SUMMARY
Constant supplies of dental epithelial cells from stem cell niches in the cervical loop enable mouse incisors to grow continuously through life. Fibroblast growth factor 10 (FGF10) has been shown to be essential for development of mouse incisors and maintenance of incisor cervical loops during prenatal development. Whether its cognate receptor, FGFR2IIIB, in the dental epithelium is required for postnatal tooth development remains unknown since Fgfr2IIIb ablation causes neonatal lethality. Here we report that tissue-specific ablation of Fgfr2 in the dental epithelium led to defective maxillary incisors that lacked ameloblasts and the enamel, and had poorly developed odontoblasts. Although the cervical loop in Fgfr2 null maxillary incisors was formed initially, it failed to continue to develop and gradually diminished soon after birth. The results suggest that the FGFR2 signaling axis plays a role in maintaining the stem cell niche required for incisor development and lifelong growth.
Keywords: growth factor, receptor tyrosine kinase, conditional gene knockout, tooth development, mouse genetics
INTRODUCTION
The fibroblast growth factor (FGF) and the FGF receptor (FGFR) have been shown to constitute reciprocal regulatory communication loops between the epithelial and mesenchymal compartments, which play an important role in tooth formation and regeneration (Kettunen et al., 2000; Harada et al., 2002; Klein et al., 2008). Mouse tooth formation starts with the budding stage at embryonic day 11 (E11), in which the oral epithelium thickens and invades in the underlying mesenchyme that becomes condensed and surrounds the epithelial cells. The budding stage is then followed by the cap and bell stages, in which different lineages of dental cells undergo proliferation and differentiation processes to form tooth crowns (Tucker and Sharpe, 2004). In the incisor, the labial epithelium, which produces differentiated ameloblasts, grows longer in length than the lingual epithelium and forms a cervical loop at the apical end of the tooth, where a population of self-renewing epithelial stem cells resides. Unlike the molars, the incisor germ continues to grow permanently without the formation of complete roots. Consequently, the incisors continuously grow through life and are regenerative (Tummers and Thesleff, 2003; Yokohama-Tamaki et al., 2006). Therefore, understanding the molecular mechanism by with stem cells in the incisor cervical loop give rise to daughter cells will shed light on understanding tooth growth and regeneration.
The FGF signaling axis consists of 22 FGF ligands and four FGFR tyrosine kinases that have multiple alternative splicing variants (McKeehan et al., 1998; Powers et al., 2000). The FGF and its cognate FGFR isoforms are tissue-specifically expressed in the stromal and epithelial compartments, which constitutes directional regulatory axes in multiple organs (Thomson, 2001; Thesleff, 2003). In tooth, expression of FGF is highly spatiotemporally specific. Fgf8 and Fgf9 are expressed in the tooth epithelium during its thickening to the early bud stage (Kettunen and Thesleff, 1998). At later stage, Fgf8 expression is diminished while Fgf9 expression is upregulated. Fgf4 expression is restricted to the enamel knot and is not seen after cap stage. In contrast, Fgf9 expression is maintained into adulthood. It is proposed that FGF4, 8, and 9 are functionally redundant in regulating adjacent mesenchymal cell proliferation and/or prevent apoptosis (Kettunen and Thesleff, 1998). On the other hand, Fgf3 and 10 are exclusively expressed in dental mesenchymal cells and promote proliferation of dental epithelial cells as well as the transit-amplifying and stem cells in the cervical loop (Harada et al., 1999; Kettunen et al., 2000; Wang et al., 2007). The observation that mice deficient in FGF10 fail to develop the cervical loop of incisors suggests that FGF10 plays a role in the maintenance of the incisor stem cell niche (Harada et al., 2002). The IIIb isoform of FGFR1 and FGFR2 are the cognate receptors for FGF3 and FGF10. Both Fgfr1IIIb and Fgfr2IIIb isoforms are expressed in the dental epithelium (Kettunen et al., 1998). Ablation of Fgfr1 in dental epithelial cells abrogates cell adherence between ameloblasts, disrupts ameloblast organization at the enamel-secretory stage, and therefore, affects the enamel formation; however it does not disrupt ameloblast differentiation (Takamori et al., 2008). Disruption of Fgfr2IIIb arrests tooth development at the bud stage, but further analyses are not available due to neonatal lethality of Fgfr2IIIb ablation (De Moerlooze et al., 2000). Besides the dental epithelium, Fgfr2IIIb is also widely expressed in other epithelial tissues. Therefore, whether FGFR2 in the tooth epithelium is required for tooth development and how ablation of Fgfr2IIIb arrests tooth development at the bud stage remain to be determined.
To circumvent this difficulty, the Cre/LoxP mediated tissue-specific recombination system was used to disrupt Fgfr2 alleles in the dental epithelium. The Fgfr2 conditional null (Fgfr2cn) mice developed defective maxillary incisors, which was attributed to failure of the Fgfr2-deficient cervical loop, a stem cell niche that gives rise to ameloblasts, to fully develop and sustain after its initial formation. The results indicate that FGFR2 signals are essential for maintenance of the stem cell niche of maxillary incisors, which is required for the incisors’ continuing growth.
Result
Ablation of Fgfr2 in the dental epithelium disrupts maxillary incisor development
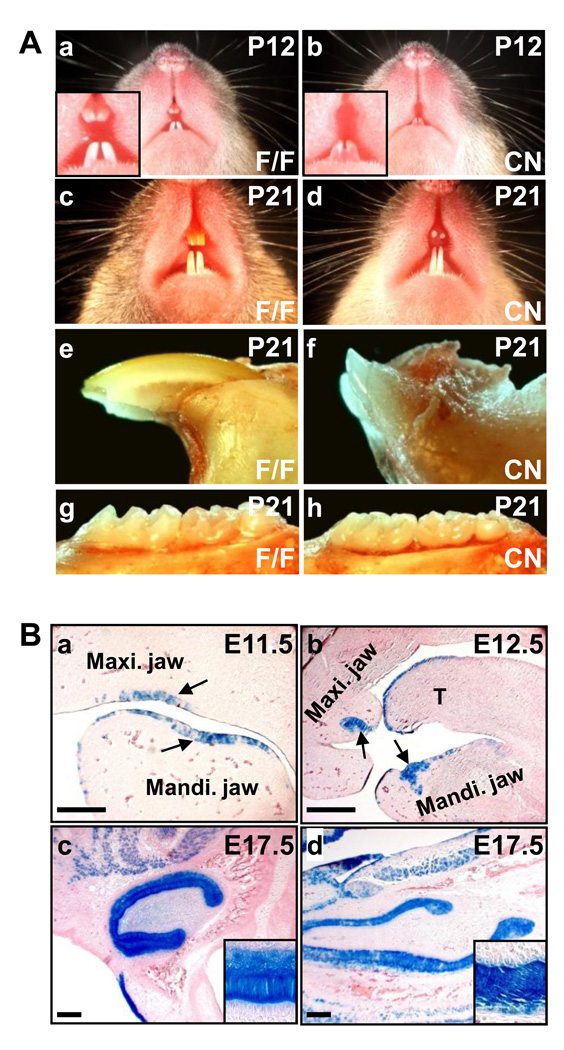
Although germline ablation of the Fgfr2IIIb isoform arrests tooth development at the bud stage (De Moerlooze et al., 2000), whether FGFR2 in the dental epithelium is required for tooth development has yet to be determined, as tooth development requires contributions from multiple tissues. To study the role of dental epithelial FGFR2 in tooth development, Fgfr2 alleles were tissue-specifically ablated in the dental epithelium at embryonic day 11.5 (E11.5) by crossing mice bearing Fgfr2flox (Yu et al., 2003) and Nkx3.1Cre knockin alleles (Lin et al., 2007). The Fgfr2cn mice were viable, but exhibited defective maxillary incisors. Among 128 mutants analyzed, 85 did not have erupted maxillary incisors. Although the rest of 43 had erupted maxillary incisors, the incisors were short and chalky white in color (Fig. 1A). Unlike the control maxillary incisor that pointed downward to the mandible, all erupted mutant maxillary incisors pointed forward (Fig. 1Ae,f). In contrast, both the mandibular incisors and the molars erupted normally and had no apparent morphological defects. H&E staining further revealed that the maxillary tooth buds were poorly developed in Fgfr2cn at postnatal day 3, while the other tooth buds had no apparent defects (Fig 2 and Supplemental Fig. 1).
Fig. 1. Loss of Fgfr2 in the dental epithelium disrupts maxillary incisor development in mouse.
(A) Defective maxillary incisors in Fgfr2cn mutant mice. The mutant maxillary incisors were short and chalky-white, although mandibular incisors and molars were normal. (B) The embryos carrying ROSA26/Nkx3.1Cre alleles collected at the indicated days were lightly fixed and stained with X-Gal. Note that Cre was expressed in the oral epithelium at E11.5 as indicated by arrows (a), thickened dental epithelium at E12.5 (b), and dental epithelial cells including ameloblasts at E17.5 in both maxillary (c) and mandibular (d) incisors. Maxi., maxillary; Mandi., mandibular; T, tongue; F/F, homozygous Fgfr2flox; CN, Fgfr2cn; scale bars, 100 µm.
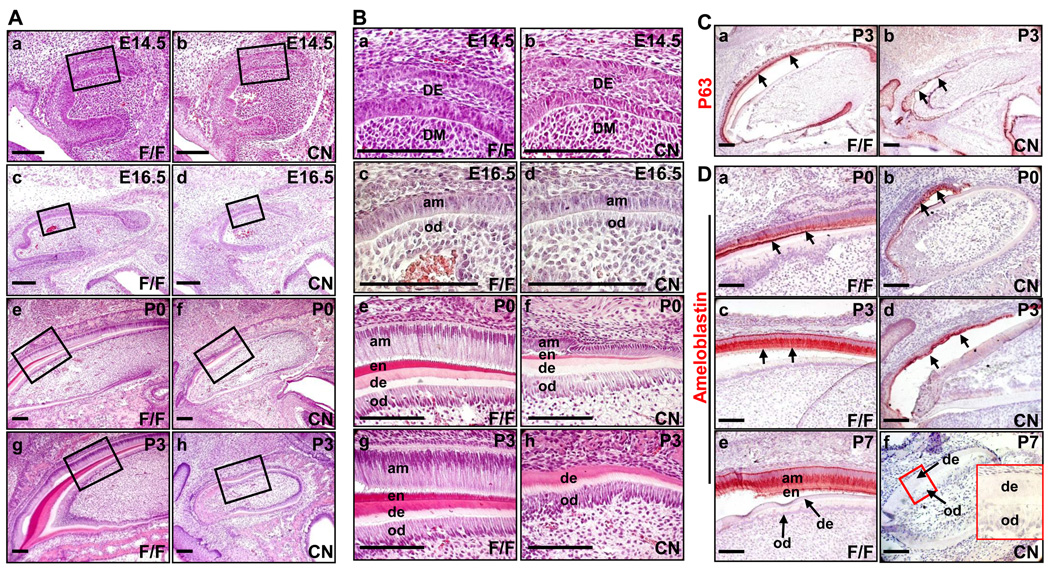
Fig. 2. Ablation of Fgfr2 causes loss of ameloblasts and disrupts odontoblast development in maxillary incisors.
(A,B) H&E staining of sagittal sections demonstrates defects in ameloblasts and odontoblasts of maxillary incisors at embryonic and neonatal stages due to the deficiency in FGFR2. Both ameloblasts and the enamel layer were lost in P3 incisors. Odontoblasts were poorly aligned, and the intensity of the dentin layer was reduced in mutant incisor. High-magnification views of boxed areas in A were shown in B. (C) Immunostaining showed that the mutant maxillary incisors had less P63 positive dental epithelial cells (indicated by arrows). (D) Immunostaining demonstrates that the mutant maxillary incisors (indicated by arrows) had reduced (P3 or earlier) or no (P7) ameloblastin-positive cells. Insert, high-magnification view of the boxed area showing the dentin and odontoblasts. am, ameloblast; de, dentin; en, enamel; od, odontoblast; DE, dental epithelium; DM, dental mesenchyme; scale bars, 100 µm.
X-Gal staining of embryos carrying Nkx3.1Cre and the R26R-lacZ reporter allele (Soriano, 1999) revealed that Nkx3.1Cre was expressed in the oral epithelium in tooth germ areas of maxillary and mandibular jaws at E11.5 (Fig. 1Ba). These cells later invaded into the surrounding mesenchyme and formed the tooth buds (Fig. 1Bb). X-Gal staining of E17.5 embryos demonstrated that Cre was expressed homogenously in the dental epithelium of maxillary (Fig. 1Bc) and mandible (Fig. 1Bd) incisors and molars (data not shown).
Ablation of Fgfr2 inhibits ameloblast and cervical loop development in the maxillary incisor
To further characterize the role of FGFR2 in maxillary incisor development, maxillary jaws were dissected and sagittal sectioned at the stages from E14.5 to postnatal day 3 for histological analyses (Fig. 2A,B). H&E staining revealed that Fgfr2cn maxillary incisor buds exhibited normal morphology at E14.5, indicating that FGFR2 signals are not essential for maxillary incisor bud formation (Fig. 2Ba,b). However, at E16.5, the ameloblasts in mutant incisors appeared to regress, especially at the areas proximal to the cervical loop where the progenitor cells that give rise to ameloblasts reside (Fig. 2Bc). The defects in ameloblasts appeared to be more severe during postnatal development (Fig. 2Bh). Most mutants lost ameloblasts and the enamel at P3, although a few mutants still had poorly developed ameloblasts at later stages.
P63, a transcription factor homologue to the P53 tumor suppressor, is expressed throughout the dental and oral epithelium and is required for tooth formation (Laurikkala et al., 2006; Rufini et al., 2006). Immunostaining with anti-P63 antibody showed that the population of P63 positive cells was dramatically reduced in Fgfr2cn maxillary incisors, further indicating defects in the Fgfr2cn dental epithelium (Fig. 2C). Ameloblastin is a major component of enamel matrix secreted by differentiated ameloblasts (Fukumoto et al., 2004; Wang et al., 2004). Although the cell population was smaller than the control, immunostaining revealed that the mutant ameloblasts still expressed ameloblastin at P0, suggesting that mutant ameloblasts still maintained differentiation characteristics and the secretory function at this stage. However, the ameloblasts in many mutant maxillary incisors were lost at P7 (Fig. 2D). No ameloblasts and enamel were found in adult mutant animals (4 weeks of age or older). The results indicate that loss of FGFR2 likely does not affect ameloblast differentiation at early developmental stages, but disrupts ameloblast homeostasis and results in loss of ameloblasts at later stages. The results are consistent with previous reports that ablation of Sprouty genes, which encode antagonists of receptor tyrosine kinase signaling, leads to ectopic enamel deposition (Klein et al., 2008), and that double ablation of Fgf3:Fgf10 causes enamel loss (Wang et al., 2007).
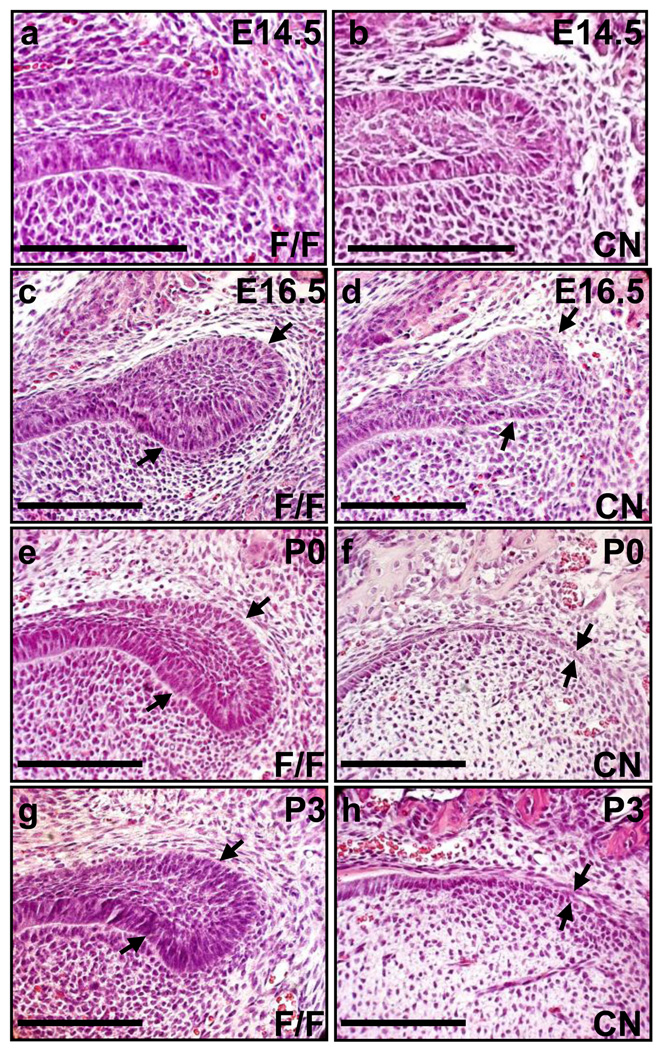
Mouse incisors are continuously growing organs; the epithelial progenitor cells located in the cervical loop at the apical end of incisors constantly give rise to ameloblasts. The results that ameloblasts in Fgfr2cn mutants were relatively normal at first but later disappeared suggest a defect in progenitors in the cervical loops. Indeed, H&E staining revealed that the cervical loops in mutants formed, although they were smaller than controls at E16.5 and were diminished at P0 (Fig. 3). As ameloblasts are derived from the cervical loop, it is possible that the failure of progenitors in the defective cervical loop to properly differentiate into ameloblasts is the causal factor of maxillary incisor defect. To test this possibility, BrdU labeling was used to assess the proliferating cells at E16.5 and E18.5 when the progenitor cells were actively engaged in proliferation. At E16.5, in control maxillary incisors, BrdU positive cells were abundant in the cervical loop, inner epithelium, and adjacent mesenchymal cells (Fig. 4A). At the same stages, however, mutant incisors only had a few cells in the cervical loop that were BrdU positive, while the inner epithelium still had many cells labeled with BrdU (Fig. 4Ab). At E18.5, most BrdU positive cells were located in the peripheral stellate reticulum where transit-amplifying cells reside in controls, while the cervical loop dramatically regressed in mutant incisors. The data suggest that the proliferation of the transit-amplifying cells was also inhibited by Fgfr2 ablation.
Fig. 3. Ablation of Fgfr2 causes loss of the cervical loop in maxillary incisors.
(A) H&E staining of sagittal sections, demonstrating that formation of the cervical loop at early stages and the loss of the cervical loop (indicated by arrows) at late stages in mutant maxillary incisors. cl, cervical loop; scale bars, 100 µm.
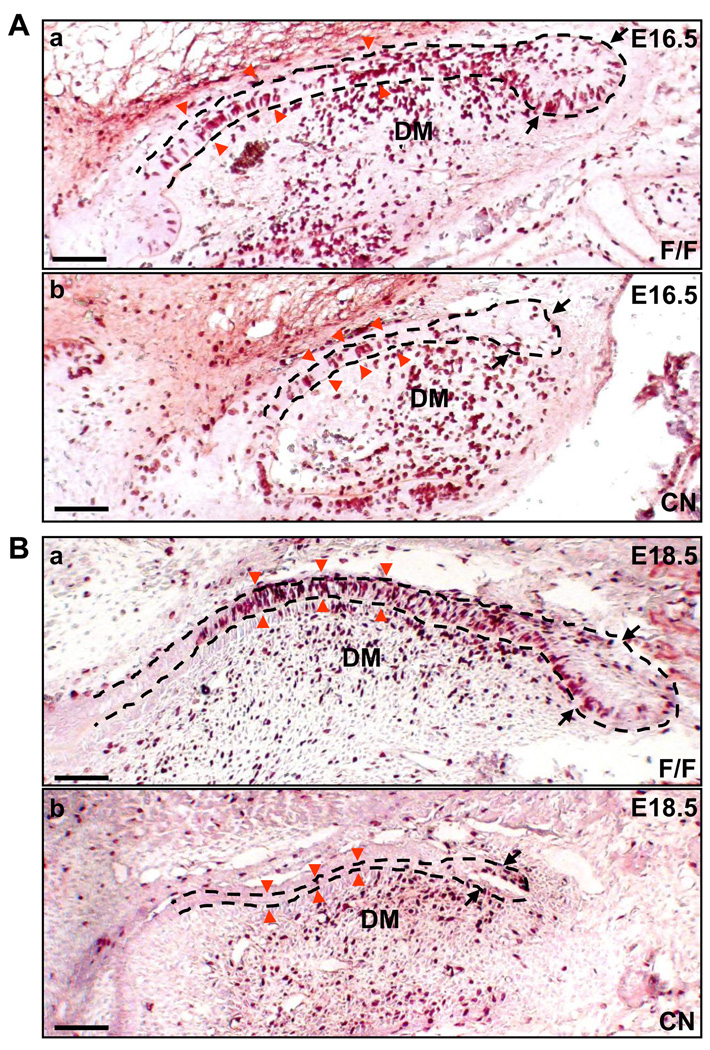
Fig. 4. Ablation of Fgfr2 alleles inhibits cell proliferation in the cervical loop.
(A) BrdU labeling of E16.5 embryos, showing that the mutant cervical loop (indicated by black arrows) was smaller and had fewer cells undergoing proliferation than did the control, whereas most of the differentiating ameloblasts in mutants (indicated by red arrow heads) were proliferating as did the control. (B) BrdU labeling of E18.5 embryos, showing that the mutant cervical loop was significantly smaller than the control and that no differentiating ameloblasts was engaged in proliferation. DM, dental mesenchyme; scale bars, 100 µm.
Ablation of epithelial Fgfr2 compromises odontoblast differentiation and function
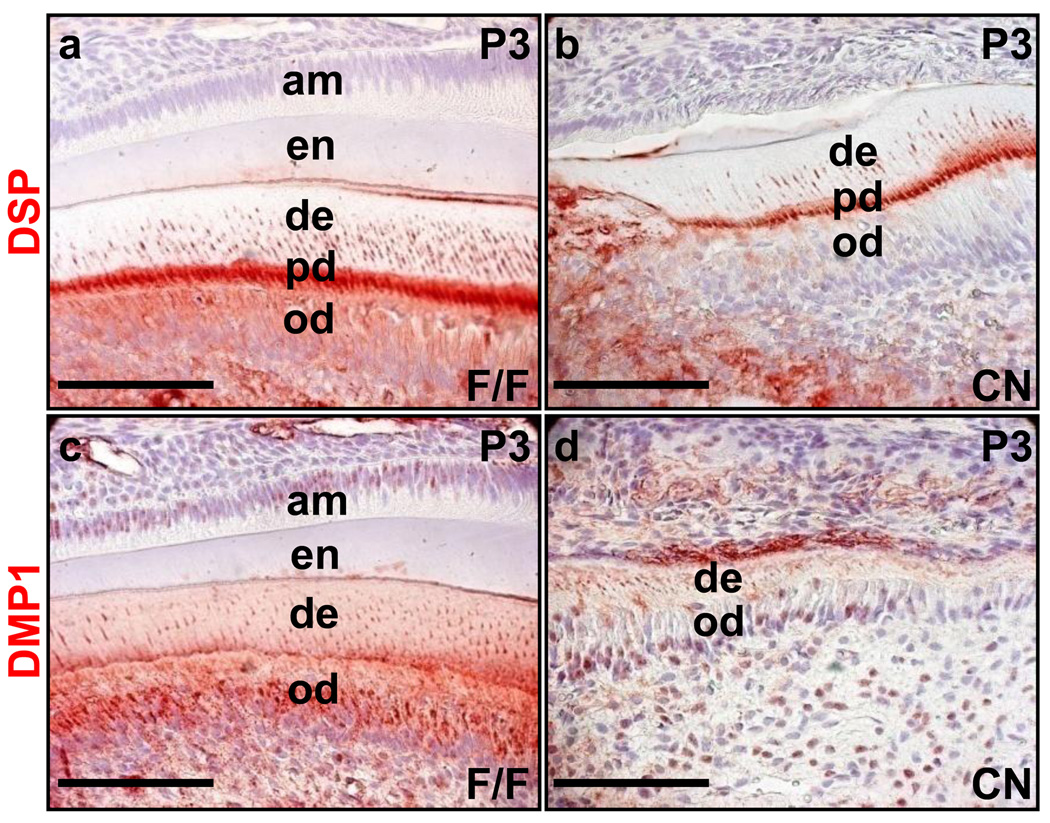
Reciprocal communications between the dental epithelium and mesenchyme are critical to tooth development and homeostasis. To investigate whether ablation of Fgfr2 in the dental epithelium also causes defects in the mesenchyme, immunostaining was carried out to assess the expression of dentin sialoprotein (DSP) and dentin matrix protein 1 (DMP1) in the maxillary incisors, both of which are characteristic proteins expressed in odontoblasts. In control incisors, DSP was located in odontoblasts, predentin, and dentinal tubules (Fig. 5a). However, DSP was detectable only in the predentin and dentinal tubules, the quantity of which was significantly reduced in the Fgfr2cn incisors (Fig. 5b). Similarly, although DMP1 staining was strong in odontoblasts, predentin, and dentinal tubules in the control incisors, only residual DMP1 staining was visible in the dentinal tubule of the Fgfr2cn incisors. These data indicate that the differentiation and function of odontoblasts are also disrupted by ablation of Fgfr2 in the dental epithelium.
Fig. 5. Ablation of Fgfr2 causes odontoblasts in maxillary incisors losing secretory function.
Immunostaining of sagittal sections of P3 incisors, showing that expression and secretion of the indicated odontoblast characteristic proteins were significantly reduced in Fgfr2cn mutant maxillary incisors. am, ameloblast; de, dentin; DMP1, dentin matrix protein 1; DSP, dentin sialoprotein; en, enamel; od, odontoblast; pd, predentin; scale bars, 100 µm.
Discussion
Continuous growth of mouse incisors requires progenitors in the cervical loop to constantly supply dental epithelial cells. Here we report that FGFR2 signals were required to sustain the existence of cervical loops. Ablation of Fgfr2 in the oral epithelium at E11 did not disrupt bud and cap formation of maxillary incisors, yet the proliferation of dental progenitor cells in mutant cervical loops was reduced, which caused the regression and eventual loss of cervical loops after E18.5. Although mutant ameloblasts developed at first and secreted ameloblastin prior to postnatal day 3, the ameloblast layer was then diminished afterwards. The results suggest that FGFR2 signals are essential for the maintenance of the cervical loop that gives rise to the dental epithelial cells necessary to lifelong incisor growth.
FGFR2 in the dental epithelium is essential to sustained proliferation of cells in the cervical loop of maxillary incisors
Different from molars, mouse incisors are lifelong regenerative tissues and have a continuous supply of dental epithelial cells from stem cells residing in the cervical loop at the apical end. Differential expression of Fgf in the incisor and molar is believed to dictate development processes between molars and incisors. At early stages, Fgf10 and Fgf3 are expressed both in incisors and molars (Harada et al., 1999). After the late bell stage, expression of Fgf10 and Fgf3 are down regulated in the molar mesenchyme. In contrast, Fgf10 is continuously expressed in the mesenchyme surrounding the apical end of incisors (Kettunen et al., 2000). Consistently, Fgf10 ablation does not affect molar formation, but disrupts the cervical loop of incisors (Harada et al., 2002). As one of the cognate receptors of FGF10, FGFR2IIIb is expressed throughout the dental epithelium (Kettunen et al., 1998). Our data demonstrates that ablation of Fgfr2 in the tooth epithelial precursors causes loss of the cervical loop of maxillary incisors, which is consistent with Fgf10 ablation. The cervical loop cells failed to proliferate vigorously, which is the likely reason that the cervical loop regressed and disappeared eventually. Interestingly, ablation of FRS2α, a proximal interactive adaptor protein recruiting multiple downstream signaling molecules to the FGFR2 kinase, with the same Cre driver did not affect cervical loop development and maintenance (data not shown), suggesting that FGFR2 regulates cervical loop development and maintenance independent of FRS2α-mediated signaling pathways.
Differential requirement of FGFR2 signals in maxillary and mandibular incisor development
Although Nkx3.1Cre is equally expressed in both mandibular and maxillary incisors, only the maxillary cervical loop was affected by Fgfr2 ablation with Nkx3.1Cre, suggesting that the requirement of FGFR2 for cervical loop homeostasis varies between the maxillary and mandibular incisors. The maxillary incisors arise from the medial nasal process originating from the lower margin of the frontal process, while the mandibular incisors arise from the mandibular arch derived from the first pharyngeal arch. The difference in tissue origins have been shown to account for a number of congenital diseases that primarily affect the central region of the upper jaw, including cleft lip and cleft palate (Avery et al., 2002). Several genes have been found to play different roles in maxillary and mandibular teeth. Dlx1 and Dlx2 double null mice lack maxillary molars but have normal mandibular molars (Thomas et al., 1997). Loss of Spry2 causes the formation of supernumerary teeth in the mandible but does not significantly affect the maxillary teeth (Klein et al., 2006). These suggest that molecular mechanisms for regulating maxillary and mandibular tooth formation are not completely identical. In this report, we also demonstrate that FGFR2 plays different roles in maxillary and mandible incisor development. Both Fgf3 and Fgf10 are expressed in the dental mesenchyme facing the dental epithelium, and Fgfr1IIIb and Fgfr2IIIb are expressed in the cervical loop epithelium of incisors (Harada et al., 1999). Although FGF3 has similar activity in activating FGFR1IIIb and FGFR2IIIb isoforms, FGF10 exhibits high activity for FGFR2IIIb than for FGFR1IIIb (Zhang et al., 2006). Therefore, future experiments are need to determine whether maxillary and mandibular incisors express a different subset of FGF and FGFR isoforms, and thus, exhibit different FGFR2 signal-dependencies for their development and maintenance of the cervical loop.
It is interesting that the mutant incisors had a wrong growth direction. Although it is possible that the change was a secondary effect resulted from malocclusion due to lack of the enamel, this is unlikely since all mutants examined (43 individuals that had erupted maxillary incisors) had the same defect, and feeding with a soft diet did not alleviate the defect (data not shown). Therefore, the phenotype is likely resulted from the defective cell proliferation caused by the Fgfr2 deletion, since the normal curvature of the incisor to posterior direction is thought to be caused by more active proliferation in the labial cervical loop than in the lingual side. Indeed, immunostaining with anti-phosphorylated histone H3 antibodies revealed that at P7, the cells in labial side were less actively engaged in proliferation than those in the lingual side of Fgfr2cn maxillary incisors, whereas in normal incisors, more cells were engaged in proliferation in the labial side than in the lingual side (supplemental Fig. 2). Further experiments are needed to systematically address the issue, as it is beyond the scope of this manuscript.
In summary, ablation of Fgfr2 in progenitor cells for the dental epithelium with Nkx3.1Cre disrupted the maintenance of the cervical loop, resulting in failure to supply daughter cells for ameloblasts during embryonic and postnatal growth of the incisors and causing maxillary incisor defects.
MATERIALS AND METHODS
Animals
All animals were housed in the Program of Animal Resources of the Institute of Biosciences and Technology, Texas A&M Health Science Center, and were handled in accordance with the principles and procedures of the Guide for the Care and Use of Laboratory Animals. All experimental procedures were approved by the Institutional Animal Care and Use Committee. The mice carrying LoxP-flanked Fgr2 alleles, the Nkx3.1Cre knock-in alleles, and the ROSA26 reporter allele, were bred as described (Lin et al., 2007). Genotypes of the mice bearing the Fgfr2flox and Fgfr2cn allele were determined by PCR analyses as described (Yu et al., 2003). Primer sequences and PCR conditions for genotyping Cre and ROSA26 reporter mice were the same as described elsewhere (Soriano, 1999; Lin et al., 2007).
Histology analysis and immunohistochemistry
The heads were collected from Fgfr2cn and Fgfr2flox embryos or mice at the ages indicated in the text. Embryonic heads were fixed with 4% paraformaldehyde (PFA)-PBS solution for 4 hours; postnatal mouse heads were decalcified and fixed with 12.5% EDTA containing 2.5% PFA for 2 weeks (solution changed twice a week) at 4 °C. Fixed tissues were serially dehydrated with ethanol, embedded in paraffin, and completely sectioned for the incisors according to standard procedures. One out of every five slides were re-hydrated and stained with hematoxylin and eosin (H&E) for scanning of general tissue structures. Immunohistochemical analyses were performed on paraffin sections mounted on Superfrost/Plus slides (Fisher Scientific, Pittsburgh, PA). The antigens were retrieved by autoclave in the Tris-HCl buffer (pH 10.0) for 5 minutes or as suggested by manufacturers of the antibodies. Goat anti-ameloblastin (R-16, 1:50 dilution), mouse anti-P63 (1:150 dilution), and rabbit anti-phosphorylated histone H3 (1:1000 dilution) antibodies were purchased from Santa Cruz (Santa Cruz, CA); mouse anti-DSP (1:50) and mouse anti-DMP1 (1:50) were generated in the Qin laboratory as described elsewhere (Baba et al., 2004a; Baba et al., 2004b). Specifically bound antibodies were detected with the ExtraAvidin Peroxidase System from Sigma (Saint Louis, MO).
For lacZ staining, the tissues were lightly fixed with 0.2% glutaraldehyde for 30 minutes, the lacZ activity was analyzed by incubation with 1 mg/ml X-Gal at room temperature overnight as described (Liu et al., 2005).
BrdU incorporation analysis
BrdU (5- bromo-2’-deoxyuridine, Sigma Co, Saint Louis, MO) was intraperitoneally injected into pregnant females (0.05 mg per 10 g body weight) at the indicated days. About 2–3 hours post injection, the mice were sacrificed and the embryos were collected for the analyses. Briefly, heads were collected, fixed in 4% PFA for 4 hours, paraffin-embedded and sectioned. The incorporated BrdU was detected on sections by immunostaining with anti-BrdU antibody (1:1000 dilution; Sigma Co, Saint Louis, MO).
Supplementary Material
Acknowledgement
We thank Drs. David M. Ornitz and Michael M. Shen for their generous supplies of Fgfr2f/f and Nkx3.1-Cre mice, respectively, as well as Dr. Wallace L. McKeehan, Young Xu, and Mary Cole for critical reading of the manuscript, and Kerstin McKeehan for excellent technical support.
The work was supported by Research Development Grant from Texas A&M Health Science Center 134402-705504, Public Health Service Grants CA096824 from the National Cancer Institute, AHA0655077Y from The American Heart Association, and DAMD17-03-0014 from the U.S. Department of Defense
Reference
- Avery JK, Steele PF, Avery N. Oral Development and Histology. In: Avery JK, editor. Oral Developmetn and Histology. New York: Thieme; 2002. pp. 21–43. [Google Scholar]
- Baba O, Qin C, Brunn JC, Jones JE, Wygant JN, McIntyre BW, Butler WT. Detection of dentin sialoprotein in rat periodontium. Eur J Oral Sci. 2004a;112:163–170. doi: 10.1111/j.0909-8836.2004.00110.x. [DOI] [PubMed] [Google Scholar]
- Baba O, Qin C, Brunn JC, Wygant JN, McIntyre BW, Butler WT. Colocalization of dentin matrix protein 1 and dentin sialoprotein at late stages of rat molar development. Matrix Biol. 2004b;23:371–379. doi: 10.1016/j.matbio.2004.07.008. [DOI] [PubMed] [Google Scholar]
- De Moerlooze L, Spencer-Dene B, Revest J, Hajihosseini M, Rosewell I, Dickson C. An important role for the IIIb isoform of fibroblast growth factor receptor 2 (FGFR2) in mesenchymal-epithelial signalling during mouse organogenesis. Development. 2000;127:483–492. doi: 10.1242/dev.127.3.483. [DOI] [PubMed] [Google Scholar]
- Fukumoto S, Kiba T, Hall B, Iehara N, Nakamura T, Longenecker G, Krebsbach PH, Nanci A, Kulkarni AB, Yamada Y. Ameloblastin is a cell adhesion molecule required for maintaining the differentiation state of ameloblasts. J Cell Biol. 2004;167:973–983. doi: 10.1083/jcb.200409077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada H, Kettunen P, Jung HS, Mustonen T, Wang YA, Thesleff I. Localization of putative stem cells in dental epithelium and their association with Notch and FGF signaling. J Cell Biol. 1999;147:105–120. doi: 10.1083/jcb.147.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada H, Toyono T, Toyoshima K, Yamasaki M, Itoh N, Kato S, Sekine K, Ohuchi H. FGF10 maintains stem cell compartment in developing mouse incisors. Development. 2002;129:1533–1541. doi: 10.1242/dev.129.6.1533. [DOI] [PubMed] [Google Scholar]
- Kettunen P, Karavanova I, Thesleff I. Responsiveness of developing dental tissues to fibroblast growth factors: expression of splicing alternatives of FGFR1, -2, -3, and of FGFR4; and stimulation of cell proliferation by FGF-2, -4, -8, and -9. Dev Genet. 1998;22:374–385. doi: 10.1002/(SICI)1520-6408(1998)22:4<374::AID-DVG7>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Kettunen P, Laurikkala J, Itaranta P, Vainio S, Itoh N, Thesleff I. Associations of FGF-3 and FGF-10 with signaling networks regulating tooth morphogenesis. Dev Dyn. 2000;219:322–332. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1062>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Kettunen P, Thesleff I. Expression and function of FGFs-4, -8, and -9 suggest functional redundancy and repetitive use as epithelial signals during tooth morphogenesis. Dev Dyn. 1998;211:256–268. doi: 10.1002/(SICI)1097-0177(199803)211:3<256::AID-AJA7>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Klein OD, Lyons DB, Balooch G, Marshall GW, Basson MA, Peterka M, Boran T, Peterkova R, Martin GR. An FGF signaling loop sustains the generation of differentiated progeny from stem cells in mouse incisors. Development. 2008;135:377–385. doi: 10.1242/dev.015081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein OD, Minowada G, Peterkova R, Kangas A, Yu BD, Lesot H, Peterka M, Jernvall J, Martin GR. Sprouty genes control diastema tooth development via bidirectional antagonism of epithelial-mesenchymal FGF signaling. Dev Cell. 2006;11:181–190. doi: 10.1016/j.devcel.2006.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurikkala J, Mikkola ML, James M, Tummers M, Mills AA, Thesleff I. p63 regulates multiple signalling pathways required for ectodermal organogenesis and differentiation. Development. 2006;133:1553–1563. doi: 10.1242/dev.02325. [DOI] [PubMed] [Google Scholar]
- Lin Y, Liu G, Zhang Y, Hu YP, Yu K, Lin C, McKeehan K, Xuan JW, Ornitz DM, Shen MM, Greenberg N, McKeehan WL, Wang F. Fibroblast growth factor receptor 2 tyrosine kinase is required for prostatic morphogenesis and the acquisition of strict androgen dependency for adult tissue homeostasis. Development. 2007;134:723–734. doi: 10.1242/dev.02765. [DOI] [PubMed] [Google Scholar]
- Liu W, Selever J, Murali D, Sun X, Brugger SM, Ma L, Schwartz RJ, Maxson R, Furuta Y, Martin JF. Threshold-specific requirements for Bmp4 in mandibular development. Dev Biol. 2005;283:282–293. doi: 10.1016/j.ydbio.2005.04.019. [DOI] [PubMed] [Google Scholar]
- McKeehan WL, Wang F, Kan M. The heparan sulfate-fibroblast growth factor family: diversity of structure and function. Prog Nucleic Acid Res Mol Biol. 1998;59:135–176. doi: 10.1016/s0079-6603(08)61031-4. [DOI] [PubMed] [Google Scholar]
- Powers CJ, McLeskey SW, Wellstein A. Fibroblast growth factors, their receptors and signaling. Endocr Relat Cancer. 2000;7:165–197. doi: 10.1677/erc.0.0070165. [DOI] [PubMed] [Google Scholar]
- Rufini A, Weil M, McKeon F, Barlattani A, Melino G, Candi E. p63 protein is essential for the embryonic development of vibrissae and teeth. Biochem Biophys Res Commun. 2006;340:737–741. doi: 10.1016/j.bbrc.2005.12.065. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Takamori K, Hosokawa R, Xu X, Deng X, Bringas P, Jr, Chai Y. Epithelial fibroblast growth factor receptor 1 regulates enamel formation. J Dent Res. 2008;87:238–243. doi: 10.1177/154405910808700307. [DOI] [PubMed] [Google Scholar]
- Thesleff I. Epithelial-mesenchymal signalling regulating tooth morphogenesis. J Cell Sci. 2003;116:1647–1648. doi: 10.1242/jcs.00410. [DOI] [PubMed] [Google Scholar]
- Thomas BL, Tucker AS, Qui M, Ferguson CA, Hardcastle Z, Rubenstein JL, Sharpe PT. Role of Dlx-1 and Dlx-2 genes in patterning of the murine dentition. Development. 1997;124:4811–4818. doi: 10.1242/dev.124.23.4811. [DOI] [PubMed] [Google Scholar]
- Thomson AA. Role of androgens and fibroblast growth factors in prostatic development. Reproduction. 2001;121:187–195. doi: 10.1530/rep.0.1210187. [DOI] [PubMed] [Google Scholar]
- Tucker A, Sharpe P. The cutting-edge of mammalian development; how the embryo makes teeth. Nat Rev Genet. 2004;5:499–508. doi: 10.1038/nrg1380. [DOI] [PubMed] [Google Scholar]
- Tummers M, Thesleff I. Root or crown: a developmental choice orchestrated by the differential regulation of the epithelial stem cell niche in the tooth of two rodent species. Development. 2003;130:1049–1057. doi: 10.1242/dev.00332. [DOI] [PubMed] [Google Scholar]
- Wang XP, Suomalainen M, Felszeghy S, Zelarayan LC, Alonso MT, Plikus MV, Maas RL, Chuong CM, Schimmang T, Thesleff I. An integrated gene regulatory network controls stem cell proliferation in teeth. PLoS Biol. 2007;5:e159. doi: 10.1371/journal.pbio.0050159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XP, Suomalainen M, Jorgez CJ, Matzuk MM, Werner S, Thesleff I. Follistatin regulates enamel patterning in mouse incisors by asymmetrically inhibiting BMP signaling and ameloblast differentiation. Dev Cell. 2004;7:719–730. doi: 10.1016/j.devcel.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Yokohama-Tamaki T, Ohshima H, Fujiwara N, Takada Y, Ichimori Y, Wakisaka S, Ohuchi H, Harada H. Cessation of Fgf10 signaling, resulting in a defective dental epithelial stem cell compartment, leads to the transition from crown to root formation. Development. 2006;133:1359–1366. doi: 10.1242/dev.02307. [DOI] [PubMed] [Google Scholar]
- Yu K, Xu J, Liu Z, Sosic D, Shao J, Olson EN, Towler DA, Ornitz DM. Conditional inactivation of FGF receptor 2 reveals an essential role for FGF signaling in the regulation of osteoblast function and bone growth. Development. 2003;130:3063–3074. doi: 10.1242/dev.00491. [DOI] [PubMed] [Google Scholar]
- Zhang X, Ibrahimi OA, Olsen SK, Umemori H, Mohammadi M, Ornitz DM. Receptor specificity of the fibroblast growth factor family. The complete mammalian FGF family. J Biol Chem. 2006;281:15694–15700. doi: 10.1074/jbc.M601252200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.