Abstract
BACKGROUND
As people with acquired immunodeficiency syndrome (AIDS) live longer due to highly active antiretroviral therapy (HAART, widely available since 1996), the overall burden of cancer may increase.
METHODS
A population-based record linkage study identified cancers in 472,378 people with AIDS (1980–2006). Using non-parametric competing risk methods, we estimated cumulative incidence of cancer across 3 calendar periods (AIDS onset in 1980–1989, 1990–1995, and 1996–2006).
RESULTS
Measured at 5-years after AIDS onset, cumulative incidence of AIDS-defining cancer (ADC) declined sharply across AIDS calendar periods (from 18% in 1980–1989, to 11% in 1990–1995, to 4.2% in 1996–2006 [i.e., HAART era]). Cumulative incidence of Kaposi sarcoma declined from 14.3% during 1980–1989 to 6.7% during 1990–1995 to 1.8% during 1996–2006. Non-Hodgkin lymphoma (NHL) cumulative incidence declined from 3.8% during 1990–1995 to 2.2% during 1996–2006; during the HAART era, NHL was the commonest (53%) ADC. Cumulative incidence of non-AIDS-defining cancer (NADC) increased from 1.1% to 1.5% with no change thereafter (1.0%, 1996–2006), in part due to declines in competing mortality. However, cumulative incidence increased steadily over time for specific NADCs (anal cancer, Hodgkin lymphoma, and liver cancer). Lung cancer cumulative incidence increased from 0.14% during 1980–1989 to 0.32% during 1990–1995, with no change thereafter.
CONCLUSIONS
We noted dramatically declining cumulative incidence of 2 major ADCs (Kaposi sarcoma and NHL) and increases in some NADCs (specifically cancers of the anus, liver, and lung, and Hodgkin lymphoma). As HIV/AIDS is increasingly managed as a chronic disease, greater attention should be focused on cancer screening and prevention.
Keywords: AIDS, anal cancer, cancer, HIV, Hodgkin lymphoma, mortality, nonparametric statistics
Mortality among human immunodeficiency virus (HIV)-infected people declined dramatically in the U.S. beginning in 1996, following widespread use of highly active antiretroviral therapy (HAART).(1–3) Despite improved overall survival, HIV-infected people have elevated cancer risk, particularly with advanced HIV infection (i.e., acquired immunodeficiency syndrome [AIDS]). Kaposi sarcoma (KS), non-Hodgkin lymphoma (NHL), and cervical cancer are considered AIDS-defining cancers (ADCs).(4) HIV-induced immune suppression leading to poor control of oncogenic viral infections plays an important role in the development of KS (due to KS-associated herpesvirus) and NHL (due to Epstein Barr virus).(5) Cervical cancer is caused by human papillomavirus.(6) HIV-infected people also have an elevated risk for other malignancies (i.e., non-AIDS-defining cancers [NADCs]), due to a high prevalence of additional viral coinfections (e.g., liver cancer caused by hepatitis C virus) and exposure to other carcinogens (e.g., lung cancer caused by tobacco).(7)
The incidence of the major ADCs (KS and NHL) steeply declined relative to widespread HAART use beginning in 1996.(8–10) However, rates of the most common NADCs (cancers of the anus, liver, and lung, and Hodgkin lymphoma) have increased or remained stable during the same period.(9–12) Prior studies have measured cancer risk in terms of incidence (e.g., rate per 100,000 person-years, or standardized to the general population), which reflects instantaneous risk and is appropriate for considering the effects of various etiologic factors or treatment (e.g., HAART) on the development of cancer. However, cancer incidence does not translate directly into cumulative risk, that is, the proportion of people with AIDS who will develop cancer over a specified time period. Cumulative incidence depends on both cancer incidence rates and mortality rates, as the occurrence of death, a competing event, precludes the occurrence of cancer.(13)
As people with AIDS live longer, their cumulative incidence of other diseases, including cancer, would be expected to increase as they contribute more person-time at risk to the development of these outcomes. The goals of the current study were to account for mortality trends while quantifying the overall burden of cancer among people with AIDS and to evaluate the impact of widespread HAART use on cancer incidence over time.
MATERIALS AND METHODS
Study design
The HIV/AIDS Cancer Match (HACM) Study is a population-based registry linkage study of people with HIV or AIDS diagnosed between 1980 and 2008 from 15 U.S. state and metropolitan regions.(8) Cases of HIV/AIDS and cancers are reportable to these registries through passive and active surveillance systems. Records of people with AIDS were then linked to cancer registry records using a probabilistic matching algorithm. Following linkage, only de-identified data are retained for analyses. Institutional review boards at participating sites approved the study.
A cohort was constructed from HACM data to evaluate cancer risk at or after AIDS onset. AIDS onset was defined using the 1993 Centers for Disease Control and Prevention definition.(4) Of N=574,242 eligible people with AIDS, individuals whose observation time ended before AIDS onset or started after AIDS onset (i.e., month 0; N=91,022) were excluded in order to make the cohort uniform at baseline. Subjects with any cancer recorded in the cancer registry prior to month 0 were also excluded (N=4,853) as were those diagnosed with AIDS prior to 1980 (N=16) and children aged less than 14 years (N=5,973), yielding a cohort of adults and adolescents with AIDS who were cancer-free before AIDS onset (N=472,378).
Information on invasive cancers was obtained from cancer registries, and malignancies were coded according to the International Classification for Diseases for Oncology.(14) Cancers were categorized by site and histology using a modification of the Surveillance, Epidemiology, and End Results (SEER) program’s “site recode with Kaposi sarcoma and mesothelioma”.(10;15) Only first cancers were considered. Vital status information was obtained from AIDS registries at the time of the linkage.
Statistical methods
Estimates of cumulative incidence (or cause-specific failure probabilities) are of clinical and public health interest, because they provide information on the probability of actually observing the event of interest (e.g., cancer) as people are followed over time. Cumulative incidence depends not only on the instantaneous risk of the event of interest but also on the risk of competing events (e.g., death) that, if they occur, preclude the development of the event of interest. In contrast, the Kaplan-Meier method provides a pure risk estimate, that is, the probability of experiencing an event of interest if all competing events could be removed from the population. This estimate is much less practically relevant, as it ignores that a subject in the real world may die before experiencing the event of interest.(13;16) We therefore computed non-parametric estimates for cumulative incidence of 3 competing outcomes: death, ADC, and NADC. The cumulative incidence function at time t estimates the probability that the event of interest (e.g. ADC) occurs before t and that it occurs before any of the competing causes of failure (e.g., death or NADC). The cumulative incidence estimates for a given cause were computed by summing the product of the overall survival function multiplied by the estimated cause-specific hazard at time t over all event times.(13)
Subjects were classified according to calendar period of AIDS onset: 1980–1989 (no or limited availability of antiretroviral therapy), 1990–1995 (monotherapy and/or dual therapy) and 1996–2006 (HAART). Observation began at AIDS onset and stopped at the event of interest, a competing event, or administrative censoring (last follow-up for cancer from the cancer registry or death according to the AIDS registry, whichever occurred first). This approach was used to estimate cumulative incidence of overall ADCs and NADCs. In additional analyses, we focused on KS and NHL, as well as the most common NADCs associated with HIV infection: cancers of the anus, liver, and lung, and Hodgkin lymphoma.(9;10) We describe the proportion of people with these cancers at AIDS onset and present cumulative incidence curves over the entire course of follow-up. We used estimates of cumulative incidence at 60 months of follow-up (5 years after AIDS onset) with corresponding 95% confidence intervals (95% CIs) for statistical testing. Five-year cumulative incidence estimates were compared using a t-test, evaluating whether incidence changed for people diagnosed with AIDS during 1980–1989, 1990–1995, and 1996–2006. Cumulative incidence at 120 months (10 years) of follow-up after AIDS onset was also compared. P-values <0.05 were considered significant.
RESULTS
Demographic characteristics of the 472,378 people included in the study are presented in Table 1. The proportion of males declined from 89.0% (1980–1989) to 75.2% (1996–2006) and the proportion of non-Hispanic whites decreased, while the proportion of non-Hispanic blacks and Hispanics increased over time. Of the major categories of HIV exposure, the proportion of men reporting male-to-male sex declined from 62.1% in 1980–1989 to 53.6% in 1996–2006, and the proportion reporting heterosexual contact increased from 3.4% to 6.3% during the same periods (Table 1).
Table 1.
Demographic Characteristics of People with AIDS in the United States, 1980–2006 (N=472,378)
| Characteristic | AIDS Diagnosis Calendar Period | ||
|---|---|---|---|
| 1980–1990 | 1990–1995 | 1996–2006 | |
| Total No. | 83789 | 213029 | 175560 |
| Sex, n (%) | |||
| Male | 74547 (89.0) | 174227 (81.8) | 132017 (75.2) |
| Female | 9242 (11.0) | 38802 (18.2) | 43543 (24.8) |
| Age in years at AIDS onset, n (%) | |||
| 15–29 | 16316 (19.5) | 33533 (15.7) | 23153 (13.2) |
| 30–39 | 39965 (47.7) | 96995 (45.5) | 71787 (40.9) |
| 40–49 | 19032 (22.7) | 459249 (27.8) | 55718 (31.7) |
| 50+ | 8476 (10.1) | 23252 (11.0) | 24902 (14.2) |
| Median | 36 | 37 | 39 |
| Race/ethnicity, n (%) | |||
| Non-Hispanic white | 43372 (51.8) | 85499 (40.1) | 50476 (28.8) |
| Non-Hispanic black | 25511 (30.5) | 80861 (38.0) | 84994 (48.4) |
| Hispanic | 14129 (16.9) | 44312 (20.8) | 37877 (21.6) |
| Other/unknown | 777 (0.8) | 2357 (1.1) | 2213 (1.3) |
| Mode of HIV exposure, n (%)a | |||
| MSM | 48394 (62.1) | 95980 (52.7) | 62330 (53.6) |
| IDU | 21150 (27.1) | 64348 (35.3) | 38613 (33.2) |
| MSM and IDU | 5736 (7.4) | 12199 (6.7) | 7996 (6.9) |
| Heterosexual | 2653 (3.4) | 9756 (5.3) | 7440 (6.3) |
Abbreviations: MSM, male-to-male sex; IDU, injection drug use.
Column percentages for mode of HIV exposure are reported for the four most common modes of exposure excluding people in the ‘other/unknown’ category (most subjects in the other/unknown category had unknown rather than other known mode of transmission).
Based on competing risk models, cumulative incidence of death (as a first event, prior to cancer) was substantial in each calendar period of AIDS onset but declined significantly at 5 years of follow-up from 70% (1980–1989) to 55% (1990–1995) to 22% (1996–2006) (Table 2). Significant declines were also noted at 120 months of follow-up (data not shown).
Table 2.
Cumulative Incidence of AIDS-defining Cancers, and Non-AIDS-defining Cancers Among People with AIDS in the United States, 1980–2006 (N=472,378)
| Occurrence of outcome as a first event | Total number of events, by calendar period of AIDS onset |
Five-year cumulative incidence (95% confidence interval), by calendar period of AIDS onset |
||||
|---|---|---|---|---|---|---|
| 1980–1989 | 1990–1995 | 1996–2006 | 1980–1989 | 1990–1995a | 1996–2006b | |
| Death | 61587 | 128148 | 41010 | 70 (69,70) | 55 (54,55) | 22 (21,22) |
| AIDS-defining cancer | 15728 | 23603 | 7570 | 18 (18,19) | 11 (11,11) | 4.2 (4.1,4.3) |
| Kaposi sarcoma | 12196 | 14597 | 3277 | 14.3 (14.1,14.6) | 6.7 (6.6,6.8) | 1.8 (1.8,1.9) |
| Non-Hodgkin lymphoma | 3463 | 8690 | 4011 | 3.9 (3.8,4.0) | 3.8 (3.7,3.9) | 2.2 (2.2,2.3) |
| Cervical cancerc | 69 | 316 | 282 | 0.63 (0.50,0.77) | 0.73 (0.70,0.82) | 0.64 (0.57,0.71) |
| Non-AIDS-defining cancer | 1056 | 4348 | 2911 | 1.1 (1.0,1.1) | 1.5 (1.5,1.6) | 1.5 (1.4,1.5) |
| Anal cancer | 29 | 328 | 196 | 0.02 (0.01,0.03) | 0.07 (0.06,0.08) | 0.09 (0.08,0.11) |
| Hodgkin lymphoma | 78 | 425 | 359 | 0.09 (0.07,0.11) | 0.15 (0.14,0.17) | 0.19 (0.17,0.21) |
| Liver cancer | 22 | 147 | 121 | 0.02 (0.01,0.03) | 0.04 (0.03,0.05) | 0.06 (0.05,0.07) |
| Lung cancer | 150 | 915 | 633 | 0.14 (0.11,0.16) | 0.32 (0.29,0.34) | 0.33 (0.30,0.36) |
Number of events corresponds to all events over the entire period of follow-up.
Cumulative incidence was calculated using competing risk methods at 60-months of follow-up, expressed as a percentage (%) of people with the specified outcome.
Cumulative incidence estimates during 1990–1995 were compared to those during 1980–1989 via a 2-sided t-test. Bolded values indicate a significant difference at P<0.05.
Cumulative incidence estimates during 1990–1995 were compared to those during 1996–2006 via a 2-sided t-test. Bolded values indicate a significant difference at P<0.05.
Analyses were restricted to women.
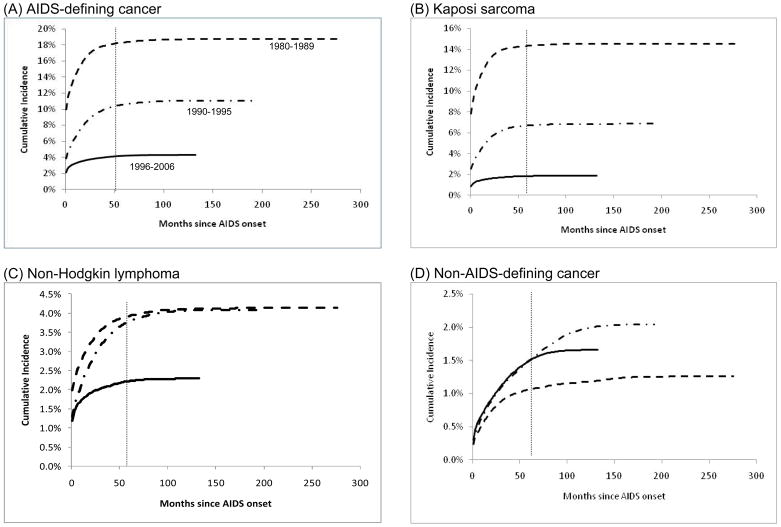
At AIDS onset, an ADC was diagnosed in 9.9% of people in 1980–1989, 3.9% of people in 1990–1995, and 2.1% of people in 1996–2006 (Figure 1A). Further, cumulative incidence of ADCs increased steeply immediately after AIDS onset for people diagnosed during 1980–1989 and 1990–1995, and to a lesser extent, during 1996–2006 (Figure 1A). Five-year cumulative incidence of ADC declined significantly with AIDS calendar time: 18% during 1980–1989, 11% during 1990–1995, and 4.2% during 1996–2006 (Table 2). Similar declines for ADCs were noted at 120 months of follow-up (Figure 1A).
Figure 1. Cumulative Incidence of AIDS-defining and non-AIDS-defining Cancer Among People with AIDS in the United States, 1980–2006.
Cumulative incidence of AIDS-defining and non-AIDS-defining cancer among people with AIDS in the United States. Results are shown for AIDS-defining cancers overall (panel A), and the 2 major AIDS-defining cancers, Kaposi sarcoma (panel B) and non-Hodgkin lymphoma (panel C). Cumulative incidence of non-AIDS-defining cancers overall is shown in panel D. Estimates are stratified by calendar year of AIDS onset: 1980–1989 (dotted line), 1990–1995 (dashed line), 1996–2006 (solid line). Cumulative incidence was estimated using competing risk time-to-event methods and is expressed as a percentage. Follow-up time is measured beginning at AIDS onset. The gray vertical line indicates cumulative incidence estimates compared at month 60 of follow-up (5-year cumulative incidence). Note vertical scales vary among the panels.
Kaposi sarcoma was the most common ADC during 1980–1989 (78%) and 1990–1995 (62%); however, during 1996–2006 NHL accounted for a majority of ADC events (53%). Cumulative incidence of KS declined across the 3 calendar periods of AIDS onset (Figure 1B, Table 2). For NHL, cumulative incidence did not change significantly in the 1990–1995 period (3.8% at 5-years after AIDS onset) relative to the 1980–1989 period (3.9%); however, NHL cumulative incidence subsequently declined to 2.2% among people diagnosed with AIDS during 1996–2006 (Figure 1C and Table 2, P<0.05). The 5-year cumulative incidence of cervical cancer among women with AIDS did not change significantly across calendar periods (0.63% during 1980–1989, 0.73% during 1990–1995, 0.64% during 1996–2006) (Table 2). Among women diagnosed with AIDS during 1996–2006, cumulative incidence of cervical cancer at 120 months of after AIDS onset was significantly lower (0.65%) relative to those diagnosed during 1990–1995 (0.80%).
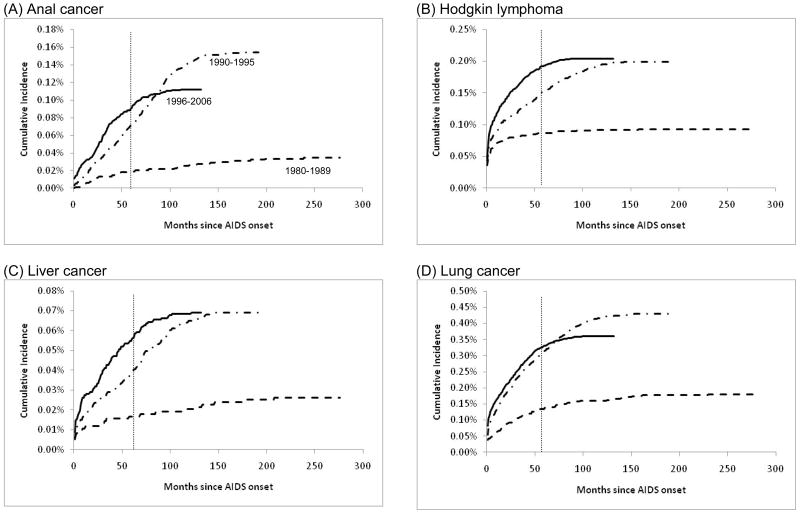
In contrast, cumulative incidence of NADCs overall increased significantly from 1980–1989 to 1990–1995 (from 1.1% to 1.5% at five years); no further change was apparent among people diagnosed with AIDS during 1996–2006 (1.5%) (Figure 1D and Table 2). Notably, however, a progressive significant increase in 5-year cumulative incidence was observed across the 3 successive calendar periods of AIDS onset for several specific NADCs (Figure 2 and Table 2). These cancers were anal cancer (5-year cumulative incidence increasing from 0.02% to 0.07% to 0.09%), Hodgkin lymphoma (0.09% to 0.15% to 0.19%), and liver cancer (0.02% to 0.04% to 0.06%). The 5-year cumulative incidence of lung cancer increased from 0.14% during 1980–1989 to 0.32% during 1990–1995, with no change subsequently (Table 2). At 120 months of follow-up, cumulative incidence of anal and lung cancer declined among people diagnosed with AIDS during 1996–2006 relative to 1990–1995, but there were no significant changes in the cumulative incidence of liver cancer and Hodgkin lymphoma between the same calendar periods (Figures 2B, 2C and 2D).
Figure 2. Cumulative Incidence of Selected Non-AIDS-defining Cancers Among People with AIDS in the United States, 1980–2006.
Cumulative incidence of selected non-AIDS-defining cancers among people with AIDS in the United States. Results are shown for anal cancer (panel A), Hodgkin lymphoma (panel B), liver cancer (panel C) and lung cancer (panel D). Estimates are stratified by calendar year of AIDS onset: 1980–1989 (dotted line), 1990–1995 (dashed line), 1996–2006 (solid line). Cumulative incidence was estimated using competing risk time-to-event methods and is expressed as a percentage. Follow-up time is measured beginning at AIDS onset. The gray vertical line indicates cumulative incidence estimates compared at month 60 of follow-up (5-year cumulative incidence). Note vertical scales vary among the panels.
DISCUSSION
In this large and nationally representative cohort of people with AIDS, significant declines in mortality over time were observed along with similar declines in the cumulative incidence of ADCs, which reflect the effects of increasingly widespread access to improved HIV therapies, including HAART (widely available since 1996). In striking contrast, however, a rise in the cumulative incidence of NADC was noted, including malignancies that occur with heightened frequency among HIV-infected people.
Major declines in mortality attributable to improved antiretroviral therapies are well documented.(1;2) HAART is effective in controlling HIV replication, leading to improved immune function and prolonged survival.(3) Declines in mortality among HIV-infected people were also observed in the U.S. prior to 1996, presumably due to increasing use of less potent antiretroviral regimens and better prophylaxis against opportunistic infections.(17) These strong trends in mortality required the application of a competing risk framework to assess the cumulative incidence of cancer. The cumulative incidence estimates in the present study correspond to the probability of observing cancer while a person with AIDS was still alive, are useful in assessing cancer risk for patients and clinicians, and can inform public health practice as a measure of cancer burden in the AIDS population.
Risk of the 2 major ADCs (KS and NHL) is elevated in the presence of immune suppression, and dramatic declines in the cumulative incidence of KS and NHL over time were demonstrated, consistent with partial immune restoration associated with HAART.(18–20) The steep rise in the cumulative incidence of KS and NHL, in the few months immediately after AIDS onset reflects that some of these cases were likely initial AIDS-defining events. Indeed, the decline across calendar periods in KS and NHL in the earliest months after AIDS can partly be attributed to the 1993 revision of the AIDS surveillance case definition, which allowed asymptomatic HIV-infected individuals with <200 CD4 cells/μL to be classified as AIDS cases.(4) Over time, the fraction of AIDS cases meeting the case definition via immunologic criteria increased due to laboratory reporting of these individuals. However, these changes in AIDS surveillance would not readily explain declines in cumulative incidence later after AIDS onset (Figure 1), and in an additional analysis excluding the earliest follow-up period after AIDS onset (months 0–3), similar declines in cumulative incidence were noted relative to widespread HAART use (data not shown).
The five-year cumulative incidence of KS and NHL declined 87% and 44%, respectively, among people diagnosed with AIDS during diagnosed during 1996–2006 (HAART era) compared with those diagnosed in the 1980s. However, NHL was the most common cancer during the most recent calendar period of AIDS. The continued occurrence of both KS and NHL suggests the need for increases in access and adherence to HAART.(21;22) Among women, the cumulative incidence of cervical cancer changed little with the widespread availability of HAART.
Overall, cumulative incidence of NADCs was low, but increased over time. This trend largely reflects the decline in mortality, which has allowed people with AIDS to live long enough to develop cancer. In particular, cumulative incidence of anal and liver cancers increased among people diagnosed with AIDS in the HAART era compared with earlier periods. It is possible that HAART-associated immune restoration, particularly late after an AIDS diagnosis, does not influence the natural history of infections with human papillomavirus (anal cancer) or hepatitis C and B viruses (liver cancer).(23–25) In addition, an increase in the cumulative incidence of Hodgkin lymphoma in the HAART era was noted. Some studies,(9;26) although not all,(27) have reported an increase in Hodgkin lymphoma incidence in the HAART period, which may reflect the complex relationship between immunosuppression and development of this malignancy.(28) We also noted a rise over time in the cumulative incidence of lung cancer; however, the burden of lung cancer was unchanged between the 2 most recent calendar periods. The excess risk of lung cancer is partly due to a high prevalence of smoking,(29) but chronic pulmonary inflammation or repeated lung infections in HIV-infected people may also be involved.(30)
Strengths of this study include its large size and inclusion of major U.S. areas affected by the HIV/AIDS epidemic. The present estimates of cumulative incidence were derived using a non-parametric competing risk framework and indicate the probability of actually observing cancer in people with AIDS. We are not aware of similar previous estimates in the field of HIV/AIDS research. A limitation is that individual-level data were lacking on important cancer co-factors such as HAART use, infection with oncogenic viruses, and smoking, which influence cancer risk. Nonetheless, we believe our estimates accurately reflect the overall cumulative incidence of cancers among people with AIDS and the impact of widespread HAART use on cancer burden over time.
While our findings can likely be generalized to the entire U.S. AIDS population, an additional limitation is that we evaluated only people with AIDS, who comprise a subset of the overall HIV-infected population. The cumulative incidence of most NADCs would be expected to be higher in HIV-infected people without AIDS, because the competing risk of death is lower in this group than among people with AIDS. Finally, our cumulative incidence estimates for people more than five years after AIDS onset should be interpreted cautiously. In particular, some people with AIDS would have migrated away from the cancer registry area, which would have led to underreporting of observed cancers.
Patterns of cancer incidence among people with AIDS in the United States are changing in the HAART era. Dramatic declines in the cumulative incidence of ADCs were noted along with an increase in the cumulative incidence of some NADCs, including those for which incidence is higher than in the general population (cancers of the anus, liver, and lung, and Hodgkin lymphoma). As HIV infection is increasingly considered with chronic disease management paradigms, greater attention should be focused on cancer screening and prevention strategies.
Acknowledgments
This research was supported by the Intramural Research Program of the National Cancer Institute.
We thank the staff at the HIV/AIDS and cancer registries at the following locations: Colorado, Connecticut, Florida, Illinois, Georgia, Los Angeles, San Diego and San Francisco, California, Massachusetts, Michigan, New Jersey, New York, New York, Seattle, Washington, Texas and Washington, D.C. We also thank Mr. Tim McNeel (Information Management Systems, Rockville, Maryland) for database management.
Footnotes
Conflicts of interest: All authors declare no conflicts of interest.
References
- 1.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–60. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 2.Palella FJ, Jr, Baker RK, Moorman AC, et al. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr. 2006;43:27–34. doi: 10.1097/01.qai.0000233310.90484.16. [DOI] [PubMed] [Google Scholar]
- 3.Detels R, Munoz A, McFarlane G, et al. Effectiveness of potent antiretroviral therapy on time to AIDS and death in men with known HIV infection duration. Multicenter AIDS Cohort Study Investigators. JAMA. 1998;280:1497–503. doi: 10.1001/jama.280.17.1497. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41:1–19. [PubMed] [Google Scholar]
- 5.IARC Monographs on the evaluation of carcinogenic risks to humans: Epstein-Barr virus and Kaposi’s sarcoma herpesvirus/human herpesvirus 8. Vol. 70. France: Lyon; 1997. [PMC free article] [PubMed] [Google Scholar]
- 6.IARC Monographs on the evaluation of carcinogenic risks to humans: human papillomaviruses. Vol. 90. France: Lyon; 2005. [Google Scholar]
- 7.Engels EA. Non-AIDS-defining malignancies in HIV-infected persons: etiologic puzzles, epidemiologic perils, prevention opportunities. AIDS. 2009;23:875–85. doi: 10.1097/QAD.0b013e328329216a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goedert JJ, Cote TR, Virgo P, et al. Spectrum of AIDS-associated malignant disorders. Lancet. 1998;20(351):1833–9. doi: 10.1016/s0140-6736(97)09028-4. [DOI] [PubMed] [Google Scholar]
- 9.Engels EA, Pfeiffer RM, Goedert JJ, et al. Trends in cancer risk among people with AIDS in the United States 1980–2002. AIDS. 2006;20:1645–54. doi: 10.1097/01.aids.0000238411.75324.59. [DOI] [PubMed] [Google Scholar]
- 10.Engels EA, Biggar RJ, Hall HI, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008;123:187–94. doi: 10.1002/ijc.23487. [DOI] [PubMed] [Google Scholar]
- 11.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370:59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 12.Patel P, Hanson DL, Sullivan PS, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann Intern Med. 2008;20(148):728–36. doi: 10.7326/0003-4819-148-10-200805200-00005. [DOI] [PubMed] [Google Scholar]
- 13.Gaynor JJ, Feuer E, Tan CC, et al. On the use of cause-specific failure and conditional failure probabilities: examples from clinical oncology data. Journal of the American Statistical Association. 1993;88:400–9. [Google Scholar]
- 14.World Health Organization. International Classification of Diseases for Oncology. 3. Geneva: World Health Organization; 2000. [Google Scholar]
- 15.SEER Cancer Statistics Review, 1975–2002. Bethesda, MD: National Cancer Institute; 2003. [Google Scholar]
- 16.Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am J Epidemiol. 2009;170:244–56. doi: 10.1093/aje/kwp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Detels R, Tarwater P, Phair JP, Margolick J, Riddler SA, Munoz A. Effectiveness of potent antiretroviral therapies on the incidence of opportunistic infections before and after AIDS diagnosis. AIDS. 2001;15:347–55. doi: 10.1097/00002030-200102160-00008. [DOI] [PubMed] [Google Scholar]
- 18.Biggar RJ, Chaturvedi AK, Goedert JJ, Engels EA. AIDS-related cancer and severity of immunosuppression in persons with AIDS. J Natl Cancer Inst. 2007;20(99):962–72. doi: 10.1093/jnci/djm010. [DOI] [PubMed] [Google Scholar]
- 19.Mbulaiteye SM, Biggar RJ, Goedert JJ, Engels EA. Immune deficiency and risk for malignancy among persons with AIDS. J Acquir Immune Defic Syndr. 2003;32:527–33. doi: 10.1097/00126334-200304150-00010. [DOI] [PubMed] [Google Scholar]
- 20.Frisch M, Biggar RJ, Engels EA, Goedert JJ. Association of cancer with AIDS-related immunosuppression in adults. JAMA. 2001;285:1736–45. doi: 10.1001/jama.285.13.1736. [DOI] [PubMed] [Google Scholar]
- 21.Cunningham WE, Markson LE, Andersen RM, et al. Prevalence and predictors of highly active antiretroviral therapy use in patients with HIV infection in the United States. J Acquir Immune Defic Syndr. 2000;25:115–23. doi: 10.1097/00042560-200010010-00005. [DOI] [PubMed] [Google Scholar]
- 22.Bangsberg DR, Moss AR, Deeks SG. Paradoxes of adherence and drug resistance to HIV antiretroviral therapy. J Antimicrob Chemother. 2004;53:696–9. doi: 10.1093/jac/dkh162. [DOI] [PubMed] [Google Scholar]
- 23.Palefsky JM, Holly EA, Efirdc JT, et al. Anal intraepithelial neoplasia in the highly active antiretroviral therapy era among HIV-positive men who have sex with men. AIDS. 2005;19:1407–14. doi: 10.1097/01.aids.0000181012.62385.4a. [DOI] [PubMed] [Google Scholar]
- 24.Chaturvedi AK, Madeleine MM, Biggar RJ, Engels EA. Risk of human papillomavirus-associated cancers among persons with AIDS. J Natl Cancer Inst. 2009;19(101):1120–30. doi: 10.1093/jnci/djp205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–76. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 26.Herida M, Mary-Krause M, Kaphan R, et al. Incidence of non-AIDS-defining cancers before and during the highly active antiretroviral therapy era in a cohort of human immunodeficiency virus-infected patients. J Clin Oncol. 2003;21:3447–53. doi: 10.1200/JCO.2003.01.096. [DOI] [PubMed] [Google Scholar]
- 27.Clifford GM, Rickenbach M, Lise M, et al. Hodgkin lymphoma in the Swiss HIV Cohort Study. Blood. 2009;113:5737–42. doi: 10.1182/blood-2009-02-204172. [DOI] [PubMed] [Google Scholar]
- 28.Biggar RJ, Jaffe ES, Goedert JJ, Chaturvedi A, Pfeiffer R, Engels EA. Hodgkin lymphoma and immunodeficiency in persons with HIV/AIDS. Blood. 2006;108:3786–91. doi: 10.1182/blood-2006-05-024109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giordano TP, Kramer JR. Does HIV infection independently increase the incidence of lung cancer? Clin Infect Dis. 2005;40:490–1. doi: 10.1086/427028. [DOI] [PubMed] [Google Scholar]
- 30.Engels EA. Inflammation in the development of lung cancer: epidemiological evidence. Expert Rev Anticancer Ther. 2008;8:605–15. doi: 10.1586/14737140.8.4.605. [DOI] [PubMed] [Google Scholar]