Abstract
A series of charge-reversal amphiphiles with different spacers separating the head group from the hydrophobic chains are described for delivery of DNA and siRNA. Among them, the amphiphiles possessing a glycine spacer (e.g., B-GlyGly) showed effective DNA transfection in CHO and NIH 3T3 cells as well as siRNA gene knockdown in HepG2 and UASMC cells. Ethidium bromide quenching assays revealed that DNA was released the fastest from the lipoplex of B-GlyGly in the presence of esterase. Also, X-ray diffraction results indicated that the DNA was located between the adjacent lipid bilayers in the lipoplex of B-GlyGly. These distinct features appear to be required for high transfection activity.
Introduction
Gene therapy has received significant attention in the past decade due to its advantages over traditional therapies. The two most common methods for gene delivery use either synthetic (1-5) or viral(6-10) vectors. Compared to viral vectors, non-viral vectors have lower toxicity, high load capacity and are easy to synthesize. Consequently, there has been a significant effort to develop and evaluate non-viral vectors, which include cationic amphiphiles and polymers(11-15) and more recently anionic amphiphiles.(16) Yet, synthetic vectors also have a number of limitations including low transfection efficiencies both in vitro and in vivo, significant cytotoxicity, and inactivation in the presence of serum. To overcome these limitations, investigators are modifying the amphiphile structure and, for small cationic amphiphiles, this entails alternative cationic head groups, linkers, and hydrophobic moieties. Examples of different cationic head groups explored include choline,(17, 18) spermine,(19) di- and tri-peptides,(20, 21) carbohydrates,(22, 23) and nucleosides.(24-32) With regards to the hydrophobic moiety, long chain ester and ether linked hydrocarbons have been extensively studied. The fluorinated analogues of cationic lipids, like DOTMA, have also been explored and these amphiphiles exhibited higher transfection activities in vitro (33) and in vivo compared to their hydrocarbon analogs.(34) In addition, researchers have used combinatorial approaches to screen large libraries of amphiphiles in order to identify key structural components of the amphiphile responsible for high transfection activity.(35, 36)
Although significant research has been conducted regarding the structure-property relationships of a wide range of cationic lipids, only a few studies can be found that systematically investigate the effect of spacers within the head group, and include cationic lipids derived from betaine glycine,(37) cationic glycolipids,(38) and cholesterol-based cationic lipids.(39) These studies, along with others, have begun to provide design parameters for optimizing the chemical structure for efficient nucleic acid delivery with minimal cytotoxicity.
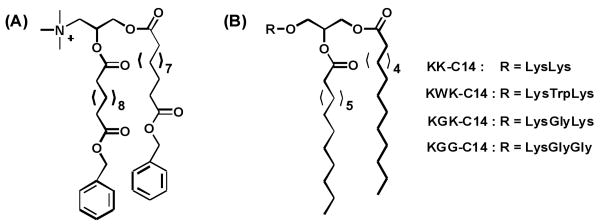
In previous research in our lab, we found that a charge-reversal amphiphile (Figure 1A) which possesses benzyl esters at the end of alkyl chains showed high transfection activity.(40) The total charge of this amphiphile was capable of switching from +1 to -1 when the benzyl esters are hydrolyzed. This charge-reversal effect was implemented to facilitate the release of DNA from the amphiphiles. In a separate study, we also found that spacing of the cationic charges within the headgroup in a series of peptide-based amphiphiles can afford improved transfection performance with amphiphiles KWK-C14, KGK-C14 and KGG-C14 showing higher transfection compared to KK-C14 (Figure 1B).(21) To take advantage of the charge-reversal effect, as well as to further understand how spacer length and composition affect transfection efficacy, here we present a study on a series of charge-reversal amphiphiles with different spacers separating the head group from the hydrophobic chains.
Figure 1.
Amphiphiles previously studied.
All the amphiphiles studied (Figure 2) possess a quaternary ammonium as a cationic head group (B) and the same alkyl chains ending with benzyl esters, but have spacers of different lengths: two, five or seven atoms. The nature of the spacers is also varied: 1) the glycine and alaine spacers are relatively rigid, hydrophilic and capable of hydrogen bonding (B-Gly, B-GlyGly, B-AlaAla; 2) the ethylene oxide spacers are flexible and hydrophilic (B-EO, B-(EO)2); and 3) the alkyl chain spacers are flexible and hydrophobic (B-(CH2)4, B-(CH2)7).
Figure 2.
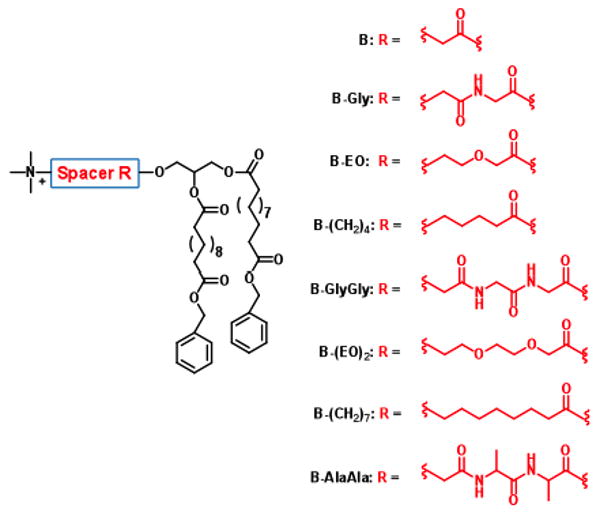
Amphiphiles with different spacers.
Results and Discussion
The synthetic routes to the different amphiphiles can be found in Scheme 1 and a detailed procedure can be found in the SI. In general, the different spacers containing the head group were first synthesized and then coupled to 1 or 2 to afford the desired products. As a representative example, the synthesis of the B-GlyGly amphiphile is described next. N,N-dimethylglycine hydrochloride and glycine benzyl ester hydrochloride were reacted in the presence of DCC and DMAP at RT for 16h. Benzyl 2-(2-(dimethylamino)acetamido)acetate was purified as yellow oil (76%). The benzyl group was removed in the presence of a catalytic amount of Pd(II) on carbon at RT under 50psi H2 for 16h to afford the intermediate, in 84% yield. The deprotected 2-(2-(dimethylamino)acetamido)acetic acid was then coupled with 2 followed by reaction with CH3I to afford the B-GlyGly amphiphile as an viscous oil in 80% yield. The characterization data for B-GlyGly, as well as all of the amphiphiles, can be found in the SI.
Scheme 1.
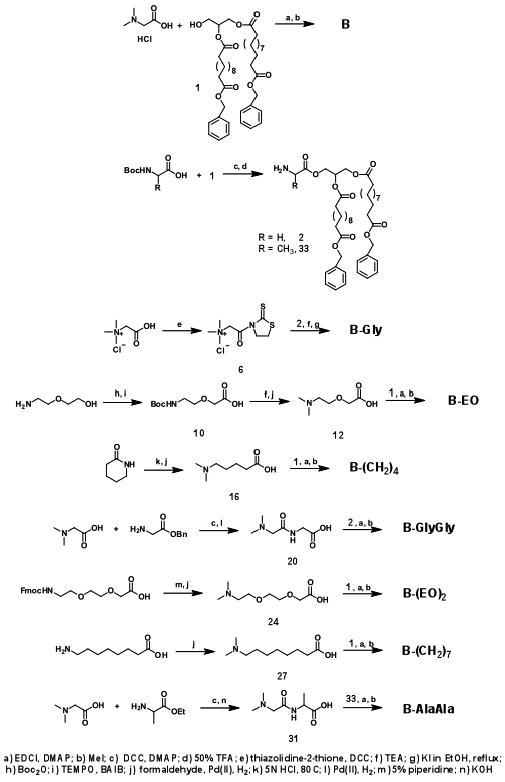
Synthesis of the cationic amphiphiles.
To determine whether the amphiphiles would bind DNA, a standard ethidium bromide (EtBr)-DNA fluorescence quenching assay was performed, as shown in Figure 3 (top). DNA binding was observed for all the amphiphiles with similar affinity. Among the amphiphiles, B-(CH2)4 and B-(CH2)7 showed the strongest binding affinity with a +/- charge ratio of 6:1, and B bound the weakest with a charge ratio of 9:1. Next, the above DNA/EtBr/amphiphile solution was incubated with esterase at pH 7.4 (100mM Tris, 100mM NaCl buffer, 300 units/mL). The intensity of fluorescence, which reflects the dissociation of the amphiphile from the DNA as a consequence of the hydrolysis of the terminal benzyl esters, increased at slightly different rates for the amphiphiles, as shown in Figure 3 (bottom). DNA was released the fastest from the B-GlyGly complex with around 30% fluorescence recovered in the first 30 minutes, while less than 10% fluorescence recovery was observed for all the other complexes. During the 4 hour period, fluorescence intensity increased slowly and reached ∼20-35% for all other complexes, except for B where the fluorescence did not change significantly.
Figure 3.
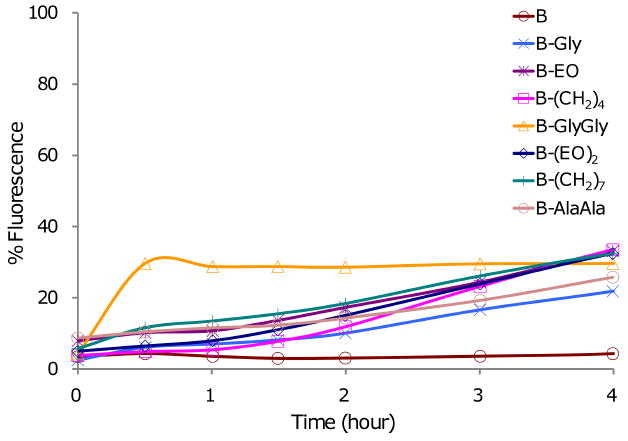
EtBr displacement assay showing the fluorescence intensity. Top: as a function of amphiphile/DNA charge ratio; bottom: as a function of time in presence of porcine liver esterase (300 units/mL).
Given their polar headgroup and long hydrophobic alkyl chains, these amphiphiles are likely to form bilayer vesicles in aqueous solution. Differential scanning calorimetry (DSC) traces of hydrated amphiphiles showed phase-transition temperatures between ≈ -22 and -15 °C, except for B which was 60.6°C (Table 1). We subsequently prepared liposome solutions of all the amphiphiles by sonicating a hydrated film of the amphiphile in an aqueous solution (see SI for details). Dynamic light scattering measurements revealed that the sizes of liposomes in the presence of DNA varied from 154 to 353 nm in pH 7.4 solution (100 mM NaCl, 100 mM Tris buffer). The stability of the liposomes over time was examined by measuring their sizes at 0, 1, 2 and 4 h (Figure 4 top). The size of liposomes formed from B-Gly, B-(EO)2 and B-(CH2)7 remained almost constant during the 4 h period, while those of other amphiphiles increased moderately. Given that these increasing rates are very small, all the liposomes appeared to be stable.
Table 1.
Phase-transition temperatures of the amphiphiles and repeat periods without and with DNA.
| Amphiphiles | Tm (°C) | Repeating period (nm) | |
|---|---|---|---|
| without DNA | with DNA | ||
| B | 60.6 | 5.9 | 6.2 |
| B-Gly | -15.4 | 5.4 | 5.6 |
| B-EO | -22.2 | 5.7 | 6.7 |
| B-(CH2)4 | -17.3 | 7.6 | 7.8 |
| B-GlyGly | -16.8 | 6.1 | 7.9 |
| B-(EO)2 | -19.6 | 6.4 | 6.5 |
| B-(CH2)7 | -14.8 | 1.4 | 5.8 |
| B-AlaAla | -19.3 | 6.4 | 7.0 |
Figure 4.
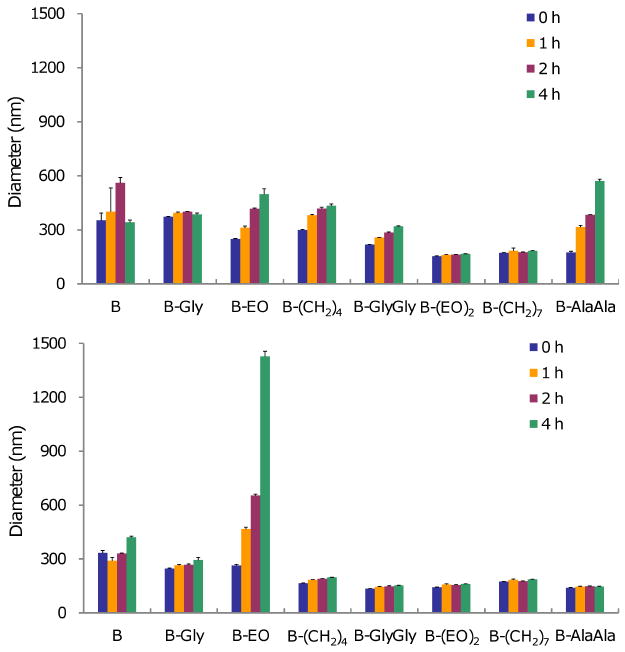
Sizes of lipoplexes (nm) at pH 7.4 (top) and pH 5 (bottom). N=3, mean±SD
Since endocytosis is one of the major cell uptake pathways for internalization of nonviral vectors,(41-44) liposomes were incubated in a solution (100 mM NaCl, 100 mM phosphate buffer) of pH 5, which resembles the pH of the endosome, and analyzed at 0, 1, 2, 4 h (Figure 4 bottom). Generally, the size of liposomes at pH 5 is slightly smaller than those at pH 7.4. During the 4 hour period, all the liposomes were stable except for the liposome formed with B-EO, which quickly increased in size. The size increased from 264 to 468 nm in 1 h, and further to 655 and 1428 nm in 2 and 4 h. These findings demonstrated that the liposomes formed from the amphiphiles were stable under pH 7.4 and mildly acidic conditions, except those formed from B-EO.
Next, to characterize the supramolecular structures, X-ray diffraction experiments were performed at 22°C on all of the samples in the absence and presence of DNA. A hydrated vesicle pellet was obtained for all samples and oriented multilayers were prepared for X-ray analysis by placing the pellets on a curved glass substrate and incubating at 66% relative humidity. All of the samples showed multiple reflections indexing as orders of lamellar repeat periods, characteristic of lipid bilayers (Table 1).
The repeat periods of all samples in the absence of DNA were between 5.4 and 7.6 nm, except for B-(CH2)7 which had a period of 1.4 nm. This period was abnormally small and could be a higher order reflection from a larger repeat period. The addition of DNA increased the repeat period for all of the assemblies. A smaller increase between 0.1 and 0.6 nm was observed for B, B-Gly, B-(CH2)4, B-(EO)2, and B-AlaAla. While, for B-EO and B-GlyGly, we observed an increase in the period of 1.0 & 1.8 nm, respectively. This larger change would be consistent with a structural model where a smectic phase was formed with the DNA chains located between the adjacent lipid bilayers within the multilamellar liposome. This structural model has been reported for complexes of DNA with DOTAP(45) and cationictriesters of phosphatidylcholine.(46) For the other samples, the repeat periods did not change significantly, indicating that DNA was not located within the lipid multilayers.
Transfection experiments using reporter gene, β-galactosidase (β-gal, pVax-LacZ1, Invitrogen) were performed with CHO and NIH 3T3 cells (Figure 5). Gene transfection results were determined after 48 h as a function of cation/anion ratios (4:1, 8:1, 12:1, 16:1).
Figure 5.
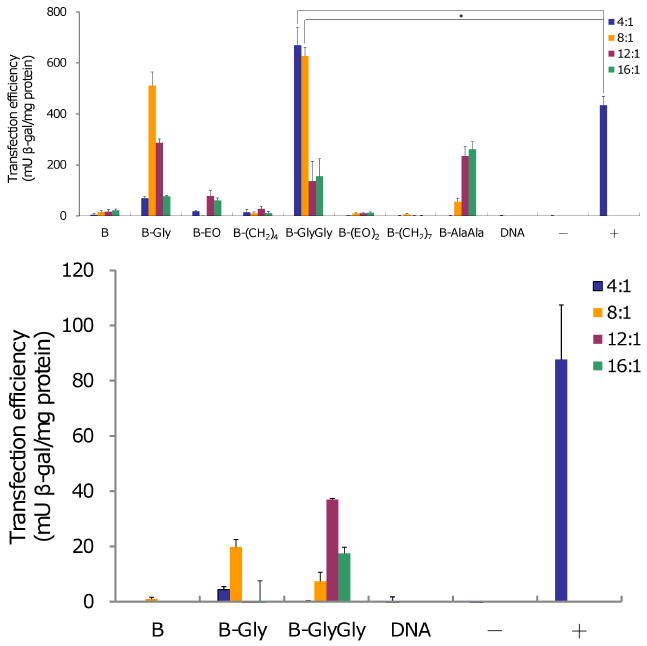
DNA transfection after 48 h in CHO (top) and NIH3T3 (bottom) cells as a function of amphiphiles and DNA molar ratio. Lipofectamine™ 2000 was used as positive control. N=3, mean±SD. * p<0.05
In CHO cells, amphiphiles B-Gly and B-GlyGly showed the highest transfection activity, amphiphile B-AlaAla exhibited moderate activity, and the other amphiphiles showed minimal transfection efficiency. B-GlyGly was significantly more active than the positive control, Lipofectamine™ 2000. However, both the amphiphiles B-Gly and B-GlyGly were less active than Lipofectamine™ 2000 in NIH 3T3 cells, but still had higher transfection efficiency compared to the other amphiphiles we tested. These results showed that those amphiphiles containing spacers that are hydrophilic, relatively rigid, and capable of hydrogen-bonding possessed the highest transfection activity. We also prepared the B-AlaAla amphiphile since this amphiphile is similar to the B-GlyGly amphiphile but the spacer is slightly more hydrophobic and rigid. The B-AlaAla amphiphile showed moderate transfection activity but less than that observed with the B-GlyGly amphiphile. To confirm that the benzyl-ester linkages on the hydrophobic chains (and the resulting charge-reversal effect) was a contributor to the gene delivery efficiency of these amphiphiles, we synthesized two additional control amphiphiles with the Gly or GlyGly spacers, possessing saturated C16 chains instead of the benzyl ester terminated hydrophobic chains. As shown in Figure 6, both amphiphiles displayed minimum transfection activity in CHO cells. This result demonstrated the importance of charge-reversal effect on the transfection. MTS assays performed with all the amphiphiles showed no significant cytotoxicity in the two cell lines (see SI).
Figure 6.
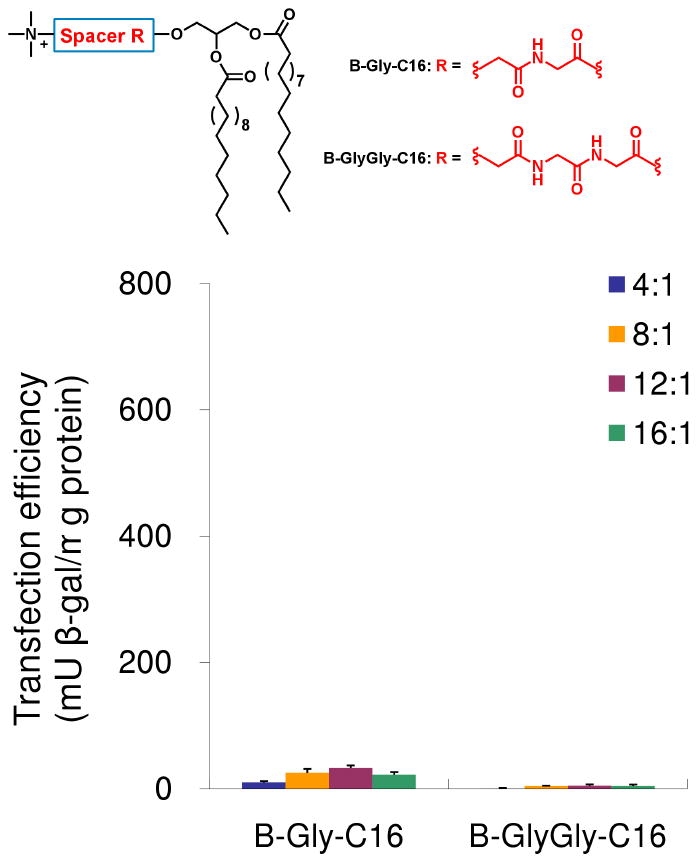
Structures of B-Gly-C16 and B-GlyGly-C16 and DNA transfection after 48 h in CHO cells as a function of amphiphiles and DNA molar ratio. Lipofectamine™ 2000 was used as positive control. N=3, mean±SD
Next, siRNA transfections were performed with the base amphiphiles B and the two best performing amphiphiles, B-Gly and B-GlyGly, using the KDalert™ GAPDH assay (Ambion) with HepG2 and UASMC cells (Figure 7) at four different cation/anion ratios (1:1, 5:1, 10:1, 15:1). NeoFX was used as the positive control. After 48 h transfection, in HepG2 cells, B and B-Gly achieved 50% gene knockdown, while B-GlyGly only achieved 20% knockdown. In UASMC cells, B and B-Gly achieved 50% gene knockdown, while B-GlyGly reached 60% knockdown. These results were comparable to those obtained with NeoFX.
Figure 7.
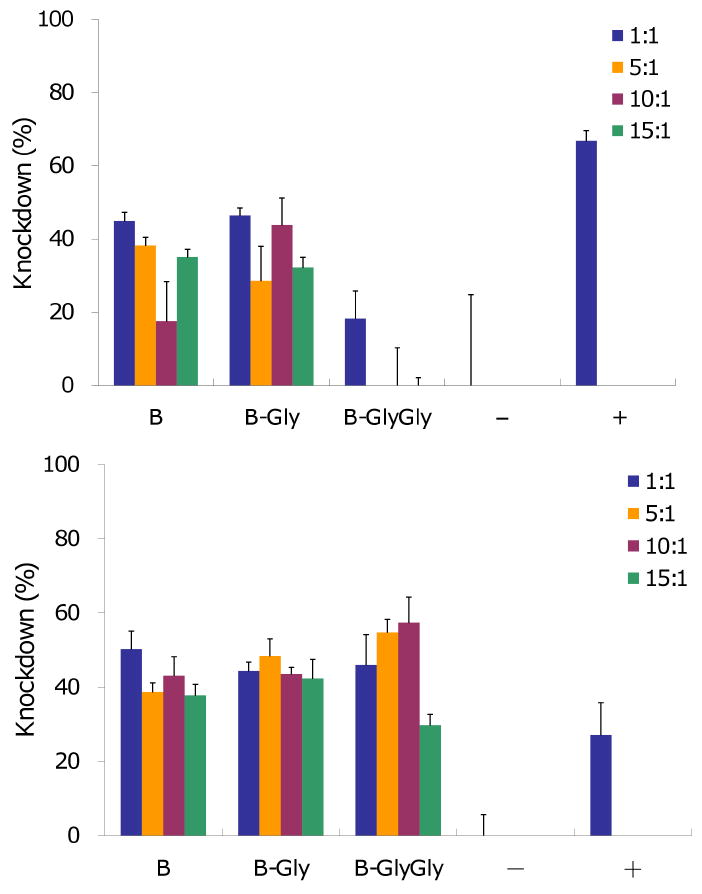
siRNA transfection after 48 h in HepG2 cells (top) and UASMC cells (bottom) as a function of amphiphiles and siRNA molar ratio, NeoFX™ was used as positive control. N=3, mean±SD
Conclusion
In summary, a series of amphiphiles with different spacers within the head group were evaluated for DNA and siRNA delivery. Among them, the amphiphiles possessing the relatively rigid and hydrophilic glycine or alaine spacers, capable of hydrogen bonding, showed effective DNA transfection in vitro. As for siRNA transfection, B, B-Gly and B-GlyGly showed gene knockdown. The binding affinity for the amphiphiles with the peptide spacers was similar to all the other amphiphiles, but DNA was released the fastest from B-GlyGly in the presence of esterase. Also, X-ray diffraction results indicate that DNA is located between the adjacent lipid bilayers in the complex formed with B-GlyGly. It is clear that many factors can influence transfection efficiency and that small perturbations in structure can have a significant effect. In this study, we found that the best performing amphiphile had a peptide linker separating the head group from the chains, complexes with DNA to afford a bilayer structure, and forms lipoplexes of 200 nm in diameter. This study highlights the importance and sensitivity of the spacer group in DNA/amphiphile assembly, liposome stability, and gene transfection activity. Continued studies with different amphiphile compositions will facilitate the development of a set of design requirements for efficient DNA and RNA delivery.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (EB005658 to M.W.G. and GM27278 to T.J.M.).
Footnotes
Supporting Information Available: Detailed synthetic procedures, preparation of liposomes, lipoplexes, and DNA and siRNA transfection protocols, and statistical analysis. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Mintzer MA, Simanek EE. Nonviral vectors for gene delivery. Chem Rev. 2009;109:259–302. doi: 10.1021/cr800409e. [DOI] [PubMed] [Google Scholar]
- 2.Ewert KK, Ahmad A, Bouxsein NF, Evans HM, Safinya CR. Non-viral gene delivery with cationic liposome-DNA complexes. Meth Mol Biol. 2008;433:159–175. doi: 10.1007/978-1-59745-237-3_10. [DOI] [PubMed] [Google Scholar]
- 3.Christine CC, Huang L. Recent advances in non-viral gene delivery. Adv Genetics. 2005;53:3–18. doi: 10.1007/4-431-27879-6_1. [DOI] [PubMed] [Google Scholar]
- 4.Wolff JA, Rozema DB. Breaking the bonds: Non-viral vectors become chemically dynamic. Mol Therapy. 2008;16:8–15. doi: 10.1038/sj.mt.6300326. [DOI] [PubMed] [Google Scholar]
- 5.Reineke TM, Grinstaff MW. Designer materials for nucleic acid delivery. Mat Res Soc Bull. 2005;30:635–639. [Google Scholar]
- 6.Deregowski P, Canalis E. Gene delivery by retroviruses. Methods in Molecular Biology. 2008;455:157–162. doi: 10.1007/978-1-59745-104-8_12. [DOI] [PubMed] [Google Scholar]
- 7.Ozawa K. Gene therapy using aav vectors. Drug Delivery System. 2007;22(6):643–650. [Google Scholar]
- 8.Moroziewicz D, Kaufman HL. Gene therapy with poxvirus vectors. Curr Opin Mol Therap. 2005;7(4):317–325. [PubMed] [Google Scholar]
- 9.Lundstrom K. Gene therapy applications of viral vectors. Tech Can Res Treat. 2004;3(5):467–477. doi: 10.1177/153303460400300508. [DOI] [PubMed] [Google Scholar]
- 10.Flotte TR. Gene therapy progress and prospects: Recombinant adeno-associated virus (raav) vectors. Gene Therapy. 2004;11(10):805–810. doi: 10.1038/sj.gt.3302233. [DOI] [PubMed] [Google Scholar]
- 11.Putnam D. Polymers for gene delivery across length scales. Nat Materials. 2006;5:439–451. doi: 10.1038/nmat1645. [DOI] [PubMed] [Google Scholar]
- 12.Yan W, Huang L. Recent advances in liposome-based nanoparticles for antigen delivery. Polymer Reviews. 2007;47:329–344. [Google Scholar]
- 13.Nicolazzi Cl, Garinot M, Mignet N, Scherman D, Bessodes M. Cationic lipids for transfection. Curr Med Chem. 2003;10:1263–1277. doi: 10.2174/0929867033457467. [DOI] [PubMed] [Google Scholar]
- 14.Luten J, van Nostrum CF, De Smedt SC, Hennink WE. Biodegradable polymers as non- viral carriers for plasmid DNA delivery. J Control Rel. 2008;126(2):97–110. doi: 10.1016/j.jconrel.2007.10.028. [DOI] [PubMed] [Google Scholar]
- 15.Green JJ, Langer R, Anderson DG. A combinatorial polymer library approach yields insight into nonviral gene delivery. Acc Chem Res. 2008;41:749–759. doi: 10.1021/ar7002336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khiati S, Pierre N, Soahary S, Grinstaff MW, Arazam N, Nallet F, Navailles L, Barthélémy P. Anionic nucleotide-lipids for in vitro DNA transfection. Bioconjugate Chem. 2009;20:1765–1772. doi: 10.1021/bc900163s. [DOI] [PubMed] [Google Scholar]
- 17.Miller AD. Cationic liposomes for gene therapy. Angew Chem Int. 1998;37:1768–1785. [Google Scholar]
- 18.Felgner PL, Gadek TR, Holm M, Roman R, Chan HW, Wenz M, Northrop JP, Ringgold GM, Danielsen M. Lipofectin: A highly efficient, lipid mediated DNA-transfection procedure. Proc Natl Acad Sci USA. 1987;84:7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Behr JP, Demeneix B, Loeffler JP, Perez-Mutul J. Efficient gene transfer into mammalian primary endocrine cells with lipopolyamine coated DNA. Proc Nat Acad Sci USA. 1989;86:6982–6986. doi: 10.1073/pnas.86.18.6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan E, Amon M, Marano RJ, Wimmer N, Kearns PS, Manolios N, Rakoczy PE, Toth I. Novel cationic lipophilic peptides for oligodeoxynucleotide delivery. Bioorg Med Chem. 2007;15:4091–4097. doi: 10.1016/j.bmc.2007.03.078. [DOI] [PubMed] [Google Scholar]
- 21.Prata CAH, Zhang X, Luo D, McIntosh TJ, Barthélémy P, Grinstaff MW. Lipophilic peptides for gene delivery. Bioconjugate Chem. 2008;19:418–420. doi: 10.1021/bc700451b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bessodes M, Dubertret C, Jaslin G, Scherman D. Synthesis and biological properties of new glycosidic cationic lipids for DNA delivery. Bioorg Med Chem Lett. 2000;10:1393–1395. doi: 10.1016/s0960-894x(00)00251-1. [DOI] [PubMed] [Google Scholar]
- 23.Herscovici J, Egron MJ, Quenot A, Leclercq F, Leforestier N, Mignet N, Wetzer B, Scherman D. Synthesis of new cationic lipids from unsaturated glycoside scaffold. Org Lett. 2001;3(12):1893–1896. doi: 10.1021/ol0159423. [DOI] [PubMed] [Google Scholar]
- 24.Gissot A, Camplo M, Grinstaff MW, Barthélémy P. Nucleoside, nucleotide and oligonucleotide based amphiphiles: A successful marriage of nucleic acids with lipids. Org Biomol Chem. 2008;6:1324–1333. doi: 10.1039/b719280k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moreau L, Barthélémy P, Maataoui ME, Grinstaff MW. Supramolecular assemblies of nucleoside-based amphiphiles. J Am Chem Soc. 2004;126:7533–7539. doi: 10.1021/ja039597j. [DOI] [PubMed] [Google Scholar]
- 26.Moreau L, Ziarelli F, Grinstaff MW, Barthélémy P. Self-assembled microspheres from f-block elements and nucleoamphiphiles. Chem Comm. 2006;15:1661–1663. doi: 10.1039/b601038e. [DOI] [PubMed] [Google Scholar]
- 27.Moreau l, Campins N, Grinstaff MW, Barthélémy P. A fluorocarbon nucleoamphiphile for the construction of actinide loaded microspheres. Tet Lett. 2006;47:7117–7120. [Google Scholar]
- 28.Barthélémy P, Prata CAH, Filocamo SF, Immoos CE, Maynor BW, Hashmi SAN, Lee SJ, Grinstaff MW. Supramolecular assemblies of DNA with neutral nucleoside amphiphiles. Chem Comm. 2005:1261–1263. doi: 10.1039/b412670j. [DOI] [PubMed] [Google Scholar]
- 29.Campins N, Dieudonné P, Grinstaff MW, Barthélémy P. Nanostructured assemblies from nucleotide based amphiphiles. New J Chem. 2007;31:1928–1934. [Google Scholar]
- 30.Chabaud P, Camplo M, Payet D, Serin G, Moreau L, Barthélémy P, Grinstaff MW. Cationic nucleoside lipids for gene delivery. Bioconjugate Chem. 2006;17:466–472. doi: 10.1021/bc050162q. [DOI] [PubMed] [Google Scholar]
- 31.Moreau L, Barthélémy P, Li Y, Luo D, Prata CAH, Grinstaff MW. Nucleoside phosphocholine amphiphile for in vitro DNA transfection. Molecular BioSystems. 2005;1:260–264. doi: 10.1039/b503302k. [DOI] [PubMed] [Google Scholar]
- 32.Arigon J, Prata CAH, Grinstaff MW, Barthélémy P. Nucleic acid complexing glycosyl nucleoside-based amphiphile. Bioconjugate Chem. 2005;16:864–872. doi: 10.1021/bc050029y. [DOI] [PubMed] [Google Scholar]
- 33.Gaucheron J, Santaella C, Vierling P. Transfection with fluorinated lipoplexes based on fluorinated analogues of dotma, dmrie and dppes. Biochim Biophys Acta – Biomembranes. 2002;1564:349–358. doi: 10.1016/s0005-2736(02)00469-8. [DOI] [PubMed] [Google Scholar]
- 34.Boussif O, Gaucheron J, Boulanger C, Santaella C, Kolbe HVJ, Vierling P. Enhanced in vitro and in vivo cationic lipid-mediated gene delivery with a fluorinated glycerophosphoethanolamine helper lipid. J Gene Med. 2001;3:109–114. doi: 10.1002/jgm.166. [DOI] [PubMed] [Google Scholar]
- 35.Akinc A, Zumbuehl A, Goldberg M, Leshchiner ES, Busini V, Hossain N, Bacallado SA, Nguyen DN, Fuller J, Alvarez R, Borodovsky A, Borland T, Constien R, de Fougerolles A, Dorkin JR, Jayaprakash KN, Jayaraman M, John M, Koteliansky V, Manoharan M, Nechev L, Qin J, Racie T, Raitcheva D, Rajeev KG, Sah DWY, Soutschek J, Toudjarska I, Vornlocher HP, Zimmermann TS, Langer R, Anderson DG. A combinatorial library of lipid-like materials for delivery of rnai therapeutics. Nat Biotechnology. 2008;26:561–569. doi: 10.1038/nbt1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Akinc A, Lynn DM, Anderson DG, Langer R. Parallel synthesis and biophysical characterization of a degradable polymer library for gene delivery. J Am Chem Soc. 2003;125:5316–5323. doi: 10.1021/ja034429c. [DOI] [PubMed] [Google Scholar]
- 37.Gilot D, Miramon ML. Cationic lipids derived from glycine betaine promote efficient and non-toxic gene transfection in cultured hepatocytes. J Gene Med. 2002;4:415–427. doi: 10.1002/jgm.279. [DOI] [PubMed] [Google Scholar]
- 38.Mahidhar YV, Rajesh M, Chaudhuri A. Spacer-arm modulated gene delivery efficacy of novel cationic glycolipids: Design, synthesis, and in vitro transfection biology. J Med Chem. 2004;47:3938–3948. doi: 10.1021/jm030464i. [DOI] [PubMed] [Google Scholar]
- 39.Reynier P, Briane D, Coudert R, Fadda G, Bouchemal N, Bissieres P, Taillandier E, Cao A. Modifications in the head group and in the spacer of cholesterol-based cationic lipids promote transfection in melanoma b16-f10 cells and tumours. J Drug Targeting. 2004;12(1):25–38. doi: 10.1080/10611860410001683040. [DOI] [PubMed] [Google Scholar]
- 40.Prata CAH, Zhao Y, Barthelemy P, Li Y, Luo D, McIntosh TJ, Lee SJ, Grinstaff MW. Charge-reversal amphiphiles for gene delivery. J Am Chem Soc. 2004;126:12196–12197. doi: 10.1021/ja0474906. [DOI] [PubMed] [Google Scholar]
- 41.Friend DS, Papahadjopoulos D, Debs RJ. Endocytosis and intracellular processing accompanying transfection mediated by cationic liposomes. Biochim Biophys Acta. 1996;1278:41–50. doi: 10.1016/0005-2736(95)00219-7. [DOI] [PubMed] [Google Scholar]
- 42.Labat-Moleur F, Steffan AM, Brisson C, Perron H, Feugeas O, Furstenberger P, Oberling F, Brambilla E, Behr JP. An electron microscopy study into the mechanism of gene transfer with lipopolyamines. Gene Therapy. 1996;3:1010–1017. [PubMed] [Google Scholar]
- 43.Zuhorn IS, Kalicharan R, Hoekstra D. Lipoplex-mediated transfection of mammalian cells occurs through the cholesterol-dependent clathrin-mediated pathway of endocytosis. J Biol Chem. 2002;277:18021–18028. doi: 10.1074/jbc.M111257200. [DOI] [PubMed] [Google Scholar]
- 44.Wrobela I, Collins D. Fusion of cationic liposomes with mammalian cells occurs after endocytosis. Biochim Biophys Acta. 1995;1235(2):296–304. doi: 10.1016/0005-2736(95)80017-a. [DOI] [PubMed] [Google Scholar]
- 45.Rädler JO, Koltover I, Salditt T, Safinya CR. Structure of DNA-cationic liposome complexes: DNA intercalation in multilamellar membranes in distinct interhelical packing regimes. Science. 1997:810–814. doi: 10.1126/science.275.5301.810. [DOI] [PubMed] [Google Scholar]
- 46.MacDonald RC, Ashley GW, Shida MM, Rakhmanova VA, Tarahovsky YS, Kennedy MT, Pozharski EV, Baker KA, Jones RD, Rosenzweig HS, Choi KL, Qiu R, McIntosh TJ. Physical and biological properties of cationic triesters of phosphatidylcholine. Biophys J. 1999;77:2612–2629. doi: 10.1016/S0006-3495(99)77095-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.