Abstract
Objective
Disturbances in sleep continuity are common among individuals with major depressive disorder (MDD) and can impact the course of depression and response to treatment. Several studies have examined depressive symptom severity among sleep disordered patients with obstructive sleep apnea (OSA). In contrast little is known about OSA in patients with MDD. The goal of this study was to examine the frequency and predictors of OSA in a sample of individuals with comorbid MDD and insomnia.
Method
Participants were 51 individuals who enrolled in a treatment study on insomnia and depression, met criteria for MDD and comorbid insomnia, and underwent an overnight polysomnography evaluation. An Apnea Hypopnea Index (AHI) ≥15 events per hour was used as a cut-off score for OSA. Regression analyses were conducted to examine clinical and demographic predictors of OSA severity as measured by the AHI.
Results
The results revealed that 39% of the sample met criteria for OSA. The OSA group had significantly higher Body-Mass Index (BMI) scores and a significantly greater proportion of men. Regression analysis revealed that male gender, older age, and higher BMI were significant predictors of OSA severity. Neither depression severity nor insomnia severity were significant predictors.
Conclusion
These findings indicate that the frequency of OSA is higher among individuals with comorbid MDD and insomnia than was previously found among people with either MDD or insomnia alone. In addition, previously identified predictors of OSA (male gender, older age, and high BMI) also apply to this population.
Keywords: Depression, Insomnia, Sleep Apnea, Sleep, Mood Disorders
INTRODUCTION
Major Depressive Disorder (MDD) is a significant problem with a lifetime prevalence between 3% and 17% worldwide (1). Among the symptoms of MDD is a complaint of sleep disturbance, including insomnia, characterized by difficulty initiating and/or maintaining sleep, or hypersomnia, characterized by excessive daytime sleepiness or increased need for sleep. In most mental health clinical settings, these self-reported sleep complaints are rarely followed by objective sleep evaluations. Whereas self-reported sleep symptoms are sufficient for diagnosing insomnia (2), a treatable occult sleep disorder, such as Obstructive Sleep Apnea (OSA), might also be present and that could contribute to the report of poor sleep quality. OSA is a sleep related breathing disorder (SRBD) characterized by upper airway obstruction that is often associated with sleep continuity disturbance and daytime sleepiness. The diagnosis of OSA requires an overnight polysomnograhpy (PSG) recording with the scoring of respiratory events. The standard PSG montage allows identification of two types of respiratory events apneas, a cessation of breathing during sleep that lasts for at least 10 seconds, and hypopneas, a reduction in breathing during sleep that lasts for at least 10 seconds and is associated with an oxygen desaturation > 3% or an arousal (3). A more elaborate montage using esophageal pressure can also identify respiratory effort related arousals, an increased respiratory effort during sleep leading to an arousal (3). The number of apneas and hypopneas per hour provides an Apnea-Hypopnea Index (AHI). The number of apneas, hypopneas, and respiratory effort related arousals per hour provides a Respiratory Disturbance Index (RDI). According to the International Classification of Sleep Disorders (ICSD-2), OSA diagnosis is made when clinical symptoms (daytime sleepiness, waking with a choking or gasping sensation, or bed partner complaints of loud snoring or witnessed apneas) are present and PSG reveals five or more respiratory events or if PSG reveals fifteen or more respiratory events with or without clinical symptoms. Estimates of the prevalence of OSA range widely depending on definition of OSA and method of assessment. A large epidemiological study conducted in the United States using in-home PSG found that the prevalence of OSA, defined by AHI ≥ 15 in the general population was 18% (4).
There is emerging evidence of a link between OSA and depression. Research has found that patients with OSA have elevated levels of depressive symptoms (5–10), with one study reporting a significant positive correlation between OSA severity, as measured by RDI, and depressive symptom severity (11). Although the relationship between OSA and depression is still unclear, these studies suggest that the presence of OSA is associated with the presence of depressive symptoms. Many individuals with OSA might not be aware of this sleep disturbance and might not be cognizant of reporting the symptoms of OSA to their health care provider. Unfortunately, few studies have examined the prevalence of OSA among patients with MDD. PSG studies conducted on small samples of patients with MDD reported that 15% of psychiatric inpatients with MDD were found to have any apneas during the night and 18% of older adults with MDD were found to have an AHI > 5 (12, 13). In an epidemiological population-based study, Ohayon (14) found that 18% of individuals with a diagnosis of MDD also met the DSM-IV defined SRBD diagnosis. Since DSM-IV diagnosis of OSA relies entirely on clinical symptoms, this study likely underestimated the prevalence of PSG defined OSA with an AHI ≥ 15. Beyond these prevalence data, very few studies have examined the clinical presentation of people with MDD and comorbid OSA. Reynolds et al. (1982) reported that MDD patients with OSA were older and 8 out of 13 patients with OSA also had complaints of insomnia. Deldin and colleagues (15) found that greater psychomotor agitation was associated with greater severity of OSA as measured by a variety of indices of OSA severity. Unfortunately, these two studies were limited by small sample sizes. Age, gender, obesity, and hypertension have been linked to OSA and MDD independently, but the relative importance of these variables as predictors of OSA in an MDD sample is unknown (14).
Underscoring the clinical relevance of disturbed sleep among depressed patients is the fact that poor sleep and abnormal sleep architecture can negatively impact the course of depression and response to treatment (16–18). Disturbed sleep is also a common residual symptom of treated MDD (19) and therefore places patients at risk for a protracted course of this chronic episodic disorder. To date, most of the studies of sleep in MDD focused on indices of sleep continuity, and EEG based-measures of sleep, largely ignoring OSA. Given that OSA can disrupt sleep has negative consequences, early detection of an occult sleep disorder among individuals with depression could aid in appropriate referral and/or treatment, possibly improving refractory insomnia and other symptoms of depression. In the present study, individuals with MDD and insomnia completed an overnight PSG evaluation for OSA as part of the screening process for a treatment study on depression and insomnia. The aims of this study were to examine the frequency of OSA in a well characterized depressed sample and to identify patient characteristics associated with OSA severity in this population.
METHOD
Participants
The present analyses were conducted secondary to the primary outcome analyses of a randomized controlled trial comparing the efficacy of adding cognitive behavioral therapy for insomnia versus a quasi-desensitization control condition to antidepressant medication for individuals with comorbid MDD and insomnia (20). Participants were recruited from primary care offices and mental health outpatient clinics using flyers and brochures, and through newspaper advertisements. Those who expressed an interest in the study completed a series of screening procedures (described below). The present study was conducted on the 51 participants who completed the screening process for the parent study. In this sample, the average age was 48.28 years (SD = 12.32) and 57% of the sample was female. The ethnic distribution was as follows: 66.7% Caucasian, 19.6% Asian/Pacific Islander, 5.9% African/American, and 5.9% Middle-Eastern. One participant was identified as multi-racial. The protocol was approved by the Human Subjects Committee at Stanford University and informed consent was obtained from all participants.
Main Study Criteria
The inclusion criteria for the main study were as follows: 1) Age between 18 and 75 years, 2) Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) criteria for MDD (21), 3) score of at least 14 on the 17-item Hamilton Rating Scale for Depression (HRSD-17; (22) 4) DSM-IV-TR criteria for insomnia including: a) a complaint of difficulty initiating or maintaining sleep for at least 1 month, b) the sleep disturbance caused clinically significant distress or impairment in social, occupational, or other important areas of functioning, c) the sleep disturbance does not occur exclusively during the course of another sleep disorder, and d) the sleep disturbance was not due to the direct physiological effects of a substance. In addition, a two-week baseline sleep diary must indicate sleep onset latency > 30 minutes and/or wake after sleep onset > 30 minutes per night at least 3 nights per week and total sleep time ≤ 6.5 hours at least 3 times per week; and 5) no psychotropic or hypnotic medication for at least 14 days (45 days for fluoxetine) prior to the screening visit.
Exclusion criteria for the main study were as follows: 1) Current active suicidal potential, psychotic features, or having received ECT or vagal nerve stimulation treatment during the last year, 2) seasonal pattern of MDD, 3) history of treatment with escitalopram or failing at least two SSRIs, 4) conditions incompatible with the study medication escitalopram (e.g., pregnancy or lactation, not using a reliable birth control method, history of seizure disorder, presence of diseases, and conditions that produce altered metabolism or hemodynamic responses, and hepatic or renal dysfunction), 5) current ongoing psychotherapy, pharmacotherapy, alternative therapy, or any other treatment of claimed efficacy for depression or insomnia including any over-the-counter medications or herbs (e.g., melatonin, valerian, kava, hop extract, St John wort, SAMe), 6) Ten or more arousals per hour of sleep related to respiratory events (apneas and hypopneas); 7) Ten or more periodic limb movement events per hour during sleep; 8) ICSD-2 criteria for circadian rhythm disorder, parasomnia, narcolepsy, or other primary sleep disorder, 9) uncontrolled medical conditions, 10) co-morbid psychiatric conditions other than MDD, 11) abnormal thyroid function or abnormal urine drug screen, and 12) inadequate English language fluency.
Procedures and Measures
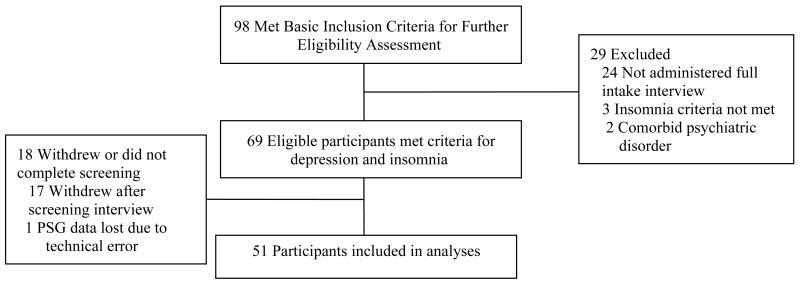
The screening process was conducted in three sequential phases: 1) telephone screen, 2) in-person interviews, and 3) overnight ambulatory polysomnography (see Figure 1). First, those who contacted study personnel to express an interest in participating were administered a 30-minute telephone screening interview, which assessed gross eligibility for the main study. This interview consisted of a script for describing the study and a brief interview to gather preliminary information on sleep/wake patterns over the past month, symptoms of depression over the past month, current psychiatric and medical conditions, and current medications. Those who were suspected of not meeting study criteria (see above), were not interested in the study, or were unable to commit to the study protocol were excluded from further screening. A total of 98 participants completed the initial telephone interview and qualified to continue with the second phase, the in-person interview.
Figure 1.
Patient Flow for Inclusion in the Study
The second screening phase began with obtaining written informed consent to participate in the study followed by an assessment that included several semi-structured interviews and questionnaires. First, the Structured Clinical Interview for the DSM-IV-TR (SCID; (23) was administered via a computer-assisted SCID interview using PsychManager™ programs for SCID-I and SCID-II for cluster B personality disorders (MHS Inc., 1998) to evaluate the presence of current MDD and to rule out other AxisI disorders according to DSM -IV criteria.. To evaluate depression severity, the HRSD-17 (22) was administered by trained interviewers. In addition participants completed the Beck Depression Inventory-II (BDI-II), a self-report validated measure that provides a quantitative index of the severity of depressive symptoms (24), and provided demographic information (e.g., gender, age, ethnicity, race, height and weight). Next, the Duke Structured Interview for Sleep Disorders was administeredto evaluate the presence of insomnia and to rule out circadian rhythm sleep disorders, parasomnia, and narcolepsy. This is a SCID-like structured interview for establishing sleep disorders diagnoses. Evidence of good inter-rater agreement has been reported for primary insomnia (r =.46) and breathing-related sleep disorder (r =.75) as defined in the DSM-IV (25). A total of 69 participants met eligibility criteria for both MDD and insomnia and all exclusion criteria (see above), except those requiring a PSG (respiratory events and limb movements). These participants were scheduled for the third phase of screening.
The third phase of screening consisted of the overnight PSG to screen for the presence of underlying sleep disorders, including periodic limb movement disorder and OSA. A standard diagnostic montage was used with recording channels that included five EEG leads (C3/A2, C4/A1, Fz/A1+A2, O1/A2, O2/A1), two EOG leads, a submentalis (surface) EMG, two ECG lead, intercostal/diaphragmatic (surface) EMG, bilateral anterior tibialis EMG, airflow (nasal cannula linked to pressure transducer) and oral thermocouple respiratory efforts (uncalibrated inductive plethysmography), thoracic and abdominal displacement (inductive plethysmography bands), finger pulse oximeter, snoring (microphone) and a position sensor. The EEG, ECG and EMG signals were recorded with a sampling rate of 1000 Hz, with all other signals digitized at 100 Hz. Appropriate bandpass filters were used for all signals. Signals were collected using an ambulatory computerized polysomnography unit, Safiro™ by Compumedics™. A registered sleep technologist scored the polysomnography data using standard criteria for sleep staging (26) and quantifying respiratory events and periodic limb movements following established criteria (3, 27). In this protocol, only apneas and hypopneas were scored, and thus the AHI was used as an index of respiratory events. A total of 52 participants completed the final screening with technical difficulties leading to the loss of one participant’s data. As a result, the present analyses were conducted on the sample of the 51 participants who had usable PSG data. Participants who met all eligibility criteria were subsequently randomized for the main study. Please see (28) for full description of main study procedures.
RESULTS
Presence of OSA
The respiratory data from the PSG revealed that 16 participants had an AHI < 5, 15 participants had an AHI between 5 and 15, 12 participants had an AHI between 15 and 25, and 8 participants had an AHI > 25. In the present study, the AHI criteria for OSA was based on the International Classification of Sleep Disorders-2 (29), requiring an AHI ≥ 15 as a cut-off for OSA. Therefore, the sample was subdivided into two groups: those with an AHI ≥ 15, referred to as the OSA group and those with AHI < 15, referred to as the non-OSA (nOSA) group. The mean AHI for the entire sample was 14.85 (SD = 16.45) with 20 participants (39%) in the OSA group. Chi-square analyses, conducted on categorical variables, revealed a gender difference, χ2 (1) = 6.41, p < .05, with significantly more men (59% of the sample) than women (24% of the sample) having AHI ≥ 15. A series of t-tests were conducted on continuous variables, including the sleep parameters derived from the PSG. Results revealed a significant difference between the groups on BMI (p < .05), with the OSA group having significantly higher BMI compared to the nOSA group. In addition, the OSA group was older, reported lower BDI and HRSD-17 scores, had more wake time after sleep onset, and had lower sleep efficiency relative to the nOSA group, but these differences did not reach statistical significance (.05 < p < .10). Table 1 summarizes means and standard deviations of the variables by group.
Table 1.
Comparison between OSA (AHI ≥15) and nOSA (AHI < 15) groups
| Variable | nOSA (n = 31) | OSA (n = 20) | Total (n = 51) | p | |||
|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | ||
| Age (years) | 45.61 | 13.16 | 52.42 | 9.82 | 48.28 | 12.32 | .050 |
| BMI | 25.20 | 4.20 | 30.26 | 9.07 | 27.18 | 6.93 | .028* |
| BDI Total | 29.77 | 9.95 | 24.42 | 9.77 | 27.74 | 10.12 | .069 |
| BDI Somatic | 17.40 | 6.41 | 14.00 | 5.21 | 6.15 | 0.88 | .059 |
| BDI Cognitive | 9.84 | 4.20 | 7.95 | 5.37 | 4.72 | 0.67 | .171 |
| HRSD-17 | 21.10 | 3.65 | 19.20 | 4.35 | 4.01 | 0.56 | .099 |
| HRSDsleep | 4.00 | 1.21 | 4.20 | 1.47 | 4.08 | 1.31 | .599 |
| PSG Data | |||||||
| TST (min) | 381.60 | 77.35 | 379.95 | 62.05 | 380.95 | 71.09 | .937 |
| SOL (min) | 19.89 | 23.78 | 25.58 | 32.33 | 22.12 | 27.29 | .473 |
| WASO (min) | 50.00 | 41.82 | 78.58 | 63.33 | 61.21 | 52.65 | .085 |
| SE (%) | 82.73 | 10.58 | 76.24 | 13.47 | 80.18 | 12.10 | .061 |
| NWAK | 14.58 | 7.46 | 16.95 | 9.08 | 15.51 | 8.13 | .314 |
| REM Latency (min) | 78.17 | 32.51 | 90.40 | 55.17 | 83.06 | 42.92 | .329 |
| Stage 1% | 7.18 | 4.18 | 9.28 | 7.90 | 8.00 | 5.94 | .221 |
| Stage 2% | 65.83 | 9.41 | 64.82 | 9.74 | 65.43 | 9.46 | .714 |
| Stage 3% | 3.69 | 4.95 | 2.73 | 4.01 | 3.31 | 4.59 | .468 |
| Stage 4% | 1.33 | 4.70 | 1.61 | 3.96 | 1.44 | 4.39 | .826 |
| REM% | 21.98 | 8.82 | 21.57 | 4.89 | 21.82 | 7.47 | .829 |
| Apnea Index | 0.63 | 1.37 | 7.63 | 8.32 | 3.37 | 6.28 | .001* |
| Hypopnea Index | 4.85 | 4.10 | 21.76 | 13.08 | 11.48 | 12.02 | .001* |
| Apnea-Hypopnea Index | 5.47 | 4.63 | 29.39 | 17.67 | 14.85 | 16.44 | .001* |
| Average min SaO2 (%) | 95.32 | 1.78 | 94.45 | 1.91 | 94.98 | 1.86 | .102 |
| PLM Index | 0.86 | 2.90 | 4.12 | 12.79 | 2.14 | 8.36 | .176 |
p < .05
Note. A series of t-tests were conducted to determine statistical significance.
Given the differences between the OSA and nOSA groups in gender distribution, gender differences in each of the variables above were tested using independent samples t-tests. Compared to me, women had significantly higher BDI score (M=30.30, SD=9.63 vs. M=23.90, SD=9.84, p < .05), greater percent of stage 2 (M=68.09, SD=8.66 vs. M=61.64, SD=9.45; p < . 05), and higher average minimum oxygen desaturation (M=95.47, SD=1.59 vs. M=94.29, SD=2.03; p < .05).
Predictors of OSA Severity
A standard linear regression analysis was conducted to identify predictors of OSA severity as indicated by the AHI. To account for a non-normal distribution of AHI in this sample, a log transformation, LN(AHI + 1), was used as the dependent variable. The independent variables were selected based on theoretical grounds and included known predictors of OSA (gender, age, BMI) and clinical features, depression and insomnia severity, to determine if these clinical features are unique predictors of OSA in this sample. For this analysis, depression severity was defined as the BDI score minus the sleep item (BDI-S) and insomnia severity was defined as the sum of the three insomnia items of the HRSD (HRSDsleep). The latter variable has been established as a valid measure of insomnia severity in patients with MDD (30). Each predictor variable was centered around its median before being entered into the model (31). The results revealed that the overall model was significant (F = 6.28, df = 5, 44, p < .001) accounting for 42% of the variance (adjusted R2 = .35) in OSA severity. Gender, age, and BMI were found to be significant predictors of AHI (p < .05). Specifically, male status, older age, and higher BMI scores were associated with higher AHI. Neither BDI-S nor HRSDsleep were found to be significant. Please see Table 2 for summary of the regression analysis.
Table 2.
Summary of Regression for Screening Variables Predicting AHI
| Variable | B | SEB | B | Zero-order correlations | semipartial correlations |
|---|---|---|---|---|---|
| Gender* | .83 | .44 | .37 | .37 | .33 |
| Age* | .04 | .01 | .42 | .38 | .39 |
| BMI* | .03 | .02 | .27 | .41 | ..26 |
| BDI-S | −.01 | .01 | −.07 | −.22 | −.06 |
| HAMsleep | .05 | .11 | .06 | .00 | .05 |
p < .05
Note. Transformation of AHI was performed using LN (AHI + 1). Overall model was significant (F = 6.28, df = 5, 44, p < .001, R2 = .42).
DISCUSSION
This study investigated the presence and predictors of OSA among individuals with comorbid MDD and insomnia. The findings in this study suggest that the co-occurrence of insomnia and depression increases the risk of OSA. The frequency of OSA with AHI ≥ 15 in this sample (39%) is considerably higher than previously reported prevalence rates of 18% for the general population (4), 15–18% among individuals with depression (12, 14), and 29% among older adults with insomnia (32, 33). When considering the middle-age cohort in this sample, the occurrence of OSA in men (59%) and women (24%) is considerably higher in comparison to a large-scale epidemiological study on a similar age cohort (30–60 year old) in the United States in which 9% of men and 4% of women had an AHI ≥ 15 (34). A similar study of 30–70 year-olds in Spain also found lower population prevalence of OSA, with 14% in men and 7% in women having an AHI ≥ 15 (35). Furthermore, the possibility that these findings underestimate the true prevalence of OSA in patients with MDD and insomnia cannot be ruled out as some individuals with an existing diagnosis of OSA and those with probable OSA were excluded during the telephone screen phase. Our finding suggests that MDD patients with co-morbid insomnia have a higher risk for OSA than the general population and should receive strong consideration for a PSG evaluation.
The PSG data also confirmed the self-reported symptoms of insomnia in this sample. Wake time after sleep onset was over 78 minutes and sleep efficiency was under 80%. Although comparisons between the OSA and nOSA groups revealed only a trend towards significance on these two variables, this pattern is in the same direction as the results from a study of OSA in people with insomnia that found lower PSG-defined sleep efficiency, more total wake time, and less total sleep time compared with people who had OSA only (36). It is important to note that these individuals were not misdiagnosed with insomnia; instead these were individuals who actually met criteria for insomnia and whose OSA diagnosis might have been missed without a PSG study.
As is the case in the general population (4), male gender, older age, and elevated BMI scores were significant predictors of OSA severity in this sample of patients with MDD and insomnia. Gender and BMI were also previously found to be predictors of AHI in a sample of older adults with insomnia (32). Neither the severity of depressive symptoms nor the severity of insomnia symptoms were significant predictors of OSA severity. This suggests that mental health providers evaluating patients who complain of mood and sleep disturbances should consider the possibility of OSA, particularly in patients of male gender, older age, and high BMI.
Interestingly, those in the OSA group reported relatively lower depression severity as measured by a self report questionnaire (BDI-II) and clinical interview (HRSD-17) compared to the nOSA group. The relationship between depression symptom severity and OSA severity has not previously been examined in MDD samples but it has been examined in sleep apnea samples with mixed results. The majority of studies on OSA patients have found a positive relationship between AHI and depression symptom severity (e.g., (11, 37)). However, one study found an inverse relationship in that higher levels of depressive symptoms was associated with milder oxygen desaturation in a sample of OSA patients (38). It is unclear why those with AHI ≥ 15 in the present sample of depressed individuals had lower depression scores. Additional research is needed to elucidate the relationship between symptoms of depression and OSA.
Several limitations of this study need to be acknowledged. First, the sample size was modest and only included individuals who expressed an interest in participating in a clinical study on depression and insomnia. Therefore, no comparison group was available for individuals without depression, insomnia, or both. Second, the inclusion/exclusion criteria of the main study preclude generalizability of the results to MDD patients who do not experience insomnia, to MDD patients with other comorbid disorders, and to other psychiatric samples. Instead, these results are meant to inform a specific sample of individuals with MDD and insomnia. Also, some variables such as hypertension and neck girth that have previously been identified as predictors of OSA were not available in this study. Finally, the present study used a PSG-defined cut-off score of AHI ≥ 15 to determine OSA diagnosis. While this score is commonly used in research studies (4, 32) and is recommended by the ICSD-2 (29), it precludes generalizability to other sleep related breathing disorders such as those associated with central sleep apnea, and those requiring the assessment of respiratory effort, not provided in this study.
Our findings add to the emerging literature on the relationship between OSA and depression with several important clinical implications. It has been suggested that OSA is under-diagnosed in MDD, possibly hindering treatment response (39). It is possible that detection and appropriate treatment of OSA can aid in the treatment of depression. For example, in a recent study the initiation of CPAP therapy led to a significant decrease in depression symptom severity in an OSA sample (40). Also, evaluation for OSA can yield important information when pharmacological treatment for depression and insomnia is considered. One study found that 30% of patients with undiagnosed OSA were prescribed sedating medications, with physicians who were not sleep specialists more likely to prescribe these medications (41). Moreover, an association was found between the use of sedating medication and increased motor vehicle accidents for individuals with severe OSA (41). Collectively, the accumulating evidence indicates, the need to consider regular screening of OSA among individuals with depression and insomnia, especially among men, older adults, and obese individuals. Since individuals with OSA might not volunteer symptoms suggestive of possible OSA in the context of a psychiatric evaluation, it is particularly important for clinicians to specifically inquire about the symptoms of OSA and consider a referral to a sleep center for a diagnostic sleep study when indicated. Further research examining the association between OSA and MDD and the clinical relevance of this comorbidity are needed. Studies of potential pathways (e.g., serotonin, metabolic syndrome) linking OSA, depression, and insomnia might yield strategies for more effective treatments for these comorbid conditions.
Acknowledgments
This research was supported by a grant form the National Institute of Mental Health (NIMH), grant number MH066131 awarded to the last author. Portions of the data from this study were presented at the annual meeting of the Associated Professional Sleep Societies, Denver, CO, June 2005. The authors wish to thank Tasha Kalista, Chai-Yu Cardell, Viola Arias, and Linda Saefke for their contribution to the research presented in this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Andrade L, Caraveo-Anduaga JJ, Berglund P, Bijl RV, De Graaf R, Vollebergh W, Dragomirecka E, Kohn R, Keller M, Kessler RC, Kawakami N, Kilic C, Offord D, Ustun TB, Wittchen HU. The epidemiology of major depressive episodes: results from the International Consortium of Psychiatric Epidemiology (ICPE) Surveys. Int J Methods Psychiatr Res. 2003;12:3–21. doi: 10.1002/mpr.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Littner M, Hirshkowitz M, Kramer M, Kapen S, Anderson WM, Bailey D, Berry RB, Davila D, Johnson S, Kushida C, Loube DI, Wise M, Woodson BT. Practice parameters for using polysomnography to evaluate insomnia: an update. Sleep. 2003;26:754–60. doi: 10.1093/sleep/26.6.754. [DOI] [PubMed] [Google Scholar]
- 3.American Academy of Sleep Medicine Task Force. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 4.Young T, Shahar E, Nieto FJ, Redline S, Newman AB, Gottlieb DJ, Walsleben JA, Finn L, Enright P, Samet JM. Predictors of sleep-disordered breathing in community-dwelling adults: the Sleep Heart Health Study. Arch Intern Med. 2002;162:893–900. doi: 10.1001/archinte.162.8.893. [DOI] [PubMed] [Google Scholar]
- 5.Borak J, Cieslicki J, Szelenberger W, Wilczak-Szadkowska H, Koziej M, Zielinski J. Psychopathological characteristics of the consequences of obstructive sleep apnea prior to and tree months after CPAP. Psychiatr Pol. 1994;28:33–44. [PubMed] [Google Scholar]
- 6.Derderian SS, Bridenbaugh RH, Rajagopal KR. Neuropsychologic symptoms in obstructive sleep apnea improve after treatment with nasal continuous positive airway pressure. Chest. 1988;94:1023–7. doi: 10.1378/chest.94.5.1023. [DOI] [PubMed] [Google Scholar]
- 7.Edinger JD, Carwile S, Miller P, Hope V, Mayti C. Psychological status, syndromatic measures, and compliance with nasal CPAP therapy for sleep apnea. Percept Mot Skills. 1994;78:1116–8. doi: 10.2466/pms.1994.78.3c.1116. [DOI] [PubMed] [Google Scholar]
- 8.Engleman HM, Martin SE, Deary IJ, Douglas NJ. Effect of continuous positive airway pressure treatment on daytime function in sleep apnoea/hypopnoea syndrome. Lancet. 1994;343:572–5. doi: 10.1016/s0140-6736(94)91522-9. [DOI] [PubMed] [Google Scholar]
- 9.Flemons WW, Tsai W. Quality of life consequences of sleep-disordered breathing. J Allergy Clin Immunol. 1997;99:S750–6. doi: 10.1016/s0091-6749(97)70123-4. [DOI] [PubMed] [Google Scholar]
- 10.Kales A, Caldwell AB, Cadieux RJ, Vela-Bueno A, Ruch LG, Mayes SD. Severe obstructive sleep apnea--II: Associated psychopathology and psychosocial consequences. J Chronic Dis. 1985;38:427–34. doi: 10.1016/0021-9681(85)90138-9. [DOI] [PubMed] [Google Scholar]
- 11.Aloia MS, Arnedt JT, Smith L, Skrekas J, Stanchina M, Millman RP. Examining the construct of depression in obstructive sleep apnea syndrome. Sleep Med. 2005;6:115–21. doi: 10.1016/j.sleep.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Reynolds CF, 3rd, Coble PA, Spiker DG, Neil JF, Holzer BC, Kupfer DJ. Prevalence of sleep apnea and nocturnal myoclonus in major affective disorders: clinical and polysomnographic findings. J Nerv Ment Dis. 1982;170:565–7. doi: 10.1097/00005053-198209000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Reynolds CF, 3rd, Kupfer DJ, Taska LS, Hoch CC, Sewitch DE, Restifo K, Spiker DG, Zimmer B, Marin RS, Nelson J, et al. Sleep apnea in Alzheimer’s dementia: correlation with mental deterioration. J Clin Psychiatry. 1985;46:257–61. [PubMed] [Google Scholar]
- 14.Ohayon MM. The effects of breathing-related sleep disorders on mood disturbances in the general population. J Clin Psychiatry. 2003;64:1195–200. doi: 10.4088/jcp.v64n1009. quiz, 1274–6. [DOI] [PubMed] [Google Scholar]
- 15.Deldin PJ, Phillips LK, Thomas RJ. A preliminary study of sleep-disordered breathing in major depressive disorder. Sleep Med. 2006;7:131–9. doi: 10.1016/j.sleep.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 16.Buysse DJ, Reynolds CFr, Houck PR, Perel JM, Frank E, Begley AE, Mazumdar S, Kupfer DJ. Does lorazepam impair the antidepressant response to nortriptyline and psychotherapy? J Clin Psychiatry. 1997;58:426–432. doi: 10.4088/jcp.v58n1003. [DOI] [PubMed] [Google Scholar]
- 17.Dew MA, Reynolds CF, Houck PR, Hall M, Buysse DJ, Frank E, Kupfer DJ. Temporal profiles of the course of depression during treatment. Predictors of pathways toward recovery in the elderly. Arch Gen Psychiatry. 1997;54:1016–1024. doi: 10.1001/archpsyc.1997.01830230050007. [DOI] [PubMed] [Google Scholar]
- 18.Winokur A, Reynolds CF. The effects of antidepressants and anxiolitics on sleep physiology. Primary Psychiatry. 1994;1:22–27. [Google Scholar]
- 19.Nierenberg AA, Keefe BR, Leslie VC, Alpert JE, Pava JA, Worthington JJ, Rosenbaum JF, Fava M. Residual symptoms in depressed patients who respond acutely to fluoxetine. J of Clin Psychiatry. 1999;60:221–225. doi: 10.4088/jcp.v60n0403. [DOI] [PubMed] [Google Scholar]
- 20.Manber R, Edinger JD, Gress JL, San Pedro-Salcedo MG, Kuo TF, Kalista T. Cognitive behavioral therapy for insomnia enhances depression outcome in patients with comorbid major depressive disorder and insomnia. Sleep. 2008;31:489–95. doi: 10.1093/sleep/31.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.American Psychiatric Association. The Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: 2000. [Google Scholar]
- 22.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;25:55–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.First MB, Spitzer RL, Gibbon MG, Williams JBW. The Structured Clinical Interview for DSM-IV. New York: Biometrics Research Department, New York State Psychiatric Institute; 1994. [Google Scholar]
- 24.Beck AT, Steer RA, Brown GK. Beck Depression Inventory. San Antonio: The Psychological Corporation; 1996. [Google Scholar]
- 25.Carney CE, Edinger JD, Olsen MK, Stechchak KM, Krystal AD, Wyatt JK. Inter-rater reliability for Insomnia Diagnoses Derived From the Duke Structured Interview for Sleep Disorders. Sleep. 2008;31:A250. [Google Scholar]
- 26.Kales A, Rechtschaffen A, University of California Los Angeles. Brain Information Service., NINDB Neurological Information Network (U.S.). A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Allan Rechtschaffen and Anthony Kales, editors. Bethesda, Md.,, U. S. National Institute of Neurological Diseases and Blindness, Neurological Information Network, 1968
- 27.American Sleep Disorder Association. Recording and scoring leg movements. The Atlas Task Force. Sleep. 1993;16:748–59. [PubMed] [Google Scholar]
- 28.Manber R, Edinger J, Gress J, San Pedro-Salcedo M, Kuo T, Kalista T. Cognitive behavioral therapy for Iinsomnia enhances depression outcome in patients with comorbid major depressive disorder and insomnia. Sleep. doi: 10.1093/sleep/31.4.489. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.American Academy of Sleep Medicine. The International Classification of Sleep Disorders-2. Rochester, MN: 2005. [Google Scholar]
- 30.Manber R, Blasey C, Arnow B, Markowitz JC, Thase ME, Rush AJ, Dowling F, Koscis J, Trivedi M, Keller MB. Assessing insomnia severity in depression: comparison of depression rating scales and sleep diaries. J Psychiatr Res. 2005;39:481–8. doi: 10.1016/j.jpsychires.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 31.Aiken LS, West SG. Multiple Regression: Testing and Interpreting Interactions. Newbury Park, CA: Sage Publications; 1991. [Google Scholar]
- 32.Lichstein KL, Riedel BW, Lester KW, Aguillard RN. Occult sleep apnea in a recruited sample of older adults with insomnia. J Consult Clin Psychol. 1999;67:405–10. doi: 10.1037//0022-006x.67.3.405. [DOI] [PubMed] [Google Scholar]
- 33.Gooneratne NS, Gehrman PR, Nkwuo JE, Bellamy SL, Schutte-Rodin S, Dinges DF, Pack AI. Consequences of comorbid insomnia symptoms and sleep-related breathing disorder in elderly subjects. Arch Intern Med. 2006;166:1732–8. doi: 10.1001/archinte.166.16.1732. [DOI] [PubMed] [Google Scholar]
- 34.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The Occurrence of Sleep-Disordered Breathing among Middle-Aged Adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 35.Duran J, Esnaola S, Rubio R, Iztueta A. Obstructive Sleep Apnea-Hypopnea and Related Clinical Features in a Population-based Sample of Subjects Aged 30 to 70 Yr. Am J Respir Crit Care Med. 2001;163:685–689. doi: 10.1164/ajrccm.163.3.2005065. [DOI] [PubMed] [Google Scholar]
- 36.Smith S, Sullivan K, Hopkins W, Douglas J. Frequency of insomnia report in patients with obstructive sleep apnoea hypopnea syndrome (OSAHS) Sleep Med. 2004;5:449–56. doi: 10.1016/j.sleep.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 37.Bardwell WA, Moore P, Ancoli-Israel S, Dimsdale JE. Fatigue in obstructive sleep apnea: driven by depressive symptoms instead of apnea severity? Am J Psychiatry. 2003;160:350–5. doi: 10.1176/appi.ajp.160.2.350. [DOI] [PubMed] [Google Scholar]
- 38.McCall WV, Harding D, O’Donovan C. Correlates of depressive symptoms in patients with obstructive sleep apnea. J Clin Sleep Med. 2006;2:424–6. [PubMed] [Google Scholar]
- 39.Schroder CM, O’Hara R. Depression and Obstructive Sleep Apnea (OSA) Ann Gen Psychiatry. 2005;4:13. doi: 10.1186/1744-859X-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwartz DJ, Karatinos G. For individuals with obstructive sleep apnea, institution of CPAP therapy is associated with an amelioration of symptoms of depression which is sustained long term. J Clin Sleep Med. 2007;3:631–5. [PMC free article] [PubMed] [Google Scholar]
- 41.Lu B, Budhiraja R, Parthasarathy S. Sedating medications and undiagnosed obstructive sleep apnea: physician determinants and patient consequences. J Clin Sleep Med. 2005;1:367–71. [PubMed] [Google Scholar]