Summary
Ovarian clear cell carcinoma (CCC) is a unique type of ovarian cancer characterized by distinct clinicopathological and molecular features. CCC is considered to be a highly malignant disease because it is resistant to conventional chemotherapy, and, when presented at advanced stages, has a dismal overall survival. Identifying and characterizing biomarkers associated with its malignant behavior is fundamental toward elucidating the mechanisms underlying its aggressive phenotype. In this study, we performed immunohistochemical analysis on 89 CCCs to assess their expression of Rsf-1 (HBXAP), a chromatin remodeling gene frequently amplified and overexpressed in several types of human cancer. We found that 73 (82%) of 89 CCCs expressed Rsf-1 and most importantly, there was a statistically significant correlation between Rsf-1 immunostaining intensity and two disease parameters: advanced stage (p= 0.008) and status of retroperitoneal lymph node metastasis (p= 0.023). However, there was no correlation between Rsf-1 expression and patient age, peritoneal tumor dissemination, or overall survival. In conclusion, a higher expression level of Rsf-1 is associated with advanced clinical stage and lymph node metastasis in CCC. Our data suggest that Rsf-1 participates in tumor progression in CCC, and indicates that the contribution of Rsf-1 to disease aggressiveness deserves further study.
Introduction
Ovarian clear cell carcinoma (CCC) represents less than 10% of ovarian cancers in the United States, but occurs more frequently in Asian women (1, 2). Multivariate analysis on a large series of CCC shows that women with CCC present at a younger age and at earlier clinical stages as compared to high-grade (conventional) serous carcinoma, the most common and lethal type of ovarian cancer (1). Approximately 50% of CCCs present as stage I diseases (3, 4) and, despite being diagnosed at an early stage, are generally considered to be highly malignant (5). Morphological and molecular studies have demonstrated that many CCCs develop in a stepwise fashion from endometriosis through atypical endometriosis to overt CCC (6–10). In fact, CCC is the most common ovarian carcinoma associated with endometriosis. There has been increased enthusiasm for identifying markers that are predictive of the clinical outcome in CCC patients. This is because CCC typically presents with stage I or II disease, and prognostic markers could have an impact on clinical decision making in the management of CCC patients, such as administration of adjuvant chemotherapy. For example, IGF2BP3 (IMP3) expression has been reported to be an independent marker of reduced disease-specific survival in CCC, but not in high-grade serous or endometrioid carcinomas of the ovary (11). Similarly, enhanced expression of annexin A4 in CCC and its association with chemoresistance to carboplatin have been recently reported (12).
To further identify markers that are associated with poor prognosis in CCC and to explore the molecular mechanisms that account for the aggressive behavior of CCC, we determined the correlation between immunoreactivity of Rsf-1, also known as HBXAP, and clinical outcome in primary CCCs. We focused on Rsf-1 (HBXAP) because the encoded protein participates in chromatin remodeling, and this gene has been identified as an amplified gene with a tumor-promoting potential in several types of neoplastic diseases including ovarian high-grade serous carcinoma (13, 14, 15). Our analysis showed that higher expression levels of Rsf-1 (HBXAP) were associated advanced stage disease and retroperitoneal lymph node metastasis. The current study provides new evidence of the biological significance of Rsf-1 expression in CCC.
Materials and methods
Tissue samples
Formalin-fixed and paraffin-embedded CCC tissues were obtained from the Department of Pathology at the University of Tokyo Hospital. A total of 89 cases of primary CCCs were retrieved from the archives, and hematoxylin and eosin (H&E) stained slides were reviewed to confirm the diagnosis based on the most recent criteria of the World Health Organization. The CCC tissues were arranged in tissue microarrays (Beecher Instruments, Silver Spring, MD) with duplicate 2 mm tissue cores obtained from the tumor area in each CCC. The collection of clinical specimens was in compliance of guideline of tissue procurement at the University of Tokyo Hospital.
Clinical information of patients with ovarian clear cell carcinoma
We reviewed the medical records from all 89 CCC patients; data obtained included demographics, age at the time of diagnosis, preoperative diagnosis, clinical stage, and survival time after treatment. None of the patients underwent preoperative chemotherapy or radiotherapy. The correlations of Rsf-1 expression with the following clinical variables were evaluated: age, stage of carcinoma (stage I/II vs. stage III/IV), peritoneal dissemination, retroperitoneal lymph node metastasis, and death rate. Stage of carcinoma was assessable in 67 cases in which the appropriate staging procedures were performed; the remaining 22 cases were not included in the staging analysis due either to incomplete surgical procedures or to missing data. Staging was in accordance with the standards of the International Federation of Gynecology and Obstetrics (FIGO). Comprehensive evaluation of peritoneal dissemination that included microscopic examination of the omentum, peritoneal wall and mesentery soft tissues was performed in 79 cases. Retroperitoneal lymph node dissection was performed in 70 cases. Follow-up information included overall survival and cancer-related death. The follow-up interval was calculated from the date of surgery to the date of death or last clinical evaluation. The mean follow-up interval was 50 months (range 1–196 months).
Immunohistochemistry
The method of immunohistochemistry and scoring of immunoreactivity for Rsf-1 expression were previously described (13, 14). Briefly, 4 µm sections were cut from the tissue microarray blocks. Antigen retrieval was performed on deparaffinized sections by steaming them in citrate buffer (pH 6.0). A monoclonal anti-Rsf-1 antibody, clone 5H2/E4 (Upstate, Lake Placid, NY), was used at an optimal dilution of 1:2000 as previously determined (13, 14) and a monoclonal anti-NAC1 antibody was used at a dilution of 1:250 (16). The sections were incubated with the antibodies for 2 hours at room temperature, followed by the EnVision+ System (DAKO, Carpinteria, California) using the peroxidase method. An isotype-matched control antibody (MN-4) was used in parallel (17). Our previous studies had shown that the distribution of Rsf-1 immunoreactivity was always homogeneous within a tumor; therefore, we used an intensity score ranging from 0 to 4+ to evaluate Rsf-1 immunoreactivity in tumors as previously described (14). A positive reaction for both Rsf-1 and NAC1 was defined as discrete localization of the chromogen in the nuclei. The tissues were scored in a blinded fashion without the knowledge of clinical information.
Rsf-1 gene knockdown using small hairpin RNA
Ovarian clear cell adenocarcinoma cell lines, ES2 and JHOC5, were used in this study. ES2 was obtained from the American Type Culture Collection (Rockville,, MD, USA); JHOC5 was a kind gift from Dr. Kentaro Nakayama, Shimane University, Japan. Both cell lines used in this study were cultured in RPMI 1640 containing 5% fetal bovine serum.
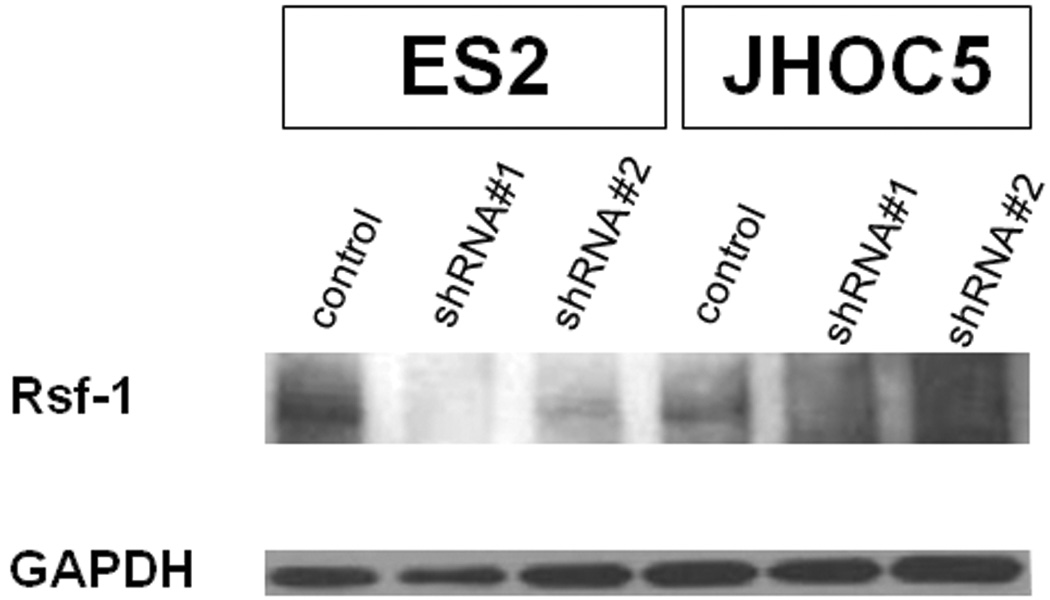
In order to confirm the specificity of the anti-Rsf-1 antibody used for immunohistochemistry, we performed Rsf-1 knockdown by transduction of two small hairpin RNAs (shRNA) and evaluated the knockdown efficiency by Western blot. The antibody specificity was indicated by reduced protein expression corresponding to Rsf-1 after gene knockdown based on western blot analysis using the same anti-Rsf-1 antibody as used in immunohistochemistry. We used lentivirus carrying the Rsf-1 shRNA sequence templates (CCGGCCAGTTCTGAAC TTTGAAGATCTCGAGATCTTCAAAGTTCAGAACT) and (CCGGCTTCTGAGA CAAAGGGTTCTACTCGAGTAGAACCCTTTGTCTCAGA), and a control shRNA sequence template, which were inserted into the lentiviral plasmid (pLKO.1-puro). Cells were washed and harvested 24 hours after transfection for protein and mRNA extraction.
For Western blot analysis, protein lysates were separated by 4% to 20% Tris-glycine gel electrophoresis and transferred onto polyvinylidene difluoride membranes using a semidry apparatus (Bio-Rad). After blocking, membranes were incubated with the anti-Rsf-1 (clone 5H2/E4) primary antibody at 4°C overnight followed by incubation with horseradish peroxidase (HRP)-conjugated secondary antibody. Protein bands were detected with Amersham ECL Western blotting detection reagents (GE Healthcare). Antibody reacting to anti-GAPDH was used to evaluate the amount of GAPDH as a loading control. Western blot analysis showed a reduced protein band corresponding to Rsf-1 in cells transfected with Rsf-1 shRNA as compared to control shRNA transfected cells, indicating the specificity of the anti-Rsf-1 antibody (Fig. 1).
Fig. 1.
Rsf-1 expression in ovarian clear cell carcinoma cell lines, ES2 and JHOC5
Western blot analysis showed a reduced protein band corresponding to Rsf-1 protein in Rsf-1 specific shRNA transfected cells as compared to control shRNA transfected cells, indicating the specificity of the anti-Rsf-1 antibody.
Statistical Analysis
Statistical analysis was performed using the χ2-test. Overall survival of CCC cases was calculated using the Kaplan-Meier method, and statistical analyses were performed using the log-rank test. Statistical analyses were performed with StatView 5.0 software (SAS Institute, Cary, NC) and P < 0.05 was considered statistically significant.
Results
Expression of Rsf-1 in ovarian clear cell carcinomas
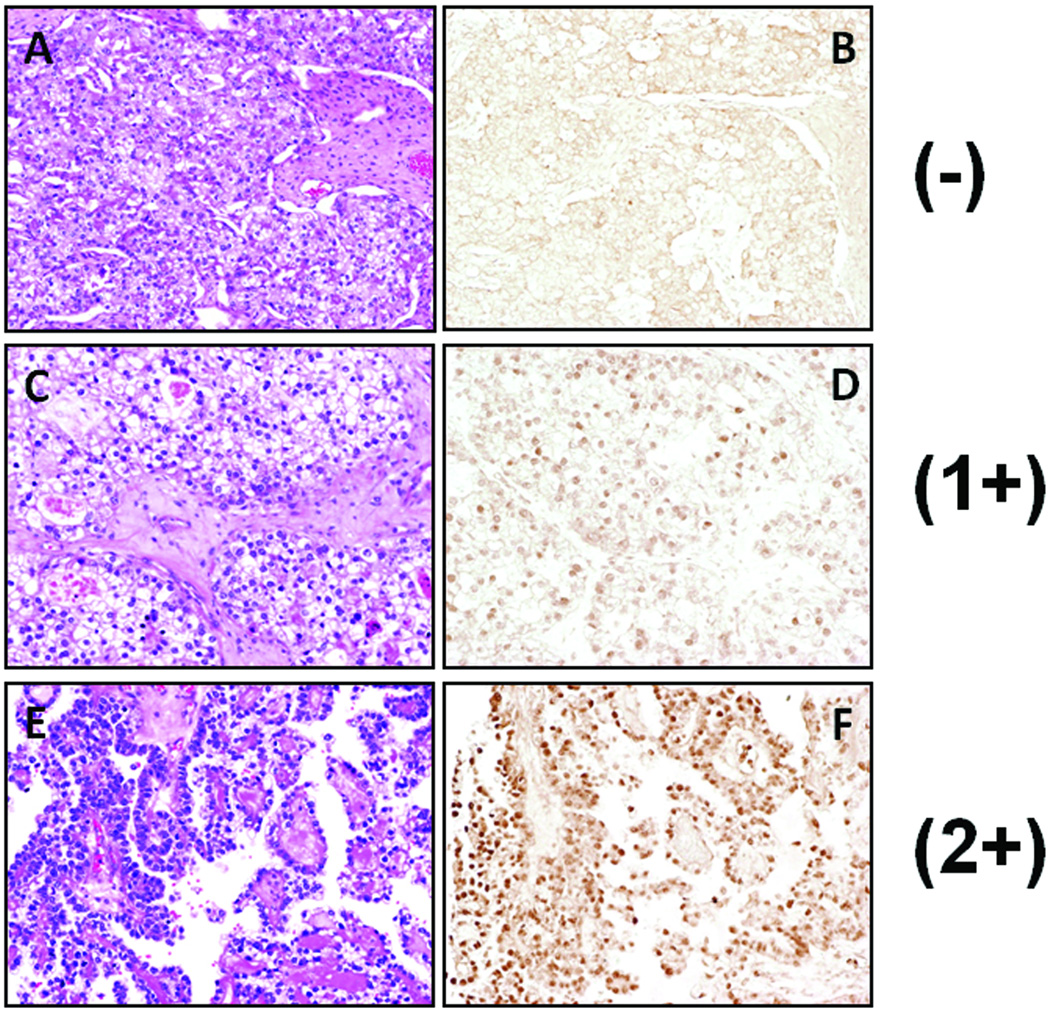
Results of Rsf-1 immunohistochemistry in CCCs are summarized in Table 1. Rsf-1 immunoreactivity was detected exclusively in nuclei of almost all tumor cells. Positive immunoreactivity of Rsf-1 was observed in 73 (82%) of 89 cases. Specifically, 16 (18%), 53 (60%), and 19 (21%) of 89 cases had a staining score of 0, 1+, and 2+, respectively. Only one case exhibited intense nuclear staining (3+). Histological features in representative cases with different Rsf-1 immunostaining intensities including 0, 1+, and 2+ are shown in Fig. 2. There was no correlation between Rsf-1 expression and histological pattern and nuclear atypia of the CCC cases.
Table 1.
Rsf-1 expression in ovarian clear cell carcinoma
| Immunostaining intensity score |
Number of cases | % |
|---|---|---|
| 0 | 16 | 18 |
| 1+ | 53 | 60 |
| 2+ | 19 | 21 |
| 3+ | 1 | 1 |
| 4+ | 0 | 0 |
| Total | 89 | 100 |
Fig. 2.
Rsf-1 immunoreactivity in representative ovarian clear cell carcinomas
(A, B) Microscopic view of an ovarian clear cell carcinoma showing negative immunoreactivity for Rsf-1. (C, D) A case of ovarian clear cell carcinoma with 1+ immunostaining intensity for Rsf-1. (E, F) A clear cell carcinoma with 2+ immunoreactivity for Rsf-1. A, C and E: hematoxylin and eosin stained sections; B, D and F: Rsf-1 stained sections.
Correlation of Rsf-1 expression with clinical features
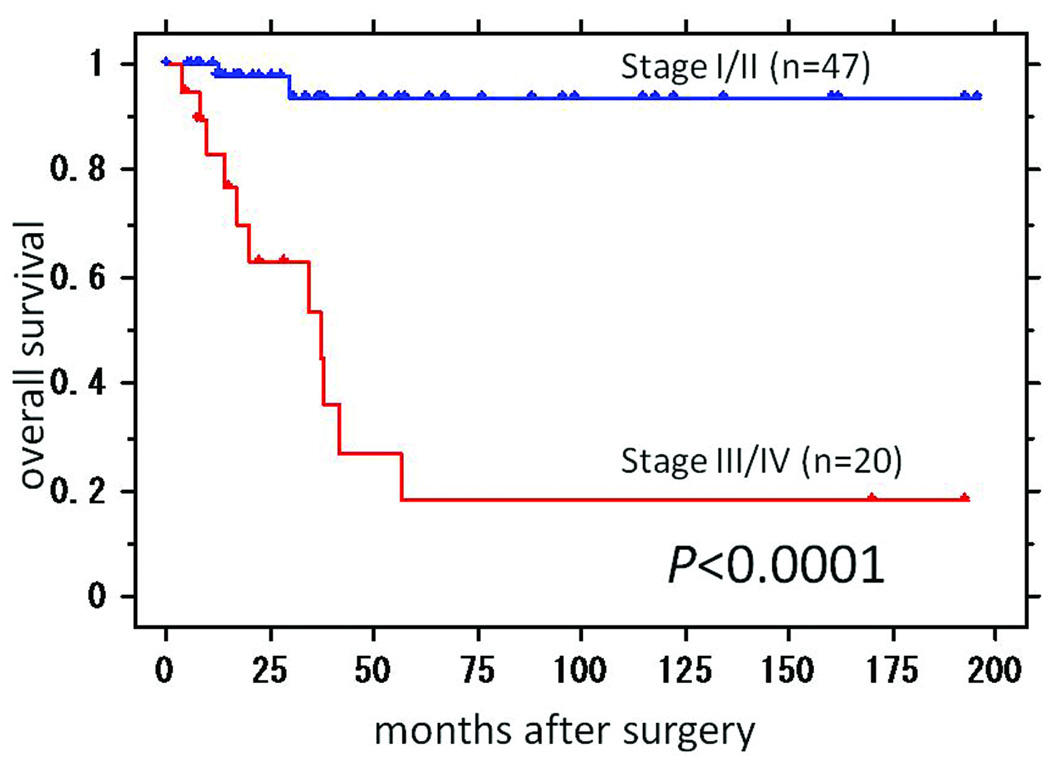
Since Rsf-1 expression has been reported to play a tumor-promoting role in ovarian cancer, we analyzed the possible correlation of Rsf-1 expression with clinical characteristics in CCC (Table 2). Statistically significant correlations were observed between Rsf-1 immunostaining intensity (score > 1) and lymph node involvement (P = 0.023). Furthermore, Rsf-1 immunostaining intensity (score > 1) was associated with advanced stage disease (Stage III/IV) (P = 0.0088). In fact, none of the Rsf-1 negative cases presented at an advanced stage. The frequency of peritoneal dissemination was higher in Rsf-1 positive cases (12/65), compared to Rsf-1 negative cases (1/14) but the difference was not statistically significant (P = 0.3). As a control we also assessed the expression of Nac1, a nuclear protein involved in transcription regulation, in the same set of CCC. We found that there was no significant association of Nac1 expression and any clinical feature including presentation stage or lymph node metastasis status (p > 0.1) (data not shown). Kaplan-Meier analyses were performed to determine if there was a correlation between Rsf-1 expression and clinical outcome. We first assessed the association between tumor stage and overall survival in CCCs, and demonstrated that stage III/IV cases (n = 20) had a poorer prognosis than stage I/II cases (n = 47) (P < 0.0001) (Fig. 3). However, Kaplan-Meier analysis did not reveal a significant difference in survival between Rsf-1 positive and negative cases (P = 0.42).
Table 2.
Correlation of Rsf-1 expression with clinical characteristics in ovarian clear cell carcinomas
| Clinical characteristics | Rsf-1 (HBXAP) expression | ||
|---|---|---|---|
| Positive | Negative | P | |
| Age (n=89) | |||
| ≥ 50 | 46 (79%) | 12 (21%) | |
| < 50 | 27 (87%) | 4 (13%) | 0.36 |
| Stage (n = 67) | |||
| I, II | 34 (72%) | 13 (28%) | |
| III, IV | 20 (100%) | 0 (0%) | 0.0088* |
| Peritoneal dissemination (n = 79) | |||
| Negative | 53 (80%) | 13 (20%) | |
| Positive | 12 (92%) | 1 (8%) | 0.30 |
| Lymph node metastasis (n = 70) | |||
| Negative | 40 (73%) | 15 (27%) | |
| Positive | 15 (100%) | 0 (0%) | 0.023* |
| Survival status (n = 89) | |||
| Alive | 58 (81%) | 14 (19%) | |
| Deceased | 15 (88%) | 2 (12%) | 0.46 |
statistically significant
Fig. 3.
Kaplan-Meier survival curve analysis shows that patients with stage III/IV ovarian clear cell carcinoma have a significantly worse overall survival rate than those with stage I/II ovarian clear cell carcinoma (P< 0.0001).
Discussion
An increase in DNA copy number at the chromosome 11q13.5 locus containing Rsf-1 (HBXAP) is detected in several types of human cancer including ovarian high-grade serous carcinoma. Rsf-1 (HBXAP) encodes for a cellular nuclear protein that binds to hSNF2H (18), forming a chromatin remodeling protein complex called RSF (Remodeling and Spacing Factor) (19, 20). Rsf-1 (HBXAP) has been shown to function as a histone chaperone in the nuclei while its binding partner, hSNF2H, possesses nucleosome-dependent ATPase activity (21). The Rsf-1/hSNF2H complex (RSF complex) mediates ATP-dependent chromatin remodeling, which alters the chromatin structure or positioning of nucleosomes (20). At the cellular level, RSF participates in chromatin remodeling in response to a variety of growth signals and environmental cues. Such nucleosome remodeling is required for transcriptional activation or repression (22, 23, 24), DNA replication (25), and cell cycle progression (26).
In this study, we used a well characterized anti-Rsf-1 antibody to study the expression pattern of Rsf-1 in CCC, and provided new evidence that expression of Rsf-1 was associated with advanced clinical stages and with the status of lymph node metastasis in CCC. The findings suggest a biological role for Rsf-1 in disease aggressiveness in this type of ovarian carcinoma. Interestingly, we have previously reported that chromosome 11q13.5 amplification and overexpression in cases of ovarian high-grade serous carcinoma contributes to shorter overall survival compared to cases without amplification. A possible mechanism was thought to be related to the de novo paclitaxel resistance rendered by Rsf-1 overexpression (27). Although Kaplan-Meier survival analysis did not show statistically significant difference between Rsf-1 positive CCC cases and Rsf-1 negative CCC cases, long-term prognosis of Rsf-1 positive cases appears to be slightly worse than Rsf-1 negative cases. However, the number of Rsf-1 negative CCC cases in our series was relatively small, and we believe that analysis in larger series on CCCs is required to conclude if Rsf-1 overexpression predicts worse overall survival in CCCs. Furthermore, our study suggests a potential use of Rsf-1 immunoreactivity as a biomarker that may prove useful for predicting clinical outcomes in primary CCC, including higher clinical stages, and for predicting the risk of developing lymph node metastasis. To this end, several proteins including IGF2BP3 (IMP3) (11) and annexin A4 (12) have been reported as new markers associated with treatment outcomes in CCC. Thus, a panel of different markers including Rsf-1 could be tested in future clinical trials to determine their potential to be used in the management of CCC patients.
In the current report, we observed that, with a single exception, the immunostaining intensity score of Rsf-1 was less than 3+ in all cases analyzed. This finding provides an independent confirmation of our previous observation in another, smaller set of CCC samples in which we demonstrated that the majority of CCCs showed an immunostaining intensity score of 1+ or 2+ (14). In fact, the percentage of Rsf-1 positive and negative cases is very similar between the current and previous reports. Moreover, analysis of SNP arrays performed on affinity-purified CCC specimens did not show an increase in DNA copy number at chromosome 11q13.5, indicating that Rsf-1 is rarely amplified in CCC (28). The above findings in CCC are in sharp contrast to those in high-grade serous carcinoma (14), and underscore the distinct molecular pathways in developing CCC and high-grade serous carcinoma (reviewed in (29)). It is also noteworthy that endometrioid and mucinous carcinomas of the ovary express Rsf-1 much less frequently as compared to CCCs and high-grade serous carcinoma. Only 49% of endometrioid carcinomas and 48% of mucinous carcinomas were Rsf-1 positive, and the intensity scores of positive cases were mostly 1+ and 2+.
In conclusion, using immunohistochemistry with an Rsf-1 specific antibody we demonstrated that the presence of Rsf-1 immunoreactivity is significantly associated with advanced stage and lymph node metastasis in primary CCCs. Our findings suggest Rsf-1 expression may contribute to disease aggressiveness in CCC, and warrant further study of the biological role of Rsf-1 in progression of CCC.
Acknowledgments
This study was supported by NIH/NCI grant CA129080 and the International Training Program from the Japan Society for the Promotion of Science.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chan JK, Teoh D, Hu JM, Shin JY, Osann K, Kapp DS. Do clear cell ovarian carcinomas have poorer prognosis compared to other epithelial cell types? A study of 1411 clear cell ovarian cancers. Gynecol Oncol. 2008;109:370–376. doi: 10.1016/j.ygyno.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 2.Ushijima K. Current status of gynecologic cancer in Japan. J Gynecol Oncol. 2009;20:67–71. doi: 10.3802/jgo.2009.20.2.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takano M, Kikuchi Y, Yaegashi N, et al. Clear cell carcinoma of the ovary: a retrospective multicentre experience of 254 patients with complete surgical staging. Br J Cancer. 2006;94:1369–1374. doi: 10.1038/sj.bjc.6603116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mizuno M, Kikkawa F, Shibata K, et al. Long-term follow-up and prognostic factor analysis in clear cell adenocarcinoma of the ovary. J Surg Oncol. 2006;94:138–143. doi: 10.1002/jso.20251. [DOI] [PubMed] [Google Scholar]
- 5.Jenison EL, Montag AG, Griffiths CT, et al. Clear cell adenocarcinoma of the ovary: a clinical analysis and comparison with serous carcinoma. Gynecol Oncol. 1989;32:65–71. doi: 10.1016/0090-8258(89)90852-4. [DOI] [PubMed] [Google Scholar]
- 6.Veras E, Mao TL, Ayhan A, et al. Cystic and adenofibromatous clear cell carcinomas of the ovary: distinctive tumors that differ in their pathogenesis and behavior: a clinicopathologic analysis of 122 cases. Am J Surg Pathol. 2009;33:844–853. doi: 10.1097/PAS.0b013e31819c4271. [DOI] [PubMed] [Google Scholar]
- 7.Fukunaga M, Nomura K, Ishikawa E, Ushigome S. Ovarian atypical endometriosis: its close association with malignant epithelial tumours. Histopathology. 1997;30:249–255. doi: 10.1046/j.1365-2559.1997.d01-592.x. [DOI] [PubMed] [Google Scholar]
- 8.Erzen M, Rakar S, Klancnik B, Syrjanen K. Endometriosis-associated ovarian carcinoma (EAOC): an entity distinct from other ovarian carcinomas as suggested by a nested case-control study. Gynecol Oncol. 2001;83:100–108. doi: 10.1006/gyno.2001.6382. [DOI] [PubMed] [Google Scholar]
- 9.Sato N, Tsunoda H, Nishida M, et al. Loss of heterozygosity on 10q23.3 and mutation of the tumor suppressor gene PTEN in benign endometrial cyst of the ovary: possible sequence progression from benign endometrial cyst to endometrioid carcinoma and clear cell carcinoma of the ovary. Cancer Res. 2000;60:7052–7056. [PubMed] [Google Scholar]
- 10.Marquez RT, Baggerly KA, Patterson AP, et al. Patterns of gene expression in different histotypes of epithelial ovarian cancer correlate with those in normal fallopian tube, endometrium, and colon. Clin Cancer Res. 2005;11:6116–6126. doi: 10.1158/1078-0432.CCR-04-2509. [DOI] [PubMed] [Google Scholar]
- 11.Kobel M, Xu H, Bourne PA, et al. IGF2BP3 (IMP3) expression is a marker of unfavorable prognosis in ovarian carcinoma of clear cell subtype. Mod Pathol. 2009;22:469–475. doi: 10.1038/modpathol.2008.206. [DOI] [PubMed] [Google Scholar]
- 12.Aoki D, Oda Y, Hattori S, et al. Overexpression of class III beta-tubulin predicts good response to taxane-based chemotherapy in ovarian clear cell adenocarcinoma. Clin Cancer Res. 2009;15:1473–1480. doi: 10.1158/1078-0432.CCR-08-1274. [DOI] [PubMed] [Google Scholar]
- 13.Shih IM, Sheu JJ, Santillan A, et al. Amplification of a chromatin remodeling gene, Rsf-1/HBXAP, in ovarian carcinoma. Proc Natl Acad Sci U S A. 2005;102:14004–14009. doi: 10.1073/pnas.0504195102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mao TL, Hsu CY, Yen MJ, et al. Expression of Rsf-1, a chromatin-remodeling gene, in ovarian and breast carcinoma. Hum Pathol. 2006;37:1169–1175. doi: 10.1016/j.humpath.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 15.Shih IM, Davidson B. Pathogenesis of ovarian cancer: clues from selected overexpressed genes. Future Oncol. 2009;5:1641–1657. doi: 10.2217/fon.09.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakayama K, Nakayama N, Davidson B, et al. A BTB/POZ protein, NAC-1, is related to tumor recurrence and is essential for tumor growth and survival. Proc Natl Acad Sci U S A. 2006 Dec 5;103:18739–18744. doi: 10.1073/pnas.0604083103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shih IM, Nesbit M, Herlyn M, et al. A new Mel-CAM (CD146)-specific monoclonal antibody, MN-4, on paraffin-embedded tissue. Mod Pathol. 1998;11:1098–1106. [PubMed] [Google Scholar]
- 18.Sheu JJ, Choi JH, Yildiz I, et al. The Roles of Human Sucrose Nonfermenting Protein 2 Homologue in the Tumor-Promoting Functions of Rsf-1. Cancer Res. 2008;68:4050–4057. doi: 10.1158/0008-5472.CAN-07-3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LeRoy G, Loyola A, Lane WS, Reinberg D. Purification and characterization of a human factor that assembles and remodels chromatin. J Biol Chem. 2000;275:14787–14790. doi: 10.1074/jbc.C000093200. [DOI] [PubMed] [Google Scholar]
- 20.Loyola A, Huang J-Y, LeRoy G, et al. Functional Analysis of the Subunits of the Chromatin Assembly Factor RSF. Mol Cell Biol. 2003;23:6759–6768. doi: 10.1128/MCB.23.19.6759-6768.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aihara T, Miyoshi Y, Koyama K, et al. Cloning and mapping of SMARCA5 encoding hSNF2H, a novel human homologue of Drosophila ISWI. Cytogenet Cell Genet. 1998;81:191–193. doi: 10.1159/000015027. [DOI] [PubMed] [Google Scholar]
- 22.Shamay M, Barak O, Shaul Y. HBXAP, a novel PHD-finger protein, possesses transcription repression activity. Genomics. 2002;79:523–529. doi: 10.1006/geno.2002.6717. [DOI] [PubMed] [Google Scholar]
- 23.Shamay M, Barak O, Doitsh G, Ben-Dor I, Shaul Y. Hepatitis B virus pX interacts with HBXAP, a PHD finger protein to coactivate transcription. J Biol Chem. 2002;277:9982–9988. doi: 10.1074/jbc.M111354200. [DOI] [PubMed] [Google Scholar]
- 24.Vignali M, Hassan AH, Neely KE, Workman JL. ATP-dependent chromatin-remodeling complexes. Mol Cell Biol. 2000;20:1899–1910. doi: 10.1128/mcb.20.6.1899-1910.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flanagan JF, Peterson CL. A role for the yeast SWI/SNF complex in DNA replication. Nucleic Acids Res. 1999;27:2022–2028. doi: 10.1093/nar/27.9.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cosma MP, Tanaka T, Nasmyth K. Ordered recruitment of transcription and chromatin remodeling factors to a cell cycle- and developmentally regulated promoter. Cell. 1999;97:299–311. doi: 10.1016/s0092-8674(00)80740-0. [DOI] [PubMed] [Google Scholar]
- 27.Choi JH, Sheu JJ, Guan B, et al. Functional analysis of 11q13.5 amplicon identifies Rsf-1 (HBXAP) as a gene involved in paclitaxel resistance in ovarian cancer. Cancer Res. 2009;69:1407–1415. doi: 10.1158/0008-5472.CAN-08-3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuo K, Mao T, Feng Y, et al. DNA copy number profiles in affinity-purified ovarian clear cell carcinoma. Clin Cancer Res. 2010 April 1; doi: 10.1158/1078-0432.CCR-09-2105. 2010 issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cho KR, Shih IM. Ovarian cancer. Annu Rev Pathol Mech Dis. 2009;4:287–313. doi: 10.1146/annurev.pathol.4.110807.092246. [DOI] [PMC free article] [PubMed] [Google Scholar]