Abstract
This treatment-development study is a Stage I evaluation of an intervention that combines mindfulness meditation with cognitive-behavior therapy for insomnia (CBT-I). Thirty adults who met research diagnostic criteria for Psychophysiological Insomnia (Edinger et al., 2004) participated in a 6-week, multi-component group intervention using mindfulness meditation, sleep restriction, stimulus control, sleep education, and sleep hygiene. Sleep diaries and self-reported pre-sleep arousal were assessed weekly while secondary measures of insomnia severity, arousal, mindfulness skills, and daytime functioning were assessed at pre-treatment and post-treatment. Data collected on recruitment, retention, compliance, and satisfaction indicate that the treatment protocol is feasible to deliver and is acceptable for individuals seeking treatment for insomnia. The overall patterns of change with treatment demonstrated statistically and clinically significant improvements in several nighttime symptoms of insomnia as well as statistically significant reductions in pre-sleep arousal, sleep effort, and dysfunctional sleep-related cognitions. In addition, a significant correlation was found between the number of meditation sessions and changes on a trait measure of arousal. Together, the findings indicate that mindfulness meditation can be combined with CBT-I and this integrated intervention is associated with reductions in both sleep and sleep-related arousal. Further testing of this intervention using randomized controlled trials is warranted to evaluate the efficacy of the intervention for this population and the specific effects of each component on sleep and both psychological and physiological arousal.
Keywords: Insomnia, Mindfulness meditation, Cognitive-behavior therapy, Arousal, Treatment-development
Insomnia is a highly prevalent problem with an estimated 10 percent of the general population experiencing both nighttime and daytime symptoms that would qualify for a diagnosis of insomnia (National Institutes of Health State of the Science Conference Statement, 2005). The current standard for non-pharmacological treatments of insomnia is a multi-component cognitive-behavior therapy for insomnia (CBT-I) which typically consists of one or more behavioral components such as sleep restriction, stimulus control, or relaxation training along with a cognitive component such as sleep education or cognitive restructuring (Morin, Bastein, & Savard, 2003). Treatment outcome studies have found that CBT-I is efficacious in reducing sleep onset latency (SOL) and wake time after sleep onset (WASO) and improving sleep efficiency (Edinger, Wohlgemuth, Radtke, Marsh, & Quillian, 2001a; Jacobs, Pace-Schott, Stickgold, & Otto, 2004; Morin, Colecchi, Stone, Sood, & Brink, 1999; Sivertsen et al., 2006). While these findings indicate the benefits of CBT-I on sleep parameters, the impact of CBT-I on other domains related to sleep, such as quality of life, daytime functioning, and sleep-related arousal are less clear.
One domain that merits further attention in insomnia treatment studies is sleep-related arousal. Although elevations in arousal have been identified as a contributing factor in the development and maintenance of insomnia, there is no consensus definition on this construct. Studies investigating psychological aspects of sleep-related arousal have found that people with insomnia report higher levels of pre-sleep rumination (Nicassio, Mendlowitz, Fussell, & Petras, 1985) and a more negative tone of sleep-related cognitions (Kuisk, Bertelson, & Walsh, 1989) relative to good sleepers. It has also been hypothesized that CBT-I reduces sleep-related arousal by changing maladaptive beliefs and attitudes that are believed to maintain the arousal (Morin, 1993). Collectively, the level of pre-sleep cognitive activity, the tone of these cognitions, and the content of these thoughts (e.g., beliefs and attitudes) might all represent aspects of sleep-related arousal. Therefore, there is a need for studies to include different measures to assess changes in arousal associated with treatment.
An intriguing mind-body intervention that appears to be particularly relevant for reducing cognitive and somatic arousal is a short-term group treatment program known as mindfulness-based stress reduction (MBSR). Originally developed by Jon Kabat-Zinn (1990), the MBSR program teaches participants the principles of mindfulness (non-judging, patience, beginner’s mind, trust, non-striving, acceptance, and letting go) and its practice. In this context, mindfulness is defined as non-judgmental present focused awareness (Kabat-Zinn, 1990). It is cultivated through formal meditation practice (e.g., body scan, sitting meditation) and informal applications of the principles of mindfulness to daily life. Unlike cognitive therapy (Beck, Rush, Shaw, & Emery, 1979) which challenges dysfunctional thoughts that lead to maladaptive behaviors, MBSR teaches patients to change their relationship with thoughts and feelings by developing an objective, compassionate, and inquisitive approach to thoughts and feelings. This shift in perspective can lead to enhancement of self-regulation, cognitive and emotional flexibility, and decreased experiential avoidance (see Shapiro, Carlson, Astin, & Freedman, 2006 for further discussion). In addition, mindfulness meditation is often associated with relaxation but it is contrasted from behavioral relaxation techniques in that the goal of mindfulness meditation is not to induce relaxation of the body but rather to cultivate awareness and stay present with whatever thoughts or body sensations arise in the moment (Kabat-Zinn, 1990). For example, progressive muscle relaxation consists of tensing and relaxing different muscle groups in a sequence throughout the body whereas the body scan meditation consists of a sequence of observing sensations across the body without moving the body or actively attempting to change tension levels (Kabat-Zinn, 1990).
Since its inception, MBSR has been used in the treatment of a variety of disorders including chronic pain, generalized anxiety disorder, fibromyalgia, psoriasis, cancer, and depression (Grossman, Niemann, Schmidt, & Walach, 2004; Kabat-Zinn, 2003; Majumdar, Grossman, Dietz-Waschkowski, Kersig, & Walach, 2002). Significant reductions in self-reported cognitive and somatic anxiety following MBSR have been reported for outpatients referred for a variety of medical conditions including chronic pain, hypertension, and gastrointestinal disorders (Kabat-Zinn, Chapman, & Salmon, 1997) with durable effects lasting up to three years (Miller, Fletcher, & Kabat-Zinn, 1995). A recent meta-analysis on MBSR (Grossman et al., 2004) reported a medium effect size (d=.50) for pre-to-post treatment effects on mental health variables (e.g., anxiety, depression) while a separate review (Baer, 2003) concluded that MBSR meets criteria as a “probably efficacious” treatment for improving psychological functioning based on definitions for empirically supported treatments.
There are several theoretical and practical reasons for considering mindfulness meditation as a treatment for insomnia. First, the principles of mindfulness, such as letting go, acceptance, and non-striving, are theoretically congruent with the goal of reducing sleep-related arousal. For example, one of the instructions for stimulus control involves getting out of bed to do a self-soothing activity if not sleeping within 15-20 minutes. Implicit in these instructions are the principles of letting go of the need to stay in bed in order to fall asleep, accepting that sleep may not come immediately, and finding an activity that is non-striving for sleep. In fact, insomnia patients who completed CBT-I reported that learning to accept their current sleep state and accepting that sleep cannot be forced were among the most helpful components of treatment (Manber, Hydes, & Kuo, 2004). Thus, a mindfulness-based approach provides a conceptual framework for targeting sleep-related arousal that could compliment the behavioral components of CBT-I that target the nighttime symptoms of insomnia. Second, mindfulness meditation encourages a shifting of one’s relationship to cognitions rather than challenging and changing the content of one’s thoughts, as would be the case in traditional CBT-I. In this manner, the mindful approach encourages participants to observe their thoughts and relate to them with greater clarity and perspective (Shapiro et al., 2006). This approach might be more appealing to patients than challenging beliefs and attitudes, as is currently advocated in most CBT-I protocols.
A few preliminary studies have investigated the impact of mindfulness meditation on sleep disruption (Shapiro, Bootzin, Figueredo, Lopez, & Schwartz, 2003; Britton, Shapiro, Penn, & Bootzin, 2003; Heidenreich, Tuin, Pflug, Michal, & Michalak, 2006). Though these studies varied in methodology and the population sampled, the findings indicate that mindfulness meditation as a single intervention might not yield strong effects on sleep. However, more promising results were found in a recent pilot study (Bootzin & Stevens, 2005) investigating a multi-component intervention including mindfulness meditation along with several sleep and stress management techniques for adolescents who complained of insomnia, daytime sleepiness, and had a substance abuse history. Participants who completed the program (42% of the sample) reported improvements in SOL, sleep efficiency, number of awakenings, total sleep time, and sleep quality as well as reductions in daytime sleepiness and worry.
Although these studies found some evidence for effects on sleep, it is unclear if mindfulness meditation has any effects on sleep-related arousal, since these studies did not include measures of arousal. Given that two studies found effects on reducing worry (Bootzin & Stevens, 2005; Britton et al., 2003), measuring sleep-related arousal might help to uncover the effects of mindfulness meditation on an important characteristic of insomnia. From a theoretical standpoint, combining mindfulness meditation with CBT-I could lead to a more potent intervention for insomnia by targeting both arousal and nocturnal wakefulness. With the goal of eventually testing this hypothesis, the present study is a preliminary evaluation of an integrated intervention for treating adults with Psychophysiological Insomnia using mindfulness meditation, sleep restriction, stimulus control, sleep hygiene, and sleep education. The specific aims of this pilot project were: 1) to evaluate the feasibility and acceptability of this treatment protocol for the purposes of developing and refining the treatment, and 2) to gather evidence for the treatment effects on sleep and sleep-related arousal. These findings are intended to determine if a more methodologically rigorous testing of the efficacy of this intervention in future studies is warranted and to guide the design of future studies in this area.
Method
STUDY DESIGN
Since this treatment protocol has never been tested for this population, the Stage Model of Behavioral Therapies was followed for treatment development (Rounsaville, Carroll, & Onken, 2001). Specifically, this study fits the Stage I criteria of the model and follows several recommendations for research activities at this stage, including: 1) use of an outlined version of the treatment manual with a small number of cases without a control condition; 2) identification of one or two outcome measures a priori along with a larger number of exploratory process and outcome measures to help understand the treatment; and 3) use of focus groups to obtain qualitative feedback. Therefore, an open-trial, pre-to post-treatment design was deemed most appropriate for this stage of treatment development. The intervention was conducted in a group format with 7-8 participants per group. A broad range of measures were administered to assess changes in sleep, arousal, mindfulness, and emotional distress during the intervention. The study protocol was approved by the Institutional Review Board at Stanford University and all participants provided written informed consent to participate in the study.
PARTICIPANTS
Recruitment
Participants were recruited using flyers posted around the Stanford University campus including bulletin boards in common areas, the student health center, and the Stanford Sleep Disorders Clinic. In addition, electronic advertisements were placed on internet bulletin boards for the San Francisco Bay area. Several participants were also referred from other on-going studies in the Department of Psychiatry at Stanford University.
Selection criteria
Participants for this study included adults between 18-65 years with Psychophysiological Insomnia, a subtype of primary insomnia that is characterized by learned or conditioned arousal (American Academy of Sleep Medicine, 2005). This population was selected to match the goals of investigating sleep-related arousal. The specific inclusion criteria followed recommended guidelines for insomnia research (Edinger et al., 2004; Lichstein, Durrence, Taylor, Bush, & Riedel, 2003): 1) SOL, WASO, or terminal wakefulness (TWAK) >30 minutes at least 3 nights per week or complaints of non-restorative sleep at least 3 nights per week despite adequate circumstances for sleep; 2) complaint of daytime impairment or distress (e.g., fatigue, impaired functioning); 3) evidence of heightened arousal (e.g., inability to shut off mind, physical tension); and 4) symptoms persisting for at least 1 month. The exclusion criteria included: 1) symptoms suggestive of another sleep disorder reported during the interview; 2) untreated current mood, anxiety, or psychotic disorder; 3) current medical condition requiring treatment not supported by this study; and 4) frequent use of alcohol within two hours before bedtime. Although use of sleep facilitating medications (e.g., hypnotic, anxiolitic, sedating anti-depressant) was not an exclusion criterion, only six participants reported use of these medications with three participants agreeing to discontinue the medications during the study and three agreeing to maintain a constant dose throughout the study.
Screening procedures
Participants who expressed an interest in the study were first given a brief screening over the telephone to evaluate general eligibility for the study. Those who passed this screening were scheduled for a baseline visit that included written informed consent to participate in the study, a semi-structured interview to assess the inclusion and exclusion criteria above, administration of pre-treatment questionnaires, and instructions for keeping a sleep diary. Participants who met all criteria for the study were then contacted via email or telephone to schedule the first treatment session.
MEASURES
Sleep diaries
Self-reports of sleep patterns were completed each morning using structured sleep diaries. The sleep variables derived from the diary are listed in Table 1 and follow the recommendations for standardized research assessment of insomnia (Buysse, Ancoli-Israel, Edinger, Lichstein, & Morin, 2006). In addition, ratings of sleep quality, daytime sleepiness, and daytime fatigue were assessed using a 10-point Likert scale (1 to 10). Sleep diaries are considered the standard of practice for the self-reported prospective measurement of sleep in research and clinical practice (Buysse et al., 2006; Smith, Nowakowski, Soeffing, Orif, & Perlis, 2003). In the present study, diaries were collected during each weekly session with the last diary collected one week after the final session. Total wake time (TWT) was used as the primary outcome measure for sleep given the heterogeneous complaints of both sleep onset and sleep maintenance difficulties in this sample as well as the increasing use of TWT as a primary outcome measure in recent studies on CBT-I (Sivertsen et al., 2006; Khalsa, 2004; Strom, Pettersson, & Andersson, 2004).
Table 1.
Nocturnal variables derived from sleep diaries
| Variable | Abbreviation | Definition and method of extraction |
|---|---|---|
| Time of lights out | LO | Time indicated as the intended beginning of the sleep period |
| Sleep onset latency | SOL | Time it takes to fall asleep from LO |
| Number of awakenings | NWAK | Number of awakenings during the night |
| Wake after sleep onset | WASO | Amount of time awake after sleep onset and before final awakening |
| Time of Final awakening | FA | Time indicated as final awakening with no more subsequent sleep. |
| Time out of bed | TOB | Time indicated as the intended end of the sleep period (i.e., rising from bed to start the day). |
| Terminal wakefulness | TWAK | Time from FA to TOB. |
| Total wake time | TWT | Total amount of time awake during the night, calculated as TWT = SOL + WASO + TWAK |
| Time in bed | TIB | Intended sleep period calculated as the time between LO and TOB. |
| Total sleep time | TST | Total time asleep calculated as TIB - TWT. |
| Sleep efficiency | SE | Proportion of time asleep during sleep period, calculated as TST/TIB×100. |
Insomnia Severity Index (ISI)
The Insomnia Severity Index (ISI) is a brief 5-item scale that has been used as both a screening and outcome measure in insomnia treatment research (Bastien, Vallieres, & Morin, 2001). It assesses the severity of both nighttime and daytime symptoms of insomnia over the past week. The scale has adequate internal consistency (Cronbach’s alpha=.74 to .78) with evidence supporting concurrent, predictive, and content validity (Bastien et al., 2001). In the present study, the ISI was used as an outcome measure and given at both pre-treatment and post-treatment with the total score used to assess the global severity of insomnia.
Meditation diaries
In addition to the sleep items, prospective self-report data on the frequency and duration of meditation activity were completed daily following the second session when meditations were introduced. These data were collected to monitor the extent of daily practice of mindfulness meditation.
Kentucky Inventory of Mindfulness Skills (KIMS)
The KIMS is a 39-item self-report measure developed by Baer and colleagues (2004) to measure mindfulness skills. This scale has adequate to good internal consistency (coefficient alpha=.83 to .91) and evidence for validity was supported (Baer et al., 2004). The KIMS was given at pre-treatment and post-treatment to evaluate changes in mindfulness skills.
Pre-Sleep Arousal Scale (PSAS)
The PSAS is a 16-item self-report measure that assesses somatic and cognitive arousal in the period prior to sleep. The scale is organized into two subscales (somatic and cognitive arousal) and a 5-point Likert scale (1 “not at all” to 5 “extremely”) is used to rate the extent to which each item is experienced. Adequate internal consistency has been reported for the PSAS (alpha=.76 and .81 for the somatic and cognitive scales respectively) and elevated PSAS scores have been found to be associated with sleep-onset difficulties (Nicassio et al., 1985). In this study, a time frame of the past week was used. The PSAS was collected on a weekly basis and the total score was used as the primary outcome measure for arousal.
Hyperarousal Scale (HAS)
The HAS is a 26-item self-report measure developed to assess arousal tendencies (Regestein, Pavlova, & Casares, 1996). A 4-point Likert scale (1 “not at all” to 4 “extremely”) is used to rate each of the 26-items. The HAS has been found to differentiate people with insomnia from normal sleepers and is hypothesized to measure trait arousal (Pavlova et al., 2001). In the present study, the HAS was given at pre-treatment and post-treatment as a measure of arousal tendencies.
Dysfunctional Beliefs and Attitudes about Sleep (DBAS)
The DBAS is a 30-item scale that assesses sleep-related cognitions. DBAS scores have been found to be elevated among older people with insomnia compared to good sleepers (Morin, Stone, Trinkle, Mercer, & Remsberg, 1993). In addition, reductions in DBAS scores have been found to correlate with improvements in sleep with CBT-I (Edinger, Wohlgemuth, Radtke, Marsh, & Quillian, 2001b; Morin, Blais, & Savard, 2002). In the present study, the DBAS was given at pre-treatment and post-treatment and the total score was used to evaluate changes in sleep-related cognitions.
Glasgow Sleep Effort Scale (GSES)
The GSES is a 7-item self-report measure of sleep effort during the past week scored on a 3-point Likert scale (0, 1, 2). The scale has adequate internal consistency (Cronbach’s alpha=.77) with evidence discriminating insomnia patients from good sleepers (Broomfield & Espie, 2005). In the present study, the GSES was given at pre-treatment and post-treatment as a state measure of arousal associated with sleep effort.
Positive and Negative Affect Schedule (PANAS)
The PANAS consists of a 10-item positive affect (PA) scale and a 10-item negative affect (NA) scale used as a brief assessment of mood (Watson, Clark, & Tellegen, 1988). Participants are asked to rate the extent to which they have experienced each of the items on a 5-point Likert scale (1 “Very little/not at all” to 5 “Extremely”). The two scales have high internal consistency (PA alpha=.86 to .90; NA alpha=.84 to .87), are stable over a 2-month period, and are largely uncorrelated. In the present study, participants were asked to base their ratings over the past week at pre-treatment and at post-treatment.
Insomnia Treatment Satisfaction Scale (ITSS)
The ITSS is a scale developed at the Stanford Sleep Disorders Clinic for evaluating the satisfaction and impact of insomnia group treatments in the clinic. Adapted from Seligman (1995), the scale consists of 9 items about satisfaction with various aspects of treatment and outcome. In addition, participants are asked to rate the degree of helpfulness for 27 components of treatment on a 4-point scale (0 “Not at all helpful”, 1 “slightly”, 2 “moderately”, 3 “very helpful”). In the present study, the item on overall satisfaction was used as an indicator of treatment satisfaction and the ratings on the helpful components were used to evaluate the perceived usefulness of the various components of treatment. The ITSS was administered and collected during the final treatment session.
PROCEDURES
General format
Following the screening procedures, each group began once a block of 7-10 participants qualified for the study. Four groups with 7-8 participants each were conducted across a 12-month period. All groups were led by the first author, who has completed formal training in both mindfulness meditation and CBT-I, with supervision from the second and third authors.
Intervention
The intervention consisted of an integration of the mindfulness training and exercises from the MBSR program (Kabat-Zinn & Santoreli, 2004) and the behavioral components and sleep education from the CBT-I programs adopted for a group format (Manber & Kuo, 2002; Morin, 1993). The importance of both components for improving sleep and decreasing arousal (e.g., coping with sleep-related distress) were emphasized as an integrative theme throughout the intervention. The treatment was delivered in six weekly sessions lasting 90-120 minutes per session. The first session consisted of introductions by each group member, followed by an overview of the treatment program, sleep education, and an introduction to the principles of mindfulness meditation. Sessions two through five consisted of a combination of mindfulness meditations, sleep restriction, and stimulus control. Each session began with guided formal meditation followed by group discussion. Meditation exercises conducted during the session included breathing meditation, body scan, walking meditation, and eating meditation. Participants were instructed to practice the meditation for at least 30 minutes per day, five days per week. Initially, during the skill acquisition phase, participants were instructed to practice the meditation no later than two hours before bedtime. Once regular daytime practice had been established, the application of mindfulness meditation during sleepless periods at night was discussed and encouraged.
Following the formal mindfulness meditation component, the remainder of each session was spent on the behavioral recommendations for insomnia. During weeks two and three, behavioral instructions for stimulus control and sleep restriction were introduced and each participant was given an individualized program to follow. The program consisted of a prescribed time in bed (TIBrx) based upon the participant’s total sleep time reported in the pre-treatment sleep diary along with stimulus control instructions that include maintaining a consistent wake time, not going to bed until sleepy, and getting out of bed if not falling asleep (or falling back asleep) in 20 minutes. During the remaining weeks, the latter part of the session was spent adjusting the TIBrx based on each participant’s progress and a discussion of any difficulties with the program encountered since the last session. The final session consisted of a discussion of relapse prevention for insomnia and continuation of mindfulness practices.
DATA ANALYSIS PLAN
Guided by the aims of a Stage I treatment development study (Rounsaville et al., 2001) the data analysis plan consisted of an assessment of treatment-related variables to evaluate the feasibility and acceptability of this study followed by an assessment of treatment-outcome variables to evaluate the effects of the intervention on sleep, arousal, psychological functioning, and mindfulness. To assess treatment-related variables, measures of compliance were derived from the sleep and meditation diaries and treatment satisfaction was derived from the ITSS and focus group. Analyses on treatment-outcome variables were conducted using an intent-to-treat sample consisting of the 30 participants who attended the first session with the last observation carried forward for drop-outs. This approach is more conservative than conducting analyses on treatment completers and minimizes the potential bias of early termination due to treatment failure. To assess the effects of the intervention, paired-samples t tests were conducted to compare pre-treatment and post-treatment means on each variable of interest. Since this was a pilot study, careful consideration was given to balancing both Type I and Type II errors (Schoenfeld, 1980). For the primary outcome measures (TWT and PSAS), a simple Bonferroni correction (p=.025) was applied to control for Type I error due to multiple comparisons. For the remaining measures (k=14), a sequential Bonferroni procedure (p≤α/k, p≤α/(k-1)...) was employed to minimize Type I error in a less conservative manner than the simple Bonferroni procedure (see Zhang, Quan, Ng, & Stepanavage, 1997). In addition, effect sizes (Cohen’s d) were calculated using the formula (Mpost - Mpre)/SDpost to pre change to provide an indication of the magnitude of the effects (Grissom & Kim, 2005). When possible, indices of clinical significance were calculated to allow a meaningful interpretation of the results. To explore the relationship between meditation, sleep, and arousal, post-hoc correlations between these variables were examined.
Results
PARTICIPANTS
Recruitment
Participant flow is documented in Figure 1. A total of 45 participants provided written consent to participate in the study and completed the screening interview. Five participants failed to qualify for the study (two for suspected sleep apnea, one for suspected Restless Leg Syndrome, one for an anxiety disorder, and one for a suspected thyroid disorder) and eight participants withdrew prior to treatment (five withdrew to receive other treatments for insomnia and three cited time constraints). Two participants qualified for the study but failed to attend the intervention. Thus, 30 participants began the intervention with each group consisting of seven to eight participants.
FIGURE 1.
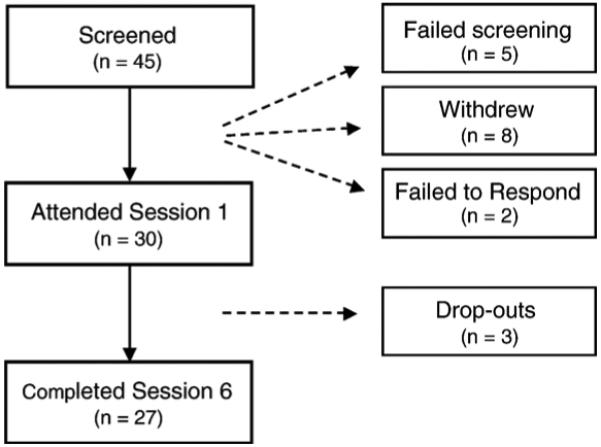
Flow diagram of participant recruitment and attendance.
Participant characteristics
The average age of the 30 participants was 36.4 years (SD=14.1; range=19-62 years) and 60% were female. The ethnic distribution was as follows: 63% Caucasian, 17% Asian, 10% Hispanic, 3% African-American, 7% other/did not respond. All participants had a high school education or above, with an average of 16.6 years of education (range=12-22 years). With regards to marital status, 60% of the participants were single and 40% were married. The duration of the current episode of insomnia ranged from 1 to 480 months, with a mean of 84.8 months and a median of 48 months.
TREATMENT-RELATED VARIABLES
Attendance
From a total of 30 participants who began the intervention, three participants withdrew before the third session and were considered drop-outs (one citing time commitment issues, one citing a lack of interest in the treatment protocol, and one participant provided no reason for discontinuing treatment). Of the remaining 27 participants, those who attended the first and last session and had no more than two absences were considered completers of the intervention. Among these completers, 18 out of 27 participants attended all six sessions, seven participants were absent for one session, and two participants were absent for two sessions.
Compliance with treatment
Compliance with treatment was assessed using the sleep and meditation diary data collected throughout the study. First, compliance with the behavioral components was assessed on TIB, a measure of compliance with sleep restriction, and TOB, a measure of compliance with the stimulus control instructions of maintaining a consistent wake time. These two measures of adherence were previously found to be associated with better treatment outcomes (Riedel & Lichstein, 2001). In the present study, deviations scores between the actual time in bed and the prescribed time in bed (TIB - TIBrx) were calculated as an index of compliance with sleep restriction for each night after instructions for TIBrx were given in session two. On average, participants were staying in bed 21.95 minutes (SD=38.32 minutes) longer than recommended. Deviation scores were also calculated for the difference between the actual time out of bed and the prescribed time out of bed (TOB - TOBrx) as an index of compliance with maintaining a stable wake time. On average, participants were staying in bed 30.46 minutes (SD=36.42 minutes) past the recommended TOB. Second, the total number of meditation sessions and the average duration of these meditation sessions were calculated as indices of compliance with the meditation exercises. The average number of meditations was 21.9 sessions throughout the intervention (SD=14.39) and the average duration of each session was 16.09 minutes (SD=5.84). With a goal of five meditation sessions per week, each lasting 30 minutes, 17 out of 30 participants (57%) reported an average of at least five sessions per week but no participant averaged more than 28 minutes per session throughout the intervention.
Satisfaction with treatment
Using data from the ITSS, two participants reported “completely satisfied”, 13 participants reported “very satisfied”,9 participants reported “fairly well satisfied”, 2 reported “somewhat dissatisfied” and 1 reported “very dissatisfied” with treatment. In addition, the helpful components survey revealed that the cognitive components (e.g., learning more about sleep) received the highest rating (M=2.14, SD=.68), followed by the mindfulness (M=2.04, SD=.78), and behavioral (M=2.00, SD=.90) components, all of which had an average rating of at least moderately helpful. The non-specific (M=1.92, SD=.84) and sleep hygiene (M=1.68, SD=.96) components were rated lowest. Qualitative feedback provided during the focus group revealed that participants enjoyed the overall intervention but also expressed an interest in increasing the number of meditation exercises, having more guidance on the meditation exercises in session, and connecting mindfulness meditation with sleep through anecdotes or theoretical discussions.
TREATMENT-OUTCOME VARIABLES
Sleep
Paired-samples t-tests conducted on sleep diary data revealed several pre to post treatment changes (see Table 2). First, a significant reduction in TWT, the primary outcome measure for sleep, was found, t(29)=6.39, p<.001. On secondary measures, significant reductions in TIB, t(29)=5.32, p<.001, and NWAK, t(29)=3.35, p<.01, were found along with improvements in sleep efficiency, t(29)=6.20, p<.001. No significant changes in TST or sleep quality were found.
Table 2.
Pre-treatment to post-treatment changes
| Measures | Pre-Treatment |
Post-Treatment |
df | t | p | d | ||
|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | |||||
| Sleep | ||||||||
| TWT (min) | 107.91 | 60.68 | 53.47 | 41.61 | 29 | 6.39 | <.001* | -1.17 |
| SOL | 39.09 | 35.47 | 20.82 | 22.06 | 29 | 4.58 | <.001* | -0.84 |
| WASO | 43.95 | 42.75 | 19.11 | 24.72 | 29 | 3.38 | .002 | -0.62 |
| TWAK | 24.87 | 20.73 | 13.54 | 15.92 | 29 | 3.17 | .004 | -0.58 |
| TST (min) | 377.23 | 62.36 | 372.51 | 62.20 | 29 | 0.60 | .553 | -0.11 |
| TIB (min) | 484.45 | 72.14 | 425.40 | 56.14 | 29 | 5.32 | <.001* | -0.97 |
| SE (%) | 78.54 | 10.57 | 87.62 | 9.75 | 29 | 6.20 | <.001* | 1.13 |
| NWAK | 2.21 | 1.79 | 1.22 | 1.35 | 27 | 3.35 | .002* | -0.61 |
| Sleep quality | 5.73 | 1.32 | 6.12 | 1.37 | 27 | 2.38 | .025 | 0.45 |
| Pre-sleep arousal | ||||||||
| Total | 34.73 | 8.94 | 28.03 | 9.02 | 29 | 5.48 | <.001* | -1.00 |
| Cognitive | 21.10 | 6.05 | 16.47 | 6.06 | 29 | 5.20 | <.001* | -0.95 |
| Somatic | 13.63 | 4.33 | 11.57 | 4.26 | 29 | 3.78 | .001 | -0.69 |
| Secondary measures | ||||||||
| Insomnia severity index | 14.90 | 4.72 | 9.57 | 5.41 | 28 | 7.09 | <.001* | -1.32 |
| Dysfunctional beliefs and attitudes about sleep | 113.27 | 31.79 | 84.93 | 40.49 | 29 | 5.77 | <.001.* | -1.05 |
| Hyperarousal scale | 66.50 | 10.00 | 63.83 | 8.93 | 29 | 2.53 | .017 | -0.46 |
| Kentucky inventory of mindfulness skills | 129.93 | 11.78 | 131.83 | 16.19 | 29 | 0.97 | .340 | 0.18 |
| Glasgow sleep effort scale | 6.72 | 2.97 | 3.66 | 2.70 | 28 | 5.19 | <.001* | -0.96 |
| Positive and negative affect schedule (Positive) | 31.53 | 6.46 | 32.80 | 6.77 | 29 | 0.99 | .328 | 0.18 |
| Positive and negative affect schedule (Negative) | 18.30 | 5.76 | 16.77 | 5.20 | 29 | 2.00 | .055 | -0.37 |
| Daytime sleepiness | 3.88 | 1.93 | 3.80 | 1.96 | 27 | .26 | .795 | -.05 |
| Daytime tiredness | 4.10 | 1.58 | 4.16 | 1.86 | 27 | .30 | .770 | .05 |
p<.05 with bonferroni adjustment for multiple comparisons.
Note. Sleep quality ranges from 1 (very poor) to 10 (excellent). Daytime sleepiness and tiredness are rated from 1 to 10 with higher numbers reflecting higher levels of sleepiness/tiredness respectively. For Cohen’s d a positive value indicates an increase from pre to post treatment while a negative value indicates a decrease from pre to post treatment.
To explore the pattern of changes in nocturnal sleep and wakefulness during the intervention, weekly averages were plotted for TWT and TST in Figures 2 and 3 respectively. As depicted in Figure 2, a linear trend was observed for the average TWT from baseline to week six and a line of best fit was calculated for these data, which revealed an average reduction of 9.35 minutes of TWT per week. In Figure 3, a quadratic trend was observed for weekly TST with an inflection point at week three. To describe this pattern, two separate lines of best fit were calculated. The first line was calculated from baseline to week three, indicating an average decrease of 10.82 minutes of TST per week during the first portion of the intervention. A second line was calculated from week three to week six, revealing an average increase of 7.83 minutes of TST per week.
FIGURE 2.
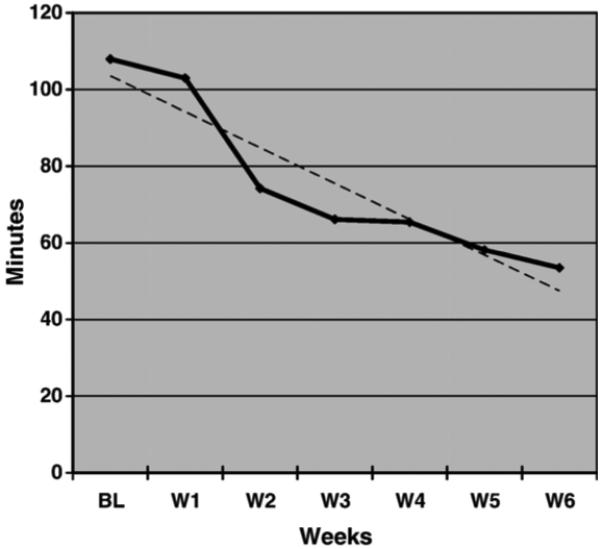
Total Wake Time in minutes across weeks with the line of best fit (y=-9.35×+112.85) indicating a linear trend for reduction in total wake time at night.
FIGURE 3.
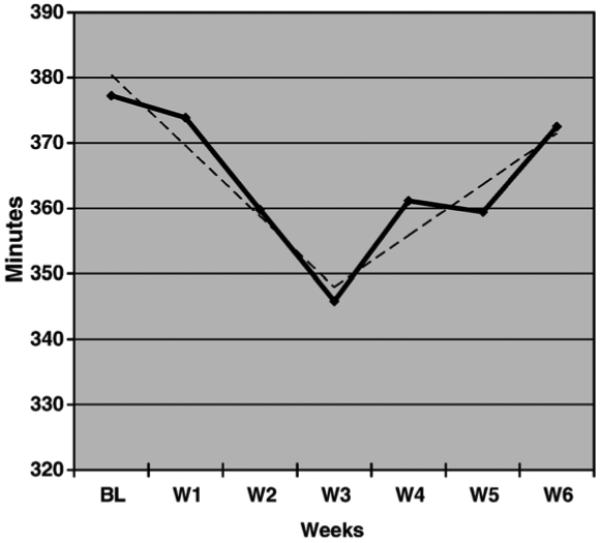
Total Sleep Time in minutes across weeks with one line of best fit (y=-10.82×+391.24) indicating decreasing sleep time from baseline to week 3 and another line of best fit (y=7.83+340.16) indicating increasing sleep time from week 3 to week 6.
Pre-sleep arousal
Paired-samples t-tests conducted on the total PSAS, the primary outcome measure for arousal, revealed significant pre-treatment to post-treatment reductions, t(29)=5.48, p<.001. As shown in Table 2, reductions were also found on both the somatic subscale and cognitive subscale. To explore weekly changes in pre-sleep arousal, weekly averages of total PSAS scores were plotted and a line of best was calculated for these data (see Figure 4). The pattern revealed an average weekly decrease of 0.98 points on the PSAS.
FIGURE 4.
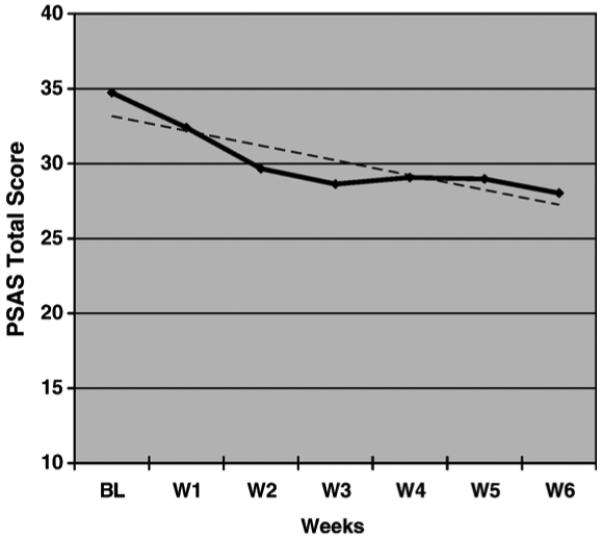
Pre-Sleep Arousal scores across weeks with the line of best fit (y=-.98×+34.15) indicating a linear trend for reduction in pre-sleep arousal.
Secondary outcome measures
The means, standard deviation, and effect sizes of the secondary measures are summarized in Table 2. A significant reduction from pre-treatment to post-treatment was found on global insomnia severity as measured by the ISI, t(28)=7.09, p<.001. Significant reductions were also found on the DBAS, t(29)=5.77, p<.001, indicating a decrease in negative sleep-related cognitions and the GSES, t(29)=5.19, p<.001, indicating a decrease in sleep-related effort. No significant changes were found on the KIMS, HAS, PANAS, or ratings of daytime sleepiness or tiredness.
Relationship between meditation and measures of sleep and arousal
To explore the relationship between mindfulness meditation and sleep and arousal, Pearson correlations were examined between meditations (total amount, total number), pre to post treatment changes in sleep variables (TWT, TST, SE, NWAK, SQ), and pre to post treatment changes in arousal variables (PSAS, HAS). The total number of meditations was significantly correlated with HAS (r=-.38, p< .05). No other significant correlations were found for the other measures.
CLINICAL SIGNIFICANCE
The clinical significance of the intervention was assessed using three methods: 1) quantitative criteria for insomnia on the final diary, 2) 50% reduction of TWT from baseline, and 3) established cutoff scores for insomnia severity, measured by the post-treatment ISI. Using the quantitative criteria of insomnia recommended by Lichstein and colleagues (2003), the percent of participants whose sleep diary met these criteria (SOL>30 minutes or WASO>30 minutes, at least 3 times per week) at the last available observation were computed. Only 4 out of the 30 participants (13%) met this threshold for the nocturnal symptom of insomnia (two participants with SOL>30 minutes and two with WASO>30 minutes). In other words, 87% of the sample would no longer satisfy the inclusion criteria for the study at the end of treatment. Using the second index of clinical significance, a 50% or greater reduction of TWT from baseline, 15 out of the 30 participants in the sample had a clinically significant reduction in TWT at the end of treatment with the average reduction of 54.4 minutes of total wake time (SD=46.67) from pre-treatment to the last available observation. Finally, using validated cut-off scores on the ISI (Bastien et al., 2001), 10 of the 14 participants (71%) who were above the cutoff score of 14 at baseline were below the cutoff score at post-treatment. Only two participants remained above the cutoff score at post-treatment and two did not provide post-treatment data. The rest of the sample was below the cutoff score at baseline and post treatment.
Discussion
The results from this preliminary investigation suggest that mindfulness meditation can be successfully combined with CBT-I in one integrated treatment package. The findings indicate the treatment protocol is feasible to deliver and is an acceptable treatment for individuals seeking treatment for insomnia. The overall patterns of change with treatment demonstrate improvements in nighttime symptoms of insomnia, reductions in pre-sleep arousal, and reductions in sleep-related distress. In addition, an association was found between the practice of meditation and reduction in trait hyperarousal. Together, these findings support further testing to evaluate the efficacy of the intervention and the specific contributions of mindfulness meditation using more rigorous controlled designs.
The first aim of this study was to gather evidence for the feasibility and acceptability of the treatment protocol. In contrast to the high attrition rate reported for adolescents (Bootzin & Stevens, 2005), recruitment and attendance for this study indicate that adults seeking treatment for insomnia can be adequately recruited and retained for this combined intervention. Overall compliance with the recommended sleep schedules was good. On average, participants spent an additional 22 minutes in bed beyond the recommended TIB and did not get out of bed until 30 minutes past the recommended TOB. These variances are similar to previously reported compliance with sleep restriction for older adults (Riedel & Lichstein, 2001). Compliance with meditation was moderate, with 57% of participants practicing at least 5 meditation sessions per week but no participant was able to maintain an average of 30 minutes per session. Unfortunately, few studies have examined compliance with the home practice of mindfulness meditation. One study (Majumdar et al., 2002) that reported compliance data for MBSR in a German sample reported a similar number of meditation sessions per week, but the German sample maintained meditation sessions that were nearly twice as long (32 minutes) as reported in this sample (16 minutes). Given the qualitative feedback from the focus groups, it is possible that compliance could be improved by providing more guidance on meditations during sessions and strengthening methods for practicing meditation at home (e.g., providing CDs). Overall satisfaction with the treatment protocol was high and the hypothesized active ingredients (mindfulness, cognitive, and behavioral components) all received an average rating of moderately helpful or better. Together, these findings have contributed to modifications in the treatment protocol and a revised treatment manual to be used for testing in a controlled study.
The second aim of this study was to gather evidence for the treatment effects on sleep and sleep-related arousal. Statistically significant improvements were found on the primary outcome measure for sleep, TWT, and the secondary sleep measures, NWAK, TIB, sleep efficiency, and ISI. Based on Cohen’s (1988) guidelines for interpreting effect sizes, the magnitude of these within-subject effect sizes were large for TWT, TIB, ISI, and sleep efficiency and moderate for NWAK and sleep quality. The magnitude of the within-subject, pre to post treatment changes in this study were similar to within-subject changes for CBT-I reported previously for TWT (Sivertsen et al., 2006), SOL (Jacobs et al., 2004), and WASO (Edinger et al., 2001a; Morin et al., 1999). In addition, this integrated treatment package combining mindfulness and CBT-I showed larger effects on SOL compared to previous studies using only mindfulness meditation (Britton et al., 2003; Heidenreich et al., 2006) and similar effects on WASO compared to the MBSR group reported by Britton and colleagues (2003). Importantly, the statistically significant effects found in this study were also clinically significant. Half of the completers in this sample experienced a 50% or greater reduction in TWT and examination of weekly changes in TWT revealed a linear pattern with an average weekly reduction of 9.35 minutes over the six weeks. At the end of treatment, only 13% of completers met quantitative criteria for insomnia and all but two participants scored below the cut-off for clinically significant insomnia on the ISI. The pattern of change in weekly TST (Figure 3) revealed a reduction in TST between baseline and week three, that was likely due to the initial sleep restriction instructions, followed by a gradual increase over the last three weeks of treatment to levels near baseline. This quadratic pattern is typical of CBT-I protocols that employ sleep restriction and stimulus control (Perlis, Jungquist, Smith, & Posner, 2005) and suggest that further improvements are likely to emerge beyond the six week period. In summary, this integrative intervention demonstrated several significant improvements in sleep among people with insomnia that are more robust compared to mindfulness-based interventions alone.
The effects of the intervention on arousal included a large effect size on the PSAS and a medium effect size on HAS, suggesting that the intervention exerted stronger effects on pre-sleep arousal compared to trait hyperarousal. This conclusion is also supported by the secondary findings of large effect sizes on measures that might reflect aspects of sleep-related arousal, including sleep effort and beliefs and attitudes about sleep. Considering the significant correlation found between the number of meditation sessions and changes in HAS, the present findings provide some preliminary evidence that mindfulness meditation might be associated with reductions in arousal among people with insomnia. Since this pilot study was not designed to study this mechanism, future studies should consider designs that explicitly investigate the nature of this relationship. Moreover, further efforts to clarify the construct of sleep-related arousal will aid future research in selecting or developing an appropriate measure for capturing changes associated with treatment.
This intervention demonstrated smaller effects on other domains of daytime functioning. No significant change was found on positive affect and a small effect size was observed on negative affect, which was less than the effect sizes previously observed for mental health variables following MBSR alone (Grossman et al., 2004). When considered with the non-significant results for the KIMS, these findings suggest that the degree of exposure to mindfulness meditation may not have been sufficient for obtaining the full benefits typically associated with mindfulness meditation. In addition, no significant changes were found on daytime sleepiness or tiredness. Although it is unclear if the current items from the sleep diaries were sufficiently sensitive to measure these constructs, previous studies have failed to find consistent differences between insomnia patients and controls on sleepiness and fatigue (Krystal, 2007). Further research should continue to investigate methods for measuring daytime functioning among people with insomnia.
The interpretation of the study findings should be made within the confines of several important limitations. First, these results should be viewed as a Stage I pilot study and it would be premature to derive clinical implications at this point. Without a control group the possibility that the observed effects are due to regression towards the mean or to spontaneous recovery cannot be ruled out. In addition, it is of note that the small sample does not represent the diverse population of people with insomnia in terms age, ethnicity, racial distribution, and insomnia severity. Second, the present design precludes testing of the specific effects of meditation compared to the CBT-I components. Although the correlational evidence suggests an association between the practice of meditation and arousal, future studies using constructive, comparative, or dismantling designs would be useful to uncover the specific effects of meditation and the components of CBT-I. Finally, this study only employed self-report measures of sleep and arousal. It is unclear if sleep-related arousal can be accurately captured using self-report measures and the possibility of demand characteristics cannot be ruled out in the context of an open trial in which participants are aware of the conditions and targets of treatment. Therefore, future studies should employ objective measures of cortical arousal (e.g., polysomnography) or autonomic arousal (e.g., skin conductance response) to remove such potential bias. Nevertheless, the current pilot study provides initial support for integrating mindfulness meditation into CBT-I. It is hoped that this pilot study along with future controlled trials will help elucidate the clinical benefits of using a mindfulness approach to treat Psychophysiological Insomnia.
Acknowledgments
This project was supported in part by a National Institutes of Health, National Research Service Award (MH19938) awarded to the first author. Portions of the data from this study were presented at the 2006 meeting for the Associated Professional Sleep Societies in Salt Lake City, UT. The authors would like to thank Dr. Allison Harvey for providing consultation on this project and to Dr. Richard Bootzin for allowing us to review a treatment manual that served as a model for this intervention. The authors are also grateful to Jennalee Cord for providing assistance with data management during this study.
Contributor Information
Jason C. Ong, Department of Psychiatry and Behavioral Sciences, Stanford University Medical Center
Shauna L. Shapiro, Department of Counseling Psychology, Santa Clara University
Rachel Manber, Department of Psychiatry and Behavioral Sciences, Stanford University Medical Center.
References
- American Academy of Sleep Medicine . The International Classification of Sleep Disorders-2. Author; Rochester, MN: 2005. [Google Scholar]
- Baer RA. Mindfulness training as a clinical intervention: A conceptual and empirical review. Clinical Psychology Science and Practice. 2003;10:125–143. [Google Scholar]
- Baer RA, Smith GT, Allen KB. Assessment of Mindfulness by Self-Report: The Kentucky Inventory of Mindfulness Skills. Assessment. 2004;11:191–206. doi: 10.1177/1073191104268029. [DOI] [PubMed] [Google Scholar]
- Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Medicine. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive Therapy of Depression. Guilford; New York: 1979. [Google Scholar]
- Bootzin RR, Stevens SJ. Adolescents, substance abuse, and the treatment of insomnia and daytime sleepiness. Clinical Psychology Review. 2005;25:629–644. doi: 10.1016/j.cpr.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Britton WB, Shapiro SL, Penn PE, Bootzin RR. Treating insomnia with mindfulness-based stress reduction. Sleep. 2003;26:A309. [Google Scholar]
- Broomfield NM, Espie CA. Towards a valid, reliable measure of sleep effort. Journal of Sleep Research. 2005;14:401–407. doi: 10.1111/j.1365-2869.2005.00481.x. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Ancoli-Israel S, Edinger JD, Lichstein KL, Morin CM. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29:1155–1173. doi: 10.1093/sleep/29.9.1155. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Lawrence Earlbaum Associates; Hillsdale, NJ: 1988. [Google Scholar]
- Edinger JD, Wohlgemuth WK, Radtke RA, Marsh GR, Quillian RE. Cognitive behavioral therapy for treatment of chronic primary insomnia: a randomized controlled trial. Journal of the American Medical Association. 2001;285:1856–1864. doi: 10.1001/jama.285.14.1856. [DOI] [PubMed] [Google Scholar]
- Edinger JD, Wohlgemuth WK, Radtke RA, Marsh GR, Quillian RE. Does cognitive-behavioral insomnia therapy alter dysfunctional beliefs about sleep? Sleep. 2001;24:591–599. doi: 10.1093/sleep/24.5.591. [DOI] [PubMed] [Google Scholar]
- Edinger JD, Bonnet MH, Bootzin RR, Doghramji K, Dorsey CM, Espie CA, et al. Derivation of research diagnostic criteria for insomnia: report of an American Academy of Sleep Medicine Work Group. Sleep. 2004;27:1567–1596. doi: 10.1093/sleep/27.8.1567. [DOI] [PubMed] [Google Scholar]
- Grissom RJ, Kim JJ. Effect Sizes for Research: A Broad Practical Approach. Lawrence Erlbaum Associates; Mahwah, NJ: 2005. [Google Scholar]
- Grossman P, Niemann L, Schmidt S, Walach H. Mindfulness-based stress reduction and health benefits. A meta-analysis. Journal of Psychosomatic Research. 2004;57:35–43. doi: 10.1016/S0022-3999(03)00573-7. [DOI] [PubMed] [Google Scholar]
- Heidenreich T, Tuin I, Pflug B, Michal M, Michalak J. Mindfulness-based cognitive therapy for persistent insomnia: a pilot study. Psychotherapy and Psychosomatics. 2006;75:188–189. doi: 10.1159/000091778. [DOI] [PubMed] [Google Scholar]
- Jacobs GD, Pace-Schott EF, Stickgold R, Otto MW. Cognitive behavior therapy and pharmacotherapy for insomnia: a randomized controlled trial and direct comparison. Archives of Internal Medicine. 2004;164:1888–1896. doi: 10.1001/archinte.164.17.1888. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J. Full catastrophe living: Using the wisdom of your body and mind to face stress, pain, and illness. Delacorte Press; New York: 1990. [Google Scholar]
- Kabat-Zinn J. Mindfulness-based interventions in context: Past, present, and future. Clinical Psychology Science and Practice. 2003:144–156. [Google Scholar]
- Kabat-Zinn J, Chapman A, Salmon P. Relationship of cognitive and somatic components of anxiety to patient preferences for different relaxation techniques. Mind/Body Medicine. 1997;2:101–109. [Google Scholar]
- Kabat-Zinn J, Santoreli S. Mindfulness-Based Stress Reduction Professional Training. Center for Mindfulness in Medicine, Health Care, and Society; 2004. [Google Scholar]
- Khalsa SBS. Treatment of Chronic Insomnia with Yoga: A Preliminary Study with Sleep-Wake Diaries. Applied Psychophysiology and Biofeedback. 2004;29:269–278. doi: 10.1007/s10484-004-0387-0. [DOI] [PubMed] [Google Scholar]
- Krystal AD. Treating the health, quality of life, and functional impairments in insomnia. Journal of Clinical Sleep Medicine. 2007;3:63–72. [PubMed] [Google Scholar]
- Kuisk LA, Bertelson AD, Walsh JK. Presleep cognitive hyperarousal and affect as factors in objective and subjective insomnia. Perceptual Motor Skills. 1989;69:1219–1225. doi: 10.1177/00315125890693-228. [DOI] [PubMed] [Google Scholar]
- Lichstein KL, Durrence HH, Taylor DJ, Bush AJ, Riedel BW. Quantitative criteria for insomnia. Behavior Research and Therapy. 2003;41:427–445. doi: 10.1016/s0005-7967(02)00023-2. [DOI] [PubMed] [Google Scholar]
- Majumdar M, Grossman P, Dietz-Waschkowski B, Kersig S, Walach H. Does mindfulness meditation contribute to health? Outcome evaluation of a German sample. Journal of Alternative and Complementary Medicine. 2002;8:719–730. doi: 10.1089/10755530260511720. discussion 731-715. [DOI] [PubMed] [Google Scholar]
- Manber R, Hydes N, Kuo T. What aspects of cognitive-behavioral therapy for insomnia do patients find helpful? Sleep. 2004;27:A282. [Google Scholar]
- Manber R, Kuo TF. Cognitative-behavioral therapies for insomnia. In: Lee-Choing L, Sateia J, Carskadon MA, editors. Sleep Medicine. Hanley and Belfus; Philadelphia: 2002. pp. 177–185. [Google Scholar]
- Miller JJ, Fletcher K, Kabat-Zinn J. Three-year follow-up and clinical implications of a mindfulness meditation-based stress reduction intervention in the treatment of anxiety disorders. General Hospital Psychiatry. 1995;17:192–200. doi: 10.1016/0163-8343(95)00025-m. [DOI] [PubMed] [Google Scholar]
- Morin CM. Insomnia: Psychological Assessment and Management. The Guilford Press; New York: 1993. [Google Scholar]
- Morin CM, Stone J, Trinkle D, Mercer J, Remsberg S. Dysfunctional beliefs and attitudes about sleep among older adults with and without insomnia complaints. Psychology and Aging. 1993;8:463–467. doi: 10.1037//0882-7974.8.3.463. [DOI] [PubMed] [Google Scholar]
- Morin CM, Colecchi C, Stone J, Sood R, Brink D. Behavioral and pharmacological therapies for late-life insomnia: a randomized controlled trial. Journal of the American Medical Association. 1999;281:991–999. doi: 10.1001/jama.281.11.991. [DOI] [PubMed] [Google Scholar]
- Morin CM, Blais F, Savard J. Are changes in beliefs and attitudes about sleep related to sleep improvements in the treatment of insomnia? Behavior Research and Therapy. 2002;40:741–752. doi: 10.1016/s0005-7967(01)00055-9. [DOI] [PubMed] [Google Scholar]
- Morin CM, Bastien C, Savard J. Current Status of cognitive-behavior therapy for insomnia: evidence for treatment effectiveness and feasibility. In: Perlis ML, Lichstein KL, editors. Treating Sleep Disorders: Principles and Practice of Behavioral Sleep Medicine. John Wiley and Sons, Inc; Hoboken, NJ: 2003. pp. 262–285. [Google Scholar]
- National Institutes of Health State of the Science Conference Statement Manifestations and Management of Chronic Insomnia in Adults. Sleep. 2005;28:1049–1057. doi: 10.1093/sleep/28.9.1049. [DOI] [PubMed] [Google Scholar]
- Nicassio PM, Mendlowitz DR, Fussell JJ, Petras L. The phenomenology of the pre-sleep state: the development of the pre-sleep arousal scale. Behavior Research and Therapy. 1985;23:263–271. doi: 10.1016/0005-7967(85)90004-x. [DOI] [PubMed] [Google Scholar]
- Pavlova M, Berg O, Gleason R, Walker F, Roberts S, Regestein Q. Self-reported hyperarousal traits among insomnia patients. Journal of Psychosomatic Research. 2001;51:435–441. doi: 10.1016/s0022-3999(01)00189-1. [DOI] [PubMed] [Google Scholar]
- Perlis ML, Jungquist C, Smith MT, Posner P. Cognitive Behavioral Treatment of Insomnia: A Session-by-Session Guide. Springer; New York, New York: 2005. [Google Scholar]
- Regestein Q, Pavlova M, Casares F. Validation of the hyperarousal scale in primary insomnia subjects. Sleep Research. 1996;25:344. [Google Scholar]
- Riedel BW, Lichstein KL. Strategies for evaluating adherence to sleep restriction treatment for insomnia. Behavior Research and Therapy. 2001;39:201–212. doi: 10.1016/s0005-7967(00)00002-4. [DOI] [PubMed] [Google Scholar]
- Rounsaville BJ, Carroll KM, Onken LS. A stage model of behavioral therapies research: Getting started and moving on from stage I. Clinical Psychology: Science and Practice. 2001;8:133–142. [Google Scholar]
- Schoenfeld D. Statistical considerations for pilot studies. International Journal of Radiation Oncology, Biology, Physics. 1980;6:371–374. doi: 10.1016/0360-3016(80)90153-4. [DOI] [PubMed] [Google Scholar]
- Seligman ME. The effectiveness of psychotherapy. The Consumer Reports study. American Psychologist. 1995;50:965–974. doi: 10.1037//0003-066x.50.12.965. [DOI] [PubMed] [Google Scholar]
- Shapiro SL, Bootzin RR, Figueredo AJ, Lopez AM, Schwartz GE. The efficacy of mindfulness-based stress reduction in the treatment of sleep disturbance in women with breast cancer: An exploratory study. Journal of Psychosomatic. 2003:85–91. doi: 10.1016/s0022-3999(02)00546-9. [DOI] [PubMed] [Google Scholar]
- Shapiro SL, Carlson LE, Astin JA, Freedman B. Mechanisms of Mindfulness. Journal of Clinical Psychology. 2006;62:373–386. doi: 10.1002/jclp.20237. [DOI] [PubMed] [Google Scholar]
- Sivertsen B, Omvik S, Pallesen S, Bjorvatn B, Havik OE, Kvale G, et al. Cognitive behavioral therapy vs zopiclone for treatment of chronic primary insomnia in older adults: a randomized controlled trial. Journal of the American Medical Association. 2006;295:2851–2858. doi: 10.1001/jama.295.24.2851. [DOI] [PubMed] [Google Scholar]
- Smith LJ, Nowakowski S, Soeffing JP, Orif HJ, Perlis ML. The measurement of sleep. In: Perlis ML, Lichstein KL, editors. Treating Sleep Disorders: Principles and Practice of Behavioral Sleep Medicine. John Wiley and Sons, Inc; Hoboken, NJ: 2003. pp. 29–73. [Google Scholar]
- Strom L, Pettersson R, Andersson G. Internet-Based Treatment for Insomnia: A Controlled Evaluation. Journal of Consulting and Clinical Psychology. 2004;72:113–120. doi: 10.1037/0022-006X.72.1.113. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Zhang J, Quan H, Ng J, Stepanavage ME. Some statistical methods for multiple endpoints in clinical trials. Controlled Clinical Trials. 1997;18:204–221. doi: 10.1016/s0197-2456(96)00129-8. [DOI] [PubMed] [Google Scholar]


