Abstract
Rationale
Heart valves develop from precursor structures called cardiac cushions, an endothelial-lined cardiac jelly that resides in the inner side of the heart tube. The cushions are then invaded by cells from different sources, undergo a series of complicated and poorly understood remodeling processes, and give rise to valves. Disruption of the fibroblast growth factor (FGF) signaling axis impairs morphogenesis of the outflow tract (OFT). Yet, whether FGF signaling regulates OFT valve formation is unknown.
Objective
To study how OFT valve formation is regulated and how aberrant cell signaling causes valve defects.
Methods and results
By employing mouse genetic manipulation, cell lineage tracing, ex vivo heart culture, and molecular biology approaches, we demonstrated that FGF signaling in the OFT myocardium upregulated Bmp4 expression, which then enhanced smooth muscle differentiation of neural crest cells (NCCs) in the cushion. FGF signaling also promoted OFT myocardial cell invasion to the cushion. Disrupting FGF signaling interrupted cushion remodeling with reduced NCCs differentiation into smooth muscle and less cardiomyocyte invasion, and resulted in malformed OFT valves.
Conclusions
The results demonstrate a novel mechanism by which the FGF-BMP signaling axis regulates formation of OFT valve primordia by controlling smooth muscle differentiation of cushion NCCs.
Keywords: FGF, BMP, heart development, NCC differentiation, cardiac valve defect
Congenital valve defects occur in 2–3% of the population. Cardiac valves developed from cardiac cushions through a series of complicated and poorly defined processes. At early stages, the cardiac cushions are composed of extracellular matrix lined by endothelial cells. The cushions are invaded by endothelial-derived mesenchymal cells after completing the endothelial-mesenchymal-transition (EMT) and neural crest cells (NCCs) from pharyngeal arches 1. The cushions subsequently undergo a series of remodeling processes. A portion of the proximal cushion undergoes remodeling and forms valve primordia and their supporting apparatus. The valve primordia are then gradually transformed from primary bulbous swollen cushions into thinly tapered valve leaflets 1. Although cushion formation has been extensively studied, how valve primordia are defined and how valve remodeling is regulated remain unclear. It has been proposed that proximal outflow tract (OFT) myocardial cells receive inductive signals and migrate into the cushion. This process, termed as myocardialization 2, subsequently induces surrounding mesenchymal cells to undergo muscle differentiation. These newly differentiated myocardial cells further induce their adjacent mesenchymal cells to differentiate until the entire mesenchymal structure is muscularized. Although required for OFT septation 2, whether this process is required for valve formation is unclear.
The fibroblast growth factor (FGF) signaling axis has been shown to play important roles in OFT cushion formation. Yet, its role in OFT valve formation remains to be characterized. The FGF family consists of 18 receptor-binding members that regulate a broad spectrum of cellular activities 3, and play important roles in heart development 4. Among them, FGF4 has been shown to regulate valve precursor cell proliferation and differentiation 5–7, and FGF8 contributes to OFT valve morphogenesis 8, 9. FGF elicits its regulatory activities via activating FGF receptor (FGFR) tyrosine kinases encoded by four highly homologous genes. FGFR substrate 2α (FRS2α) is a broadly expressed adaptor protein that is required for the FGF to activate MAP and PI3 kinase pathways, the two major pathways in the FGF signaling cascade 10–12. Frs2α null embryos die between embryonic (E) 7.0–7.5 days 13. Ablation of Frs2α, or double ablation of Fgfr1/Fgfr2, in heart progenitor cells disrupts endocardial EMT and NCC deployment to the OFT, resulting in OFT alignment and septation defects 14. Ablation of Fgf8 also leads to OFT alignment and septation defects 9. Gain of function and dominant negative mutations of SHP2 that directly bind to FRS2α and mediate FGF signaling to the MAPK pathway causes enlarged valves in Noonan Syndrome and LEOPARD syndrome patients, respectively 15, 16.
In this report, we demonstrated that ablation of Frs2α, or double ablation of Fgfr1/Fgfr2, in heart progenitor cells led to enlarged OFT valves and bicuspid aortic valves (BAV). FGF regulated Bmp4 expression in the myocardium via AP1 transcription factor binding sites located upstream of the Bmp4 coding sequence. Disrupting FGF signaling diminished Bmp4 expression in the OFT myocardium and reduced smooth muscle (SM) differentiation of cushion NCCs, thus, leaving excessive undifferentiated NCC-derived mesenchymal cells within valve primordia. Treating in vitro cultured heart explants with BMP4 partially rescued the defects. The results demonstrate a novel role of NCC differentiation orchestrated by the FGF-BMP signaling axis during OFT valve formation.
Methods
All animals were housed in the Program for Animal Resources of the Institute of Biosciences and Technology, and were handled in accordance with the principles and procedures of the Guide for the Care and Use of Laboratory Animals. All experimental procedures were approved by the Institutional Animal Care and Use Committee.
Results
Disruption of FGF signaling leads to enlarged OFT valves
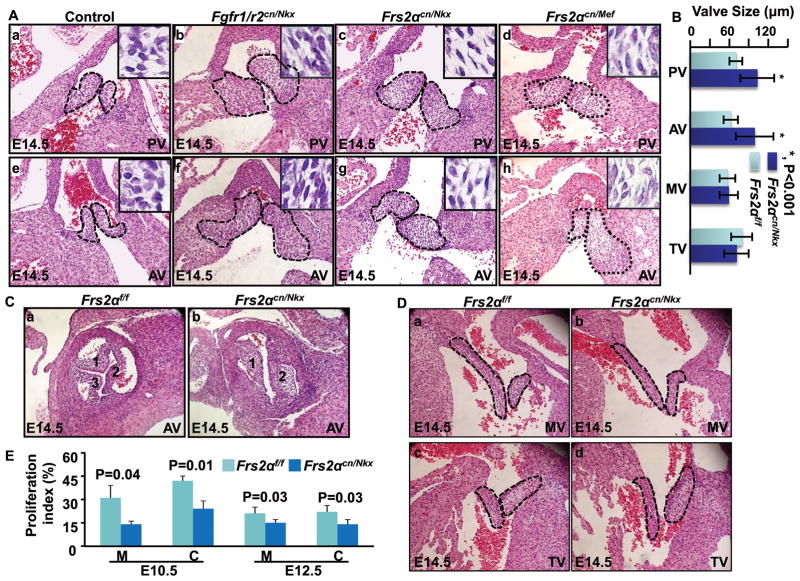
Disruption of FGF signaling in mouse second heart field (SHF) progenitors compromises OFT cushion formation and results in OFT alignment and septation defects 9, 14. Since OFT cushions also give rise to both pulmonary and aortic valves, we then investigated whether ablating FGF signaling also affected OFT valve development. H&E staining of E14.5 embryo sections revealed that Fgfr1 and Fgfr2 double conditional ablations in cardiac progenitor cells with Nkx2.5Cre (Fgfr1/r2cn/Nkx) led to enlarged OFT valves (Fig. 1A). Individual ablation of Fgfr1 or Fgfr2 failed to cause such defects (data not shown), suggesting redundant activities of the two FGFRs in regulating OFT valve development. Since FRS2α is the major adaptor protein linking the FGFR to MAP kinase and PI3K/AKT pathways, we then ablated Frs2α alleles in the same domain with Nkx2.5Cre (Frs2αcn/Nkx) or in the SHF with Mef2CCre (Frs2αcn/Mef). Similar to Fgfr1/r2cn/Nkx, both Frs2αcn/Nkx and Frs2αcn/Mef embryos exhibited enlarged OFT valves (Fig. 1A). The phenotype remained obvious in neonatal hearts (Online Figure I). The average diameters of pulmonary and aortic valves were 70±10 μm and 62±11 μm in E14.5 control embryos and 103±26 μm and 99±29 μm in Frs2αcn/Nkx embryos, respectively (Fig. 1B). About 20% of Frs2αcn/Nkx embryos had BAVs (Fig. 1C). All valve defects were associated with OFT septation defects, indicating that OFT cushion remodeling was affected. No apparent defects were found in mitral valves and tricuspid valves (Fig. 1D). Cell fate mapping experiments showed that atrioventricular valves were only composed of endothelial lineage cells, while OFT valves had both endothelium- and NCC-derived cells (Online Figure. II), suggesting that the defects were likely associated with NCC-derived mesenchymal cells.
Figure 1. Disruption of the FGF signaling axis leads to OFT valve hyperplasia.
A, Transverse sections of E14.5 embryos were H&E stained to demonstrate enlarged OFT valves. Pulmonary and aortic valves are highlighted with dotted lines. Inserts are enlarged pictures of the valve. B, The embryos were serially sectioned, and the valve dimensions were measured in every five sections. Averages of three largest values were calculated from each embryo. Data represent an average dimension of at least 10 valves and are expressed as means ± standard deviation. C, Coronal sections of embryos were H&E stained demonstrating bicuspid aortic valves in E14.5 Frs2αcn/Nkx embryos. D, Transverse sections of E14.5 embryos were H&E stained demonstrating atrioventricular valves. E, BrdU incorporation demonstrating compromised cell proliferation in Frs2αcn/Nkx OFTs. Percentages of positive cells in the OFT myocardium and cushions from four embryos were calculated and expressed as means ± standard deviations. Fgfr1/r2f/f, Fgfr1 and Fgfr2 double floxed embryos; Fgfr1/r2cnNkx, Fgfr1/Fgfr2 double conditional ablation with Nkx2.5Cre; Frs2αflox, Frs2α floxed, Frs2αcn/Nkx, Frs2α conditional ablation with Nkx2.5Cre; Frs2αcn/Mef, Frs2α conditional ablation with Mef2CCre; PV, pulmonary valve; AV, aortic valve; MV, mitral valve; TV, tricuspid valve; C, cushion; M, myocardium.
Defects in OFT cushion cell recruitment, proliferation, and apoptosis are not causal factors for enlarged valves in Frs2αcn OFT
Both Frs2αcn/Nkx and Frs2αcn/Mef embryos had enlarged OFT valves (Fig. 1A). Yet, only Frs2αcn/Nkx embryos show reduced endothelial and NCC contributions to OFT cushions and have small OFT cushions 14. Thus, the valve defect likely did not result from cushion insufficient recruitment of endothelium- and NCC-derived cells into OFT cushions. Since the enlarged Frs2αcn OFT valves had more mesenchymal cells at E14.5 day, BrdU labeling was used to determine whether mutant cushions had increased cell proliferation activities. Both mesenchymal and myocardial cells in Frs2αcn/Nkx OFT cushions actually had reduced proliferation at E10.5 and E12.5 (Fig. 1E), which was consistent with our previous report 14. This suggests that enlarged valves are not caused by proliferation defects.
To test whether the valve enlargement was caused by apoptosis defects, TUNEL assays were employed to assess cushion cell apoptosis at E13.5. Compared with control Frs2αf/f OFT cushions, apoptotic cell numbers in Frs2αcn/Nkx OFT cushions were significantly reduced (Fig. 2A, B). Consistently, Fgfr1/r2cn/Nkx OFT cushions also exhibited reduced apoptosis at E13.5 (Fig. 2C), suggesting that the FGF might be involved in regulating OFT cushion cell apoptosis. Surprisingly, most apoptotic cells were not located at the valve primordia. Instead, they were located beneath the valve primordia, suggesting that enlarged valves were not directly caused by reduced apoptosis. Moreover, Frs2αcn/Mef OFT that did not exhibit increased apoptosis in the cushion (Fig. 2D), nevertheless, still had enlarged OFT valves (Fig. 1A), further indicating that OFT valve hyperplasia was not caused by defective apoptosis. Consistent with previous findings in chicken models 17, cell lineage tracing revealed that only NCC-, but not endocardium-, derived cushion mesenchymal cells underwent apoptosis (Online Figure III).
Figure 2. Disruption of FGF signaling reduces OFT cushion cell apoptosis during remodeling.
A&B, Apoptosis in E13.5 OFT cushions. Representative sections from anterior to posterior sides were shown in A. Averages of total apoptotic cell numbers in all sections from three individuals were calculated and shown in B as means ± standard deviations. C. Apoptotic cells in Fgfr1/r2Nkx, Frs2αcn/Mef, and control embryos at E13.5.
Ablation of Frs2α causes defects in cushion myocardialization and smooth muscle differentiation, leaving excessive undifferentiated mesenchyme within the OFT valve primordia
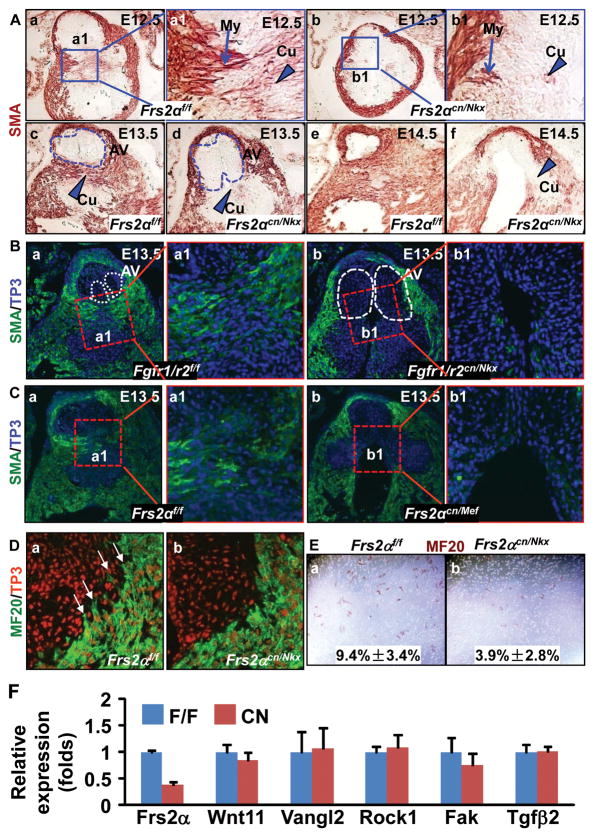
Unlike wildtype cells that were round, Frs2αcn/Nkx OFT valve cells were spindle-like (Fig. 1A, inserts), suggesting differentiation defects. We then investigated whether aberrant cushion cell differentiation caused the valve remodeling defects. During this stage, proximal OFT myocardial cells, which express SM α-actin (SMA) and sarcomeric myosin, invade the cushion and induce adjacent mesenchymal cells to undergo SM differentiation 18–20. Immunostaining with anti-SMA antibodies revealed that at E12.5, the SMA positive myocardial cells protruded into proximal OFT cushions of wildtype embryos; some cells in the cushion also expressed low level SMA (Fig. 3Aa). In Frs2αcn/Nkx embryos, however, the myocardial cells failed to extend into the cushion, and SMA expressing cushion mesenchymal cells was reduced (Fig. 3Ab), indicating defects in both myocardial cell invasion and mesenchymal cell differentiation. At E13.5, increased SMA positive cells appeared in control OFT cushions, which separated OFT cushions into two parts and, thus, defined the valve primordia (Fig. 3Ac). However, the SMA positive cells in Frs2αcn/Nkx OFT cushions were reduced and failed to separate valve primordia from the rest of the cushion, leaving excessive mesenchymal cells within the valve (Fig. 3Ad). At E14.5, myocardialization and SM differentiation of the proximal outlet septum were almost completed in control cushions; the valve was sculpted, and the aorta was separated from the right ventricle by muscular tissues (Fig. 3Ae). In contrast, the outlet septum in Frs2αcn/Nkx remained unmuscularized, and the valve still remained bulbous and swollen (Fig. 3Af). Consistently, similar defects were found in Fgfr1/r2cn/Nkx and Frs2αcn/Mef embryos (Fig. 3B&C), further confirming the role of FGF signaling in this process.
Figure 3. Ablation of the FGF signaling axis leads to defective myocardialization and SM differentiation in OFT cushions.
A-C, Transverse sections of embryos were immunostained with anti-SMA antibody demonstrating defects in myocardialization and SM differentiation. The specifically bound antibodies were visualized either with peroxidase activities (A) or fluorescent dyes (B&C). Arrows indicate migrating myocardial cells (My), and arrow heads indicate cushion mesenchymal cells (Cu) undergoing SM differentiation. Dotted lines outline the valve primordia. To-Pro3 (TP3) was used for nuclear counterstaining. D, Immunostaining with MF20 antibody revealed that the myocardial cells in control OFT were elongated and had long lamellipodia extending into the cushion mesenchyme as indicated by arrows (a); reduced lamellipodia were observed in Frs2αcn/Nkx OFT myocardial cells (b). E, Myocardial cells isolated from E13.5 OFT migrating through the membranes were identified by immunostaining with MF20 antibody (c–d). The ratios of migrated MF-20 positive cells over total MF-20 positive cells were calculated from 3 individual samples and were expressed as means ± standard deviations (e). F, Real-time RT-PCR analyses of E12.5 OFTs. Data are normalized to GAPDH and expressed as folds of changes over wide type samples.
Immunostaining with anti-sarcomeric myosin MF20 antibody was carried out to further dissect whether the unmuscularized OFT cushions were caused by defective myocardialization or SM differentiation. In control OFT, the myocardial cells next to cushions were elongated and had lamellipodia extending into the cushion mesenchyme (Fig. 3Da). In contrast, Frs2αcn/Nkx OFT myocardial cells were round and almost had no lamellipodia extending into the cushion (Fig. 3Db), suggesting compromised inward migration of OFT myocardial cells. To confirm this, cells from E13.5 proximal OFTs were isolated for transwell migration assays, and the myocardial cells that migrated through the membrane were identified by sarcomeric myosin expression. It was apparent that Frs2αcn/Nkx OFTs had fewer cells migrating through the membrane than controls (Fig. 3E), indicating that FRS2α-mediated signals promoted myocardial cell migration in a cell autonomous manner. Focal adhesion kinase (FAK) and Vangl2 mediated noncanonical Wnt signaling pathways are involved in regulating OFT myocardium migration 18, 21. However, expressions of FAK, Rock1, Vangl2, and Wnt11 were not changed in E12.5 Frs2αcn/Nkx OFT (Fig. 3F), suggesting that the FGF regulated OFT myocardium migration through other pathways.
FRS2α-mediated signals are required for regulating SM differentiation of cushion mesenchymal cells in the OFT
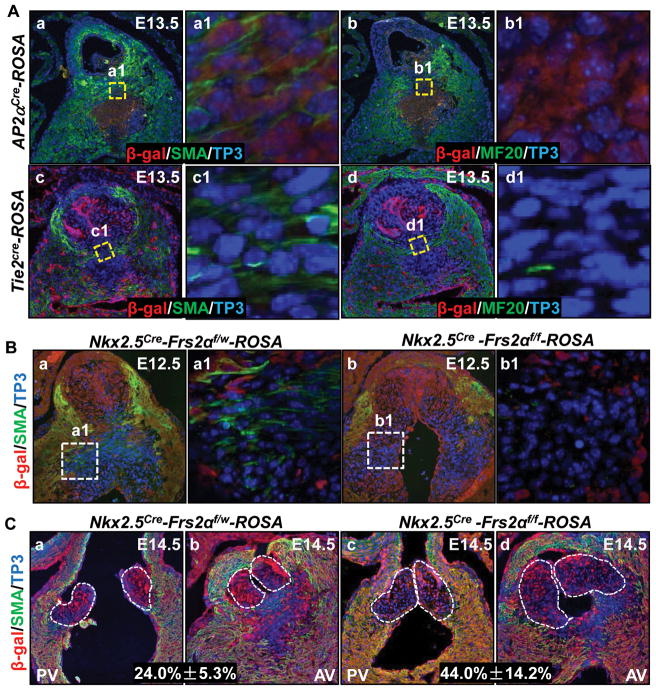
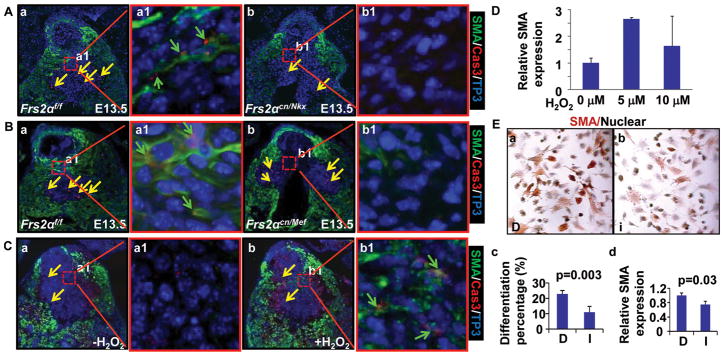
The OFT cushion mesenchyme mainly consists of both endocardium- and NCC- derived cells. To determine how FRS2α-mediated signals regulated cushion cell differentiation and the origin of cushion muscular cells, cell fate mapping was carried out with ROSA26 reporter mice 22 and Tie2cre driver that was expressed in the endocardium 23 or Ap2αcre that was expressed in NCCs 24. Immunostaining revealed that most mesenchymal cells in proximal OFT cushions were labeled by Ap2αcre, but not Tie2cre, suggestive NCC-origin of the cells. These NCC-derived cells were SMA positive and MF20 negative (Fig. 4Aa&b), whereas endothelial-derived cells were both SMA and MF20 negative (Fig. 4Ac&d), indicating that NCC-, but not endothelial-, derived cells in the OFT cushions underwent SM differentiation. All MF-20 positive cells located at the junction of the OFT myocardium and cushion were β-galactosidase negative in both Tie2cre-ROSA26 and Ap2αcre-ROSA26 mice, suggesting that these cells were neither from the endothelium nor from NCCs, but from the myocardium (Fig. 4Ab&d). Double staining of β-gal and SMA of E12.5 Nkx2.5Cre-Frs2αflox/W-ROSA26 embryos revealed that all SMA positive OFT cushion mesenchymal cells were β-gal negative (Fig. 4Ba). Since Nkx2.5Cre is expressed in both OFT myocardial and endocardial cells, the results confirmed that these SMA-positive mesenchymal cells were neither myocardial nor endocardial derived. Furthermore, since most proximal OFT cushion mesenchymal cells were NCC-origin, these β-gal negative and SMA positive cells were most likely derived from NCCs. This population of cells were severely reduced in Nkx2.5Cre-Frs2αf/f-ROSA26 OFT cushions (Fig. 4Bb), indicating that NCC differentiation was disrupted in mutant cushions. Furthermore, both β-gal and SMA negative cells in OFT valves were increased from 17.1%±5 in Nkx2.5Cre-Frs2αf/w-ROSA26 embryos to 26.7%±10 in Nkx2.5Cre-Frs2αf/f-ROSA26 embryos at E14.5 (Fig. 4C), suggesting that more undifferentiated NCCs were left in mutant than in control valve primordia. Since moderate caspase-3 activity is required for stem cell differentiation 25–27, we subsequently investigated whether caspase-3 activation levels underlie cushion mesenchymal differentiation and apoptosis. Double immunostaining revealed that wildtype OFT cushions had many mesenchymal cells exhibiting moderate cleaved caspase-3 and SMA staining at E13.5 (Fig. 5Aa, green arrows). Frs2αcn/Nkx OFT cushions had reduced caspase-3 positive (strongly and moderately) and SMA positive cells (Fig. 5A); Frs2αcn/Mef OFT cushions only had reduced moderate caspase-3 and SMA double positive cells (Fig. 5B), suggesting that apoptotic and differentiated OFT cushion cells are different subpopulations. To confirm that caspase-3 activity played a key role in cushion cell differentiation, ex vivo cultured E12.5 embryonic hearts were treated with H2O2 to induce caspase-3 activation. As expected, H2O2 treatment induced caspase-3 activation at both high and moderate levels and SMA expression (Fig. 5C). More importantly, increased SMA expression was found in cells exhibiting moderate caspase-3 activities. Real-time RT-PCR analyses also revealed that SMA expression was increased by H2O2 treatments (Fig. 5D). Consistently, inhibition of caspase-3 significantly suppressed expression of SMA in NCCs isolated from pharyngeal arches 1 and 2 of E10.5 embryos, as revealed by both immunostaining and real-time RT-PCR analyses (Fig. 5E). The results further demonstrated that appearance of moderate caspase-3 activity induced OFT cushion cells to differentiate.
Figure 4. Ablation of Frs2α in heart progenitor cells compromises NCC-derived cushion cells undergoing SM differentiation.
A, Transverse sections of embryos were immunostained with anti-β galactosidase and anti-SMA or MF20 antibodies as specified. B&C, Double immunostaining of transverse embryo sections revealed that SMA expression in β-gal negative NCCs was reduced in Frs2αcn/Nkx OFT cushions (B) and valves (C). SMA, SM α-actin. Percent of β-gal negative cells was scored from serial sections of Frs2αcn/Nkx and control OFT valves and was expressed as means ± standard variations.
Figure 5. Reduced moderate caspase-3 activation in NCC-derived cushion mesenchymal cells in the Frs2αcn/Nkx OFT.
A-C, Transverse sections of embryos were double immunostained with anti-SMA and anti-caspase-3 (cleaved) antibodies. Samples in panel C were overnight-cultured E12.5 hearts after being treated with 5 μM H2O2 for 30 min. Cells with SMA and moderate levels of caspase-3 (can only be detected with 40X or 63X objectives) are indicated with green arrows, and those with strong caspase-3 activation (can be detected with 10X objectives) were indicated with yellow arrows; Cas3, caspase-3. D, E12.5 hearts were treated with H2O2 at indicated concentrations for 30 min and then cultured overnight. Total RNA was isolated from OFTs, and SMA expression was analyzed with real-time RT-PCR. Data were normalized with GAPDH, calculated from three replicate samples, and expressed as folds of increase. E, NCCs isolated from pharyngeal arch 1 and 2 of E10.5 embryos were treated with caspase-3 inhibitor (I) or DMSO solvent (D) for 24 hours. Expression of SMA was analyzed with immunostaining with anti-SMA antibody (a-c), or with real-time RT-PCR. The percent of SMA positive cells was calculated from 5 randomly selected views. Data are means ± standard deviations of three replicate samples.
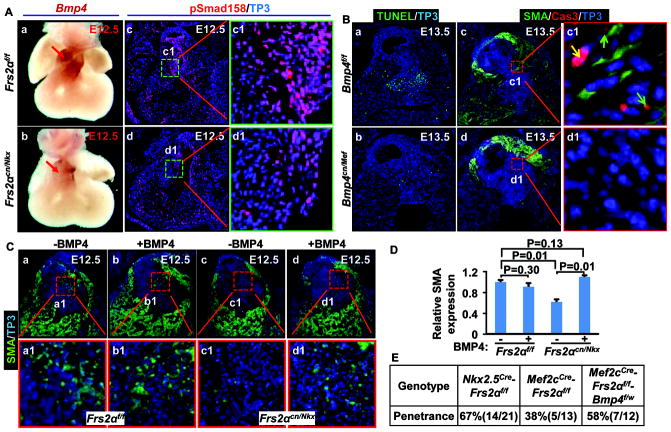
The BMP4 pathway mediates FGF signals in regulating OFT cushion cell differentiation
Since both Nkx2.5cre and Mef2ccre were not expressed in NCCs, FRS2α-mediated signals in regulating NCC-derived cushion mesenchymal differentiation must be non-cell autonomous and mediated by other signaling pathways. Furthermore, ablation of Frs2α in endothelial cells with Tie2cre did not cause apparent defects 14, therefore, the mediating signals likely were derived from the OFT myocardium. BMP4 has been shown to control NCC apoptosis 28; ablation of Bmp4 also leads to valve hyperplasia 29. Since Bmp4 expression in the SHF is regulated by FRS2α-mediated signaling pathways 14, we then tested whether BMP4 was the downstream pathway that mediated FGF signals in regulating OFT cushion remodeling. Wholemount in situ hybridization demonstrated that Bmp4 expression was reduced in the Frs2αcn/Nkx OFT myocardium at E12.5 (Fig. 6A). Consistently, phosphorylation of Smad1/5/8, downstream targets of the BMP receptor, was dramatically reduced in Frs2αcn/Nkx cushion mesenchymal cells (Fig. 6A). Ablation of Bmp4 with Mef2ccre also reduced apoptosis, expression of SMA, and activation of caspase-3 in OFT cushion mesenchymal cells (Fig. 6B). Together, it is suggested that BMP4 is the downstream mediator of FGF for regulating OFT cushion remodeling. To test this possibility, E12.5 hearts were cultured in the presence or absence of BMP4 overnight. Both immunostaining and real-time RT-PCR analyses revealed that BMP4 increased SMA expression in Frs2αcn/Nkx OFT (Fig. 6C&D) suggesting that BMP4 treatment rescued the defects. Ablation of one Bmp4 allele in Frs2αcn/Mef embryos increased the penetrance of valve hyperplasia from 38% to 58% (Fig. 6E), further suggesting that BMP4 mediated FGF signaling in regulating OFT cushion remodeling.
Figure 6. FRS2α-mediated signals regulate OFT cushion remodeling via BMP4.
A, Whole-mount in-situ hybridization with E12.5 embryos demonstrates reduced Bmp4 expression in Frs2αcn/Nkx OFT (panels a and b). Red arrows indicate Bmp4 expression. Panels c and d, transverse sections of E12.5 embryos were immunostained with antibody against phosphorylated Smad1/5/8. B, Apoptotic cells in transverse sections of E13.5 embryos were detected with TUNEL assays (a, b). Panels c and d, double immunostaining of transverse sections of E13.5 embryos with anti-SMA and anti-caspase-3 antibodies. Green arrows indicate cells with moderate caspase-3 activation and SMA expressions, and yellow arrow indicates strong caspase-3 activation. C, Embryonic hearts collected at E12.5 were cultured overnight in the presence or absence of 10 ng/ml of BMP4. Paraffin sections of the hearts were then immunostained with anti-SMA antibodies. D, Real-time RT-PCR analyses of SMA expression in ex vivo cultured OFTs. Data were normalized to GAPDH and expressed as folds of changes over untreated samples. E, Ablation of one Bmp4 allele increases the penetrance of OFT valve defects in Frs2αcn/Mef embryos.
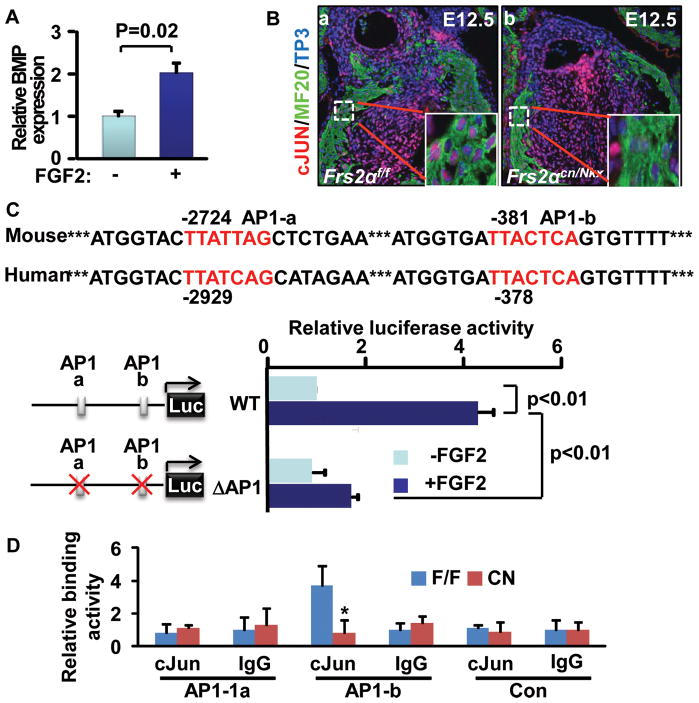
To determine whether Bmp4 expression was regulated by the FGF, in vitro cultured hearts were treated with FGF2, and the Bmp4 expression was assessed with real-time RT-PCR. The results demonstrated that treating the heart with FGF2 significantly increased Bmp4 expression (Fig. 7A). As a main downstream pathway of FRS2α, the MAP kinase cascade activates AP1 transcription activity by inducing c-Jun and c-Fos phosphorylation. Immunostaining revealed that c-Jun nuclear localization was reduced in the Frs2αcn/Nkx OFT myocardium (Fig. 7B), indicating compromised c-Jun activation. The upstream non-coding sequence of the Bmp4 allele has two candidate AP1 binding sites that potentially can be regulated by FGF signals. Thus, the 3.6 Kb upstream non-coding sequence of the Bmp4 allele was cloned into a luciferase reporter plasmid. Luciferase activity analyses revealed that FGF2 promoted expression of the reporter; deletion of the AP1 binding sites diminished the response (Fig. 7C). Furthermore, in vivo ChIP assays demonstrated that interaction of c-Jun and the proximal, but not the distal, AP1 binding site was reduced in mutant OFTs (Fig. 7D). Together, the data suggest that the FGF signaling axis in the OFT myocardium regulates Bmp4 expression via activation of AP1 to control cushion NCC differentiation during OFT cushion remodeling.
Figure 7. The FGF signaling axis regulates Bmp4 expression via activating the AP1 transcription activity.
A, E12.5 heart explants were cultured in the presence or absence of 10 ng/ml FGF2 overnight, and Bmp4 expression was analyzed with real-time RT-PCR. Data were normalized to GAPDH and expressed as folds of increase over untreated samples. B, Transverse sections of E12.5 embryos were immunostained with anti-c-JUN antibody, showing reduced c-JUN nuclear localization in the OFT myocardium of Frs2αcn/Nkx embryos. Inserts are high-magnification views of the boxed areas. OFT cushions are highlighted with dotted lines. C, AP1-binding sites in the Bmp4 allele were required for FGF2 stimulation. Panel a, luciferase reporter constructs. Panel b, luciferase reporter transfected C2C12 cells were incubated overnight in the presence or absence of 10 ng/ml FGF2, and the luciferase activity was analyzed. Data are means and standard deviation from 3 replicate wells. ΔAP, AP1 site deleted mutant. D, E12.5 hearts were subjected to ChIP analyses with anti-C-Jun antibody. Pulled-down DNA fragments were analyzed with real-time PCR and expressed as folds of changes over untreated samples.
Discussion
In this report, we demonstrated that FRS2α-mediated signaling in the myocardium regulated OFT cushion remodeling and defined valve primordia via BMP4. Ablating this signaling axis disrupted cushion myocardialization and SM differentiation, and caused valve enlargement. The results demonstrate a novel FGF-BMP signaling axis from the OFT myocardium to the cushion mesenchyme regulating OFT cushion NCC differentiation, which plays a key role in OFT valve primordium formation. The findings are rather surprising since previous studies show that ablation of FGF signaling leads to small OFT cushions, which give rise to the OFT valves and septum 9, 14.
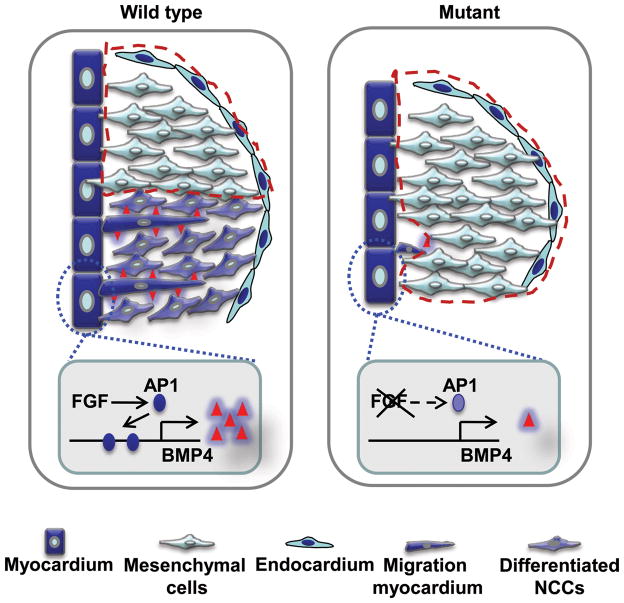
We further demonstrated that the FGF promoted NCC differentiation non-cell autonomously via BMP4. The SM differentiation of cushion mesenchymal cells separated them into two populations: one underwent SM differentiation and contributed to OFT septation; the other did not undergo muscle differentiation and contributed to OFT valves (Fig. 8). At E14.5, only a few undifferentiated NCCs remained in WT valves. In mutant mice, however, the disrupted SM differentiation left many undifferentiated NCC-derived mesenchymal cells in the cushions and caused enlarged valves, even though total cushion mesenchymal cells were reduced (Fig. 8). In some severe cases that had OFT septation defects, the aortic and pulmonary valve primordia were not separated, resulting in a large fused valve.
Figure 8. A working model of FGF signaling in OFT valve formation.
Defective cushion NCC differentiation fails to define valve primordia and leaves excessive cells within valve primordia, which results in a large valve. Valve primordia are outlined with red lines.
Although it has been reported that mature OFT valve cells are largely derived from the endocardium (de Lange et al., 2004; Jiang et al., 2000), the part of OFT cushions that gave rise to valves and the underlying region were mainly comprised of NCC-derived mesenchymal cells at E13.5 (Fig. S2). These NCC lineage cells then either underwent apoptosis or SM differentiation (Figs. 4&5), leaving mainly endothelial lineage cells in OFT valves. The data are consistent with previous findings that no endocardial lineage cells in OFT valves undergo apoptosis and that the endocardial lineage replaces NCCs during late stages of valve formation 30. Frs2αcn/Nkx OFT valves had significantly increased non-Nkx2.5 lineage cells (Fig. 4C), suggesting that more NCC-lineage contributed to mutant OFT valves. Unlike OFT cushions, AV cushions only had the endocardium lineage mesenchymal cells (Fig. S2). This likely explains why AV valves were not affected by ablation of FGF signaling.
Mutations in SM α-actin are associated with familial BAV defects 31, implying that defective SM differentiation of NCCs may contribute to BAV. However, although NCC differentiation was disrupted in both Frs2αcn/Nkx and Frs2αcn/Mef OFT cushions, only Frs2αcn/Nkx embryos exhibited BAV defects, indicating that BAV defects in Frs2α mutants were not caused by disrupted NCC differentiation. Since cushion mesenchymal apoptosis was disrupted in Frs2αcn/Nkx, but not Frs2αcn/Mef, OFT cushions, it is possible that reduced apoptosis contributed to failure in separating the valve leaflets from each other.
FRS2α has four GRB2 and two SHP2 binding sites. SHP2 is a ubiquitously expressed protein tyrosine phosphatase that plays a central role in the MAP kinase and other signaling pathways 32, 33. Gain-of-function of SHP2 enhances MAPK activation and promotes the EMT and mesenchymal cell proliferation; dominant negative SHP2 suppresses MAPK activation and fails to arrest the proliferation of mesenchymal cells at later stages 34. Thus, a narrow range of MAPK activity is required for embryonic development. Ablation of Frs2α in heart progenitor cells reduces MAPK activation in the myocardium. Here, we demonstrated that deletion of Frs2α led to reduced AP1 transcription activity, resulting in reduced Bmp4 expression in the myocardium, inhibited cushion NCC differentiation, and caused valve hyperplasia. The results reveal a novel mechanism that FGF signals in the OFT myocardium negatively regulate the valve size through promotion of NCC differentiation.
The regulatory mechanism underlying SM differentiation of OFT cushion NCCs remains poorly understood. It has been shown that transient and moderate caspase-3 activities promote stem cell differentiation 25. Consistently, only those cells with moderate caspase-3 activity in OFT cushions undergo SM differentiation. Ablation of Frs2α compromised BMP4 signaling in OFT cushion cells and reduced the population of cells with SMA expression and moderate caspase-3 activity. Therefore, the results suggest that BMP4 is an upstream regulator for caspase-3 activation in OFT cushion NCCs. It remains unanswered why these cushion mesenchymal cells have different caspase-3 activities. One possibility is that differentiated NCCs are insensitive to BMP4 stimulation, and undifferentiated NCCs are BMP sensitive and will undergo apoptosis when they receive sustained BMP4 stimulation. However, further experimentation is needed to test this hypothesis.
In conclusion, we demonstrated a novel FGF-BMP signaling axis between the OFT myocardium and cushion mesenchyme that regulated OFT cushion myocardialization and SM differentiation during cushion remodeling and that the two processes play an important role in defining OFT valve primordia. The results shed new light on understanding how perturbation of cell signaling and NCC differentiation lead to congenital heart valve defects.
Supplementary Material
Acknowledgments
We thank Drs. Juha Partanen and David Ornitz for their generosity to share the Fgfr1- floxed and Fgfr2-floxed mice, and Mary Cole for critical reading of the manuscript.
Source of Funding
NIH-CA96824 from the NCI to FW, 2R01DE12324 and R01HL093484 to JFM, and AHA0655077Y to FW and 09PRE2010130 from the American Heart Association to JZ.
Abbreviations footnote
- BMP
bone morphogenetic proteins
- EMT
endothelial-mesenchymal transition
- FGF
fibroblast growth factor
- FGFR
FGF receptor
- FRS2α
FGFR substrate 2α
- FHF
first heart field
- NCCs
neural crest cells
- OFT
outflow tract
- SHF
second heart field
- SM
smooth muscle
- SMA
smooth muscle actin
Footnotes
Disclosures
None
References
- 1.Armstrong EJ, Bischoff J. Heart valve development: endothelial cell signaling and differentiation. Circulation research. 2004;95:459–470. doi: 10.1161/01.RES.0000141146.95728.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van den Hoff MJ, Kruithof BP, Moorman AF. Making more heart muscle. Bioessays. 2004;26:248–261. doi: 10.1002/bies.20006. [DOI] [PubMed] [Google Scholar]
- 3.McKeehan WL, Wang F, Luo Y. Handbook of Cell Signaling. 2. I. New York: Academic/Elsevier Press; 2009. The fibroblast growth factor (FGF) signaling complex. [Google Scholar]
- 4.Chien KR, Domian IJ, Parker KK. Cardiogenesis and the complex biology of regenerative cardiovascular medicine. Science (New York, NY) 2008;322:1494–1497. doi: 10.1126/science.1163267. [DOI] [PubMed] [Google Scholar]
- 5.Lincoln J, Alfieri CM, Yutzey KE. BMP and FGF regulatory pathways control cell lineage diversification of heart valve precursor cells. Developmental biology. 2006;292:292–302. doi: 10.1016/j.ydbio.2005.12.042. [DOI] [PubMed] [Google Scholar]
- 6.Sugi Y, Ito N, Szebenyi G, Myers K, Fallon JF, Mikawa T, Markwald RR. Fibroblast growth factor (FGF)-4 can induce proliferation of cardiac cushion mesenchymal cells during early valve leaflet formation. Developmental biology. 2003;258:252–263. doi: 10.1016/s0012-1606(03)00099-x. [DOI] [PubMed] [Google Scholar]
- 7.Zhao B, Etter L, Hinton RB, Jr, Benson DW. BMP and FGF regulatory pathways in semilunar valve precursor cells. Dev Dyn. 2007;236:971–980. doi: 10.1002/dvdy.21097. [DOI] [PubMed] [Google Scholar]
- 8.Ilagan R, Abu-Issa R, Brown D, Yang YP, Jiao K, Schwartz RJ, Klingensmith J, Meyers EN. Fgf8 is required for anterior heart field development. Development (Cambridge, England) 2006;133:2435–2445. doi: 10.1242/dev.02408. [DOI] [PubMed] [Google Scholar]
- 9.Park EJ, Watanabe Y, Smyth G, Miyagawa-Tomita S, Meyers E, Klingensmith J, Camenisch T, Buckingham M, Moon AM. An FGF autocrine loop initiated in second heart field mesoderm regulates morphogenesis at the arterial pole of the heart. Development. 2008;135:3599–3610. doi: 10.1242/dev.025437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kouhara H, Hadari YR, Spivak-Kroizman T, Schilling J, Bar-Sagi D, Lax I, Schlessinger J. A lipid-anchored Grb2-binding protein that links FGF-receptor activation to the Ras/MAPK signaling pathway. Cell. 1997;89:693–702. doi: 10.1016/s0092-8674(00)80252-4. [DOI] [PubMed] [Google Scholar]
- 11.Xu H, Lee KW, Goldfarb M. Novel recognition motif on fibroblast growth factor receptor mediates direct association and activation of SNT adapter proteins. J Biol Chem. 1998;273:17987–17990. doi: 10.1074/jbc.273.29.17987. [DOI] [PubMed] [Google Scholar]
- 12.Ong SH, Lim YP, Low BC, Guy GR. SHP2 associates directly with tyrosine phosphorylated p90 (SNT) protein in FGF-stimulated cells. Biochemical and biophysical research communications. 1997;238:261–266. doi: 10.1006/bbrc.1997.7272. [DOI] [PubMed] [Google Scholar]
- 13.Hadari YR, Gotoh N, Kouhara H, Lax I, Schlessinger J. Critical role for the docking-protein FRS2 alpha in FGF receptor-mediated signal transduction pathways. Proc Natl Acad Sci U S A. 2001;98:8578–8583. doi: 10.1073/pnas.161259898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J, Lin Y, Zhang Y, Lan Y, Lin C, Moon AM, Schwartz RJ, Martin JF, Wang F. Frs2alpha-deficiency in cardiac progenitors disrupts a subset of FGF signals required for outflow tract morphogenesis. Development (Cambridge, England) 2008;135:3611–3622. doi: 10.1242/dev.025361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen B, Bronson RT, Klaman LD, Hampton TG, Wang JF, Green PJ, Magnuson T, Douglas PS, Morgan JP, Neel BG. Mice mutant for Egfr and Shp2 have defective cardiac semilunar valvulogenesis. Nat Genet. 2000;24:296–299. doi: 10.1038/73528. [DOI] [PubMed] [Google Scholar]
- 16.Krenz M, Yutzey KE, Robbins J. Noonan syndrome mutation Q79R in Shp2 increases proliferation of valve primordia mesenchymal cells via extracellular signal-regulated kinase 1/2 signaling. Circulation research. 2005;97:813–820. doi: 10.1161/01.RES.0000186194.06514.b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poelmann RE, Mikawa T, Gittenberger-de Groot AC. Neural crest cells in outflow tract septation of the embryonic chicken heart: differentiation and apoptosis. Dev Dyn. 1998;212:373–384. doi: 10.1002/(SICI)1097-0177(199807)212:3<373::AID-AJA5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 18.Phillips HM, Murdoch JN, Chaudhry B, Copp AJ, Henderson DJ. Vangl2 acts via RhoA signaling to regulate polarized cell movements during development of the proximal outflow tract. Circulation research. 2005;96:292–299. doi: 10.1161/01.RES.0000154912.08695.88. [DOI] [PubMed] [Google Scholar]
- 19.van den Hoff MJ, Moorman AF, Ruijter JM, Lamers WH, Bennington RW, Markwald RR, Wessels A. Myocardialization of the cardiac outflow tract. Developmental biology. 1999;212:477–490. doi: 10.1006/dbio.1999.9366. [DOI] [PubMed] [Google Scholar]
- 20.van den Hoff MJ, Kruithof BP, Moorman AF, Markwald RR, Wessels A. Formation of myocardium after the initial development of the linear heart tube. Developmental biology. 2001;240:61–76. doi: 10.1006/dbio.2001.0449. [DOI] [PubMed] [Google Scholar]
- 21.Hakim ZS, DiMichele LA, Doherty JT, Homeister JW, Beggs HE, Reichardt LF, Schwartz RJ, Brackhan J, Smithies O, Mack CP, Taylor JM. Conditional deletion of focal adhesion kinase leads to defects in ventricular septation and outflow tract alignment. Molecular and cellular biology. 2007;27:5352–5364. doi: 10.1128/MCB.00068-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 23.Kisanuki YY, Hammer RE, Miyazaki J, Williams SC, Richardson JA, Yanagisawa M. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Developmental biology. 2001;230:230–242. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- 24.Macatee TL, Hammond BP, Arenkiel BR, Francis L, Frank DU, Moon AM. Ablation of specific expression domains reveals discrete functions of ectoderm-and endoderm-derived FGF8 during cardiovascular and pharyngeal development. Development (Cambridge, England) 2003;130:6361–6374. doi: 10.1242/dev.00850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abdul-Ghani M, Megeney LA. Rehabilitation of a contract killer: caspase-3 directs stem cell differentiation. Cell stem cell. 2008;2:515–516. doi: 10.1016/j.stem.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 26.Janzen V, Fleming HE, Riedt T, Karlsson G, Riese MJ, Lo Celso C, Reynolds G, Milne CD, Paige CJ, Karlsson S, Woo M, Scadden DT. Hematopoietic stem cell responsiveness to exogenous signals is limited by caspase-3. Cell stem cell. 2008;2:584–594. doi: 10.1016/j.stem.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fujita J, Crane AM, Souza MK, Dejosez M, Kyba M, Flavell RA, Thomson JA, Zwaka TP. Caspase activity mediates the differentiation of embryonic stem cells. Cell stem cell. 2008;2:595–601. doi: 10.1016/j.stem.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Graham A, Francis-West P, Brickell P, Lumsden A. The signalling molecule BMP4 mediates apoptosis in the rhombencephalic neural crest. Nature. 1994;372:684–686. doi: 10.1038/372684a0. [DOI] [PubMed] [Google Scholar]
- 29.McCulley DJ, Kang JO, Martin JF, Black BL. BMP4 is required in the anterior heart field and its derivatives for endocardial cushion remodeling, outflow tract septation, and semilunar valve development. Dev Dyn. 2008;237:3200–3209. doi: 10.1002/dvdy.21743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Lange FJ, Moorman AF, Anderson RH, Manner J, Soufan AT, de Gier-de Vries C, Schneider MD, Webb S, van den Hoff MJ, Christoffels VM. Lineage and morphogenetic analysis of the cardiac valves. Circulation research. 2004;95:645–654. doi: 10.1161/01.RES.0000141429.13560.cb. [DOI] [PubMed] [Google Scholar]
- 31.Guo DC, Pannu H, Tran-Fadulu V, Papke CL, Yu RK, Avidan N, Bourgeois S, Estrera AL, Safi HJ, Sparks E, Amor D, Ades L, McConnell V, Willoughby CE, Abuelo D, Willing M, Lewis RA, Kim DH, Scherer S, Tung PP, Ahn C, Buja LM, Raman CS, Shete SS, Milewicz DM. Mutations in smooth muscle alpha-actin (ACTA2) lead to thoracic aortic aneurysms and dissections. Nature genetics. 2007;39:1488–1493. doi: 10.1038/ng.2007.6. [DOI] [PubMed] [Google Scholar]
- 32.Chan G, Kalaitzidis D, Neel BG. The tyrosine phosphatase Shp2 (PTPN11) in cancer. Cancer metastasis reviews. 2008;27:179–192. doi: 10.1007/s10555-008-9126-y. [DOI] [PubMed] [Google Scholar]
- 33.Feng GS. Shp2-mediated molecular signaling in control of embryonic stem cell self-renewal and differentiation. Cell research. 2007;17:37–41. doi: 10.1038/sj.cr.7310140. [DOI] [PubMed] [Google Scholar]
- 34.Edouard T, Montagner A, Dance M, Conte F, Yart A, Parfait B, Tauber M, Salles JP, Raynal P. How do Shp2 mutations that oppositely influence its biochemical activity result in syndromes with overlapping symptoms? Cell Mol Life Sci. 2007;64:1585–1590. doi: 10.1007/s00018-007-6509-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.