Abstract
Male Drosophila fruit flies acquire and defend territories in order to attract females for reproduction. Both, male-directed agonistic behavior and female-directed courtship consist of series of recurrent stereotypical components. Various studies demonstrated the importance of species-specific sound patterns generated by wing vibration as being critical for male courtship success. In this study we analyzed the patterns and importance of sound signals generated during agonistic interactions of male Drosophila melanogaster. In contrast to acoustic courtship signals that consist of sine and pulse patterns and are generated by one extended wing, agonistic signals lack sine-like components and are generally produced by simultaneous movements of both wings. Though intra-pulse oscillation frequencies (carrier frequency) are identical, inter-pulse intervals are twice as long and more variable in aggression signals than in courtship songs, where their precise temporal pattern serves species recognition. Acoustic signals accompany male agonistic interactions over their entire course but occur particularly often after tapping behavior which is a major way to identify the gender of the interaction partner. Since similar wing movements may either be silent or generate sound and wing movements with sound have a greater impact on the subsequent behavior of a receiver, sound producing wing movements seem to be generated intentionally to serve as a specific signal during fruit fly agonistic encounters.
Key words: acoustic signals, temporal patterns, frequency analysis, agonistic behavior, courtship, ethogram
Introduction
Species in the genus Drosophila show a considerable range of complex, context dependent behaviors consisting of stereotyped components. As two examples of intraspecific interactions, courtship and agonistic behavior were described in Drosophila almost a century ago1 and a large literature concerning different forms of courtship behavior in various subgroups of Drosophila is available (reviewed in refs. 2–4). Courtship of certain Drosophilid species includes so called “love songs” generated through vibrations of one extended wing by the males in close proximity to a conspecific female.5–7 These songs have been demonstrated to contain species-specific spectro-temporal patterns.8 Sounds are perceived by sensory neurons of Johnston's organ in the second segment of the fly antennae9,10 and the resonance frequency has been demonstrated to vary in a non-linear manner with sound intensity, thereby improving sensitivity for low song intensities.11–14
Courtship of Drosophila consists of a series of different behavioral steps,4,15,16 initiated by a male's orientation movement towards a female. Tapping of the female abdomen or thorax with one of the forelegs of a male is usually followed by extension of the wing located nearest to the female and the production of a courtship song through rapid vibration. These actions are followed by licking of the genitalia of the female and, finally, a copulation attempt by the male. The function of the courtship song is to slow down locomotion and increase receptivity of the female.17,18 Courtship songs of Drosophila melanogaster consist of two parts with distinct patterns, the pulse song and the sine song.19 Pulse songs are trains of single pulses consisting of one to three cycles that are produced with interpulse intervals (IPI, critical for species recognition and the effect on female behavior) of around 35 ms and a carrier frequency with energy between 200–280 Hz.20,21 The sine song is a continuous sinusoidal humming with a carrier frequency of approximately 160 Hz.22 Female flies can perform “rejection buzzes” coupled with kicking movements or extrusion of the ovipositor in order to fend off male copulation attempts if the male is not accepted as a mating partner.23,24
Like other organisms, Drosophila displays aggressive behavior to acquire or secure important resources including food, territory and mating partners.25,26 Although agonistic behavior of fruit flies was originally described by Sturtevant,1 it received relatively little attention for a long period of time. Fueled by a general interest in uncovering the genetic basis of the neural mechanisms underlying inherited behavior (reviewed in ref. 27) and the availability of sophisticated genetic and molecular methods, the agonistic behavior of Drosophila males and females has emerged recently as a prominent area of investigation.16,28–31 Like courtship, agonistic behavior consists of series of recurrent stereoptypical actions. The patterns observed have been associated with different levels of aggression and their frequency and duration have been used to quantify the intensities of fights.16 The characterization of distinct behavioral acts, especially of displays without physical interaction, still may be incomplete, however, as far as the signals used and their impact on the subsequent behavior of an opponent. As an example, the information content of the behavioral pattern generally described as “wing flicks”, which summarizes various kinds of vigorous wing movements in front of a rival,28,32 may differ according to the particular situation, the precise performance of the movement and the combination of wing flicks with other signals. While it is known that some of these movements can produce acoustic signals26,32,33 their characteristics and particular role in inter-male interactions have not been studied in detail.
In this study we recorded and analyzed sounds produced by males of Drosophila melanogaster during agonistic encounters and compared them with courtship songs. In addition, we determined when during aggressive encounters, acoustic signals were produced and whether these signals have an impact on the subsequent behavior in order to evaluate their information content.
Results
Sound recordings.
Drosophila melanogaster males readily compete for limited resources such as food and females and for acquisition and defense of territories. Their agonistic behavior consists of series of encounters that include a variety of stereo-typed offensive and defensive components.16,28,39,40 Agonistic interactions were continuously accompanied by acoustic signals of both opponents in all male-male pairings observed in this study (Fig. 1). In contrast to courtship songs of D. melanogaster, which have been analyzed in detail by various laboratories (see Introduction for refs.), only few studies on the structure of acoustic signals that this species generates during agonistic encounters exist (reviewed in refs. 26 and 33).
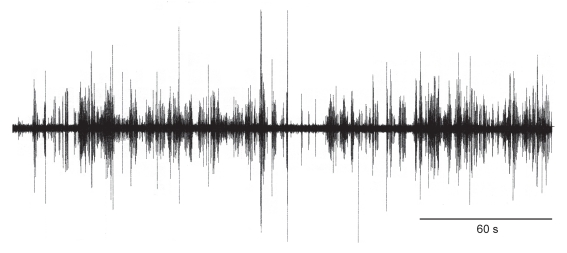
Figure 1.
Oscillogram of acoustic signals generated by two fighting Drosophila melanogaster males. During the 4-minute recording period, the males engaged in multiple encounters that included the behavioural acts approach, wing threat, wing flick, fencing, tussling, boxing, retreat and chasing.
To visualize the differences between courtship and aggressive acoustic signals, Figures 2 and 3 show oscillograms of sounds produced by male D. melanogaster during courtship of a female (Fig. 2) and during agonistic interactions with another male (Fig. 3).
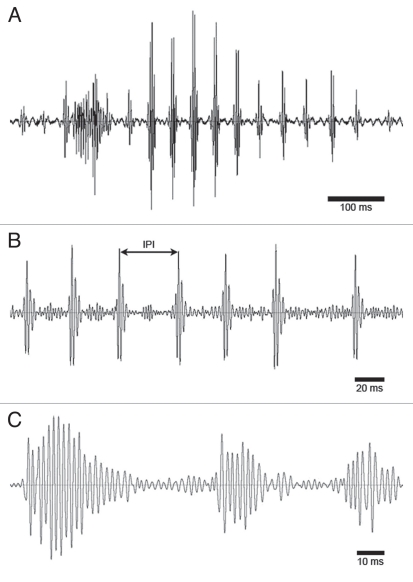
Figure 2.
Typical oscillograms of acoustic signals produced by male D. melanogaster in the course of courting a female. (A) Short (0.7 s) sequence of a courtship song with sine song in the beginning, followed by pulse song. (B) Train of pulses from a pulse song. Each pulse consist of three to four cycles; CF ∼340 Hz, IPI vary between 30 and 50 ms. (C) Sine song consisting of three distinct sine bouts; CF ∼500 Hz.
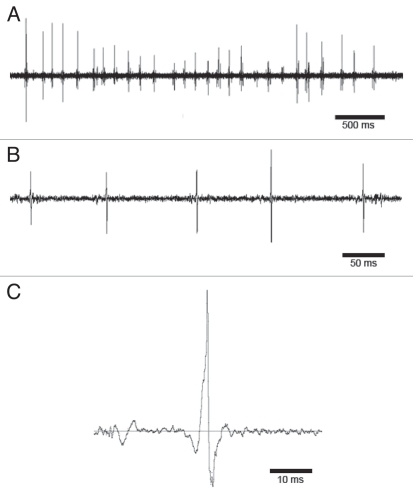
Figure 3.
Oscillograms of acoustic signals produced by male D. melanogaster during agonistic encounters. (A) Sequence of pulses produced by a male while interacting with the opponent. CF ∼300 Hz. (B) Higher magnification of a row pulses. Each pulse consists of 1–3 cycles; CF ∼300 Hz, IPIs vary between 90 and 110 ms. (C) One single pulse consisting of one cycle.
Courtship songs serve to slow down moving females and promote their copulatory readiness. They are generated by vibration of one extended wing and include two different patterns, the sine song and the pulse song (Fig. 2A). Pulse songs consist of individual pulses that are produced in regular sequences (IPIs vary between 30 and 50 ms) and typically consist of 3–4 cycles (Fig. 2B). Sine songs consist of bouts of larger numbers of sinusoidal wing oscillations that mediate a more prolonged hum (Fig. 2C). Sine songs and regular pulse songs were the only sounds produced by males in the presence of a female. Females occasionally produced rejection buzzes to interfere with male copulation attempts (not shown).
In contrast to courtship songs, acoustic signals that accompany agonistic interactions are usually generated by vibration of both extended wings. Figure 3A shows an oscillogram of a series of pulse-like acoustic signals generated by one male while it chased its opponent. Individual pulses consisted of 1–3 cycles and the IPIs in this particular recording varied between 90 and 110 ms (Fig. 3B and C).
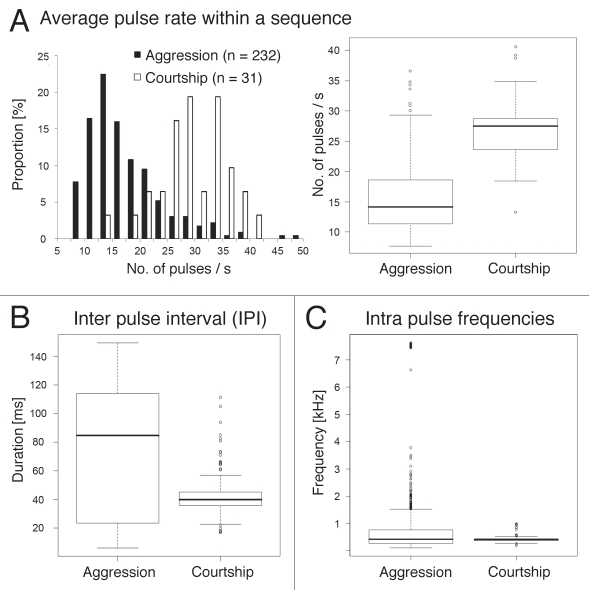
A major difference between courtship songs and acoustic signals during aggression was the lack of sine-like sounds during agonistic interactions. A comparison of pulse songs generated in both behavioral situations revealed additional differences (summarized in Fig. 4). Pulse rates within trains that contained at least four pulses (as those shown in Figs. 2A and 3A) varied over different ranges (Fig. 4A) and the median of pulse rates of aggression signals (14.1 s−1) was significantly different from the median of pulse rates of courtship pulse songs (27.5 s−1; Chi square test, p = 3.3 × 10−8). The IPI, a characteristic of courtship songs that is critical for species recognition and courtship success, was found to be much more variable in aggression signals (average IPI: 76 ± 45 ms, n = 1,170, median: 85 ms) than in courtship pulse songs (average IPI: 43 ± 14 ms, n = 196, median: 40 ms) (Fig. 4B), suggesting that the temporal arrangement of pulses may be less important in aggression signals. The average carrier frequencies of both song types were almost identical (median aggression song: 0.419 kHz; median courtship pulse song: 0.403 kHz) although variability was again larger in the aggression signals (Fig. 4C). A number of pulses generated during agonistic interactions contained high frequency components (up to 7.5 kHz), which are unlikely to result from the typical mode of sound production by wing vibration. Pulses with frequency components above 2 kHz were detected in 19 out of 205 evaluated agonistic songs but could not be associated with any particular condition of one or both opponents. Similar high-frequency acoustic signals were also recorded from wing-ablated D. melanogaster males (not shown). Since D. melanogaster auditory organs are tuned to lower frequencies (≤800 Hz depending on sound intensity), it is presently unknown whether these high frequency pulses are accidentally generated by movements accompanying agonistic interactions or may serve as a previously undescribed sound signal.
Figure 4.
Comparison of acoustic signals produced during male/male aggression and male/female courtship. (A) Relative distribution and box plot representation of average pulse rates within pulse sequences that contained at least four pulses. A pulse sequence contained series of pulses with an IPI of less than 150 ms. Median of aggression songs: 14.12 s−1, median of courtship songs: 27.48 s−1, Chi square test: χ2M = 30.51, p = 3.3 × 10−8. (B) Duration of IPI. Median of aggression songs: 85 ms (n = 1170), median of courtship songs: 40 ms (n = 196). Statistically different; Mann-Whitney U test, p < <10−4 (C) Intra pulse frequency. Median of aggression songs: 0.419 kHz (n = 1526), median of courtship songs: 0.403 kHz (n = 205). Not statistically different; Mann-Whitney U test, p = 0.56.
Both, courtship and agonistic behavior include the sequential performance of particular components in male D. melanogaster. In contrast to courtship, where sound production is restricted to the period between recognition of the female and her mating state by touching her body with the fore legs (“tapping”) and mounting the female, sounds are continuously produced during the progression of agonistic interactions from low to high intensities.
Ethogram and dyad analysis of agonistic behavior.
In order to associate sound production with particular components of agonistic behavior and to determine whether these sounds function as acoustic signals that influence the subsequent progress of fighting, an ethogram that included wing movements with accompanying sound production as one behavioral act was generated. Similar to previous studies by various authors, though not identical since sound production had not been included in other studies, ten behavioral acts were selected that were clearly distinguishable and appeared with sufficient frequencies to enable statistical evaluation (Table 1). Behavioral acts that promoted aggressive interactions (approach, chase), or involved close contact (boxing, tussling), were regarded as offensive while all forms of retreat were regarded as defensive behaviors.
Table 1.
Ethogram of male flies during agonistic encounters
| Behavior | Abbr. | Description |
| Approach | A | One fly advances towards the other; usually offensive |
| Chasing | Ch | One fly chases after the other; offensive |
| Courtship associated behaviour | CAB | Courtship elements exhibited towards a male (like licking or copulation attempts) |
| Doing nothing | DN | No (re)action visible |
| High-level aggression | HLA | A combination of several aggressive and offensive interactions between flies, including fencing, lunging, head butts, holding, tussling and boxing |
| Retreat | R | An animal tries to escape from the opponent either by walking, running or flying away; defensive |
| Tapping | T | A fly extends one leg and touches the body (legs, thorax, abdomen) of the opponent |
| Wing movements | WM | Includes every silent distinct movements of the wings like wing flick, wing waving, etc., excluding wing threats |
| Wing movements with sound | WMS | Includes the same movements like WM but they are accompanied by sound (usually structured sound pulses) |
| Wing threats | WT | Both wings raised to an angle of approx. 45° from the horizontal body axis; offensive when flies are facing each other, defensive if otherwise |
A matrix was generated that included all dyads consisting of a particular behavioral act by one fly and the subsequent behavior of the other fly. The matrix (Table 2) reflects how often a particular behavioral act of one fly was followed by a particular action of the other. The numbers in brackets represent the expected occurrence of the respective dyad if the ten behavioral acts of the ethogram had equal probability to follow the initial act of the opponent. Cell-wise examinations of the observed frequencies (Freeman-Tukey-deviates) revealed dyads occurring significantly more (light grey) or less (dark grey) often than expected in relation to the total numbers of their appearance. In line with an earlier study,16 some dyadic sequences of behavioral acts appeared with significantly enhanced or reduced frequencies in comparison with the relative proportion of their occurrence. To mention just a few: high level aggression only emerges when the opponent responds in the same way, approach of one male promotes approach of the opponent and retreat stimulates chasing and wing threats in the opponent.
Table 2.
Transition matrix showing the amount of observed two-act sequences (dyads)
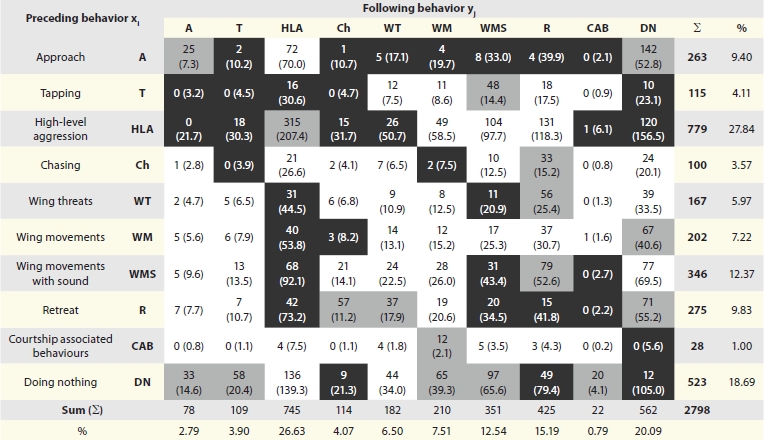 |
The preceding behavior xi is shown in rows, the following behavior in columns, numbers in brackets are expected values. Chi-square tests resulted in non-random distribution of the transitions with p < 0.0001. Light grey marks the cells for which Freeman-Tukey-deviates suggested significant overrepresentation, dark grey marks cells, which are under-represented and white cells are within the statistically expected range, assuming random distribution.
Wing movements with sound (WMS), the focus of the present study, were characteristically embedded into sequences of behavioral acts. They were unlikely to accompany approach but were highly likely stimulated in flies that were tapped by the opponent (Table 2). In particular, abdominal tapping invariably led to WMS (roughly five times more often than expected; data not shown), while tapping the opponent's thorax and legs was not particularly stimulating to initiate WMS. Since tapping another fly's body with the fore legs in order to recognize its gender is a male-specific behavior,4,41 a tapped male will also identify its counterpart as a male opponent. Therefore, aggression sounds are generated right after identification of a second fly as a male opponent. While Tapping was followed by WMS in 41% of its occurrences, high level aggression, chasing, wing movements and rarely observed courtship associated behaviors of one fly were followed by WMS of the other fly in 13%, 10%, 8% and 18% of their overall occurrences. WMS appeared less often than predicted by equal probability of subsequent behaviors in response to approach (3%), wing threats (7%), retreat (7%) and sound producing wing movements of the opponent (9%).
Following retreat of the opponent, wing movements with sound production were reduced. Silent wing movements were equal probability occurrences and the frequency of silent wing threats even was enhanced. This suggests that sounds may serve as acoustic signals in particular situations and are not an invariant by-product of wing movements. In line with this, sound generating wing movements frequently induced retreat of the opponent while silent wing movements were not particularly effective in this respect. A consequence of inducing retreats is the reduction of high-level aggression, which requires the willingness of both opponents to escalate. WMS also reduced the occurrence of courtship-associated behaviors and suppressed sound production by the opponent.
Dyads containing the act “doing nothing”, which had to be included to generate properly alternating actions and reactions of opponents, were not included in these considerations since it is not clear whether “doing nothing” is a true behavioral act or just the result of the rapid performance of two different acts by the same fly.
Information science analysis.
Using the information content of the transition matrix describing the frequency of occurrence of behavioral actions and their transitions, we were able to perform an information-theoretical analysis of the impact of the behavior of one fly on the subsequent behavioral action of the other. Table 3 provides an overview of the information gained by observing a certain behavior of one opponent (H), the information remaining unknown about the following behavior of the other opponent (conditional information), the reduction of uncertainty regarding the following action (normalized transmission) and the relative contribution of a behavioral action to the subsequent activity.
Table 3.
Results of the information-theoretical analysis of agonistic inter-male interactions in D. melanogaster
| Parameter | Transition | |
| xi→yj | ||
| Information H(X) | 2.932 bit | |
| Information H(Y) | 2.868 bit | |
| Conditional Information H(Y/X) | 2.638 bit | |
| Normalized Transmission t(X;Y) | 10.0% | |
| Relative Contribution P(xi) J(xi;Y) of the Preceding Behavior | ||
| Doing nothing | 0.073 | |
| Approach | 0.069 | |
| Retreat | 0.043 | |
| High level aggression | 0.037 | |
| Tapping | 0.025 | |
| Wing threat | 0.012 | |
| Courtship associated behavior | 0.011 | |
| Chasing | 0.009 | |
| Wing movements with sound | 0.009 | |
| Silent wing movements | 0.007 | |
Parameters are calculated for the transitions from one behavior xi to any other behavior yj. Behaviors are arranged according to their impact on the subsequent behavior of the opponent. Higher values represent a larger influence on the following action. For details about parameters, see also references 53 and 54.
The values H(X) and H(Y) for information gained when observing an act xi out of X (or yj out of Y) are high (2.93 and 2.87, respectively) concerning the maximal possible values (Hmax (X) = log2n = log210 ≈ 3,3 bit) for ten possible behavioral actions. This demonstrates that the information transferred from the sender of a signal to the receiver is large as is the uncertainty about the behavior displayed by the sender before it is observed.
That H(X) and H(Y) differ slightly from each other is likely due to small differences in probabilities P(xi) and P(yj) that result from unequal numbers of observed instances of xi and yj.
The conditional information remaining unknown about the following behavior Y is high and close to H(Y) if the preceding behavior X is known. This suggests only weak constraints between X and Y because many different behaviors yj can be observed after a certain behavior xi. The normalized transmission t(X;Y), derived from the conditional information H(Y/X), gives the amount of uncertainty about Y that is reduced by knowing X and therefore also provides some insights into the constraints between subsequent actions. In the case of inter-male aggression t(X;Y) is 10%, which corresponds to the high value of H(Y/X). This means that, overall, observing a behavior X only slightly reduces the uncertainty about which following act will be observed as a response.
Of more interest than the general values described above, is the impact of a particular behavioral act on the subsequent behavior of an opponent. These values are listed as relative contributions of proceeding to following behavioral acts in Table 3. High numbers represent strong influences on subsequent behavioral acts while low numbers indicate that the preceding behavior has only a weak influence on the opponent's response.
As already suggested by the results shown in the contingency matrix (Table 2), “approach” (A) had the largest impact on the subsequent behavior. This was expected since the approach of one fly (particularly to start an aggressive encounter) should be an important signal for another fly. Next in the order of impact on the actions of a receiver were “retreat” (R) leading to termination of an encounter, and the physical interactions “high level aggression” (HLA) and “tapping” (T). The latter two cannot be ignored by an opponent and therefore usually stimulate a reaction. The lowest impact on the response of an opponent is exerted by “wing movements” (WM). Sound generating wing movements (WMS) are slightly more effective in influencing interactions between opponents. This suggests that the acoustical component of WMS may indeed represent a signal used to transfer information from one fly to the other.
Discussion
Sexual and agonistic behaviors have been analyzed in considerable detail in various Drosophila species. Both behaviors consist of stereotyped components whose duration, intensity and exact sequence depend on the interplay of at least two individuals. Making use of the genetic amenability of Drosophila, certain genetic factors that determine the performance of these gender-specific social behaviors have been identified.29,30,32,42 Details about how genetic information determines the formation of the precise neural circuits involved in controlling species- and gender-specific behaviors remain unknown.
Like many other insects, Drosophila species generate acoustic communication signals during both reproductive and agonistic behavior. In this study, we recorded courtship and agonistic songs of wild type Drosophila melanogaster and identified parameters that are different between the two song types. Courtship songs are generated by vibrations of one extended wing and include two different patterns, sine song and pulse song. Pulses usually consist of 3–4 cycles and are generated at regular sequences with species typical IPIs43–45 or regular oscillations of IPI durations.8,44,46 In contrast, a particular posture for sound production, like the extended wing seen during courtship, was not observed during agonistic encounters. Instead sounds seemed to be generated by both wings (although the exact mechanism remains elusive until sound recordings are performed with accompanying high-speed video recordings). Aggression songs consisted exclusively of pulses that contained fewer cycles (1–3) and were generated with longer and more irregular intervals without a narrow range of IPIs. Similar patterns of agonistic pulse like songs that interrupted courtship songs were previously observed when two male D. melanogaster competed for one female.26 Differences between complex and highly regular courtship songs and less regular aggression songs also have been observed in some species of grasshoppers and crickets. The reason for this may lie in the need to transmit different information to different recipients. Courtship songs signal species identity to the female, thereby preventing hybridization between sympatric species43,44,47,48 and, possibly contributing to sexual selection by displaying features of male quality.49,50 Aggression songs accompany the territorial behaviors intended to acquire or secure potential resources. In Drosophila melanogaster a food source that attracts females for reproduction and oviposition is defended against competitors. The competitors however, may be of different sympatric species. Acoustic signals in this context, therefore, may not be required to display species identity using complex and accurately produced patterns, but may just signal aggressive intention to competitors competing for the same resource.
Acoustic signals also appear to represent an additional component of Drosophila melanogaster agonistic behavior. All agonistic encounters observed in the present study were almost continuously accompanied by pulsed sounds. Sound production was especially prominent following identification of the opponent as a male, either by tapping or being tapped with the fore legs. This and the observation that flies were able to produce similar wing movements with or without sounds, suggest that sound production represents an additional behavioral component used during agonistic interactions and is not just a by-product of other agonistic activities. Acoustic signals represent an additional part of the information that is continuously exchanged between opponents, thereby complementing visual threat signals, direct physical interactions and chemosensory cues.16,51,52
We also staged small numbers of fights between pairs of females without analyzing them in detail. In contrast to fights between males, fights between females were completely silent most of the time, except for a few short sound bouts towards the end of agonistic interactions. Females share some of the behavioral patterns seen in fights between males (e.g., fencing and lunging) but other patterns are sex selective (females perform head butts and shoves but no lunging, boxing and tussling). Also in contrast to males, female fights do not result in the establishment of hierarchical relationships.28 Thus the almost continuous production of pulsed acoustic signals seems to be another male specific component of D. melanogaster agonistic behavior.
We included sound generating wing movements (WMS) into the ethogram of Drosophila melanogaster agonistic behavior and analyzed the occurrence of this behavioral component within the sequences of previously analyzed activities. As demonstrated by the contingency table (Table 2), WMS were rarely associated with the approach of flies to each other, but were frequently induced by tapping. Tapping is thought to serve species- and sexdetermination by contact chemosensory information.4 Since only males tap other flies with their fore legs, this likely serves as yet another way that flies recognize their counterparts as males.
All behavioral components that included wing-derived signals (wing threat, wing movements with sound, silent wing movements) inhibited higher levels of aggression. Wing threats and sound producing wing movements both induced the retreat of the opponent, while silent wing movements were not particularly effective in this regard. Since retreat and escalation to high-level aggression are mutually exclusive, wing derived signals could serve as additional factors used in deciding the outcome of individual encounters during fights. Wing threats and sound producing wing movements can mediate de-escalation of fight intensity. Thereby they can help to reduce the transition to higher levels of fight intensity like boxing and tussling. This also has been recognized by earlier studies.16
To investigate further the role of the sounds produced during wing movements, we applied information theory to the behavioral outcomes shown in Table 2 to compute the contribution of single behavioral acts to the overall transmission of information during agonistic encounters. This approach incorporates all possible behavioral dyads and may reveal restrictions imposed by one behavioral pattern on the possible behavioral patterns displayed by a receiver. The results enabled us to investigate the effect of a sender's preceding behavior on the receiver (‘strength of a signal’) on the basis of information gained by the preceding action and thereby also by the amount of uncertainty reduced for the following behavior when the preceding one is known. Table 3 lists these contributions for each behavior in the ethogram. Since the values given indicate average relative contributions for a particular behavior to the next one, they are merely qualitatively interpreted with respect to the ranking of their impact.
The behavioral patterns of particular interest in this study, WMS (wing movements plus sounds) and WM (silent wing movements), showed a relatively small contribution to the overall transmission of information (0.009 and 0.007, respectively), indicating that these acts had relatively little impact on the following behavior. Thus wing movements are not particularly strong signals when compared to actions like approach, retreat or high level aggression. Since wing movements accompanied by sounds scored slightly higher than silent wing movements, these probably transmitted more (or different) information than the visual signal contributed by the same wing movements without sound (perhaps comparable to someone in a crowd either just waving in your direction or waving and calling your name at the same time). Thus, acoustics produced by D. melanogaster may be considered as communication signals, though they may only be effective in a cross-modal summation with corresponding wing movements serving as visual signals. In the cases examined here, such signals have little or no impact on other, stronger behavioral signals, like highly aggressive ones. Instead they seem to function as somewhat ‘subtle’ signs used to reduce aggression or unwanted contact in an encounter. This assumption is supported by the reduction of high level aggression and the increase of retreats seen after sound producing wing movements (but not after silent wing movements) and the high amount of sound producing wing movements after an animal is tapped by an opponent (Table 2; WMS→HLA, WMS→R and T→WMS).
Having demonstrated that D. melanogaster males use wing-derived sounds as acoustic signals during agonistic encounters, future studies on more territorial fruit fly species also should be performed. In addition, a detailed analysis of female rejection sounds23,24 and the acoustic signals generated during the final phases of female fights should help to establish a collection of gender- and situation- specific (courtship, agonistic, rejection) acoustic patterns in fruit flies.
Materials and Methods
Flies.
All experiments were conducted with laboratory stocks of wild type Drosophila melanogaster CantonS (CS). The animals were reared in 175 ml breeding vials (Greiner Bio-One GmbH) on approximately 2-cm thick layers of commercial Nekton-Drosophila-food concentrate (Gunter Enderle Nekton-Produkte, Pforzheim, Germany) prepared with tap water and vinegar. Rearing conditions included 25°C temperature, 65% relative humidity and 16:8 hours light:dark cycles.
Imagos were collected soon after eclosion and isolated in small glass jars (Scherf Präzision Europa GmbH; height: 3.5 cm; Ø = 2.5 cm) containing a few drops of food. Both males and females were held separately for two days. On the third day they were transferred to a new jar containing only water on a patch of filter paper and kept for another day in order to increase their readiness to subsequently aggregate on a small food patch in the arena.
All recordings of behavior and acoustic signals were performed between one and two hours after the fly's subjective “sunrise” in a sound proof chamber at 25°C ± 1°C. This period coincides with a phase of high locomotor activity that starts shortly before dawn.34
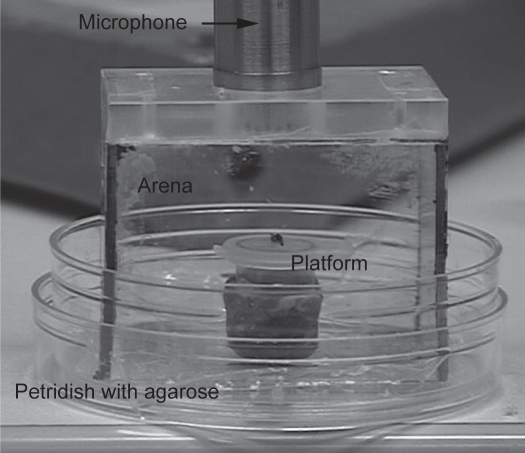
The arena.
Fly encounters were staged in an “arena” (Fig. 5), a square glass chamber built from microscope slides (dimensions: 4 cm length, 2.5 cm width and 2.5 cm height). This arena was placed on a petri dish (Ø = 5.5 cm), which contained a layer (5 mm) of 1% agarose to maintain constant humidity. The top of the arena consisted of a 5 mm thick Plexiglas plate with a hole in the middle (Ø = 1.8 cm) where the microphone could be inserted.
Figure 5.
Experimental setup for sound recording and videotaping of D. melanogaster agonistic interactions.
In the centre of the arena, directly beneath the microphone, the lid of an Eppendorf-cup on top of a small cube of modeling wax (1 cm in height) served as the stage for fly encounters. A small drop of fresh baker's yeast with a few grains of saccharose was placed in the middle of this “stage” to attract the flies and bring them into close vicinity to each other and to the microphone.
Sound and video recordings.
Two male flies were transferred into the arena via the hole at the top to interact for 30 minutes. The hole was sealed with a microphone (Brüel & Kjær, type 4133), connected to a series of pre- and main amplifiers (Brüel & Kjær, type 2669 and type 5935). The amplified sound signals were directly digitized via an Ensoniq AudioPCI ES1371 soundcard (Creative Labs) and stored as 22 kHz, 16 bit, mono wave-files on a windows PC. Data acquisition was supported by the program Cool Edit 2000 (Syntrillium Software).
Simultaneously with sound recordings, agonistic behavior was videotaped with a camcorder (Canon MV10), equipped with two macro lenses (Hama GmbH & Co., KG, +1 and +2 dioptres, respectively) for further magnification. The videotaped sequences were digitized via a Pinnacle DV500 Plus video card and further processed with the software Adobe Premiere 6.0.
Processing of sound- and video-files.
To synchronize the videos of the behavior with the corresponding high quality recordings of acoustic signals and their visual display as real-time oscillograms, the sound signals were first replayed with Cool Edit 2000 and a screen capture of the running oscillogram was taken with SnagIt7 (TechSmith). The oscillograms then were synchronized with the video recordings by alignment of optical and acoustical trigger signals generated during the original recordings. Using the video-editing software VirtualDub (www.virtualdub.org) and the frame server AviSynth (www.avisynth.org), videos of fly behavior, captured oscillograms and fly-generated sounds were stacked and merged into one data file. This file was subsequently used to associate sound signals with particular behavioral components and analyze their role in fly aggressive behavior.
Analysis of behavior.
Flies performed several encounters during the 30 minutes observation periods. Encounters started with the approach of one or both flies to a distance below two body lengths and were terminated either by one fly retreating or when both flies performed no visible activity at all for 2 seconds (reviewed in ref. 16). Behavioral actions within individual encounters were analyzed and used to build an ethogram of the flies' agonistic behavior (Table 1). Sequences of different actions during agonistic encounters were divided into two-act sequences (dyads) consisting of an action of one fly and the immediate reaction of the other. Since the flies did not strictly alternate their behavioral actions but also performed certain acts repeatedly while the opponent displayed no visible behavioral response, the act “doing nothing” was introduced in such cases to establish properly alternating dyads (reviewed in refs. 35–37).
For the analysis of agonistic behavior in D. melanogaster, 194 inter-male encounters with a total of approximately 1,500 single actions were split into two-act sequences (preceding action of one fly and following action of the other) and transferred to transition matrices (contingency tables). In order to test for non-randomness of the behavioral patterns Chi-square tests were applied. Likelihood-ratio tests (G-statistics) and Freeman-Tukey deviates were calculated to identify transitions that occurred more or less often than predicted by chance. These tests were performed using a collection of public domain Java Applets for the analysis of behavioral data by Robert Huber (available at http://caspar.bgsu.edu/~software/Java/Grinders.html).
To evaluate the behavioral actions accompanied by sounds and to determine whether the sound itself served as a communication signal with an impact on the receiver's subsequent behavior, the contingency tables were also used to calculate specific parameters based on information theory (reviewed in refs. 36 and 39). These parameters were:
H(X) as the entropy or information (as a measure of the average amount of uncertainty) received when a particular behavioral act xi out of the repertoire X (which represents all behaviors contained in the ethogram) is performed by the initiator of a dyad as the preceding act.
This parameter is calculated by using the formula
in which the repertoire X is made up of n categories of distinct behavioral acts x (x1, x2, … xn) and P(xi) describes the probability that the act xi out of X is performed.
Y represents all behaviors contained in the ethogram that potentially could follow the behavioral act x of the opponent fly. H(Y) describes the entropy or information received when an act yj out of the repertoire Y (which in this case equals X, since two male flies should have the same behavioral potential) is performed as the following act.
The information H(X) [or H(Y)] as defined above gives a measure for the average reduction of uncertainty if one behavioral act is performed. If the second behavior of a dyad is completely determined H(X) equals 0. If, in the other extreme, all acts are equiprobable reactions, the information gained by observing an act xi is maximal and reaches. Thus, the higher H(X), the more diverse and complex the sequence of behaviors can be.
While H(X) provides only an average measure of information of single actions, it is also possible to calculate the information for multivariable conditions, i.e., for the sequential behavior of two animals (data not shown here). This joint information
reflects the average amount of information of a dyadic behavioral sequence where P(xiyj) represents the probability that the act xi is followed by the act yj.
The joint information is then used to determine the conditional information remaining unknown about the following act if the preceding one is known. You can also address H (Y/X) = H (X, Y) − H (X) as the conditional uncertainty of the following act when the preceding one is known. This measure can never exceed H(Y) and equals H(Y) if the knowledge of the preceding behavior does not have any impact on the following behavior. If the conditional uncertainty or information reaches zero, the following act is totally determined by the preceding one.
To estimate the impact of a preceding action on the following behavior the normalized transmission
was calculated. Expressed in percentages, this value shows the extent to which uncertainty of Y is reduced by knowing X. The higher t(X;Y) the more knowledge you gain about the possible following act knowing the preceding one and the tighter these two are linked. Therefore, the normalized transmission could be interpreted as a measure of relatedness between X and Y.
To further investigate the constraints from a certain preceding behavior to the following (to measure the signal's strength), the relative contribution of distinct actions to the overall transmission, as defined by reference 35 as:
was determined. In this equation, J(xi,Y) describes the contribution of an act xi to a following act and P(yi/xi) states the conditional probability of an action yj if xi is known. P(xi)/(xi,Y) is thus the weighted average of the information transmitted from the sender performing xi towards the receiver.
The parameters described above, especially the last one, the relative contribution of a preceding behavior to the subsequent one, was used to ascertain whether the sound produced during agonistic interactions by one fly serves as a meaningful signal, as conveyor of information and thus as part of inter-individual communication.
Acknowledgements
Ralf Heinrich thanks Yick-Bun Chan and Steven P. Nilsen of the Kravitz laboratory at Harvard Medical School for their introduction to fruit fly handling during the initiation phase of the project. This work was partially supported by grants from the National Institute of General Medical Sciences (http://www.nigms.nih.gov/; GM-067645; GM-074675 to Edward A. Kravitz). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Sturtevant AH. Experiments on sex recognition and the problem of sexual selection in Drosophila. J Anim Behav. 1915;5:351–366. [Google Scholar]
- 2.Bastock M, Manning A. The courtship of Drosophila melanogaster. Behaviour. 1955;8:86–111. [Google Scholar]
- 3.Cobb M, Connolly K, Burnet B. Courtship behavior in the melanogaster species subgroup of Drosophila. Behaviour. 1985;95:203–231. [Google Scholar]
- 4.Greenspan RJ, Ferveur JF. Courtship in Drosophila. Annu Rev Genet. 2000;34:205–232. doi: 10.1146/annurev.genet.34.1.205. [DOI] [PubMed] [Google Scholar]
- 5.Shorey HH. Nature of the sound produced by Drosophila melanogaster during courtship. Science. 1962;137:677–678. doi: 10.1126/science.137.3531.677. [DOI] [PubMed] [Google Scholar]
- 6.Schilcher FV. The function of pulse and sine song in the courtship of Drosophila melanogaster. Anim Behav. 1976;24:622–625. [Google Scholar]
- 7.Hall JC. The mating of a fly. Science. 1994;264:1702–1714. doi: 10.1126/science.8209251. [DOI] [PubMed] [Google Scholar]
- 8.Kyriacou CP, Hall JC. The function of courtship song rhythms in Drosophila. Anim Behav. 1982;30:794–801. [Google Scholar]
- 9.Ewing AW. Complex courtship songs in the Drosophila funebris species group: escape from an evolutionary bottleneck. Anim Behav. 1979;27:343–349. [Google Scholar]
- 10.Eberl DF, Hardy RW, Kernan MJ. Genetically similar transduction mechanisms for touch and hearing in Drosophila. J Neurosci. 2000;59:81–88. doi: 10.1523/JNEUROSCI.20-16-05981.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Göpfert MC, Robert D. The mechanical basis of Drosophila audition. J Exp Biol. 2002;205:1199–1208. doi: 10.1242/jeb.205.9.1199. [DOI] [PubMed] [Google Scholar]
- 12.Göpfert MC, Robert D. Motion generation by Drosophila mechanosensory neurons. Proc Natl Acad Sci USA. 2003;100:5514–5519. doi: 10.1073/pnas.0737564100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kernan MJ. Mechanotransduction and auditory transduction in Drosophila. Pflugers Arch. 2007;454:703–720. doi: 10.1007/s00424-007-0263-x. [DOI] [PubMed] [Google Scholar]
- 14.Tauber E, Eberl DF. Acoustic communication in Drosophila. Behav Processes. 2003;63:197–210. [Google Scholar]
- 15.Markow TA, Hanson SJ. Multivariate analysis of Drosophila courtship. Proc Natl Acad Sci USA. 1981;78:430–434. doi: 10.1073/pnas.78.1.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen S, Lee AY, Bowens NM, Huber R, Kravitz EA. Fighting fruit flies: a model system for the study of aggression. Proc Natl Acad Sci USA. 2002;99:5664–5668. doi: 10.1073/pnas.082102599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ewing AW. Functional aspects of Drosophila courtship. Biol Rev. 1983;58:275–292. [Google Scholar]
- 18.Crossley SA, Bennet-Clark HC, Evert HT. Courtship song components affect male and female Drosophila differently. Anim Behav. 1995;50:827–839. [Google Scholar]
- 19.Bennet-Clark HC, Dow M, Ewing AW, Manning A, von Schilcher F. Courtship stimuli in Drosophila melanogaster. Behav Genetics. 1976;6:93–95. doi: 10.1007/BF01065681. [DOI] [PubMed] [Google Scholar]
- 20.Cowling DE, Burnet B. Courtship songs and genetic control of their acoustic characteristics in sibling species of the Drosophila melanogaster subgroup. Anim Behav. 1981;29:924–935. [Google Scholar]
- 21.Rybak F, Aubin T, Moulin B, Jallon JM. Acoustic communication in Drosophila melanogaster courtship: Are pulse- and sine-song frequencies important for courtship success? Canadian J Zool. 2002;80:987–996. [Google Scholar]
- 22.Wheeler DA, Fields WL, Hall JC. Spectral analysis of Drosophila courtship songs: D. melanogaster, D. simulans and their interspecific hybrid. Behav Genet. 1988;18:675–703. doi: 10.1007/BF01066850. [DOI] [PubMed] [Google Scholar]
- 23.Ewing AW, Bennet-Clark HC. The courtship songs of Drosophila. Behaviour. 1968;31:288–301. [Google Scholar]
- 24.Paillette M, Ikeda H, Jallon JM. A new acoustic signal of the fruit-flies Drosophila simulans and D. melanogaster. Bioacoustics. 1991;3:247–254. [Google Scholar]
- 25.Kravitz EA, Huber R. Aggression in invertebrates. Curr Opin Neurobiol. 2003;13:736–743. doi: 10.1016/j.conb.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 26.Tauber E, Eberl DF. The effect of male competition on the courtship song of Drosophila melanogaster. J Insect Behavior. 2002;15:109–120. [Google Scholar]
- 27.Baier A, Wittek B, Brembs B. Drosophila as a new model organism for the neurobiology of aggression? J Exp Biol. 2002;205:1233–1240. doi: 10.1242/jeb.205.9.1233. [DOI] [PubMed] [Google Scholar]
- 28.Nilsen SP, Chan YB, Huber R, Kravitz EA. Gender-selective patterns of aggressive behavior in Drosophila melanogaster. Proc Natl Acad Sci USA. 2004;101:12342–12347. doi: 10.1073/pnas.0404693101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vrontou E, Nilsen SP, Demir E, Kravitz EA, Dickson BA. fruitless regulates aggression and dominance in Drosophila. Nat Neurosci. 2006;9:1469–1471. doi: 10.1038/nn1809. [DOI] [PubMed] [Google Scholar]
- 30.Dierick HA, Greenspan RJ. Molecular analyses of flies selected for aggressive behavior. Nat Genet. 2006;38:1023–1031. doi: 10.1038/ng1864. [DOI] [PubMed] [Google Scholar]
- 31.Dierick HA, Greenspan RJ. Serotonin and neuropeptide F have opposite modulatory effects on fly aggression. Nat Genet. 2007;39:678–682. doi: 10.1038/ng2029. [DOI] [PubMed] [Google Scholar]
- 32.Villella A, Gailey DA, Berwald B, Ohshima S, Barnes PT, Hall JC. Extended reproductive roles of the fruitless gene in Drosophila melanogaster revealed by behavioral analysis of new fru mutants. Genetics. 1997;147:1107–1130. doi: 10.1093/genetics/147.3.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Popov AV, Peresleni AI, Ozerskii PV, Shchekanov EE, Savvateeva-Popova EV. The role of the flabellar and ellipsoid bodies of the central complex of the brain of Drosophila melanogaster in the control of courtship behavior and communicative sound production in males. Neurosci and Behav Physiol. 2005;35:741–750. doi: 10.1007/s11055-005-0118-x. [DOI] [PubMed] [Google Scholar]
- 34.Helfrich-Forster C. The circadian system of Drosophila melanogaster and its light input pathways. Zoology. 2002;105:297–312. doi: 10.1078/0944-2006-00074. [DOI] [PubMed] [Google Scholar]
- 35.Steinberg JB, Conant RC. An information analysis of the intermale behaviour of the grasshopper Chortophaga viridifasciata. Anim Behav. 1974;22:617–627. [Google Scholar]
- 36.Steinberg JB. Information theory as an ethological tool. In: Hazlett BA, editor. Quantitative Methods in the Study of Animal Behavior. New York: Academic Press; 1977. pp. 47–74. [Google Scholar]
- 37.Oden N. Partitioning dependence in nonstationary behavioral sequences. In: Hazlett BA, editor. Quantitative Methods in the Study of Animal Behavior. New York: Academic Press; 1977. pp. 203–220. [Google Scholar]
- 38.Shannon CE, Weaver W. The Mathematical Theory of Communication. Urbana: University of Illinois Press; 1949. [Google Scholar]
- 39.Hoffmann AA. A laboratory study of male territoriality in the sibling species Drosophila melanogaster and D. similans. Anim Behav. 1987;35:807–818. [Google Scholar]
- 40.Hoyer SC, Eckart A, Herrel A, Zars T, Fischer SA, Hardie SL, et al. Octopamine in male aggression of Drosophila. Curr Biol. 2008;18:159–167. doi: 10.1016/j.cub.2007.12.052. [DOI] [PubMed] [Google Scholar]
- 41.Manoli DS, Baker BS. Median bundle neurons coordinate behaviours during Drosophila male courtship. Nature. 2004;430:564–569. doi: 10.1038/nature02713. [DOI] [PubMed] [Google Scholar]
- 42.Lee G, Hall JC. A newly uncovered phenotype associated with the fruitless gene of Drosophila melanogaster: aggression-like head interactions between mutant males. Behav Genet. 2000;30:263–275. doi: 10.1023/a:1026541215546. [DOI] [PubMed] [Google Scholar]
- 43.Bennet-Clark HC, Ewing AW. Pulse interval as a critical parameter in courtship song of Drosophila melanogaster. Anim Behav. 1969;17:755–759. [Google Scholar]
- 44.Ritchie MG, Halsey EJ, Gleason JM. Drosophila song as a species-specific mating signal and the behavioural importance of Kyriacou and Hall cycles in D. melanogaster song. Anim Behav. 1999;58:649–657. doi: 10.1006/anbe.1999.1167. [DOI] [PubMed] [Google Scholar]
- 45.Moulin B, Rybak F, Aubin T, Jallon JM. Compared ontogenesis of courtship song components of males from the sibling species, D. melanogaster and D. simulans. Behav Genet. 2001;31:299–308. doi: 10.1023/a:1012231409691. [DOI] [PubMed] [Google Scholar]
- 46.Alt S, Ringo J, Talyn B, Bray W, Dowse H. The period gene controls courtship song cycles in Drosophila melanogaster. Anim Behav. 1998;56:87–97. doi: 10.1006/anbe.1998.0743. [DOI] [PubMed] [Google Scholar]
- 47.Elsner N, Popov AV. Neuroethology of acoustic communication. Adv Insect Physiol. 1978;13:229–355. [Google Scholar]
- 48.Helversen Ov, Helversen Dv. Forces driving co-evolution of song and song recognition in grasshoppers. Prog Zool. 1994;39:253–284. [Google Scholar]
- 49.Gray DA. Female house crickets, Acheta domesticus, prefer the chirps of large males. Anim Behav. 1997;54:1553–1562. doi: 10.1006/anbe.1997.0584. [DOI] [PubMed] [Google Scholar]
- 50.Klappert K, Reinhold K. Acoustic preference functions and sexual selection on the male calling song in the grasshopper Chorthippus biguttulus. Anim Behav. 2003;65:225–233. [Google Scholar]
- 51.Svetec N, Ferveur JF. Social experience and pheromonal perception can change male-male interactions in Drosophila melanogaster. J Exp Biol. 2005;208:891–898. doi: 10.1242/jeb.01454. [DOI] [PubMed] [Google Scholar]
- 52.Rybak F, Sureau G, Aubin T. Functional coupling of acoustic and chemical signals in the courtship behaviour of the male Drosophila melanogaster. Proc R Soc B. 2002;269:695–701. doi: 10.1098/rspb.2001.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hazlett BA. Quantitative Methods in the Study of Animal Behaviour. New York: Academic Press; 1977. [Google Scholar]
- 54.McCowan B, Hanser SF, Doyle LR. Quantitative tools for comparing animal communication systems: information theory applied to bottlenose dolphin whistle repertoires. Anim Behav. 1999;100:409–419. doi: 10.1006/anbe.1998.1000. [DOI] [PubMed] [Google Scholar]