Abstract
We previously showed that transplantation with interleukin-21 receptor (IL-21R) gene-deficient splenocytes resulted in less severe GVHD than was observed with wild type splenocytes. We now here sought to find mechanism(s) explaining this observation. Recipients of donor CD4+ T cells lacking IL-21R exhibited diminished GVHD symptoms, with reduced inflammatory cell infiltration into the liver and intestine, leading to prolonged survival. After transplantation, CD4+ T cell-numbers in the spleen were reduced and mixed lymphocyte reaction and cytokine production by CD4+ T cells were impaired. These results suggest that IL-21 might promote GVHD through enhancing production of effector CD4+ T cells. Moreover, we found that CD25 depletion altered neither the impaired mixed lymphocyte reaction in vitro nor the ameliorated GVHD symptoms in vivo. Thus, the attenuated GVHD might be caused by an impairment of effector T-cell differentiation itself rather than by an increase in regulatory T-cells and suppression of effector T-cells.
Keywords: Cytokine, GVHD, Interleukin-21, IL-21
Introduction
Interleukin-21 (IL-21) was discovered as a co-stimulatory cytokine for T-cell proliferation and NK-cell expansion in vitro (1,2). IL-21 is produced by activated CD4 T-cells (1), and its receptor is expressed on T, B, and NK cells (1,3). It has also been reported that IL-21 suppresses dendritic cell function (4) and increases hematopoietic progenitor cells (5). IL-21 is known to play critical roles in immunoglobulin production (6), whereas reports have differed regarding its contributions to Th1-, Th2-, and Th17-mediated effects and differentiation (6–15). Regarding Th17, IL-21 contributes to Th17 differentiation but may not be required for this process (7,9,14,15). A relationship between IL-21 and autoimmune disease has been established. Overexpression of IL-21 induces inflammation, and in an SLE model mouse (the BXSB.6-Yaa+/J mouse) with high serum levels of IL-21 (16), development of disease is abrogated when these mice are crossed to IL-21R KO mice (17). In addition, autoimmune nonobese diabetic (NOD) mice do not develop diabetes in the absence of IL-21 signaling (18–20).
Graft-versus-host disease (GVHD) is a major complication following hematopoietic stem cell transplantation (21), sometimes with a fatal outcome. Previously, we showed that transplantation with interleukin-21 receptor (IL-21R) gene-disrupted splenocytes resulted in less severe GVHD than was seen with wild type splenocytes (22). We sought to elucidate the mechanism(s) for this observation and now demonstrate dysregulated effector function of activated CD4+ T cells in IL-21R−/− mice.
Materials and Methods
Mice
IL-21R−/− and IL-17−/− mice were generated previously (6,23). Both were C57BL/6 background. Both male and female mice were used as donors. C57BL/6-DBA2-F1 male mice were purchased from Clea Japan (Tokyo, Japan). All mice used in experiments were 6–12 weeks old. All mice were housed in a Jichi Medical University mouse facility, which is regulated by an intramural small animal committee, and were treated in accordance with the university guidelines.
In vitro T-cell stimulation and mixed lymphocyte reaction
Cells were cultured in RPMI1640 (Invitrogen, Carlsbad, CA) supplemented with 10 % fetal calf serum (Sigma, St. Louis, MO), 2 mM L-glutamine (Invitrogen), 50 μM 2-mercaptoethanol (Sigma), 0.1 mg/mL streptomycin, and 100 U/mL penicillin G (Invitrogen). Non-specific pan T-cell stimulation was performed using anti-CD3/CD28 beads for three days according to the manufacturer’s instructions (Dynal Biotech ASA, Oslo, Norway). Allo-antigen-specific T-cell stimulation was induced by co-cultivation of CD4 T-cells with 30 Gy irradiated splenocytes from C57BL/6-DBA2-F1 mice for four days.
GVHD models
To eliminate effects of wild type T cells in BM, we used IL-21R−/− BM. We compared transplantations with IL-21R−/− vs. wild type CD4+ T cells. C57BL/6-DBA2-F1 mice were irradiated with 11 Gy and injected intravenously with 5 × 106 IL-21R−/− BM and 5 × 106 purified CD4+ T cells from either wild type or IL-21R−/− mice. The cells were purified using CD4-microbeads and AutoMACS® (Milltenyi Biotec K.K., Tokyo, Japan; purity was >80–90 %).
Pathological analysis
Two weeks after transplantation, mice were sacrificed and liver, skin, and intestine were subjected to formalin fixation, paraffin embedding, excision and hematoxylin-eosin staining. Photographs were taken with an Olympus BX51 microscope using X400 magnification.
Flow cytometric analysis
Fc-block® (BD Biosciences, San Jose, CA) was used to prevent non-specific antibody binding to Fc receptors. Antibodies to CD4 (RM4-5), CD8 (Ly-2), CD25 (7D4), H-2b (AF6-88.5), H-2d (SF1-1.1), IFN-γ (XMG1.2), and TNF-α (MP6-XT22) were purchased from BD Biosciences and anti-Foxp3 (FJK-16a) was from eBioscience (San Diego, CA). Intracellular staining was performed with Cytofx/Cytoperm® kit (BD Biosciences) according to the manufacturer’s instruction. Cells were stimulated with anti-mouse CD3/CD28 beads for 5 hours in the presence of GolgiStop® (BD Biosciences). For Foxp3 intracellular staining, the stimulation was omitted. A LSR flow cytometer (BD Biosciences) was used for data collection, and data were analyzed using CellQuest software (BD Biosciences).
Enzyme linked immunosorbent assay (ELISA)
ELISA kits for IL-2, IL-4, and IFN-γ were from BD Biosciences and ELISA kits for IL-21, IL-17, TNF-α, and TGF-β1 were from R&D Systems (Minneapolis, MN). Concentrations were determined according to the manufacturer’s instructions.
CD25 depletion in vitro and in vivo
In vitro purification of CD4+ T cells and depletion of the CD25+CD4+ subpopulation was performed by cell-sorting using a FACSAria® (BD Biosciences), which yielded highly pure populations (>98%). In vivo CD25 depletion was performed by injecting anti-CD25 antibody as described previously (24, 25). Briefly, a hybridoma producing anti-CD25 antibody (PC61, American Type Culture Collection) was cultured in serum-free medium (PFHM-II from Invitrogen) and the antibody was purified from supernatant by ammonium sulfate precipitation and a PD10 column (GE Healthcare, UK). The purified product was quantified by the Bradford assay (Bio-Rad, Hercules, CA) at OD595 and 1 mg was injected intraperitoneally weekly from day 0 for 3 weeks. Control rat non-specific IgG was purchased from Invitrogen.
RT-quantitative PCR
At day 21 after bone marrow transplantation, CD25-negative CD4+ T cells were purified by cell-sorting from either recipients of wild type or IL-21R−/− CD4+ T cells, and RNA was isolated (RNeasy®, Qiagen, Valencia, CA), reverse transcribed using SuperScript® First-Strand Synthesis System for RT-PCR (Invitrogen), and PCR-amplifed using TaqMan® Gene Expression Assay’s primer for mouse Foxp3 (Mm00475156) and β-actin (Mm00607939) and an ABI Prism 7700 sequence detection System (PE Applied Biosystems, Foster, CA, USA).
Statistical analysis
Kaplan-Meier plots were used to compare survival rates. The Logrank test was used to evaluate p values. Statistical analyses were performed using “Stat Mate ver. 6” (ATMS, Tokyo, Japan). The Student’s t-test was used and all error bars in this study are S.D., unless otherwise specified.
Results
Purified CD4+ T cell-transplantation and pathological analysis
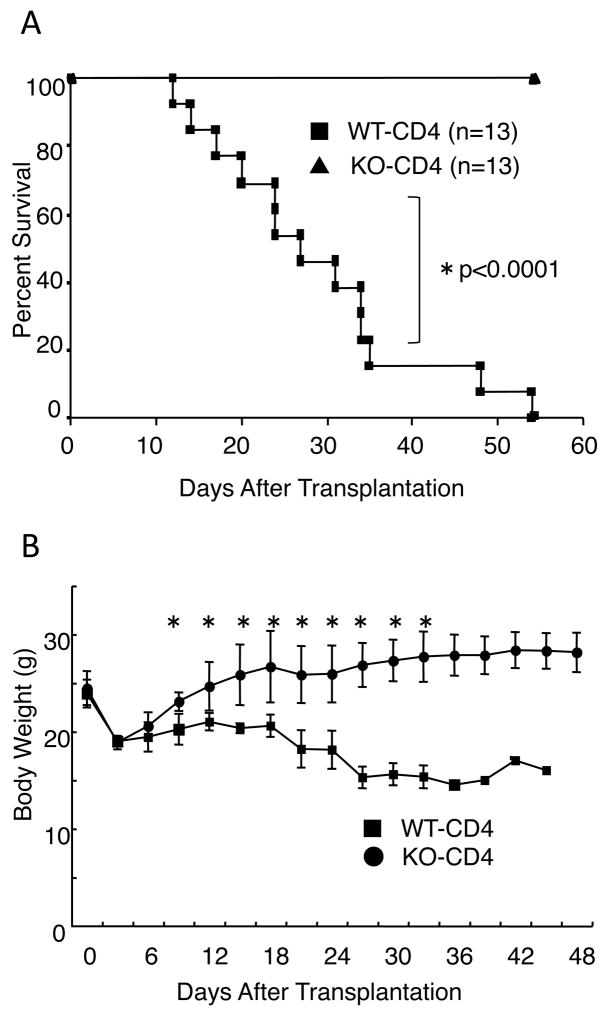
As noted above, decreased GVHD was observed when we transplanted IL-21R-deficient as compared to wild type bulk splenocytes (22). Although we sought to find molecular mechanism(s) for the ameliorated GVHD, no clue was immediately evident from the transplantation experiments performed (22). Thus, here we used purified CD4+ T cells instead of bulk splenocytes in this study, hoping to augment the differences observed. We used a well-known model of CD4+ T cell-mediated GVHD (26), in which C57BL/6 mice were donors and C57BL/6-DBA2-F1 mice were recipients. In this model, the difference between wild type and IL-21R−/− cells indeed appeared to be greater than in the previous experiments with bulk splenocytes (22). All recipients of wild type CD4+ T cells died within 55 days whereas those receiving IL-21R−/− CD4+ T cells survived during this time period (Fig. 1A). Moreover, recipients of IL-21R−/− CD4+ T cells recovered from body weight loss by day 14 but those receiving wild type CD4+ T cells did not recover and moreover continued to lose body weight (Fig. 1B). In recipients of IL-21R−/− CD4+ T cells, pathological analysis showed markedly reduced infiltration into region surrounding bile ducts and portal veins, and into the interstitial region of small intestine, as compared to the greater infiltration observed in recipients of wild type CD4+ T cells (Fig. 2, upper and middle panels). Apoptotic bodies near the surface area of crypts in the small intestine could barely be seen in recipients of IL-21R−/− CD4+ T cells, in contrast to recipients of wild type CD4+ T cells, in which apoptotic bodies were evident (Fig. 2, middle panel, see arrowheads). No significant difference was observed in skin pathology among recipients of wild type CD4+ and IL-21R−/− CD4+ T cells. These results suggested that IL-21 might be essential for CD4-mediated GVHD, at least in this setting.
Figure 1.
A role for IL-21 in CD4+ T cell-mediated GVHD. (A) Survival of recipients of wild type and IL-21R−/− CD4+ T cells. C57BL/6-DBA2-F1 mice were irradiated with 11 Gy and received 5 × 106 IL-21R−/− BM with 5 × 106 wild type or IL-21R−/− CD4+ T cells. Shown are combined data from two independent experiments. A total of 13 recipients each for wild type and IL-21R−/− CD4+ T cells were analyzed. P values were calculated by Logrank test. (B) Body weight after bone marrow transplantation. Asterisks denote statistical significance (p<0.05) by the Student’s t-test.
Figure 2.
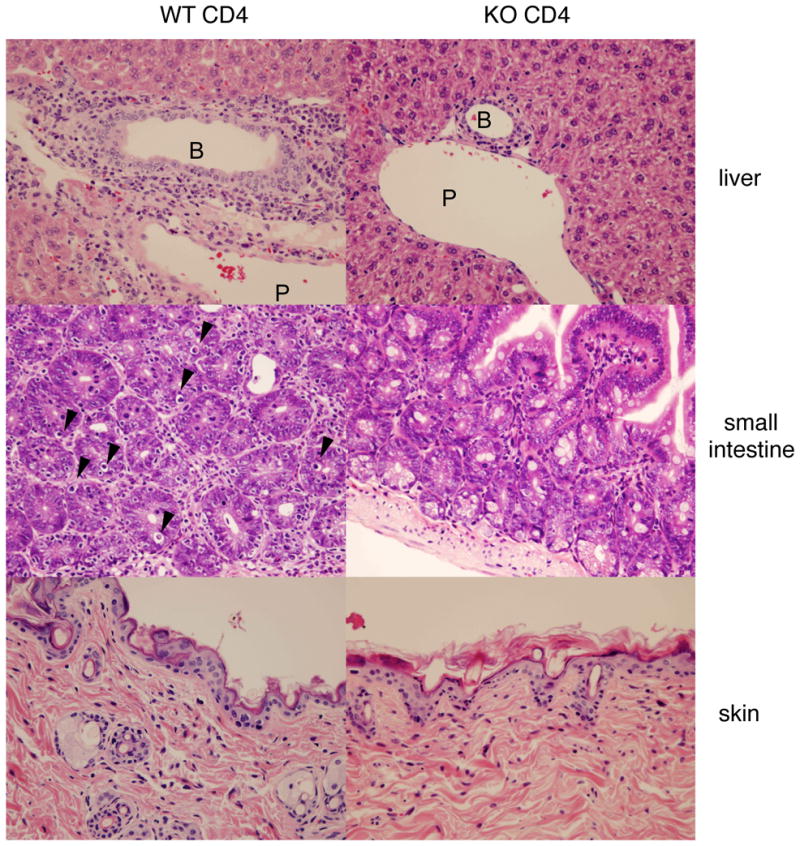
Pathological analysis of recipients. H&E staining of liver, small intestine, and skin are shown (Original magnification was × 400). In recipients of wild type CD4+ T cells, cell infiltration around portal vein (P), around bile duct (B), and into interstitial region in small intestine are evident. Arrowheads in small intestine indicate apoptotic bodies near the surface of crypts. These changes were barely seen in recipients of IL-21R−/− CD4+ T cells. Skin did not show any significant difference between recipients of wild type and IL-21R−/− CD4+ T cells. Shown is a representative result of 6 mice analyzed in each group. Only one recipient of IL-21R−/− CD4+ T cell showed apoptotic bodies in the lumens of intestine and infiltration around the bile duct and portal vein, as was observed in the recipients of wild type CD4+ T cells.
Normal cytokine production by the splenocytes after transplantation is dependent on IL-21
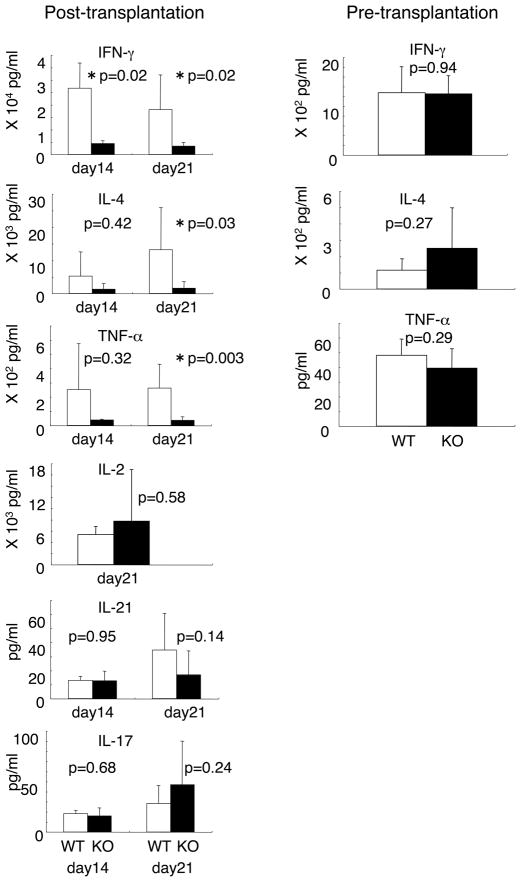
The above observations suggested that IL-21-mediated donor CD4+ T cell activation was involved in the exacerbation of GVHD. Because we could not find any significant difference in serum cytokine concentrations after transplantation (Supplemental Fig. S1), we assessed T cell differentiation by cytokine production in the presence of cellular stimulation. Interestingly, at day 14 and 21 after transplantation, bulk splenocytes from recipients of IL-21R−/− CD4+ T cell exhibited defective cytokine production, with decreased levels of IFN-γ, TNF-α, and IL-4; in contrast, levels of IL-2, IL-17, and IL-21 were not significantly diminished (Fig. 3, left six panels, p values are indicated). Before transplantation, IL-21R−/− CD4+ T cells did not show any significant defect in IFN-γ, IL-4, or TNF-α production (Fig. 3, right three panels), suggesting that the defect was acquired after transplantation. This defect in effector T cell function might represent a mechanism for the difference in the development of GVHD by mice receiving wild type versus IL-21R−/− CD4+ T cells.
Figure 3.
Cytokine production by bulk splenocytes before and after CD4+ T cell-transplantation. At day 14 and 21 after transplantation, splenocytes (5 × 105) were taken and stimulated with anti-CD3/CD28 antibodies for 18 hours. Concentrations of cytokines in the supernatants were determined by ELISA. In total 12–13 recipients of wild type CD4+ T cells and 10 recipients of IL-21R−/− CD4+ T cells were analyzed. Prior to transplantation, 5 wild type and 8 IL-21R−/− mice were analyzed. P values were calculated by the Student’s t-test. Asterisks denote statistical significance (p<0.05). At day 14–21 after transplantation, the proportion of donor cells in the spleen was >95% in our settings.
CD4+ T cells were responsible for the low production of cytokines
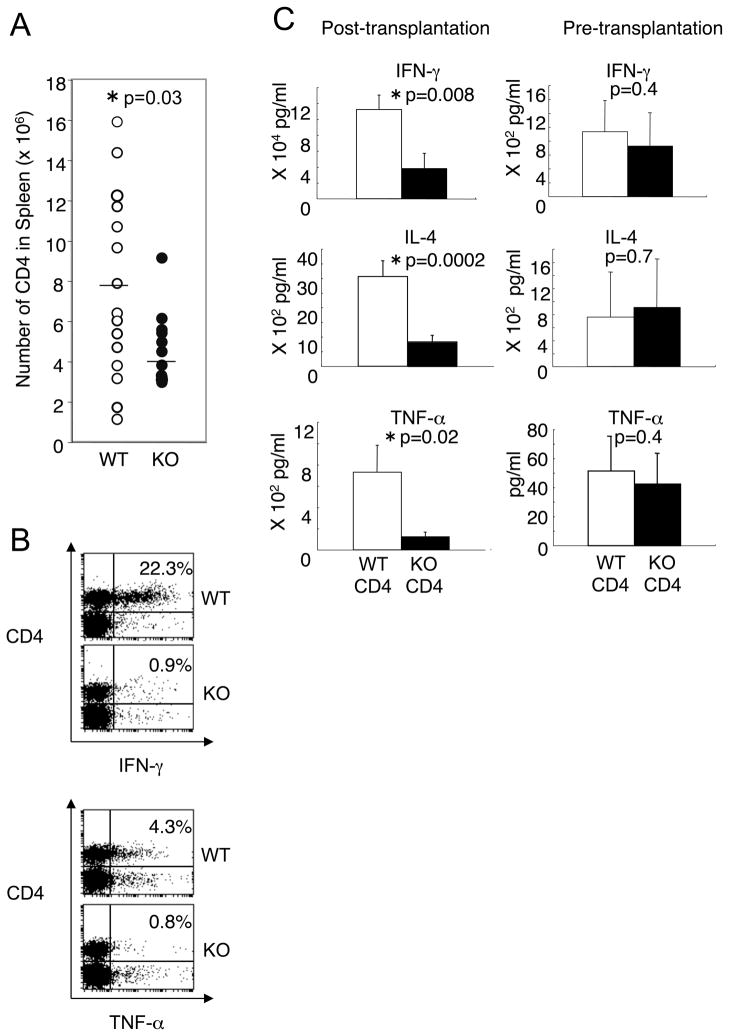
To elucidate the basis for diminished cytokine production, we examined the number of donor CD4+ T cells in the spleen at day 14–21 after transplantation. The number of donor H-2Kd-negative CD4+ T cells was significantly lower in recipients of IL-21R−/− CD4+ T cells than in recipients of wild type CD4+ T cells (Fig. 4A, p=0.03, Welch t-test, n=15 vs 12), although the ranges overlapped. As it is thought that donor T cells proliferate in secondary lymphoid organs such as the spleen and then infiltrate into target organs (27), the reduced number of CD4+ T cells in the spleen is consistent with the reduced infiltration into the liver and small intestine, as shown above (Fig. 2). To identify the cells responsible for defective cytokine production, we performed intracellular staining and ELISA with purified CD4+ T cells. After anti-CD3/CD28 stimulation, the proportion of IFN-γ+ and TNF-α+ cells in splenic CD4+ T cells was lower in recipients of IL-21R−/− CD4+ T cells than in those receiving wild type CD4+ T cells (Fig. 4B). Moreover, post-transplantation, the levels of IFN-γ, TNF-α, and IL-4 production were significantly diminished with splenic purified CD4+ T cells from recipients of IL-21R−/− CD4+ T cells as compared to those receiving wild type CD4+ T cells (Fig. 4C, left three panels). Before transplantation, IL-21R−/− CD4+ T cells did not show any defect in IFN-γ, TNF-α, and IL-4 production (Fig. 4C, right panels).
Figure 4.
Cytokine production by splenic CD4+ T cells before and after transplantation. (A) Absolute number of donor H-2Kd-negative CD4+ T cells in the spleen. The number of donor CD4+ T cells was determined by multiplying the number of splenocytes by the percentage of H-2Kd-negative CD4+ T cells. Each dot depicts the number of donor CD4+ T cells in a mouse. Lines in the middle of dots indicate the average. Total mice analyzed were 15 recipients of wild type CD4+ T cells and 12 recipients of IL-21R−/− CD4+ T cells. (B) Intracellular staining of splenocytes after anti-CD3/CD28 stimulation. Splenocytes (1 × 106) were stimulated with anti-CD3/CD28 antibodies for 5–6 hours and stained with either anti-IFN-γ or anti-TNF-α antibody in combination with anti-CD4 antibody. A total of three recipients in each group were analyzed and a representative result is shown. (C) Cytokine production by CD4+ T cells in vitro. At day 14 or 21 after transplantation, splenic CD4+ T cells (5 × 105) were purified and stimulated with anti-CD3/CD28 antibodies for 18 hours. Concentrations of cytokines in the supernatants were determined by ELISA. Twelve mice were analyzed in each group after transplantation. Total mice analyzed before transplantation were 5–6 wild type and 8–9 IL-21R−/− mice. Asterisks denote statistical significance (p<0.05).
IL-17 production and GVHD induced by IL-17−/− CD4+ T cells
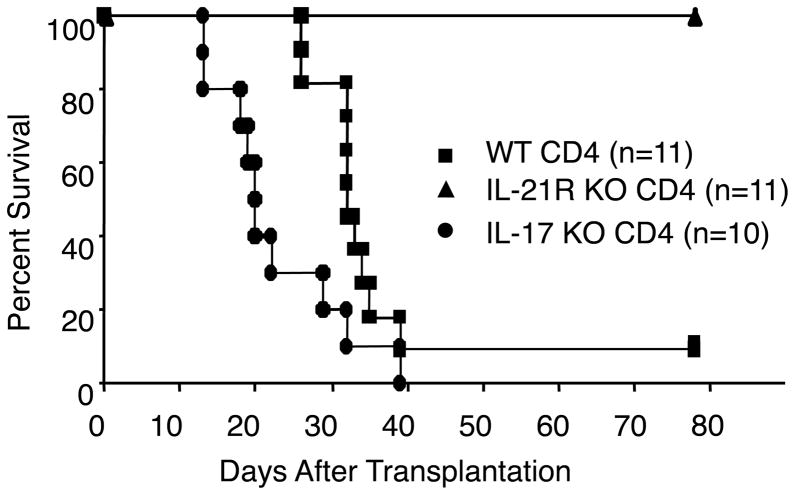
Although IL-21 is not essential for Th17 differentiation, IL-21 can promote Th17 differentiation. To evaluate the effect of IL-21−/− CD4+ T cell-transplantation on IL-17 production, we measured IL-17 after transplantation. As shown in Fig 3, bottom panel, bulk splenocytes from recipients of IL-21R−/− CD4+ T cells produced comparable amounts of IL-17 at day 14 and 21 after transplantation, as compared to mice receiving wild type CD4+ T cells. Moreover, we found that IL-17−/− CD4+ T cells induced lethal GVHD analogous to wild type CD4+ T cells (if anything, death occurred earlier), suggesting that IL-17 is dispensable for this process, in contrast to the essential role of IL-21 as indicated by the survival of mice receiving IL-21R−/− CD4+ T cells (Fig. 5).
Figure 5.
IL-17−/− CD4+ T cells induced lethal GVHD. Survival of recipients of wild type (WT), IL-21R−/− (IL-21RKO), or IL-17−/− (IL-17KO) CD4+ T cells. Lethally irradiated (11 Gy) C57BL/6-DBA2-F1 mice were transplanted with 5 × 106 IL-21RKO-BM and 5 × 106 WT (filled square), IL-21R KO (filled triangle), or IL-17 KO (filled circle) CD4+ T cells. The data represent the combined result of two independent experiments.
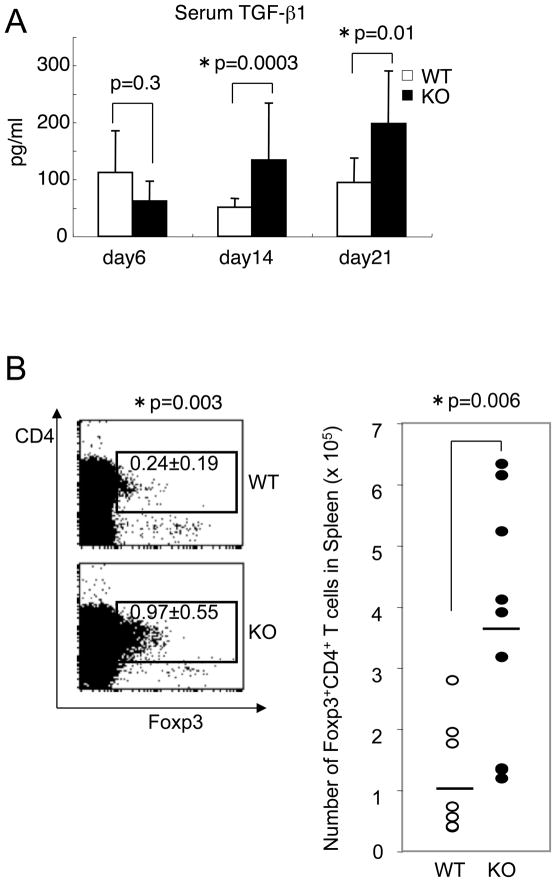
Regulatory T cell (Treg) number in spleen
We next determined the serum concentration of the major immunosuppressive cytokine, TGF-β1 at day 6–21 after transplantation. We found an increase of TGF-β1 only after transplantation (Fig. 6A, p=0.0003 at day 14, p=0.01 at day 21, Student’s t-test). In splenocytes from recipients of IL-21R−/− CD4+ T cells, the production of TGF-β1 and IL-10 by in vitro T cell stimulation was not up-regulated and in fact tended to be diminished (Supplemental Fig. S2), suggesting that the increase of serum TGF-β1 might be due to cells other than T cells. As naïve T cells can differentiate into regulatory T (Treg) cells in the presence of TGF-β1 (28) and it was reported that IL-21−/− T cells were predisposed to differentiate into Treg (8), we also investigated whether more Treg cells were induced in recipients of IL-21R−/− CD4+ T cells. The proportion of splenic Foxp3+CD4+ Treg phenotype cells in recipients of IL-21R−/− CD4+ T cells was higher than that in recipients of wild type CD4+ T cells, but the total percentage was still only ~1% (Fig. 6B, left panel). The absolute number was ~4 folds higher but the actual number was only ~4 × 105 out of the total number of splenocytes, ~4 × 107 (Fig. 6B, right panel). In contrast to post-transplantation, pre-transplantation-splenocytes from IL-21R−/− mice did not show an increase of Foxp3+CD4+ T cells as compared to cells from wild type mice (Supplemental Fig. S3), suggesting that the increased Treg after transplantation was an induced Treg during GVHD reaction. For that reason, we did not deplete CD25+ cells prior to transplantation.
Figure 6.
Increase of splenic Treg cells. (A) Up-regulation of serum TGF-β1. Serum TGF-β1 concentrations at the indicated day after transplantation were determined by ELISA per the manufacturer’s instruction. Three samples from recipients of wild type CD4+ T cells and four samples from recipients of IL-21R−/− CD4+ T cells at day 6, 23 samples from recipients of wild type CD4+ T cells and 25 samples from recipients of IL-21R−/− CD4+ T cells at day 14, and 8 samples from recipients of wild type CD4+ T cells and 7 samples from recipients of IL-21R−/− CD4+ T cells at day 21 were analyzed. Asterisks denote statistical significance (p<0.05). (B) The percentage and absolute number of splenic Foxp3+CD4+ regulatory T cells at day 14 after transplantation. The left panel shows a representative flow cytometric result from 8–9 similar samples. The right panel indicates the number of all samples; the averages are indicated by the horizontal bars.
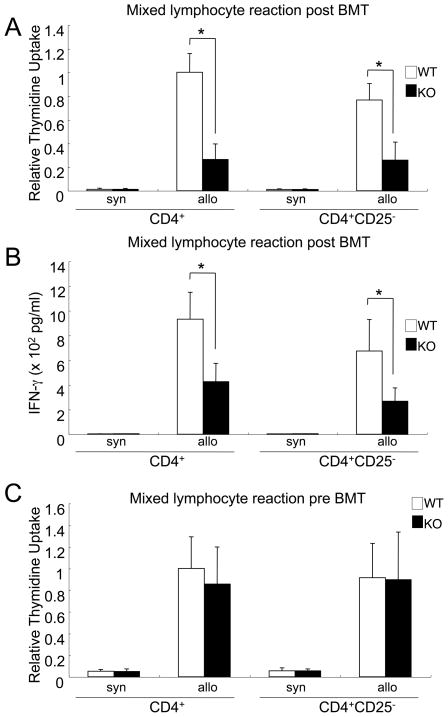
CD25 depletion did not restore the suppressed allo-reaction in vitro and did not exacerbate the ameliorated GVHD
To investigate the importance of Treg cells in diminishing GVHD, we performed a mixed lymphocyte reaction, which corresponds to allo-reaction in vitro, with or without CD25+CD4+ T cells. As Foxp3 is an intracellular protein and Foxp3 staining cannot be used to purify or deplete Treg cells, anti-CD25 antibody is widely used for this purpose (9, 29–32). The impaired mixed lymphocyte reaction of IL-21R−/− CD4+ T cells after transplantation was not restored by CD25 depletion (Fig. 7A), nor was the impaired IFN-γ production by IL-21R−/−CD4+ T-cells in a mixed lymphocyte reaction (Fig. 7B). Moreover, analogous to cytokine production by anti-CD3/CD28 stimulation (Fig. 3), IL-21R−/− CD4+ T cells before transplantation were not defective for allo-reaction (Fig. 7C).
Figure 7. An impaired CD4 allo-reaction is not dependent on CD25+CD4+ T cells.
CD4 allo-reaction in vitro was impaired after transplantation, and this impairment was not restored by CD25+ T cell depletion. (A) At day 14 after transplantation, 1 × 105 sorter-purified splenic CD4+ or CD25-negative CD4+ T cells (>98% purity) were cultured with 4 × 105 irradiated allogeneic C57BL/6-DBA2-F1 splenocytes for 4 days. The cells were pulsed with 1 μCi of [3H]-thymidine for the last 24 hours. Relative thymidine uptake to the value of wild type CD4+ T cells is depicted. (B) Culture was the same as in (A), but IFN-γ concentrations in the supernatants were determined by ELISA. (C) Sorter-purified splenic CD4+ or CD25-negative CD4+ cells from non-transplanted mice were cultured with irradiated allogeneic C57BL/6-DBA2-F1 splenocytes. Relative thymidine uptake to the number of wild type CD4+ T cells is depicted. Asterisks denote statistical significance (p<0.05).
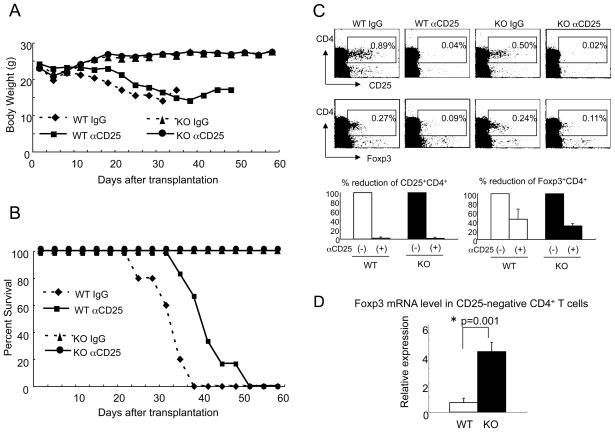
Consistent with the in vitro experiments above, CD25+ depletion in vivo did not alter the severity of GVHD in recipients of IL-21R−/− CD4+ T cells, without altering the body weight loss and survival (Fig. 8A and 8B). In contrast, the severity of GVHD in recipients of wild type CD4+ T cells appeared to be slightly diminished by CD25+ depletion (Fig. 8A and 8B). In this condition, as previously reported (30), the depletion efficacy of CD25+CD4+ T cells was more than 95 % and that of Foxp3+CD4+ T cells was at least 50 % (Fig. 8C upper and lower panels). Interestingly, Foxp3 expression was higher in CD25-negative CD4+ T cells from recipients of IL-21R−/− CD4+ T cells than from recipients of wild type CD4+ T cells (Fig. 8D). Together with results in vitro (Fig. 7), this suggests a relationship between unresponsiveness of CD25-negative CD4+ T cells and higher expression of Foxp3.
Figure 8. The ameliorated GVHD induced by IL-21R−/− CD4+ T cells is not dependent on CD25+CD4+ T cells.
The ameliorated GVHD induced by IL-21R−/− CD4+ T cells was not exacerbated by depletion of CD25+CD4+ T cells. Body weight (A) and survival (B) of recipients are shown. Comparison between recipients of wild type CD4+ T cells and IL-21R−/− CD4+ T cells, and further comparison with and without anti-CD25 antibody treatment were performed. Control antibody used was non-specific rat IgG. (C) Splenic CD25+CD4+ T cells and splenic Foxp3+CD4+ T cells at day 14 after transplantation with or without anti-CD25 antibody treatment were analyzed by flow cytometry (upper panels). The lower panels indicate the mean of percent reduction of CD25+CD4+ and Foxp3+CD4+ cells from three similar results. (D) Foxp3 mRNA level in CD25-negative CD4+ T cells at day 21 after transplantation. Cell-sorter purified CD25-negative CD4+ T cells were subjected to mRNA purification, reverse-transcriptase-treatment, and Taqman® quantitative PCR. Relative value to β-actin was denoted.
Discussion
Here, we have reported evidence indicating that IL-21 is critical for the pathogenesis of CD4+ T cell-mediated GVHD, at least in part due to effects on CD4 differentiation. In this study, we focused on CD4+ T cell-mediated GVHD; a role for IL-21 in CD8+ T cell-mediated GVHD remains to be investigated.
We found a profound defect in T cell effector function only after transplantation, although serum cytokine concentrations had no obvious difference. According to these results, T cell differentiation into Th1 and Th2 cells appeared to be altered in the absence of IL-21 during GVHD. Cytokines are believed to have both positive and negative roles in GVHD. For example, whereas T cells from IFN-γ deficient mice developed more severe GVHD (33–35), T cells from Stat4 (Th1) deficient mice exhibited less severe GVHD than T cells from wild type mice with less severe colitis (36). In contrast to IFN-γ−/− T cells, T cells from IL-4 deficient mice induced less severe GVHD (34); analogously, T cells from Stat6 (Th2) deficient mice induced less severe GVHD than those from wild type mice (36). T cells from TNF-α deficient mice developed less severe GVHD with less severe colitis (37). Our data suggest a strong correlation between the defect in effector function in recipients of IL-21R−/− CD4+ T cells and the attenuated phenotype of GVHD, indicating a role for IL-21 in this process.
IL-21 as well as IL-6 induces Th17 differentiation in the presence of TGF-β, suggesting a possible involvement of IL-17 in the phenotype we observed. However, our results with IL-17−/− CD4+ T cells demonstrated that IL-17 was dispensable for CD4+ T cell-mediated GVHD, indicating that the attenuated GVHD in recipients of IL-21R−/− CD4+ T cells was not due to an IL-17-related defect. During the preparation of this manuscript, a role for IL-17 in GVHD was reported (38–40). These reports varied but one report suggested that the lack of IL-17 promotes GVHD (38). Another report suggested that IL-17−/− CD4+ T cells can ameliorate GVHD only at the beginning of GVHD, which suggested a promoting effect for IL-17 at an early stage of GVHD (39). The third report suggested that ex vivo differentiated Th17 cells induced skin and lung GVHD (40). Thus, the role of IL-17 may be complex and dependent on the specific experimental conditions.
Because there are reciprocal relationships between Th1/Th2 and regulatory T cell differentiation (41–43) and between IL-21 and regulatory T cell differentiation (8), we investigated the level of Treg cells in the spleens of recipients. Foxp3+CD4+ T cells were increased in percentage and absolute number but still represented only ~1% of splenocytes. Regarding the relationship between the defective effector T cells function and the increased number of Treg cells, it is possible that increased Treg cells suppress functional effector T-cells, but alternatively, it is also possible that effector differentiation itself is defective and the resulting effector T cells cannot respond to allo-antigen, analogous to the situation in T cell anergy, and that the increased Treg cell number is also a result of a dysregulated differentiation. Our results here might be more consistent with the latter possibility, given that Treg depletion by anti-CD25 treatment did not alter the results in vitro and in vivo, although the efficiency of depletion of Foxp3+CD4+ T cells in vivo was incomplete. It is also conceivable that the up-regulation of Foxp3 in CD25-negative CD4+ T cells (which would not be removed by CD25+ depletion) in the absence of IL-21 signaling might result in unresponsiveness or poor-responsiveness of effector T cells, and also that more than one mechanism can contribute to the attenuated GVHD.
Supplementary Material
Footnotes
This work was supported in part by grants from the Ministry of Health, Labor and Welfare of Japan, by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and by the Intramural Research Program of the National Heart, Lung and Blood Institute, National Institutes of Health (Bethesda, MD).
Disclosures
Drs. Katsutoshi Ozaki and Warren J. Leonard are inventors on patents and patent applications related to IL-21.
References
- 1.Parrish-Novak J, Dillon SR, Nelson A, Hammond A, Sprecher C, Gross JA, Johnston J, Madden K, Xu W, West J, Schrader S, Burkhead S, Heipel M, Brandt C, Kuijper JL, Kramer J, Conklin D, Presnell SR, Berry J, Shiota F, Bort S, Hambly K, Mudri S, Clegg C, Moore M, Grant FJ, Lofton-Day C, Gilbert T, Rayond F, Ching A, Yao L, Smith D, Webster P, Whitmore T, Maurer M, Kaushansky K, Holly RD, Foster D. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. 2000;408:57–63. doi: 10.1038/35040504. [DOI] [PubMed] [Google Scholar]
- 2.Spolski R, Leonard WJ. Interleukin-21: basic biology and implications for cancer and autoimmunity. Annu Rev Immunol. 2008;26:57–79. doi: 10.1146/annurev.immunol.26.021607.090316. [DOI] [PubMed] [Google Scholar]
- 3.Ozaki K, Kikly K, Michalovich D, Young PR, Leonard WJ. Cloning of a type I cytokine receptor most related to the IL-2 receptor beta chain. Proc Natl Acad Sci USA. 2000;97:11439–114344. doi: 10.1073/pnas.200360997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brandt K, Bulfone-Paus S, Foster DC, Rückert R. Interleukin-21 inhibits dendritic cell activation and maturation. Blood. 2003;102:4090–4098. doi: 10.1182/blood-2003-03-0669. [DOI] [PubMed] [Google Scholar]
- 5.Ozaki K, Hishiya A, Hatanaka K, Nakajima H, Wang G, Hwu P, Kitamura T, Ozawa K, Leonard WJ, Nosaka T. Overexpression of IL-21 induces expansion of hematopoietic progenitor cells. Int J Hemat. 2006;84:224–230. doi: 10.1532/IJH97.06036. [DOI] [PubMed] [Google Scholar]
- 6.Ozaki K, Spolski R, Feng CG, Qi CF, Cheng J, Sher A, Morse HC, 3rd, Liu C, Schwartzberg PL, Leonard WJ. A critical role for IL-21 in regulating immunoglobulin production. Science. 2002;298:1630–1634. doi: 10.1126/science.1077002. [DOI] [PubMed] [Google Scholar]
- 7.Zhou L, I, Ivanov I, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 8.Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM, Dong C. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 9.Korn T, Bettelli E, Gao W, Awasthi A, Jäger A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wurster AL, V, Rodgers L, Satoskar AR, Whitters MJ, Young DA, Collins M, Grusby MJ. Interleukin 21 is a T helper (Th) cell 2 cytokine that specifically inhibits the differentiation of naive Th cells into interferon gamma-producing Th1 cells. J Exp Med. 2002;196:969–977. doi: 10.1084/jem.20020620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pesce J, Kaviratne M, Ramalingam TR, Thompson RW, Urban JF, Jr, Cheever AW, Young DA, Collins M, Grusby MJ, Wynn TA. The IL-21 receptor augments Th2 effector function and alternative macrophage activation. J Clin Invest. 2006;116:2044–2055. doi: 10.1172/JCI27727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fröhlich A, Marsland BJ, Sonderegger I, Kurrer M, Hodge MR, Harris NL, Kopf M. IL-21 receptor signaling is integral to the development of Th2 effector responses in vivo. Blood. 2007;109:2023–2031. doi: 10.1182/blood-2006-05-021600. [DOI] [PubMed] [Google Scholar]
- 13.Strengell M, Sareneva T, Foster D, Julkunen I, Matikainen S. IL-21 up-regulates the expression of genes associated with innate immunity and Th1 response. J Immunol. 2002;169:3600–3605. doi: 10.4049/jimmunol.169.7.3600. [DOI] [PubMed] [Google Scholar]
- 14.Coquet JM, Chakravarti S, Smyth MJ, Godfrey DI. IL-21 is not essential for Th17 differentiation or experimental autoimmune encephalomyelitis. J Immunol. 2008;180:7097–7101. doi: 10.4049/jimmunol.180.11.7097. [DOI] [PubMed] [Google Scholar]
- 15.Sonderegger I, Kisielow J, Meier R, King C, Kopf M. IL-21 and IL-21R are not required for development of Th17 cells and autoimmunity in vivo. Eur J Immunol. 2008;38:1833–1838. doi: 10.1002/eji.200838511. [DOI] [PubMed] [Google Scholar]
- 16.Ozaki K, Spolski R, Ettinger R, Kim HP, Wang G, Qi CF, Hwu P, Shaffer DJ, Akilesh S, Roopenian DC, Morse HC, 3rd, Lipsky PE, Leonard WJ. Regulation of B cell differentiation and plasma cell generation by IL-21, a novel inducer of Blimp-1 and Bcl-6. J Immunol. 2004;173:5361–5371. doi: 10.4049/jimmunol.173.9.5361. [DOI] [PubMed] [Google Scholar]
- 17.Bubier JA, Sproule TJ, Foreman O, Spolski R, Shaffer DJ, Morse HC, 3rd, Leonard WJ, Roopenian DC. A critical role for IL-21 receptor signaling in the pathogenesis of systemic lupus erythematosus in BXSB- Yaa mice. Proc Natl Acad Sci U S A. 2009;106:1518–1523. doi: 10.1073/pnas.0807309106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.King C, Ilic A, Koelsch K, Sarvetnick N. Homeostatic expansion of T cells during immune insufficiency generates autoimmunity. Cell. 2004;117:265–277. doi: 10.1016/s0092-8674(04)00335-6. [DOI] [PubMed] [Google Scholar]
- 19.Spolski R, Kashyap M, Robinson C, Yu Z, Leonard WJ. IL-21 signaling is critical for the development of type I diabetes in the NOD mouse. Proc Natl Acad Sci USA. 2008;105:14028–14033. doi: 10.1073/pnas.0804358105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sutherland AP, Van Belle T, Wurster AL, Suto A, Michaud M, Zhang D, Grusby MJ, von Herrath M. IL-21 is required for the development of type 1 diabetes in NOD mice. Diabetes. 2009;58:1144–1155. doi: 10.2337/db08-0882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shlomchik WD. Graft-versus-host disease. Nat Rev Immunol. 2007;7:340–352. doi: 10.1038/nri2000. [DOI] [PubMed] [Google Scholar]
- 22.Meguro A, Ozaki K, Oh I, Hatanaka K, Matsu H, Tatara, Sato RK, Leonard WJ, Ozawa K. IL-21 is critical for graft-versus-host disease in a mouse model. Bone Marrow Transplant. 2010;45:723–729. doi: 10.1038/bmt.2009.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakae S, Komiyama Y, Nambu A, Sudo K, Iwase M, Homma I, Sekikawa K, Asano M, Iwakura Y. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002;17:375–387. doi: 10.1016/s1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- 24.Sakoda Y, Hashimoto D, Asakura S, Takeuchi K, Harada M, Tanimoto M, Teshima T. Donor-derived thymic-dependent T cells cause chronic graft-versus-host disease. Blood. 2007;109(4):1756–64. doi: 10.1182/blood-2006-08-042853. [DOI] [PubMed] [Google Scholar]
- 25.Aoyama K, Koyama M, Matsuoka K, Hashimoto D, Ichinohe T, Harada M, Akashi K, Tanimoto M, Teshima T. Improved outcome of allogeneic bone marrow transplantation due to breastfeeding-induced tolerance to maternal antigens. Blood. 2009;113(8):1829–33. doi: 10.1182/blood-2008-05-155283. [DOI] [PubMed] [Google Scholar]
- 26.Teshima T, Hill GR, Pan L, Brinson YS, van den Brink MR, Cooke KR, Ferrara JL. IL-11 separates graft-versus-leukemia effects from graft-versus-host disease after bone marrow transplantation. J Clin Invest. 1999;104:317–325. doi: 10.1172/JCI7111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beilhack A, Schulz S, Baker J, Beilhack GF, Wieland CB, Herman EI, Baker EM, Cao YA, Contag CH, Negrin RS. In vivo analyses of early events in acute graft-versus-host disease reveal sequential infiltration of T-cell subsets. Blood. 2005;106:1113–1122. doi: 10.1182/blood-2005-02-0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 29.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64. [PubMed] [Google Scholar]
- 30.Liesz A, Suri-Payer E, Veltkamp C, Doerr H, Sommer C, Rivest S, Giese T, Veltkamp R. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med. 2009;15(2):192–9. doi: 10.1038/nm.1927. [DOI] [PubMed] [Google Scholar]
- 31.Fujita S, Sato Y, Sato K, Eizumi K, Fukaya T, Kubo M, Yamashita N, Sato K. Regulatory dendritic cells protect against cutaneous chronic graft-versus-host disease mediated through CD4+CD25+Foxp3+ regulatory T cells. Blood. 2007;110(10):3793–803. doi: 10.1182/blood-2007-04-086470. [DOI] [PubMed] [Google Scholar]
- 32.Radojcic V, Pletneva MA, Yen HR, Ivcevic S, Panoskaltsis-Mortari A, Gilliam AC, Drake CG, Blazar BR, Luznik L. STAT3 signaling in CD4+ T cells is critical for the pathogenesis of chronic sclerodermatous graft-versus-host disease in a murine model. J Immunol. 2010;184:764–74. doi: 10.4049/jimmunol.0903006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang YG, Dey BR, Sergio JJ, Pearson DA, Sykes M. Donor-derived interferon gamma is required for inhibition of acute graft-versus-host disease by interleukin 12. J Clin Invest. 1998;102:2126–2135. doi: 10.1172/JCI4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murphy WJ, Welniak LA, Taub DD, Wiltrout RH, Taylor PA, Vallera DA, Kopf M, Young H, Longo DL, Blazar BR. Differential effects of the absence of interferon-gamma and IL-4 in acute graft-versus-host disease after allogeneic bone marrow transplantation in mice. J Clin Invest. 1998;102:1742–1748. doi: 10.1172/JCI3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang H, Asavaroengchai W, Yeap BY, Wang MG, Wang S, Sykes M, Yang YG. Paradoxical effects of IFN-{gamma} in graft-vs.-host (GVH) disease reflect promotion of lymphohematopoietic GVH reactions and inhibition of epithelial tissue injury. Blood. 2009;113:3612–3619. doi: 10.1182/blood-2008-07-168419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nikolic B, Lee S, Bronson RT, Grusby MJ, Sykes M. Th1 and Th2 mediate acute graft-versus-host disease, each with distinct end-organ targets. J Clin Invest. 2000;105:1289–1298. doi: 10.1172/JCI7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmaltz C, Alpdogan O, Muriglan SJ, Kappel BJ, Rotolo JA, Ricchetti ET, Greenberg AS, Willis LM, Murphy GF, Crawford JM, van den Brink MR. Donor T cell-derived TNF is required for graft-versus-host disease and graft-versus-tumor activity after bone marrow transplantation. Blood. 2003;101:2440–2445. doi: 10.1182/blood-2002-07-2109. [DOI] [PubMed] [Google Scholar]
- 38.Yi T, Zhao D, Lin CL, Zhang C, Chen Y, Todorov I, LeBon T, Kandeel F, Forman S, Zeng D. Absence of donor Th17 leads to augmented Th1 differentiation and exacerbated acute graft-versus-host disease. Blood. 2008;112:2101–10. doi: 10.1182/blood-2007-12-126987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kappel LW, Goldberg GL, King CG, Suh DY, Smith OM, Ligh C, Holland AM, Grubin J, Mark NM, Liu C, Iwakura Y, Heller G, van den Brink MR. IL-17 contributes to CD4-mediated graft-versus-host disease. Blood. 2009;113:945–952. doi: 10.1182/blood-2008-08-172155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carlson MJ, West ML, Coghill JM, Panoskaltsis-Mortari A, Blazar BR, Serody JS. In vitro differentiated TH17 cells mediate lethal acute graft-versus-host disease with severe cutaneous and pulmonary pathology. Blood. 2009;113:1365–1374. doi: 10.1182/blood-2008-06-162420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wei J, Duramad O, Perng OA, Reiner SL, Liu YJ, Qin FX. Antagonistic nature of T helper 1/2 developmental programs in opposing peripheral induction of Foxp3+ regulatory T cells. Proc Natl Acad Sci U S A. 2007;104:18169–18174. doi: 10.1073/pnas.0703642104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mantel PY, Kuipers H, Boyman O, Rhyner C, Ouaked N, Rückert B, Karagiannidis C, Lambrecht BN, Hendriks RW, Crameri R, Akdis CA, Blaser K, Schmidt-Weber CB. GATA3-driven Th2 responses inhibit TGF-beta1-induced FOXP3 expression and the formation of regulatory T cells. PLoS Biol. 2007;5:e329. doi: 10.1371/journal.pbio.0050329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hill JA, Hall JA, Sun CM, Cai Q, Ghyselinck N, Chambon P, Belkaid Y, Mathis D, Benoist C. Retinoic acid enhances Foxp3 induction indirectly by relieving inhibition from CD4+CD44hi Cells. Immunity. 2008;29:758–770. doi: 10.1016/j.immuni.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.