Abstract
Violence and aggression are public health problems that can benefit from ongoing research into risk reduction and prevention. Current developmental theories of violence and aggression emphasize biological and psychosocial factors, particularly during adolescence. However, there has been less focus on understanding the interactive, multiplicative effects of these processes. Furthermore, little attention has been given to the pre-, peri-, and postnatal periods, where prevention and intervention may yield effective results. Early health risk factors that influence negative behavioral outcomes include prenatal and postnatal nutrition, tobacco use during pregnancy, maternal depression, birth complications, traumatic brain injury, lead exposure, and child abuse. There is an ample literature to suggest that these early health risk factors may increase the likelihood of childhood externalizing behaviors, aggression, juvenile delinquency, adult criminal behavior, and/or violence. This paper proposes an early health risk factors framework for violence prediction, built on existing developmental theories of criminal behavior and supported by empirical findings. This framework addresses gaps in the adolescent psychopathology literature and presents a novel conceptualization of behavioral disturbance that emphasizes the pre-, peri-, and post-natal periods, when a child’s development is critical and the opportunity for behavioral and environmental modification is high. Implications for such a framework on violence prevention programs are discussed.
Keywords: Health Risk Factors, Alcohol during Pregnancy, Tobacco during Pregnancy, Toxicity and Drugs During Pregnancy, Head Injury During Early Childhood, Teenage Pregnancy, Birth Complications, Malnutrition of the Pregnant Mother, Malnutrition During Early Postnatal Years, Brain Dysfunction, Aggression, Child Behavior Problems, Violence Prevention, Protective Factors, Mediating Factors, Biosocial Interaction
1. Introduction
Childhood aggression, teenage delinquency, and adult violent acts are being increasingly viewed as a public health issue. Identifying early risk factors is an important first step in preventing violence – a major societal problem. Despite decades of research into social and biological risk factors for antisocial and aggressive behavior in children and adults (Farrington et al., 2000; Virkkunen et al., 1995; Webster-Stratton & Hammond, 1999), very little is known about effects of early childhood health factors on these outcomes. More specifically, research into the causes of antisocial and violent behavior has been hindered by three key empirical, conceptual, and methodological gaps in the literature.
1.1 Gaps in the Current Literature
The main empirical gap is that the role of early childhood health risk factors, such as prenatal factors, has been relatively neglected in the child and adolescent psychopathology literature. Recent research indicates that the prenatal stage of child development may be a key period in which effective interventions can potentially prevent later aggressive and violent behavior (Liu & Wuerker, 2005). Despite this, there remains a dearth of literature explicitly discussing the unique impact of prenatal contributors to these outcomes.
The second gap which is conceptual in nature is that to date theoretical frameworks of early risk factors for violence and aggression have not effectively integrated social and biological factors. Furthermore, health risk factors have been largely ignored from these frameworks. One exception is Raine et al. (1997), who more than a decade ago proposed a biosocial interaction model for understanding antisocial behavior. It is clear that childhood externalizing behaviors and adult violence are both byproducts of multiple processes. Violent behavior in adults and externalizing behavior in children are complex phenomena, and it is likely that neither biological nor psychosocial perspectives alone can adequately explain these outcomes. Considering early health factors along with biosocial processes provides a multi-perspective, cross-disciplinary approach that may improve the empirical foundation of the literature and lead to the development of more effective interventions.
The third gap which is methodological in nature concerns lack of prospective longitudinal studies on early health risk factors for negative behavioral outcomes. In the past, studies on childhood antisocial and aggressive behavior were mainly correlational and based on cross-sectional study designs or retrospective investigations. Prospective, longitudinal studies, while still correlational, can help tease out the temporal ordering of variables and assess whether the data are consistent with a causal model, particularly when structural equation modeling is used. For instance, a 15-year follow-up study on the impact of nurse home visits during prenatal and early childhood periods found that nurse-visited children displayed significantly fewer behavioral problems (e.g., arrests, running away, convictions) later in life than children who were not nurse-visited (Olds et al., 1998). Such studies provide compelling evidence that tackling early health risk factors may effectively reduce later aggressive and antisocial behavior and that longitudinal approaches can be used successfully to investigate these effects.
1.2 Current Theoretical Models and the Need for a New Approach
Some of the most important research on developmental theories on violence and aggression has emanated from the work of Moffitt, Farrington, and Fergusson. Moffitt’s dual pathway developmental theory of criminal behavior (Moffitt, 1993; Moffitt et al., 2002) categorizes antisocial adolescents according to their age of onset of deviant behavior (i.e., “early starters” versus “late starters”). “Early starters” are more likely to develop into life-course persistent (LCP) offenders rather than adolescent-limited offenders (as with “late starters”). Individuals in the LCP group are characterized by a combination of temperamental (e.g., negative emotionality) and neurological (e.g., inattention, hyperactivity, low IQ, low resting heart rate) antecedents that begin early in life and give rise to deviant behavior that steadily continues throughout adolescence, especially when combined with environmental deficits such as poor parenting. For instance, Moffitt et al., (2002) previously reported that childhood-onset delinquency had the strongest association with psychopathic personality traits, mental-health problems, substance dependence, greater number of children within the family, family financial problems, parental work problems, and drug-related and violent criminal behavior. Poor neurological status has also predicted male delinquency that began before age 13 years and persisted at high levels afterwards (Moffitt et al., 2006).
Farrington’s integrated cognitive antisocial potential theory (Farrington, 2005) parallels Moffitt’s framework in that it too emphasizes the predictability and developmental trajectories of criminal behavior, with particular attention paid to temperamental and neurological influences. His theory considers an individual’s antisocial potential (AP), or likelihood of engaging in antisocial behaviors, as well as cognitive thought processes that predispose to antisocial behavior. Farrington differentiates individuals with long-term AP from those with short-term AP, much like Moffitt’s LCP and adolescent-persistent distinction. Similar to Moffitt’s findings among LCP individuals, Farrington has reported that individuals with long-term AP are more likely to come from unstable family environments, exhibit lower IQ, display poor attention and hyperactivity, and have poor socialization skills. Indeed, Farrington noted that the most important childhood (ages 8-10 years) predictors of later delinquency were childhood antisocial behavior, impulsivity, low IQ and attainment, family criminality, poverty, large family size, and poor parental child-rearing behavior (Farrington 1995, 2000, 2001). What makes his approach particularly unique, however, is that it is based on data collected on multigenerational sources (Farrington & Walsh, 2007), allowing for a more thorough examination of both individual and family-related antecedents, such as poor parental supervision, parental conflict, and parental antisocial behaviors.
Support for these theories has come in part from important empirical testing by Fergusson and colleagues (Fergusson & Horwood 2001, 2002; Fergusson et al., 1994), who used longitudinal trajectory models to apply Moffitt’s and Farrington’s approaches and identify potential future offenders. Based on structural equation modeling, they observed that early childhood conduct problems predicted later delinquency and accounted for much of the relationship between IQ/academic achievement and delinquency (Fergusson and Horwood, 2001). These findings are consistent with those of Moffitt and Caspi (2001) who reported that childhood-onset delinquency – though not adolescent-onset delinquency – was associated with inadequate parenting, neurocognitive problems, and temperament and behavior problems during childhood. In a 21-year longitudinal study, Fergusson and Horwood (2002) identified male gender, parental criminality, parental conflict, novelty seeking, low IQ, and low self esteem as being most predictive of an offending trajectory.
While research from Moffitt, Farrington, and Fergusson has been influential in the field of developmental psychopathology, there is room for improvement. The children in Moffitt’s studies were all over 3 years of age. Similarly, Farrington’s samples were ages 10 years and older, and Fergusson’s samples were all ages 8 years and older. The theoretical model presented in this paper - the early health risk factor model - builds upon the work of these researchers by taking an integrated approach that includes health, biological, and psychosocial variables to better understanding predictors of negative behaviors, but it is unique in its application to early childhood. It is specifically designed to focus on the early childhood trajectory years and beyond) and developmental outcomes, and in this way can be conceptualized as an extension of Moffitt’s LCP model (Moffitt, 1993; Moffitt et al., 2002). Second, while Moffitt’s and Farrington’s models recognize the importance of biological as well as psychosocial factors, they do not necessarily emphasize the interactive effects between these variables. This is an important consideration, as possible synergistic effects may further increase the risk for negative outcomes beyond that of each of the risk factors independently. This framework addresses this oversight by proposing a greater focus on the interrelationships between a child’s biological and environmental background.
The field may benefit from a conceptual framework that both supports replicated findings about violence and aggression risk factors in children and adolescents while at the same time addressing the above described gaps, thus providing a relatively new perspective on understanding, predicting, and preventing these outcomes. This paper therefore introduces a new framework of early childhood health risk factors that stresses the multiplicative effects of biological, psychosocial, and environmental factors. The focus on early childhood health risk factors also helps inform the development of violence prevention programs, which may reduce childhood behaviors and possible prevent their escalation into more serious juvenile delinquency and adult crime. This framework is presented below, followed by a brief review of recent empirical finding to support this proposal.
2. An Early Health Conceptual Framework for Violence Study
2.1 Overview of the Conceptual Framework
The World Health Organization (WHO) defines health risk factors as behaviors and conditions that increase the likelihood for major injury, disease, or death (Ezzati et al., 2004). Among the 20 health risk factors that the WHO identifies as being of primary importance, preventable health risk factors include low childhood and maternal weight, tobacco and alcohol use, iron deficiency, obesity, high cholesterol and blood pressure, and unsafe sexual practices. These factors have considerable impact on longevity and quality of life, as worldwide estimates suggest they contribute to more than 29 million deaths annually (Ezzati et al., 2004).
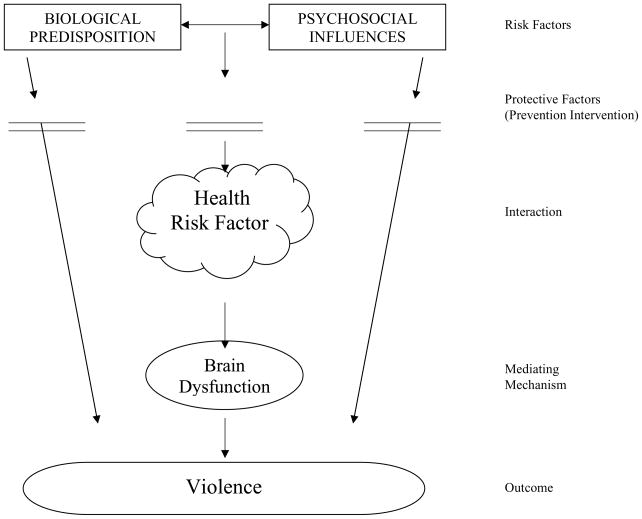
This proposal for an early health risk factor framework (Figure 1) is built upon the author’s previous model of child externalizing behavior (Liu, 2004) which was derived from a biosocial model of violence (Raine et al., 1997). In this proposed new framework, health risk factors are defined as byproducts of biological and psychosocial processes during the pre-, peri-, and postnatal period and are hypothesized to be predictors of adverse outcomes, including childhood externalizing behavior, juvenile delinquency, and adult violence. Of note, the early health risk factors outlined in this framework focuses only on those which occur during prenatal period and childhood, are known to impair brain functions, and which would consequently be expected to influence behavior. Specifically, more negative behavioral conditions are hypothesized to be derived from prenatal insults or toxins, including fetal alcohol syndrome, and emerge on the basis of neural maldevelopment that results in cognitive dysfunction (Bishop & Rutter, 2009), in turn predisposing to life-course-persistent anti-social behavior (Moffitt, 1993). As a result, not all early health risk factors can be exhaustively encompassed since some are still lacking empirical evidence demonstrating their relationship to neurological deficits and violent/aggressive behaviors. In this framework, biological factors by themselves are hypothesized to directly give rise to behavioral outcomes. Similarly, social risk factors can independently and directly lead to externalizing behaviors. Importantly, the framework emphasizes that the interaction between the biological and psychosocial risk factors, which develops early health risk factors, along with potential mediators, may account for the relationship between the predictors and the expected outcomes.
Figure 1.
Health Risk Factor for Violence
Further discussion of the components of this proposed framework follows below, with subsequent descriptions of the empirical evidence supporting each component. Early childhood health risk factors assumed to increase the risk of childhood externalizing behavior and adult violence include exposure to alcohol, tobacco, toxicity and drugs during pregnancy; head injury during early childhood; teenage pregnancy; birth complications; and malnutrition both of the pregnant mother and the child in early postnatal years. This empirical review does not aim to cover all these health factors and will instead focus on those of greatest relevance to the proposed framework (summarized in Table 1).
Table 1.
Review of Early Health Risk Factors that May Increase Risk of Childhood Aggression
| Key Health Risk Factors | Possible Mechanism | Key Intervention/Prevention Strategy |
|---|---|---|
| Smoking during pregnancy | Nicotine and carbon monoxide impairment of the noradrenergic system, decreased dopamine and Serotonin Impaired basal ganglia, cerebral cortex and cerebellar cortex; reduction in brain glucose; retardation in brain growth, structural deficits to the corpus callosum |
Assess smoking status of all expecting or prospective mothers; individual smoking cessation counseling, such as Quit Works, a counseling and referral service used in Massachusetts; pharmacotherapy, such as nicotine patch, gum, inhalers, sprays (Centers For Disease Control and Prevention, 2006); early prevention programs in schools focused on dealing with peer pressure to smoke and building self-esteem (Botvin et al., 1995). |
| Birth complications (preeclampsia, preterm birth, gestational diabetes) | Direct brain injury (e.g. forceps delivery) Indirect brain damage due to hypoxia (e.g., cord compression) with anoxia and selective damage to the hippocampus Perinatal asphyxia leads to damage in the brainstem, thalamus, basla ganglia, parasagittal areas, and hippocampus as well as impaired intellectual abilities, slower academic progress, and poorer visual-motor integration |
Prenatal care, prenatal vitamins, ultrasound, patient education |
| Alcohol, drug abuse during pregnancy | Structural deficits in the corpus callosum Brain growth retardation |
Screening prospective and expecting mothers for drug use/abuse, abuse cessation education, referral for treatment and support, drug counseling, group counseling (National Institute on Drug Abuse, 2009), early prevention programs in schools focused on dealing with peer pressure to take drugs, and building self- esteem (Botvin et al., 1995) |
| Teenage pregnancy | Decreased family stability Increased risk of poverty Increased risk of child abuse Increased risk of poor school performance |
Education of parents, children and teachers aimed at improving social outcomes (academic achievement, resisting peer pressure, developing positive alternatives) and strengthening children’s support system (Hawkins et al., 1999) |
| Maternal Depression | Decreased responsiveness to children Increased risk of affective and behavioral problems, such as hostility, lack of warmth, and disengagement from the child (Gelfand et al., 1990) |
Screening and follow-up, such as community-based support programs (Knitzer, 2008); education and awareness promotion; family support. |
| Malnutrition | Reduction in brain cells Disturbed neurochemistry function (altered neurotransmitter regulation with decreased serotonin and dopamine, altered hormone regulation) Exacerbation of neurotoxins |
Nutritional screening for diagnosis, nutritional intake, and anthropometric measures (child weight, height, head circumference, with values charted at each follow-up); referral to dietician, primary care provider, or speech/language therapist (for swallowing, chewing difficulty) as needed; education on adequate nutrition; food intake diary (McCarthy et al., 2008; Royal College of Nursing, 2006) |
| Lead exposure | Neurotoxicity Decrease in Prefrontal Cortex gray matter volume Alterations in neurotransmitters Inhibition of NMDA receptor |
Blood lead level (BLL) screening, lead risk assessment of home, education, information on sources of lead, follow-up with healthcare provider (Wisconsin Council on Families and Children, 2000) nutrition intervention |
| Head injury | Release of inflammatory mediators that contribute to cell-death cascades or post-synaptic receptor modifications Release of free radicals Excessive glutamate, excitotoxicity from calcium and zinc influx into neurons, dysfunctional mitochondria, separation of axons from cell body, microcirculatory derangements, break-down of blood-brain barrier due to astrocyte foot process swelling (Park et al., 2008), degenerative brain changes (Boll and Barth, 1983 as cited by Bijur, 1990) Damage to prefrontal region that normally controls and regulates reactions generated by limbic system Injuries are associated with sensory and motor deficits, language difficulties, and memory and attention dysfunctions. |
Follow-up care with healthcare provider, educate on prevention in the home (by use of bicycle helmets, car seats or booster seats, gates on stairways and doors, window guards, no wheeled baby walkers) (Schutzman, 2008; Park et al., 2008) |
| Child abuse | Traumatic brain injury (e.g., shaken baby syndrome), which can result in diffuse axonal injury, subdural and/or subarachnoid hemorrhage Hypoxic/ischemic damage that can lead to cerebral edema and then cerebral atrophy and/or infarction, chronic extra-axial fluid collection, cystic encephalomalacia (Kairys et al., 2001) Low family functioning, poor stability, and security within the family |
Education on child development and discipline, social services assistance, screening and treatment of alcohol, drug abuse and psychological problems, family planning, accessible healthcare (Bethea, 1999) |
| Maternal stress | Increased cortisol, which can affect the expression of thousands of genes in fetal brain cells, so elevated levels of maternal cortisol may impact fetal brain development Down regulation in placental 11β-HSD2, increasing fetal exposure to cortisol (Glover et al., 2009; O’Donnell et al., 2009 Mairesse et al., 2007) Reductions in measured PI (pulsatility index) value in the middle cerebral artery and a higher measured PI value in the umbilical artery of the fetus, which is considered a sign of fetal hypoxia (Sjostrom et al., 1997) HPA axis regulation of the fetus; changes in HPA axis activity are associated with physical and mental disorders (Entringer et al. 2009) |
Minimize stress, including seeking psychological therapy is needed; seek support from friends and family to reduce stress during pregnancy; consume adequate protein (in pregnant rats, a 50% reduction in protein intake was associated with a 33% drop in placental 11β-HSD2 activity) |
2.2 Overview of Components of the Framework
2.2.1 Biological Risk Factors
During the pre-, peri-, and postnatal periods, pertinent biological risk factors include both genetic and maternal pathophysiological processes that can impede fetal growth and development (Liu & Wuerker, 2005). While many early biological risk factors during the pre-and postnatal period may be linked to childhood externalizing behaviors, subsequent sections briefly review only a few of the most important factors, including maternal malnutrition, smoking during pregnancy, and birth complications.
2.2.2 Psychosocial Risk Factors
Psychosocial risk factors have been hypothesized to be associated with an increased risk for negative behavioral outcomes. Research on psychosocial risk factors has focused on child abuse (Widom, 1989), marital conflict (Margolin, 1988), poor parenting (Hodgins et al., 2001), poor child rearing (Webster-Stratton & Hammond 1999), domestic violence (Stoff et al., 1997), intimate partner violence (Campbell et al., 2007), and community violence (Scarpa & Haden, 2006). Other important considerations include low socioeconomic status, high psychosocial stress, negative attitudes about pregnancy, pregnancy during adolescence, and psychiatric disorders including substance abuse.
2.2.3 Protective Factors (Prevention and Intervention)
In this framework, protective factors are proposed to be introduced as early as before health risk factors are developed, and can serve to reduce biological vulnerability and intervene psychosocial influence each individually as well as to break down the interaction effects which produce early health risk factors. Protective factors increase one’s resilience, enhance resistance to risk, and strengthen individual defenses against the development of negative outcomes. The prenatal developmental period is intuitively an important time in which to focus on preventative measures because the developing fetus is highly vulnerable to influences from the environment such as maternal nutrition, toxins, and other health-related variables. Thus, proper prenatal care, including regular physical examinations, proper nutrition, general healthy lifestyle, avoidance of toxic chemicals (e.g., tobacco and substance use), as well as strong parental bonding and family harmony, are vital for risk reduction and increasing the chance of a positive outcome despite risk exposure.
2.2.4 Health Risk Factors: The Interaction between Biology and the Psychosocial Environment
Etiology of child externalizing behavior, delinquency, and adult violence is complex, and it is likely that both genes and environment contribute (Baker et al., 2008). An interaction refers to the effects of two risk factors are not merely additive, but are instead multiplicative. The framework argues that the likelihood of early health factors leading to later externalizing behavior would be strongly increased when biological risk factors combine with social risk factors. For example, birth complications, an early health risk factor which has been repeated found to predispose children to later violence (Liu et al., 2009) can be due to both biological risk factors (e.g. poor nutrition) and psychosocial risk factors (e.g. lack of prenatal care). The argument that psychosocial and biological factors interact to influence behavior is also support by other studies. Maternal cigarette smoking has been found to interact with paternal cigarette smoking to increase the risk of juvenile offending (Gibson & Tibbetts, 2000). Rasanen et al. (1999) found that maternal smoking combined with social risk factors (teenage pregnancy, single family status, and unwanted pregnancy), increased the odds ratios for violent offending by up to 9-fold for non-persistent offenses and up to 14-fold for persistent offenses. Furthermore, Caspi et al. (2002) also found that maltreated children with a genotype conferring high levels of monoamine oxidase A (MAO-A) expression were less likely to develop antisocial problems. Kim-Cohen et al. (2006) then further reported that mental health problems are significantly stronger in males with the genotype conferring low MAOA activity.
Recently, epigenetics - the study of inherited alterations in gene expression brought about by mechanisms other than changes in the underlying DNA sequence – has gained increasing attention in health risk factor research as recognition of multiple influences on maternal-fetal pathways has emerged. Epigenetic factors appear important in predicting behavioral outcomes as they may impinge on the neurodevelopment of the fetus’ growing brain. For example, exposure to environmental chemicals during the prenatal period may affect gene transcription, which subsequently influences the development of epigenetic disorders (Fushiki, 2009). Given that biology and the social environment are intertwined and may even have synergistic effects as opposed to independent effects, they are collectively defined unitarily as “health risk factors”.
This framework also recognizes the reciprocal interaction between biological and psychosocial risk factors. The reciprocal interaction is thus defined because psychosocial factors can give rise to biological risk factors, just as biological risk factors can predispose certain individuals to social risk factors. For example, teenage pregnancy as one component of social adversity is generally viewed as a psychosocial risk factor, but it could be that genetic/biological traits (e.g. high hormone levels) may predispose some teenager to engage in sexual activities and become pregnant. Another example of reciprocal interaction is that substance abuse is often viewed as a social-behavioral problem (Curran et al., 2000), but it could also be argued that individuals who abuse drugs and alcohol have a genetic/biological vulnerability to such behavior (Raine et al., 1997).
Thus, this framework proposes that early health risk factors, which results from the interaction between biological and psychosocial risk factors, are critically important in predisposition of later aggression, hyperactivity, and delinquency.
2.2.5 The Mediating Factor of the Brain
Related to the concept of interactive effects, resulting in early health risk factors, is mediating effects, which help explain the mechanism of action when an outcome is observed. Low IQ is among the most-replicated cognitive mediating factors for behavior problems (Hirachi & Hindelang, 1977; Moffitt, 1990). As discussed previously, findings from Liu et al. (2009) document that low IQ mediates the relationship between birth complications and externalizing behaviors. Other authors have similarly found that low IQ mediates the relationship between prenatal biological factors and externalizing outcomes, including such factors as nutrition (Liu, 2004; Liu et al., 2005), maternal tobacco exposure (Weitzman et al., 2002), and maternal alcohol use (Korkman et al., 2003). Furthermore, longitudinal studies have suggested that neuropsychological deficits characterize child and adult violence and antisocial behavior (Raine, 2002), and early neurocognitive deficits precede the onset of these antisocial behaviors (Moffitt, 1990).
Spatial IQ is defined as the non-verbal ability to perform complex visuo-spatial tasks (such as putting blocks together to form a pre-specified pattern, work one’s way bthrough a maze) and is related to problem solving abilities in mathematics, engineering, architecture, science, art, and games. Low spatial IQ at age 3, as well as low verbal and spatial IQ at age 11, predicted Life-Course-Persistent antisocial behavior in community children (Raine et al., 2002). Similar results, showing that impairments in spatial abilities, but not verbal abilities, have been identified among antisocial children (Spelz et al., 1999). A possible mechanism by which low IQ in general functions as a mediating factor could be through its numerous psychosocial influences, including decreased bonding with parents, school failure, occupational failure, and low self-esteem – all of which increase the likelihood for antisocial behavior (Liu et al., 2010).
Regarding specific components of intelligence, verbal IQ on the widely-used Wechsler scales has been more strongly correlated with achievement than performance IQ (Kaufman & Lichtenberger, 2006). The predictive relationship between low verbal intelligence and later criminal behavior may reflect low academic achievement and school difficulties in general.
The relationship between spatial abilities and antisocial behavior may be due to right hemispheric damage, which would result in visuospatial deficits. Right hemisphere dominance aids in facial recognition and orientation, attention, and arousal in infants and young children. Impairment to this brain hemisphere could hinder child-parent bonding and affective processing and regulation (Raine et al., 2005). As such, in this proposed framework cognition is hypothesized to mediate the biosocial risk on externalizing and violent behaviors.
2.2.6 Impact of Early Health Risk Factors on Neurodevelopment and Subsequent Behaviors
Raine (2002) and Raine et al. (2002) present evidence from brain imaging, neuropsychological, and neurological studies that uniformly suggest damage or dysfunction to the prefrontal cortex as a significant predisposition to antisocial behavior. Low IQ and neurocognitive deficits manifested in the right hemisphere, and specifically in the visual and motor cortices, appear particularly important. These deficits can impair a child’s ability to establish visual and physical connections with the parents, thereby reducing parental bonding. Individuals who show significant instrumental aggression have shown evidence of amygdala dysfunction, resulting in resistance to aversive conditioning and passive avoidance learning (Blair, 2004).
The processes that can induce neurocognitive deficits are numerous. Sowell et al. (2007) collected functional MRI data showing that children with heavy prenatal alcohol exposure exhibit functional abnormalities in the left medial temporal and dorsal prefrontal lobes and difficulties, areas which are pertinent in verbal learning. De Haan et al. (2006) as well as others (Sie et al., 2000) have put forth imaging evidence that perinatal asphyxia in term birth infants can lead to damage to the brainstem, thalamus, basal ganglia, parasagittal areas, and hippocampus. In an earlier study by Robertson et al. (1989), among infants who survived peri-natal asphyxia 25% showed significant neurological impairments, including encephalopathy, which has been correlated with impaired general intellectual abilities, slower academic progress, and poorer visual-motor integration in school-aged children. Non-accidental head injury, including shaken-baby syndrome, can cause diffuse neurological insult particularly to the prefrontal cortex. It is no surprise therefore that these injuries are significantly associated with a range of negative neurological outcomes, including a greater incidence of convulsions and subsequent hypoxia; sensory processing and motor deficits; language difficulties; and memory, attention, and other executive dysfunction (Ashton, 2010). Of note, infants and very young children appear to be especially prone to these negative outcomes (Ashton, 2010). Bellinger et al (2008) reports that lead exposure may preferentially inhibit functioning in several areas of the brain, including the ventrolateral prefrontal cortex, anterior cingulate cortex, postcentral gyri, inferior parietal lobule, and cerebellum. More recently, brain imaging studies show that exposure to lead during childhood is associated with decreased gray matter volume in adulthood, especially in portions of the prefrontal cortex which are responsible for mood regulation and decision-making (Cecil et al., 2008), hich in turn predisposes these individuals to violent and criminal behavior. Consequently, lead exposure has been associated with outcomes related to poor impulse control, including violent offending, substance use, and ADHD (Bellinger et al., 2008).
3. Empirical Evidence of Health Risk Factors during Prenatal and Early Childhood
The following section briefly reviews the current empirical evidence supporting components of this framework. This includes the following health risk factors: prenatal maternal smoking; malnutrition; lead exposure; birth complications; childhood head injury; maternal depression and stress; and child abuse. While these do not exhaustively represent all health risk factors of importance to behavioural outcomes, they reflect factors that are most salient, are well-supported by the literature and, to a large degree are amenable to prevention and intervention strategies.
3.1 Smoking during Pregnancy
There is increasing evidence recognizing a significant association between smoking during pregnancy and later conduct disorder and violent offending (Ashford et al., 2008; D’Onofrio et al., 2010; Gibson & Tibbetts, 2000; Raine, 2002; Rasanen et al., 1999, Wakschlag et al., 2009), even after controlling for demographic, parental, and perinatal risk confounds (Brennan et al, 1999, 2002,). In a large birth cohort of males (N = 4169), Brennan et al. (1999) found a two-fold increase in adult violent offending among offspring of mothers who smoked 20 or more cigarettes per day during pregnancy, with an increasing dose-response relationship between number of cigarettes smoked and rates of violence. Furthermore, a recent study has shown that even second-hand exposure to cigarette smoking in the mother has been associated with increased externalizing psychopathology in the child (Gatzke-Kopp & Beauchaine, 2007). The exact mechanism through which maternal tobacco exposure predisposes to behavioral problems in offspring is unclear. However, carbon monoxide and nicotine have been implicated in numerous areas of declining neurofunctioning, including noradrenergic disruption and serotonin and dopamine regulation (Muneoka et al., 1997); reductions in brain glucose (Eckstein et al., 1997); and basal ganglia, cerebral cortex, and cerebellar cortex dysfunction (Olds, 1997; Raine, 2002).
3.2 Malnutrition
Nutrition factors play an active and critical role in brain development during pregnancy and early childhood. Human studies suggest that a deficiency in macronutrients (e.g. protein), micronutrients (e.g. the trace elements zinc and iron), and a component of omega-3 fatty acids (docosahexaenoic acid or DHA – a long-chain essential fatty acid) can disturb brain functioning and thus further predispose to behavior disorders in children (Breakey, 1997; Fishbein, 2001; Lister et al., 2005; Liu & Raine, 2006; Werbach, 1992). Neugebauer et al., (1999) found that the male offspring of mothers severely malnourished during the first and second trimesters of pregnancy had 2.5 times the normal rate of antisocial personality disorder in adulthood. Postnatally, prospective findings indicate that children with protein, zinc, iron, and vitamin B deficiencies at age 3 years exhibited greater antisocial, aggressive, and/or hyperactive behaviors at ages 8, 11, and 17 years and the effects are mediated by cognitive deficits (Liu et al., 2004; Liu et al., 2003). Zinc deficiency has been specifically associated with greater violence and aggression (Liu et al., 2004; Watts, 1990) as well as hyperactivity (Brophy, 1986). In animal models, monkeys and rats deprived of the amino acid tryptophan show higher rates of aggression (Bjork et al., 1999), possibly due to the role of tryptophan in synthesizing serotonin, which has been shown to be reduced in individuals with impulsivity and violent behaviors (Virkkunmen et al., 1995; Werbach, 1995). Low iron has also been linked to aggression, conduct disorder, and juvenile delinquency (Rosen et al., 1985; Werbach, 1995). Furthermore, iron supplementation was shown improve cognitive and behavioral outcomes among non-iron deficient children with attention-deficit hyperactivity disorder (Sever et al., 1997).
The precise mechanism of action in these relationships is not well understood. There is speculation that proteins or minerals play a role in either regulating neurotransmitters or hormones, or else exacerbate neurotoxins, and in doing so predispose to brain dysfunction, aggression, and hyperactivity (Coccaro et al., 1997; Ferris & Grisso, 1996; Liu & Raine, 2006). Despite these promising findings, the research literature on malnutrition and externalizing behaviors remains both limited and controversial (Liu et al., 2004).
3.3 Lead Exposure
The toxic effects of lead on neurodevelopment are well-known, along with their consequent impact on behavior and development (Chen & Rogan, 2005; Dietrich et al., 2004; Koller et al., 2004; Needleman et al., 1996;Bellinger et al., 2005). Both animal studies (Delville, 1999) and human studies have found that higher lead levels measured either in the bone or blood are significantly related to aggressive and delinquent behavior in childhood (Burns et al., 1999; Needleman et al., 1996) as well as in early adulthood (Wright et al., 2008). Monkeys exposed to lead show patterns of behavioral impairment resembling the deficits of children with attention deficit hyperactivity disorder (ADHD) (Rice, 2000). Burns et al. (1999) found that school children from a lead-smelting community had increased externalizing behavior problems. This effect remained after controlling for a number of confounding variables, including quality of the child’s home environment, maternal psychopathology, and the child’s IQ. Sciarillo et al. (1992) found that children with elevated blood lead had behavior problems scores that were 2.7 times those of the controls. In addition, even children with low lead exposure may suffer from impaired cognitive performance (Koller et al., 2004). For example, Needleman (1996) found that children with elevated bone lead levels but no symptoms of lead toxicity showed higher aggression and delinquency scores compared to low-lead counterparts (Needleman, 1996). More recently, Wright et al. (2008) found that elevated blood lead concentrations during the prenatal and postnatal are associated with higher rates of criminal arrests in early adulthood. Nevertheless, studies in this area are to date few and limited, with many studies limited by small sample size or restriction to males only.
It is recognized that elevated blood lead concentrations are more common among children living in poverty, likely accounting for the mediating role of socioeconomic status between lead and behavioral outcomes (ATSDR, 1992; Hubbs-Tait et al., 2005). Environmental toxicity, micronutrients deficiency, and negative social environments may all produce adverse cognitive and behavioral outcomes (Moore, 2005), underscoring the importance of recognizing combined “health risk factors” (i.e. early health, biological, and psychosocial factors).
3.4 Birth Complications
Birth complications have been associated with antisocial behavior, particularly in the context of mitigating factors such as socioeconomic status and family stress. A large sample (N=4,269) of Danish males demonstrated an association between birth complications and violent behavior in adulthood when combined with early maternal rejection of the child (Raine et al., 1994). This has been replicated in four other international samples (Sweden, Finland, Canada, and United States). Prospective longitudinal research has similarly linked obstetric complications in the context of disadvantaged familial environment to increased adult violent crime (Piquero & Tibbetts, 1999). The interaction between pregnancy complications and poor parenting also predicts to adult violence (Hodgins, Kratzer, & Mcneil, 2001), as does the interaction between increased serious obstetric complications and family adversity (Arsenault et al., 2002). For instance, in a sample of Finnish males (N=5,587), the interaction between perinatal risk and being an only child raised the odds of adult violent offending by a factor of 4.4 (Kemppainen et al., 2001).
Recent evidence suggests that cognition might represent a possible third variable accounting for the relationship between birth complications and later externalizing behavior. Liu et al. (2009) examined the influence of birth complications in predisposing to externalizing behaviors at age 11 years. Birth complications included prenatal factors such as hypertension, low blood albumin, bleeding, and other illness (e.g., diabetes, pneumonia, asthma, and epilepsy). Perinatal factors consisted of breech fetal positioning, premature rupture of membrane, fetal distress, and use of instrument delivery (e.g., forceps, Cesarean section). Postnatal factors included cyanosis and treatment with oxygen. Among a sample of nearly 1,800 children, low IQ was significantly associated with both birth complications and externalizing behavior and was found to mediate the birth complications-externalizing behavior relationship. Birth complications may directly contribute to brain dysfunction by impeding proper neurodevelopment. For example, hypoxia selectively damages the hippocampal formation, thereby impairing the limbic system, a brain area repeatedly documented by brain imaging research to be associated with aggression regulation and disinhibition (Liu & Wuerker, 2005; Raine, 2002).
3.5 Head Injury
The literature linking even minor head injuries to violent behavior has consistently implicated traumatic brain injury in children in increasing emotional dysregulation and behavioral problems, including externalizing behaviors (Hatzitaskos et al., 1994; Andrews et al. 1998; Hatzitaskos et al., 1994; Max et al., 1998). Findings from neuropsychological assessments indicate significantly greater frontal and temporal dysfunction among antisocial individuals as compared to controls (Moffitt, 1990). Neuropsychological impairment may in part be a function of poor maternal prenatal conditions (e.g., alcohol and tobacco exposure). Furthermore, brain imaging studies have found that aggressive prisoners exhibit significantly reduced glucose metabolism in the prefrontal cortex relative to matched controls (Raine et al., 1997a). While findings are variable, antisocial and violent individuals as a group appear to be more likely to demonstrate neurocognitive deficits relative to non-violent controls.
3.6 Maternal Depression
Maternal depression has been consistently found to increase the potential for negative behaviors in the offspring (Hoffman et al., 2006; Phelan et al., 2007). In one sample, maternal depression was associated with a six-fold increase in the risk for childhood externalizing behavior (Mohan et al., 1998). More recently, a large-scale longitudinal study found that maternal depression was significantly associated with externalizing behavior boys at the age of 24 months, but by the time the children entered first grade, the relationship was stronger amongst girls (Blatt-Eisengart et al., 2009). This is consistent with other researchers who have noted a moderating effect of gender on the relationship between maternal depression and externalizing behavior (Burt et al., 2005; Wall and Holden, 1994). The contribution of maternal mental health on childhood aggression may be mediated by family factors, such as intra-family conflict and degree of spousal support (Malik et al., 2007) or other maternal characteristics, such as hostile and controlling behaviors (Marchand et al., 2002).
3.7 Maternal Stress
Although not outlined in the proposed framework, maternal stress should also be recognized as an important, overarching component of health risk factors as it can result from any of the above risk factors. Prenatal maternal stress is viewed as exposure of a pregnant woman to distress - either psychosocial or environmental - which induces biochemical changes in the mother that subsequently adversely affect the fetus’ growing organ systems, including the brain. As a multifaceted factor, prenatal stress has been studied in various forms, such as chronic anxiety (Sjostrom et al., 1997) and psychosocial stress (Entringer et al., 2009). Associations have been found between prenatal stress and low birth weight (Wadhwa et al., 2001). Additionally, findings from animal studies have linked prenatal stress to neurodevelopmental modifications in rats (Glover et al., 2009). However, while recent research has demonstrated an association between prenatal stress and the developing fetus (particularly the development of the hypothalamic-pituitary-adrenal, or HPA, axis, - Glover et al., 2009) the link between prenatal stress and behavior remains largely unresolved.
Prenatal stress can be measured through biomarkers, such as cortisol level measurements, or through self-report, such as the Spielberger State-Trait Anxiety Inventory (STAI). Maternal anxiety as measured by the STAI was thought to be related to a down regulation in placental 11β-HSD2, an enzyme that protects the fetus from high levels of circulating maternal cortisol (Glover et al., 2009). High cortisol levels are associated with adverse birth outcomes and may alter fetal development and subsequent adult health (Evans et al., 2008). Furthermore, prenatal stress can be denoted retrospectively. Study shows that experiencing a major negative life event was found to be associated with an alteration in the fetus’ hypothalamic-pituitary-adrenal axis functioning (Entringer, et al., 2009). Such changes are associated with physical and mental disorders, and the study authors concluded that psychosocial stress has a strong impact on an offspring’s mental and behavioral development. Prenatal stress, including exposure to toxicants such as cocaine during pregnancy, has been shown to be linked to elevated salivary cortisol stress reactivity in school-aged children (Lester et al., 2010), and Susman et al. (2010) found that high cortisol reactivity in early adolescent children was directly related to antisocial and rule- breaking behavior.
3.8 Child Abuse
It is not surprising that child abuse and neglect are strongly associated with a host of negative outcomes, including behavioral disturbance in adolescence and later adulthood (Widom, 1989; Teicher, 2000). Abused and neglected children were nearly five times as likely to be arrested for juvenile delinquency, with Caucasian youth demonstrating a staggering 20-fold increase in risk for juvenile arrest for violent crime (English et al., 2002). Moreover, the odds of arrest for criminal behavior as an adult were twice as high amongst victims of abuse. These trends were demonstrated in both males and females. Child maltreatment has also been linked to other negative outcomes, such as drug use and psychiatric illness (Kelly et al., 1997; U.S. Department of Health and Human Services, 2008), which may indirectly increase the odds for aggression and violent behavior. In a review of the long-term consequences of exposure to abuse, Teicher (2000) identified four possible pathways that may explain such findings, including abnormalities in limbic functioning (predisposing to inhibition failure and poor emotional regulation), left hemispheric damage to the hippocampus (resulting in memory impairment), decreased activation of cross-hemispheric connections, and dysregulation of emotion and attention resulting from impairment to the vernis of the cerebellum. From a psychosocial perspective, low self-esteem – a common feature of abuse victims (U.S. Department of Health and Human Services) – has been found to significantly increase the odds of aggression over time (Donnellan et al., 2005).
4. Significance of the Framework and Implications
While the proposed framework should be viewed as heuristic, it may nevertheless provide an improved conceptual framework to better understand and prevent violent and externalizing behavior in children and adolescents. Research is needed in order to test the hypotheses suggested by this framework. Nonetheless, the significance and potential contribution made by the framework can be viewed at four levels.
First, violence is a problem that exacts significant social, psychological, and economic tolls. Childhood externalizing behavior is a well-documented predisposition to adult violence (Farrington, 1989, 2001; Ferris et al., 1996; Surgeon General, 2001). Increased awareness of contributing factors to childhood externalizing behavior can therefore help better elucidate the etiology of violence and crime. Second, the development of interventions to reduce aggression in children hinges on the ability to identify early risk factors that are amenable to change. For instance, Webster-Stratton and Hammon (1997) found that parental training that results in improved competence and maternal parenting skills was associated with a reduction in childhood conduct problems. Consequently, research on risk factors may help inform the development of such prevention programs. Additionally, because violence is considered a public health problem, prevention research plays a vital role in informing public policy and acquiring the economic support needed by local, state, and national agencies to implement violence prevention programs. From a theoretical perspective, the early health risk factor framework emphasizes interactive effects between biological and psychosocial processes, which allows for the examination of health risk factors from the main effect perspective as well as the interactive or biosocial perspective. Thus, it may help provide a clearer theoretical framework for future research on violence prediction and prevention.
Lastly, from a clinical perspective, there has been little recognition of the ways in which general medicine can contribute to violence prevention, but the framework’s emphasis on the early childhood developmental period may help facilitate greater discussion about the role of public health prevention initiatives, including those among general medicine. Health promotion programs implemented early in the child’s life, such as pre- and post-natal home visits by nurses, have been shown to play an important role in reducing future negative behaviors, including juvenile delinquency (Olds et al., 1998). Olds and colleagues (1998) found that children of mothers who received nurse home visits during the pre- and postnatal period displayed strikingly fewer antisocial behaviors at age 15 years than children of mothers who did not receive such visits, including 55% fewer arrests and 80% fewer criminal convictions. Nurse-visited children also had significantly lower rates of cigarette and alcohol use (Olds et al., 1998). Health visitation and wellness programs may be particularly useful for at-risk individuals, such as single and low-income mothers.
While there may certainly be a role for general medicine in helping to shape future child behavior, it should be noted that there are individual differences in susceptibility to early risk factors. For instance, given the same level of lead exposure in the same environment, some children do not develop high blood lead levels or behavioral problems, while others clearly do. These individual differences might reflect effects of protective or ameliorative factors, such as nutrition and parenting. More studies need to be conducted to understand why some risk factors do not uniformly result in adverse outcomes. Along these lines, one shortcoming of this proposed framework is that while interactions between the factors described earlier likely result in violent and aggressive behavior, there is a lack of empirical data to explain the underlying mechanisms of action in these interactions. Further research is needed before definitive conclusions can be made about cause-and-effect relationships and the underlying mechanisms.
Use of public prevention programs may be particularly well-suited to the early childhood developmental period. Tremblay’s research on the prevention of criminal behavior, in which he characterizes the preschool years as being “the best window of opportunity” for successfully intervening to reduce physical aggression (Tremblay, 2002), suggests that optimal results come with three important caveats. First, the intervention should be applied early on in the child’s life rather than waiting until adolescence. Second, it should addresses multiple risk factors rather than a single factor. Third, it should be conducted over an extended period of time (Tremblay & Craig, 1995). Specifically, targeting parenting behaviors, socially disruptive behaviors, and cognitive skills is advocated. Such an intervention might include teaching children verbal skills to mediate their use of physical aggression, helping young children learn to delay gratification, and teaching children cooperative skills to improve social interactions (Tremblay & Craig, 1995). School- and home-based programs can effectively apply such interventions or work closely with parents to impart these skills to children early on, before aggressive behavior escalates throughout the developmental trajectory.
The question of “nature versus nurture” has long been debated and continues to be controversial even today. In the past, sociologists have placed a great emphasis on social factors, while biological psychologists argued that violent behavior is rooted in pathophysiology. By focusing on the early developmental period, this framework serves as an extension of Moffitt’s LCP pathway, and the emphasis of this framework on interaction effects is consistent with the multifactorial approach (i.e., developmental, psychosocial, and environmental) described by Moffitt, Farrington, Raine, Fergusson, and others. This framework’s conceptualization is easily adaptable to prevention efforts, such as those suggested by Tremblay, as early health risk factors are amenable to intervention, as indicated by findings for Olds’ longitudinal study. By concentrating on early, modifiable risk factors, health professionals can play a greater role in secondary and tertiary prevention. This symbolizes a new and exciting interface between the contributions of scientists, educators, and practitioners alike, and may help address the longstanding public health concern of how to prevent violence.
Research Highlights.
This paper proposes an early health risk factors framework for violence prediction and prevention, built on existing developmental theories by Moffitt, Farrington, Raine, and Fergusson, of criminal behavior and supported by empirical findings.
This framework focuses only on the early health factors known to impair brain functions and which would consequently predispose to aggressive and violent behavior.
The “health risk factors” integrate biosocial interactive processes and protective factors, and may help facilitate discussion about the role of violence study and prevention research.
Acknowledgments
Funding: This study is supported by NIH/NIEHS K01-ES015 877 and R01-ES018858.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrews TK, Rose FD, Johnson DA. Social and behavioral effects of traumatic brain injury in children. Brain Injury. 1998;12(2):133–138. doi: 10.1080/026990598122755. [DOI] [PubMed] [Google Scholar]
- Arsenault L, Tremblay RE, Boulerice B, Saucier JF. Obstetrical complications and violent delinquency: Testing two developmental pathways. Child Development. 2002;73(2):496–508. doi: 10.1111/1467-8624.00420. [DOI] [PubMed] [Google Scholar]
- Ashford J, van Lier Pol AC, Timmermans M, Cuijpers P, Koot HM. Prenatal smoking and internalizing and externalizing problems in children studied from childhood to late adolescence. Journal of the American Academy of Child & Adolescent Psychiatry. 2008;47(7):779–787. doi: 10.1097/CHI.0b013e318172eefb. [DOI] [PubMed] [Google Scholar]
- Ashton R. Practitioner Review: Beyond shaken baby syndrome: what influences the outcomes for infants following traumatic brain injury? Journal of Child Psychology and Psychiatry. 2010;51(9):967–980. doi: 10.1111/j.1469-7610.2010.02272.x. [DOI] [PubMed] [Google Scholar]
- ATSDR Impact of Lead-Contaminated Soil on Public Health. S. Department of Health and Human Service; 1992. [Google Scholar]
- Baker LA, Raine A, Liu J, Jacobson KC. Differential genetic and environmental influences on reactive and proactive aggression in children. Journal of Abnormal Child Psychology. 2008;36:1265–1278. doi: 10.1007/s10802-008-9249-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger DC. Neurological and behavioral consequences of childhood lead exposure. Public Library of Science Medicine. 2008 May 27;5(5):e115. doi: 10.1371/journal.pmed.0050115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger DC, Hu H, Kalaniti K, Thomas N, Rajan P, Sambandam S, Ramaswamy P, Balakrishnan K. A pilot study of blood lead levels and neurobehavioral function in children living in Chennai, India. International Journal of Occupational and Environmental Health. 2005;11(2):138–143. doi: 10.1179/oeh.2005.11.2.138. [DOI] [PubMed] [Google Scholar]
- Bethea L. Primary prevention of child abuse. American Family Physicians. 1999 1999 March 15; Retrieved February 17, 2010 from http://www.aafp.org/afp/990315ap/1577.html. [PubMed]
- Bijur PE, Haslum M, Golding J. Cognitive and behavioral sequelae of mild head injury in children. Pediatrics. 1990;86(3):337–344. [PubMed] [Google Scholar]
- Bishop D, Rutter M. Neurodevelopmental Disorders: Conceptual Issues. In: Rutter M, Bishop DVM, Pine DS, Scott S, Stevenson J, Taylor E, Thapar A, editors. Rutter’s Child and Adolescent Psychiatry. 5. Oxford, UK: Blackwell Publishing Ltd; 2009. [Google Scholar]
- Bjork J, Dougherty D, Moeller F, Cherek D, Swann A. The effects of tryptophan depletion and loading on laboratory aggression in men: time course and a food-restricted control. Psychopharmacology. 1999;142(1):24–30. doi: 10.1007/s002130050858. [DOI] [PubMed] [Google Scholar]
- Blair RJR. The roles of orbital frontal cortex in the modulation of antisocial behavior. Brain and Cognition. 2004;55(1):198–208. doi: 10.1016/S0278-2626(03)00276-8. [DOI] [PubMed] [Google Scholar]
- Blatt-Eisengart I, Drabick DA, Monahan KC, Steinberg L. Sex differences in the longitudinal relations among family risk factors and childhood externalizing symptoms. Developmental Psychology. 2009;45(2):491–502. doi: 10.1037/a0014942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boll TJ, Barth J. Mild head injury. Psychiatric Developments. 1983;1(3):263–75. [PubMed] [Google Scholar]
- Botvin GJ, Baker E, Dusenbury L, Botvin EM, Diaz T. Long-term follow-up results of a randomized drug abuse prevention trial in a white middle-class population. Journal of the American Medical Association. 1995;273(14):1106–1112. [PubMed] [Google Scholar]
- Breakey J. The role of diet and behavior in childhood. Journal of Pediatric Child Health. 1997;33(3):190–194. doi: 10.1111/j.1440-1754.1997.tb01578.x. [DOI] [PubMed] [Google Scholar]
- Brennan PA, Grekin ER, Mednick SA. Maternal smoking during pregnancy and adult male criminal outcomes. Archives of General Psychiatry. 1999;56(3):215–219. doi: 10.1001/archpsyc.56.3.215. [DOI] [PubMed] [Google Scholar]
- Brennan PA, Grekin ER, Mortensen EL, Mednick SA. Relationship of maternal smoking during pregnancy with criminal arrest and hospitalization for substance abuse in male and female adult offspring. The American Journal of Psychiatry. 2002;159(1):48–54. doi: 10.1176/appi.ajp.159.1.48. [DOI] [PubMed] [Google Scholar]
- Brophy MH. Zinc and childhood hyperactivity. Biological Psychiatry. 1986;21(7):704–705. doi: 10.1016/0006-3223(86)90137-x. [DOI] [PubMed] [Google Scholar]
- Burns JM, Baghurst PA, Sawyer MG, McMichael AG, Tong SL. Lifetime low-level exposure to environmental lead and children’s emotional and behavioral development at ages 11–13 years. The Port Pirie Cohort Study. American Journal of Epidemiology. 1999;149(8):740–749. doi: 10.1093/oxfordjournals.aje.a009883. [DOI] [PubMed] [Google Scholar]
- Burt KB, Van Dulmen MH, Carlivati J, Egeland B, Sroufe LA, Forman DR, Appleyard K, Carlson EA. Mediating links between maternal depression and offspring psychopathology: the importance of independent data. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2005;46(5):490–499. doi: 10.1111/j.1469-7610.2004.00367.x. [DOI] [PubMed] [Google Scholar]
- Campbell JC, Glass N, Sharps PW, Laughon K, Bloom T. Intimate partner homicide: review and implications of research and policy. Trauma Violence Abuse. 2007;8(3):246–269. doi: 10.1177/1524838007303505. [DOI] [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, Taylor A, Poulton R. Role of genotype in the cycle of violence in maltreated children. Science. 2002;2;297(5582):851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Cecil KM, Brubaker CJ, Adler CM, Dietrich KN, Altaye M, Egelhoff JC, Wessel S, Elangovan I, Hornung R, Jarvis K, Lanphear BP. Decreased brain volume in adults with childhood lead exposure. Public Library of Science Medicine. 2008;5(5):112. doi: 10.1371/journal.pmed.0050112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. A Practical Guide to Working with Health-Care Systems on Tobacco-Use Treatment. Atlanta, GA: US: 2006. [Google Scholar]
- Assessment and Intervention: A child with lead poisoning. Wisconsin Council on Families and Children, 2000. 2000;Chapter 8 Retrieved February 17, 2010 from http://dhs.wi.gov/lead/doc/Chap8NrsgCase.pdf.
- Chen A, Rogan W. Improving behavior of lead-exposed children: micronutrient supplementation, chelation, or prevention. The Journal of Pediatrics. 2005;147(5):570–571. doi: 10.1016/j.jpeds.2005.08.044. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, Kavoussi RJ, Hauger RL. Serotonin function and antiaggressive response to fluoxetine: A pilot study. Biological Psychiatry. 1997;42(7):546–552. doi: 10.1016/S0006-3223(97)00309-0. [DOI] [PubMed] [Google Scholar]
- Curran GM, White HR, Hansell S. Personality, environment, and problem drug use. Journal of Drug Issues. 2000;30(2):375–405. [Google Scholar]
- De Haan M, Wyatt JS, Roth S, Vargha-Khadem F, Gadian D, Mishkin M. Brain and cognitive-behavioural development after asphyxia at term birth. Developmental Science. 2006;9(4):350–358. doi: 10.1111/j.1467-7687.2006.00499.x. [DOI] [PubMed] [Google Scholar]
- Delville Y. Exposure to lead during development alters aggressive behavior in golden hamsters. Neurotoxicology and Teratology. 1999;21(4):445–449. doi: 10.1016/s0892-0362(98)00062-2. [DOI] [PubMed] [Google Scholar]
- Dietrich KN, Ris MD, Succop PA, Berger OG, Bornschein RL. Early exposure to lead and juvenile delinquency. Neurotoxicoly & Teratology. 2001;23(6):511–518. doi: 10.1016/s0892-0362(01)00184-2. [DOI] [PubMed] [Google Scholar]
- Donnellan MB, Trzesniewski KH, Robins RW, Moffitt TE, Caspi A. Low Self-Esteem Is Related to Aggression, Antisocial Behavior, and Delinquency. Psychological Science. 2005;16(4):328–335. doi: 10.1111/j.0956-7976.2005.01535.x. [DOI] [PubMed] [Google Scholar]
- D’Onofrio BM, Singh AL, Iliadou A, Lambe M, Hultman CM, Grann M, Neiderhiser JM, Långström N, Lichtenstein P. Familial confounding of the association between maternal smoking during pregnancy and offspring criminality: A population-based study in sweden. Archives of General Psychiatry. 2010;67(5):529–538. doi: 10.1001/archgenpsychiatry.2010.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstein LW, Shibley IJ, Pennington JS, Carver FM, Pennington SN. Changes in brain glucose levels and glucose transporter protein isoforms in alcohol- or nicotine-treated chick embryos. Brain Research Developmental Brain Research. 1997;103(1):59–65. doi: 10.1016/s0165-3806(97)00117-x. [DOI] [PubMed] [Google Scholar]
- English DJ, Widom CS, Brandford C. Childhood victimization and delinquency, adult criminality, and violent criminal behavior: A replication and extension. Final report presented to the National Institute of Justice, Grant No. 97-IJ-CX-0017.2002. [Google Scholar]
- Entringer S, Kumsta R, Hellhammer DH, Wadhwa PD, Wüst S. Prenatal exposure to maternal psychosocial stress and HPA axis regulation in young adults. Hormones and Behavior. 2009;55(2):292–298. doi: 10.1016/j.yhbeh.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Evans LM, Myers MM, Monk C. Pregnant women’s cortisol is elevated with anxiety and depression - but only when comorbid. Archives of Women’s Mental Health. 2008;11(3):239–248. doi: 10.1007/s00737-008-0019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzati M, Lopez AD, Rodgers A, Murray CJL, editors. Comparative Quantification of Health Risks: Global and Regional Burden of Diseases Attributable to Selected Major Risk Factors. Vol. 1. World Health Organization; Geneva, Switzerland: 2004. [Google Scholar]
- Farrington DP, Welsh BC. Saving Children from a Life of Crime: Early Risk Factors and Effective Interventions. Oxford: Oxford University Press; 2007. [Google Scholar]
- Farrington DP. The Integrated Cognitive Antisocial Potential (ICAP) Theory. In: Farrington DP, editor. Integrated Developmental and Life-Course Theories of Offending. New Brunswick, NJ: Transaction; 2005. pp. 73–92. [Google Scholar]
- Farrington DP. Early predictors of adolescent aggression and adult violence. Violence and Victims. 1989;4(2):79–100. [PubMed] [Google Scholar]
- Farrington DP. Development of Offending and Anti-social Behaviour From Childhood: Key Findings From the Cambridge Study in Delinquent Development. Journal of Child Psychology. 1995;360(6):929–964. doi: 10.1111/j.1469-7610.1995.tb01342.x. [DOI] [PubMed] [Google Scholar]
- Farrington DP. Psychosocial predictors of adult antisocial personality and adult convictions. Behavioral Sciences and the Law. 2000;18(5):605–622. doi: 10.1002/1099-0798(200010)18:5<605::aid-bsl406>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Farrington DP. Predicting adult official and self-reported violence. In: Pinard G-F, Pagani L, editors. Clinical assessment of dangerousness: Empirical contributions. New York, NY: Cambridge University Press; 2001. pp. 66–88. [Google Scholar]
- Fergusson DM, Horwood LJ. Male and female offending trajectories. Development and Psychopathology. 2002;14:159–177. doi: 10.1017/s0954579402001098. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ, Lynskey MT. The childhoods of multiple problem adolescents: A 15 year longitudinal study. Journal of Child Psychology and Psychiatry. 1994;35:1123–1140. doi: 10.1111/j.1469-7610.1994.tb01813.x. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ. Early Disruptive Behavior, IQ, and Later School Achievement, and Delinquent Behavior. Journal of Abnormal Child Psychololgy. 1995;23(2):183–199. doi: 10.1007/BF01447088. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ. The Christchurch Health and Development Study: review of findings on child and adolescent mental health. Australian and New Zealand Journal of Psychiatry. 2001;35:287–296. doi: 10.1046/j.1440-1614.2001.00902.x. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Grisso T, editors. Understanding aggressive behavior in children. New York, NY: New York Academy of Sciences; 1996. [Google Scholar]
- Fishbein D. Biobehavioral Perspectives in Criminology. Belmont, CA: Wadsworth/Thomson Learning; 2001. [Google Scholar]
- Fushiki S. Effects of environmental chemicals on brain development, with a reference to epigenetic gene. No To Hattatsu. 2009;41(3):219–223. [PubMed] [Google Scholar]
- Gatzke-Kopp L, Beauchaine T. Direct and passive prenatal nicotine exposure and the development of externalizing psychopathology. Child Psychiatry and Human Development. 2007;38:255–269. doi: 10.1007/s10578-007-0059-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand DM, Teti DM. The effects of maternal depression on children. Clinical Psychology Review. 1990;10(3):329–353. [Google Scholar]
- Gibson CL, Tibbetts SG. A biosocial interaction in predicting early onset of offending. Psychological Reports. 2000;86(2):509–518. doi: 10.2466/pr0.2000.86.2.509. [DOI] [PubMed] [Google Scholar]
- Glover V, Bergman K, Sarkar P, O’Connor TG. Association between maternal and amniotic fluid cortisol is moderated by maternal anxiety. Psychoneuroendocrinology. 2009;34(3):430–435. doi: 10.1016/j.psyneuen.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Hatzitaskos P, Lewis DO, Yeager CA, Trujillo K. The documentation of central nervous system insults in violent offenders. Juvenile and Family Court Journal. 1994;45(3):29–37. [Google Scholar]
- Hawkins JD, Catalano RF, Kosterman R, Abbott R, Hill KG. Preventing adolescent health-risk behaviors by strengthening protection during childhood. Archives of Pediatrics and Adolescent Medicine. 1999;153(3):226–234. doi: 10.1001/archpedi.153.3.226. [DOI] [PubMed] [Google Scholar]
- Hodgins S, Kratzer L, McNeil TF. Obstetric complications, parenting, and risk of criminal behavior. Archives of General Psychiatry. 2001;58(8):746–752. doi: 10.1001/archpsyc.58.8.746. [DOI] [PubMed] [Google Scholar]
- Hoffman C, Crnic KA, Baker JK. Maternal Depression and Parenting: Implications for Children’s Emergent Emotion Regulation and Behavioral Functioning. Parenting: Science and Practice. 2006;6(4):271–295. [Google Scholar]
- Hubbs-Tait L, Nation J, Krebs N, Bellinger D. Neurotoxicants, Micronutrients, and Social Environments. Individual and Combined Effects on Children’s Development. Psychological Science in the Public Interest. 2005;6(3):57–121. doi: 10.1111/j.1529-1006.2005.00024.x. [DOI] [PubMed] [Google Scholar]
- Kairys S, Alexander R, Block R, Everett V, Hymel K, Jenny C. Shaken Baby Syndrome: Rotational Cranial Injuries—Technical Report. Pediatrics. 2001;108(1):206–210. doi: 10.1542/peds.108.1.206. [DOI] [PubMed] [Google Scholar]
- Kaufman AS, Lichtenberger EO. Assessing adolescent and adult intelligence. New York: John Wiley & Sons; 2006. [Google Scholar]
- Kelly TP, McArdle P. Using the Achenbach Child Behaviour Checklist in the differential diagnosis of disruptive behaviour disorders. Irish Journal of Psychological Medicine. 1997;14(4):136–138. [Google Scholar]
- Kemppainen L, Jokelainen J, Jaervelin MR, Isohanni M, Raesaenen P. The one-child family and violent criminality: A 31-year follow-up study of the Northern Finland 1966 birth cohort. American Journal of Psychiatry. 2001;158(6):960–962. doi: 10.1176/appi.ajp.158.6.960. [DOI] [PubMed] [Google Scholar]
- Kim-Cohen J, Caspi A, Taylor A, Williams B, Newcombe R, Craig IW, Moffitt TE. MAOA, maltreatment, and gene-environment interaction predicting children’s mental health: new evidence and a meta-analysis. Mol Psychiatry. 2006;11(10):903–13. doi: 10.1038/sj.mp.4001851. [DOI] [PubMed] [Google Scholar]
- Knitzer J, Theberge S, Johnson K. Reducing maternal depression and its impact on young children: toward a responsive early childhood policy framework. Project Thrive, issue brief No. 2. 2008 Retrieved February 17, 2010 from http://www.nccp.org/publications/pdf/text_791.pdf.
- Koller K, Brown T, Spurgeon A, Levy L. Recent developments in low-level lead exposure and intellectual impairment in children. Environmental Health Perspectives. 2004;112(9):987–994. doi: 10.1289/ehp.6941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkman M, Kettunen S, Autti-Rämö I. Neurocognitive impairment in early adolescence following prenatal alcohol exposure of varying duration. Child Neuropsychology. 2003;9(2):117–128. doi: 10.1076/chin.9.2.117.14503. [DOI] [PubMed] [Google Scholar]
- Lester BM, Lagasse LL, Shankaran S, Bada HS, Bauer CR, Lin R, Das A, Higgins R. Prenatal cocaine exposure related to cortisol stress reactivity in 11-year-old children. The Journal of Pediatrics. 2010;157(2):288–295. doi: 10.1016/j.jpeds.2010.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister JP, Blatt GJ, Debassio WA, Kemper TL, Tonkiss J, Galler JR, Rosene DL. Effect of prenatal protein malnutrition on numbers of neurons in the principal cell layers of the adult rat hippocampal formation. Hippocampus. 2005;15(3):393–403. doi: 10.1002/hipo.20065. [DOI] [PubMed] [Google Scholar]
- Liu J. Childhood externalizing behavior-theory and implication. Journal of Child and Adolescent Psychiatric Nursing. 2004;17(3):93–103. doi: 10.1111/j.1744-6171.2004.tb00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Li L, Fang F. Psychometric properties of the Chinese version of the Parental Bonding Instrument. International Journal of Nursing Studies. 2010 doi: 10.1016/j.ijnurstu.2010.10.008. [Epub ahead of Print], (PMID: 21094942) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Raine A. The effect of childhood malnutrition on externalizing behavior. Current Opinion in Pediatrics. 2006;18(5):565–570. doi: 10.1097/01.mop.0000245360.13949.91. [DOI] [PubMed] [Google Scholar]
- Liu J, Wuerker A. Biosocial Bases of Violence: Implications for Nursing Research. International Journal of Nursing Studies. 2005;42(2):229–41. doi: 10.1016/j.ijnurstu.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Liu J, Raine A, Venables P, Mednick SA. Malnutrition at age 3 years and lower cognitive ability at age 11: A prospective longitudinal study. Archives of Pediatrics & Adolescent Medicine. 2003;157:593–600. doi: 10.1001/archpedi.157.6.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Raine A, Venables P, Mednick SA. Malnutrition at age 3 years predisposes to externalizing behavior problems at ages 8, 11 and 17 years. American Journal of Psychiatry. 2004;161(11):2005–2013. doi: 10.1176/appi.ajp.161.11.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Raine A, Wuerker A, Venables P, Mednick SA. The association of birth complications and externalizing behavior in early adolescents: Direct and mediational effects. Journal of Research on Adolescence. 2009;19(1):93–111. doi: 10.1111/j.1532-7795.2009.00583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mairesse J, Lesage J, Breton C, Bréant B, Hahn T, Darnaudéry M, Dickson SL, Seckl J, Blondeau B, Vieau D, Maccari S, Viltart O. Maternal stress alters endocrine function of the feto-placental unit in rats. American Journal of Physiology. Endocrinology and Metabolism. 2007;292(6):E1526–33. doi: 10.1152/ajpendo.00574.2006. [DOI] [PubMed] [Google Scholar]
- Malik NM, Boris NW, Heller SS, Harden BJ, Squires J, Chazan-Cohen R, Beeber LS, Kaczynski KJ. Risk for maternal depression and child aggression in Early Head Start families: A test of ecological models. Infant Mental Health Journal. 2007;28(2):171–191. doi: 10.1002/imhj.20128. [DOI] [PubMed] [Google Scholar]
- Marchand JF, Hock E, Widaman K. Mutual Relations Between Mothers’ Depressive Symptoms and Hostile-Controlling Behavior and Young Children’s Externalizing and Internalizing Behavior Problems. Parenting. 2002;2(4):335–353. [Google Scholar]
- Margolin G, John RS, Gleberman L. Affective responses to conflictual discussions in violent and nonviolent couples. Journal of Consulting and Clinical Psychology. 1988;56(1):24–33. doi: 10.1037//0022-006x.56.1.24. [DOI] [PubMed] [Google Scholar]
- Max JE, Castillo CS, Robin DA, Lindgren SD, Smith WL, Jr, Sato Y, Arndt S. Posttraumatic stress symptomatology after childhood traumatic brain injury. Journal of Nervous and Mental Disease. 1998;186(10):589–596. doi: 10.1097/00005053-199810000-00001. [DOI] [PubMed] [Google Scholar]
- McCarthy S, Pufulete M, Whelan K. Factors associated with knowledge of genetics and nutritional genomics among dietitians. Journal of Human Nutrition and Dietetics. 2008;21(6):547–554. doi: 10.1111/j.1365-277X.2008.00913.x. [DOI] [PubMed] [Google Scholar]
- Moffitt TE. Adolescence-limited and life-course-persistent antisocial behaviour: A developmental taxonomy. Psychological Review. 1993;100:674–701. [PubMed] [Google Scholar]
- Moffitt TE. The neuropsychology of delinquency: A critical review. In: Tonry M, Morris N, editors. Crime and justice: A review of research. Chicago: University of Chicago Press; 1990. pp. 99–169. [Google Scholar]
- Moffitt TE, Caspi A. Childhood Predictors Differentiate Life-Course-Persistent and Adolescent-limited Antisocial Pathways Among Males and Females. Development and Psychophathology. 2001;13:355–375. doi: 10.1017/s0954579401002097. [DOI] [PubMed] [Google Scholar]
- Moffitt TE, Caspi A, Harrington H, Milne B. Males on the Life-course-persistent and Adolescent-limited Antisocial Pathways: Follow-up at Age 26 Years. Development and Psychopathology. 2002;14:179–207. doi: 10.1017/s0954579402001104. [DOI] [PubMed] [Google Scholar]
- Moffitt TE, Lynam DR, Silva PA. Neuropsychological Tests Predicting Persistent Male Delinquency. Criminology. 2006;32(2):277–300. [Google Scholar]
- Mohan D, Fitzgerald M, Collins C. The relationship between maternal depression (antenatal and pre-school stage) and childhood behavior problems. Irish Journal of Psychological Medicine. 1998;15(1):10–13. [Google Scholar]
- Moore C. An Unhealthy Start in Life-What Matters Most? Psychological Science in the Public Interest. 2005;6(3):i–ii. doi: 10.1111/j.1529-1006.2005.00023.x. [DOI] [PubMed] [Google Scholar]
- Muneoka K, Ogawa T, Kamei K, Muraoka S, Tomiyoshi R, Mimura Y, Kato H, Suzuki MR, Takigawa M. Prenatal nicotine exposure affects the development of the central serotonergic system as well as the dopaminergic system in rat offspring: Involvement of route of drug administrations. Brain Research Developmental Brain Research. 1997;102(1):117–126. doi: 10.1016/s0165-3806(97)00092-8. [DOI] [PubMed] [Google Scholar]
- National Institute on Drug Abuse. Principles of Drug Addiction Treatment: A research-based Guide. 2. National Institutes of Health, US Department of Health and Human Services; 2009. [Last updated April 24, 2009]. Retrieved February 22, 2010 from http://www.drugabuse.gov/PODAT/PODATIndex.html. [Google Scholar]
- Needleman HL, Riess JA, Tobin MJ, Biesecker GE. Bone lead levels and delinquent behavior. Journal of the American Medical Association. 1996;275(5):363–369. [PubMed] [Google Scholar]
- Neugebauer R, Hoek HW, Susser E. Prenatal exposure to wartime famine and development of antisocial personality disorder in early adulthood. Journal of the American Medical Association. 1999;282(5):479–481. doi: 10.1001/jama.282.5.455. [DOI] [PubMed] [Google Scholar]
- O’Donnell K, O’Connor TG, Glover V. Prenatal Stress and Neurodevelopment of the Child: Focus on the HPA Axis and Role of the Placenta. Developmental Neuroscience. 2009;31(4):285–292. doi: 10.1159/000216539. [DOI] [PubMed] [Google Scholar]
- Olds D. Tobacco exposure and impaired development: A review of the evidence. Mental Retardation and Developmental Disabilities Research Reviews. 1997;3(3):257–269. [Google Scholar]
- Olds D, Henderson CR, Jr, Cole R, Eckenrode J, Kitzman H, Luckey D, Pettitt L, Sidora K, Morris P, Powers J. Long-term effects of nurse home visitation on children’s criminal and antisocial behavior: 15-year follow-up of a randomized controlled trial. Journal of the American Medical Association. 1998;280(14):1238–1244. doi: 10.1001/jama.280.14.1238. [DOI] [PubMed] [Google Scholar]
- Park E, Bell JD, Baker AJ. Traumatic brain injury: Can the consequences be stopped? Canadian Medical Association Journal. 2008;178(9):1163–1170. doi: 10.1503/cmaj.080282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan K, Khoury J, Atherton H, Kahn RS. Maternal depression, child behavior, and injury. Injury Prevention. 2007;13(6):403–408. doi: 10.1136/ip.2006.014571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piquero A, Tibbetts S. The impact of pre/perinatal disturbances and disadvantaged familial environment in predicting criminal offending. Studies on Crime & Crime Prevention. 1999;8(1):52–70. [Google Scholar]
- Raine A, Moffitt TE, Caspi A, Loeber R, Stouthamer-Loeber M, Lynam D. Neurocognitive Impairments in Boys on the Life-Course Persistent Antisocial Path. Journal of Abnormal Psychology. 2005;114(1):38–49. doi: 10.1037/0021-843X.114.1.38. [DOI] [PubMed] [Google Scholar]
- Raine A. Annotation: The role of prefrontal deficits, low autonomic arousal and early health factors in the development of antisocial and aggressive behavior in children. Journal of Child Psychology & Psychiatry & Allied Disciplines. 2002;43(4):417–434. doi: 10.1111/1469-7610.00034. [DOI] [PubMed] [Google Scholar]
- Raine A, Brennan P, Mednick SA. Birth complications combined with early maternal rejection at age 1 year predispose to violent crime at age 18 years. Archives of General Psychiatry. 1994;51(12):984–988. doi: 10.1001/archpsyc.1994.03950120056009. [DOI] [PubMed] [Google Scholar]
- Raine A, Brennan P, Farrington D, Mednick SA. Biosocial basis of violence. New York: Plenum Press; 1997. [Google Scholar]
- Raine A, Lencz T, Taylor K, Hellige JB, Bihrle S, LaCasse L, Lee M, Ishikawa S, Colletti P. Corpus callosum abnormalities in psychopathic antisocial individuals. Archives of General Psychiatry. 2003;60:1134–1142. doi: 10.1001/archpsyc.60.11.1134. [DOI] [PubMed] [Google Scholar]
- Raine A, Venables PH, Mednick SA. Low resting heart rate at age 3 years predisposes to aggression at age 11 years: Evidence from the Mauritius Child Health Project. Journal of the American Academy of Child & Adolescent Psychiatry. 1997a;36(10):1457–1464. doi: 10.1097/00004583-199710000-00029. [DOI] [PubMed] [Google Scholar]
- Raine A, Yaralian PS, Reynolds C, Venables PH, Mednick SA. Spatial but not verbal cognitive deficits at age 3 years in persistently antisocial individuals. Development & Psychopathology. 2002;14:25–44. doi: 10.1017/s0954579402001025. [DOI] [PubMed] [Google Scholar]
- Rasanen P, Hakko H, Isohanni M, Hodgins S, Jarvelin MR, Tiihonen J. Maternal smoking during pregnancy and risk of criminal behavior among adult male offspring in the northern Finland 1996 birth cohort. American Journal of Psychiatry. 1999;156:857–862. doi: 10.1176/ajp.156.6.857. [DOI] [PubMed] [Google Scholar]
- Rice D. Parallels between attention deficit hyperactivity disorder and behavioral deficits produced by neurotoxic exposure in monkeys. Environmental Health Perspectives. 2000;108(3):405–408. doi: 10.1289/ehp.00108s3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson CM, Finer NN, Grace MG. School performance of survivors of neonatal encephalopathy associated with birth asphyxia at term. Journal of Pediatrics. 1989;114:343–449. doi: 10.1016/s0022-3476(89)80132-5. [DOI] [PubMed] [Google Scholar]
- Rosen GM, Deinard AS, Schwartz S, Smith C, Stephenson B, Grabenstein B. Iron deficiency among incarcerated juvenile delinquents. Journal of Adolescent Health Care. 1985;6(6):419–423. doi: 10.1016/s0197-0070(85)80045-0. [DOI] [PubMed] [Google Scholar]
- Royal College of Nursing. Malnutrition: what nurses working with children and young people need to know and do. An RCN position statement. London, UK: Royal College of Nursing; 2006. [Google Scholar]
- Scarpa A, Haden SC. Community Violence Victimization and Aggressive Behavior: The Moderating Effects of Coping and Social Support. Aggressive Behavior. 2006;32(5):502–515. [Google Scholar]
- Schutzman S. [Last updated June 9, 2008];Patient Information: head injury in children and adolescents. 2008 From UpToDate For Patients online. Retrieved February 17, 2010 from http://www.uptodate.com/patients/content/topic.do?topicKey=~vEAPHNqmCOJqF1t.
- Sciarillo WG, Alexander G, Farrell KP. Lead exposure and child behavior. American Journal of Public Health. 1992;82(10):1356–60. doi: 10.2105/ajph.82.10.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sever Y, Ashkenazi A, Tyano S, Weizman A. Iron treatment in children with attention deficit hyperactivity disorder. A preliminary report. Neuropsychobiology. 1997;35(4):178–180. doi: 10.1159/000119341. [DOI] [PubMed] [Google Scholar]
- Sie LTL, Van Der Knapp MS, Oosting J, De Vries LS, Lafeber HN, Valk J. MR patterns of hypoxic-ischemic brain damage after prenatal, perinatal or posnatal asphyxia. Neuropediatrics. 2000;31:128–136. doi: 10.1055/s-2000-7496. [DOI] [PubMed] [Google Scholar]
- Sjöström K, Valentin L, Thelin T, Maršál K. Maternal anxiety in late pregnancy and fetal hemodynamics. European Journal of Obstetrics & Gynecology and Reproductive Biology. 1997;74(2):149–155. doi: 10.1016/s0301-2115(97)00100-0. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Lu LH, O’Hare ED, McCourt ST, Mattson SN, O’Connor MJ, Bookheimer SY. Functional magnetic resonance imaging of verbal learning in children with heavy prenatal alcohol exposure. NeuroReport. 2007;18(7):635–639. doi: 10.1097/WNR.0b013e3280bad8dc. [DOI] [PubMed] [Google Scholar]
- Speltz ML, DeKlyen M, Calderon R, Greenberg MT, Fisher PA. Neuropsychological characteristics and test behaviors of boys with early onset conduct problems. Journal of Abnormal Psychology. 1999;108:315–325. doi: 10.1037/0021-843X.108.2.315. [DOI] [PubMed] [Google Scholar]
- Stoff D, Breiling J, Maser J. Handbook of Antisocial Behavior. New York: Wiley; 1997. [Google Scholar]
- Teicher MH. Wounds that time wouldn’t heal: The neurobiology of childhood abuse. Cerebrum. 2000;2(4):50–67. [Google Scholar]
- Tremblay RE. Development of physical aggression from early childhood to adulthood. In: Tremblay RE, Barr RG, Peters RDeV, editors. Encyclopedia on Early Childhood Development [online] Montreal, Quebec: Centre of Excellence for Early Childhood Development; 2002. [Accessed [November 16, 2010]]. pp. 1–6. Available at: http://www.child-encyclopedia.com/documents/TremblayANGxp.pdf. [Google Scholar]
- U.S. Department of Health and Human Services. Youth violence: A report of the Surgeon General. Washington, DC: Author; 2001. [Google Scholar]
- U.S. Department of Health and Human Services. Long-Term Consequences of Child Abuse and Neglect. 2008 Retrieved from http://www.childwelfare.gov/pubs/factsheets/long_term_consequences.pdf.
- Virkkunen M, Goldman D, Nielsen DA, Linnoila M. Low brain serotonin turnover rate (low CSF5-HIAA) and impulsive violence. Journal of Psychiatry and Neuroscience. 1995;20(4):271–275. [PMC free article] [PubMed] [Google Scholar]
- Wadhwa PD, Sandman CA, Garite TJ. The neurobiology of stress in human pregnancy: implications for prematurity and development of the fetal central nervous system. Progress in Brain Research. 2001;133:131–142. doi: 10.1016/s0079-6123(01)33010-8. [DOI] [PubMed] [Google Scholar]
- Wakschlag L, Kistner E, Pine D, Biesecker G, Pickett K, Skol A, Dukic V, Leventhal B, Cox N, Burns J, Wright R, Cook E. Interaction of prenatal exposure to cigarettes and MAOA genotype in pathways to youth antisocial behavior. Molecular Psychiatry, advance online publication. 2009 doi: 10.1038/mp.2009.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall JE, Holden EW. Aggressive, assertive, and submissive behaviors in disadvantaged, inner-city preschool children. Journal of Clinical Child & Adolescent Psychology. 1994;23(4):382–390. [Google Scholar]
- Watts DL. Trace elements and neuropsychological problems as reflected in tissue mineral analysis (TMA) patterns. Journal of Orthomolecular Medicine. 1990;5(3):159–166. [Google Scholar]
- Webster-Stratton C, Hammond M. Treating children with early-onset conduct problems: A comparison of child and parent training interventions. Journal of Consulting and Clinical Psychology. 1997;65(1):93–109. doi: 10.1037//0022-006x.65.1.93. [DOI] [PubMed] [Google Scholar]
- Webster-Stratton C, Hammond M. Marital conflict management skills, parenting style, and early-onset conduct problems: processes and pathways. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 1999;40(6):917–927. [PubMed] [Google Scholar]
- Weitzman M, Byrd RS, Aligne CA, Moss M. The effects of tobacco exposure on children’s behavioral and cognitive functioning: implications for clinical and public health policy and future research. Neurotoxicology and Teratology. 2002;24(3):397–406. doi: 10.1016/s0892-0362(02)00201-5. [DOI] [PubMed] [Google Scholar]
- Werbach M. Nutritional Influences on Aggressive Behavior. Journal of Orthomolecular Medicine. 1995;7(1):45–51. [Google Scholar]
- Werbach MR. Nutritional influences on aggressive behavior. Journal of Orthomolecular Medicine. 1992;7(1):45–51. [Google Scholar]
- Widom CS. Does violence beget violence ? A critical examination of the literature. Psychological Bulletin. 1989;106(1):3–28. doi: 10.1037/0033-2909.106.1.3. [DOI] [PubMed] [Google Scholar]
- Wright JP, Dietrich KN, Ris MD, Hornung RW, Wessel SD, Lanphear BP, Ho M, Rae MN. Association of prenatal and childhood blood lead concentrations with criminal arrests in early adulthood. Public Library of Science Medicine. 2009;5(5):101. doi: 10.1371/journal.pmed.0050101. [DOI] [PMC free article] [PubMed] [Google Scholar]