Abstract
Background
Malaria infections are a major public health problem in Africa and prompt treatment is one way of controlling the disease and saving lives.
Methods
This cluster-randomised controlled community intervention conducted in 2003–2005 aimed at improving early malaria case management in under five children. Health workers were trained to train community-based women groups in recognizing malaria symptoms, providing first-line treatment for uncomplicated malaria and referring severe cases. Evaluation was through a pre- (2004) and a post-intervention survey (2005). Anaemia prevalence was the primary outcome.
Results
1715 children aged 6–59 months were included in the pre-intervention survey and 2169 in the post-intervention survey. The prevalence of anaemia decreased significantly from 37% [95% CI 34.7–39.3] to 0.5% [95% CI 0.2–0.7] after the intervention (p<0.001); slightly more in the intervention (from 43.9% to 0.8%) than in the control (30.8% to 0.17%) group (p=0.038). Fever and reported fever decreased significantly and the mean body weight of the children increased significantly over the study period in both control and intervention groups.
Conclusion
The decrease in anaemia was significantly associated with the intervention, whereas the fever and body weight trends might be explained by other malaria control activities or seasonal/climate effects in the area. The community intervention was shown to be feasible in the study context.
Keywords: malaria, Tanzania, randomised controlled trial, community intervention, sulfadoxine/pyrimethamine
Introduction
Every year about one million people die from malaria worldwide, mostly underfive children living in Sub-Saharan Africa1. In Tanzania, about 30% of all deaths in these children are directly attributed to malaria2. In East Africa, the proportion of childhood deaths attributed to malaria increased from 18 to 37 % from 1982–89 to 1990–98, mainly thought to be due to the intensification of chloroquine resistance1. In high transmission areas malaria causes anaemia, often severe, adding to its magnitude as a public health problem3. Malnutrition and underweight has also been linked to malaria, with most recent studies agreeing that improved nutritional status reduces the severity of malaria episodes and results in fewer malaria deaths4.
Although there is a growing commitment to the fight against malaria in recent years, adequate delivery of existing interventions remains one of the main challenges for the weak health systems of Sub-Saharan Africa5. Effective treatment of malaria episodes is a fundamental pillar of the malaria control strategy. Due to development of chloroquine (CQ) resistance, Tanzania changed its first line malaria treatment from CQ to sulfadoxine/pyrimethamine (SP) in August 20016.
Another challenge in sub-Saharan Africa is the fact that most children never make it to formal health care but often get treated by their guardians at home instead7. In addition, low quality of care in Tanzanian health facilities has been reported6. These realities have led to advocacy for home-based management of fever as an extension of the health system. Such a strategy was spearheaded by Uganda where antimalarials for treatment of fevers, so-called “homapak” (CQ+SP), were distributed by community volunteers, called drug distributors8. Home-based management of fever is meant to ensure prompt treatment of malaria and thus reduce progression to a severe disease, thus avoiding difficult referrals and deaths. Evaluation of the “homapak” program shows promising improvement of treatment practice9 although the sustainability remains a challenge.
Few other studies have tried to measure the effects of complex malaria treatment interventions at the community level in endemic areas. One study in Ethiopia measured the effectiveness of mothers treating malaria episodes in their young children and was able to show a major reduction in all-cause mortality and malaria-specific mortality attributed to the intervention10. In an intervention study in Burkina Faso training mothers and supplying community health workers with antimalarials was associated with a significant reduction in malaria morbidity, but mortality was not measured11.
Also, evidence of the capacity of mothers in Guinea Bissau to adequately administer antimalarial treatment to children in the home12 has led many policy makers and financers to support the undertaking and implementation of this strategy in African communities. A commitment from an African summit held in Abuja in 2000 was to ensure that by 2005 at least 60% of malaria episodes would have access to safe, effective and affordable treatment within 24 hours of symptom onset13. This has further prompted home based malarial management strategies. However, studies conducted in Kenya, The Gambia and Zaire using village health volunteers for community malaria treatment were unable to show major effects of the intervention on morbidity and mortality in young children14.
The human resource issue has recently become a top priority15 but little research has been performed on what kind of training interventions are most effective to teach non-medical personnel in providing care. Most reviews of successful interventions to improve care have shown that multifaceted interventions are more likely to be effective16.
This project is an EU INCO-DEV funded collaboration between the Karolinska Institute (Sweden), Heidelberg University (Germany), Muhimbili University College of Health Sciences (Tanzania) and Centre de Recherche en Santé de Nouna (Burkina Faso) called the MAMOP project (Improving the management of childhood MAlaria: an experiment to bridge the gap between MOthers and health care Providers). It is a controlled malaria community intervention with a pre-post design conducted in rural Burkina Faso and Tanzania in 2002–2004. The objective of the MAMOP study was to develop an intervention to improve first line case management of malaria in underfive children through primary caretakers in collaboration with local women groups and existing health centres and to evaluate its feasibility and effectiveness on anaemia, fever and malaria prevalence. The project has been registered as a clinical trial on ISRCTN as ISRCTN34104704
Methods
Study area and population
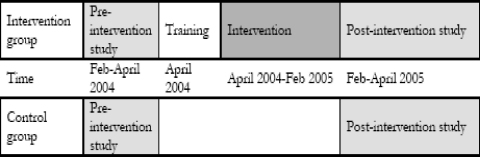
The study was implemented in Mkuranga district, Coast Region of Tanzania. The district capital is located about 60km south of Dar es Salaam. Mkuranga is one of the poorest districts of Tanzania; it is holoendemic for malaria with peak transmission in January and June. It had a population of 187,428 according to the 2003 census17. For this study, training of health staff and women group leaders took place in April 2004. The intervention itself was fully implemented from May 2004 until February 2005, with a pre- and post-intervention survey conducted in February to May 2004 and February to May 2005, respectively.
Administratively, a Tanzanian district is divided into divisions that in turn are divided into wards. The Mkuranga district consists of 31 wards, 11 of which were excluded from the study sampling to avoid interference with other intervention projects in this area (HIV project by AMREF and initiative on trachoma by TRACHOMA). From the remaining 20 wards, 10 were randomly selected for the MAMOP project in a computerised randomisation (in Excel®), names of which were got through the district authorities. The intervention was implemented in five randomly chosen wards (Mkenge, Kiparang'anda, Tambani, Kitomondo, Kiwambo) and the five remaining wards (Kimanzichana, Mkuranga (incl Finzi part of town), Dondwe, Sotele, Kisiju) were controls. From each of the selected wards, one village was randomly selected for the study. All households in the selected villages were included in the study. No villages were closer than 10 km to each other. There was no blinding in the study design.
Study design
The study was designed as a cluster-randomised controlled effectiveness trial. The primary outcome of the study was the proportion of moderate/severe anaemia (haemoglobin < 8 g/L by colour scale) in children aged 6–59 months. Secondary outcomes included proportions of measured fever (Axillary temp > 37.5 °C), malaria prevalence and reported fever during the last 48 hours, mean malaria parasite densities mean haemoglobin values, and mean weight. These endpoints were assessed at the cross-sectional pre- and post-intervention surveys.
Sample size
To be able to detect a 10% difference in anaemia prevalence between the intervention and control group with 80% power and at a significance level of 5%, 960 under-five children were required. As the households were used as units of analysis and as around 80% of all households include an under-five child, about 1200 households had to be sampled. We decided to include all households in the sampled villages, making the sample size slightly larger.
The intervention
The intervention had a number of different components. It follows the training of trainers approach18. The main components of the intervention were:
1. Training of health workers
The health workers of all health facilities in the MAMOP study area were trained in the principles of malaria case management, including clinical diagnosis, treatment and follow-up. The teaching consisted of four days of lectures combined with presentation and case studies. During three days following the theoretical training, the health workers were exposed to practical management of suspected malaria cases in the out patient department of the district hospital.
2. Training of women leaders
Two of the trained health workers were selected to train 36 women leaders selected by the MAMOP intervention communities together with members of the research team. Women leaders were nominated by village groups and 36 of them were selected by the research team based on certain inclusion criteria: (i) completed primary school (ii) member of already existing women groups (iii) resident of the selected villages and expected to live there during the MAMOP study period. Twenty-five of the trained women were later selected for the intervention. The training followed the same model as the health worker training, but with a focus on identifying fever cases that should be treated as suspected uncomplicated malaria by the women leaders or referred to health facilities as suspected severe malaria or other diseases requiring formal health care treatment. Similar to the health worker training, the women leaders saw patients at the district hospital as part of the training. During the study period the women leaders were given a monthly allowance equivalent to 20 USD. The women were trained to treat children with SP as a single dose by age, as specified by the Tanzanian national treatment guidelines. In addition, all children received standard doses of paracetamol over the first two days, also by age as stated in the Tanzanian national guidelines. The women were advised to directly supervise the intake of SP and the first dose of paracetamol, and to visit the sick child again on the second and third day. In case of danger signs (convulsions, altered consciousness, lethargy, unable to drink or breast feed, vomiting everything, unable to stand/sit due to weakness) at any time point or ongoing fever at the end of the treatment time, the women had to refer the child to the health centre.
3. Sensitisation of the communities on the aim of the study
The village leaders were informed about the intervention jointly with representatives from the district health authorities, the research team and the trained health workers. The village leaders informed hamlet (sub-division of village) leaders who in turn called for a community meeting together with the trained women leaders to inform and discuss about the intervention with the community. All households were informed about the MAMOP intervention by the hamlet leaders and all mothers later attended a community information meeting. Mothers with febrile children were clearly advised to go to the house of women leaders for early treatment.
4. Drug supply to women leaders by trained health workers
Every two weeks the women leaders were visited by health workers in order to renew drug supplies combinedwith continued supervision as described below. Drugs were given free of charge by the trained women to the mothers/caretakers.
5. Continuous supervision of health workers and women leaders
Two health workers working at Mkuranga District Hospital supervised and checked the performance of the women leaders during visits to the villages every two weeks using standardised check-lists and collected forms about diagnosed and treated cases. Every four weeks supervision meetings with the women leaders and health workers together were also conducted at the Mkuranga district hospital by the research team. The study progress was discussed, problems identified and handled and the involved health workers and women leaders asked to raise problems encountered. These meetings were conducted during a total of nine months.
Study evaluation
A pre-intervention survey was conducted in February to May 2004, and a post-intervention survey was done in February to May 2005 (Fig 1). All households with under fives (this was assessed by help of the village secretaries) in the 10 study villages were visited in a household-survey and interviewed using a standard questionnaire. The questionnaire included information on socio-demographics, economic status of the household and care-seeking behaviour for febrile disease in under fives. Only one child, the one that last had a febrile episode, was studied in each household. In all households the mother was given a number assigned to the underfive child that had last had a febrile episode. The mother took the child who had received a number to the “field lab”. In the field lab a clinical officer and a nurse received the children/mothers and interviewed them using another questionnaire. They also assessed the health condition of the child, weighed and measured temperature of all children. Spring scales from which the children hung were used for weighing, and for the children who could step on the scales non-digital scales were used. Common mercury thermometers, normally used in Tanzanian health facilities, were used for measuring temperature.
Figure 1.
Overview of intervention design
The child was brought on to a technician who undertook the following investigations:
Thick blood smear: All children who came to the field lab
Filter paper for detection of Heamoglobin levels according to the WHO colour scale method
Exclusion criteria: severe illness of the child (i.e. presenting with convulsions, altered consciousness, lethargy, unable to drink or breast feed, vomiting everything, or unable to stand/sit due to weakness), reported HIV infection.
Laboratory investigations
All blood samples both pre- and post-intervention were processed by the same experienced laboratory technician. Haemoglobin levels were analysed using the WHO colour scale method19. In the preintervention survey the colour scale showing haemoglobin levels in g/dL was used and in the post-intervention survey the colour scale showing the haemoglobin results in %. We tested the validity of these two methods in a sub-study at MUCHS after the data collection and have converted all results to g/dL for comparability.
Thick and thin blood smears were taken and Giemsa stained for detection and determination of malaria parasites. The numbers of asexual parasites were counted against 200 white blood cells (WBCs) in thick films. Parasite densities (asexual parasites/ml blood) were calculated assuming a leukocyte count of 8000 WBC/ml blood. A blood film was considered negative when examination of 500 WBC fields did not show the presence of asexual forms of P. falciparum. Blood slides were re-read by an external laboratory technician.
Statistical analysis
The responses from the questionnaires were precoded and data entered into Epi-Info and later transferred to Statistica® for analysis. Logistic regression with systematic inclusion and exclusion based on significances was performed with the following predictors: level of education of the guardian, age of the guardian, weight-for-height z-scores of the under five, whether the household was in an intervention or control village, and whether the result was pre or post intervention.
Ethical considerations
The protocol was approved by the Tanzanian Commission of Science and Technology (reference number: HQ.9A.ID NO.71.99). Community consent was sought during village meetings in all study villages. During the following visits to individual households, caretakers were asked for their oral consent after having received detailed information from the study physician about all risks and benefits of the study. They were informed that they could withdraw from the study at any time and without disadvantage.
Results
Intervention data
The twenty-five women leaders treated 2675 children during the 12-month period of the intervention. Each woman saw from 23 to 217 children, the average was 105 children per woman. The mean age of the children was 29.1 months (SD 17.5) and 48.1% of them were boys. Of these children, 39 (1.5%) were older than 59 months, the oldest of them 88 months. Only children aged 6-59 months were included in the analysis. More than 94% of the children had sought care with fever as the main symptom. Other common main symptoms given as the reason for seeking care were cough, diarrhoea, runny nose, vomiting and headache, each of them comprising less than 1% of the sick children. 97.8 % of the children seen by the trained women were given SP and paracetamol, 1.6 % and 0.6% were given a drug and referred to a health facility or referred immediately, respectively.
Socio-demographic characteristics of survey participants
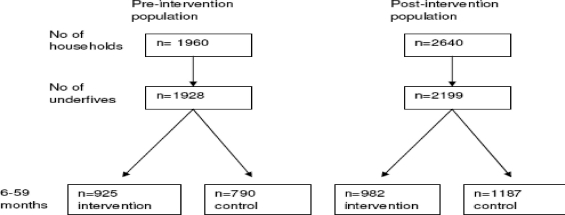
A total of 1928 under fives were included in the pre-intervention and 2199 in the post-intervention survey (figure 2, table 1).
Figure 2.
Study population in the pre- and post-intervention. 213 and 30 of the children in the pre- and post-intervention surveys, respectively, were younger than 6 months
Table 1.
Sociodemographic characteristics of study children (6–59 months) and their mothers
| Pre-intervention | Post-intervention | |||
| Intervention(n=925) | Control(n=790) | Intervention(n=982) | Control(n=1187) | |
| Mothers | ||||
| Age (years) | 28,2 (SD + 7,4) | 28,0 (SD + 7,8) | 29,3 (SD + 8,3) | 27,9 (SD + 8,1) |
| Education | ||||
| Primary education | 39% | 50% | 49,9% | 58,6% |
| Secondary education | 1% | 1% | 0,3% | 2,0% |
| No education | 60% | 49% | 49,8% | 39,4% |
| 6–59 months old children | ||||
| Mean age (months) | 29,0 (SD + 17,4) | 30,4 (SD + 16,8) | 30,4 (SD + 16,0) | 29,5 (SD + 15,4) |
| Median age(IQR) | 24(28) | 27(32) | 28(32) | 27(26) |
| % Females | 55% | 51% | 51,7% | 51,0% |
Effects of the intervention on primary and secondary outcomes
Table 2 shows the main outcomes from the pre-intervention and post-intervention surveys, respectively. A significantly lower prevalence of anaemia (p<0.001) was observed in the post-intervention survey compared to the pre-intervention period (0.5% [95% CI 0.2–0.7] vs. 37% [95% CI 34.7–39.3]) and the decrease was significantly larger in the intervention (from 43.9% to 0.8%) compared to the control (30.8% to 0.17%) group (p=0.038). Overall, fever prevalence (axillary temperature > 37.5°C) and reported fever episodes during the last 48 hours were also significantly lower and mean body weight was significantly higher during the post-intervention compared to the pre-intervention survey (p<0.001), but there were no significant differences between control and intervention groups (p=0.204). We also calculated weight-for-age z-scores according to the WHO standards20 and these were also significantly higher overall after the intervention (p<0.001) but not different between the intervention and control groups (p=0.12). Pre-intervention, 25.8% of the study children had Plasmodium falciparum parasites detected in their blood smears (Table 2). Unfortunately, due to logistic difficulties, we could not perform a validation of the parasitaemia results post-intervention. We therefore chose not to present the post-intervention results.
Table 2.
Outcome measures in the pre- and post-intervention population
| Intervention villages | Control villages | |||
| pre(n=925) | post(n=982) | pre(n=790) | post(n=1187) | |
| Temperature (°C) [CI] | 36.7 [36.68–36.78] | 36.8 [36.8–36.85] | 36.6 [36.56–36.61] | 36.8 [36.8–36.84] |
| Percentage with temp > 37,5 °C | 11.7% | 2.1% | 7.1% | 1.1% |
| Body weight (Kg) (SD) | 10.5 (4.1) | 12.0 (3.4) | 10.8 (2.8) | 12.0 (3.3) |
| Reported fever (last 48 hrs) | 8% | 2.5% | 8% | 1.5% |
| Blood slide positive for parasites | 31.2% | * | 19.5% | * |
| Parasite density (geometric mean) | 2016 | * | 1793 | * |
| Haemoglobin (g/dL) [CI](SD) | 8.1 [7.96–8.14](1.3) | 10.6 [10.52–10.67](1.2) | 8.6 [8.5–8.7](1.5) | 11.0 [10.96–11.1](1.3) |
| Percentage Hb <8 g/dL(moderate and severe anaemia) |
43.9% [40.7–47.1] | 0,8% [0.24–1.36] | 30.8% [27.6–34] | 0.17% [0–0.4] |
| Percentage Hb < 5g/dL (severe anaemia) |
0.11% [0–0.32] | 0% | 0.25% [0–0.6] | 0% |
SD= standard deviation *Not performed due to technical difficulties
We performed multivariable analyses to see what factors could have an effect on anaemia, adjusting for age of mother, education of mother, and weight-for-age z-scores of the child. Pre-intervention, only a low weight-for-age z-score of the child was independently associated with anaemia (p<0.002). Post-intervention there were too few patients with anaemia to be able to do the same analysis. We also performed a multivariable analysis on the weight-for-age z-scores controlling for the baseline differences and found no significant effects of the intervention on the increased bodyweight (p=0.436).
Discussion
This intervention was shown to be feasible under programme conditions. The main finding was an overall reduction in the prevalence of anaemia over time, which was slightly, but significantly larger in the intervention compared to the control group. This overall effect is likely caused by two major changes in malaria treatment and prevention in Tanzania which were introduced in parallel to the MAMOP intervention. First of all, Tanzania changed its first line antimalarial from CQ to SP in August 2001, briefly before this study started. Secondly, at around the same time, a national intervention took place which provided subsidised insecticide-treated mosquito nets (ITN) to all pregnant women21. It is thus very likely that these developments have led to a larger availability of a more effective malaria treatment (SP) both in the intervention and control clusters. Moreover, it has clearly been shown that ITNs reduce malaria-related anaemia in children22. It is also possible that a trend of increased food security contributed to the increased haemoglobin levels. Because of the above mentioned concurrent interventions, it is likely that our intervention effect was diluted and that a significant effect could have been seen with a larger sample size.
Although there was a significant decrease in anaemia prevalence in our study, the change attributable to the intervention was relatively small. In the MAMOP study performed in Burkina Faso there was also a decrease in anaemia prevalence, but with no difference between the intervention and control group23. It has previously been shown that a change in malaria treatment policy may be warranted after unsatisfactory haemoglobin recovery with treatment24 and it has been reported that differences in response only becomes apparent when high resistance levels are seen25.
In addition to those already mentioned, there could be several reasons for lack of effect of our intervention. Firstly, there could have been contamination between the intervention and control areas. Secondly, the educational intervention itself could have been ineffective. At professional level our intervention consisted mainly of lectures and theoretical case studies without any follow-up training. This form of training has been shown to be less effective than e.g. multifaceted interventions16,26. However, our intervention did contain elements known to increase the effect of interventions, such as face-to-face outreach visits. Thirdly, there could have been faults in the sensitization process of the community where it is important that the appropriate groups are targeted and that all persons involved indecision making are informed. Studies show that interventions need to be adapted to a specific context27.
A surprising finding in this study was the dramatic increase in body-weight in the children after the intervention, however there was no difference between the intervention and control groups. The increase in body weight is most probably associated with a reduction in the incidence and severity of malaria episodes over time. It is less probable that the nutritional status of the population should have increased so dramatically in one year.However a marked reduction from 1999 to 2005 in the proportion of underweight children has been seen in a national survey from Tanzania28.
There are some limitations to this study. Differences in the malaria transmission intensity between the two survey time points is another possible confounder of our findings. It might well be possible that transmission intensity was lower before the post-intervention survey compared to before the pre-intervention survey. Unfortunately, many blood slides for parasitaemia post-intervention were missing and the counts could not be verified. We therefore chose not to include this parameter in the results. Moreover, anaemia might not be a very good outcome measurement in malaria community trials as there are other important determinants of anaemia development, particularly malnutrition .This intervention study was performed under “real world conditions”. This kind of research involves taking advantage of the actual context where whatever we are interested in occurs30, but also presents a number of challenges concerning logistics of intervention implementation and data collection. This is one of the reasons why we have not been able to present all results originally aimed for in this project.
Conclusion
We have shown the feasibility of a complex malaria community intervention bridging the gap between the health workers at the peripheral health centres and the mothers of young children in individual households through women groups. Such an approach is promising given the lack of access to formal health services in much of rural Sub-Saharan Africa and the still high burden of malaria in these areas. However, with the introduction of Artemisinine-based Combination Therapy (ACT) the administration of the more complex dosage regimens in this setting needs special consideration. Furthermore, additional research is needed on the quality of the trained women.s diagnoses and on the interaction between the trained women and mothers before the strategy can be recommended as policy.
Acknowledgements
The project was financed by the EC-INCO-DEV (project no IC A4-CT-2001-10010) and by Sida-SAREC grants (Bil Th106, SWE-2002-063 and SWE-2005-30). We thank all MAMOP team members in Burkina Faso, Germany, Sweden and Tanzania for collaboration and constructive criticism. We thank all the parents/guardians and health care providers for their co-operation and participation in the study. We are grateful to James Fulgence (Department of Clinical Pharmacology, MUHAS) for assisting in the collection of blood samples in the field and to Mr Membi (MUHAS) for microscopy for malaria parasitaemia. We also thank Dr Max Petzold (Nordic School of Public Health) for help with Statistical analysis and advice and Dr Annika Janson (Karolinska Institutet) for help with evaluating the WHO haemoglobin colour scale method in Dar es Salaam.
References
- 1.Korenromp EL, Williams BG, Gouws E, Dye C, Snow RW. Measurement of trends in childhood malaria mortality in Africa: an assessment of progress toward targets based on verbal autopsy. Lancet Infect Dis. 2003 Jun;3(6):349–358. doi: 10.1016/s1473-3099(03)00657-1. PubMed. [DOI] [PubMed] [Google Scholar]
- 2.MoH-Tanzania . Morbidity and mortality statistics: Health information and Research section, Planning & Policy department. Tanzania: Ministry of Health; 1999. Contract No.: Document Number. [Google Scholar]
- 3.Greenwood B. Aymptomatic malaria infections - do they matter? Parasitol Today. 1987;3:206–214. doi: 10.1016/0169-4758(87)90061-5. [DOI] [PubMed] [Google Scholar]
- 4.Caulfield LE, Richard SA, Black RE. Undernutrition as an underlying cause of malaria morbidity and mortality in children less than five years old. American Journal of Tropical Medicine and Hygiene. 2004;71(suppl 2):55–63. [PubMed] [Google Scholar]
- 5.Kouyaté B, Sie A, Ye M, De Allegri M, Muller O. The great failure of malaria control in Africa: a district perspective from Burkina Faso. PLoS Med. 2007 Jun;4(6):e127. doi: 10.1371/journal.pmed.0040127. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eriksen J, Tomson G, Mujinja P, Warsame M, Jahn A, Gustafsson LL. Assessing health worker performance in malaria case management of underfives at health facilities in a rural Tanzanian district. Tropical Medicine & International Health. 2007;12(1):52–61. doi: 10.1111/j.1365-3156.2006.01753.x. PubMed. [DOI] [PubMed] [Google Scholar]
- 7.Breman JG. The ears of the hippopotamus: manifestations, determinants, and estimates of the malaria burden. American Journal of Tropical Medicine and Hygiene. 2001 Jan–Feb;64(1–2 Suppl):1–11. doi: 10.4269/ajtmh.2001.64.1. [DOI] [PubMed] [Google Scholar]
- 8.MoH-Uganda, author. Implementation guidelines for the Home Based Management of fever strategy. MoH, Uganda Kampala: WHO/UNICEF/BASCIS/DISH; 2002. Contract No.: Document Number|. [Google Scholar]
- 9.Nsungwa-Sabiiti J, Peterson S, Pariyo G, Ogwal-Okeng J, Petzold MG, Tomson G. Home-based management of fever and malaria treatment practices in Uganda. Trans R Soc Trop Med Hyg. 2007 Dec;101(12):11991–11207. doi: 10.1016/j.trstmh.2007.08.005. PubMed. [DOI] [PubMed] [Google Scholar]
- 10.Kidane G, Morrow RH. Teaching mothers to provide home treatment of malaria in Tigray, Ethiopia: a randomised trial. Lancet. 2000 Aug 12;356(9229):550–555. doi: 10.1016/S0140-6736(00)02580-0. PubMed. [DOI] [PubMed] [Google Scholar]
- 11.Pagnoni F, Convelbo N, Tiendrebeogo J, Cousens S, Esposito F. A community-based programme to provide prompt and adequate treatment of presumptive malaria in children. Trans R Soc Trop Med Hyg. 1997 Sep–Oct;91(5):512–517. doi: 10.1016/s0035-9203(97)90006-7. [DOI] [PubMed] [Google Scholar]
- 12.Kofoed PE, Lopez F, Aaby P, Hedegaard K, Rombo L. Can mothers be trusted to givemalaria treatment to their children at home? Acta Tropica. 2003 Apr;86(1):67–70. doi: 10.1016/s0001-706x(03)00018-4. PubMed. [DOI] [PubMed] [Google Scholar]
- 13.Nsungwa-Sabiiti J, Tomson G, Pariyo G, Ogwal-Okeng J, Peterson S. Community effectiveness of malaria treatment in Uganda—a long way to Abuja targets. Ann Trop Paediatr. 2005 Jun;25(2):91–100. doi: 10.1179/146532805X45683. [DOI] [PubMed] [Google Scholar]
- 14.Delacollette C, Van der Stuyft P, Molima K. Using community health workers for malaria control: experience in Zaire. Bull World Health Organ. 1996;74(4):423–430. [PMC free article] [PubMed] [Google Scholar]
- 15.Travis P, Bennett S, Haines A, Pang T, Bhutta Z, Hyder AA, et al. Overcoming health-systems constraints to achieve the Millennium Development Goals. Lancet. 2004 Sep 4–10;364(9437):900–906. doi: 10.1016/S0140-6736(04)16987-0. [DOI] [PubMed] [Google Scholar]
- 16.Davis DA, Thomson MA, Oxman AD, Haynes RB. Changing physician performance. A systematic review of the effect of continuing medical education strategies. JAMA. 1995 Sep 6;274(9):700–705. doi: 10.1001/jama.274.9.700. PubMed. [DOI] [PubMed] [Google Scholar]
- 17.NBoST, author. United Republic of Tanzania: 2002 Population and Housing Census. General report. Dar es Salaam: National Bureau of Statistics, Central Census Office, President.s Office for Planning and Privatization; 2003. Jan, 2003Contract No.: Document Number|. [Google Scholar]
- 18.Evans B, Armstrong D, Weinman J, Elliott L. Training trainers: a new approach for community medicine. Public Health. 1990 Jan;104(1):3–8. doi: 10.1016/s0033-3506(05)80339-7. PubMed. [DOI] [PubMed] [Google Scholar]
- 19.Stott GJ, Lewis SM. A simple and reliable method for estimating haemoglobin. Bull World Health Organ. 1995;73(3):369–373. PubMed. [PMC free article] [PubMed] [Google Scholar]
- 20.WHO, author. WHO Anthro (version 3.1, June 2010) 3.1 ed. Geneva: WHO; 2010. PubMed. [Google Scholar]
- 21.Mushi AK, Schellenberg JR, Mponda H, Lengeler C. Targeted subsidy for malaria control with treated nets using a discount voucher system in Tanzania. Health Policy Plan. 2003 Jun;18(2):163–171. doi: 10.1093/heapol/czg021. PubMed. [DOI] [PubMed] [Google Scholar]
- 22.Abdulla S, Schellenberg JA, Nathan R, Mukasa O, Marchant T, Smith T, et al. Impact on malaria morbidity of a programme supplying insecticide treated nets in children aged under 2 years in Tanzania: community cross sectional study. BMJ. 2001 Feb 3;322(7281):270–273. doi: 10.1136/bmj.322.7281.270. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kouyate B, Some F, Jahn A, Coulibaly B, Eriksen J, Sauerborn R, et al. Process andeffects of a community intervention on malaria in rural Burkina Faso: randomized controlled trial. Malar J. 2008;7:50. doi: 10.1186/1475-2875-7-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ekvall H, Premji Z, Bjorkman A. Chloroquine treatment for uncomplicated childhood malaria in an area with drug resistance: early treatment failure aggravates anaemia. Trans R Soc Trop Med Hyg. 1998 Sep–Oct;92(5):556–560. doi: 10.1016/s0035-9203(98)90913-0. [DOI] [PubMed] [Google Scholar]
- 25.Bloland PB, Lackritz EM, Kazembe PN, Were JB, Steketee R, Campbell CC. Beyond chloroquine: implications of drug resistance for evaluating malaria therapy efficacy and treatment policy in Africa. J Infect Dis. 1993 Apr;167(4):932–937. doi: 10.1093/infdis/167.4.932. PubMed. [DOI] [PubMed] [Google Scholar]
- 26.Ross-Degnan D, Laing RO, Santoso B, Ofori-Adjei D, Lamoureux C, Hogerzeil HV. Improving pharmaceutical use in developing countries: a critical review of experience and lack of experience; 1st International Conference for Improving Use of Medicines; 1997; Chiang Mai, Thailand. 1997. [Google Scholar]
- 27.Chalker J, Ratanawijitrasin S, Chuc NT, Petzold M, Tomson G. Effectiveness of a multicomponent intervention on dispensing practices at private pharmacies in Vietnam and Thailand—a randomized controlled trial. Soc Sci Med. 2005 Jan;60(1):131–141. doi: 10.1016/j.socscimed.2004.04.019. PubMed. [DOI] [PubMed] [Google Scholar]
- 28.Masanja H, de Savigny D, Smithson P, Schellenberg J, John T, Mbuya C, et al. Child survival gains in Tanzania: analysis of data from demographic and health surveys.[see comment] Lancet. 2008;371(9620):1276–1283. doi: 10.1016/S0140-6736(08)60562-0. PubMed. [DOI] [PubMed] [Google Scholar]
- 29.Müller O, Traore C, Jahn A, Becher H. Severe anaemia in west African children: malaria or malnutrition? Lancet. 2003 Jan 4;361(9351):86–87. doi: 10.1016/S0140-6736(03)12154-X. PubMed. [DOI] [PubMed] [Google Scholar]
- 30.Robson C. Real World Research - A resource for social scientists and practicioner-reserachers (2nd edition) Second edition. Blackwell publishing; 2002. [Google Scholar]