Abstract
Definitive evidence on the impact of MnSOD/SOD2-deficiency and the consequent effects of high flux of mitochondrial reactive oxygen species (ROS) on pre-natal/pre-adult development has yet to be reported for either Drosophila or mice. Here we report that oocytes lacking maternal SOD2 protein develop into adults just like normal SOD2-containing oocytes suggesting that maternal SOD2-mediated protection against mitochondrial ROS is not essential for oocyte viability. However, the capacity of SOD2-null larvae to undergo successful metamorphosis into adults is negatively influenced in the absence of SOD2. We therefore determined the impact of a high superoxide environment on cell size, progression through the cell cycle, cell differentiation and cell death and found no difference between SOD2-null and SOD2+ larva and pupa. Thus loss of SOD2 activity clearly has no effect on pre-adult imaginal tissues. Instead, we found that the high mitochondrial superoxide environment arising from the absence of SOD2 leads to the induction of autophagy. Such autophagic response may underpin the resistance of pre-adult tissues to unscavenged ROS. Finally, while our data establish that SOD2 activity is less essential for normal development, the mortality of Sod2−/− neonates of both Drosophila and mice suggests that SOD2 activity is indeed essential for the viability of adults. We therefore asked if the early mortality of SOD2-null young adults could be rescued by activation of SOD2 expression. The results support the conclusion that the early mortality of SOD2-null adults is largely attributable to the absence of SOD2 activity in the adult per se. This finding somewhat contradicts the widely held notion that failure to scavenge the high volume of superoxide emanating from the oxidative demands of development would be highly detrimental to developing tissues.
Key words: mitochondria, MnSOD, development, autophagy, Drosophila, ROS
Introduction
The growth and development of multicellular eukaryotes is fueled by oxygen (O2)-dependent high-energy metabolism. Indeed, the extent to which aerobic organisms go to insure adequate oxygen supply to developing young attests to the host of injurious effects that can arise from oxygen deprivation during development. Ironically, the pathways of oxygen utilization in mitochondrial respiration and oxidative phosphorylation also generate copious amounts of the unstable superoxide radical1 which is rapidly and spontaneously reduced to other reactive oxygen species (ROS) of which some are highly toxic and cause irreparable cellular damage. Accretion of such unrepaired ROS-mediated cellular damage is causally implicated in many human diseases and in processes linked to organismal aging.2–5 Moreover, accumulation of ROS-damaged mitochondria puts the organism at a much higher risk for a variety of pathophysiological conditions and for reduced cognitive and physiological functions underpinning overall fitness.6,7
Much of what we know about the role of ROS in multicellular eukaryotes has come from studies of adult-stage organisms. Some of the most informative of these studies have focused on the phenotypic consequences of genetic deficiency for enzymes that function to detoxify specific ROS. For example, genetic ablation of SOD2, a manganese-containing superoxide dismutase and the principal scavenger of mitochondrial superoxide, negatively affects the survival of organisms as diverse as yeast,8,9 Drosophila10,11 and mice.12,13 SOD2-deficient pups from SOD2 mutant strains of mice present a multitude of cell and tissue damage culminating in complete neonatal mortality.12,13 Importantly, present evidence suggests that the lack of SOD2 does not overtly affect the prenatal health or viability of Sod2−/− mouse embryos when compared to their SOD2-positive (Sod2+/+, Sod2+/−) littermates.13
Likewise, SOD2-null mutants of Drosophila exhibit complete adult mortality within a day of pupal eclosion.10 However, definitive evidence on the impact of SOD2-deficiency on pre-natal/preadult development has yet to be reported for either Drosophila or mice. Here we report data, which lead to the conclusion that pre-adult development in Drosophila is less susceptible to high ambient mitochondrial superoxide arising from the complete absence of SOD2. These results, together with the mammalian data, above, allow us to hypothesize that while SOD2-mediated scavenging of mitochondrially-generated superoxide is in fact required for post-natal (post-eclosion) survival in animal taxa as widely divergent as mammals and holometabolous insects, its function is less required for normal prenatal (pre-eclosion) development. If confirmed, this hypothesis would serve to identify an important transition between pre-and post-natal (post-eclosion) development in animals generally in the generation and/or metabolism ROS.
Results
Maternally contributed SOD 2 is not essential for embryogenesis.
Earlier studies showed that Drosophila oocytes normally accumulate a large store of Sod2 transcripts.14 To investigate whether embryos contain SOD2 protein and if so, whether it is of maternal vs. embryonic origin, we used a SOD2-specific antibody to identify SOD2 protein in Sod2−/− and Sod2+/− embryos produced from Sod2+/− females. Figure 1 shows the presence of copious amounts of SOD2 protein in both Sod2−/− and Sod2+/− embryos, indicating that some if not all of the SOD2 protein in embryos is of maternal origin. A plausible explanation for the stockpiling of SOD2 by oocytes is to provide protection from oxidative damage until the zygotic transcription of Sod2 begins. To test this possibility, we made germ cell (oocyte) clones devoid of maternal SOD2 and followed the development of these eggs to adulthood (Fig. 2). To our surprise, oocytes lacking maternal SOD2 protein develop into adults and the recovery of a large number of Sod2n283 adult animals (total of 67) proves this point beyond any doubt (Fig. 2C). We therefore conclude that maternal SOD2-mediated protection against mitochondrial ROS is not essential during the process of oocyte development.
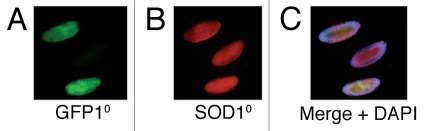
Figure 1.
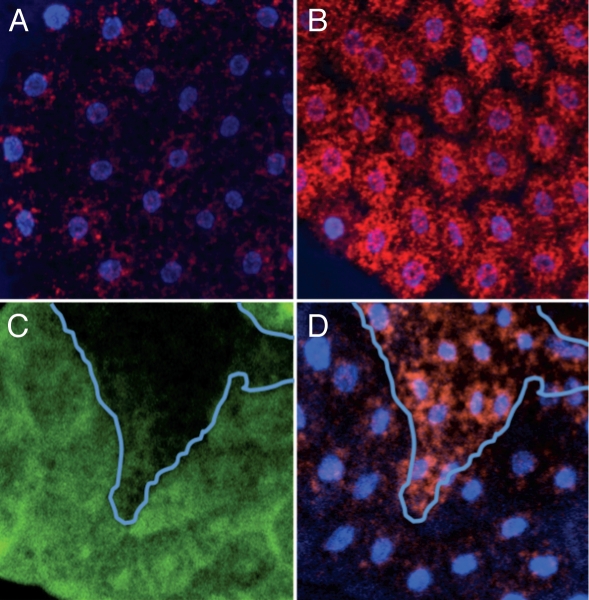
Maternal transmission of SOD2 protein. Shown here are three developing embryos obtained from a heterozygous Sod2n283/+ mother. (A) Two of the three embryos express GFP by virtue of a GFP-marked, Sod2+ Balancer chromosome. The GFP negative embryo should be SOD2-null (Sod2n283/Sod2n283). (B) The same embryos stained with anti-SOD2 antibody (red). The presence of SOD2 protein in the Sod2n283/Sod2n283 embryo, indicates maternal transmission from the SOD2n283/+ mother. (C) Merge with DAP I (blue). Genetic constructs are described in the Materials and Methods section.
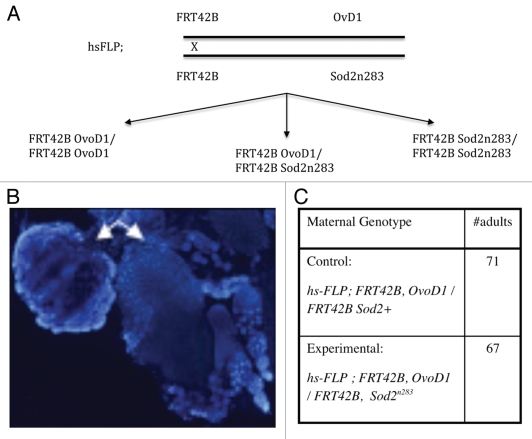
Figure 2.
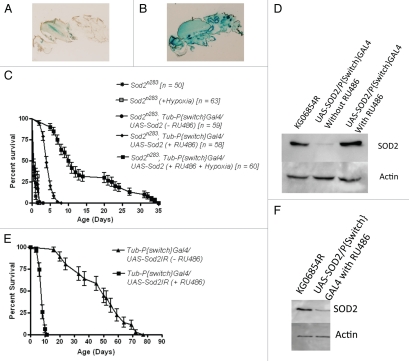
Making SOD2-null oocytes. (A) Mitotic recombination was induced in premeiotic germ cells using a standard FLP-based recombination system. (B) Success of the recombination system is demonstrated when ovarioles on the left appear dystrophic and carry no functional eggs due to the presence of the dominant OvoD1 mutation. Ovarioles on the right are (Sod2n283/Sod2n283) and grow normally within a fully formed germarium. Ovaries are stained with DAPI. (C) Maternal genotypes indicate that if adult animals appeared from the experimental mother then they must arise from the Sod2n283 eggs, since the OvoD1 carrying eggs will degenerate. That sufficient (67) adult progeny were recovered from the Sod2n283 eggs indicates that absence of SOD2 in the oocytes does not impede fertilization and subsequent development to adulthood. Beyond this, the actual numbers of adult animals recovered in the control (71) and the experimental (67) crosses bear no quantitative significance.
We then asked how long maternal SOD2 actually persists during development. By western analysis, we are unable to detect maternal SOD2 from the third larval instar onwards (Fig. 3). We then determined the capacity of SOD2-null larvae to undergo successful metamorphosis into adults. Interestingly, complete lack of SOD2 has some influence on metamorphosis as evident from the failure of significant number of Sod2n283 pupae to turn into adult flies (Table 1). However, Sod2n283/+ heterozygotes show no significant influence on metamorphosis (Table 1).
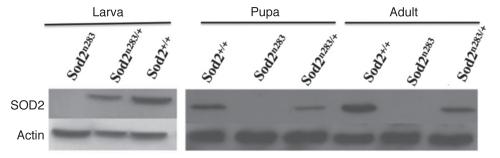
Figure 3.
Developmental loss of maternal SOD2. Western analysis with anti-SOD2 antibody shows that maternal SOD2 does not persist into the third larval instar onwards in Sod2n283 progeny derived from a Sod2n283/CyO, GFP stock.
Table 1.
Pre-adult development in the absence of SOD2
| Larvae | Pupae | Adults | |||||||
| Dead | Alive | Total | Dead | Alive | Total | Dead | Alive | Total | |
| KG0654R (Control) | 0 | 250 | 250 | 0 | 250 | 250 | 23 | 227 | 250 |
| Sod2n283/Cyo | 0 | 250 | 250 | 4 | 246 | 250 | 33 | 213 | 246 |
| Sod2n283 | 0 | 250 | 250 | 16 | 234 | 250 | 37 | 197 | 234 |
At 95% confidence interval significantly less number of adults emerged out from Sod2n283 pupae compared to KG08654R control (χ2 = 4.87, p = 0.05). However, no significant difference in adult metamorphosis was noted between the KG08654 and Sod2n283/Cyo (χ2 = 2.199, p = 0.05) and between the Sod2n283/Cyo and Sod2n283 (χ2 = 0.553, p = 0.05).
Development and differentiation are not perturbed in high ROS environment.
We then asked if we could find evidence of cell or tissue damage, which although perhaps insufficient to hinder development, would be evidence of ROS-mediated pathology. Dihydroxy ethidium (DHE) is an efficient and selective detector of superoxide in vivo, which acts by emitting a red fluorescence in the presence of superoxide.15 DHE staining of fat body, brain and eye discs tissues revealed that steady-state levels of tissue superoxide are significantly enhanced in SOD-null larva and pupa as compared to the controls (Fig. 4A–D).
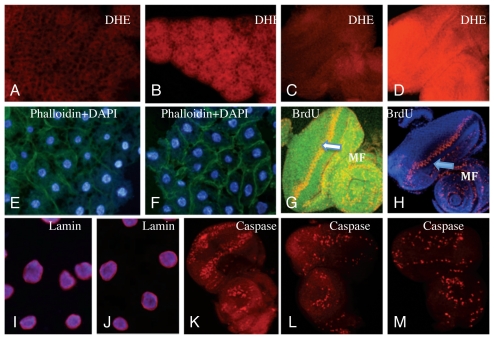
Figure 4.
Assessment of superoxide content and tissue damage in SOD2-null larvae. (A–D) DHE staining of developing fat body (A and B) and eye disc (C and D) from Sod2n283 larvae indicates high steady-state levels of superoxide compared to Sod2+ controls. (A and C) KG06854R (Sod2+); (B and D) Sod2n283. Phaloidin staining, which demarcates cell boundaries, shows similar cell sizes in Sod2n283 (E) and Sod2+ control (F). BrdU incorporation, which identifies the S-phase of the cell cycle, demonstrates that cell cycle progression in the morphogenetic furrow (arrow heads) appears normal in Sod2+ control (G) and Sod2n283 eye discs (H). Induction of apoptosis. The nuclear lamin, which appears as a purple halo around the nucleus, remains intact in Sod2n283 (J) compared to the Sod2+ control (I). The induction of apoptosis by activation of the hid gene expression in the eye disc generates an abundance of caspase positive cells as shown by the use of an activated caspase antibody (K). The same activated caspase antibody picks up only a few caspase positive cells in Sod2n283 (M), which is no more than in the Sod2+ control (L).
To evaluate the pathological potential of this high superoxide environment in vivo, we compared tissues from SOD2-null and SOD2+ larvae and pupae using four different parameters of cell size, cell growth and differentiation, cell death and autophagy. To determine the impact of a high superoxide environment on cell size, we used phalloidin staining of fat body from SOD2-null and SOD2+ larva (Fig. 4E and F) and pupa: no apparent difference in cell size was detected. To determine the effect on cell growth and differentiation, we used Drosophila eye disc tissue, which develops from 6–23 epithelial cells that are set aside at the cellular blastoderm stage. These disc mother cells proliferate to form up to 9,700 cells by late third instar larval stage and give rise to all ommatidial precursors.16 The proliferative capacity of ommatidial cells from SOD2-null and SOD2+ larvae was compared following BrdU incorporation. Our data indicate that neither the progression of the cell cycle nor the cell differentiation events are affected by the absence of SOD2 (Fig. 4G and H). In addition to the eye disc we also compared cell proliferation in the brain and found no effect of the lack of SOD2 on brain cell proliferation (not shown).
We then looked for an association between the high superoxide environment in tissues of SOD2-null larvae and in situ cell death using two well defined programmed cell death reporters for Drosophila: breakdown of nuclear Lamin protein17 and activation of cellular caspase activity.18 Fat body cells obtained from SOD2-null larvae show no signs of nuclear lamin degradation; the lamin signal appeared as a well-defined halo around the nuclei similar to the control (Fig. 4I and J). With regard to caspase activation, the cell death effecter gene hid19 causes extensive cell death, therefore many cells appeared positive for active caspase (Fig. 4K). Some natural caspase activity appears in the developing eye disc of Drosophila in a temporally restricted pattern.18 SOD2-null eye discs exhibit a level of caspase activity equivalent to the controls, thereby indicating no additional cell death activity arising from the absence of SOD2 (Fig. 4L and M). In similar experiments, no additional caspase activity was seen in the Sod2-null brain (not shown). In summary, our data suggests that the high superoxide environment of SOD2-null tissues does not promote cell death activity in developing tissues.
Unscavenged mitochondrial superoxide promotes autophagy.
Stress conditions such as starvation or oxidative stress are known to promote autophagy,20–24 a process that also happens naturally during metamorphosis.25,26 Autophagy, which is mediated through lysozomes, is fundamentally a catabolic process for eliminating or recycling damaged macromolecules within cells.27–30 We anticipated that the high flux of superoxide radicals might cause protein damage and consequently autophagic induction in Sod2n283. Upon induction of autophagy, lysosomal vesicles and autophagosomes appear as punctate structures in the cytoplasmic compartment when stained with lysotracker, a lysosome specific fluorescent dye.23 We used lysotracker staining to monitor induction of autophagy in SOD-null tissue. Fat body cells from SOD2-null larvae show marked accumulation of lysosomal puncta compared to the SOD2+ control where fewer autophagic vesicles are evident (Fig. 5A and B). To further establish this point, Sod2−/− cell clones were generated in a background of Sod2+/− cells in the fat body tissue by mitotic recombination. Lysotracker staining shows that the lysosomal puncta are restricted to the Sod2−/− clone (Fig. 5C and D) confirming that high levels of unscavenged mitochondrial superoxide lead to autophagy.
Figure 5.
Absence of SOD2 induces autophagy. Lysotracker staining shows few lysosomal puncta in the control fat body cells from third instar larvae (A) where as numerous lysotracker positive cells appear in the Sod2n283 (B). Sod2n283 cell clones (GFP negative area) are induced by mitotic recombination (C). Lysotracker positive cells appear heavily in Sod2n283 cell clones (D) suggesting that absence of SOD2 induces autophagy.
SOD2 activity is essential for adult viability.
While the above data establish that lack of SOD2 activity has only a limited effect on developmental processes, it is equally well established from the premature deaths of Sod2−/− neonates that SOD2 activity is essential for adult viability. In principle, the inviability of SOD2-null adults could originate either from (a) latent damage to imaginal tissues arising from inadequate superoxide scavenging during larval and pupal life, or from (b) a vital requirement for SOD2 activity that arises within and is confined specifically to post-eclosion adults. Observations presented above already eliminated the first possibility. In order to investigate the second possibility we activated ubiquitous expression of SOD2 in newly-eclosed SOD2-null (Sod2n283) adults. Using a Tubulin-P[Switch] GAL4 driver, GAL4-dependent expression of a UAS-linked Sod2+ transgene was induced by feeding the P[Switch] activator, RU486 (Fig. 6A and B) and the expression of SOD2 protein in Sod2n283; Tubulin-P[Switch]/UAS-SOD2 adults was confirmed (Fig. 6D). Activation of SOD2 expression in Sod2n283 adults improves their survival compared to the un-induced controls of the same genotype (Fig. 6C). On average, Sod2n283;Tubulin-P[Switch]GAL4/UAS-SOD2 adults survive up to five days (mean life span), with a maximum survival up to eight days (Fig. 6C). This is a marked improvement over the survival of Sod2n283 with mean and maximum life spans of 0.5 and one day, respectively.10 This data supports the conclusion that adult viability is dependent on SOD2 activity in the adult per se. It is worth noting that activation of SOD2 expression in Sod2n283 adults does not fully restore normal lifespan. One possible reason could be that SOD2 is not completely non-essential during metamorphosis, which is now documented (Table 1). The other reason may be due to the inadequacy of the P[Switch] system to fully restore SOD2 activity during acute demand or due to the possible contribution of injury in pre-adult imaginal tissues is not discernible from this data. Clearly, SOD2 activity in the post-eclosion adult per se is required for normal adult lifespan.
Figure 6.
Activation of SOD2 expression in Tub P[Switch]GAL4/UAS-Sod2 in Sod2n283 adults improves survival. (A) Background levels of LacZ reporter without RU486 feeding of Tub P[Switch]GAL4/UAS-LacZ flies and (B) induction of lacZ expression in all tissues following RU486 feeding demonstrates the adult restricted expression of the P[Switch] element. (C) Activation of SOD2 expression in Sod2n283 with the Tub P[Switch] driver extends the mean adult life span of these flies from less than 24 hrs to about five days. Further extension of Sod2n283 adult survival is possible by keeping the SOD2 activated flies under hypoxia (5% O2). These flies now survive up to 21 days (mean life span). Neither hypoxia nor RU486 feeding alone significantly influences the survival of Sod2n283 adults. (D) Evidence from western blot analysis of SOD2 expression in Sod2n283 following RU486 feeding. (E) Suppression of SOD2 expression in adults by activating a UAS-SOD2IR (Sod2RNAi) with the Tub P[Switch]GAL4 driver leads to a significant reduction in adult life span. (F) Significant suppression of SOD2 expression in Sod21R.
To investigate this point further, we used the gene switch system in conjunction with Sod2RNAi11 to inactive the expression of SOD2 in Sod2+ adults. Activation of an UAS-SOD2IR transgene with the Tubulin-P[Switch]GAL4 driver in newly-eclosed adults produces a sharp reduction in SOD2 expression (Fig. 6F) and a precipitous early adult mortality equivalent to that seen in SOD2-null mutant adults (Fig. 6E). Together, these manipulations of SOD2 expression support the conclusion that the early mortality of SOD2-null adults is largely if not fully attributable to the absence of SOD2 activity in the adult per se and definitely not to the latent effect of the lack of SOD2 activity in pre-adult imaginal tissues.
We believe that the feeding-dependent mechanism of GeneSwitch induction remains the main obstacle toward a complete rescue of Sod2n283 life span because a critical but unavoidable window of non-protection will always exist between eclosion, feeding and activation of SOD2 expression. We therefore attempted to circumvent this issue by reducing the ambient oxygen level for Sod2n283; Tubulin-P[Switch]GAL4/UAS-SOD2 flies which were simultaneously fed with RU486. The hypoxic condition by itself has little impact on the survival of Sod2n283 adults (Fig. 6) same as pointed out earlier by Wicks et al.31 but following the activation of SOD2 with RU486, these flies can now live up to 25 days (average life span) under hypoxic environment, presumably due to reduced oxygen metabolism (Fig. 6). This observation further reinforces that the amount of ROS production can directly influence the adult life span.
Discussion
The presumed importance of the antioxidant protection system in the oocyte as well as during embryonic development has not been examined carefully with molecular genetic tools. Existing information suggests that mammalian embryos generate little mitochondrial ROS and therefore less oxidative stress, until mitochondrial oxidative phosphorylation begins at the blastula stage when embryos switch to high energy metabolism from a low energy state.32,33 Through the use of genetic manipulation, we demonstrate here that SOD2 depleted oocytes are viable, can be fertilized, undergo embryogenesis and complete development. If the same is true for mammalian oocytes, then our observations clarify how a transplanted SOD2−/− ovary in a Sod2+/+ mouse host can give rise to viable progeny.34 We are therefore left with an enigma: the high abundance SODs in Drosophila oocytes and by extension in murine oocytes, appear to be completely dispensable for normal development.14,35–37
We then inquired how postembryonic development reacts to a high level of unscavenged mitochondrial ROS. Lack of SOD2 activity in Sod2n283 leaves plenty of unscavenged ROS in post-embryonic tissues yet no overt cellular damage could be identified. Because tissue replenishment is a general characteristic of Dipteran insect metamorphosis,48 it could be argued that in Sod2n283 ROS-damaged tissues are replenished during the process of metamorphosis thereby enabling their survival. That we found no net difference in damage between tissues that undergo histolysis (like the fat body) and the non-replaceable tissues such as the eye and the brain argues against this mechanism. So, we looked for some means of tissue protection, which led to the finding of disproportionate autophagy in Sod2n283 larval tissues. In light of our data, we consider it the possibility that ROS-mediated induction of autophagy provides protection of larval tissues against oxidative damage as has been shown in other experimental systems.38 While prior studies have indicated a possible connection between autophagy and oxidative stress20,24 here we show that autophagy is indeed induced in pre-adult tissue in vivo in response to a high ROS environment. Thus, Sod2n283 could offer a model for exploring the cellular mechanism of oxidative stress-mediated autophagic induction.
According to this model, SOD2 protection is clearly essential for adult viability. To examine this possibility further, we activated SOD2 expression in newly eclosed Sod2n283 adults and observed a modest improvement in survival. The failure to observe more robust improvement in adult viability could reflect limitations in the GeneSwitch system (e.g., lag in feeding response time) or it could reflect in vivo limitations of the model itself.
In terms of the biological efficacies of free radicals in vivo, an interesting corollary is emerging between the protection offered by SOD2 and a second parallel pathway for ROS protection system in Drosophila, which consists of Thioredoxin reductase and GSH. Loss of either of these later enzymes causes severe developmental defects both in mice and Drosophila alike.39–42 One can also include in this list the complexI mutant that is known to cause high ROS production leading to serious developmental defects.15 Why then loss of SOD2 is principally related to the adult survival only? We propose that SOD2 loss of function effects are principally superoxide mediated, where as complex1, GSH and Thr have an active SOD enzyme system available that is capable of generating all kinds of ROS (H2O2, OH·- etc.,) at a much faster pace so the damaging effects are far more potent. Admittedly we need to prove this hypothesis further by measuring specific ROS production during development and during adult life.
Materials and Methods
Making SOD2−/− oocytes and fat body cells.
Fly stocks carrying P[w[+mW.hs] = FRT(w[hs])]G13 insertion at 42B were recombined with Sod2n283 to create FRT42B Sod2n283 chromosome. Somatic recombination was induced in females of the genotype hs-FLP; FRT42B Sod2n283/FRT42B OvoD1 following activation of the Flipase enzyme with heat shock (1 hour at 37°C) applied during the early larval stage. Once mature, eggs that are laid by this female should be Sod2n283 because all homozygous and heterozygous OvoD1 eggs will degenerate.43 Mature females were crossed with males, wild-type or Sod2n283/+. Thus the effects of germ line depletion of SOD2 activity was monitored by counting the number of adults produced from the Sod2n283 eggs. Three cohorts each of experimental and control crosses were set up carrying five males and five females in each cohort.
To make Sod2−/− clones in the fat body tissue, Sod2n283 was recombined with the FRT42D and somatic recombination was induced during second larval instar with heat shock.
Tissue preparation and immuno staining.
Dissected tissues were incubated in DHE reagent (Dihydroxy Ethidium; Invitrogen Inc.,) to detect in vivo superoxide generation. 30 uM of DHE was dissolved in Schneider's medium.15 For BrdU labeling tissues were incubated in BrdU solution (75 ug of BrdU in 1 ml PBS) for 45 minutes followed by detection with an anti-BrdU antibody (1:200 in PBSA). Caspase activation and nuclear lamin was detected with an activated caspase antibody (Cell Signaling at 1:100 in PBSA), Anti-LaminDmo antibody (DSHB, Iowa State University). For Phalloidin staining, dissected fat bodies were incubated in Phalloidin (Sigma), washed and mounted. For the purpose of embryo staining, flies of the genotype Sod2n283/CyO, GFP were mated inter se. Embryos were collected and processed for antibody staining. Tissues were mounted with Vectashield (Vector Laboratories) and observed under either a confocal microscope (Nikon Model: EZ-C1 Eclipse) or regular fluorescence microscope (Olympus). Tissue histochemical studies were performed with a minimum of ten individuals per experiment. As a routine practice all microscopic observations were confirmed by observing a specific effect at least in ten different fields.
Improving the adult viability of Sod2n283.
SOD2 expression was turned on in Sod2n283; UAS-SOD2/TubulinP[Switch]Gal4 adult flies by keeping them in a media spread with RU486 solution (Sigma M8046; 5 mg/ml).44 We put our best efforts to transfer them to RU486 medium within ∼1 hour after eclosion. Surviving adults were counted each day. Data analysis was done in Prism Software (V3.02 Graph Pad Incorporated). Tubulin-P[Switch] is a generous gift from Scott Pletcher lab. The UAS-Sod2 overexpression and UAS-Sod2IR lines were obtained from John Phillips' lab.
Western immunoblot analysis.
Performed according to previously published procedures.45,46
Determination of eclosion ratio.
A Sod2n283/CyO, Twist-GFP stock was constructed. All homozygous Sod2n283 embryo, larva and pupa from this stock are GFP negative where as the heterozygotes appeared as GFP positive. Eclosion ratios were compared between homozygote null (Sod2n283), heterozygotes (Sod2n283/CyO) and KG06854R control by collecting 250 larvae from each genotype and the number of larvae successfully transitioning to the pupal and adult stages were recorded. KG06854R shares the same genetic background as Sod2n283.47 Chi-square independence test was performed at 95% confidence interval by feeding the data into the University of Kansas web site http://www.people.ku.edu/~preacher/chisq/chisq.html.
Acknowledgements
Authors are thankful to Dr. Scott Pletcher and Prof. John Phillips. Acknowledgements are due to the Howard Hughes Medical Research Scholars Core Facility in HU Biology Department and Drosophila Stock Center in Bloomington, Indiana for providing fly stocks for this project. Work supported by NIH grants 2U54 NS039407-06A1 (NINDS) and 1R15 AG025754-01 (NIA) awarded to Atanu Duttaroy.
Abbreviations
- MnSOD
manganese superoxide dismutase
- ROS
reactive oxygen species
References
- 1.Boveris A, Cadenas E. Mitochondrial production of superoxide anions and its relationship to the antimycin insensitive respiration. FEBS letters. 1975;54:311–314. doi: 10.1016/0014-5793(75)80928-8. [DOI] [PubMed] [Google Scholar]
- 2.Andersen JK. Oxidative stress in neurodegeneration: cause or consequence? Nature Med. 2004;10:18–25. doi: 10.1038/nrn1434. [DOI] [PubMed] [Google Scholar]
- 3.Ballinger SW. Mitochondrial dysfunction in cardiovascular disease. Free Radic Biol Med. 2005;38:1278–1295. doi: 10.1016/j.freeradbiomed.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 4.Cadenas E, Davies KJ. Mitochondrial free radical generation, oxidative stress and aging. Free Radic Biol Med. 2000;29:222–230. doi: 10.1016/s0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- 5.Fukui H, Moraes CT. The mitochondrial impairment, oxidative stress and neurodegeneration connection: reality or just an attractive hypothesis? Trends Neurosci. 2008;31:251–256. doi: 10.1016/j.tins.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pieczenik SR, Neustadt J. Mitochondrial dysfunction and molecular pathways of disease. Exp Mol Pathol. 2007;83:84–92. doi: 10.1016/j.yexmp.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 7.Wallace DC. Animal models for mitochondrial disease. Methods Mol Biol. 2002;197:3–54. doi: 10.1385/1-59259-284-8:003. [DOI] [PubMed] [Google Scholar]
- 8.O'Brien KM, Dirmeier R, Engle M, Poyton RO. Mitochondrial protein oxidation in yeast mutants lacking manganese-(MnSOD) or copper- and zinc-containing superoxide dismutase (CuZnSOD): evidence that MnSOD and CuZnSOD have both unique and overlapping functions in protecting mitochondrial proteins from oxidative damage. J Biol Chem. 2004;279:51817–51827. doi: 10.1074/jbc.M405958200. [DOI] [PubMed] [Google Scholar]
- 9.Unlu ES, Koc A. Effects of deleting mitochondrial antioxidant genes on life span. Ann NY Acad Sci. 2007;1100:505–509. doi: 10.1196/annals.1395.055. [DOI] [PubMed] [Google Scholar]
- 10.Duttaroy A, Paul A, Kundu M, Belton A. A Sod2 null mutation confers severely reduced adult life span in Drosophila. Genetics. 2003;165:2295–2299. doi: 10.1093/genetics/165.4.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirby K, Hu J, Hilliker AJ, Phillips JP. RNA interference-mediated silencing of Sod2 in Drosophila leads to early adult-onset mortality and elevated endogenous oxidative stress. Proc Natl Acad Sci USA. 2002;99:16162–16167. doi: 10.1073/pnas.252342899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lebovitz RM, Zhang H, Vogel H, Cartwright J, Jr, Dionne L, Lu N, et al. Neurodegeneration, myocardial injury and perinatal death in mitochondrial superoxide dismutase-deficient mice. Proc Natl Acad Sci USA. 1996;93:9782–9787. doi: 10.1073/pnas.93.18.9782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Y, Huang TT, Carlson EJ, Melov S, Ursell PC, Olson JL, et al. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nature Gene. 1995;11:376–381. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- 14.Duttaroy A, Parkes T, Emtage P, Kirby K, Boulianne GL, Wang X, et al. The manganese superoxide dismutase gene of Drosophila: structure, expression and evidence for regulation by MAP kinase. DNA Cell Biol. 1997;16:391–399. doi: 10.1089/dna.1997.16.391. [DOI] [PubMed] [Google Scholar]
- 15.Owusu-Ansah E, Yavari A, Mandal S, Banerjee U. Distinct mitochondrial retrograde signals control the G1-S cell cycle checkpoint. Nature Gene. 2008;40:356–361. doi: 10.1038/ng.2007.50. [DOI] [PubMed] [Google Scholar]
- 16.Wolff T, Ready DF. Cell death in normal and rough eye mutants of Drosophila. Development. 1991;113:825–839. doi: 10.1242/dev.113.3.825. [DOI] [PubMed] [Google Scholar]
- 17.Martin DN, Baehrecke EH. Caspases function in autophagic programmed cell death in Drosophila. Development. 2004;131:275–284. doi: 10.1242/dev.00933. [DOI] [PubMed] [Google Scholar]
- 18.Yu SY, Yoo SJ, Yang L, Zapata C, Srinivasan A, Hay BA, Baker NE. A pathway of signals regulating effector and initiator caspases in the developing Drosophila eye. Development. 2002;129:3269–3278. doi: 10.1242/dev.129.13.3269. [DOI] [PubMed] [Google Scholar]
- 19.Kurada P, White K. Ras promotes cell survival in Drosophila by downregulating hid expression. Cell. 1998;95:319–329. doi: 10.1016/s0092-8674(00)81764-x. [DOI] [PubMed] [Google Scholar]
- 20.Girardot F, Monnier V, Tricoire H. Genome wide analysis of common and specific stress responses in adult Drosophila melanogaster. BMC Genomics. 2004;5:74. doi: 10.1186/1471-2164-5-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Develop Cell. 2004;6:463–477. doi: 10.1016/s1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- 22.Lum JJ, DeBerardinis RJ, Thompson CB. Autophagy in metazoans: cell survival in the land of plenty. Nature Rev. 2005;6:439–448. doi: 10.1038/nrm1660. [DOI] [PubMed] [Google Scholar]
- 23.Scott RC, Schuldiner O, Neufeld TP. Role and regulation of starvation-induced autophagy in the Drosophila fat body. Develop Cell. 2004;7:167–178. doi: 10.1016/j.devcel.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 24.Xiong Y, Contento AL, Nguyen PQ, Bassham DC. Degradation of oxidized proteins by autophagy during oxidative stress in Arabidopsis. Plant Physiol. 2007;143:291–299. doi: 10.1104/pp.106.092106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cecconi F, Levine B. The role of autophagy in mammalian development: cell makeover rather than cell death. Develop Cell. 2008;15:344–357. doi: 10.1016/j.devcel.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melendez A, Neufeld TP. The cell biology of autophagy in metazoans: a developing story. Development. 2008;135:2347–2360. doi: 10.1242/dev.016105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rubinsztein DC. The roles of intracellular protein-degradation pathways in neurodegeneration. Nature. 2006;443:780–786. doi: 10.1038/nature05291. [DOI] [PubMed] [Google Scholar]
- 29.Vellai T, Takacs-Vellai K, Sass M, Klionsky DJ. The regulation of aging: does autophagy underlie longevity? Trends Cell Biol. 2009;19:487–494. doi: 10.1016/j.tcb.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams A, Jahreiss L, Sarkar S, Saiki S, Menzies FM, Ravikumar B, Rubinsztein DC. Aggregate-prone proteins are cleared from the cytosol by autophagy:therapeutic implications. Curr Topics Develop Biol. 2006;76:89–101. doi: 10.1016/S0070-2153(06)76003-3. [DOI] [PubMed] [Google Scholar]
- 31.Wicks S, Bain N, Duttaroy A, Hilliker AJ, Phillips JP. Hypoxia rescues early mortality conferred by superoxide dismutase deficiency. Free Radic Biol Med. 2009;46:176–181. doi: 10.1016/j.freeradbiomed.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 32.Dumollard R, Carroll J, Duchen MR, Campbell K, Swann K. Mitochondrial function and redox state in mammalian embryos. Semin Cell Dev Biol. 2009;20:346–353. doi: 10.1016/j.semcdb.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 33.Leese HJ. Quiet please, do not disturb: a hypothesis of embryo metabolism and viability. Bioessays. 2002;24:845–849. doi: 10.1002/bies.10137. [DOI] [PubMed] [Google Scholar]
- 34.Matzuk MM, Dionne L, Guo Q, Kumar TR, Lebovitz RM. Ovarian function in superoxide dismutase 1 and 2 knockout mice. Endocrinology. 1998;139:4008–4011. doi: 10.1210/endo.139.9.6289. [DOI] [PubMed] [Google Scholar]
- 35.Donnay I, Knoops B. Peroxiredoxins in gametogenesis and embryo development. Subcell Biochem. 2007;44:345–355. doi: 10.1007/978-1-4020-6051-9_16. [DOI] [PubMed] [Google Scholar]
- 36.Evsikov AV, Graber JH, Brockman JM, Hampl A, Holbrook AE, Singh P, et al. Cracking the egg: molecular dynamics and evolutionary aspects of the transition from the fully grown oocyte to embryo. Genes Dev. 2006;20:2713–2727. doi: 10.1101/gad.1471006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guerin P, El Mouatassim S, Menezo Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum Reprod Update. 2001;7:175–189. doi: 10.1093/humupd/7.2.175. [DOI] [PubMed] [Google Scholar]
- 38.Simonsen A, Cumming RC, Brech A, Isakson P, Schubert DR, Finley KD. Promoting basal levels of autophagy in the nervous system enhances longevity and oxidant resistance in adult Drosophila. Autophagy. 2008;4:176–184. doi: 10.4161/auto.5269. [DOI] [PubMed] [Google Scholar]
- 39.Conrad M, Jakupoglu C, Moreno SG, Lippl S, Banjac A, Schneider M, et al. Essential role for mitochondrial thioredoxin reductase in hematopoiesis, heart development and heart function. Mol Cell Biol. 2004;24:9414–9423. doi: 10.1128/MCB.24.21.9414-9423.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jakupoglu C, Przemeck GK, Schneider M, Moreno SG, Mayr N, Hatzopoulos AK, et al. Cytoplasmic thioredoxin reductase is essential for embryogenesis but dispensable for cardiac development. Mol Cell Biol. 2005;25:1980–1988. doi: 10.1128/MCB.25.5.1980-1988.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Missirlis F, Phillips JP, Jackle H. Cooperative action of antioxidant defense systems in Drosophila. Curr Biol. 2001;11:1272–1277. doi: 10.1016/s0960-9822(01)00393-1. [DOI] [PubMed] [Google Scholar]
- 42.Shi ZZ, Osei-Frimpong J, Kala G, Kala SV, Barrios RJ, Habib GM, et al. Glutathione synthesis is essential for mouse development but not for cell growth in culture. Proc Natl Acad Sci USA. 2000;97:5101–5106. doi: 10.1073/pnas.97.10.5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oliver B, Perrimon N, Mahowald AP. The ovo locus is required for sex-specific germ line maintenance in Drosophila. Genes Dev. 1987;1:913–923. doi: 10.1101/gad.1.9.913. [DOI] [PubMed] [Google Scholar]
- 44.Osterwalder T, Yoon KS, White BH, Keshishian H. A conditional tissue-specific transgene expression system using inducible GAL4. Proc Natl Acad Sci USA. 2001;98:12596–12601. doi: 10.1073/pnas.221303298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Godenschwege T, Forde R, Davis CP, Paul A, Beckwith K, Duttaroy A. Mitochondrial superoxide radicals differentially affect muscle activity and neural function. Genetics. 2009;183:175–184. doi: 10.1534/genetics.109.103515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paul A, Belton A, Nag S, Martin I, Grotewiel MS, Duttaroy A. Reduced mitochondrial SOD displays mortality characteristics reminiscent of natural aging. Mech Ageing Dev. 2007;128:706–716. doi: 10.1016/j.mad.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paul A, Duttaroy A. Genomic regions responsible for manganese superoxide dismutase regulation in Drosophila melanogaster. Aging Cell. 2003;2:223–231. doi: 10.1046/j.1474-9728.2003.00056.x. [DOI] [PubMed] [Google Scholar]
- 48.Ashburner M. Function and structure of polytene chromosomes during insect development. In: Beament JWL, editor. Advances in Insect Physiology. London: Academic Press Inc., Ltd; 1970. [Google Scholar]