Abstract
Background
The incidence of sleeping sickness is still considerable in the Komo Mondah focus, in spite of case-detection strategy. A combined strategy that associated both mass screening and vector control is effective for the control of the disease. In the perspective of a targeted vector control in main transmission sites, we have carried out an entomological survey in the epicentre of the focus.
Objectives
To determine tsetse flies distribution, human-fly contact point and eventually risk factors for acquisition of the disease.
Methods
“Vavoua” traps were set for Glossina in four biotopes selected after an interview of HAT patients concerning their working places. Tsetse were captured and dissected. DNA from organs was analysed by PCR for trypanosome infections. The origin of blood meals was determined by ELISA.
Results
The focus is infested by three species of Glossina: G. palpalis palpalis (1149: 91.85%) found in all biotopes; G. fuscipes fuscipes (85: 6.79%) and G. caliginea (17: 1.36%) found in water spots and landing stages. They are infected by three subgenera of trypanosomes and only G. palpalis palpalis is infected by human trypanosomes. G. fuscipes fuscipes is infected by T. brucei sl and G. caliginea is not infected. Flies are absent at the periphery of houses except in one village. Only 29.20% of blood meals were from humans. Landing stages built in swamp mangrove are presenting the higher index of epidemiological risk and populations are exposed to the disease when they go to the area for taking their fishing boats.
Conclusion
Swamp mangrove would be targeted in priority during a vector control campaign.
Keywords: Transmission, Human African Trypanosomiasis, Glossina, Gabon
Introduction
The occurrence of Human African Trypanosomiasis (HAT) in an area is acutely determined by the presence of three factors: the parasite (Trypanosoma), the vector (Glossina or tsetse fly) and the human host. Tsetse populations are influenced mainly by densityindependent factors such as temperature and humidity, which in turn depend on vegetation cover. Fly densities are determined by the availability of hosts and of suitable habitats. The effect of tsetse population density on disease transmission is controversial, since the relationships between transmission and fly density clearly differ in different endemic areas1. It is known that the closeness of human-fly contact is particularly important for the transmission of Trypanosoma brucei gambiense, and it is probably a major factor in the distribution of the disease2,3. Although the transmission modalities of HAT are one of the fundamental characteristics of a given focus, they are often unknown or neglected. Many entomological factors are involved in the transmission of the disease such as teneral state, density and mobility of Glossina4. When associated to others as human behaviour5 and the presence of an animal reservoir6, these factors contribute to maintain the disease in an area.
The Komo Mondah focus is located in the distribution area of Glossina palpalis palpalis Robineau-Desvoidy, 1830 in Central Africa. It is an old sleeping sickness focus. In 1922, the incidence of the disease in the area was 0.78%7. Forty years later, some authors8 reported that 69 HAT cases were detected in the Estuaire province where the Komo Mondah focus is located. The situation remained less known up to year 1991 when an evaluation scientific expedition of the International Scientific Council for Trypanosomiasis Research and Control “ISCTRC”9 revealed that 45 new cases were detected in the country with 95.6% coming from the Estuaire province. From 2003 to 2006, more than thirty new cases were actively detected per year in the focus by the medical team of the national sleeping sickness control program (Programme National de Lutte contre la Trypanosomiase Humaine Africaine “PNLTHA”, unpublished). The Komo Mondah focus provides the 65% of the overall HAT cases diagnosed in the whole country. The incidence of the disease is still considerable in spite of casedetection strategy put in place by the national control program. It is known that a strategy that associated both mass screening and vector control is effective for the control of sleeping sickness. Little is known about entomological features of HAT in the Komo Mondah focus and as far as we are aware, only long-standing publications10,11 are available. This focus is sitting astride humid forest and swamp mangrove where trapping is quite difficult. In the perspective of a targeted vector control in main transmission sites, we have carried out an entomological survey in the epicentre of the focus.
Methods
Survey zone
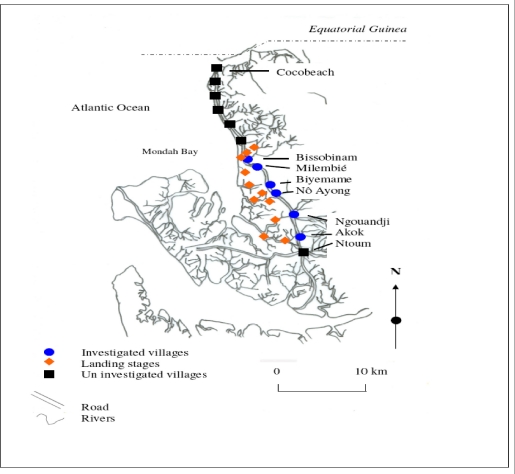
The survey zone, located in coastal plain, extends over six villages (epicentre of the focus) from Ntoum to Cocobeach : Akok (00°51'N, 009°74'E), Ngouandji (00°58'N, 009°70'E), Nô Ayong (00°62'N, 009°68'E), Biyemame (00°65'N, 009°66'E), Milembié (00°67'N, 009°64'E) and Bissobinam (00°95'N, 009°58'E) (fig. 1).
Figure 1.
The Human African Trypanosomiasis focus of Komo Mondah
This focus is of a great epidemiological interest because it is opposite to the HAT Kogo focus of Equatorial Guinea, less than 5 kilometresfar (by maritime way) from Cocobeach. There are two kinds of vegetations: mangrove at the coastline and tropical rain forest between sea and villages. The climate is equatorial type with four seasons: two dry seasons from December to January and from May to September and two rainy seasons from October to November and from February to April. Fishing is the main activity in the area and there are many landing stages (where fishers tie their boat up) located for the most part, at more than six kilometres from the village, except in Bissobinam and Nô Ayong. Houses are built out of wood along the main road in hamlets. Some families have pitched camp near landing stages for drying and/or smoking fish. Livestock farming is scarce in the area. Villagers are rearing mainly goats, poultry, cats and dogs.
Data collection
The survey was carried out during the schools holidays of March because some families living in Libreville used to spend their holidays in the area and are exposed to the disease. Records of HAT patients actively or passively diagnosed from 2002 to 2007, living in the study area and treated at N. Kembo hospital (Libreville), healed or not, were reviewed, and relevant demographic data (name, village of residence, age, gender and the year of the first diagnosis) collected. A follow-up of the patients was then undertaken with the help of the chief of the village and a village nurse. They were informed about the purpose of the survey and asked for consent. Then, they were asked to respond to a standard questionnaire concerning their activities during the last two years prior to diagnosis of the disease since only those ones were easy to remember. Typical activities were as follows: fishing, farming, hunting, forestry activity, domestic activities related to water such as bathing, laundry and search of water for household use.
Entomological survey
After the interview, all working places were prospected and four biotopes were retained for trapping: border of the village, water spots, farms (food crops) and landing stages. “Vavoua” traps12 were then set out in each village, from the border of houses to the coastal area.
Each trap site was cleared to ensure reasonable visibility of the trap and traps were examined twice a day, at 10 a.m. and at 4 p.m., during four days. Data on the species and sex of tsetse captured in each trap were recorded according to the computerbased identification key for tsetse flies13. Flies were dissected 24h after capture in a drop of sterile 0.9% saline water on a microscope slide under a magnifying glass. Midgut, proboscis and salivary glands were examined under a light microscope at a 400X magnification, for the search of trypanosomes. Organs, both infected and uninfected were then transferred separately into microfuge tubes containing 50µl of 70% ethanol and stored at 4°C in the field and at −20°C in the laboratory until use for PCR analysis. Teneral flies were identified by the residual sac from the larval stage in the midgut14. Blood meals from flies were collected on Whatman n°4 papers and stored with desiccant in dark and dry conditions until analysis by ELISA.
PCR amplification
DNA was extracted from organs using the “ReadyAmp® Genomic DNA Purification System” (Promega) Kit as previously described15. The supernatant collected was used directly for PCR or stored at −20°C. Three pairs of specific primers for Trypanosoma congolense “forest type”, Trypanosoma brucei s.l. and Trypanosoma vivax were used16,17. Amplification was done on 25 µl of mixture containing 5 µl of DNA extract, 10 mM Tris-HCl (pH 9), 50 mM KCl, 3 mM MgCl2, 200 µM dNTP (dTTP, dATP, dCTP and dGTP), 1 µM oligonucleotide primers and 0.25 units of Taq (Thermos Aquaticus) DNA polymerase (Appligene-Oncor, USA). Positive (2.5 ng of reference DNA) and negative (without DNA) controls were then included in each set of experiments. The reaction mixtures were overlaid of paraffin oil to prevent evaporation. One denaturing step at 94°C was programmed before 30 amplification cycles. Each cycle contained a denaturing step at 94°C for 1 min, annealing at 60°C for 1 min and extension at 72°C for 1 min. The Taq DNA polymerase was added during the first cycle when temperature reached 60°C (hot-start PCR). The last elongation step was extended to 15 min. The amplified products were analysed on 1.5% agarose gel containing ethidium bromide and visualised by UV light. Samples positives to T. brucei sl were then amplified with primers specific for T. brucei gambiense group 118. Amplification was performed as previous apart from annealing which was at 62°C. PCR test was repeated on all PCR positive and also on some negative samples randomly selected.
ELISA
ELISA was performed as previously described19; human, pig, goat and sheep antibodies were tested. Origin of a sample was determined when its optic density was higher than the mean of optic densities of negative controls of which was added for two times the standard-deviation.
Data analysis
The human-fly contact and the index of epidemiological risk were calculated respectively as follows20,21: With k a constant equal to 632 in farms and 623 at the edge of villages, n the number of human blood meals, C the number of captured flies; a is a constant equal to 1.23 in farms and 0.63 at the edge of villages, P the number of used traps, j the number of trapping days and t the number of teneral flies.
Data were analysed using the computer statistical package for the social sciences (SPSS) for Windows version 12.0. The incidence of HAT according to sex and age was used to test any relationship with age and gender. Overall data were compared using a chi-squared test.
Results
The study sample included 106 HAT patients diagnosed from 2002 to 2007 and present in the area at the time of the survey. We didn't keep out people living in Libreville and take in account only their activities while being in the study area. HAT patient's age were ranged from 9 to 76 years old. Most of them were fishermen (74.47%). Only few individuals were housewives (3.77%) or civil servants (1.88%) (table 1). Few of them were originating from Cameroon (5.7%) and Equatorial Guinea (10.4%) which are endemic countries of sleeping sickness but they were settled in Gabon for more than ten years and had never stayed in a known HAT focus other than the Komo Mondah focus. The number of HAT male patients 68/106 (64.2%) was significantly higher than females 38/106 (35.8%) (p=0.02, 95% confidence interval [95% CI] 0.36–0.90) and there were no significant differences between different age groups.
Table 1.
Population sample (HAT cases) by age, sex and main activity
| Less than or equal to 20 | 21 – 40 | 41 – 60 | Greater than or equal to 60 | |||||
| Males | Females | Males | Females | Males | Females | Males | Females | |
| Fishing | 14 | 7 | 8 | 5 | 12 | 10 | 20 | 3 |
| Studying | 10 | 7 | 3 | 1 | 0 | 0 | 0 | 0 |
| Civil service | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 |
| Housework | 0 | 1 | 0 | 1 | 0 | 2 | 0 | 0 |
Distribution of the vectors and infection rate
A total of 1251 tsetse flies were captured in the six villages; Glossina were absent at the border of all villages, except in Bissobinam; in Akok and Ngouandji, they were also absent from farms. On the opposite, they were present in water spots and landing stages in all villages screened. Apparent density per trap (ADT) was significantly different per biotopes and per villages (chi2=12.14;p<0.05). The highest ADT was observed in landing stages in Bissobinam (table 2). Three species and sub-species of Glossina were captured: Glossina palpalis palpalis (1149; 91.85%), Glossina fuscipes fuscipes Newstead, 1911 (85; 6.79%) and Glossina caliginea Austen, 1929 (17; 1.36%). G. palpalis palpalis was found in all biotopes while G. caliginea and G. fuscipes fuscipes were found only in water spots and landing stages. Teneral flies of G. palpalis palpalis were identified in water spots and landing stages of all villages and also in farms in Bissobinam (table 2); those of G. fuscipes fuscipes were found only in landing stages in Akok. No G. caliginea teneral fly was captured.
Table 2.
Apparent density of Glossina and teneral flies per trap (ADT)
| Villages Biotopes | Akok | Ngouandji | Nô Ayong | Biyemame | Milembié | Bissobinam |
| Border of the village | 0 | 0 | 0 | 0 | 0 | 0.75 a /0 |
| Water spots | 1.25a/0.07b | 1.08a /0.11b | 1.06a /0.08b | 1.31a /0.29b | 1.19a /0.20b | 2.62a /0.25b |
| Farms (food crops) | 0 | 0 | 0.25a /0 | 2.13a /0 | 1.0a /0 | 2.12a /0.17b |
| Landing stages | 1.4a /0.27b | 1.67a /0.22b | 17.25a /1.17b | 4.58a /0.89b | 8.38a/0.75b | 20.16a /0.96b |
Apparent density per trap of Glossina
Apparent density per trap of tenerals
Females were more captured (sex ratio=0.63) and significantly more infected than males (chi2=21.23; p<0.05). 104 Glossina of the total dissected flies (550) were found infected by the three species of trypanosomes tested: Trypanosoma brucei sl (65/104; 62.5%), T. congolense “forest type” (14/104; 13.46%) and T. vivax (11/104; 10.58%) with 13.46% (14/104) of mixed infections of T. brucei sl and T. congolense.
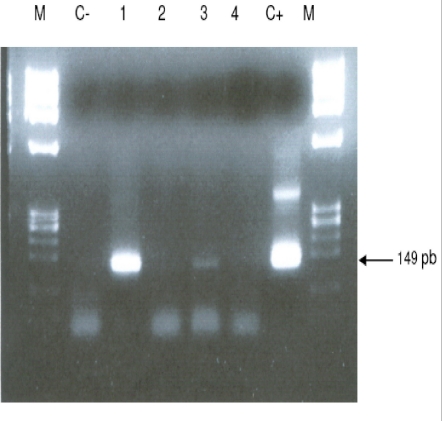
T. brucei sl was found in all biotopes and 18.5% (12/65) of T. brucei gambiense group 1 (fig. 2) were distinguished from T. brucei sl. This sub-specie was identified only in landing stages of Nô Ayong, Biyemame and Bissobinam. G. palpalis palpalis were infected by all species of trypanosomes identified, G. fuscipes fuscipes was infected only by T. brucei sl and G. caliginea was not found infected.
Figure 2.
Ethidium stained gel showing PCR products of an infected fly with T. brucei gambiense group 1 (TRBPA 1/2) primer sets
Feeding behaviour and index of epidemiologic risk
Feeding behaviour was not significantly different between males and females (chi2=0.00; p>0.05). No blood meal was identified from pigs and sheep, while 29.20% (40/137) of blood meals were from humans, 4.38% (6/137) from goats and 66.44% (91/137) were unidentified. Most (95/137) of blood meals were from G. palpalis palpalis and 32.63% (31/91) of these blood meals originated from humans, while G. caliginea didn't feed on humans (table 3).
Table 3.
Origin of blood meals of Glossina
|
G. palpalis palpalis n=95 |
G. fuscipes fuscipes n= 33 |
G. caliginean =9 |
||||
| Blood meals |
% | Blood meals |
% | Blood meals |
% | |
| Human | 31 | 32.63 | 9 | 27.27 | 0 | 0 |
| Goats | 2 | 2.11 | 2 | 6.06 | 2 | 22.22 |
| Undetermined | 62 | 65.26 | 22 | 66.67 | 7 | 77.78 |
| Total | 95 | - | 33 | - | 9 | - |
Higher index of epidemiologic risk was found in landing stages of all villages investigated (table 4). In water spots of Ngouandji and Biyemame, index was low and no risk was observed in farms, except in Bissobinam.
Table 4.
Index of epidemiologic risk per biotopes and per villages
| Akok | Ngouandji | Nô Ayong | Biyemame | Milembié | Bissobinam | |
| Border of villages | 0 | 0 | 0 | 0 | 0 | 0 |
| Water spots | 0 | 0.00068 | 0 | 0.0031 | 0 | 0 |
| Farms (food crops) | 0 | 0 | 0 | 0 | 0 | 0.019 |
| Landing stages | 0.0029 | 0.020 | 1.005 | 0.72 | 0.13 | 0.85 |
Discussion
Fly densities are determined by the availability of hosts and suitable habitats1. The apparent density per trap (ADT) remained relatively low (less than 5 Glossina/trap/day) in all biotopes of all villages, except landing stages of Nô Ayong and Bissobinam where it was more than 15 Glossina/trap/day. Absence of pigs in the area could justify the low density of flies22. Besides, the survey was carried during rainy season where environmental conditions are not suitable for tsetse flies. High ADT of Glossina observed in landing stages of these villages could be due to the numerous of landing stages in these areas. We have noticed that the more frequent the number of landing stages, the more important the Glossina density. One could think that the presence of tsetse flies around Bissobinam village is due to the proximity of river which offers suitable environmental conditions for tsetse.
Three species of Glossina were captured: Glossina palpalis palpalis, Glossina caliginea, and Glossina fuscipes fuscipes. These results agree with observations made by previous authors10,11,23 who showed the predominance of G. palpalis palpalis in the area. The presence of G. caliginea only in water spots and landing stages would be due to their alliance withmangrove forest. G. palpalis palpalis was found in all biotopes while G. fuscipes fuscipes were found only in water spots and landing stages. An author has observed a progressive replacement of G. fuscipes quanzensis by G. palpalis palpalis in Congo24. It is difficult for us to assert that G. palpalis palpalis is pushing away G. fuscipes fuscipes from the Komo Mondah focus only in the regards of our results, though G. palpalis palpalis and G. fuscipes fuscipes were not simultaneously caught in the same biotope. It is known that G. fuscipes fuscipes is rarely found far from open water or riverine habitats3 while G. palpalis palpalis is highly mobile and maintain a close association with villages25. The presence of teneral flies in water spots and landing stages indicates that these areas are reproduction sites of tsetse flies; reproduction sites of G. f. fuscipes were observed only in some landing stages of Akok village. Infected tsetse flies were found in landing stages of all villages investigated; they were also found in water spots of Biyemame and Bissobinam. G. palpalis palpalis was infected by human trypanosomes and seems to be the main vector of HAT in the focus. A tsetse fly must be teneral to become infected26. Since G. fuscipes fuscipes was not infected by T. b. gambiense and teneral captured only in Akok where no human trypanosomes were identified, one can conclude that this specie is not involved in the transmission of disease, but it can be a potential vector since HAT cases are diagnosed in this village. Mixed infections were observed only in landing stages which are natural habitats for wild animals. These results were also observed in some HAT foci of Cameroon17. A recent study showed that such a feature depends more on the vectorial capacities of flies than concurrent trypanosome infections in the host127. Infection rate of tsetse flies in a given biotope is in relation with hosts present in this biotope28. The presence of animal's trypanosomes (Nannomonas, Duttonella, Trypanozoon) in this focus links with the number of feeds taken upon animals. In fact, only 29.2% of blood meals were from humans. As origins of more than 65% of blood meals were unidentified, feeding behaviour of Glossina in this focus is not well elucidated. A previous study has described the presence of many reptiles: big lizards (Varanus niloticus) and crocodiles (Crocodylus cataphractus, Osteolemus tetraspis) upon which Glossina feed23. Domestic and wild animals can harbour human trypanosomes29,30 and are more or less involved in the epidemiology of sleeping sickness. The importance of blood meals coming from animals suggests the need to determine the role animals play in the epidemiological cycle of sleeping sickness in the Komo Mondah focus. This test will be performed by heteroduplex technique which is more exact than ELISA31. The presence of T. congolense could be in relation with goats since it has been observed that Glossina feed sometimes upon them32; however many others animals such as Suidae (Warthog, Potamochoerus) can harbour Nannomonas. It seems that landing stages are main transmission sites of the disease in the focus. Human contracts the disease during his presence in this biotope when he is taking fishing boats and this could explain the fact that fishing is the main activity of 75% of HAT cases. A low index of epidemiologic risk was observed in water spots of Ngouandji and Biyemame. According to the PNLTHA, high incidence of the disease is observed in Bissobinam (PNLTHA, unpublished). Bissobinam is the only village where tsetse flies were caught in all biotopes and where index of epidemiologic risk was also observed in farms. This village is more irrigated than others and the presence of water alone in an endemic area increases the risk for contracting the disease by more than three folds33.
Conclusion
Glossina palpalis palpalis is the main vector of sleeping sickness in the Komo Mondah focus. Tsetse flies are rare around villages and human contracts the disease essentially when he goes to the swamp mangrove for boarding or landing before or after fishing; landing stages being more hazardous biotopes. Glossina feed more upon animals and three trypanosomes subgenera are present. A vector control would be targeted in priority in swamp mangrove where are located main transmission sites of the disease.
Acknowledgments
We thank administrative and sanitary authorities of Komo-Mondah and Noya for their hospitality and help. We are grateful to villagers for their collaboration during this research. Technical assistance was provided by the technical staff of PNLTHA/Gabon and PSR-THA/OCEAC.
References
- 1.WHO, author. Report of a WHO Expert Committee. Geneva: World Health Organisation; 1998. Control and surveillance of african trypanosomiasis. Technical Report Series; n° 881. [PubMed] [Google Scholar]
- 2.Nash TAM. Tsetse flies in British West Africa. London: Colonial Office, HMSO; 1948. pp. 1–75. [Google Scholar]
- 3.Leak SGA. Tsetse biology and ecology. Their role in the epidemiology and control of trypanosomiasis. New York, USA: CABI Publishing; 1999. [Google Scholar]
- 4.Laveissière C, Couret D, Staak C, Hervouet JP. Glossina palpalis et ses hôtes en secteur forestier de Côte d'Ivoire. Relations avec l'épidémiologie de la trypanosomiase humaine. Cah ORSTOM, Sér Ent Méd Parasitol. 1985;23:167–185. (Fre). [Google Scholar]
- 5.Hervouët JP, Laveissière C. Ecologie humaine et maladie du sommeil en Côte d'Ivoire. Cahiers ORSTOM, série Entomologie Médicale et Parasitologie. 1987;34:101–111. (Fre). [Google Scholar]
- 6.Mehlitz D. Les réservoirs animaux de la maladie du sommeil. 1985. Document OMS, TRY/EC/WP/85.
- 7.Sicé A. La trypanosomiase humaine au Gabon. Organisation du laboratoire et de la station de traitement des trypanosomés de Libreville. Annales de Médecine et de pharmacie coloniales. 1922;20:151–158. (Fre). [Google Scholar]
- 8.Gateff C, Fouchet M. La trypanosomiase dans la région de l'Estuaire du Gabon, épidémiologie. Méd Trop. 1968;28:177–198. (Fre). [PubMed] [Google Scholar]
- 9.ISCTRC, author. Formulaire pour l'évaluation du contrôle et de la surveillance de la trypanosomiase humaine africaine, Gabon-21ème réunion. Yamoussokro: Côte d'Ivoire; 1991. [Google Scholar]
- 10.Challier A. Recommandations pour une campagne de lutte Rapport final Organisation mondiale de la santé, Bureau Régional de l'Afrique. 1970. Enquête sur les glossines des foyers de trypanosomiase humaine en République du Gabon - Prospection des gïtes de l'Estuaire et de l'Ogooué Maritime. (Fre). [Google Scholar]
- 11.Mboumba M. Rapport final du Service National d'Assainissement. 1977. Lutte contre les glossines au Gabon, foyer de l'Estuaire. (Fre). [Google Scholar]
- 12.Laveissière C, Grébaut P. Recherches sur les pièges à glossines (Diptera; Glossinidae). Mise au point d'un modèle économique: le piège ‘Vavoua’. Trop Med Parasitol. 1990;41:185–192. (Fre). [PubMed] [Google Scholar]
- 13.Brunhes J, Cuisance D, Geoffroy B, Hervy JP. Logiciel d'identification et d'enseignement. Montpellier, France: Editions ORSTOM; 1998. Les glossines ou mouches tsé-tsé. (Fre). [Google Scholar]
- 14.Laveissière C. Détermination de l'êge des glossines ténérales. Cah ORSTOM, sér Ent Méd Parasitol. 1975;13:3–11. (Fre). [Google Scholar]
- 15.Penchenier L, Dumas V, Grébaut P, Reifemberg JM, Cuny G. Improvement of blood and fly gut processing for diagnosis of Trypanosomosis. Parasite. 1996;4:387–389. doi: 10.1051/parasite/1996034387. [DOI] [PubMed] [Google Scholar]
- 16.Masiga DK, Smith JA, Hayes P, Bromidge JT, Gibson WC. Sensitive detection of trypanosomes in tsetse flies by DNA amplification. Int J Parasitol. 1992;22:909–918. doi: 10.1016/0020-7519(92)90047-o. [DOI] [PubMed] [Google Scholar]
- 17.Morlais I, Grébaut P, Bodo JM, Djoha S, Cuny G. Characterization of trypanosome infection by polymerase chain reaction (PCR) amplification in wild tsetse flies in Cameroon. Parasitology. 1998;116:547–554. doi: 10.1017/s0031182098002625. [DOI] [PubMed] [Google Scholar]
- 18.Herder S, Simo G, Nkinin S, Njiokou F. Identification of trypanosomes in wild animals from Southern Cameroon using the Polymerase Chain Reaction (PCR) Parasites. 2002;9:345–349. doi: 10.1051/parasite/2002094345. [DOI] [PubMed] [Google Scholar]
- 19.Beier JC, Perkins PV, Kords J, Diggs D, Gargam TP, Koech DK. Blood meal identification by direct enzyme-linked immunosorbent assay (ELISA) tested on anopheles (Diptera: Culicidea) in Kenya. J Med Entomol. 1988;25:19–16. doi: 10.1093/jmedent/25.1.9. [DOI] [PubMed] [Google Scholar]
- 20.Laveissière C, Sane B, Meda HA. Measurement of risk in endemic areas of human african trypanosomiasis in Côte d'Ivoire. Trans R Soc Trop Med Hyg. 1994;88:645–648. doi: 10.1016/0035-9203(94)90207-0. [DOI] [PubMed] [Google Scholar]
- 21.Laveissière C, Grébaut P. Risk index of sleeping sickness transmission: simplification of the calculation. Insect Sci Applic. 2003;23(2):99–102. [Google Scholar]
- 22.Kagbadouno M, Camara M, Bouyer J, Hervouet JP, Courtin F, Jamonneau V, et al. Tsetse elimination: its feasibility in the historical sleeping sickness focus of Loos islands, Guinea. Parasite. 2009;16(1):29–35. doi: 10.1051/parasite/2009161029. [DOI] [PubMed] [Google Scholar]
- 23.Lancien J, Sarda J, Ekouague L. Rapport final ORSTOM. 1985. Bekale - Une première expérience de lutte par piégeage contre les mouches tsé-tsé, dans la mangrove gabonaise. [Google Scholar]
- 24.Gouteux JP. Un cas d'exclusion géographique chez les glossines : l'avancée de G. palpalis palpalis vers Brazzaville (Congo) au détriment de G. palpalis quanzensis. Insect Sci Applic. 1992;13(1):59–67. [Google Scholar]
- 25.Baldry DAT. Local distribution and ecology of Glossina palpalis and Glossina tachinoides in forest foci of west african human trypanosomiasis, with special reference to associations between peri-domestic tsetse and their hosts. Ins Sci Appl. 1980;1:85–93. [Google Scholar]
- 26.Dukes HL. Studies on the factors that may influence the transmission of the polymorphic trypanosomes by tsetse. IX. On the infectivity to Glossina of the trypanosome in the blood of mammal. Ann Trop Med Parasitol. 1935;29:131–143. [Google Scholar]
- 27.Van Den Bossche P, De Deken R, Brandt J, Geerts S, Geysen D, Berkvens D. The transmission of mixed Trypanosoma brucei brucei/T. congolense infections by tsetse (Glossina morsitans morsitans) Veterinary Parasitology. 2004;119(3):147–153. doi: 10.1016/j.vetpar.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 28.Nekpeni EB, Eouzan JP, Dagnogo M. Infection de Glossina palpalis palpalis (Diptera, Glossinidae) par les trypanosomes en zone forestière de Gagnoa en Côte d'Ivoire. Trop Med Parasitol. 1991;42:399–403. (Fre). [PubMed] [Google Scholar]
- 29.Njiokou F, Laveissière C, Simo G, Nkinin S, Grébaut P, Cuny G, Herder S. Wild fauna as a probable animal reservoir for Trypanosoma brucei gambiense in Cameroon. Journal of molecular epidemiology and evolutionary genetics in infectious diseases. 2006;6(2):147–153. doi: 10.1016/j.meegid.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 30.Simo G, Asonganyi T, Nkinin SW, Njiokou F, Herder S. High prevalence of Trypanosome brucei gambiense group 1 from the Fontem sleeping sickness focus in Cameroon. Veterinar y Parasitology. 2006;139(1):57–66. doi: 10.1016/j.vetpar.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 31.Njiokou F, Simo G, Nkinin S, Laveissière C, Herder S. Infection rate of Trypanosoma brucei sl, T. congolense “forest type”, T. vivax and T. simae in small wild vertebrates in south Cameroon. Acta Tropica. 2004;62:139–146. doi: 10.1016/j.actatropica.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 32.Ndoutamia G, Brahim A, Nadjindoroum P, Moudaidandi G, Diimgang G, Loubadjim R. Sensibilité des chèvres Kirdimi et Sahéliennes du Tchad à la trypanosomose à Trypanosoma congolense. Revue Elev Méd Vét Pays Trop. 2000;53(1):9–15. (Fre). [Google Scholar]
- 33.Laveissière C, Hervouet JP, Couret D. Localisation et fréquence du contact hommeglossine en secteur forestier de Côte d'Ivoire: 1. Recherche des points épidémiologiquement dangereux dans l'environnement végétal. Cah ORSTOM, Sér Ent Parasitol. 1986;24:21–35. (Fre). [Google Scholar]