Abstract
Background
A substantial number of the world population especially that of the developing countries rely on herbal products to control their fertility since ancient times. Rumex steudelii Hochst is one of the traditionally used antifertility plants in Ethiopia. Previous studies showed that the methanolic root extract of the plant had reversible antifertility effect in experimental animals. However, no study had hitherto been done on the antifertility activity of the methanolic root extract of Rumex steudelii on the ovary and uterus of female albino rats.
Objective
To investigate the quantitative aspects of follicular development in the ovaries and uterine histology in cyclic female albino rats to get further information on the possible mechanism of antifertility effect of the methanolic extract of R. steudelii.
Methods
The effect of the extract on uterine histology and ovarian follicular growth was determined after oral administration of the methanolic root extract of Rumex steudelii at 2.2, 2.5, 3.0 g/kg/day doses consecutively for 30 days.
Results
The extract significantly decreasing the number of healthy small antral, Graffian follicles and corpora lutea with concomitant significant increase in the number of atretic follicles of the same stage in dose dependent manner. Treatment at 3.0 g/Kg dose level in addition caused a significant decrease in the number of healthy primary, small preantral and large preantral follicles with concomitant significant increase in the number of atretic primary follicles. The ovarian and uterine wet weights are reduced significantly. The extracts also caused a significant decrease in the epithelial cell height, myometrial and stromal thickness in a dose dependent manner.
Conclusion
The study demonstrated that the methanolic extract could cause atrophic changes in the uterus and disruption of ovarian folliculogenesis by inhibiting further development of the recruited ovarian follicles.
Keywords: Rumex steudelii, Antifertility, Folliculogenesis, Endometrium, uterus, ovary, ovarian follicles, corpus luteum, Rats
Introduction
In recent times focus on plant research has increased all over the world and a large body of evidence has been collected to show immense potentials of medicinal plants used in various traditional systems. According to recent literature review 48 out of 72 traditionally employed antifertility medicinal plants had antifertility potential1.
Rumex steudelii Hochst is one of the traditionally used antifertility plants in Ethiopia. The plant is locally named as “Tult” or “Yeberemelase” and belongs to the family polygonaceae. It is distributed as a weed in the habitats of altitude ranging from 1200 to 3900m. The root of this plant is used as an abortifacient and in the control of fertility traditionally4. The methanolic extract of this plant showed anti-implantation effect in rats as previously reported by Desta3. The preliminary antifertility study of the methanolic root extract at 2.2, 2.5, 2.8, 3.0 g/kg doses showed a dose dependent significant decrease in the number of litters2. In addition, the extract at 2.2 g/kg dose level significantly prolonged the estrus cycle in general and the diestrous phase of the estrous cycle in particular4. It was also showed that the antifertility effects of the extract were transient and reversible. The extract also caused significant decrease in the number of implantation sites and reduced serum estrogen level4. The phytochemical screening of the plant by Gebrie has showed the presence of polyphenols and phytosterols2.
Based on the traditional use of the plant and findings of the previous studies, it was speculated that the methanolic root extract of Rumex steudelii may cause its antifertility effect by disruption of follicular growth in the ovary and/or due to its direct effect on the uterine activity. Therefore, this study was undertaken to investigate the effect of methanolic root extract of Rumex steudelii on the ovarian follicle differentiation and uterine histology of female albino rats at the effective anti-fertility dose range. Counts of ovarian follicle differentiation can be used as the most sensitive indicator of female reproductive function and could predict the type of reproductive disruption that may be caused by exposure to various chemicals5.
Methods
Preparation of extract
The root parts of Rumex steudelii were collected during the month of December 2004 from Akaki district, south of Addis Ababa, Ethiopia. The plant was identified by a taxonomist and voucher sample (Herbarium number RS-2194) was deposited in the herbarium of Department of Drug Research, Ethiopian Health and Nutrition Research Institute, Addis Ababa, Ethiopia. The roots were cleaned from any extraneous materials, shade dried and ground to powder. The methanolic extraction of the root was carried out as described in the previous study2, 4. The powdered material was mixed with methanol in a percolator and extraction process was carried out by percolation method at room temperature. The extract was concentrated under vacuum in rotary evaporator to yield dark brown semi solid mass. Further drying was carried out on water bath at 40°C to yield a solid residue. The extract was reconstituted in distilled water to get the desired concentration for all tests.
Test animals
The study was conducted on mature virgin female Wistar Albino rats. Adult female and male rats were obtained from Ethiopian health and nutrition research institute, Addis Ababa, and transferred to the animal breeding house in the department of Pharmacology, Addis Ababa University. They were acclimated to the laboratory conditions for five days and allowed to breed. The female litters were isolated and maintained in separate cages. All the animals used in the experiment were bred and maintained in a standard animal house. The animals were housed in polypropylene cages and maintained in well ventilated room provided with 12:12h light and dark cycle for each 24h period at a temperature of approximately 250C. They were fed on pellets and tap water ad libitum. All experiments were conducted on matured virgin female albino rats aged 2–3 months weighing 190–210 gram, showing regular estrous cycle length (4–5 days). The phases of estrous cycle were determined by observing the vaginal smear in the morning (08: 00h to 10:00h) according to procedure described previously by Cooper et al6. The animals were then assigned randomly into four groups (Group I–IV) of 5 rats each. Group I (distilled water); Group II (2.2 g/kg of BW); Group III (2.5 g/kg of BW), Group IV (3.0 g/kg of BW).
Administration of the extract was done using intragastric tube on the basis of animal body weight. The treatment was given for 30 days to cover six regular estrous cycles. For all experiments the treatment was started when the animals were in estrus phase7. Initial body weight before treatment and final body weight at the time of sacrifice were recorded.
The study was carried out following approval from the Faculty of Medicine Institutional Review Board on the use and care of experimental animals.
Tissue collection
Twenty four hours after the last treatment all the animals were sacrificed. The ovaries and the uteri were then carefully dissected free of adhering fat and mesentery, blotted on filter paper to remove excess fluid. The wet weight of the uterus and each ovary was recorded separately to the nearest 0.0001g on electronic balance. Uteri and ovaries of the left side were used for histological studies.
Routine histological preparation
The dissected ovary and the middle third of the uterus of the left side were immersion-fixed in bouin's fluid over night at room temperature after which the tissues were transferred to ascending grades of alcohol for dehydration. The tissues were cleared with two changes of xylene for one and half hours each, transferred into two changes of molten paraffin wax I and II for one and half hour each and wax- III for overnight in an oven at 650c for infiltration. The tissues were then embedded in metal moulds containing molten paraffin wax, and orientation was done to allow coronal section for ovaries and transverse section for the uterus.
Each tissue block was serially sectioned at 6µm thickness using Leica rotary microtome (Leica RM 2125, Leica Microsystems Nussloch GmbH, Deutschland) and strips of sections were gently lowered into the surface of a warm water bath at 40°C. The floated sections were mounted on egg albumin coated microscopic slides, and put in an oven maintained at 60°C for 30–40 minutes to fix the tissue firmly on the slide. The slides were dewaxed with two changes of xylene and hydrated with decreasing alcohol concentration and then immersed in water for 5 minutes. The sectioned tissues were then stained regressively with Ehrlich's hematoxylin and counter stained with Eosin. After staining with eosin tissues were washed in tap water and dehydrated by rinsing in increasing concentration of alcohol and rinsed in xylene-I and then placed in xylene-II until mounting. Finally a drop of mountant DPX (A mixture of Distyrene, a Plasticizer, and Xylene) was placed on top of the sections and the cover slip was applied.
Light microscopy
Microscopic examination of the uterine tissue
Five representative uterine sections per animal were selected from the serial sections of the middle third segment of each left uterine horn by simple random sampling method. Measurements of epithelial cell height, stromal thickness, and myometrial thickness were done using the method described by Andriana et al8. All the changes observed in the examined tissue sections were recorded and photographed using a Leitz Dialux 20 microscope wild photoautomat MPS 51(Wild Heerbrgg Ltd., Heerburgg, Switzerland) fitted with camera containing 35-mm film. The uteri of control animals were evaluated first. The uteri in the remaining groups were evaluated blind to treatment.
Microscopic examination of the ovarian tissue
Each left ovary wasserially sectioned at 6 µm thickness. Every tenth section was photographed using a Leitz Dialux 20 microscope wild photoautomat MPS 51 fitted with camera containing 35-mm film.
Classification of ovarian follicles for analysis
Follicles were classified as primary, small preantral, large preantral, small antral and graffian according to the morphological classification scheme used by Lundey et al9. Follicles were classified as atretic if they displayed two or more of the following criteria within a single cross-section: more than two pyknotic nuclei in the granulosa cell layer, granulosa cells and cell debris within the antral cavity, fragmented oocyte and granulosa cells pulling away from the basement membrane10.
Quantitative evaluation of ovarian follicles
To quantitatively evaluate ovarian follicles, the methods described by Engelberget et al11 were used in the present study. Starting with the first serial section that contained the ovarian tissue, every tenth serial section was used for differential follicle numbers. The numbers of healthy primary, small preantral, large preantral, small antral and Graffian follicle and atretic follicles of the corresponding stages were counted from every tenth section and added to determine the total number for each. To avoid double-counting in the growing class, only those follicles that showed the nucleus of the oocyte were counted. The corpora lutea were also counted from every tenth serial section. To avoid double counting, each corpus luteum was followed throughconsecutive sections to ensure that it was only counted once.
Statistical analysis
The data were expressed as mean±SEM and analyzed using SPSS statistical software. One way analysis of variance (ANOVA) was used to assess the variation of the means among the treatments. If the variation was greater than expected by chance alone, Dunneett's multiple comparison tests was performed to compare each treatment group with the control. And the Student t-test was performed to compare body weight changes before and after treatment. Significance was established when the p value was less than 0.05.
Results
Effect on body and genital organ weight
The effect of the extract on the body and genital organ weight is illustrated in Table 1. In the control animals, there was increase in the final body weight as compared to the initial body weight but not statistically significant. Rats treated with a dose of 2.2, 2.5 and 3.0 g /kg body weight showed a slight increment in the final body weight, which was not statistically significant compared to the initial body weight. No signs of toxicity were observed for all experimental groups following treatment at all doses. Uterine and ovarian relative wet weight was reduced significantly (P<0.05) after treatment at 2.2, 2.5, 3.0 g/kg doses for 30 days compared to controls in a dose dependent manner.
Table 1.
Effect of Rumex steudelii root extract on body weight and genital organ weight of female albino rats
| Dose | Body weight | Organ weight(mg/100g) | |||
| Treatment | (g/kg) | Initial | Final | Uterus | Ovary |
| Control | - | 213.60±1.93 | 36.20±2.42 | 128±8.61 | 41.34 ± 2.75 |
| Methanol extract | 2.2 | 14.40±3.28 | 224.00±2.98 | 103±3.95* | 33.36 ± 2.05* |
| Methanol extract | 2.5 | 220.27±4.02 | 223.20±5.95 | 100±2.78** | 29.93±1.90** |
| Methanol extract | 3.0 | 219.25±1.83 | 220.60±3.98 | 0.99±5.25** | 25.40 ±1.41** |
Data recorded as Mean ± SEM N = 5
P<0.05
P < 0.01
Effect on uterine histology
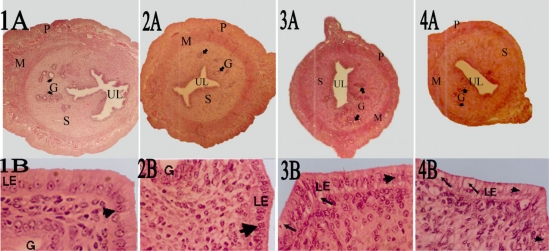
The uterine histology of the control rats showed normal features: single layered columnar epithelial cell with elongated nuclei at the base of the cells, highly folded epithelial lining, numerous and tortuous endometrial glands, edematous stroma (Fig. 1, 1A). The uterine endometrium of the extract treated animals revealed some degree of inhibition of endometrial development as evidenced by poor development of endometrial epithelium, endometrial glands and stroma. These uterine changes were dose dependent, the doses of 2.5, and 3.0 g/kg being the most effective in causing such changes in the uterus. Animals treated at a dose of 2.2 g/kg showed low cuboidal cells with sparse distribution of low columnar cells, having smaller and darker staining nuclei situated mainly at apical and middle aspects of the cells (Fig. 1, 2B). Similar changes were observed in the endometrial epithelium in animals treated at 2.5 g/kg dose with the cells appearing more close to each other. A few degenerating epithelial cells were clearly visible in the epithelial lining at this dose (Fig. 1, 3B). In animals treated at a dose of 3.0 g/kg, the endometrial epithelium appeared disorganized and contain many degenerating epithelial cells with many cavities (vacuoles) containing degenerating materials (Fig. 1, 4B).
Figure 1.
Photomicrographs of hematoxylin and eosin stained tissue cross-sections from equivalent regions of the left uterine horn of vehicle treated control rats(1A,1B) and rats treated with 2.2 (2A,2B), 2.5 (3A,3B), and 3.0 (4A, 4B) g/Kg/day doses.
Photographed at a magnification x 25
Pictures 1B, 2B, 3B, 4B photographed at a magnification x 400
M, myometrium; S, endometrial stroma; P, perimetrium; UL, uterine lumen. Pictures 1A, 2A, 3A, 4A
Note that the uterus of control rats showing numerous, irregular, tortuous and wide lumen endometrial glands (G) and extensive fold of the luminal epithelium (1A). The luminal epithelium (LE) is composed of a single layer of columnar epithelial cells with large nuclei at the basal aspect of the cells (large arrow heads in 1B). In treated rats note the dose dependent anti-uterotrophic effect of the extract, absence of extensive folding of luminal epithelium(2A, 3A, 4A) and the reduction of luminal epithelium(LE) height (2B, 3B,4B).The luminal epithelium in 2B is composed of high cuboidal epithelial cells(arrow heads in 2B). Note also that the luminal epithelium (LE) in 3B, 4B is highly disorganized and containing vacuolated cells with degenerated materials (arrows in 3B and 4B).
The quantitative assessment of epithelial cell height, stromal thicknesses and myometrial thicknesses at treatments of various doses of Rumex steudelii root extract is summarized in Table-2. Results of the uterine histomorphometry revealed that there was a dose dependent significant decrease (P < 0.05) in the epithelial cell height, stromal thickness and myometrial thickness in treated animals compared to the controls.
Table 2.
Effect of Rumex steudelii root extract on epithelial cell height, stromal thickness and myometrial thicknesses
| Dose | Thickness of different layers of the uterus (µm) | |||
| Treatment | (g/kg) | Epithelial cell height | Myometrial thickness | Stromal thickness |
| Control | - | 32.45±0.42 | 293.00±1.35 | 474.00±4.46 |
| Methanol extract | 2.2 | 29.25±0.25* | 227.75±2.48* | 451.25±3.50** |
| Methanol extract | 2.5 | 26.50±0.63** | 186.00±2.69** | 397.25±3.96** |
| Methanol extract | 3.0 | 28.25±0.74** | 178.75±1.58** | 381.25±4.89** |
Data recorded as Mean ± SEM N = 5
P<0.05
P < 0.01
Effect on the ovarian folliculogenesis
The histological observation of the ovaries of control animals revealed numerous healthy follicles at various stages of development and fresh corpus luteum (Fig.2A). Most of these follicles revealed normal features such as intact oocyte and absence of pyknotic granulosa cells in the granulosa cell layer and absence of fragmented granulosa cells and cells debris in the antral cavity (Fig.3A). There were also few atretic follicles. On the other hand, in animals treated at a dose of 2.2 g/kg healthy primary and small preantral follicles were observed frequently but the later stages of follicles large preantral, small antral follicles and Graffian follicles were less frequently observed (Fig.2B) as compared to the controls. Animals treated with 2.5 g/kg body weight dose have showed fewer preantral and antral and Graffian follicles and fewer fresh or active corpora lutea and many atretic follicles than the control (Fig. 2C). These changes were more evident in animals treated at a 3.0 g/kg dose level (Fig. 2D).
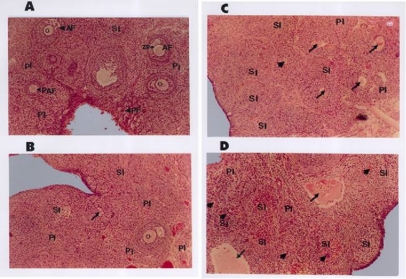
Figure 3.
Photomicrographs of hematoxylin and eosin stained representative section of the ovarian tissue illustrating the interstitial cell masses, follicular remnants and oocyte
The tissues were obtained from equivalent regions of vehicle-treated control rats (A) or rats treated with 2.2 (B), 2.5 (C), and 3.0(D) g/Kg/day. In the control note that follicles with normal (intact) oocyte (arrow heads in A) appeared frequently. Aggregates of secondary interstitial cells (SI) were more prevalent in ovaries of rats treated at 2.5 and 3.0g/Kg body weight doses (C and D). Note also the increased prevalence of necrotic oocyte (arrows) and follicular remnants or remnants of Zona pellucida (arrow heads) especially at 2.5 and 3.0g/Kg body weight dose levels(C and D). PI, primary interstitial cells; SI, secondary interstitial cells; AF, antral follicle; PAF, preantral follicle; PF, primordial follicle; ZP, zona pellucida; O, oocyte. All pictures are photographed at a magnification of X100
The granulosa lutein cells of most of the corpora lutea in the ovaries of extract treated animas showed abnormal features such as appearance of darkly stained nuclei with peripherally located nucleoli and vaculations in the cytoplasm of the cells. These changes were more manifested in animals treated at 2.5 and 3.0 g/kg body weight. It was also apparent that aggregation of secondary interstitial cells was more prevalent in the ovaries of extract treated rats especially at 2.5 and 3.0 g/kg dose level (Fig.3B, C and D). As shown in the table 3 and 4, the extract caused a statistically significant reduction in the number of small antral and Graffian follicles in animals treated with a dose of 2.2 g/kg dose level with concomitant significant increase in the number of atretic follicles of the same stage. Treatment with 2.5 g/kg dose caused a statistically significant decrease (P<0.05) in the number of healthy small preantral, large preantral, small antral, Graffian follicles and active or fresh corpora lutea with a concomitant statistically significant increase in the number of atretic follicles of the same stage. Treatment with 3.0 g/kg dose caused a statistically significant decrease (P<0.05) in the number of active or fresh corpora lutea and healthy preantral and antral and a significant increase in the number of atretic primary follicles. The result also showed a significant reduction in the total number of normal follicles in all treated groups compared to control.
Table 3.
Mean number of healthy follicles present in the ovaries of rat after treatment with Rumex steudelii root extract
| Dose | Small | Large | Small | Total no of | Corpus | |||
| Treatment | (g/kg) | Primary | preantral | preantral | Antral | Graffian | Follicles | Luteum |
| Control | - | 82.40±1.12 | 69.80±1.39 | 30.40± | 12.80± | 9.20± | 213± | 8.60± |
| 0.93 | 0.37 | 0.58 | 2.95 | 0.68 | ||||
| Methanolic extract | 2.2 | 80.80±1.07 | 67.20±1.49 | 28.00± | 10.60± | 6.40± | 199.60± | 6.60± |
| 0.71 | 0.51* | 0.51** | 0.93* | 0.24* | ||||
| Methanolic extract | 2.5 | 78.40±0.93 | 60.40± | 22.40± | 10.40± | 4.00± | 179.00± | 3.40± |
| 1.21** | 0.92* | 0.51** | 0.32** | 2.00* | 0.24** | |||
| Methanolic extract | 3.0 | 64.20±1.55** | 57.00± | 19.60± | 8.20± | 3.40± | 156.00± | 3.20± |
| 1.37** | 0.81** | 0.51** | 0.40** | 1.76* | 0.37** |
Data recorded as Mean ± SEM N = 5
P<0.05
P < 0.01
Table 4.
Mean number of atretic follicles present in the ovaries of rat after treatment with Rumex steudelii root extract
| Dose | Small | Large | Small | Graffian | Total no. of | ||
| Treatment | (g/kg) | Primary | preantral | preantral | antral | follicles | |
| Control | 10.20±0.66 | 13.80±0.37 | 7.40±0.60 | 4.60±0.40 | 3.20±0.37 | 39.20±0.73 | |
| Methanolic extract | 2.2 | 11.40±0.51 | 15.80±0.86 | 9.00±0.55 | 7.20±0.66** | 6.00±0.31** | 49.80±0.87** |
| Methanolic extract | 2.5 | 11.20±0.58 | 15.20±0.37 | 9.80±0.37* | 7.80±0.50** | 5.40±0.51** | 48.80±1.16** |
| Methanolic extract | 3.0 | 14.00±0.41** | 11.60±0.68 | 7.20±0.37 | 5.20±0.37 | 4.20±0.37 | 42.20±0.80 |
Data recorded as Mean ± SEM N = 5
P<0.05
P < 0.01
Discussion
In the present study no significant change in the final body weight was observed after 30 days of administration of Rumex steudelii root extract at all dose levels. However, a significant reduction in genital organ wet weight was noticed in dose dependent manner. Since body weight gain was not altered significantly in the extract treated rats in comparison with the controls, the histomorphological changes observed on the female reproductive system following treatment may be attributed to the effect of the extract itself on this specific system. In addition, the absence of any sign and symptom of toxicity in the extract treated animals suggests that the extract was safe to the female rats at the doses employed in the present study.
Reduction in the observed weight of the ovary and the uterus after treatment of the animals with Rumex steudelii root extract at these dose levels may be attributed to the absence or reduced availability of ovarian hormones and gonadotrophins, respectively. The study of follicular numbers can provide important information about the function of theovary, in particular the relationship between folliculogenesis and the factors that regulate it. This may be important for example in determining the relative roles of gonadothrophic and steroid hormones and local intra ovarian growth factors in regulating the survival and maturation of follicles at any stage of their development.
Atretic follicles are degenerating follicles. Ovarian follicles degenerate as a result of disruption of their growth and differentiation. Although Follicular atresia is an integral part of ovarian function, the increased number of atretic follicles and concomitant decrease in the number of preantral, antral, graffian follicles in extract treated rats as compared to control may be due to the non availability of a required amount of the extra ovarian regulators, the gonadotrophins (FSH and LH). This could be due to the effect of the extract indirectly at the level of hypothalamus or pituitary gland or directly by insensitizing the follicular receptors to the available gonadotrophins. All follicles are apparently exposed to the same fluctuations of gonadotrophins, although, not all are equally responsive, some ovulate and others become atretic. This shows the presence of intragonadal regulatory factors which modulate the effect of these major hormones. Two main intragonadal regulatory factors can be distinguished: intra-follicular and intra ovarian12. So the biologically active substances in the extract might have suppressed follicular growth in the ovaries by interfering at the level of receptors and mRNA expression of these intra ovarian and intra follicular regulating factors. Thus the extract may have anti ovulatory effect at the doses used in the present study.
The formation of the corpus luteum is a direct continuation of preovulatory follicular development. In the rabbit and many other species estradiol is the main luteotrophic hormone. Prolactin, FSH and LH contribute to the luteotrophic complex as they enhance estrogen secretion by promoting the growth of large follicles13. The decrease in the number of corpora lutea in treated rats indicates that the extract inhibited the conversion of the preovulatory follicles into corpora lutea, arresting ovulation.
Increased prevalence of secondary interstitial cell masses, necrotic oocyte and remnants of zona pellucida observed in the present study especially at 2.5 g/kg and 3.0 g/kg dose levels reflect increased follicular atresia that had occurred in the ovaries. It is well documented that secondary interstitial cells are derived from theca cells of the atretic follicles which undergo hypertrophy and persist throughout life in the ovaries14.
The quantitative findingsof the depressed folliculogenesis in this experiment correlates with the low plasma level of estradiol and extended diestrous phase of the estrous cycle in the extract treated rats at a 2.2 g/kg dose level as described in the previous study by Gebrie et al2, 4. The result observed in the present study on the histology and follicular growth of the ovaries are also in line with other studies: Treatment of female rats with petroleum ether, benzene, chloroform and alcohol extracts of Momordica charantia seed extracts caused significant decrease in the number of developing follicles, Graffian follicles and corpora lutea and an increased number of atretic follicles in rats treated with these extracts as reported by Sharanabasappa et al15. In addition, follicular degeneration was reported after administration of Malva viscus conzattii flower extract daily for 25 days16.
In the present investigation, changes of ovarian follicular growth and histology have been accompanied by prominent histological changes on the uterine endometrium of extract treated rats. The extract caused atrophic effect on the uterine tissue as revealed by a significant reduction in the epithelial cell height, stromal thickness and myometrial thickness. These changes may be mediated through the effect of the extract either directly on the uterine tissue as estrogen antagonist or indirectly by disruption of the hormonal balance on the hypothalamo-hypophysial- ovarian-uterine axis.
The appearance of vacuolated cells containing degenerating material observed in the present study at 2.5 g/kg and 3.0 g/kg dose levels may be attributed to estrogen deprivation in the blood circulation. It is known that estrogen induces a rapid increase in micro vascular permeability in the rodent uterus, leading to stromal edema and marked increase in uterine wet weight17. Compacted stroma in extract treated animals observed in the present study may be due to little or absence of estrogen in the extract treated rats.
The above changes in the uterine histology, histomorphometry after treatment with the extract at various doses may cause the endometrium infavorable for the implantation of the fertilized ovum and hence their antifertility effect. This could be correlated with the previous studies made by Gebrie et al2 who reported that the methanolic root extract caused a significant reduction in the number of liters and a significant decrease in the number of implantation sites after treatment at the effective antifertility doses. The results observed inthe present study on the histology of the uterus is in agreement with the studies made by Pal18 who had reported that treatment of rats with Sesbania sesban seeds at a dose of 250 mg/kg and 400 mg/kg for 30 days caused great reduction in endometrial height, atrophy of uterine glands, compact stroma and poor vascularity.
Conclusion
The present study demonstrated antiovulatory and anti-uterotrophic effects of the methanolic root extract of Rumex steudelii in albino rats. This effect may be mediated through direct effect of the extract on the reproductive organs by suppressing follicular growth in the ovary and/ or by disruption of the hormonal balance in the hypothalamo-hypophysial ovarian and uterine axis.
Recommendation
Further work on isolation of the active ingredients and their effect on the histology of the reproductive organs may provide further information on the possible mechanism of action of the extract. Chronic toxicity studies should also be carried out.
Acknowledgements
We are very grateful to School of Graduate Study of the Addis Ababa University and Ministry of Health of Federal Democratic Government of Ethiopia for financial support. We are alsothankful to the Ethiopian Health and Nutrition Research Institute for providing necessary laboratory facilities. We also thank the staff of the Department of Anatomy and Pharmacology for the technical support and careful handling of laboratory animals.
References
- 1.Maurya R, Srivastava S, Kulshreshta DK, Gupta CM. Traditional remedies for fertility regulation. Current Medicinal Chemistry. 2004;11:1431–1450. doi: 10.2174/0929867043365215. [DOI] [PubMed] [Google Scholar]
- 2.Gebrie E, Makonnen E, Debella A, Zerihun L. Phytochemical screening and pharmacological evaluations for the antifertility effect of the methanolic root extract of Rumex steudelii. Journal of Ethnopharmacology. 2005;96:139–143. doi: 10.1016/j.jep.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 3.Desta B. Ethiopian Traditional Herbal Drugs. Part III: Antifertility activities of 70 medicinal plants. Journal of Ethnopharmacology. 1994;44:199–209. doi: 10.1016/0378-8741(94)01187-7. [DOI] [PubMed] [Google Scholar]
- 4.Gebrie E, Makonnen E, Zerihun L, Debella A. The possible mechanisms for the antifertility action of methanolic rootextract of Rumex steudelii. African health science. 2005;5(2):119–125. [PMC free article] [PubMed] [Google Scholar]
- 5.Bolon B, Bucci TJ, Warbritton AR, Chen JJ, Mattison DR, Heindel JJ. Differential follicle counts as a screen for chemically induced ovarian toxicity in mice: Results from continuous breeding bioassays. Fundamental Applied Toxicology. 1997;39:1–10. doi: 10.1006/faat.1997.2338. [DOI] [PubMed] [Google Scholar]
- 6.Cooper RL, Goldman JM, Vandenbergh JG. Monitoring of the estrous cycle in the laboratory rodent by vaginal lavage. In: Heindel JJ, Chapin RE, editors. Methods in toxicology: female reproductive toxicology. San Diego: Academic press; 1993. pp. 45–56. [Google Scholar]
- 7.Rama Krishna Murthy D, Madhusudan Reddy C, Patil SB. Effect of benzene extract of Hibiscus rosa sinesis on the estrous cycle and ovarian activity in albino mice. Biology and Pharmaceutical Bulletin. 1997;20(7):756. doi: 10.1248/bpb.20.756. [DOI] [PubMed] [Google Scholar]
- 8.Andriana DP, Thomas HU, Benjamin RF, Peter LG, Nicholas TL, Ken MB. Bisphenol A-induced increase in uterine weight and alterations in uterine morphology in ovariectomized B6C3F1 Mice: Role of Estrogen Receptor. Toxicological science. 2000;56:332–339. doi: 10.1093/toxsci/56.2.332. [DOI] [PubMed] [Google Scholar]
- 9.Lundy T, Smith P, O' Connell A, Hudson NL, Mc Natty KP. Populations of granulosa cells in small follicles of the sheep ovary. Journal of Reproduction and fertility. 1999;155:251–262. doi: 10.1530/jrf.0.1150251. [DOI] [PubMed] [Google Scholar]
- 10.Osman P. Rate and course of atresia during follicular development in the adult cycling rat. Journal of reproduction and fertility. 1985;73:261–270. doi: 10.1530/jrf.0.0730261. [DOI] [PubMed] [Google Scholar]
- 11.Engelbregt MJT, Van Weissenbruch MM, Popp-Snijders C, Delemarre-Van de Waal HA. Delayed first cycle in intrauterine growth-related and postnatally undernourished female rats: Follicular growth and ovulation after stimulation with pregnant mare serum gonadotropin at first cycle. Journal of endocrinology. 2002;173:297–304. doi: 10.1677/joe.0.1730297. [DOI] [PubMed] [Google Scholar]
- 12.Cortvrindt R, Smith Invitro follicle growth: Achievements in Mammalian species. Reproduction of Domestic Animals. 2001;36:3–9. doi: 10.1046/j.1439-0531.2001.00261.x. [DOI] [PubMed] [Google Scholar]
- 13.Shivalingappa H, Satyanarayan ND, Purohit MG, Sharanabasappa A, Patil SB. Effect of ethanol extract of Rivea hypocrateriformis on the estrous cycle of the rat. Journal of Ethnopharmacology. 2002;82:11–17. doi: 10.1016/s0378-8741(02)00073-9. [DOI] [PubMed] [Google Scholar]
- 14.Gore-Langton RE, Armstrong DT. Follicular steroidogenesis and its control. In: Knobil E, Neil J, editors. The physiology of reproduction. New York: Raven press; 1988. pp. 331–385. [Google Scholar]
- 15.Sharanabasappa A, Vijayakumar B, Sarasweit B. Effect of Momordica charantia seed extracts on ovarian and uterine activities in Albino Rats. Pharmaceutical Biology. 2002;40(7):501–507. [Google Scholar]
- 16.Dixit VP. Effect of chronically administered Malva Viscus Conzattii flower extract on the female genital tract of gerbil, Merionis hurrianae, Jerdon. Indian Journal of Experimental Biology. 1977;15(8):1650–1652. [PubMed] [Google Scholar]
- 17.Carroll WR. Variation in the water content of the rat uterus during continuous estrogenic treatment. Endocrinology. 1945;36:266–271. [Google Scholar]
- 18.Pal SS. Fertility control of female through Sesbania Sesban Seeds. Journal of Research and Education in Indian Medicine. 1990;9(4):27–32. [Google Scholar]