (Lillesaar et al., 2009; Mueller and Wullimann, 2009; Wullimann and Mueller, 2004).
We determined whether Dp's origin is the result of such a radial migratory activity by performing longterm-BrdU experiments on larval zebrafish ranging from three to eight and nine days after fertilization. We used an antibody against Hu-proteins, a marker for newborn and differentiating neurons, as counterstain. In this way the pallium (P) was easily distinguishable from the subpallium (S) based on its differing proliferation and migration patterns (figs. 6A-D). The heterogeneous subpallium showed strong proliferative and migratory activity (figs. 6A-D) and exhibited many post-mitotic double-labeled BrdU- and Hu-cells (yellow cells nicely seen in fig. 6A). In addition, migrated subpallial neurons mono-stained for Hu-proteins (green) appeared brighter than pallial ones. The pallium displayed a great homogeneity and dimly lit up as a uniform area of weakly Hu-labeled, green neurons. Here, double-labeled BrdU- and Hu-positive cells (orange) remained in the vicinity of their dorsal proliferative zones of origin marked in red as BrdU-positive. Against this consistent green background of BrdU-negative cells, we identified individual red oval BrdU-positive cells migrating mediolaterally across the pallium (arrowheads in fig.6A-D).
Fig. 6.
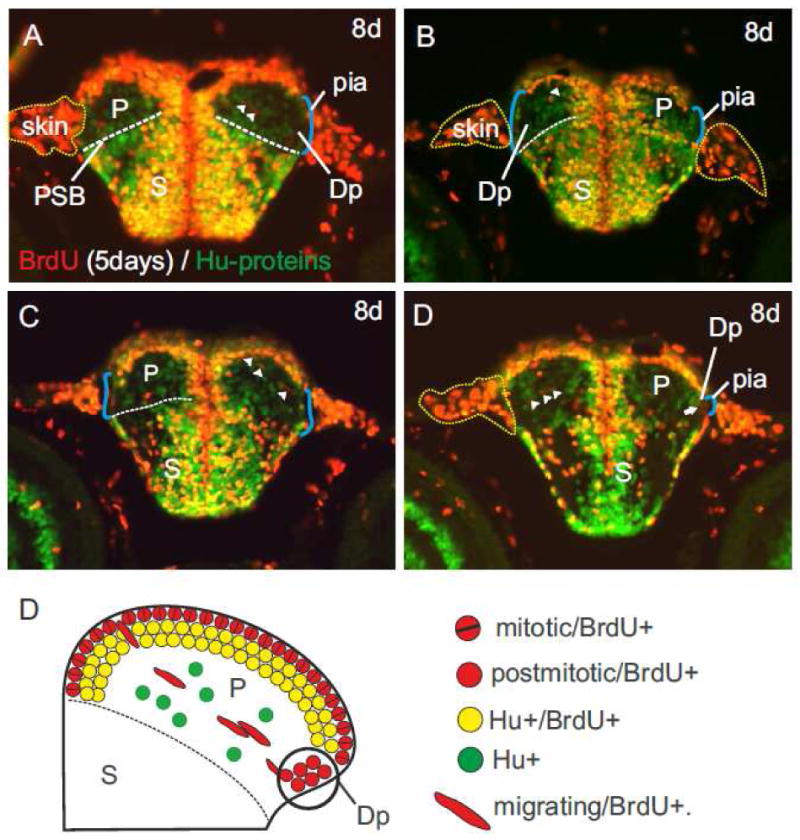
BrdU longterm pulse-chase experiments. (A-D) A pulse-chase experiment from three to eight days postfertilization (dpf) revealed distinct pallial (P) versus subpallial (S) proliferation and migration patterns. The subpallium (S) is stronger stained by BrdU and Hu-antibodies compared to the pallium and allowed to delineate the pallial-subpallial boundary (PSB). (A) Two radially migrating BrdU-positive and Hu-negative cells (arrowheads) forming a chain. (B) A migrating BrdU-positive cell (arrowhead) originating in the proliferation zone of the pallium in a medial area. (C) A chain of radially migrating BrdU-positive cells from a similar area towards the pial surface where the primordial posterior (Dp) zone forms. (D) Same brain as fig. C, one section further caudally. The primordial posterior (Dp) zone consisting of dimly red, round BrdU-positive cells close to the pial surface of the telencephalon. In contrast, the pallial proliferative zone above is formed by shiny red, BrdU-positive cells and orange BrdU-/Hu-positive cells. (E) Schematic cartoon that describes the genesis of the primordial posterior (Dp) zone as a result of radial migration of cells across the entire pallium as detected with the above described BrdU-/Hu double labeling experiments.
This chain of migrating cells originated in a lateral field of the medial (Dm) zone and moved towards the cell poor area of the primordial posterior (Dp) zone (figs. 6A-D). Once these oval shaped, red migrating cells arrived at their destination they transformed into round-shaped, red neurons building the posterior (Dp) zone (fig. 6D). In addition, the postmitotic BrdU-positive cells of Dp were distinctly different from adjacent BrdU-positive cells of the lateral (Dl) zone. Cells of Dl were double-labeled for Hu and lit up yellow while the migrated cell population of Dp appeared red only, thus, Dp lacked these yellow Hu-positive neurons. This difference in cell population reveals that Dp is not a derivative of the proliferative matrix of the lateral (Dl) zone nor of any other proliferative zone close by, as similarly suggested by Lillesaar and colleagues (Lillesaar et al., 2009). Rather, the developmental foundation of Dp is the above described radial migration of neurons across the pallium (Mueller et al., 2008; Wullimann and Mueller, 2004). The domain of origin of those migrating neurons lies laterally adjacent to the primordial medial (Dm) zone.
Now that we have identified all true pallial divisions and their possible mode of development, we can perform a topological analysis of the zebrafish pallium. We propose a new developmental model of the teleostean pallial eversion (fig. 7A) characterized by three main morphogenetic processes. First, the posterior (Dp) zone is the result of a radial migration across the entire pallium (fig. 7A1) as previously suggested (Wullimann and Mueller, 2004). Second, the central (Dc) zone including its proliferative matrix invaginates during development (fig. 7A2). Third, the lateral (Dl) zone subsequently overgrows the central (Dc) zone in a medially oriented protrusion (fig. 7A4). According to our model, the dorsal (Dd) zone is not present in zebrafish, and might not be a histogenetic unit in other teleosts. The dorsal (Dd) zone in other teleosts may be, as the sulcus ypsiloniformis (Y) in zebrafish, a corollary of the morphogenetic movements during the complex eversion process.
If we now topologically compare the teleostean telencephalon with the mouse model (fig. 1; fig. 7A-C), we conclude that the medial (Dm) zone corresponds to the ventral pallium (VP), the posterior (Dp) zone to the lateral pallium (LP), the central (Dc) zone to the dorsal (pallium) and the lateral (Dl) zone to the medial pallium (MP).
3. Discussion
In this study, we identify in zebrafish the dorsal pallial division, which topologically corresponds to the dorsal pallium of other jawed vertebrates, including the isocortex of mammals. The teleostean dorsal pallial division has been overlooked because of its obscured development. For the first time, we establish the central (Dc) zone as a histogenetic unit, one that includes its own germinative field of origin in the dorsal proliferative matrix. We hypothesize, that Dc's location at the center is the result of an invagination process caused by the protruding medial (Dm) and lateral (Dl) zones during development (fig. 7A).
Until now, the central (Dc) zone of the zebrafish pallium has not been considered a true histogenetic unit. Instead, Dc has been treated as a deeper zone of the periventricular zones like the medial (Dm), the dorsal (Dd) and the lateral (Dl) zones (Northcutt, 2006; Northcutt, 2008: Nieuwenhuys, 2009). Similarly, Dc together with periventricular parts of Dd, and Dl has been discussed as a possible homologue of the dorsal pallium (Wullimann and Mueller, 2004). These assumptions, however, must be rejected as we clearly show that the central (Dc) zone is a true histogenetic unit and, thus, a distinct entity of the adult zebrafish brain.
Our interpretation of pallial divisions—appart from Dc and Dd— fundamentally agrees with current research. The medial Dm, lateral Dl, and the posterior Dp zones of the zebrafish pallium are established homologues of the pallial amygdala, hippocampus, and piriform cortex respectively (Braford, 1995; Northcutt, 2006; Portavella et al., 2002). In regard to Dl, we agree with the suggestion that the dorsal (Dld) and ventral (Dld) zones in other teleosts than zebrafish are subdivisions of the same histogenetic unit, i.e. the medial pallium (Northcutt, 2006).
Our anatomical delineation of Dm, Dl, and Dp is furthermore supported by molecular marker distributions in the adult and developing teleostean telencephalon. For example, the interpretation of the medial (Dm) zone as the pallial amygdala is consistent with behavioral studies (Portavella et al., 2002; Portavella et al., 2004), the presence of ascending fibers from basal forebrain cholinergic cell groups, and the distribution of calretinin-positive cells (Castro et al., 2006; Mueller et al., 2004). Also, the interpretation of the lateral (Dl) zone as the teleostean hippocampus is supported by behavioral experiments and molecular marker distribution (Castro et al., 2006; Portavella et al., 2002). The posterior (Dp) zone of the teleostean pallium has been previously established as the region homologous to the piriform cortex of amphibians and other tetrapods (Braford, 1995). Dp is well distinguishable from the overlaying lateral (Dl) zone, which expresses NADPHd and parvalbumin (this study) as well as neuropeptide Y (Castro et al., 2006). Overall, what we describe as a conserved pallial organization in zebrafish is consistent with developmental gene expression patterns and mirrors the conserved molecular organization of subpallial divisions. Like in mammals, the teleostean pallium —including its dorsal pallial division— is invaded by tangentially migrating GABAergic cells that originate in a region homologous to the medial ganglionic eminence (Martyniuk et al., 2007; Mueller et al., 2006; Mueller et al., 2008; Mueller and Guo, 2009; Mueller and Wullimann, 2009; Retaux et al., 2008).
Our results have significant implications for the understanding of the pallial evolution in ray-finned fish and vertebrates in general. The identification of a histogenetic unit in zebrafish that topologically corresponds to the dorsal pallium of other vertebrates is the first evidence for a dorsal pallium in ray-finned fish. A possible dorsal pallial division in teleosts has been discussed as being convergent, because there had been no signs of a dorsal pallium in non-teleost ray-finned fish (Nieuwenhuys, 2009; Northcutt, 2006; Northcutt, 2008). For example, a dorsal pallial division has not been identified in Polypterus, the most basal group of ray-finned fish (Holmes and Northcutt, 2003; Northcutt et al., 2004; Northcutt, 2008). The identification of Dc as the possible homologue of the dorsal pallium in other vertebrates changes this situation. For example, sturgeons — a second clade of basal ray-finned fish and closely related to Polypterus — do possess a central (Dc) zone homologous to the one of teleosts (Adrio et al., 2008; Northcutt, 2008; Pinuela and Northcutt, 2007). This latter finding supports the hypothesis that a dorsal pallium is an ancestral character of ray-finned fish, homologous to those of other vertebrates.
The dorsal (Dd) zone, however, has to be excluded as a possible candidate for representing either a convergent or homologous dorsal pallium in zebrafish. We believe that Dd of other teleosts than zebrafish is a composite area formed during the invagination process described in this study.
The identification of a distinct dorsal pallial division in zebrafish and its development through eversion has implications beyond evolutionary theory. For once, we provide a stable geography of the zebrafish pallium for succeeding physiological, anatomical, and behavioral studies. Our developmental paradigm of a dorsal pallial division overgrown by the medial (Dm) and the lateral (Dl) zones and our finding of tangentially migrating cells that form the posterior (Dp) zone need to be further analyzed in future studies regarding the genetic regulation of these processes in zebrafish.
4. Material and Methods
Animal Treatments
We used 20 adult zebrafish (age 6 to 12 months) from our local breeding colony at UCSF. Our zebrafish were anesthetized with tricaine methanosulfonate (MS222, Sigma), perfused with Sörensen phosphate buffer (PB, pH 7.4), and perfusion fixed with 4% paraformaldehyde (PFA, in PB). We immediately removed the brains after perfusion and postfixed them in 4% PFA for 24 to 48 hours. Then, we washed the fixed brains three times for 15 minutes with PB and then transferred them into a solution of 30% sucrose in PB (w/v) for 16 hours (overnight). On the next day, we transferred the cryoprotected brains to Tissue-Tek® O.C.T.™ and froze them at minus 20° Celsius. We analyzed three adult brains stained against NADPHd activity and five stained against parvalbumin. Larval zebrafish, that we first treated with BrdU (see BrdU-labeling) were anesthetized with tricaine methanosulfonate (MS222) for a couple of minutes and then fixed overnight with 4% PFA. For this study we analyzed seven larval brains stained against BrdU and Hu-proteins.
NADPHd-Activity Histochemistry
We performed our histochemical detection of NADPHd-activity in adult zebafish brains according to Giraldez-Perez and colleagues (Giraldez-Perez et al., 2008). We incubated the brain sections in a solution of 0.1 M Tris buffer (pH 8.0), 0.05% Triton-X-100 (Sigma-Aldrich), 1 mM beta-NADPH (Sigma-Aldrich), and 0.8 mM nitroblue tetrazolium (Sigma-Aldrich) at 37° Celsius for 1 to 2 hours. Afterwards we rinsed the slides with the brain sections three times in phosphate buffer (PB) and fixed them for 1 hour in 4% PFA. Later we dehydrated them in a graded series of ethanol and coverslipped them with Entellan (Merck).
Parvalbumin Immunostaining
We applied a monoclonal mouse antibody against parvalbumin (MAB1572, 1:2000, Millipore) on cryosectioned brain sections (thickness 18-35 μm). We used a secondary antibody (rabbit IgG) coupled with horseradish peroxidase (Elite ABC Kit, Vectastain PK-6102) and diaminobenzidine (DAB) as chromogen as described elsewhere (Mueller et al., 2004). The DAB incubation of slides with brain sections involved heavy metal intensification (50 mg DAB plus 3 ml 1% nickel-sulfate plus 3 ml 1% cobalt chloride in 200 ml PBS). After 20 minutes of preincubation, we added 600 μm of 0.3% H2O2. DAB was allowed to react with H2O2 for 20 minutes. Later, we rinsed the slides three times in PB, dehydrated them in a graded series of ethanol, and coverslipped them with Entellan (Merck).
BrdU-labeling
We incubated 12 larval zebrafish in a 10 mM solution of BrdU (dissolved in system water) for 3 to 8 days post fertilization as described elsewhere (Mueller and Wullimann, 2002). In order to cryoprotect our sections we left our fixed larvae in 30% sucrose in PB overnight and then transferred them to Tissue-Tek® O.C.T.™ and frozen them at minus 20° Celsius. Sections with a thickness of 16 μm were prepared at a cryotome. Immunohistochemistry was performed as follows: We washed our slides with sections in PBS (3×, 10 min) and then incubated them in 4 N HCl for 20 minutes at room temperature. Afterwards, we washed the sections first with PBS (3×, 10 min) and later in PBS + 0.5% Triton (2×, 5 minutes) and PBS (3×, 10 min). Then we blocked our sections in 3% bovine serum albumine (BSA) in PBS for 30 minutes and incubated them with primary antibodies (diluted in 3% BSA-PBS) overnight at 4° Celsius. The following day, we washed our sections in PBS (3×, 10 min) and in PBS + 0.1% Triton (2×, 5 min). Later, we incubated our sections with secondary antibodies (diluted in PBS + 0.1% Triton) for 2 h, followed by washes with PBS (6×, 10 min). We coverslipped our slides using Dako fluorescent mounting medium. Our primary antibodies were anti-BrdU (rat, 1:2000, Abcam) and anti-HuC/D (mouse, 1:1500, Invitrogen). Our secondary antibodies were anti-rat 568 and anti-mouse 488 (both Alexa, 1:200 dilution). We took our images using a Zeiss compound microscope and we used Adobe Photoshop CS for our image processing.
Acknowledgments
We thank Steffi Dippold and Mimi Zeiger for their editorial work and Glenn Northcutt for comments on the manuscript. This work is supported by the NIH AA016021, NS042626, UCSF Byers Award, and Sandler Family Foundation.
List of Abbreviations
- BLA
basolateral amygdala
- Ctx
cortex
- CP
caudate putamen
- D
dorsal telencephalon (pallium)
- Dc
central zone of the dorsal telencephalon
- Dd
dorsal zone of the dorsal telencephalon
- Dl
lateral zone of the dorsal telencephalon
- Dm
medial zone of the dorsal telencephalon
- DP
dorsal pallium
- Dp
posterior zone of the dorsal telencephalon
- EN
entopeduncular nucleus
- GP
globus pallidus
- hip
hippocampus
- LGE
lateral ganglionic eminence
- lot
lateral olfactory tract
- LP
lateral pallium
- LV
lateral ventricle
- MGE
medial ganglionic eminence
- MP
medial pallium
- NT
nucleus taeniae
- OB
olfactory bulb
- P
pallium
- pirCtx
piriform cortex
- PSB
pallial-subpallial boundary
- Po
preotic region
- S
subpallium
- Sep
septum
- TV
telencephalic ventricle
- V
ventral telencephalon (subpallium)
- Vd
dorsal nucleus of the ventral telencephalon
- Vl
lateral nucleus of the ventral telencephalon
- VP
ventral pallium
- Vv
ventral nucleus of the ventral telencephalon
- Y
sulcus ypsiloniformis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References cited
- Adrio F, Anadon R, Rodriguez-Moldes I. Distribution of somatostatin immunoreactive neurons and fibres in the central nervous system of a chondrostean, the Siberian sturgeon (Acipenser baeri) Brain Res. 2008;1209:92–104. doi: 10.1016/j.brainres.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Braford MR., Jr Comparative aspects of forebrain organization in the ray-finned fishes: touchstones or not? Brain Behav Evol. 1995;46:259–74. doi: 10.1159/000113278. [DOI] [PubMed] [Google Scholar]
- Braford MR., Jr Stalking the everted telencephalon: comparisons of forebrain organization in basal ray-finned fishes and teleosts. Brain Behav Evol. 2009;74:56–76. doi: 10.1159/000229013. [DOI] [PubMed] [Google Scholar]
- Butler AB. Topography and topology of the teleost telencephalon: a paradox resolved. Neurosci Lett. 2000;293:95–8. doi: 10.1016/s0304-3940(00)01497-x. [DOI] [PubMed] [Google Scholar]
- Castro A, Becerra M, Manso MJ, Anadon R. Calretinin immunoreactivity in the brain of the zebrafish, Danio rerio: distribution and comparison with some neuropeptides and neurotransmitter-synthesizing enzymes. I. Olfactory organ and forebrain. J Comp Neurol. 2006;494:435–59. doi: 10.1002/cne.20782. [DOI] [PubMed] [Google Scholar]
- Costagli A, Kapsimali M, Wilson SW, Mione M. Conserved and divergent patterns of Reelin expression in the zebrafish central nervous system. J Comp Neurol. 2002;450:73–93. doi: 10.1002/cne.10292. [DOI] [PubMed] [Google Scholar]
- Gage SP. The Wilder Quarter-Century Book. Ithaca, NY: Comstock Publ Co.; 1883. The brain of Diemyctilis viridenscens from larval to adult life an comparison with the brain of Amia and of Petromyzon; pp. 259–313. [Google Scholar]
- Giraldez-Perez RM, Gaytan SP, Ruano D, Torres B, Pasaro R. Distribution of NADPH-diaphorase and nitric oxide synthase reactivity in the central nervous system of the goldfish (Carassius auratus) J Chem Neuroanat. 2008;35:12–32. doi: 10.1016/j.jchemneu.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Gonzalez A, Northcutt RG. An immunohistochemical approach to lungfish telencephalic organization. Brain Behav Evol. 2009;74:43–55. doi: 10.1159/000229012. [DOI] [PubMed] [Google Scholar]
- Holmes PH, Northcutt RG. Connections of the pallial telencephalon in the Senegal bichir, Polypterus. Brain Behav Evol. 2003;61:113–47. doi: 10.1159/000069750. [DOI] [PubMed] [Google Scholar]
- Levine RL, Dethier S. The connections between the olfactory bulb and the brain in the goldfish. J Comp Neurol. 1985;237:427–44. doi: 10.1002/cne.902370402. [DOI] [PubMed] [Google Scholar]
- Lillesaar C, Stigloher C, Tannhauser B, Wullimann MF, Bally-Cuif L. Axonal projections originating from raphe serotonergic neurons in the developing and adult zebrafish, Danio rerio, using transgenics to visualize raphe-specific pet1 expression. J Comp Neurol. 2009;512:158–82. doi: 10.1002/cne.21887. [DOI] [PubMed] [Google Scholar]
- Martyniuk CJ, Awad R, Hurley R, Finger TE, Trudeau VL. Glutamic acid decarboxylase 65, 67, and GABA-transaminase mRNA expression and total enzyme activity in the goldfish (Carassius auratus) brain. Brain Res. 2007;1147:154–66. doi: 10.1016/j.brainres.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Medina L, Legaz I, Gonzalez G, De Castro F, Rubenstein JL, Puelles L. Expression of Dbx1, Neurogenin 2, Semaphorin 5A, Cadherin 8, and Emx1 distinguish ventral and lateral pallial histogenetic divisions in the developing mouse claustroamygdaloid complex. J Comp Neurol. 2004;474:504–23. doi: 10.1002/cne.20141. [DOI] [PubMed] [Google Scholar]
- Medina L, Abellan A. Development and evolution of the pallium. Semin Cell Dev Biol. 2009;20:698–711. doi: 10.1016/j.semcdb.2009.04.008. [DOI] [PubMed] [Google Scholar]
- Mueller T, Wullimann MF. BrdU-, neuroD (nrd)- and Hu-studies reveal unusual non-ventricular neurogenesis in the postembryonic zebrafish forebrain. Mech Dev. 2002;117:123–35. doi: 10.1016/s0925-4773(02)00194-6. [DOI] [PubMed] [Google Scholar]
- Mueller T, Vernier P, Wullimann MF. The adult central nervous cholinergic system of a neurogenetic model animal, the zebrafish Danio rerio. Brain Res. 2004;1011:156–69. doi: 10.1016/j.brainres.2004.02.073. [DOI] [PubMed] [Google Scholar]
- Mueller T, Wullimann MF. Atlas of Early Zebrafish Brain Development: A Tool for Molecular Neurogenetics. Vol. Elsevier; 2005. [Google Scholar]
- Mueller T, Vernier P, Wullimann MF. A phylotypic stage in vertebrate brain development: GABA cell patterns in zebrafish compared with mouse. J Comp Neurol. 2006;494:620–34. doi: 10.1002/cne.20824. [DOI] [PubMed] [Google Scholar]
- Mueller T, Wullimann MF, Guo S. Early teleostean basal ganglia development visualized by zebrafish Dlx2a, Lhx6, Lhx7, Tbr2 (eomesa), and GAD67 gene expression. J Comp Neurol. 2008;507:1245–57. doi: 10.1002/cne.21604. [DOI] [PubMed] [Google Scholar]
- Mueller T, Guo S. The distribution of GAD67-mRNA in the adult zebrafish (teleost) forebrain reveals a prosomeric pattern and suggests previously unidentified homologies to tetrapods. J Comp Neurol. 2009;516:553–68. doi: 10.1002/cne.22122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller T, Wullimann MF. An evolutionary interpretation of teleostean forebrain anatomy. Brain Behav Evol. 2009;74:30–42. doi: 10.1159/000229011. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuys R. The morphogenesis and the general structure of the actinopterygian forebrain. Acta Morphol Neerl Scand. 1962;5:65–78. [PubMed] [Google Scholar]
- Nieuwenhuys R. The forebrain of actinopterygians revisited. Brain Behav Evol. 2009;73:229–52. doi: 10.1159/000225622. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuys R, Meek J. The telencephalon of actinopterygian fishes. In: P A, Jones EG, editors. Comparative Aspects of Cortical Structure. Plenum Press; New York: 1990. pp. 31–73. [Google Scholar]
- Northcutt RG. Evolution of the telencephalon in nonmammals. Annu Rev Neurosci. 1981;4:301–50. doi: 10.1146/annurev.ne.04.030181.001505. [DOI] [PubMed] [Google Scholar]
- Northcutt RG, Davis RG. Fish Neurobiology, Vol 2. Higher Brain Area and Functions. University of Michigan Press; Ann Arbor, MI: 1986. Telencephalic Organization in Ray-Finned Fishes; pp. 41–98. [Google Scholar]
- Northcutt RG, Plassmann W, Holmes PH, Saidel WM. A pallial visual area in the telencephalon of the bony fish Polypterus. Brain Behav Evol. 2004;64:1–10. doi: 10.1159/000077538. [DOI] [PubMed] [Google Scholar]
- Northcutt RG. Connections of the lateral and medial divisions of the goldfish telencephalic pallium. J Comp Neurol. 2006;494:903–43. doi: 10.1002/cne.20853. [DOI] [PubMed] [Google Scholar]
- Northcutt RG. Forebrain evolution in bony fishes. Brain Res Bull. 2008;75:191–205. doi: 10.1016/j.brainresbull.2007.10.058. [DOI] [PubMed] [Google Scholar]
- Northcutt RG. Telencephalic organization in the spotted African Lungfish, Protopterus dolloi: a new cytological model. Brain Behav Evol. 2009;73:59–80. doi: 10.1159/000204963. [DOI] [PubMed] [Google Scholar]
- Pinuela C, Northcutt RG. Immunohistochemical organization of the forebrain in the white sturgeon, Acipenser transmontanus. Brain Behav Evol. 2007;69:229–53. doi: 10.1159/000099612. [DOI] [PubMed] [Google Scholar]
- Pombal MA, Megias M, Bardet SM, Puelles L. New and old thoughts on the segmental organization of the forebrain in lampreys. Brain Behav Evol. 2009;74:7–19. doi: 10.1159/000229009. [DOI] [PubMed] [Google Scholar]
- Portavella M, Vargas JP, Torres B, Salas C. The effects of telencephalic pallial lesions on spatial, temporal, and emotional learning in goldfish. Brain Res Bull. 2002;57:397–9. doi: 10.1016/s0361-9230(01)00699-2. [DOI] [PubMed] [Google Scholar]
- Portavella M, Torres B, Salas C. Avoidance response in goldfish: emotional and temporal involvement of medial and lateral telencephalic pallium. J Neurosci. 2004;24:2335–42. doi: 10.1523/JNEUROSCI.4930-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puelles L, Kuwana E, Puelles E, Bulfone A, Shimamura K, Keleher J, Smiga S, Rubenstein JL. Pallial and subpallial derivatives in the embryonic chick and mouse telencephalon, traced by the expression of the genes Dlx-2, Emx-1, Nkx-2.1, Pax-6, and Tbr-1. J Comp Neurol. 2000;424:409–38. doi: 10.1002/1096-9861(20000828)424:3<409::aid-cne3>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Rakic P. Evolution of the neocortex: a perspective from developmental biology. Nat Rev Neurosci. 2009;10:724–35. doi: 10.1038/nrn2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retaux S, Pottin K, Alunni A. Shh and forebrain evolution in the blind cavefish Astyanax mexicanus. Biol Cell. 2008;100:139–47. doi: 10.1042/BC20070084. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Moldes I. A developmental approach to forebrain organization in elasmobranchs: new perspectives on the regionalization of the telencephalon. Brain Behav Evol. 2009;74:20–9. doi: 10.1159/000229010. [DOI] [PubMed] [Google Scholar]
- Studnička FK. Zur Lösung einiger Fragen aus der Morphologie des Vorderhirnes der Cranioten. Anat Anz. 1894:307–20. [Google Scholar]
- Wicht H, Northcutt RG. Telencephalic connections in the Pacific hagfish (Eptatretus stouti), with special reference to the thalamopallial system. J Comp Neurol. 1998;395:245–60. doi: 10.1002/(sici)1096-9861(19980601)395:2<245::aid-cne8>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Wullimann MF, Rupp B, Reichert H. Neuroanatomy of the Zebrafish Brain - A Topological Atlas. Birkhäuser Verlag; Basel: 1996. [Google Scholar]
- Wullimann MF, Rink E. Detailed immunohistology of Pax6 protein and tyrosine hydroxylase in the early zebrafish brain suggests role of Pax6 gene in development of dopaminergic diencephalic neurons. Brain Res Dev Brain Res. 2001;131:173–91. doi: 10.1016/s0165-3806(01)00270-x. [DOI] [PubMed] [Google Scholar]
- Wullimann MF, Mueller T. Teleostean and mammalian forebrains contrasted: Evidence from genes to behavior. J Comp Neurol. 2004;475:143–62. doi: 10.1002/cne.20183. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Ishikawa Y, Yoshimoto M, Xue HG, Bahaxar N, Sawai N, Yang CY, Ozawa H, Ito H. A new interpretation on the homology of the teleostean telencephalon based on hodology and a new eversion model. Brain Behav Evol. 2007;69:96–104. doi: 10.1159/000095198. [DOI] [PubMed] [Google Scholar]


