Abstract
It has been 10 years since the seminal work of Dwight Bergles and collaborators demonstrated that NG2 (nerve/glial antigen 2)-expressing oligodendrocyte progenitor cells (NG2 cells) receive functional glutamatergic synapses from neurons (Bergles et al., 2000), contradicting the old dogma that only neurons possess the complex and specialized molecular machinery necessary to receive synapses. While this surprising discovery may have been initially shunned as a novelty item of undefined functional significance, the study of neuron-to-NG2 cell neurotransmission has since become a very active and exciting field of research. Many laboratories have now confirmed and extended the initial discovery, showing for example that NG2 cells can also receive inhibitory GABAergic synapses (Lin and Bergles, 2004) or that neuron-to-NG2 cell synaptic transmission is a rather ubiquitous phenomenon that has been observed in all brain areas explored so far, including white matter tracts (Kukley et al., 2007; Ziskin et al., 2007; Etxeberria et al., 2010). Thus, while still being in its infancy, this field of research has already brought many surprising and interesting discoveries, and has become part of a continuously growing effort in neuroscience to re-evaluate the long underestimated role of glial cells in brain function (Barres, 2008). However, this area of research is now reaching an important milestone and its long-term significance will be defined by its ability to uncover the still elusive function of NG2 cells and their synapses in the brain, rather than by its sensational but transient successes at upsetting the old order established by neuronal physiology. To participate in the effort to facilitate such a transition, here we propose a critical review of the latest findings in the field of NG2 cell physiology – discussing how they inform us on the possible function(s) of NG2 cells in the brain – and we present some personal views on new directions the field could benefit from in order to achieve lasting significance.
Keywords: α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor (AMPAR), nerve/glial antigen 2 (NG2) cells, neuron, oligodendrocyte progenitor cell (OPC), postsynaptic density (PSD)
Abbreviations: AMPAR, α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor; CNP-GFP, C-type natriuretic peptide-green fluorescent protein; CNS, central nervous system; EGFP, enhanced green fluorescent protein; EPSC, excitatory postsynaptic current; GABA, γ-aminobutyric acid; GABAAR, GABA type A receptor; LTP, long-term potentiation; MBP, maltose-binding protein; NG2, nerve/glial antigen 2; NMDAR, N-methyl-d-aspartate receptor; OL, oligodendrocyte lineage; OPC, oligodendrocyte progenitor cell; PDGFRα, platelet-derived growth factor receptor α; PSD, postsynaptic density; SCP, Schwann cell progenitor
INTRODUCTION
The NG2 (nerve/glial antigen 2) is a chondroitin sulfate proteoglycan predominantly expressed in the brain by a subpopulation of glial cells called NG2 cells. These cells are classically described as OPCs (oligodendrocyte progenitor cells), since they usually co-express OPC markers such as PDGFRα (platelet-derived growth factor receptor α) and O4 (Nishiyama et al., 1996; Reynolds and Hardy, 1997), and give rise to most – if not all – oligodendrocytes in the brain (Dimou et al., 2008; Rivers et al., 2008; Zhu et al., 2008; Kang et al., 2010). The only other cells known to express NG2 in the brain are the pericytes lining blood vessels (Ozerdem et al., 2001). While pericytes are not usually considered as NG2 cells per se, they may be confused with them, especially in the absence of co-staining for other OPC markers. In the last decade, NG2 cells have generated a lot of interest among neuroscientists, because they display a combination of features unexpected in OPCs. These include: (i) an almost uniform distribution in both grey and white matter areas; (ii) a complex stellate morphology; (iii) a tendency to intimately associate with neuronal cell bodies and dendrites (Dawson et al., 2003; Butt et al., 2005); (iv) the ability to keep proliferating in the adult brain (Dawson et al., 2003; Aguirre et al., 2004; Ligon et al., 2006); and (v) a latent ability – in the context of brain injury or pathology – to give rise to astrocytes and neurons that may be recruited to areas of lesion (Belachew et al., 2003; Aguirre et al., 2007; Tamura et al., 2007; Rivers et al., 2008; Zhu et al., 2008). However, the most striking of these features – and the main focus of this review – is their ability to receive synapses from neurons.
Neuron-NG2 cell glutamatergic synapses have been originally described in NG2 cells recorded in hippocampal slices from both young postnatal mice and adult mice (Bergles et al., 2000). Since then, these synapses have been shown to be a near-universal feature of NG2 cells in all grey and white matter areas explored so far, including brainstem (Müller et al., 2009), cerebellar cortex (Lin et al., 2005), dentate gyrus (Mangin et al., 2008), cortex (Chittajallu et al., 2004; Kukley et al., 2008; Ge et al., 2009), corpus callosum (Kukley et al., 2007; Ziskin et al., 2007; De Biase et al., 2010a; Etxeberria et al., 2010) and cerebellar white matter (Karadottir et al., 2008; De Biase et al., 2010a). Additionally, NG2 cells are also able to receive GABAergic synapses (GABA is γ-aminobutyric acid) in hippocampus (Lin and Bergles, 2004), dentate gyrus (Mangin et al., 2008) and cortex (Ge et al., 2009; Tanaka et al., 2009). However, in spite of their universality, the functions of these synapses still remain completely unknown.
HOW FAR ARE WE FROM DEFINING THE FUNCTIONS OF NEURON-NG2 SYNAPSES?
For a long time, a major reason why the function of neuron-NG2 synapses has been so hard to define has been the lack of tools that would allow selective modulation of synaptic transmission in NG2 cells. However, this roadblock is now mostly cleared by the recent generation of several transgenic lines allowing specific deletion or overexpression of selected genes in NG2 cells. These lines were generated by using different gene promoters actively expressed in NG2 cells, and include the NG2-Cre, NG2-CreErt2, PDGFRα-CreErt2, CNP-Cre and PLP-Cre lines (Lappe-Siefke et al., 2003; Delaunay et al., 2008; Rivers et al., 2008; Zhu et al., 2008; De Biase et al., 2010a; Kang et al., 2010). However, in order to properly use these genetic tools, a more detailed knowledge of the identity of the postsynaptic proteins mediating synaptic currents in NG2 cells is necessary. For example, while it is known that glutamatergic EPSCs (excitatory postsynaptic currents) in NG2 cells are mediated by AMPAR (α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor), it appears that NG2 cells can express all of the four known subunits that can compose these receptors (α1–α4) (De Biase et al., 2010a). This implies that, because of possible compensatory effects between subunits, a quadruple conditional knockout of all these subunits might have to be generated in order to completely eliminate EPSCs in NG2 cells – a rather daunting task at best.
A better approach maybe to target critical postsynaptic anchoring proteins, such as PSD-95 (postsynaptic density 95) or other equivalent proteins playing similar functional roles (Feng and Zhang, 2009). However, information on the basic composition of the PSD in NG2 cells is still lacking. These challenges also apply to GABAergic synapses between neurons and NG2 cells, as the GABAAR (GABA type A receptor) subtypes expressed by NG2 cells at postsynaptic site are still undefined. Postsynaptic GABAARs in neurons are pentamers made of 2 α-subunits, 2 β-subunits and 1 γ-subunit, and based on the large number of GABAAR subunits identified so far (6 for α, 3 for β and 3 for γ) (Jacob et al., 2008), a conditional multiple knockout approach may be necessary. However, since removing all subunits from either the α or the β category is sufficient to render all postsynaptic GABAAR non-functional in a cell, this approach may remain relatively easy to achieve, especially since it is unlikely that NG2 cells co-express all possible subunit combinations. Also, as for glutamatergic synapses, a more feasible approach maybe to target critical postsynaptic anchoring proteins, such as gephyrin (Fritschy et al., 2008). But once again, we are lacking crucial information about the structure of the GABAergic PSD in NG2 cells in order to be able to successfully apply a conditional knockout strategy to understand the physiological role of these synapses.
Based on the issues described above, we believe that some groundwork will have to be done in order to characterize the composition of PSDs in NG2 cells, before we can apply genetic approaches to understand the function of synapses in these cells. However, this goal maybe reached earlier than we think, based on a recent report demonstrating removal of most if not all functional NMDARs (N-methyl-d-aspartate receptors) in NG2 cells after a single conditional knockout for the NR1 subunit utilizing a PDGFRα-CreErt2 mouse (De Biase et al., 2010b). Even though this successful cell-specific genetic/functional ablation takes advantage of the fact that NR1 alone is necessary to the function of all NMDAR subtypes, these findings demonstrate that the conditional ablation of a subtype of postsynaptic receptor can be successfully achieved in NG2 cells.
HOW ARE SYNAPTIC CURRENTS TRANSDUCED IN NG2 CELLS?
Another important area of inquiry that could help us understand the function of neuron-NG2 cell synapses relates to the transduction of synaptic currents in NG2 cells themselves.
Do NG2 cells fire action potentials in response to synaptic stimulation?
The recent report of a subpopulation of NG2 cells firing action potentials in the rat cerebellum (Karadottir et al., 2008) would suggest that a subpopulation of NG2 cells (and glia) could display functional properties more similar to neurons than previously thought – i.e. translating synaptic inputs into action potentials. However, the existence of such a subpopulation of ‘spiking’ NG2 cells remains uncertain, since a recent report by the same group found that, in contrast with what was found in rats, only few NG2 cells are able to produce regenerative spikes in mice (Clarke et al., 2010). This finding is consistent with reports by other laboratories (Chittajallu et al., 2004; De Biase et al., 2010a). Furthermore, since almost all NG2 cells are contacted by synapses, the presence of synaptic inputs cannot be correlated with their ability to fire action potentials. Therefore, it is currently unclear whether the ability of few NG2 cells to potentially generate real action potentials is relevant to the quest for the specific physiological function of synapses in NG2 cells, or is rather an attempt to use concepts ‘imported’ from neuronal physiology and apply them unaltered to glial physiology. In conclusion, based on current data, action potentials are unlikely to represent the way most NG2 cells translate synaptic inputs, particularly in the absence of any classical output for these action potentials, such as the ability to release neurotransmitters in response to depolarization.
Neuron-to-NG2 synapses and calcium entry
A current hypothesis regarding how synaptic currents are transduced in NG2 cells involves calcium entry in response to glutamate as well as GABA. This question has already been extensively discussed in recent reviews (Gallo et al., 2008; Bergles et al., 2010) and we will only summarize the main points here. In the case of glutamatergic synapses, it has been shown that postsynaptic AMPAR expressed by NG2 cells are often permeable to calcium (Bergles et al., 2000; Lin et al., 2005; Ge et al., 2006; Mangin et al., 2008). Therefore, synaptic activation of these Ca2+ permeable AMPARs would directly result in a local increase of calcium, proportional to the amplitude of the synaptic current. Another mode of calcium entry involves the hypothetical ability of both glutamatergic and GABAergic synapses to depolarize NG2 cells to a membrane potential that is sufficient to activate voltage-dependent calcium channels (Berger et al., 1992).
Since NG2 cells frequently express voltage-dependent sodium channels, it has also been proposed that the depolarization induced by the synaptic release of glutamate or GABA could be sufficient to activate these sodium channels and to induce an increase in intracellular sodium large enough to allow calcium entry via the Na+/Ca2+-exchanger (Blaustein and Lederer, 1999; Tong et al., 2009). However, except for glutamatergic synapses between climbing fibres and cerebellar NG2 cells, the degree of depolarization induced by individual neuron-NG2 cell synapses is usually small (1–5 mV). Considering that NG2 cells also exhibit a strongly hyperpolarized membrane resting potential (−80 to −100 mV), the synchronized activation of many synapses would then be necessary to activate either voltage-dependent calcium channels or sodium channels. Such synchronization may be hard to achieve, if one also takes into account the limited number of functional synapses per NG2 cell reported in most studies. Indeed, while there is evidence that the exogenous application of GABA and glutamate can induce an intracellular calcium increase in NG2 cells (Ge et al., 2006), there is still surprisingly no evidence that physiological/endogenous synaptic release of one of these neurotransmitters is able to cause a similar effect. One of the reasons why such synaptic-induced calcium increase has not yet been observed in NG2 cells may relate to the fact that Ca2+-transients do not arise in the soma, but instead are restricted to the cell processes, i.e. where neuron-NG2 cell synapses are frequently observed (Bergles et al., 2000; Kukley et al., 2007; Ziskin et al., 2007). More importantly, even if physiologically induced Ca2+ transients are demonstrated in NG2 cells, intracellular calcium is a ubiquitous second messenger, involved in a variety of distinct and complex cellular processes. Therefore, limited information on the function(s) of synaptic transmission in NG2 cells can be obtained solely based on the demonstration of Ca2+ transients elicited by synaptic activation of glutamate or GABA receptors in these cells.
In fact, we believe that, in order to understand the specific function of neuron-NG2 cell synapses, we should understand what make these cells a unique player in brain physiology – distinct from neurons and astrocytes – rather than focusing on the similarities between NG2 cells and other neural cell types. In other words, in order to understand the role of synaptic inputs in NG2 cell physiology, we first need to know more about what NG2 cells are and what specific tasks they accomplish in the brain that would necessitate such an anatomical and functional attribute.
NG2 CELL: A PROGENITOR CELL OR A FOURTH MAJOR GLIAL CELL TYPE?
NG2 cells have long been classified as a progenitor cell type, i.e. a cell displaying a rather short-lived phenotype, with the main function of giving rise to mature and fully differentiated neural cells. Both in the developing and adult brain, NG2 cells exhibit the three main features associated with a progenitor state, including the ability to: (i) actively proliferate; (ii) migrate long distances; and (iii) generate other cell types. Importantly, it has now been shown that NG2 cells can be contacted by neuron-NG2 synapses, while they are engaged in any of these progenitor-related functions.
There are currently few doubts remaining over the fact that NG2-expressing cells are the main and probably sole source of oligodendrocytes in the brain (Dimou et al., 2008; Rivers et al., 2008; Zhu et al., 2008; Kang et al., 2010), and several studies have hinted of a limited ability of these cells to give rise to both astrocytes and neurons (Belachew et al., 2003; Aguirre et al., 2007; Tamura et al., 2007; Rivers et al., 2008; Zhu et al., 2008). A recent study by our laboratory has also provided the first evidence that neuron-NG2 synapses are actively formed and regulated during spontaneous remyelination occurring after demyelination of the corpus callosum, suggesting that they may play a role in the early steps of the myelination/remyelination process (Etxeberria et al., 2010). The hypothesis of an early function of neuron-NG2 synapses during the myelination process is supported by other studies showing that NG2 cells lose their synapses as they differentiate into myelinating oligodendrocytes (De Biase et al., 2010a; Kukley et al., 2010). However, it remains to be defined whether the loss of synapses is causal or consequential to this differentiation process. Nonetheless, since electrical activity is known to influence myelination (Demerens et al., 1996), it is tempting to hypothesize that neuron-NG2 neurotransmission conveys information on axonal electrical activity to these progenitors and regulates NG2 cell lineage progression to fully mature myelinating oligodendrocytes.
It has also been clearly shown that NG2 cells represent the main proliferating cell population in the postnatal and adult brain, both in grey and white matter areas (Nishiyama et al., 2002; Dawson et al., 2003; Aguirre et al., 2004; Ligon et al., 2006). Moreover, three independent laboratories have now demonstrated that NG2 cells remain connected to neurons by functional glutamatergic and GABAergic synapses, while dividing (Kukley et al., 2008; Ge et al., 2009; Tanaka et al., 2009). Since a previous study has shown that glutamate inhibits OPC proliferation in cell culture and that an undefined endogenous source of glutamate inhibits NG2 cell proliferation in developing cerebellum (Yuan et al., 1998), it is likely that glutamatergic synapses inhibit NG2 cell proliferation in an activity-dependent manner.
Finally, NG2 cells are able to migrate over long distance, both in the postnatal brain (Levison and Goldman, 1993; Zhu et al., 2008) as well as in the adult brain (Etxeberria et al., 2010). There is evidence that NG2 cells can be contacted by glutamatergic synapses as they migrate short distances after division (Kukley et al., 2008), and that they may be able to do the same as they migrate longer distances to remyelinate axons in a demyelinated lesion (Etxeberria et al., 2010). As it has been shown that glutamate can promote OPC migration via an αv integrin/myelin proteolipid protein complex (Gudz et al., 2006), it is conceivable that these synapses could regulate OPC migration in an activity-dependent manner.
However, several other findings on NG2 cells do not fit with the pure oligodendrocyte progenitor hypothesis. First, both during development and in the adult, NG2 cells can be found at an almost uniform density in most grey and white matter areas (Butt et al., 2005). While there is a significant level of myelination in grey matter areas, one would expect a higher accumulation of OPCs in the most heavily myelinated fibre tracts. Moreover, at least half of these cells do not appear to be normally involved in generating other cell types (Rivers et al., 2008; Psachoulia et al., 2009). In fact, most NG2 cells exhibit a complex stellate morphology with many fine processes, more or less uniformly distributed around the soma territory in a 50 μm radius (Butt et al., 2005). These processes are often associated with neuronal soma and dendrites, two neuronal compartments lacking myelination (Wigley and Butt, 2009).
Therefore, while having all the hallmarks of a progenitor cell type, it has been proposed that NG2 cells represent a fourth glial cell type in the developing and adult brain, whose function(s) would not be directly related to their ability to generate oligodendrocytes. As a consequence, this ambiguous identity of NG2 cells has led some authors to propose the existence of at least two different classes of NG2 cells (Horner et al., 2002; Bakiri et al., 2009; Lytle et al., 2009): (i) a progenitor NG2 cell type exhibiting a simple bipolar morphology, a high rate of proliferation and giving rise to oligodendrocytes, and (ii) an adult NG2 glial cell type exhibiting a complex stellate morphology, a low proliferation rate and an inability to form oligodendrocytes. However, there is still limited evidence in favour of such a clear dichotomy. Moreover, the apparently diverse features of NG2 cells may not be due to the existence of several distinct subpopulations – each with a single function – but rather reflect the fact that NG2 cells may have multiple functions, which we still do not entirely understand in the context of overall brain development and physiology. Therefore, the next and last section of this review will be a speculative attempt at integrating what is currently known about NG2 cell physiology into a unifying framework.
EXPLORING NEW TERRITORIES: A POSSIBLE FUNCTION OF NG2 CELLS IN THE DEVELOPMENT OF NEURAL NETWORKS
Evolutionary considerations on NG2 cells
The only physiological role of NG2 cells identified so far is their ability to generate oligodendrocytes. In fact, there is now strong experimental evidence that NG2 cells are the main and possibly the only source of oligodendrocytes in the brain (Dimou et al., 2008; Rivers et al., 2008; Zhu et al., 2008; Kang et al., 2010). However, as we mentioned above, it is also strongly suspected that NG2 cells may have other functions in the normal physiology of the brain, although we still lack specific hypotheses of what these functions could be and whether they might be related to their established role in generating myelinating oligodendrocytes.
A potentially fruitful way to approach this question is to take an evolutionary viewpoint. Indeed, myelin and myelinating cells are a relatively recent innovation, mostly found in vertebrate nerves (Hartline and Colman, 2007). This implies that precursors of myelin-forming cells, such as oligodendrocytes and Schwann cells, should also be present in unmyelinated ancestor organisms with a function distinct from that of generating myelin, but probably close enough to lead easily into this innovation in myelinated nervous systems. Moreover, it can be hypothesized that the acquisition of a myelinating function has not completely eliminated other roles of these ancestral proto-myelinating cells in vertebrates. These other functions could therefore still be found in the large population of non-myelinating NG2 cells present in the mammalian CNS (central nervous system). In support of this hypothesis, it has been shown that in many non-myelinated organisms, such as crabs, lobsters and Drosophila, non-myelinating glial cells are indeed frequently found to closely interact with axons and to ensheath nerves without myelinating them (Hartline and Colman, 2007; Banerjee and Bhat, 2008). This anatomical relationship is reminiscent of non-myelinating Schwann cells and NG2 cells and their relationship with axons. It would be of great interest to determine whether glial cells in Drosophila or in other non-myelinated organisms can also receive synapses from neurons and whether OPCs in non-mammalian organisms displaying myelination – such as zebrafish – can receive synapses. This information would greatly help us to determine if the existence of neuron–glial cell synapses are part of the myelination process per se or have a more ancestral function in neuron–glia interactions.
Studies on axon–glia interactions in model organisms lacking myelin, such a Drosophila melanogaster, could offer important insights into some of the non-myelinating functions of NG2 cells in the mammalian brain. For example, subpopulations of glial cells which intimately interact with axons during development are known to influence axon pathfinding by expressing diffusible and membrane-bound guidance cues, including netrins and slits in Drosophila (Oland and Tolbert, 2003; Parker and Auld, 2006). Interestingly, many of these guidance cues are also known to influence axon pathfinding in mammals and, while their exact cellular origin remains to be defined, it is known that cells belonging to the OL (oligodendrocyte lineage) can express netrins (Manitt et al., 2001; Löw et al., 2008), as well as other proteins involved in axon pathfinding such as semaphorin4D (Moreau-Fauvarque et al., 2003) and ephrinB3 (Benson et al., 2005). In Drosophila, glial cells are also involved in the defasciculation of bundled group of axons by migrating and physically separating different tracts of axons that project to different targets (Klämbt et al., 1991). This process may be related to the tendency of NG2 cells to align and delimit separate groups of axons in mammalian white matter tracts well before myelination occurs (Figure 2D and later).
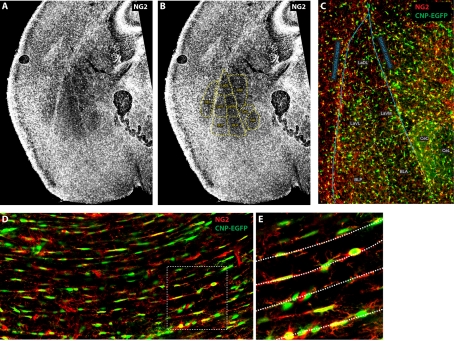
Figure 2. Distribution of NG2 cells in the amygdala and corpus callosum of a CNP-GFP (C-type natriuretic peptide-green fluorescent protein) mouse at 7 days after birth (P7).
(A–C) High resolution images taken with a standard fluorescence microscope showing the distribution of the proteoglycan NG2 (greyscale in A and B, red in C) and the fluorescent protein EGFP (enhanced green fluorescent protein) expressed under the promoter of the OL-specific protein CNPase (green in C) in a P7 mouse (coronal section). The image in (B) is identical with (A), except for the inclusion of an overlay (in yellow) showing the putative location of several amygdala nuclei, where NG2 is expressed at different levels. The higher magnification image in (C) shows that NG2+CNP-EGFP+ cells are particularly dense in the external and amygdala capsule and that their density varies for each nucleus. Astr: amygdalostriatal transition; BLA: basolateral amygdala nuclei, anterior; BLP: basolateral amygdala nuclei, posterior; CeC: central amygdala nucleus, capsular; CeL: central amygdala nucleus, lateral division; DeN: dorsal endopiriform nucleus; LaDL: lateral amygdala nuclei, dorsolateral; LaVL: lateral amygdala nuclei, ventrolateral; LaVM: lateral amygdala nuclei, ventromedian. (D, E) Single confocal image showing the distribution of NG2+/CNP-EGFP+ cells in corpus callosum (coronal section) of a P7 mouse. The image in (E) corresponds to the area surrounded by the dashed box in (D). Dashed lines in (E) indicate chains of NG2+CNP-EGFP+ cells subdividing adjacent bundles of callosal axons
It is also thought that during development, an organism goes through a sequence of steps that roughly reflect their relative order of appearance in its evolutionary history. Therefore, one could speculate ancestral non-myelinating functions of NG2 cells to be active before the myelination process starts, i.e. during embryonic and early postnatal development.
Role of NG2 cells during the formation and regeneration of neuronal networks
Because neuron-NG2 cell synapses are formed when axons are still unmyelinated (Kukley et al., 2007; Ziskin et al., 2007) and are lost as NG2 cells begin to differentiate into oligodendrocytes (Etxeberria et al., 2010; De Biase et al., 2010a; Kukley et al., 2010), it is very likely that the function of these synapses is related to the role of NG2 cells during early development. Unfortunately, while NG2 cells are clearly present during embryonic and early postnatal development, we still lack information of their role during this period. NG2 cells are generated 2 or 3 weeks before the onset of myelination, suggesting that they may be involved in early phases of postnatal development (Richardson et al., 2006; Kessaris et al., 2006). It has been shown that virally mediated ablation of OL cells during postnatal development of the cerebellar cortex disrupts its layering and alters Purkinje cell dendrite arborization and axon fasciculation (Mathis et al., 2003), demonstrating that OL cells can influence normal development of the nervous system. Moreover, during the development of the peripheral nervous system in mammals, it is known that disrupting the normal interactions between motor axon and SCPs (Schwann cell progenitors) leads to abnormal motor axon growth, pathfinding, fasciculation and muscle innervations (Riethmacher et al., 1997; Morris et al., 1999; Woldeyesus et al., 1999). Since NG2 cells and SCP share many common features, among them the ability to give rise to myelinating cells, it is plausible that NG2 cells may also exert an early influence on axon growth, pathfinding and synapse formation during the development of the CNS. While a role of NG2 cells during earlier phase of development has not yet been demonstrated, it may explain why these cells express a variety of molecules known to inhibit axon outgrowth, such as NG2 itself, Nogo and potentially other MAIs (myelin-associated inhibitors) of axon growth (Filbin, 2003).
It is known that NG2 is associated with postsynaptic AMPARs via the PSD protein GRIP (glutamate receptor-interacting protein; Stegmüller et al., 2003), suggesting not only that the NG2 proteoglycan may be enriched at neuron-NG2 cell synapse but also that the postsynaptic expression of NG2 and AMPARs may perhaps be co-regulated. This last possibility is particularly interesting because it is known that LTP (long-term potentiation) can be induced at neuron-to-NG2 cell synapses between Schaffer collateral and NG2 cells in the CA1 area (Ge et al., 2006) and that the associated increase in EPSC amplitude results from an increase in the postsynaptic expression/insertion of AMPARs. If AMPARs and NG2 are associated and co-inserted at the synapse, LTP in NG2 cells would increase local expression of NG2 at single neuron-to-NG2 cell synapses. Therefore, synaptic activity could regulate NG2 expression at the level of a single synapse, a phenomenon that could then inhibit the ability of the axon forming the synapse to grow collaterals and form additional synapses on neighbouring neurons. Importantly, it has recently been shown that Nogo and Omgp have a negative influence on LTP between Schaffer collateral and CA1 pyramidal neurons (Raiker et al., 2010). Therefore, it would be of great interest to determine whether synaptic activity between Schaffer collateral and NG2 cells can influence the expression of inhibitory factors such as NG2, Omgp or Nogo, and whether this phenomenon allows neurons to regulate the inhibitory activity of NG2 cells on their axonal and synaptic growth in an activity-dependent manner.
Until now, most of the work published on inhibitory factors expressed by NG2 cells has been focusing on their negative influence on the regeneration of axons after injury in the adult nervous system. NG2 cells undergo proliferation in response to a variety of insults, including trauma, infection and demyelination (Levine, 1994; Keirstead et al., 1998; Redwine and Armstrong, 1998; Levine and Reynolds, 1999; Wu et al., 2000; McTigue et al., 2001; Mason et al., 2001; Watanabe et al., 2002; Aguirre et al., 2007). After a stab wound or a spinal contusion, NG2 cells participate in the glial scar surrounding the lesion and inhibit the regeneration of axons from unlesioned parts of the brain into or through the lesion. While this inhibitory effect is mostly seen as detrimental – in terms of regeneration and functional recovery of injured brain regions – it might have beneficial effects outweighing disadvantages. For example, such an inhibitory action would help contain extension of the damage from the lesion to healthy parts of the brain. Indeed, mature functional networks depend on the specificity of their connection and architecture, a specificity that can only be acquired after a long and complex developmental process (Harel and Strittmater, 2006). Therefore, by preventing haphazard growth of axons and the formation of aberrant connections from and towards the damaged area (Harel and Strittmater, 2006), NG2 cells and other cells forming the glial scar would limit further destabilization of the remaining neural networks. Since it is known that in adult, axons can still form new synapses with NG2 cells after a demyelinating lesion (Etxeberria et al., 2010), it would be of great interest to determine whether in other types of injuries, regenerating axons can also from synapses onto the NG2 cells forming the glial scar.
Interestingly, a role of NG2 in delimiting injured brain regions in a pathological context may also give us clues on their normal physiological role during development and in the adult brain. In other words, both in normal and pathological conditions, NG2 cells may participate to isolate distinct brain areas from each other and regulate their connectivity.
Neuron–NG2 cell interactions: a two-way dynamic system
In order to understand the possible role that synaptic transmission plays in NG2 cells, it is important to remember that fast synaptic transmission via ionotropic receptors is only one of many ways for glial cells to detect and be influenced by neuronal activity. Therefore, its unique features should be taken into account when we consider the possible functions of neuron–NG2 cell synaptic transmission. Indeed, as mentioned in previous reviews (Gallo et al., 2008; Bergles et al., 2010), synaptic neurotransmission probably provides the best spatio-temporal resolution among all cell–cell signalling systems, allowing a cell to discriminate the activity of individual axons and synapses on a millisecond scale.
Many authors, including us (Gallo et al., 2008), have hypothesized that neuron–NG2 glutamatergic synapses may regulate NG2 cell proliferation, migration and differentiation. Indeed, glutamate is known to inhibit NG2 cell proliferation, increase their migration speed and inhibit their ability to differentiate into oligodendrocytes (Gallo et al., 1996; Yuan et al., 1998; Gudz et al., 2006). Importantly, the effects of glutamate on NG2 cell proliferation, migration and differentiation are concentration dependent. Therefore, since synchronization of presynaptic neurons results in the simultaneous release of glutamate via multiple neuron–NG2 synapses and larger glutamatergic currents (Mangin et al., 2008), one would predict that the more synchronized the axons contacting a given NG2 cell are, the more efficiently they will be able to decrease its rate of proliferation, and to increase its speed of migration, while inhibiting its ability to differentiate into an oligodendrocyte.
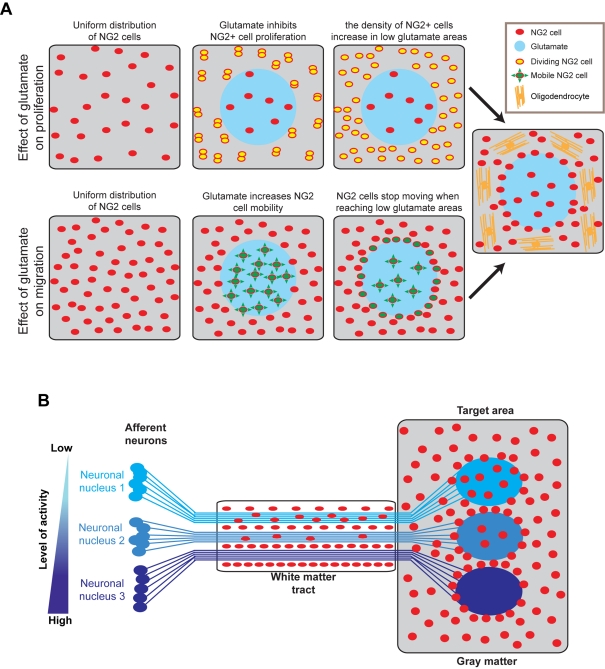
Since NG2 cells are already quite uniformly distributed in the brain (Zhu et al., 2008) when the first functional neuron–NG2 cell glutamatergic synapses are detected around P3 (Mangin et al., 2008; De Biase et al., 2010a), one could gain insights by modelling what the influence of localized and synchronized synaptic releases of glutamate on an initially uniformly distributed group of NG2 cells would be. Figure 1 illustrates such a situation, where NG2 cells (in red) are initially uniformly distributed in an area of grey or white matter before a group of axons/synapses begin to release glutamate in a synchronized fashion (in blue). Since glutamate released from these axons will locally inhibit the proliferation rate of NG2 cells in the area where activity is synchronized, the density of NG2 cells should increase at a faster rate outside of the synaptically synchronized area than inside. In terms of cell migration, the migration speed of NG2 cells should specifically increase in the area of synchronized activity. If one simply assumes that glutamate increases NG2 cell mobility in an undirected manner, NG2 cells in the synchronized/high-glutamate area should keep moving randomly until they reach by chance the non-synchronized/low-glutamate surrounding tissue where their migration speed will then be reduced. This would not only lead to a decrease in NG2 cell density inside the synchronized/high glutamate area but also to a local accumulation of NG2 cells at the interface between synchronized/high-glutamate and non-synchronized/low-glutamate areas. As the density of NG2 cells increases in non-synchronized/low-glutamate areas, their proliferation rate would progressively drop because of a density-dependent inhibitory feedback (Zhang and Miller, 1996), therefore limiting the maximum density of NG2 cells that can be attained in a given area. Ultimately, NG2 cells outside of the synchronized/high-glutamate area would be more likely to differentiate into oligodendrocytes. It is interesting to note that, in such a context, the effects of glutamate on cell proliferation and migration would synergize and contribute to a decrease in the density of NG2 cells in regions where axons tend to be synchronized in their activity.
Figure 1. Hypothetical consequences of localized and synchronized release of glutamate on NG2 cells.
(A) Cartoon illustrating how synchronized release of glutamate from a localized group of axons or synapses (blue) would affect the behaviour of uniformly distributed NG2 cells (red), taking into account the known effect of glutamate on NG2 cell proliferation (upper three panels), migration/mobility (lower three panel) and differentiation (rightmost panel) (Gallo et al., 1996; Yuan et al., 1998; Gudz et al., 2006). Note that the effects of glutamate on NG2 cell proliferation and migration/mobility would synergize to lower the density of NG2 cells in the area where glutamate is synchronously released. Ultimately, this area will display lower myelination (‘grey matter area’), while the surrounding tissue will exhibit a higher density of NG2 cells and oligodendrocytes (‘white matter area’). (B) Cartoon illustrating how NG2 cells (in red) would behave in different settings where three distinct neuronal nuclei (deep blue to light blue) exhibit a high degree of internal synchronization, but different level of overall activity. Each nucleus projects towards an adjacent target area via a shared white matter tract. In the white matter tract, NG2 cells would tend to accumulate at the boundaries between the bundles of axons corresponding to each nucleus in an activity-dependent manner. In the target grey matter area, an equivalent phenomenon would occur, where NG2 would be depleted from each target area and accumulate at their boundaries in an activity-dependent manner.
Of course, in the brain such a simplified situation is unlikely to occur and NG2 cells are more likely to encounter a series of adjacent microenvironments, each exhibiting a high level of internal synchronization, and corresponding for example to distinct neuronal nuclei, cortical columns and layers. A good example of this type of anatomical setting is the amygdala, where at least 10 adjacent distinct nuclei can be identified (Swanson and Petrovich, 1998) (Figure 2). In this context, one would expect the density of NG2 cells to be distributed inside each of these nuclei, depending on their level of activity and synchronization. NG2 cells would tend to accumulate at a higher density either in a nucleus exhibiting low neuronal activity or at the boundary between highly active nuclei, as the activity between adjacent nuclei is not synchronized (Figure 2). Such an activity-dependent accumulation of OPCs at the interface between nuclei exhibiting distinct pattern of synchronized activity could also partially explain how white matter tracts will usually form at the boundaries between neuronal nuclei, such as the amygdala capsule and the external capsule, which separate the lateral nucleus from the surrounding amygdala nuclei (Figures 2A–2C). Moreover, since NG2 cells express a number of factors inhibiting axon growth, accumulation of NG2 cells could also limit the ability of axons – and maybe even dendrites – originating from neurons in one nucleus to make aberrant contact with neurons in neighbouring nuclei. This function of NG2 cells could be linked to their ability to inhibit axon growth in the glial scar.
In a similar way, this mechanism could explain why a large white matter tract such as the corpus callosum is regularly spanned by chains of NG2 cells that would subdivide this region into smaller adjacent bundles of axons (Figures 2D and 2E). While the significance and role of these chains of NG2 cells are still unknown, they may be separating groups of callosal axons depending on their origin and targets. Indeed, the corpus callosum is principally made of inter-hemispheric cortico–cortical connections between homotopic regions of the cortex (See Figure 3B), and the axons connecting different regions of the cortex remain segregated when crossing the corpus callosum (Jouandet et al., 1986; Nakamura and Kanaseki, 1989; Wahl et al., 2007). As axonal activity would tend to be synchronized within each bundle of axons originating from the same cortical region – but not between them – NG2 cells would once again tend to accumulate at the boundaries between each functionally distinct bundle of axons, which would explain why NG2 cells adopt such a peculiar distribution pattern in corpus callosum. What could then be the function of such patterned distribution of NG2 cells?
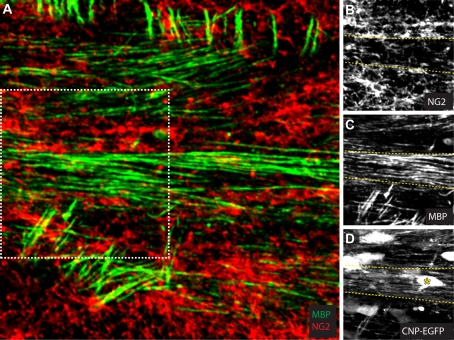
Figure 3. NG2 cells delimit myelinated bundle of axons in the corpus callosum of a P13 CNP-GFP mouse.
(A–D) Projection of a small stack of confocal images (five consecutives planes with z = 2 μm) illustrating the tendency of bundles of axons to myelinate [MBP (maltose-binding protein) staining in green in (A), grey in (C)] as a whole independently of surrounding axons. Boundaries are delimited by NG2 cells [NG2 staining in red in (A), grey in (B)] (coronal section). Images in (B–D) correspond to the area surrounded by the dashed box in (A). The dashed lines in yellow (B–D) indicate the interface between the myelinated bundle of axons labelled for MBP (C) and NG2 cells labelled for the proteoglycan NG2 (B). The image in (D) shows that bright CNP-GFP+ oligodendrocyte processes appear restricted to a single bundle. The yellow asterisk indicates the position of the cell body of one of the oligodendrocyte myelinating the axon bundle delimited by the yellow dashed lines. At this developmental stage, CNP-GFP fluorescence is difficult to detect in NG2 cells, as it is much lower than the fluorescence detected in mature oligodendrocytes.
One possibility is that NG2 cells could actually regulate axon growth and drive electrically synchronized axons originating from the same cortical area to form tight independent fascicles, while growing towards the contralateral hemisphere. For example, callosal axons originating from the somatosensory cortex are known to grow during the first postnatal days in mice, crossing the midline around P3 and reaching their target in the contralateral hemisphere around P6 (Wang et al., 2007). Importantly, it is known that, during this developmental period, callosal NG2 cells already receive functional glutamatergic synapses (De Biase et al., 2010a) and that the speed of growth of callosal axon is reduced when their electrical activity is reduced (Wang et al., 2007). Therefore, callosal axon growth would be globally decreased by inhibitory factors expressed by NG2 cells, which would push axons to organize into tighter electrically synchronized fascicles. This synchronized activity would locally reduce NG2 cell density and create NG2 cell-free territories suitable for axonal growth. Such a role in axon fasciculation would be consistent with the hypothesis that NG2 cells may also have functions similar to those of non-myelinating glial cells in Drosophila (Klämbt et al., 1991).
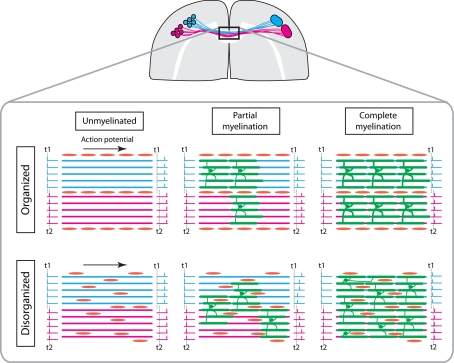
Another non-exclusive role for this patterned accumulation of NG2 cells between separate axonal bundles is that these chains of cells may have a later function in development, as they might allow myelination to occur independently in each callosal bundle of fibres. During development, it is common to see myelination to occur synchronously in isolated bundles of callosal axons, while surrounding bundles remain unmyelinated (Figure 3). Often, these myelinated bundles will be delimited by NG2 staining, suggesting that NG2 cells may have an inhibitory function in the growth of oligodendrocyte processes, forcing them to limit their myelinating activity to a single bundle of fibres. Indeed, recent evidence indicates that inhibitory factors expressed by OPCs – such as Nogo – tend to delimit the extent of the territory myelinated by individual oligodendrocytes (Chong et al., 2010). Such an organization of the myelination process may be fundamental to prevent transient de-synchronization of axons belonging to the same bundle that would occur if the myelination process were random (Figure 4). Indeed, by pushing individual oligodendrocytes to only myelinate inter-hemispheric axons in a single bundle, this organized process will allow progressive myelination and speed up conduction velocity in these axons in a synchronized manner. This will ultimately result in synchronized activation of their target areas (Figure 4). By contrast, if oligodendrocytes were allowed to randomly myelinate axons independently of their origin, this process would greatly disrupt the ability of target neurons to detect relevant correlations in their inputs, and would instead create unsynchronized activity until all callosal axons corresponding to a given cortical area are fully myelinated (Figure 4). Moreover, in the event of a demyelinating lesion, a partial and random loss of oligodendrocytes would be more likely to disrupt synchrony between axons belonging to the same bundle if the myelination process was initially random.
Figure 4. Hypothetical scenarios of myelination processes occurring either independently between functionally distinct axon bundles or in a random fashion.
In this cartoon, two groups of cortical neurons (blue and purple circles) belonging to adjacent cortical columns processing distinct types of sensory information (e.g. somatosensory information from two adjacent digits) project towards homotypic areas of the contralateral cortex via the corpus callosum. In this example, we assume that: (i) callosal axons originating from each group remain segregated (blue and purple lines); (ii) they are delimited by chains of NG2 cells (in red); and (iii) the extent of myelination by individual oligodendrocytes (in green) is limited to individual bundles by NG2 cells. However, other mechanisms could allow similar outcomes. For example, even in the absence of axon segregation and NG2 boundaries, NG2 cells differentiating into oligodendrocytes could initially identify axons belonging to the same functional group via neuron-NG2 cells synapses based on the fact these axons fire synchronously more often than axons carrying distinct types of sensory information. Whatever the exact mechanism, our central argument here is that specifying the myelination process between functionally distinct groups of axons allows to continuously preserve the temporal relationship between action potentials within each group. t1 and t2 indicate the time at which neurons belonging to each group are firing. The firing is strongly synchronized within each group by the fact that they are activated by the same sensory stimulus (e.g. one digit is stimulated at t1, the other at t2). First column from the left: before myelination occurs, assuming that the axonal length is similar for each group of axons (another argument in favour of their bundling) and that their axon diameter is identical, action potentials generated simultaneously in a given group of neurons (blue and purple bars of the left of each schema) should slowly reach their contralateral target at the same time, and their coherence is preserved. Second column from the left: once myelination starts, the conduction velocity of the axons is increased by myelination. If each oligodendrocyte cell limits its myelinating activity to a single bundle (top panel) and tends therefore to myelinate all the axons belonging to the same group at once, the conduction velocity of all axons belonging to this group will be increased in an identical manner, and the synchrony of their action potential will be preserved when they reach their target. On the other hand, if oligodendrocytes myelinate axons they myelinate randomly and as long as myelination is not complete (see third column from the left), each axon would see its conduction velocity increase by a different value and their action potential will be desynchronized once they reach their target.
Finally, such an accumulation of NG2 cells at the boundaries between groups of synchronized axons or between neuronal nuclei may be one of many pre-requisites for these cells to differentiate into oligodendrocytes in vivo. Indeed, it has been shown that the differentiation rate of NG2 cells into oligodendrocytes is increased when they are cultivated at high density (Rosenberg et al., 2008). In fact, the differentiation of NG2 cells into oligodendrocytes can be induced by mere mechanical compression of NG2 cells, using for example a high density of biologically inert beads (Rosenberg et al., 2008). Our model implies that NG2 cells would start myelinating – or remyelinating – axons once their local density have reached a critical level in the process of forming – or reforming – inhibitory barriers between functionally distinct groups of axons or neurons. Such a model would insure that myelination does not start before axon bundles and neuron groups are organized and partitioned into distinct functional groups, thereby insuring orderly myelination.
CONCLUSIONS
In this review article, we attempted to gather disparate facts known about NG2 cells and neuron-to-NG2 cell synapses into one possible unifying framework. While it is possible that much of the specific and oversimplified hypotheses provided here will be disproven by the harsh reality of biological complexity, we hope that some of the general ideas proposed may at least offer new ways to think about NG2 cells and their function in the brain. More particularly, we believe that our understanding of these cells will greatly benefit from: (i) studying their role before myelination and during early development; (ii) looking at the function of possible homologous cell types in non-myelinated organisms; (iii) examining how synchronized/non-synchronized activity in neurons influence their proliferation, mobility and differentiation; (iv) taking into account the importance of isochronicity/coherence in signal conduction and the potentially disruptive influence of a random myelination process; and (v) conceptualizing neuron–OPC interactions as a complex reciprocal system emerging from the dynamic equilibrium between cooperative and competitive influences. Because NG2 cells have a fundamental role in myelin pathologies such as multiple sclerosis and axon regeneration after brain injuries, an integrated understanding of their diverse roles during development will greatly contribute to our ability to develop comprehensive and original strategies to treat such pathologies.
REFERENCES
- Aguirre A, Chittajallu R, Belachew S, Gallo V. NG2-expressing cells in the subventricular zone are type C-like cells and contribute to interneuron generation in the postnatal hippocampus. J Cell Biol. 2004;165:575–589. doi: 10.1083/jcb.200311141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre A, Dupree JL, Mangin JM, Gallo V. A functional role for EGFR signaling in myelination and remyelination. Nature Neurosci. 2007;10:990–1002. doi: 10.1038/nn1938. [DOI] [PubMed] [Google Scholar]
- Bakiri Y, Attwell D, Káradóttir R. Electrical signalling properties of oligodendrocyte precursor cells. Neuron Glia Biol. 2009;5:3–11. doi: 10.1017/S1740925X09990202. [DOI] [PubMed] [Google Scholar]
- Banerjee S, Bhat MA. Glial ensheathment of peripheral axons in Drosophila. J Neurosci Res. 2008;86:1189–1198. doi: 10.1002/jnr.21574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barres BA. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron. 2008;60:430–440. doi: 10.1016/j.neuron.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Belachew S, Chittajallu R, Aguirre AA, Yuan X, Kirby M, Anderson S, Gallo V. Postnatal NG2 proteoglycan-expressing progenitor cells are intrinsically multipotent and generate functional neurons. J Cell Biol. 2003;161:169–186. doi: 10.1083/jcb.200210110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson MD, Romero MI, Lush ME, Lu QR, Henkemeyer M, Parada LF. Ephrin-B3 is a myelin-based inhibitor of neurite outgrowth. Proc Natl Acad Sci USA. 2005;102:10694–10699. doi: 10.1073/pnas.0504021102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger T, Schnitzer J, Orkand PM, Kettenmann H. Sodium and calcium currents in glial cells of the mouse corpus callosum slice. Eur J Neurosci. 1992;4:1271–1284. doi: 10.1111/j.1460-9568.1992.tb00153.x. [DOI] [PubMed] [Google Scholar]
- Bergles DE, Roberts JD, Somogyi P, Jahr CE. Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature. 2000;405:187–191. doi: 10.1038/35012083. [DOI] [PubMed] [Google Scholar]
- Bergles DE, Jabs R, Steinhäuser C. Neuron–glia synapses in the brain. Brain Res Rev. 2010;63:130–137. doi: 10.1016/j.brainresrev.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein MP, Lederer WJ. Sodium/calcium exchange: its physiological implications. Physiol Rev. 1999;79:763–854. doi: 10.1152/physrev.1999.79.3.763. [DOI] [PubMed] [Google Scholar]
- Butt AM, Hamilton N, Hubbard P, Pugh M, Ibrahim M. Synantocytes: the fifth element. J Anat. 2005;207:695–706. doi: 10.1111/j.1469-7580.2005.00458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chittajallu R, Aguirre A, Gallo V. NG2-positive cells in the mouse white and grey matter display distinct physiological properties. J Physiol. 2004;561:109–122. doi: 10.1113/jphysiol.2004.074252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong SYC, Rosenberg SS, Shen YA, Hahn AT, Zheng B, Zhang LI, Mcgee AW, Lu QR, Chan JR. Nogo-A establishes spatial segregation and extent of myelination during development. 2010 doi: 10.1073/pnas.1113540109. SfN Meeting 2010, San Diego, Poster 539.18/C31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke LN, Hamilton NB, Young KM, Kessaris N, Richardson WD, Attwell D. The distribution and excitability of two types of oligodendrocyte precursor cell in white and grey matter. 2010 SfN Meeting 2010 San Diego, Poster 853.21/H14. [Google Scholar]
- Dawson MR, Polito A, Levine JM, Reynolds R. NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol Cell Neurosci. 2003;24:476–488. doi: 10.1016/s1044-7431(03)00210-0. [DOI] [PubMed] [Google Scholar]
- De Biase LM, Nishiyama A, Bergles DE. Excitability and synaptic communication within the oligodendrocyte lineage. J Neurosci. 2010a;30:3600–3611. doi: 10.1523/JNEUROSCI.6000-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Biase LM, Kang SH, Potter E, Fukaya M, Srivastava I, Rowitch D, Mishina M, Bergles DE. NMDA receptor function in the oligodendrocyte lineage. 2010b In SfN Meeting 2010, San Diego, Poster 853.18/H11. [Google Scholar]
- Delaunay D, Heydon K, Cumano A, Schwab MH, Thomas JL, Suter U, Nave KA, Zalc B, Spassky N. Early neuronal and glial fate restriction of embryonic neural stem cells. J Neurosci. 2008;28:2551–2562. doi: 10.1523/JNEUROSCI.5497-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demerens C, Stankoff B, Logak M, Anglade P, Allinquant B, Couraud F, Zalc B, Lubetzki C. Induction of myelination in the central nervous system by electrical activity. Proc Natl Acad Sci USA. 1996;93:9887–9892. doi: 10.1073/pnas.93.18.9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimou L, Simon C, Kirchhoff F, Takebayashi H, Götz M. Progeny of Olig2-expressing progenitors in the gray and white matter of the adult mouse cerebral cortex. J Neurosci. 2008;28:10434–10442. doi: 10.1523/JNEUROSCI.2831-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etxeberria A, Mangin JM, Aguirre A, Gallo V. Adult-born SVZ progenitors receive transient synapses during remyelination in corpus callosum. Nat Neurosci. 2010;13:287–289. doi: 10.1038/nn.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng W, Zhang M. Organization and dynamics of PDZ-domain-related supramodules in the postsynaptic density. Nat Rev Neurosci. 2009;10:87–99. doi: 10.1038/nrn2540. [DOI] [PubMed] [Google Scholar]
- Filbin MT. Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nat Rev Neurosci. 2003;4:703–713. doi: 10.1038/nrn1195. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Harvey RJ, Schwarz G. Gephyrin: where do we stand, where do we go? Trends Neurosci. 2008;31:257–264. doi: 10.1016/j.tins.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Gallo V, Zhou JM, McBain CJ, Wright P, Knutson PL, Armstrong RC. Oligodendrocyte progenitor cell proliferation and lineage progression are regulated by glutamate receptor-mediated K+ channel block. J Neurosci. 1996;16:2659–2670. doi: 10.1523/JNEUROSCI.16-08-02659.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo V, Mangin JM, Kukley M, Dietrich D. Synapses on NG2-expressing progenitors in the brain: multiple functions? J Physiol. 2008;586:3767–3781. doi: 10.1113/jphysiol.2008.158436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge WP, Yang XJ, Zhang Z, Wang HK, Shen W, Deng QD, Duan S. Long-term potentiation of neuron–glia synapses mediated by Ca2+-permeable AMPA receptors. Science. 2006;312:1533–1537. doi: 10.1126/science.1124669. [DOI] [PubMed] [Google Scholar]
- Ge WP, Zhou W, Luo Q, Jan LY, Jan YN. Dividing glial cells maintain differentiated properties including complex morphology and functional synapses. Proc Natl Acad Sci USA. 2009;106:328–333. doi: 10.1073/pnas.0811353106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudz TI, Komuro H, Macklin WB. Glutamate stimulates oligodendrocyte progenitor migration mediated via an alphav integrin/myelin proteolipid protein complex. J Neurosci. 2006;26:2458–2466. doi: 10.1523/JNEUROSCI.4054-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harel NY, Strittmatter SM. Can regenerating axons recapitulate developmental guidance during recovery from spinal cord injury? Nat Rev Neurosci. 2006;7:603–616. doi: 10.1038/nrn1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartline DK, Colman DR. Rapid conduction and the evolution of giant axons and myelinated fibers. Curr Biol. 2007;17:R29–R35. doi: 10.1016/j.cub.2006.11.042. [DOI] [PubMed] [Google Scholar]
- Horner PJ, Thallmair M, Gage FH. Defining the NG2-expressing cell of the adult CNS. J Neurocytol. 2002;31:469–480. doi: 10.1023/a:1025739630398. [DOI] [PubMed] [Google Scholar]
- Jacob TC, Moss SJ, Jurd R. GABA(A) receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat Rev Neurosci. 2008;9:331–343. doi: 10.1038/nrn2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouandet ML, Lachat JJ, Garey LJ. Topographic distribution of callosal neurons and terminals in the cerebral cortex of the cat. Anat Embryol (Berl) 1986;173:323–342. doi: 10.1007/BF00318916. [DOI] [PubMed] [Google Scholar]
- Kang SH, Fukaya M, Yang JK, Rothstein JD, Bergles DE. NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration. Neuron. 2010;68:668–681. doi: 10.1016/j.neuron.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karadottir R, Hamilton NB, Bakiri Y, Attwell D. Spiking and nonspiking classes of oligodendrocyte precursor glia in CNS white matter. Nat Neurosci. 2008;11:450–456. doi: 10.1038/nn2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keirstead HS, Levine JM, Blakemore WF. Response of the oligodendrocyte progenitor cell population (defined by NG2 labelling) to demyelination of the adult spinal cord. Glia. 1998;22:161–170. [PubMed] [Google Scholar]
- Klämbt C, Jacobs JR, Goodman CS. The midline of the Drosophila central nervous system: a model for the genetic analysis of cell fate, cell migration, and growth cone guidance. Cell. 1991;9:331–343. doi: 10.1016/0092-8674(91)90509-w. [DOI] [PubMed] [Google Scholar]
- Kessaris N, Fogarty M, Iannarelli P, Grist M, Wegner M, Richardson WD. Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat Neurosci. 2006;9:173–179. doi: 10.1038/nn1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukley M, Capetillo-Zarate E, Dietrich D. Vesicular glutamate release from axons in white matter. Nat Neurosci. 2007;10:311–320. doi: 10.1038/nn1850. [DOI] [PubMed] [Google Scholar]
- Kukley M, Kiladze M, Tognatta R, Hans M, Swandulla D, Schramm J, Dietrich D. Glial cells are born with synapses. FASEB J. 2008;22:2957–2969. doi: 10.1096/fj.07-090985. [DOI] [PubMed] [Google Scholar]
- Kukley M, Nishiyama A, Dietrich D. The fate of synaptic input to NG2 glial cells: neurons specifically downregulate transmitter release onto differentiating oligodendroglial cells. J Neurosci. 2010;30:8320–8331. doi: 10.1523/JNEUROSCI.0854-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappe-Siefke C, Goebbels S, Gravel M, Nicksch E, Lee J, Braun PE, Griffiths IR, Nave KA. Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nat Genet. 2003;33:366–374. doi: 10.1038/ng1095. [DOI] [PubMed] [Google Scholar]
- Levine JM. Increased expression of the NG2 chondroitin-sulfate proteoglycan after brain injury. J Neurosci. 1994;4:4716–4730. doi: 10.1523/JNEUROSCI.14-08-04716.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JM, Reynolds R. Activation and proliferation of endogenous oligodendrocyte precursor cells during ethidium bromide-induced demyelination. Exp Neurol. 1999;160:333–347. doi: 10.1006/exnr.1999.7224. [DOI] [PubMed] [Google Scholar]
- Levison SW, Goldman JE. Both oligodendrocytes and astrocytes develop from progenitors in the subventricular zone of the rat forebrain. Neuron. 1993;10:201–212. doi: 10.1016/0896-6273(93)90311-e. [DOI] [PubMed] [Google Scholar]
- Ligon KL, Kesari S, Kitada M, Sun T, Arnett HA, Alberta JA, Anderson DJ, Stiles CD, Rowitch DH. Development of NG2 neural progenitor cells requires Olig gene function. Proc Natl Acad Sci USA. 2006;103:7853–7858. doi: 10.1073/pnas.0511001103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SC, Bergles DE. Synaptic signaling between GABAergic interneurons and oligodendrocyte precursor cells in the hippocampus. Nat Neurosci. 2004;7:24–32. doi: 10.1038/nn1162. [DOI] [PubMed] [Google Scholar]
- Lin SC, Huck JH, Roberts JD, Macklin WB, Somogyi P, Bergles DE. Climbing fiber innervation of NG2-expressing glia in the mammalian cerebellum. Neuron. 2005;46:773–785. doi: 10.1016/j.neuron.2005.04.025. [DOI] [PubMed] [Google Scholar]
- Löw K, Culbertson M, Bradke F, Tessier-Lavigne M, Tuszynski MH. Netrin-1 is a novel myelin-associated inhibitor to axon growth. J Neurosci. 2008;28:1099–1108. doi: 10.1523/JNEUROSCI.4906-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lytle JM, Chittajallu R, Wrathall JR, Gallo V. NG2 cell response in the CNP-EGFP mouse after contusive spinal cord injury. Glia. 2009;57:270–285. doi: 10.1002/glia.20755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangin JM, Kunze A, Chittajallu R, Gallo V. Satellite NG2+ progenitor cells share common glutamatergic inputs with associated interneurons in the mouse dentate gyrus. J Neurosci. 2008;28:7610–7623. doi: 10.1523/JNEUROSCI.1355-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manitt C, Colicos MA, Thompson KM, Rousselle E, Peterson AC, Kennedy TE. Widespread expression of netrin-1 by neurons and oligodendrocytes in the adult mammalian spinal cord. J Neurosci. 2001;21:3911–3922. doi: 10.1523/JNEUROSCI.21-11-03911.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason JL, Suzuki K, Chaplin DD, Matsushima GK. Interleukin-1beta promotes repair of the CNS. J Neurosci 2001. 2001;21:7046–7052. doi: 10.1523/JNEUROSCI.21-18-07046.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis C, Collin L, Borrelli E. Oligodendrocyte ablation impairs cerebellum development. Development. 2003;130:4709–4718. doi: 10.1242/dev.00675. [DOI] [PubMed] [Google Scholar]
- McTigue DM, Wei P, Stokes BT. Proliferation of NG2-positive cells and altered oligodendrocyte numbers in the contused rat spinal cord. J Neurosci. 2001;21:3392–3400. doi: 10.1523/JNEUROSCI.21-10-03392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau-Fauvarque C, Kumanogoh A, Camand E, Jaillard C, Barbin G, Boquet I, Love C, Jones EY, Kikutani H, Lubetzki C, Dusart I, Chédotal A. The transmembrane semaphorin Sema4D/CD100, an inhibitor of axonal growth, is expressed on oligodendrocytes and upregulated after CNS lesion. J Neurosci. 2003;23:9229–9239. doi: 10.1523/JNEUROSCI.23-27-09229.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JK, Lin W, Hauser C, Marchuk Y, Getman D, Lee KF. Rescue of the cardiac defect in ErbB2 mutant mice reveals essential roles of ErbB2 in peripheral nervous system development. Neuron. 1999;23:273–283. doi: 10.1016/s0896-6273(00)80779-5. [DOI] [PubMed] [Google Scholar]
- Müller J, Reyes-Haro D, Pivneva T, Nolte C, Schaette R, Lübke J, Kettenmann H. The principal neurons of the medial nucleus of the trapezoid body and NG2(+) glial cells receive coordinated excitatory synaptic input. J Gen Physiol. 2009;134:115–127. doi: 10.1085/jgp.200910194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H, Kanaseki T. Topography of the corpus callosum in the cat. Brain Res. 1989;485:171–175. doi: 10.1016/0006-8993(89)90679-3. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Lin XH, Giese N, Heldin CH, Stallcup WB. Co-localization of NG2 proteoglycan and PDGF alpha-receptor on O2A progenitor cells in the developing rat brain. J Neurosci Res. 1996;43:299–314. doi: 10.1002/(SICI)1097-4547(19960201)43:3<299::AID-JNR5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Watyanabe M, Yang Z, Bu J. Identity, distribution, and development of polydendrocytes: NG2-expressing glial cells. J Neurocytol. 2002;31:437–455. doi: 10.1023/a:1025783412651. [DOI] [PubMed] [Google Scholar]
- Oland LA, Tolbert LP. Key interactions between neurons and glial cells during neural development in insects. Annu Rev Entomol. 2003;48:89–110. doi: 10.1146/annurev.ento.48.091801.112654. [DOI] [PubMed] [Google Scholar]
- Ozerdem U, Grako KA, Dahlin-Huppe K, Monosov E, Stallcup WB. NG2 proteoglycan is expressed exclusively by mural cells during vascular morphogenesis. Dev Dyn. 2001;222:218–227. doi: 10.1002/dvdy.1200. [DOI] [PubMed] [Google Scholar]
- Parker RJ, Auld VJ. Roles of glia in the Drosophila nervous system. Semin Cell Dev Biol. 2006;17:66–77. doi: 10.1016/j.semcdb.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Psachoulia K, Jamen F, Young KM, Richardson WD. Cell cycle dynamics of NG2 cells in the postnatal and ageing brain. Neuron Glia Biol. 2009;5:57–67. doi: 10.1017/S1740925X09990354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiker SJ, Lee H, Baldwin KT, Duan Y, Shrager P, Giger RJ. Oligodendrocyte-myelin glycoprotein and Nogo negatively regulate activity-dependent synaptic plasticity. J Neurosci. 2010;30:12432–12445. doi: 10.1523/JNEUROSCI.0895-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redwine JM, Armstrong RC. In vivo proliferation of oligodendrocyte progenitors expressing PDGFalphaR during early remyelination. J Neurobiol. 1998;37:413–428. doi: 10.1002/(sici)1097-4695(19981115)37:3<413::aid-neu7>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Reynolds R, Hardy R. Oligodendroglial progenitors labeled with the O4 antibody persist in the adult rat cerebral cortex in vivo. J Neurosci Res. 1997;47:455–470. doi: 10.1002/(sici)1097-4547(19970301)47:5<455::aid-jnr1>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Richardson WD, Kessaris N, Pringle N. Oligodendrocyte wars. Nat Rev Neurosci. 2006;7:11–18. doi: 10.1038/nrn1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riethmacher D, Sonnenberg-Riethmacher E, Brinkmann V, Yamaai T, Lewin GR, Birchmeier C. Severe neuropathies in mice with targeted mutations in the ErbB3 receptor. Nature. 1997;389:725–730. doi: 10.1038/39593. [DOI] [PubMed] [Google Scholar]
- Rivers LE, Young KM, Rizzi M, Jamen F, Psachoulia K, Wade A, Kessaris N, Richardson WD. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat Neurosci. 2008;11:1392–1401. doi: 10.1038/nn.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg SS, Kelland EE, Tokar E, De la Torre AR, Chan JR. The geometric and spatial constraints of the microenvironment induce oligodendrocyte differentiation. Proc Natl Acad Sci USA. 2008;105:14662–14667. doi: 10.1073/pnas.0805640105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegmüller J, Werner H, Nave KA, Trotter J. The proteoglycan NG2 is complexed with alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors by the PDZ glutamate receptor interaction protein (GRIP) in glial progenitor cells.Implications for glial-neuronal signaling. J Biol Chem. 2003;278:3590–3598. doi: 10.1074/jbc.M210010200. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Petrovich GD. What is the amygdala? Trends Neurosci. 1998;21:323–331. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- Tamura Y, Kataoka Y, Cui Y, Takamori Y, Watanabe Y, Yamada H. Multi-directional differentiation of doublecortin- and NG2-immunopositive progenitor cells in the adult rat neocortex in vivo. Eur J Neurosci. 2007;25:3489–3498. doi: 10.1111/j.1460-9568.2007.05617.x. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Tozuka Y, Takata T, Shimazu N, Matsumura N, Ohta A, Hisatsune T. Excitatory GABAergic activation of cortical dividing glial cells. Cereb Cortex. 2009;19:2181–2195. doi: 10.1093/cercor/bhn238. [DOI] [PubMed] [Google Scholar]
- Tong XP, Li XY, Zhou B, Shen W, Zhang ZJ, Xu TL, Duan S. Ca(2+) signaling evoked by activation of Na(+) channels and Na(+)/Ca(2+) exchangers is required for GABA-induced NG2 cell migration. J Cell Biol. 2009;186:113–128. doi: 10.1083/jcb.200811071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl M, Lauterbach-Soon B, Hattingen E, Jung P, Singer O, Volz S, Klein JC, Steinmetz H, Ziemann U. Human motor corpus callosum: topography, somatotopy, and link between microstructure and function. J Neurosci. 2007;27:12132–12138. doi: 10.1523/JNEUROSCI.2320-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CL, Zhang L, Zhou Y, Zhou J, Yang XJ, Duan SM, Xiong ZQ, Ding YQ. Activity-dependent development of callosal projections in the somatosensory cortex. J Neurosci. 2007;27:11334–11342. doi: 10.1523/JNEUROSCI.3380-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Toyama Y, Nishiyama A. Differentiation of proliferated NG2-positive glial progenitor cells in a remyelinating lesion. J Neurosci Res. 2002;69:826–836. doi: 10.1002/jnr.10338. [DOI] [PubMed] [Google Scholar]
- Wigley R, Butt AM. Integration of NG2-glia (synantocytes) into the neuroglial network. Neuron Glia Biol. 2009;5:21–28. doi: 10.1017/S1740925X09990329. [DOI] [PubMed] [Google Scholar]
- Woldeyesus MT, Britsch S, Riethmacher D, Xu L, Sonnenberg-Riethmacher E, Abou-Rebyeh F, Harvey R, Caroni P, Birchmeier C. Peripheral nervous system defects in ErbB2 mutants following genetic rescue of heart development. Genes Dev. 1999;13:2538–2548. doi: 10.1101/gad.13.19.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Miller RH, Ransohoff RM, Robinson S, Bu J, Nishiyama A. Elevated levels of the chemokine GRO-1 correlate with elevated oligodendrocyte progenitor proliferation in the jimpy mutant. J Neurosci. 2000;20:2609–2617. doi: 10.1523/JNEUROSCI.20-07-02609.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Eisen AM, McBain CJ, Gallo V. A role for glutamate and its receptors in the regulation of oligodendrocyte development in cerebellar tissue slices. Development. 1998;125:2901–2914. doi: 10.1242/dev.125.15.2901. [DOI] [PubMed] [Google Scholar]
- Zhang H, Miller RH. Density-dependent feedback inhibition of oligodendrocyte precursor expansion. J Neurosci. 1996;16:6886–6895. doi: 10.1523/JNEUROSCI.16-21-06886.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Bergles DE, Nishiyama A. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development. 2008;135:145–157. doi: 10.1017/S1740925X09000015. [DOI] [PubMed] [Google Scholar]
- Ziskin JL, Nishiyama A, Rubio M, Fukaya M, Bergles DE. Vesicular release of glutamate from unmyelinated axons in white matter. Nat Neurosci. 2007;10:321–330. doi: 10.1038/nn1854. [DOI] [PMC free article] [PubMed] [Google Scholar]