Abstract
Objective:
A lower level of education often co-occurs with alcohol problems, but factors underlying this co-occurrence are not well understood. Specifically, whether these outcomes share part of their underlying genetic influences has not been widely studied. Educational level also reflects various environmental influences that may moderate the genetic etiology of alcohol problems, but gene–environment interactions between educational attainment and alcohol problems are unknown.
Method:
We studied the two nonmutually exclusive possibilities of common genetic influences and gene–environment interaction between alcohol problems and low education using a population-based sample (n = 4,858) of Finnish young adult twins (Mage = 24.5 years, range: 22.8–28.6 years). Alcohol problems were assessed with the Rutgers Alcohol Problem Index and self-reported maximum number of drinks consumed in a 24-hour period. Years of education, based on completed and ongo-ing studies, represented educational level.
Results:
Educational level was inversely associated with alcohol problems in young adulthood, and this association was most parsimoniously explained by overlapping genetic influences. Independent of this co-occurrence, higher education was associated with increased relative importance of genetic influences on alcohol problems, whereas environmental factors had a greater effect among twins with lower education.
Conclusions:
Our findings suggest a complex relationship between educational level and alcohol problems in young adulthood. Lower education is related to higher levels of alcohol problems, and this co-occurrence is influenced by genetic factors affecting both phenotypes. In addition, educational level moderates the importance of genetic and environmental influences on alcohol problems, possibly reflecting differences in social-control mechanisms related to educational level.
Educational level is a key component of socioeconomic status and strongly relates to a multitude of medical conditions, health behaviors, and mortality (Adler et al., 1994). Substance use disorders, including alcohol dependence, are more prevalent among those with more truncated educational achievement (Jacobi et al., 2004; Kes-sler et al., 2005; Suvisaari et al., 2009). Longitudinal studies have highlighted interconnections between developmental patterns of alcohol problems and educational outcomes, suggesting both that poor school success predicts drinking problems later in life and that heavy drinking in adolescence predicts lower education (Crum et al., 2006; Kessler et al., 1995; Pitkänen et al., 2008; Swendsen et al., 2009).
In addition to possible causal influences between alcohol problems and low educational achievement, there may be common underlying factors that influence both outcomes, thus giving rise to their co-occurrence. One such factor might be genetic background, as individual differences both in alcohol-related and educational outcomes arise, in part, because of differences in genetic makeup (Agrawal and Lyn-skey, 2008; Baker et al., 1996; Dick et al., 2009a; Johnson et al., 2009a; Silventoinen et al., 2004). Alcohol and other substance use disorders are associated with poorer cognitive abilities (Beatty et al., 2000; Latvala et al., 2009; Tapert and Brown 2000), and we have found evidence of partial genetic overlap between poorer verbal cognitive ability and alcohol dependence symptoms in early adulthood (Latvala et al., in press). Because cognitive abilities are highly predictive of the level of education to be attained (Deary et al., 2007) and because genetic factors contribute to this association (Bartels et al., 2002; Johnson et al., 2006), the question arises whether the co-occurrence of alcohol problems and low education is also, in part, the result of genetic influences common to these behavioral outcomes. This perspective ex-tends the prevailing approaches to the relationship between socioeconomic status and health outcomes, namely the social causation, social selection, and interactionist perspectives (Conger and Donnellan, 2007), which would argue, respectively, that low education leads to alcohol problems, alcohol problems lead to low education, or educational level and alcohol problems reciprocally influence each other.
Genetic and environmental influences on variation in a trait of interest, or covariation of two or more traits, can be assessed in twin studies using the different degree of genetic relatedness between identical (monozygotic [MZ]) and fraternal (dizygotic [DZ]) twins (Boomsma et al., 2002). Besides demonstrating the presence of genetic influences, however, twin studies are increasingly beginning to address the phenomenon of differing genetic influences conditional on environmental variation, or gene–environment interaction (Heath et al., 2002; Sher et al., 2010; van der Zwaluw and Engels, 2009). Pioneering studies found the heritability (i.e., proportion of phenotypic variance explained by genetic variance) of different indices of alcohol use to be dependent on various environmental contexts, such as marital status (Heath et al., 1989), religious upbringing (Koopmans et al., 1999), and urban versus rural residency (Dick et al., 2001; Rose et al., 2001). More recent studies have reported, for example, enhanced genetic influences on adolescent substance use in environments with lower parental monitoring and more substance-using friends (Dick et al., 2007a, 2007b).
Educational level is related to many facets of an individual's environment throughout the life span, ranging from chemical exposures to interpersonal relations (Evans and Kantrowitz, 2002; Gallo et al., 2006). It is thus conceivable that education might also have a moderating effect on the genetic etiology of alcohol problems. For example, it might be posited that education-related differences in homogenizing environmental influences, such as social norms, modify the importance of genetically influenced characteristics of the individual—an example of social context as a control mechanism (Shanahan and Hofer, 2005). However, this and other possible types of gene–environment interaction effects between education and alcohol problems have not been extensively studied.
Finland is a Nordic country whose educational system offers public schooling of uniform quality (Organisation for Economic Co-Operation and Development, 2007) without tuition fees, rendering educational opportunities virtually independent of financial and other family backgrounds. This feature, combined with another—that only a small proportion of the population totally abstains from alcohol (Hela-korpi et al., 2009)—makes Finland an informative setting for a genetic study of educational level in relation to alcohol problems. We used data from Finnish twins in early adulthood to examine the two nonmutually exclusive scenarios of common genetic influences and gene–environment interaction between alcohol problems and low education.
Method
Sample and measures
The present study is based on the Wave IV questionnaire survey of FinnTwin16, a population-based longitudinal study of five consecutive birth cohorts (1975–1979) of Finnish twins (Kaprio et al., 2002; Rose et al., 1999). FinnTwin16 was initiated in 1991 when the 1975 cohort was sequentially enrolled in 10 mailouts during 1–2 months following the twins' 16th birthdays. Baseline questionnaire data collection was completed in 1996 with pairwise response rates exceeding 88%, yielding baseline data from 2,733 twin pairs. Subsequent follow-up assessments were made at ages 17, 18.5, and approximately 25 years. The first three waves were tightly controlled for age, in appreciation of the rapid development of alcohol use in adolescence. In young adulthood, the surveys were telescoped into a 30-month period, with each birth year assessed in a 6-month window during 2000–2002 (Kaprio et al., 2002). The baseline and follow-up assessments included surveys of health habits and attitudes, symptom checklists, personality scales, and social relationships. Zygosity was determined on the basis of a well-validated questionnaire, containing items on the twins' similarity and confusability, completed by both co-twins and their parents at the baseline (Kaprio et al., 2002). Wave IV data of the outcomes of interest were available for a total of 4,974 individuals from 2,671 twin pairs (from 838 MZ, 879 same-sex DZ, and 954 opposite-sex DZ pairs). Of the sample, 54.8% were females, and the mean age was 24.5 years (range: 22.8–28.6 years). The data collection procedures of FinnTwin16 were approved by the Ethical Committee of the Faculty of Medicine, University of Helsinki, and by the Institutional Review Board of Indiana University. Participants provided written informed consent.
Alcohol problems were assessed with the Rutgers Alcohol Problem Index (RAPI), a self-report measure of alcohol-related problems experienced during the previous 12 months (White and Labouvie, 1989). The original RAPI has 23 items. In the FinnTwin16 young adult data collection, the item on whether alcohol use interfered with school work or examination preparation was omitted, creating a 22-item Finnish adaptation of RAPI with four response options. The internal consistency of this adapted version in the FinnTwin16 sample was as good (Cronbach's α = .90) as that of the original RAPI (Cronbach's α = .92) (White and Labouvie, 1989). The full RAPI scale without missing items was available for 4,260 twins from complete twin pairs (425 female MZ, 280 male MZ, 373 same-sex female DZ, 312 same-sex male DZ, and 740 opposite-sex DZ pairs) and 561 individual twins (68 MZ females, 60 MZ males, 68 DZ females from same-sex pairs, 119 DZ males from same-sex pairs, 173 DZ females from opposite-sex pairs, and 73 DZ males from opposite-sex pairs).
As another indicator of alcohol problems, we used a self-reported estimate of maximum number of alcoholic drinks consumed in a 24-hour period during the lifetime (maximum drinks). This measure has been used in genetic studies as a quantitative phenotype closely related to diagnosis of alcohol dependence (Saccone et al., 2005). We had data on maximum drinks for 4,048 twins from complete twin pairs (407 female MZ, 264 male MZ, 348 same-sex female DZ, 303 same-sex male DZ, and 702 opposite-sex DZ pairs) and 519 individual twins (55 MZ females, 54 MZ males, 59 DZ females from same-sex pairs, 110 DZ males from same-sex pairs, 173 DZ females from opposite-sex pairs, and 68 DZ males from opposite-sex pairs).
Information on the attained level of education was available as categorical classifications of each participant's completed and ongoing studies. Using this information, a variable representing the estimated total years of education was created. This was done on the basis of the standard duration of each type of education. In the Finnish educational system, compulsory education continues through Grade 9 (age 16). Secondary education is divided into vocational (nonacademic) and academic secondary education (high school), which typically takes 2 and 3 years to complete, respectively. Tertiary education is provided by polytechnic schools and universities, lasting typically 3.5 and 5 years, respectively. Polytechnic schools train professionals in various fields in response to labor market needs, whereas universities conduct scientific research and provide the highest levels of education. To enter tertiary education, academic secondary education is generally required, although some exceptions exist. For the participants who still had their studies underway when completing the young adult questionnaire, ongoing studies were taken into account by using half of the standard duration of the type of education in question as an average estimate of years studied. For example, individuals who reported having completed academic secondary education and were currently studying in the university were thus given the value 14.5 (9 + 3 + 2.5) for years of education. Information on education was available for 4,516 twins from complete twin pairs (448 female MZ, 298 male MZ, 393 same-sex female DZ, 336 same-sex male DZ, and 783 opposite-sex DZ pairs) and 453 individual twins (45 MZ females, 47 MZ males, 50 DZ females from same-sex pairs, 100 DZ males from same-sex pairs, 159 DZ females from opposite-sex pairs, and 52 DZ males from opposite-sex pairs).
The data included 116 individuals (2.3% of the sample) who could be classified as probable lifetime abstainers based on their responses throughout the data collection. These individuals were excluded from all analyses to avoid the assumption that a single unidimensional distribution encompasses both initiation of alcohol use and problem drinking. Thus, the final sample contained 4,858 twin individuals (2,414 complete pairs and 30 individual twins).
Statistical analysis
The association of educational level with RAPI and maximum drinks was studied with linear regression models, adjusting for familial clustering of the data (Williams, 2000). As a result of strong positive skewness, Box-Cox transformations of mean RAPI and maximum drink scores were used in the analyses, but values of untransformed variables are presented as descriptive information. For a first estimation of the presence of genetic influences on these traits and their covariation, twin and cross-twin cross-trait correlations were compared in different zygosity groups. In these comparisons, larger correlations within MZ than DZ pairs suggest the presence of genetic influences. Based on within-pair analyses, we proceeded into biometrical twin modeling.
In basic biometrical twin models, variance in a trait is partitioned into additive genetic influences (A), common environmental influences (C), and unique environmental influences (E) (Neale and Cardon, 1992). Additive genetic influences represent the sum of the individual effects of each gene on the phenotype, and thus correlate 1.0 between MZ twins, who share all their genes identical by descent, and 0.5 between DZ twins, who share, on average, 50% of their segregating genes. Common environmental influences refers to all environmental influences that make twins in a pair more similar to each other, and, by definition, correlate 1.0 between both MZ and DZ twins. Unique environmental influences, in contrast, are environmental influences that affect only one member of the twin pair. They are uncorrelated between both MZ and DZ twins, making co-twins thus more dissimilar.
In all twin modeling, the significance of each parameter in the model is tested by dropping the parameter and evaluating the change in −2 log likelihood between the initial model and the nested submodel. Model comparisons are made with a likelihood ratio chi-square test, and a significant change in the chi-square value indicates that dropping the parameter significantly decreases model fit, suggesting that the parameter should be retained in the model (Neale and Cardon, 1992).
Modeling was initiated with standard univariate analysis for each of the outcomes using the full sample including opposite-sex DZ pairs. These models yield estimates of additive genetic, common environmental, and unique environmental influences on the outcomes, and also enable testing the presence of quantitative and qualitative sex differences in genetic influences. Following that initial phase, trivariate Cholesky decomposition models for education and the alcohol variables (RAPI and maximum drinks) were estimated. In multivariate analysis, the association between traits is modeled by decomposing the phenotypic covariance of the variables into proportions accounted for by A, C, and E effects. The degree of association of the genetic factors influencing the traits is estimated as the genetic correlation between the latent genetic factors for the two traits. Common and unique environmental correlations are estimated in a similar fashion.
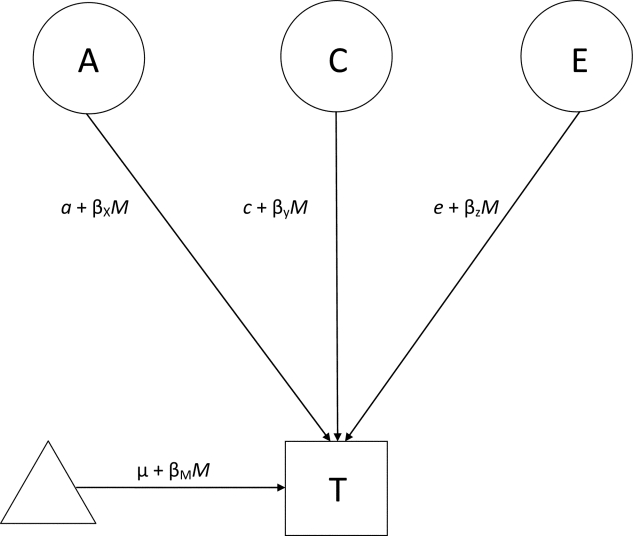
Gene-environment interaction effects between educational level and the alcohol problem variables were investigated with univariate moderation models in which a standardized variable of years of education served as a moderator for RAPI and maximum drinks. These models are extensions of the standard univariate model, modified to include a moderation component (Purcell, 2002). As shown in Figure 1, in addition to the standard paths a, c, and e, which indicate the additive genetic effect, the common environmental effect, and the unique environmental effect, respectively, a moderation coefficient β is included on each of these paths. In the moderation model, the additive genetic value is a linear function of the moderator variable M (educational level in the present models), represented by the equation a + βXM, where βX, an unknown parameter to be estimated, represents the magnitude of the moderating effect. A βX that is significantly different from zero is taken as evidence for a moderation effect on additive genetic influences. Moderation effects on common and unique environmental influences, βY and βZ, are estimated similarly. The pathway μ + βMM models the main effects of the moderator variable on the outcome. Importantly, this pathway also includes any covariance between the moderator and the outcome, including genetic correlation. Moderation effects are thus not confounded by possible common genetic influences on the moderator (education in the present study) and outcome variables (RAPI and maximum drinks). All genetic modeling in the present study was performed in Mx (Neale et al., 2006), a structural equation modeling program developed specifically for analyzing twin and family data.
Figure 1.
Moderation model (shown for one twin of the pair only). Depicted as circles, the latent variables A, C, and E indicate additive genetic, common environmental, and unique environmental influences, respectively, on the trait (T) of interest. The triangle indicates the mean of T. The paths a, c, and e indicate the magnitude of each latent variance component's effect on the trait. Each path includes a β term, which indicates the moderation coefficient for the moderator variable M.
Results
Descriptives
Distributions of educational level, RAPI, and maximum drinks in twin individuals, stratified by zygosity and sex, are shown in Table 1. Nearly half the sample (44.9%) reported completed or ongoing polytechnic or university (tertiary) education, whereas only 5.0% reported no studies beyond compulsory education. Women were more highly educated than men (p < .001), and in both sexes, MZ twins had a slightly higher education than DZ twins (p < .05). Robust sex differences were also observed in the alcohol variables, with men having higher values than women (both variables: p < .001). The twin types were also found to differ in these variables, with DZ twins scoring higher than MZ twins (RAPI: p < .05; maximum drinks: p < .001).
Table 1.
Distribution of educational level and alcohol problems in twin individuals across zygosity and sex
| Variable | MZF (n = 920) | DZF (n = 1,732) | MZM (n = 626) | DZM (n = 1,580) | Total (n = 4,858) |
| Education, M (SD) | 13.6(2.4) | 13.3 (2.6) | 13.0 (2.3) | 12.8(2.3) | 13.2 (2.5) |
| RAPI, M (SD) | 4.6 (6.2) | 4.5 (6.1) | 6.7 (8.1) | 7.6 (8.0) | 5.8 (7.2) |
| Max. drinks, M (SD) | 11.6(6.2) | 11.9(6.2) | 21.1 (10.2) | 21.8(9.8) | 16.2 (9.4) |
Notes: Dizygotic female (DZF) and dizygotic male (DZM) individuals in the table are drawn from both same-sex and opposite-sex DZ pairs. MZF = monozygotic females; MZM = monozygotic males; RAPI = Rutgers Alcohol Problem Index; max. drinks = maximum number of drinks consumed in a 24−hour period.
Table 2 gives the phenotypic correlations between years of education, RAPI, and maximum drinks in men and women. The correlations between education and the alcohol problem variables were of modest size but highly significant in both sexes. As an example of education-related differences in alcohol problems, the mean of RAPI was 11.4 (95% CI [9.3, 13.5]) among men with compulsory education only, compared with 6.4 (95% CI [5.9, 6.8]) in those with tertiary education, and the numbers of reported maximum drinks in these educational categories were 25.0 (95% CI [22.7, 27.3]) and 20.4 (95% CI [19.9, 21.0]), respectively. Twin and cross-twin cross-trait correlations between years of education and alcohol problems are given in Table 3 and 4, respectively. In most cases, they were larger within MZ than DZ twin pairs, suggesting genetic influences on these traits.
Table 2.
Phenotypic problems correlations between educational level and alcohol problems
| Education | RAPI | Max. drinks | |
| Education | 1 | −.06** | −.08** |
| RAPI | −.09*** | 1 | 49*** |
| Max. drinks | −.07** | 1 .38*** | 1 |
Notes: Correlations for males are below and those for females above the diagonal. RAPI = Rutgers Alcohol Problem Index; max. drinks = maximum number of drinks consumed in a 24-hour period.
p <.01;
p <.001.
Table 3.
Twin correlations for educational level and alcohol problems
| MZF | SS-DZF | MZM | SS-DZM | OS-DZ | |
| Education | .58 | .44 | .68 | .47 | .31 |
| RAPI | .51 | .22 | .48 | .22 | .15 |
| Max. drinks | .44 | .29 | .48 | .15 | .18 |
Notes: MZF = monozygotic females; SS-DZF = dizygotic females from same-sex pairs; MZM = monozygotic males; SS-DZM = dizygotic males from same-sex pairs; OS-DZ = opposite-sex pairs; RAPI = Rutgers Alcohol Problem Index; max. drinks = maximum number of drinks consumed in a 24-hour period.
Table 4.
Cross-twin cross-trait correlations between educational level and alcohol problems
| Females |
Males |
|||||
| Education | RAPI | Max. drinks | Education | RAPI | Max. drinks | |
| Education | — | −.07 | −.15 | — | −.02 | −.04 |
| RAPI | −.03 | — | .13 | −.08 | — | .12 |
| Max. drinks | –.01 | .28 | — | −12 | .31 | — |
Notes: Monozygotic correlations are below and same-sex dizygotic correlations above the diagonal. The correlations were calculated using the double-entry method. RAPI = Rutgers Alcohol Problem Index; max. drinks = maximum number of drinks consumed in a 24-hour period
Genetic modeling
Standardized estimates of additive genetic, common environmental, and unique environmental influences on educational level, RAPI, and maximum drinks are presented in Table 5. Constraining the additive genetic, common environmental, and unique environmental paths equal in males and females did not significantly decrease model fit for RAPI, χ2Δ(3) = 2.53, p = .47, whereas a significant decrease for education, χ2Δ(3) = 25.15, p < .001, and maximum drinks, χ2Δ(3) = 90.73, p < .001, was observed. Constraining the genetic correlation to .5 in opposite-sex DZ twins was statistically possible for RAPI, χ2Δ(1) = 0.63, p = .426, and maximum drinks, χ2Δ(1) = 0.001, p = .975, but not for education, χ2Δ(1) = 7.69, p < .01, suggesting qualitative sex differences in the genetic influences on educational level. The best fitting models for RAPI and maximum drinks did not include common environmental influences, χ2Δ(1) < 0.01, p > .99, χ2Δ(2) = 0.59, p = .75, whereas removing the C component significantly decreased model fit for education, χ2Δ(2) = 22.17, p < .001.
Table 5.
Standardized estimates [95% confidence intervals] of additive genetic (A), common environmental (C), and unique environmental (E) influences on educational level and two alcohol problem measures from univariate models
| Females |
Males |
|||||
| A | C | E | A | C | E | |
| Education | .32 [.15–.50] | .30 [.14–.45] | .37 [.33–.43] | .41 [.24–.60] | .28 [.11–.43] | .31 [.26–.36] |
| RAPI | .48 [.43–.53] | — | .52 [.47–.57] | .48 [.43–.53] | — | .52 [.47–.57] |
| Max. drinks | .44 [.37–.51] | — | .56 [.49–.63] | .45 [.37–.53] | — | .55 [.47–.63] |
Notes: RAPI = Rutgers Alcohol Problem Index; max. drinks = maximum number of drinks consumed in a 24-hour period.
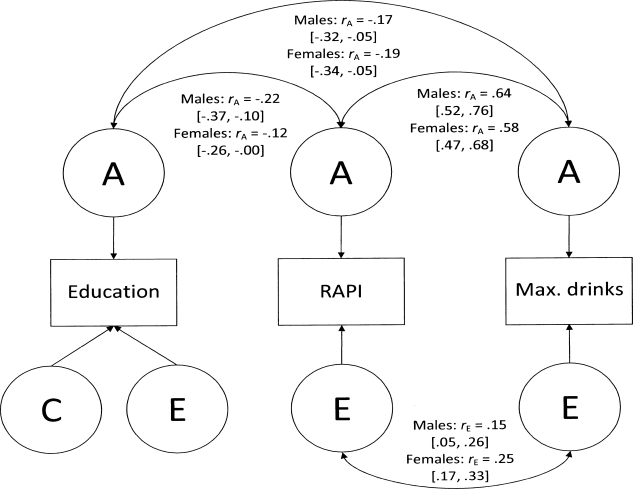
The significance of additive genetic, common environmental, and unique environmental correlation between educational level and the two alcohol problem variables was tested in trivariate models separately by sex. For both alcohol problem variables and education, covariance resulting from correlated genetic influences was significant in both sexes (females: p < .05 for education and RAPI, p < .01 for education and maximum drinks; males: p < .01 for education and RAPI, p < .05 for education and maximum drinks), whereas covariance resulting from correlated environmental influences could be removed from the models without statistically significant decrease in model fit. In contrast, both additive genetic and unique environmental sources of covariance contributed significantly to the association between RAPI and maximum drinks in both sexes (females: p < .001 for rA and rE; males: p < .001 for rA and p < .01 for rE). Figure 2 summarizes the genetic and environmental contributions to the covariance between years of education, RAPI, and maximum drinks.
Figure 2.
Genetic and environmental contributions to the covariance between education and two alcohol problem variables. The figure shows the estimates of additive genetic correlation (rA) and unique environmental correlation (rE) and their 95% confidence intervals (in brackets) from the final, best fitting trivariate Cholesky decomposition for years of education, the Rutgers Alcohol Problem Index (RAPI) scores, and maximum number of drinks consumed in a 24-hour period (max. drinks) separately for the sexes. Depicted as circles, the latent variables A, C, and E indicate additive genetic, common environmental, and unique environmental variance components.
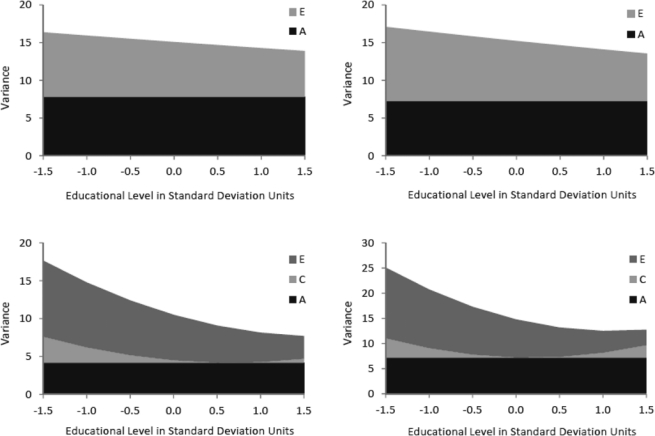
In the univariate moderation models, significant moderation effects were found for both alcohol problem variables in both sexes. For RAPI, educational level moderated unique environmental influences such that higher education was related to decreased unique environmental variance (females: p < .05; males: p < .01), whereas moderation effects on A and C influences were not statistically significant. For maximum drinks, significant moderation effects on both common and unique environmental paths were detected (females: p < .001 for both effects; males: p < .01 for moderation on C, p < .001 for moderation on E). Higher education was also related to decreased unique environmental variance in maximum drinks, whereas the effect on common environmental influences was more complex. An increase in C variance was found related to both low and high levels of education, whereas C variance was reduced close to zero at the mean of the education distribution. This nonlinear change in variance occurred because the moderating effect changed the direction of the C effect on maximum drinks from negative at low educational level to positive at high educational level.
As a result of these moderating effects, additive genetic influences explained a larger proportion of variance in both alcohol variables in those with higher education, whereas common and unique environmental influences were more important in twins with lower education. For example, the heritability of RAPI in men increased from .29 at low education (1.5 SD below the mean) to .56 at high education (1.5 SD above the mean). These moderating effects of educational level on RAPI and maximum drinks are shown graphically in Figure 3.
Figure 3.
Additive genetic (A), common environmental (C), and unique environmental (E) variance components of the Rutgers Alcohol Problem Index scores (top panel) and maximum number of drinks in a 24-hour period (bottom panel) in females (left) and males (right) as a function of educational level in standard deviation units.
Discussion
Using data from a population-based sample of Finnish twins in early adulthood, we present evidence of genetic correlation and gene–environment interaction between educational level and two indicators of alcohol problems. As anticipated, twins with lower education reported significantly more problems related to alcohol use within the last 12 months and higher numbers of consumed alcoholic drinks in a 24-hour period during the lifetime. Biometrical twin modeling suggested that genetic factors influence this co-occurrence, with a proportion of the genetic variation that increases the risk for alcohol problems also predisposing to attaining lower education. Consistent with earlier studies (Agrawal and Lynskey, 2008; Baker et al., 1996; Dick et al., 2009a; Johnson et al., 2009a; Silventoinen et al., 2004), heritability estimates of educational level and alcohol problems were moderate, ranging from 32% to 48%. In addition, independently of this genetically influenced co-occurrence, educational level also moderated the environmental influences specific to alcohol problems. As a result, the relative importance of genetic influences on both indicators of alcohol problems was greater among those with a higher level of education.
Our results extend the currently scarce genetically informed research on the relationship between alcohol use behaviors and education. Two recent studies reported on genetic correlation and gene–environment interaction between these phenomena, respectively, but neither study assessed both with the same sample. In their multivariate analysis of young adult data from the Minnesota Twin Family Study, Johnson et al. (2009b) reported overlapping genetic influences on education and an alcohol use composite, including symptoms of alcohol abuse/dependence (diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Revised [DSM-III-R]; American Psychiatric Association, 1987) and maximum number of drinks. Their multivariate model also included IQ, assessed in adolescence, and most of the shared genetic variance with alcohol use in fact reflected both IQ and education. A large proportion of the covariance of IQ, education, and alcohol use also seemed to be the result of overlapping common environmental influences, but the authors concluded that their sample of 626 twin pairs lacked sufficient statistical power to distinguish between genetic and common environmental influences in the multivariate setting. Timberlake et al. (2007), on the other hand, investigated the effects of college attendance on drinking behaviors, and because their sample included twins and siblings, they could model gene–environment interaction. Results suggested that college students exhibited greater genetic influence on quantity of alcohol consumed per drinking episode—a finding parallel to the present gene–environment interaction results. However, as discussed by the authors, the experiences and drinking-promoting influences related to college attendance in the United States may be quite specific to that environmental context, such as participation in fraternities/sororities and various athletic programs. Thus, our findings in Finnish young adults are likely to reflect, at least partly, different mechanisms of moderation by education.
Studies on the relationship between education and other substance use-related outcomes are also relevant to the present findings. Genetically informed studies on education and substance use other than alcohol are equally few in number, however. McCaffery et al. (2008) reported smoking initiation to have a negative correlation with educational attainment in male twins, and this correlation was explained by an overlap in both genetic and environmental influences. Educational attainment also significantly moderated the variance in smoking initiation, with higher education also being associated with reduced variance in that study, but whether this interaction occurred with genetic or environmental components could not be resolved. In the present sample, smoking correlated with alcohol problems and also had an inverse association with education, suggesting that the present findings might be at least partly replicated with smoking.
Our results suggest that, at least in Finland, where educational opportunities are relatively equal, genetic factors contribute to the association between alcohol problems and low education. Although the present modeling results with cross-sectional data cannot rule out possible causal relations between these outcomes, they do indicate that genetically influenced individual differences should be considered as one possible mechanism underlying the associations between components of socioeconomic status and health behaviors (Conger and Donnellan, 2007). General intelligence has been suggested as one such factor underlying socioeconomic inequalities in health (Der et al., 2009; Gottfredson, 2004). Importantly, the present multivariate modeling results mirrored our previous results on the inverse association between verbal intelligence and alcohol problems (Latvala et al., in press), suggesting that the genetic correlation between education and alcohol problems might also encompass general intelligence, as was the case in the study by Johnson et al. (2009b), discussed above. Interestingly, a recent study on a large, population-based sample of Swedish male twins reported a strong inverse association between smoking status and IQ, and found no support for a causal relationship between these traits (Wennerstad et al., 2010). However, despite their strong correlation, intelligence and education also seem to have independent associations with health outcomes (Batty et al., 2009; Lager et al., 2009).
The present gene–environment interaction analyses indicated that higher education was associated with reduced unique environmental variance in alcohol problems, whereas there was no direct moderation on additive genetic variance. Educational level also moderated the common environmental variance component in maximum drinks. These moderation effects were similar in men and women, and they resulted in increased relative importance of genetic influences on alcohol problems in those with higher education. This finding may seem contradictory, as higher education was related to lower level of alcohol problems. It is important to realize, however, that the moderation models were adjusted for education, so that the genetic and environmental influences estimated, as well as their moderation effects, concern only variation in alcohol problems that is independent of educational level. This was accomplished by including the main effect of the moderator variable in the means model. This feature of the model also makes sure that the moderation effect is not an artifact produced by genetic correlation (Purcell, 2002), which was found to explain the association between educational level and alcohol problems in the present study.
What might be the environmental factors related to higher education that lead to higher heritability of alcohol problems? In Finland as elsewhere, education is related to generally better prospects in life, including less unemployment, better working conditions, higher salaries, better neighborhood quality, and better health (Evans and Kantrowitz, 2002; Havén, 1999). One especially important environmental correlate of higher education in Finland is urban residency (Havén, 1999). Previous studies in Finnish twins have reported increased heritability of drinking behaviors and behavior problems in adolescence in urban environments, whereas common environmental factors seem to be more important in rural environments (Dick et al., 2001, 2009b; Rose et al., 2001). These findings have been interpreted as reflecting higher levels of social control and structural constraints placed on people in more rural environments, whereas urban environments are presumed to allow individual, genetically influenced behavioral characteristics to be more freely expressed (Shanahan and Hofer, 2005).
In the present sample of young adults, education was indeed strongly related to urban residency: 86% of those with at least academic secondary education reported urban residency, whereas the proportion was 58% for those with lower education. There were other notable "environmental" differences, as well. Those young adults with less than academic secondary education were more often married or cohabiting (68% vs. 50%), were more likely to have children (24% vs. 6%), and were more likely to be working (and not, e.g., studying; 60% vs. 35%) at the time of the current assessment as young adults. All these differences in the personal environment and life situation might contribute to the increased importance of genetic influences and reduced environmental influences in those with higher education. For example, besides urban residency, also being married has been associated with less genetic influence on alcohol consumption (Heath et al., 1989). Importantly, all these features also seem compatible with the scenario of less social control related to higher level of education.
Limitations of the present study include the fact that only a relatively crude estimate of years of education was available. However, we conducted the analyses also using an ordinal variable created from the original categorical classifications of completed and ongoing studies, and a similar pattern of results as reported here was found in both multi-variate and moderation analyses. Second, a large proportion of the sample still had their studies underway when completing the young adult questionnaire, but this information was taken into account in the variable for years of education. Further, as a result of strong positive skewness, Box-Cox transformed alcohol problem variables were used in the analyses. This is potentially problematic, as it is known that variable transformations can result in artifactual interaction effects (Purcell, 2002). This does not seem to have been the case in the present study, as similar moderation effects were detected also using the raw untransformed alcohol variables. For example, the heritability of the raw RAPI scores increased from 0.20 at low education (−1.5 SD units) to 0.53 at high education (+ 1.5 SD units) in men, and similarly from 0.17 to 0.44 in women.
A further limitation is that gene–environment interaction effects and genetic correlation were not modeled simultaneously using the moderated Cholesky approach (Purcell 2002). The more simple univariate moderation approach was chosen because of limited statistical power to reliably detect specific moderation effects on shared and nonshared genetic and environmental influences on education and alcohol problems when the phenotypic associations between these traits were weak. The moderated Cholesky model has also been criticized for potentially producing spurious interaction effects (Rathouz et al., 2008). Importantly, simulations by Purcell (2002) suggested that the presence of genetic correlation between the moderator and outcome variables does not lead to artificial interaction effects when the main effect of the moderator is included in the univariate moderation model, as was done in the present analyses.
A final limitation is that diagnoses of alcohol use disorders were not available. A subsample (n = 602) of the present data did in fact provide information on DSM-III-R alcohol dependence (Latvala et al., in press). In that subsample, the alcohol problem indicators used in the present study, RAPI and maximum drinks, had moderate positive correlations with the number of alcohol dependence criteria met (r = .55 and r = .50, respectively). RAPI scores in late adolescence robustly predicted alcohol diagnoses in early adulthood, with the odds ratio of outcome alcohol diagnosis per unit increase in adolescent RAPI exceeding 10 (Dick et al., in press). In the present study, RAPI and maximum drinks were moderately correlated, and shared genes explained approximately 80% of this correlation in men and 70% in women.
In conclusion, the present study of a population-based sample of Finnish twins suggests a complex relationship between educational level and alcohol problems in young adulthood. Lower education is related to significantly higher levels of alcohol problems, and this co-occurrence is influenced by genetic factors that both increase the risk for alcohol problems and predispose to lower educational attainment. Independent of this co-occurrence, higher educational level is associated with increased relative importance of genetic influences on alcohol problems, whereas common and unique environmental influences play a more important role in young adults with lower education, possibly reflecting differences in social control mechanisms related to educational level. All in all, these results underline the importance of studying the complex interplay of genetic and environmental influences on substance use behaviors.
Footnotes
This study was supported by National Institute on Alcohol Abuse and Alcoholism grants AA-12502, AA-00145, andAA-09203 awarded to Richard J. Rose, and AA-15416 awarded to Danielle M. Dick; Academy of Finland grants 100499 and 118555 awarded to Jaakko Kaprio. Jaakko Kaprio is also supported by the Centre of Excellence in Complex Disease Genetics of the Academy of Finland
References
- Adler NE, Boyce T, Chesney MA, Cohen S, Folkman S, Kahn RL, Syme SL. Socioeconomic status and health. The challenge of the gradient. The American Psychologist. 1994;49:15–24. doi: 10.1037//0003-066x.49.1.15. [DOI] [PubMed] [Google Scholar]
- Agrawal A, Lynskey MT. Are there genetic influences on addiction: Evidence from family, adoption and twin studies. Addiction. 2008;103:1069–1081. doi: 10.1111/j.1360-0443.2008.02213.x. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (3rd ed., rev.) Washington, DC: Author; 1987. [Google Scholar]
- Baker LA, Treloar SA, Reynolds CA, Heath AC, Martin NG. Genetics of educational attainment in Australian twins: Sex differences and secular changes. Behavior Genetics. 1996;26:89–102. doi: 10.1007/BF02359887. [DOI] [PubMed] [Google Scholar]
- Bartels M, Rietveld MJH, Van Baal GCM, Boomsma DI. Heritability of educational achievement in 12-year-olds and the overlap with cognitive ability. Twin Research. 2002;5:544–553. doi: 10.1375/136905202762342017. [DOI] [PubMed] [Google Scholar]
- Batty GD, Wennerstad KM, Smith GD, Gunnell D, Deary IJ, Tynelius P, Rasmussen F. IQ in early adulthood and mortality by middle age: Cohort study of 1 million Swedish men. Epidemiology. 2009;20:100–109. doi: 10.1097/EDE.0b013e31818ba076. [DOI] [PubMed] [Google Scholar]
- Beatty WW, Tivis R, Stott HD, Nixon SJ, Parsons OA. Neuropsychological deficits in sober alcoholics: Influences of chronicity and recent alcohol consumption. Alcoholism: Clinical and Experimental Research. 2000;24:149–154. [PubMed] [Google Scholar]
- Boomsma D, Busjahn A, Peltonen L. Classical twin studies and beyond. Nature Reviews. Genetics. 2002;3:872–882. doi: 10.1038/nrg932. [DOI] [PubMed] [Google Scholar]
- Conger RD, Donnellan MB. An interactionist perspective on the socioeconomic context of human development. Annual Review of Psychology. 2007;58:175–199. doi: 10.1146/annurev.psych.58.110405.085551. [DOI] [PubMed] [Google Scholar]
- Crum RM, Juon HS, Green KM, Robertson J, Fothergill K, Ensminger M. Educational achievement and early school behavior as predictors of alcohol-use disorders: 35-year follow-up of the Woodlawn Study. Journal of Studies on Alcohol. 2006;67:75–85. doi: 10.15288/jsa.2006.67.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deary IJ, Strand S, Smith P, Fernandes C. Intelligence and educational achievement. Intelligence. 2007;35:13–21. [Google Scholar]
- Der G, Batty GD, Deary IJ. The association between IQ in adolescence and a range of health outcomes at 40 in the 1979 US National Longitudinal Study of Youth. Intelligence. 2009;37:573–580. doi: 10.1016/j.intell.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Aliev F, Viken R, Kaprio J, Rose RJ. Rutgers Alcohol Problem Index (RAPI) scores at age 18 predict alcohol dependence diagnoses seven years later. Alcoholism: Clinical and Experimental Research. doi: 10.1111/j.1530-0277.2010.01432.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Bernard M, Aliev F, Viken R, Pulkkinen L, Kaprio J, Rose RJ. The role of socioregional factors in moderating genetic influences on early adolescent behavior problems and alcohol use. Alcoholism: Clinical and Experimental Research. 2009a;33:1739–1748. doi: 10.1111/j.1530-0277.2009.01011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Pagan JL, Holliday C, Viken R, Pulkkinen L, Kaprio J, Rose RJ. Gender differences in friends' influences on adolescent drinking: A genetic epidemiological study. Alcoholism: Clinical and Experimental Research. 2007a;31:2012–2019. doi: 10.1111/j.1530-0277.2007.00523.x. [DOI] [PubMed] [Google Scholar]
- Dick DM, Prescott C, McGue M. The genetics of substance use and substance use disorders. In: Kim YK, editor. Handbook of behavior genetics. New York: Springer; 2009b. pp. 433–453. [Google Scholar]
- Dick DM, Rose RJ, Viken RJ, Kaprio J, Koskenvuo M. Exploring gene–environment interactions: Socioregional moderation of alcohol use. Journal of Abnormal Psychology. 2001;110:625–632. doi: 10.1037//0021-843x.110.4.625. [DOI] [PubMed] [Google Scholar]
- Dick DM, Viken R, Purcell S, Kaprio J, Pulkkinen L, Rose RJ. Parental monitoring moderates the importance of genetic and environmental influences on adolescent smoking. Journal of Abnormal Psychology. 2007b;116:213–218. doi: 10.1037/0021-843X.116.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans GW, Kantrowitz E. Socioeconomic status and health: The potential role of environmental risk exposure. Annual Review of Public Health. 2002;23:303–331. doi: 10.1146/annurev.publhealth.23.112001.112349. [DOI] [PubMed] [Google Scholar]
- Gallo LC, Smith TW, Cox CM. Socioeconomic status, psychosocial processes, and perceived health: an interpersonal perspective. Annals of Behavioral Medicine. 2006;31:109–119. doi: 10.1207/s15324796abm3102_2. [DOI] [PubMed] [Google Scholar]
- Gottfredson LS. Intelligence: Is it the epidemiologists' elusive "fundamental cause" of social class inequalities in health? Journal of Personality and Social Psychology. 2004;86:174–199. doi: 10.1037/0022-3514.86.1.174. [DOI] [PubMed] [Google Scholar]
- Havén H. Helsinki, Finland: Statistics Finland; 1999. Education in Finland 1999: Statistics and indicators. [Google Scholar]
- Heath AC, Jardine R, Martin NG. Interactive effects of genotype and social environment on alcohol consumption in female twins. Journal of Studies on Alcohol. 1989;50:38–48. doi: 10.15288/jsa.1989.50.38. [DOI] [PubMed] [Google Scholar]
- Heath AC, Todorov AA, Nelson EC, Madden PAF, Bucholz KK, Martin NG. Gene-environment interaction effects on behavioral variation and risk of complex disorders: The example of alcoholism and other psychiatric disorders. Twin Research. 2002;5:30–37. doi: 10.1375/1369052022875. [DOI] [PubMed] [Google Scholar]
- Helakorpi S, Paavola M, Prättälä R, Uutela A. Health behaviour and health among the Finnish adult population, spring 2008. Helsinki, Finland: National Institute for Health and Welfare; 2009. [Google Scholar]
- Jacobi F, Wittchen H-U, Hölting C, Höfler M, Pfister H, Müller N, Lieb R. Prevalence, co-morbidity and correlates of mental disorders in the general population: Results from the German Health Interview and Examination Survey (GHS) Psychological Medicine. 2004;34:597–611. doi: 10.1017/S0033291703001399. [DOI] [PubMed] [Google Scholar]
- Johnson W, Deary IJ, Iacono WG. Genetic and environmental transactions underlying educational attainment. Intelligence. 2009a;37:466–478. doi: 10.1016/j.intell.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson W, Hicks BM, McGue M, Iacono WG. How intelligence and education contribute to substance use: Hints from the Minnesota Twin Family Study. Intelligence. 2009b;37:613–624. doi: 10.1016/j.intell.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson W, McGue M, Iacono WG. Genetic and environmental influences on academic achievement trajectories during adolescence. Developmental Psychology. 2006;42:514–532. doi: 10.1037/0012-1649.42.3.514. [DOI] [PubMed] [Google Scholar]
- Kaprio J, Pulkkinen L, Rose RJ. Genetic and environmental factors in health-related behaviors: studies on Finnish twins and twin families. Twin Research. 2002;5:366–371. doi: 10.1375/136905202320906101. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Foster CL, Saunders WB, Stang PE. Social consequences of psychiatric disorders: I. Educational attainment. The American Journal of Psychiatry. 1995;152:1026–1032. doi: 10.1176/ajp.152.7.1026. [DOI] [PubMed] [Google Scholar]
- Koopmans JR, Slutske WS, van Baal GCM, Boomsma DI. The influence of religion on alcohol use initiation: Evidence for Genotype × Environment interaction. Behavior Genetics. 1999;29:445–453. doi: 10.1023/a:1021679005623. [DOI] [PubMed] [Google Scholar]
- Lager A, Bremberg S, Vågerö D. The association of early IQ and education with mortality: 65 year longitudinal study in Malmö. Sweden. BMJ. 2009;339:b5282. doi: 10.1136/bmj.b5282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latvala A, Castaneda AE, Perälä J, Saarni SI, Aalto-Setälä T, Lön-nqvist J, Tuulio-Henriksson A. Cognitive functioning in substance abuse and dependence: A population-based study of young adults. Addiction. 2009;104:1558–1568. doi: 10.1111/j.1360-0443.2009.02656.x. [DOI] [PubMed] [Google Scholar]
- Latvala A, Tuulio-Henriksson A, Dick DM, Vuoksimaa E, Viken RJ, Suvisaari J, Rose RJ. Genetic origins of the association between verbal ability and alcohol dependence symptoms in young adulthood. Psychological Medicine. doi: 10.1017/S0033291710001194. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffery JM, Papandonatos GD, Lyons MJ, Koenen KC, Tsuang MT, Niaura R. Educational attainment, smoking initiation and lifetime nicotine dependence among male Vietnam-era twins. Psychological Medicine. 2008;38:1287–1297. doi: 10.1017/S0033291707001882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical modeling. 7th ed. Richmond, VA: Department of Psychiatry, Virginia Commonwealth University; 2006. [Google Scholar]
- Neale MC, Cardon LR. Methodology for genetic studies of twins and families. Dordrecht, The Netherlands: Kluwer Academic; 1992. [Google Scholar]
- Organisation for Economic Co-operation and Development. PISA 2006: Science competencies for tomorrow's world: Volume 1. Analysis. Paris, France: Author; 2007. [Google Scholar]
- Pitkänen T, Kokko K, Lyyra AL, Pulkkinen L. A developmental approach to alcohol drinking behaviour in adulthood: A follow-up study from age 8 to age 42. Addiction. 2008;103(Suppl. 1):48–68. doi: 10.1111/j.1360-0443.2008.02176.x. [DOI] [PubMed] [Google Scholar]
- Purcell S. Variance components models for gene–environment interaction in twin analysis. Twin Research. 2002;5:554–571. doi: 10.1375/136905202762342026. [DOI] [PubMed] [Google Scholar]
- Rathouz PJ, Van Hulle CA, Rodgers JL, Waldman ID, Lahey BB. Specification, testing, and interpretation of gene-by-measured-environment interaction models in the presence of gene–environment correlation. Behavior Genetics. 2008;38:301–315. doi: 10.1007/s10519-008-9193-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose RJ, Dick DM, Viken RJ, Kaprio J. Gene-environment interaction in patterns of adolescent drinking: Regional residency moderates longitudinal influences on alcohol use. Alcoholism: Clinical and Experimental Research. 2001;25:637–643. [PubMed] [Google Scholar]
- Rose RJ, Kaprio J, Winter T, Koskenvuo M, Viken RJ. Familial and socioregional environmental effects on abstinence from alcohol at age sixteen. Journal of Studies on Alcohol, Supplement. 1999;13:63–74. doi: 10.15288/jsas.1999.s13.63. [DOI] [PubMed] [Google Scholar]
- Saccone SF, Saccone NL, Neuman RJ, Rice JP. Genetic analysis of the maximum drinks phenotype. BMC Genetics. 2005;6(Suppl. 1):S124. doi: 10.1186/1471-2156-6-S1-S124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanahan MJ, Hofer SM. Social context in gene–environment interactions: Retrospect and prospect. The Journals of Gerontology. Series B: Psychological Sciences and Social Sciences 60. 2005;Spec. No. 1:65–76. doi: 10.1093/geronb/60.special_issue_1.65. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Dick DM, Crabbe JC, Hutchison KE, O'Malley SS, Heath AC. Consilient research approaches in studying Gene x Environment interactions in alcohol research. Addiction Biology. 2010;15:200–216. doi: 10.1111/j.1369-1600.2009.00189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silventoinen K, Sarlio-Lähteenkorva S, Koskenvuo M, Lahelma E, Kaprio J. Effect of environmental and genetic factors on education-associated disparities in weight and weight gain: A study of Finnish adult twins. The American Journal of Clinical Nutrition. 2004;80:815–822. doi: 10.1093/ajcn/80.4.815. [DOI] [PubMed] [Google Scholar]
- Suvisaari J, Aalto-Setälä T, Tuulio-Henriksson A, Härkänen T, Saarni SI, PeralaX, Lonnqvist X. Mental disorders in young adulthood. Psychological Medicine. 2009;39:287–299. doi: 10.1017/S0033291708003632. [DOI] [PubMed] [Google Scholar]
- Swendsen J, Conway KP, Degenhardt L, Dierker L, Glantz M, Jin R, Kessler RC. Socio-demographic risk factors for alcohol and drug dependence: The 10-year follow-up of the national comorbid-ity survey. Addiction. 2009;104:1346–1355. doi: 10.1111/j.1360-0443.2009.02622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapert SF, Brown SA. Substance dependence, family history of alcohol dependence and neuropsychological functioning in adolescence. Addiction. 2000;95:1043–1053. doi: 10.1046/j.1360-0443.2000.95710436.x. [DOI] [PubMed] [Google Scholar]
- Timberlake DS, Hopfer CJ, Rhee SH, Friedman NP, Haberstick BC, Lessem XM, Hewitt XK. College attendance and its effect on drinking behaviors in a longitudinal study of adolescents. Alcoholism: Clinical and Experimental Research. 2007;31:1020–1030. doi: 10.1111/j.1530-0277.2007.00383.x. [DOI] [PubMed] [Google Scholar]
- van der Zwaluw CS, Engels RCME. Gene-environment interactions and alcohol use and dependence: Current status and future challenges. Addiction. 2009;104:907–914. doi: 10.1111/j.1360-0443.2009.02563.x. [DOI] [PubMed] [Google Scholar]
- Wennerstad KM, Silventoinen K, Tynelius P, Bergman L, Kaprio J, Rasmussen F. Associations between IQ and cigarette smoking among Swedish male twins. Social Science & Medicine. 2010;70:575–581. doi: 10.1016/j.socscimed.2009.10.050. [DOI] [PubMed] [Google Scholar]
- White HR, Labouvie EW. Towards the assessment of adolescent problem drinking. Journal of Studies on Alcohol. 1989;50:30–37. doi: 10.15288/jsa.1989.50.30. [DOI] [PubMed] [Google Scholar]
- Williams RL. A note on robust variance estimation for cluster-correlated data. Biometrics. 2000;56:645–646. doi: 10.1111/j.0006-341x.2000.00645.x. [DOI] [PubMed] [Google Scholar]