Currently, 24 active antiretroviral agents are available for use in the United States. These agents target and interrupt an array of HIV functions, including reverse transcription, proteolysis, envelope fusion, and integration [1]. When used in a variety of combinations, these agents have greatly reduced the morbidity and mortality associated with natural HIV infection in the United States [2]. In this issue of Clinical Infectious Diseases, Geretti et al. [3] demonstrate in an observational study that modern combination antiretroviral therapy works, even when the infecting virus is non–subtype B HIV-1. Specifically, this study examined the rates of viral suppression with antiretroviral therapy during infection with subtypes A, C, and D and with the circulating recombinant form (CRF) 02_AG. This is important, because modern antiretroviral therapy was developed on the basis of investigations of subtype B HIV-1 infection, even though this subtype accounts for only 12% of worldwide infections and is almost nonexistent in many regions [4].
HIV-1 DIVERSITY
HIV is a lentivirus with zoonotic origins that can be traced to the simian immunodeficiency virus that infects chimpanzees for HIV-1 and sooty mangabeys for HIV-2 [5–7]. Phylogenetic analyses of viral isolates suggest 3 independent transmission events of primate simian immunodeficiency viruses to humans, each establishing distinct groups of HIV-1: M (main), O (outlier), and N (new). There are currently 9 recognized HIV-1 pure subtypes (A–D, F–H, J, and K) and 43 CRFs [8–10]. In many places in the world where multiple subtypes cocirculate, up to 10% of HIV-1 infections occur with unique recombination forms (URFs), owing to the ease and frequency with which HIV-1 recombines during dual infection [8, 9, 11–13]. Formally, subtypes are defined as phylogenetically separate clusters of strains, and HIV-1 sequences that are the result of recombination among pure subtypes are defined as CRFs if there are at least 3 independent viral strains that show the same recombination structure and as URFs otherwise [10]. The nomenclature of subtypes and recombinant forms is continually evolving (pun intended) as molecular epidemiology and surveillance of HIV-1 throughout the world improves.
The HIV-1 genome is ~9.8 kb long (compared with ~3 gigabases in the human genome) and is composed of 9 genetic coding regions, each performing a variety of structural, virologic, and immunologic evasion functions [14]. These coding regions vary in their genetic diversity across group M subtypes. For instance, the env gene, which encodes for the envelope protein, can be >30% different between any 2 subtypes [15], whereas the region that encodes the integrase protein is usually only 3%–8% genetically distant [10]. The high genetic diversity of the HIV-1 M group is due to a number of factors, including mutation rates close to the evolutionary speed limit [16], high recombination rates [17], vigorous heterogeneous cellular [18] and humoral [19] immune responses that leave an imprint on the viral genome [20], and complex population dynamics [21]. Despite this enormous genetic diversity, each subtype, CRF, and URF of the HIV-1 M group can cause the characteristic immunosuppression in the form of AIDS, leading to death. However, the extent of clinically meaningful phenotypical differences among the subtypes in pathogenicity (such as neurologic, cardiovascular, or renal damage), transmissibility, the ability to evade host immune responses, or cellular tropism remains uncertain.
ANTIRETROVIRAL THERAPY AND DRUG RESISTANCE
Because the HIV epidemics found in the wealthiest countries of the world involve predominantly subtype B virus, the development of antiretroviral therapy has been heavily based on the virology of this subtype [22–25]. Similarly, most investigation into HIV drug resistance has been limited to subtype B virus, and little information in genotypic resistance patterns or the rate of resistance development is available for other subtypes [26]. Correspondingly, HIV sequences deposited in public databases and used for retrospective research are disproportionately of subtype B. For instance, the Stanford Drug Resistance Database [27] currently contains ~17,300 subtype B reverse-transcriptase sequences but only ~10,400 sequences of all other subtypes combined, even though non–subtype B accounts for ~88% of all HIV-1 infections worldwide [4].
Owing to a high replication rate and error-prone reverse transcription, all subtypes, CRFs, and URFs of HIV-1 can rapidly develop mutations that confer decreased susceptibility (or resistance) to every available antiretroviral therapy agent [27–30]. The rate of developing resistance-associated mutations (RAMs) depends on the maintenance of protein function, replicative fitness advantage in the presence of drug, and ease of the mutational change, which are all influenced by the genetic background of the infecting virus [25]. For example, a single nucleotide substitution from the wild-type codon found in subtype C can generate the mutation V106M, which is associated with non-nucleoside reverse-transcriptase inhibitor resistance, but at least 2 substitutions are needed for the wild-type subtype B codon (figure 1). Table 1 provides other examples of previously characterized RAMs for which the most direct mutational pathway from wild-type codon to RAM is shorter in non-B subtypes. In addition, the observed pattern of RAMs can be different for the same antiretroviral therapy agent among different subtypes, especially for mutations outside the reverse-transcriptase- and protease-coding regions [31], although previous work by Kantor et al. [32] found that HIV-1 resistance patterns are similar across subtypes.
Figure 1.
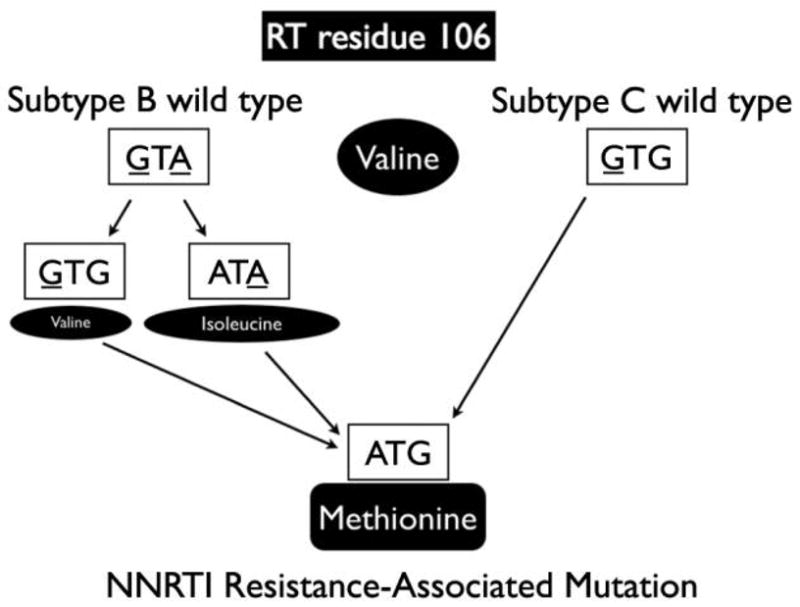
Different pathways to acquire a V106M mutation in reverse transcriptase (RT), which is associated with nonnucleoside reverse-transcriptase inhibitor (NNRTI) resistance from wild-type codons of human immunodeficiency virus type 1 subtype B and subtype C.
Table 1.
Positions in HIV-1 pol where the direct mutational pathway from consensus (wild type) to a resistance-associated mutation (RAM) is shorter in some non-B subtypes than in subtype B.
| Position in pol | ART class | RAM residuea | Shortest mutational pathway | |
|---|---|---|---|---|
| Subtype B | Non-B subtypeb | |||
| PR V82 | PI | S T |
GTC→TCC GTC→AGC |
G, CRF14: ATC→AGC G, CRF14: ATC→ACC |
| RT D67 | NRTI | N | GAC→AAC | CRF04: AAC |
| RT K70 | NRTI | G R |
AAA→GGA AAA→AGA |
CRF04: AGA→GGA CRF04: AGA |
| RT V106 | NNRTI | M | GTA→ATG | C: GTG→ATG |
| RT V179 | NNRTI | E | GTT→GAG | G, CRF02, CRF14: GTG→GAG |
| RT T215 | NRTI | F Y |
ACC→TTC ACC→TAC |
CRF04: TCC→TTC CRF04: TCC→TAC |
| IN E157 | INI | Q | GAA→CAA | CRF03: CAA |
NOTE. Consensus sequences based on the reference sets from Los Alamos National Laboratory (http://www.hiv.lanl.gov). ART, antiretroviral therapy; CRF, circulating recombinant form; IN, integrase; INI, integrase inhibitor; NNRTI, nonnucleoside reverse-transcriptase inhibitor; NRTI, nucleoside reverse-transcriptase inhibitor; PI, protease inhibitor; PR, protease coding region; RT, reverse-transcriptase coding region.
Single-letter amino acid code.
Non-B subtypes are underlined.
As antiretroviral therapy use becomes more prevalent worldwide, drug-resistant viral strains are likely to increase in frequency among all subtypes because of both de novo adaptation and transmission of resistant variants [33]. Transmitted drug resistance is especially concerning because most non–subtype B infections occur in parts of the world where viral load monitoring during antiretroviral therapy is either unavailable or not routinely used [34]. Even in Australia, Canada, and the United States, where viral loads are regularly available, multidrug-resistant HIV-1 strains currently comprise up to 6% of new HIV infections [33, 35]. In addition, viral subtypes often circulate in geographic regions with distinct local populations [36], and therefore the efficacy of antiretroviral therapy among populations may be more influenced by the different host genetics or nutrition (e.g., drug adsorption and metabolism) or cultural differences (e.g., medication use and adherence) [37] more so than the subtype of the infecting virus.
SUMMARY AND RECOMMENDATIONS
As demonstrated in the work of Geretti et al. [3], modern antiretroviral therapy works well for the handful of non–subtype B viruses investigated so far and will certainly save lives, independent of the subtype of the infecting virus. To evaluate how infecting subtype influences treatment response as antiretroviral therapy is rolled out to the rest of the world, where most HIV-1 infections are not subtype B, we will need to (1) maintain robust and upto-date subtype determination procedures [38], (2) routinely monitor for virologic failure of antiretroviral therapy with subsequent surveillance for drug resistance in worldwide populations [34], (3) develop genotypic methods that reliably detect RAMs in sequences of all subtypes, (4) characterize antiretroviral therapy susceptibility (phenotype) for RAMs by subtype [31], and (5) evaluate the impact of host characteristics of the infected population on antiretroviral therapy efficacy and viral subtype. Together, these procedures will help inform antiretroviral therapy guidelines among various populations infected with different subtypes.
Footnotes
Potential conflicts of interest. S.L.K.P. and D.M.S.: no conflicts.
References
- 1.Hammer SM, Eron JJ, Jr, Reiss P, et al. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society-USA panel. JAMA. 2008;300:555–70. doi: 10.1001/jama.300.5.555. [DOI] [PubMed] [Google Scholar]
- 2.Palella FJ, Jr, Baker RK, Moorman AC, et al. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr. 2006;43:27–34. doi: 10.1097/01.qai.0000233310.90484.16. [DOI] [PubMed] [Google Scholar]
- 3.Geretti AM, Harrison L, Green H, et al. The effect of HIV-1 subtype on virological and immunological response to starting highly active antiretroviral therapy. Clin Infect Dis. 2009;48:1296–1305. doi: 10.1086/598502. (in this issue) [DOI] [PubMed] [Google Scholar]
- 4.Hemelaar J, Gouws E, Ghys PD, Osmanov S. Global and regional distribution of HIV-1 genetic subtypes and recombinants in 2004. AIDS. 2006;20:W13–23. doi: 10.1097/01.aids.0000247564.73009.bc. [DOI] [PubMed] [Google Scholar]
- 5.Clavel F, Guetard D, Brun-Vezinet F, et al. Isolation of a new human retrovirus from West African patients with AIDS. Science. 1986;233:343–6. doi: 10.1126/science.2425430. [DOI] [PubMed] [Google Scholar]
- 6.Keele BF, Van Heuverswyn F, Li Y, et al. Chimpanzee reservoirs of pandemic and nonpandemic HIV-1. Science. 2006;313:523–6. doi: 10.1126/science.1126531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Heuverswyn F, Li Y, Neel C, et al. Human immunodeficiency viruses: SIV infection in wild gorillas. Nature. 2006;444:164. doi: 10.1038/444164a. [DOI] [PubMed] [Google Scholar]
- 8.Perrin L, Kaiser L, Yerly S. Travel and the spread of HIV-1 genetic variants. Lancet Infect Dis. 2003;3:22–7. doi: 10.1016/s1473-3099(03)00484-5. [DOI] [PubMed] [Google Scholar]
- 9.Robertson DL, Anderson JP, Bradac JA, et al. HIV-1 nomenclature proposal. Science. 2000;288:55–6. doi: 10.1126/science.288.5463.55d. [DOI] [PubMed] [Google Scholar]
- 10. [Accessed 21 January 2009];HIV sequence database. 2007 July; Available at: http://www.hiv.lanl.gov.
- 11.McCutchan FE, Hoelscher M, Tovanabutra S, et al. In-depth analysis of a heterosexually acquired human immunodeficiency virus type 1 superinfection: evolution, temporal fluctuation, and intercompartment dynamics from the seronegative window period through 30 months postinfection. J Virol. 2005;79:11693–704. doi: 10.1128/JVI.79.18.11693-11704.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris ME, Serwadda D, Sewankambo N, et al. Among 46 near full length HIV type 1 genome sequences from Rakai District, Uganda, subtype D and AD recombinants predominate 1. AIDS Res Hum Retroviruses. 2002;18:1281–90. doi: 10.1089/088922202320886325. [DOI] [PubMed] [Google Scholar]
- 13.Peeters M. [Accessed 21 January 2009];Recombinant HIV sequences: their role in the global epidemic. Available at: http://www.hiv.lanl.gov/content/index.
- 14.Frankel AD, Young JA. HIV-1: fifteen proteins and an RNA. Annu Rev Biochem. 1998;67:1–25. doi: 10.1146/annurev.biochem.67.1.1. [DOI] [PubMed] [Google Scholar]
- 15.Gao F, Morrison SG, Robertson DL, et al. Molecular cloning and analysis of functional envelope genes from human immunodeficiency virus type 1 sequence subtypes A through G. J Virol. 1996;70:1651–67. doi: 10.1128/jvi.70.3.1651-1667.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeldovich KB, Chen P, Shakhnovich EI. Protein stability imposes limits on organism complexity and speed of molecular evolution. Proc Natl Acad Sci U S A. 2007;104:16152–7. doi: 10.1073/pnas.0705366104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rhodes T, Wargo H, Hu WS. High rates of human immunodeficiency virus type 1 recombination: near-random segregation of markers one kilobase apart in one round of viral replication. J Virol. 2003;77:11193–200. doi: 10.1128/JVI.77.20.11193-11200.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brumme ZL, Brumme CJ, Carlson J, et al. Marked epitope- and allele-specific differences in rates of mutation in human immunodeficiency type 1 (HIV-1) Gag, Pol, and Nef cytotoxic T-lymphocyte epitopes in acute/early HIV-1 infection. J Virol. 2008;82:9216–27. doi: 10.1128/JVI.01041-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frost SD, Wrin T, Smith DM, et al. Neutralizing antibody responses drive the evolution of human immunodeficiency virus type 1 envelope during recent HIV infection. Proc Natl Acad Sci USA. 2005;102:18514–9. doi: 10.1073/pnas.0504658102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore CB, John M, James IR, Christiansen FT, Witt CS, Mallal SA. Evidence of HIV-1 adaptation to HLA-restricted immune responses at a population level. Science. 2002;296:1439–43. doi: 10.1126/science.1069660. [DOI] [PubMed] [Google Scholar]
- 21.Poon AF, Kosakovsky Pond SL, Bennett P, Richman DD, Leigh Brown AJ, Frost SD. Adaptation to human populations is revealed by within-host polymorphisms in HIV-1 and hepatitis C virus. PLoS Pathog. 2007;3:e45. doi: 10.1371/journal.ppat.0030045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomson MM, Najera R. Molecular epidemiology of HIV-1 variants in the global AIDS pandemic: an update. AIDS Rev. 2005;7:210–24. [PubMed] [Google Scholar]
- 23.Geretti AM. HIV-1 subtypes: epidemiology and significance for HIV management. Curr Opin Infect Dis. 2006;19:1–7. doi: 10.1097/01.qco.0000200293.45532.68. [DOI] [PubMed] [Google Scholar]
- 24.Finzi D. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 25.Wong JK, Hezareh M, Gunthard HF, et al. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278:1291–5. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- 26.Kantor R, Katzenstein D. Drug resistance in non-subtype B HIV-1. J Clin Virol. 2004;29:152–9. doi: 10.1016/S1386-6532(03)00115-X. [DOI] [PubMed] [Google Scholar]
- 27.Rhee SY, Gonzales MJ, Kantor R, Betts BJ, Ravela J, Shafer RW. Human immunodeficiency virus reverse transcriptase and protease sequence database. Nucleic Acids Res. 2003;31:298–303. doi: 10.1093/nar/gkg100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pillay V, Pillay C, Kantor R, Venter F, Levin L, Morris L. HIV type 1 subtype C drug resistance among pediatric and adult South African patients failing antiretroviral therapy. AIDS Res Hum Retroviruses. 2008;24:1449–54. doi: 10.1089/aid.2008.0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sylla M, Chamberland A, Boileau C, et al. Characterization of drug resistance in antiretroviral-treated patients infected with HIV-1 CRF02_AG and AGK subtypes in Mali and Burkina Faso. Antivir Ther. 2008;13:141–8. [PubMed] [Google Scholar]
- 30.Nyombi BM, Holm-Hansen C, Kristiansen KI, Bjune G, Muller F. Prevalence of reverse transcriptase and protease mutations associated with antiretroviral drug resistance among drug-naive HIV-1 infected pregnant women in Kagera and Kilimanjaro regions, Tanzania. AIDS Res Ther. 2008;5:13. doi: 10.1186/1742-6405-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martinez-Cajas JL, Pant-Pai N, Klein MB, Wainberg MA. Role of genetic diversity amongst HIV-1 non-B subtypes in drug resistance: a systematic review of virologic and biochemical evidence. AIDS Rev. 2008;10:212–23. [PubMed] [Google Scholar]
- 32.Kantor R, Katzenstein DA, Efron B, et al. Impact of HIV-1 subtype and antiretroviral therapy on protease and reverse transcriptase genotype: results of a global collaboration. PLoS Med. 2005;2:e112. doi: 10.1371/journal.pmed.0020112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Little SJ, Holte S, Routy JP, et al. Antiretroviral-drug resistance among patients recently infected with HIV. N Engl J Med. 2002;347:385–94. doi: 10.1056/NEJMoa013552. [DOI] [PubMed] [Google Scholar]
- 34.Smith DM, Schooley RT. Running with scissors: using antiretroviral therapy without monitoring viral load. Clin Infect Dis. 2008;46:1598–600. doi: 10.1086/587110. [DOI] [PubMed] [Google Scholar]
- 35.Liu L, May S, Richman DD, et al. Comparison of algorithms that interpret genotypic HIV-1 drug resistance to determine the prevalence of transmitted drug resistance. AIDS. 2008;22:835–9. doi: 10.1097/QAD.0b013e3282f5ff71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gilbert MT, Rambaut A, Wlasiuk G, Spira TJ, Pitchenik AE, Worobey M. The emergence of HIV/AIDS in the Americas and beyond. Proc Natl Acad Sci U S A. 2007;104:18566–70. doi: 10.1073/pnas.0705329104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.King J, Aberg JA. Clinical impact of patient population differences and genomic variation in efavirenz therapy. AIDS. 2008;22:1709–17. doi: 10.1097/QAD.0b013e32830163ad. [DOI] [PubMed] [Google Scholar]
- 38.Holguin A, Lopez M, Soriano V. Reliability of rapid subtyping tools compared to that of phylogenetic analysis for characterization of human immunodeficiency virus type 1 non-B subtypes and recombinant forms. J Clin Microbiol. 2008;46:3896–9. doi: 10.1128/JCM.00515-08. [DOI] [PMC free article] [PubMed] [Google Scholar]


