Abstract
G protein-gated inwardly rectifying potassium (GIRK) channels hyperpolarize neurons in response to the activation of many G-protein coupled receptors and thus control the excitability of neurons through GIRK-mediated self-inhibition, slow synaptic potentials and volume transmission. GIRK channel function and trafficking are highly dependent on their subunit composition. Pharmacological investigations of GIRK channels and studies in animal models suggest that GIRK activity has an important role in physiological responses, including pain perception and memory modulation. Moreover, abnormal GIRK function has been implicated in altering neuronal excitability and cell death that may be important in the pathophysiology of human diseases such as epilepsy, Down’s syndrome, Parkinson’s disease and drug addiction. GIRK channels may therefore prove to be a valuable new therapeutic target for treating these health problems.
Introduction
Much of the interplay of excitatory and inhibitory signals required for normal neuronal function occurs in the dendrites of neurons1. Detailed electron and light microscopic studies show that fast excitatory inputs are mediated by ionotropic glutamate receptors such as NMDARs and AMPARs that are located in the postsynaptic density at the head of the spine, whereas inhibitory synapses typically form on the soma and dendritic shafts1. The fast inhibitory signals are mediated by ionotropic GABAA/glycine receptors. In addition to this fast inhibition, a slower inhibitory postsynaptic potential (sIPSC) exists that is mediated by G protein-activated inwardly rectifying potassium (GIRK or Kir3) channels that are located perisynaptically outside the postsynaptic density in the spine and also on the shaft2. This review discusses recent findings on the physiology, function and dysfunction of GIRK channels in the brain.
Physiology of GIRK channels
GIRK channels are members of a large family of inwardly rectifying potassium channels (Kir1 – Kir7). The term “inward rectification” refers to a change in slope of the current-voltage relationship at the reversal potential (i.e. the zero current level, which occurs at the equilibrium potential for K+ - EK). Therefore the outward current is smaller (but not zero) than the inward current (Figure 1A), due to occlusion of the central pore by intracellular Mg2+ and polyamines3. Under physiological conditions the resting membrane potential of a typical neuron is positive to EK, and the small outward K+ current through GIRK channels decreases the excitability of a neuron (Figure 1A, large arrow). Different types of neurotransmitters, such as acetylcholine, dopamine, opioids, serotonin, somatostatin, adenosine and GABA, activate these channels by stimulating their cognate G protein-coupled receptors (GPCRs), which in turn couple specifically to pertussis toxin (PTX) sensitive heterotrimeric G proteins that activate GIRK channels4. Whether the activated Gα subunit or Gβγ dimer directly opened GIRK channels was the focus of an animated debate in the 1980s (see Box 1). Now, a preponderance of evidence supports the conclusion that Gβγ dimers released from PTX-sensitive (Gαi/Gαo) G proteins bind directly to GIRK channels causing them to open5-9 (Box 1).
Figure 1. Physiology of GIRK channels in the mammalian brain.
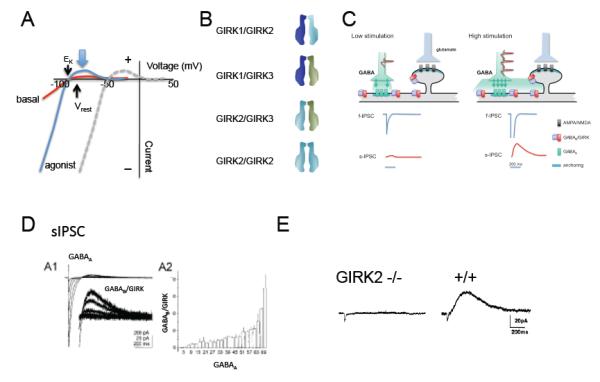
A, Hypothetical example of inwardly rectifying potassium currents through GIRK channels. Current (I) is plotted as a function of voltage (V) (I-V plot). With physiological levels of extracellular K, the current reverses (zero current potential) near −90 mV at the equilibrium potential for K (EK). The basal current (red) and agonist-induced (blue) currents show inward rectification (large inward (−) and small outward current (+)). The outward flow of current near the resting potential of the cell (large arrow) hyperpolarizes the neuron’s membrane potential. With higher extracellular potassium (e.g. 20 mM KCl), a concentration commonly used to study GIRK channels, the I-V relation shifts to the right to the new EK (dashed line), demonstrating a universal property of inwardly rectifying K channels. B, Three primary GIRK subunits exist in brain and form heterotetramers (GIRK1-GIRK2, GIRK2-GIRK3, GIRK1-GIRK3) and homotetramers (GIRK2-GIRK2)3,19-23. Less is known about GIRK4 in the brain. C, Generation of the slow IPSC. Two levels of synaptic activity are shown. Low stimulation of a GABA-ergic neuron releases GABA at levels sufficient to only activate GABAA channels (left panel). Higher stimulation (right panel) releases more GABA that accumulates in the synaptic cleft and diffuses to neighboring GABAB/GIRK complexes, located on the shaft and dendritic spine118-120. The resulting hypothetical fast and slow IPSCs from these two scenarios are shown below. D, Electrophysiological recording of the fast IPSC (GABAA) and slow IPSC (GABAB-GIRK) from a hippocampal slice (taken with permission from ref119). The relationship between the GABAA and GABAB response is non-linear (A2), suggesting accumulation of GABA is needed to activate GABAB receptors. E, The GABAB receptor dependent sIPSC is absent in the hippocampus of mice lacking GIRK2 channels (taken with permission from ref27).
Box 1. The great debate of the 1980s: activation of GIRK channels by Gβγ or Gα G proteins?
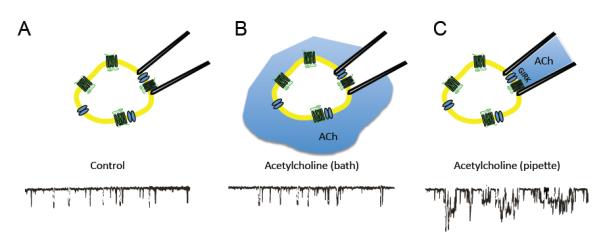
Over 20 years ago, an impassioned debate arose over the mechanism of GIRK channel activation. At that time, the canonical pathway for G protein signaling involved exchange of GTP for GDP on the Gα subunit, and subsequent Gα-GTP dependent activation or inhibition of catalytic enzymes, such as adenylyl cyclase or phospholipase C. Cardiac GIRK channels, referred to as IKACh, on the other hand were hypothesized to be activated directly by G proteins through a membrane delimited pathway4,166. One of the first hints of a novel signaling pathway came from studies using patch-clamp recordings from rabbit atrial cells: bath application of acetylcholine (ACh) did not alter GIRK channel activity recorded in cell-attached patches (panel b), but when ACh was included in the recording pipet (panel c), GIRK channel activity greatly increased (taken with permission from ref166). These results suggested that activation of GIRK channels by G protein coupled receptors occurs through proteins anchored to the membrane, and not through freely diffusible cytoplasmic 2nd messengers.
Two different groups examined whether GIRK channels were activated directly by G proteins. One group showed that application of purified native or recombinant Gα pre-activated with GTPγS (Gα-GTPγS) led to robust GIRK channel activation167,168. Another group found that application of purified Gβγ subunits, and not Gα-GTPγS, directly activated GIRK channels5. Thus, there was agreement on the direct activation of GIRK channels by G proteins but little consensus on whether Gα or Gβγ subunits mediated receptor activation. With the cloning of GIRK subunits and improved protein expression and purification techniques, two papers in 1994 provided new evidence for Gβγ-dependent activation. In one study, expression of recombinant Gβγ subunits led to sustained activation of GIRK channels, which was attenuated by a novel type of Gβγ binding protein, βARK7. A second study showed that different combinations of recombinant and purified Gβγ dimers directly activated GIRK channels6. Today, there is agreement that Gβγ subunits activate GIRK channels and, although there have been no further experiments demonstrating that Gα activates GIRK channels, Gαi/o G proteins are still considered important regulators of GIRK channels affecting receptor specificity and basal channel activity82,84,85,92,99,100,169.
Building neuronal GIRK channels
Mammals express four GIRK channel subunits (GIRK1-4 or Kir3.1-3.4). In the brain, GIRK1-GIRK3 subunits are common (Figure 1B), while GIRK4 expression is low and therefore does not significantly contribute to cerebral GIRK currents10. The three splice variants of GIRK2 that are expressed in the brain, differ in the length of the C-terminal domain: GIRK2c contains a PDZ-binding motif that is absent in GIRK2a and GIRK2b11-14. For clarification, GIRK2a (Kir3.2a) has been previously referred to as GIRK214 and GIRK2-115, while GIRK2c has been previously referred to as KATP-216, GIRK2A-114,15 and BIR117. GIRK1 splice variants exist in the brain but have not been investigated in detail18 and no splice variants have been reported for GIRK3 and GIRK4. GIRK subunits assemble into tetrameric channels in both heterologous expression systems (e.g. Xenopus oocytes, HEK-293 cells) and native tissues3,19-23. Because GIRK1 and GIRK3 subunits are unable to form functional channels on the plasma membrane, they form heterotetrameric channels (GIRK1/3; GIRK2/3)20,24,25 (Figure 1B). GIRK2, on the other hand, is unique because it can form homotetramers11,22,23 and can assembles with other GIRK subunits to form heterotetramers, such as GIRK1/2 and GIRK2/3.
Biochemical and molecular genetic experiments indicate that the predominant form of GIRK channels in the brain is a heterotetramer of GIRK1 and GIRK2 subunits19. Whether asymmetric arrangements of GIRK channel heterotetramers, such as a GIRK1-GIRK1-GIRK2-GIRK3, exist in neurons is still not known. Studies with GIRK knockout mice demonstrate that GIRK2 plays a primary role in generating GIRK currents in neurons (Table 1); mice lacking GIRK2 channels exhibit little or no GIRK current in a number of brain regions (Figure 1D, Table 1), including the hippocampus, cerebellum, substantia nigra, locus coeruleus and VTA26-32. Interestingly, GIRK3 knockout mice are indistinguishable from wild-type controls in some behavioral tests33 (e.g. open-field motor activity and motor-coordination) and have similar agonist-induced currents but exhibit less severe withdrawal from sedatives34 and reduced cocaine self-administration35. These findings suggest GIRK3 may have a regulatory role for GIRK signaling in the brain.
Table 1.
Summary of GIRK knockout mice
| GIRK knockout |
Phenotype | Generator | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Agonist-evoked GIRK current | Behavioral | ||||||||
| Brain | Heart | ||||||||
| VTA | SNc | CA1 | LC | Spinal cord L II |
Cereb ellum |
Atrium | |||
| GIRK1−/− | nc 26 | nc 28 | ↓ 28 | ↓ 31 | ↓ 172 | • Reduced score in anxiety behavior testing 33 | 172 | ||
| GIRK2−/− | ↓ 26 | ↓ 28 | ↓ 27,28 | ↓29,30 | ↓ 31 | ↓ 32 | 145 | ||
| GIRK3−/− | ↓ 26 | nc 28 | nc 28 | nc 29 ↓ 30 |
nc 31 | 29 | |||
| GIRK2−/− GIRK3−/− |
↓ 26 | ↓ 28 | ↓ 29,30 | 29 | |||||
| GIRK4−/− | ↓ 175 | • Impaired performance in the Morris water maze 10 | 175 | ||||||
Single-channel recordings reveal that the biophysical properties of heterotetrameric and homotetrameric channels differ. Whereas GIRK2 homotetrameric channels have short opening times (<0.5 ms mean open time), the heterotetrameric channels (e.g. GIRK1/GIRK2, GIRK1/GIRK3 or GIRK1/GIRK4) display longer opening times (~1-2 ms mean open times)3,11,23,25,36. The significance of different single channel kinetics is not clear but the direct association of G protein Gβγ subunits with GIRK channels might alter the frequency of long openings, giving rise to large receptor-activated currents37,38.
Interestingly, the single-channel conductances for channels expressed in heterologous expression systems (38-40 pS) are fairly uniform compared to those observed in neurons, which reveal a variety of brain region-specific differences (see Table 2). In native tissues, the subunit composition is not known but single-cell RT-PCR data have recently provided information about possible types of heterotetramers (Table 2). Differences in conductance between native and heterologously expressed GIRK channels suggest that the subunit composition of native channels may not be symmetric (e.g. two GIRK1, one GIRK2 and one GIRK3) or that there are channels composed of currently unknown subunits. More detailed single channel and biochemical studies are needed to resolve these differences.
Table 2.
Brain G protein-gated Inward Rectifiers
| Brain Region | Single-channel conductance | Composition |
|---|---|---|
| hippocampal pyramidal111,176,177 | 33-38 pS | GIRK1, 2a, 2c, 3 |
| substantia nigra41,178 | 56 pS | GIRK2a, 2c |
| locus coeruleus179,180,182 | 30 & 45 pS | GIRK1, 2, 3, 4 |
| nucleus basalis181,182 | 32-39 pS | GIRK1,2,3 4 |
| ventral tegmental area43 | unknown | GIRK1, 2c, 3 |
| medial prefrontal cortex57 | 30 pS | unknown |
GIRK motifs: trafficking and binding partners
A number of intrinsic amino acid sequences have been identified that control the intracellular trafficking of GIRK channels. GIRK2 contains a strong ER export signal (acidic residues) as well as an internalization (“VL”) motif39, enabling this subunit to form homotetramers or heteroteramers. GIRK1, on the other hand, lacks an ER export signal and must associate with another GIRK subunit (e.g. GIRK2 or GIRK3) to express on the plasma membrane24,39.. Although GIRK3 cannot form functional homotetramers, it can coassemble with GIRK1 or GIRK2 to form functional heterotetramers11,20,23,25. Unique to GIRK3, however, is a lysosomal targeting sequence (“YWSI”) that promotes degradation of GIRK channels39. Both GIRK2c and GIRK3 contain a class I PDZ binding motif, and interact directly with a novel PDZ-containing trafficking protein, sorting nexin 27 (SNX27)40. Association of SNX27 with GIRK3-containing channels leads to a reduction of GIRK signaling on the plasma membrane, most likely by promoting its internalization40. Whether GIRK2c and GIRK3 also interact with classical PDZ containing proteins, such as PSD95/SAP97, in neurons is unclear41,42. The interplay of these trafficking motifs suggests that GIRK2 plays a primary role in forming native GIRK currents while GIRK3 may regulate the availability of GIRK channels on the plasma membrane.
Though most neurons express GIRK1, 2 and 3, dopamine (DA) neurons of the SN and VTA do not express GIRK1-containing GIRKs. In SN dopamine neurons, for example, GIRK channels are comprised of GIRK2a/2c subunits41. In the VTA dopamine neurons, some GIRK channels are heterotetramers of GIRK2c/GIRK343. It is not well understood why DA neurons express a unique combination of GIRK subunits but GIRK2/3 channels are less sensitive to Gβγ subunits and exhibit a higher EC50 for coupling to GABAB receptors than GIRK1-containing channels20,43. This difference in sensitivity has been linked to important changes in the output of the drug reward pathway (see below).
Several modulators have been described that alter GIRK channel activity, including Na+44,45, ethanol46-48 and phosphorylation by PKA49,50 and PKC kinases51-57. Both Na+ and ethanol appear to stimulate GIRK channels through a specific binding site on the channel (Figure 2, see below). PKC-dependent phosphorylation decreases while PKA-dependent phosphorylation enhances channel activity49-57 (Figure 2C). In addition to these modulators, changes in the levels of the membrane phospholipid phosphatidylinositol 4,5 bisphosphate (PIP2), can regulate the activity of GIRK channels58-60. Stimulation of GPCRs that couple to Gq G proteins stimulate phospholipase C (PLC) and deplete plasma membrane levels of PIP2, leading to desensitizing GIRK currents 55,61,62 (but see ref63). Activation of PLC also leads to stimulation of PKC, enabling cross-talk of these two pathways (PIP2 depletion and PKC phosphorylation) in the regulation of GIRK channels55,56.
Figure 2. Structural insights into gating and the formation of a macromolecular GIRK signaling complex.
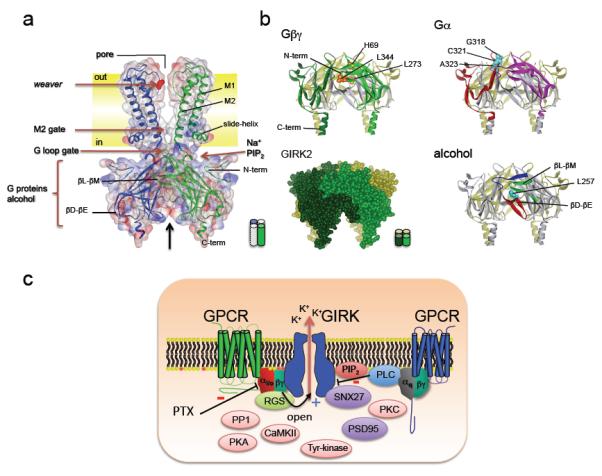
A, A structure (ribbon model superimposed with space filled model) of the Kirbac1.3/GIRK1 chimeric channel72 is shown in a typical lipid bilayer (‘out’: extracellular) This structure contains the cytoplasmic domains (N- and C-termini) of GIRK1, and the transmembrane domains (M1, M2) and pore region of the bacterial inward rectifier (Kirbac1.3) channel (PDB:2QKS). The structure of the GIRK1 cytoplasmic domain in this full-length chimeric protein is similar to those of other inward rectifiers 64-66. The K+ selectivity filter is the site of the weaver mutation22,133,134. The regions implicated in Na+ and PIP2 association are shown58-60,66,91. Two channel gates formed by the M2 transmembrane domain and cytoplasmic G-loop form a physical barrier to ion permeation65,67,68,88-90. Note the constriction formed by the G-loop gate65. B, Modulation sites are shown from a side-view perspective in two adjacent subunits. The regions implicated in Gβγ activation (light and dark green β-strands; H69, L273 and L344: Yellow)9,75-82, Gαi/o association (red and pink β-strands, G318, C321 and A323 - blue)80,84,85, and ethanol-dependent activation (N-terminus:blue; βD-βE sheet: yellow; βL-βM sheet: green; L257:red)46 are shown. The sites are mapped on structure of GIRK2 (PDB:2E4F)66 C), Schematic shows a macromolecular signaling complex that contains a GPCR, which couples to PTX-sensitive Gαi/o G proteins, a GIRK channel, an RGS protein, SNX27, and possibly PSD95. Gβγ opens GIRK channels (+). Modulators of GIRK channels are also shown, including tyrosine kinase, CaMKII kinase, PKA/PKC kinases, and PP1 phosphatase49-57,129,130,171. A second GPCR that couples to the Gq pathway is shown. Activation of this pathway stimulates PLC, which leads to activation of PKC, and depletion of PIP2, both of which reduce (−) GIRK channel activity55,61,62. Pertussis toxin (PTX) inhibits (−) activation of GIRK channels through Gαi/o G proteins4.
Molecular and Structural Insights into GIRK function
Although a high-resolution three-dimensional structure of a full-length GIRK channel is currently not available, structures of the cytoplasmic domains of GIRK1, GIRK2 and a G protein-insensitive inward rectifier (IRK1) have been solved64-66. Comparison of these structures highlights a conserved secondary structure for inwardly rectifying potassium channels, consisting of 14 beta strands and two alpha helices (Figure 2A,B). These cytoplasmic high resolution structures have revealed a new structure, the G loop formed by the βH-βI sheet65, that is important in channel gating65,67,68, and identified several amino acids involved in regulating inward rectification and K+ binding64,69-71. A recent 3D structure of a full-length chimeric channel, containing the transmembrane domains and pore of a bacterial inward rectifier (KirBac1.3) channel, and the cytoplasmic N- and C-terminal domains of GIRK1 has been solved in two different conformations72 revealing the relative position of the pore (selectivity filter), G-loop and M2 gates, and the cytoplasmic interaction sites (Figure 2A). Notably, these crystals revealed two different positions of the G loop, which have been tentatively ascribed to putative open and closed states of the channel72. Though more studies are needed, a structural view of GIRK channel gating is emerging that involves the N-terminal slide-helix73, K+ and polyamine binding sites69,70,71,74, and movement of the M2 and G loop gates65,69,71,72,73.
Having high-resolution structures of GIRK channels provides key information on the structural context of sites previously identified for G protein regulation. GIRK channels are predominantly closed at resting membrane potentials (agonist-independent, basal activity) and become activated upon stimulation of PTX-sensitive Gi/o G proteins (receptor-induced current). Both forms of activation involve binding of Gβγ directly to the GIRK channel8,9,36,75. Functional and biochemical studies have identified sequences in the N- and C-terminal domains of GIRK1-4 that are involved in both agonist-independent and receptor-induced Gβγ activation9,75-82 (Figure 2A,B). In particular, a Leu (GIRK1-L333, GIRK2-L344, GIRK4-L339) in the C-terminal domain of GIRK channels (βL-βM sheet) appears to have a critical role in the Gβγ-dependent activation of GIRK channels77,80,81. Similarly, functional and biochemical studies have pinpointed Gα binding domains in the N- and C-terminal domains of GIRK channels82-85,99.
Mapping these G protein-interaction sites on the high-resolution structures provides clues to possible mechanisms of Gβγ gating (Figure 2A,B). For example, many of the regions implicated in Gβγ activation fall on the external side of the cytoplasmic domains, along an interface formed by two adjacent subunits (Figure 2B). Originating from different subunits, sequences from the N-terminal domain and the βD-βE and βL-βM sheets in the C-terminal domain contribute to this interface. An interaction between the N- and C-terminal domains of two subunits has been shown to be important for assembly and gating of GIRK channels 86. Recently, a hydrophobic pocket has been identified in this subunit interface and implicated in G protein-dependent activation of GIRK channels46. FRET (fluorescence resonance energy transfer, see Box 2) measurements further reveal that Gβγ activation elicits a structural change, possibly a rotation, in the GIRK N- and C-terminal domains87. Systematic mutagenesis studies have indicated that specific amino acids in the M2 transmembrane domain88-90 and G-loop65,67,68 form a barrier to ion conduction in the closed state; thus conformational changes triggered in the cytoplasmic domains must couple to the channel’s gates to open the channel. For example, the binding of Gβγ subunits to the channel could induce a conformational change involving the βL-βM sheets (containing the Leu implicated in Gβγ activation), the N-terminal domain, and the slide-helix, which, in turn, is relayed to the M2 and G-loop gates near the membrane. High-resolution structures of Gβγ or Gαβγ complexed with full-length GIRK channels will be necessary to clarify our understanding of the molecular and structural mechanism underlying Gβγ activation and to identify amino acids in the Gβγ and Gα subunits that interact with GIRKs.
Box 2. Spectroscopic studies provide evidence for dynamic GIRK/GPCR macromolecular complex.
High-resolution spectroscopic studies measuring fluorescence resonance energy transfer (FRET) have been used to investigate the spatial proximity of proteins within the macromolecular complex. FRET occurs when two fluorophores with overlapping spectral properties are aligned and <100 Å away from each other. If there is movement in the protein such that the distance (or orientation) between the two fluorophores changes, then FRET will increase when they move closer or decrease when they move apart. In a macromolecular complex, one would predict that if the proteins are physically close then FRET measurements can be used to determine whether two specific proteins are closer than 100 Å of each other. FRET studies have indicated that some GPCRs pre-couple with G proteins and channels94,96,101 (but see170). GABAB receptors and RGS proteins also produce detectable FRET with GIRK channels26,102. Using a similar technique, a significant bioluminescence resonance energy transfer (BRET) signal has been detected between tagged β2 adrenergic receptors and GIRK channels96. Recently, a FRET study using TIRF microscopy (Total Internal Reflection Fluorescence) and tagged GIRK channels and Gβγ subunits surprisingly found that FRET efficiency decreased upon receptor activation92 – one would have expected FRET to increase as Gβγ and GIRK channels come closer together. These findings support the model that the Gαβγ heterotrimer associates directly with GIRK channel at rest and that Gβγ moves within a restricted region around the channel during activation. Collectively, these studies have provided important evidence for the existence of a macromolecular signaling complex containing GIRK channels, GPCRs, G proteins and RGS proteins.
High-resolution structures of inwardly rectifying K+ channels have also enabled visualization of amino acids implicated in PIP2 binding as well as possible gating mechanisms for ethanol and Na+ modulation. Clusters of basic (positively charged) amino acids of the cytoplasmic domain58-60 are positioned in close proximity to the plasma membrane, where they are poised to interact directly with negatively changed membrane phospholipids and couple with the two gates (M2 and G loop) (Figure 2A). Recently, a hydrophobic pocket formed by the N-terminal domain, and the C-terminal βD-βE and βL-βM sheets has been identified as a site for ethanol activation of GIRK channels46. Bulky amino acid substitutions of a Leu located in the βD-βE sheet of the hydrophobic pocket decreases ethanol-dependent activation46 (Figure 2B). A structural analysis of GIRK2 has also provided a possible mechanism for Na+ dependent regulation of GIRK channels: Na+ may promote PIP2 binding to GIRK channels by breaking a hydrogen bond formed between an aspartate in the βC-βD sheet and an arginine66,91. These high-resolution protein structures have provided important snapshots of inward rectifiers in different conformational states as well as provided detailed maps for the location of functional domains. The next wave of high-resolution structural analysis should provide a more precise 3-D model of the conformational changes that underlie the gating mechanisms of inwardly rectifying channels.
A macromolecular signaling complex for GIRK channels
Over the last few years, several studies have provided generated for the existence of a macromolecular signaling complex on the plasma membrane. This complex is postulated to contain G proteins, GPCRs, GIRK channels and regulatory proteins92-97 (see review by ref2) (Figure 2C). First, because GIRK channels remain open as long as Gβγ subunits are available, Gα subunits are expected to remain near the channel to terminate GIRK channel activation, through formation of the inactive Gαβγ heterotrimer (Box 1). Second, PTX-sensitive Gα subunits can associate directly with GIRK channels and alter channel gating84,85,98,99. In fact, Gα may also be involved in establishing receptor specificity such that stimulation of GPCRs that couple to only PTX-sensitive G proteins activate GIRK channels84,100. Third, spectroscopic studies with fluorescently tagged GPCRs, G proteins and regulator of G protein signaling (RGS) proteins, have provided details on the spatial relationship between proteins within this macromolecular signaling complex26,92,94,96,101,102 (see Box 2). Interestingly, spectroscopic studies with Gαiβγ heterotrimers have suggested that G protein activation involves a conformational rearrangement of the Gαi and Gβγ subunits, in contrast to the canonical model whereby Gα and Gβγ subunits physically separate103. The ability to undergo rearrangement, however, may be unique to Gαi, since other studies suggest Gαo may dissociate104-107. Together, these studies support a view that GIRK channels reside in a macromolecular signaling complex, governed by protein-protein interactions, limited diffusion and lipid domains2.
Recent studies have begun to investigate the regulation and localization of GIRK channels expressed in their native environment. In cerebellar granule cells, biochemically restricting the movement of μ opioid receptors does not alter the rate of GIRK activation, revealing that these signaling proteins are localized in membrane compartments and are not freely diffusing in the membrane97. Using neuronal PC12 cells, it was discovered that chronic stimulation of endogenous M2 muscarinic (M2R) receptors leads to down regulation of both the receptors and GIRK channels108, indicating M2R and GIRK are co-regulated in a complex. Furthermore, the GABABR2 subunit has been shown to dimerize with the M2R and form an even larger functional M2R/GABABR2/GIRK heteromeric complex109. Electron microscopic (EM) immunohistochemical studies with brain tissue provide information on the localization of native GIRK channels. In hippocampus, GIRK channels are expressed on the dendritic shafts of hippocampal neurons110, which has been confirmed by cell-attached patch-clamp recordings111. In addition to the shaft, immuno-gold labeling show a tight association of GIRK channels and GABAB receptors on dendritic spines110. In the cerebellum, immunoelectron microscopy studies show that GIRK2/GIRK3 channels are localized in the postsynaptic density, while GIRK1/GIRK3 channels are perisynaptic112. It is tempting to speculate that the subcellular localization may correlate with the Gβγ affinity (GIRK2/GIRK3; lower EC50) in neurons.
Future immunoelectron or super-resolution microscopy studies may reveal additional details on the localization of GIRK channels, GABAB receptors, RGS and G proteins in neurons, that provide further insights into their function. Discussions concerning the existence of a GIRK macromolecular signaling complex in vivo still continue, however. Additional studies will need to clarify the role of collision coupling and whether changes in GIRK signaling occur at the level of the GPCR/GIRK macromolecular complex or individual components in the signaling pathway.
Reducing excitability: physiological roles for GIRK channels
The physiological activation of GIRK channels can shape the neuronal network behavior in many areas of the brain at different levels. The basal activity of GIRK channels contributes to the resting potential of neurons, shifting the membrane voltage by ~−8 mV27. This hyperpolarization of the resting membrane potential decreases electrical excitability. In addition, receptor activation of GIRK channels provides another level of inhibition, to which three different changes in signaling can be generally ascribed.
Neuronal self-inhibition
Some neurons release a neurotransmitter to activate Gi/o-coupled receptors and in turn GIRK channels on their own dendrites (autaptic transmission), causing self-inhibition (Figure 3A). For example, a train of action potentials fired by low threshold spiking (LTS) interneurons of the cortex results in long-lasting hyperpolarization. This change in excitability occurs in a cell-autonomous way through endocannabinoids released from dendrites and the activation of CB1 receptors coupled to GIRK channels on the very same dendrites113. This modulation of LTS neurons may lead to long-lasting changes in cortical networks owing to altered glutamate transmission in pyramidal neurons. Dopamine neurons also exhibit a form of autaptic inhibition. Here, dendodendritic release of dopamine activates dopamine D2 receptors coupled to GIRK channels, leading to suppression of firing114.
Figure 3. Three types of neuronal signaling pathways for GIRK channels in the brain.
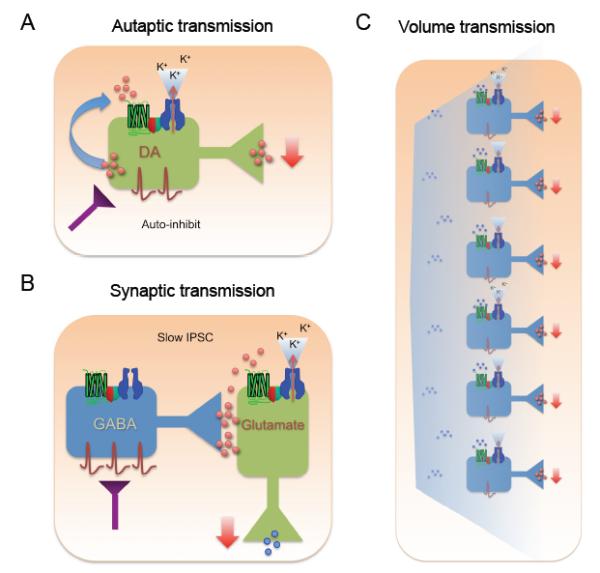
A) In autaptic synapses, neurotransmitters are released by and bind to the same neuron, stimulating GPCRs and activating GIRK channels. The net effect of such an autapic synapse is a reduction in the release of neurotransmitter (negative feedback). B) The slow IPSC. High stimulation triggers release of GABA that spills over to neighboring GABAB /GIRK complexes, located on the dendritic spine and shaft. C) Network modulation. GIRK channels involved in volume transmission. When a neurotransmitter is released from many neurons, the ambient concentration rises and diffuses to activate GIRK channels on target neurons. This leads to reduced network activity.
Neuron-to-neuron inhibition
Activation of postsynaptic GABAB receptors115, D2 receptors116 and group II metabotropic glutamate receptors mGluRs117 by transmitters released from neighboring neurons (synaptic transmission) may also activate GIRK channels. For GABAergic synapses, GABA released spontaneously or driven by a single action potential produces a fast inhibitory postsynaptic potential, mediated by GABAA channels (Figure 1C - left panel, 3B). By contrast, strong or repetitive stimulation is required to elicit the slow inhibitory potential, suggesting that GABA released into the synaptic cleft diffuses and activates peri-synaptic GABAB receptors coupled to GIRK channels (Figure 1C-right panel, 3B)118-120. Thus, GABAB receptors/GIRK channels could be positioned directly adjacent to synaptically localized GABAA receptors or in a neighboring dendritic spine (for review, see ref121). The slow time course of outward current through GIRK channels therefore correlates with the time required for diffusion of the neurotransmitter and is not limited by the speed of G protein activation. The reuptake/degradation of the neurotransmitter and the limited space for diffusion may affect the amplitude of the slow IPSC, thus regulating the degree of inhibition. In the hippocampus, generation of the slow IPSC is important for regulating the rhythmic activity of the network119. In midbrain DA neurons, slow activation of GIRKs via D2 receptors originates from local pooling of dendrodendritically released DA114,122. Future studies are needed to demonstrate a physiological role for the slow IPSC in vivo. In addition to postsynaptic activation, recent characterizations of GABAB receptor mediated presynaptic inhibition has revealed a component that is sensitive to a GIRK-specific channel inhibitor tertiapin123, suggesting a presynaptic role for GIRK channels in inhibiting neurotransmitter release2,124,125. Thus, GABA may inhibit pre-synaptic release concurrent with postsynaptic hyperpolarization.
Network-level inhibition
The regulation of ambient levels of several endogenous GPCR agonists (e.g. adenosine, somatostatin) may exert a modulatory effect on large-scale neuronal networks through GIRK channel activation. This large-scale effect of neuromodulators is known as volume transmission. Moderate activation of GIRK channels in a population of neurons would be expected to dampen membrane excitability (Figure 3C). For instance, somatostatin acting via SST5 receptors may alter the oscillation behavior of thalamic networks through post-synaptic activation of GIRK channels, along with presynaptic inhibition126. Similarly endogenous adenosine may suppress gamma oscillations in the hippocampus127, possibly due to the selective expression of A1 receptors on pyramidal neurons, which also activate GIRK channels. Dopamine concentrations are difficult to measure after synaptic or dendritic release128 but can be modeled in the extracellular space based on known diffusion properties and uptake efficiency of surrounding cells. The transmitter encounters predominantly extrasynaptic DA autoreceptors on DA axons and heteroreceptors on neighboring cells. In this model the sphere of influence for high affinity DA receptors has a volume of several tens of μm3, and may therefore affect thousands of synapses of many neurons simultaneously. Conditional transgenic mouse lines or cell specific knockouts of GIRK channel subunits are among the future studies that are required to specifically investigate the role of GIRK channels in signaling pathways beyond the direct hyperpolarization.
Moving channels to modulate GIRK
Recently, a plasticity of the slow IPSCs in response to GABAB and D2 receptor activation has been observed (Figures 3b, 4). For example, depolarization of the postsynaptic neurons to bring the membrane potential close to 0 mV, increases the GABAB receptor-mediated IPSC several fold for the duration of the experiment129. This increased IPSC is independent of the activation of GABAB receptors, but dependent on NMDAR activation, similar to hippocampal NMDAR-dependent LTP of AMPAR-mediated EPSCs. It has been suggested that GIRK currents become larger because additional channels are inserted130. Indeed, the activation of NMDA receptor in cultured dissociated hippocampal neurons increases the level of the surface expression of GIRK1 and GIRK2 channel in the soma and dendrites within minutes130. This insertion requires protein phosphatase-1-mediated dephosphorylation of a GIRK2 serine residue (Ser-9) that promotes channel recycling. Increased expression of GIRK channels would reduce excitability, dampening signaling of hippocampal pyramidal neurons. For example, GIRK channel activation is implicated in the maintenance of LTP at glutamatergic synapses. In mice lacking GIRK2, the depotentiation of LTP in hippocampal neurons is impaired because adenosine A1 receptor fail to activate of GIRK channels 131.
In contrast, activation of dopamine D2 receptors causes a long-term depression (LTD) of the DA-dependent slow IPSC 116. This LTD is inhibited by the presence of the calcium chelator BAPTA in the recordings pipette, and seems therefore to rely on a cell-autonomous postsynaptic effect (Figure 3A). This reduction in inhibition would be expected to increase burst firing and DA release in the terminal projections in vivo. Less is known concerning the mechanism of this plasticity but could involve desensitization of D2 receptors, modulation of G-protein function and/or GIRK channel availability (as GIRK channels can rapidly redistribute).
Taken together, GIRK channels play a key role in many types of neuronal communication and, as will be outlined in the following sections, modification of GIRK channels can modulate CNS function in health and disease (Figure 4).
Figure 4. GIRK channels are implicated in different disease states.
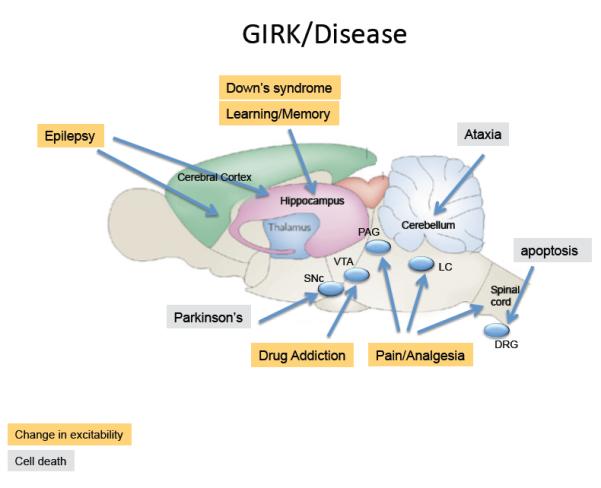
Schematic shows saggital view of a rat brain highlighting regions where GIRK channels have been implicated in brain neuronal disorders or diseases due to changes in excitability (yellow) or cell death (grey). Changes in GIRK activity throughout the brain may contribute to: Epilepsy: GIRK2 knockout mice develop spontaneous convulsions and show a propensity for generalized seizures145. Similar pro-convulsive effects are observed with GIRK channel inhibitor tertiapin when administered intrathecally.]148. Down’s Syndrome & Learning and Memory: Two mouse models for Down’s syndrome, the Ts65Dn149 and the Ts1Cje DS150, contain a gene duplication for Girk2 and show larger slow IPSC mediated by GABAB receptors151, impairments in LTP and enhancement of LTD151. Although all neurons are affected, this phenotype may involve mostly hippocampal functions. Drug Addiction: GIRK knockouts show impairment in self-administration of cocaine35, altered response to GHB26, less severe withdrawal from sedatives34 and reduced conditioned place taste aversion for ethanol158. Changes in the function of the mesolimbic system, involving the VTA, are thought to underlie these changes. Pain: Removing GIRK2/GIRK3 reduces the potency (coupling efficiency), but leaves efficacy (maximal response) of opioid-analgesia intact30,137, effects most likely involving the peri-acqueductal grey and spinal cord. Chronic neuropathic pain can lead to tyrosine phosphorylation of GIRK1 subunits, which reduces basal GIRK activity171. Ataxia/Parkinson’s: In the weaver mouse, GIRK2 channels contain a mutation that eliminates K+ selectivity and leads to degeneration of midbrain SNc DA neurons and cerebellar granular neurons161. The gain-of-function phenotype in DA neurons is of clinical interest due to its similarity to the degeneration in Parkinson’s disease. In dorsal root ganglion (DRG) cells, activation of the neurotrophin receptor p75 NTR increases levels of PIP2, which activates GIRK2 channels and signals to promote programmed cell death via K+ efflux-induced apoptosis164.
GIRK channels in pain perception and analgesia
GIRK channels have been implicated in pain perception. This notion is based on studies with mice carrying mutations in GIRK channels, and the activation of GIRK channels by endogenous pain modulators such as endorphins, endocannabinoids and analgesic drugs.
GIRK channels were first implicated in pain perception when opioid analgesia was tested in weaver mice that carry a spontaneous mutation in the pore region of GIRK2132 leading to a loss of K+ selectivity and G-protein insensitivity (GIRK2wv)22,133,134. Upon receptor activation GIRK2wv depolarizes rather than hyperpolarizes neurons. Morphine and a selective κ opioid agonist (U-50488) that are known to activate GIRK2-containing channels through opioid receptors reduce pain perception in wild-type mice, but not in the weaver mice135. The interpretation of the opioid-dependent behaviors, however, is confounded by the loss of neurons in the substantia nigra and cerebellum136.
Subsequent studies have taken advantage of GIRK channel knockout mice (see Table 1). In GIRK2−/− mice, for example, the dose-response curve for morphine and clonidine (an α2AR agonist) analgesia is shifted to higher concentrations, while the maximal effect was preserved137. This finding was confirmed in a recent study using a GIRK2/3 double knockout mouse30; in these mice the potency (coupling efficiency) of opioid-analgesia was reduced, but their efficacy (maximal response) was intact.
Knockout studies also suggest that GIRK channels may account for gender difference in nociception. The higher pain threshold in males, measured as a longer latency for tail withdrawal to radiant heat, is abolished in GIRK2−/− mice137, indicating that GIRK channel activation may account for some of the gender differences in normal pain perception. Further evidence for this notion came from a study that showed the analgesic effects of ethanol, oxotremorine (muscarinic receptor agonist), baclofen (GABAB receptor agonist), clonidine (α2 receptor agonist) and WIN 55,212-2 (cannabinoid receptor agonist) reduced or eliminated in male but not female GIRK2−/− mice138. Studies using mice lacking GIRK1 or GIRK2 channels show a blunted response to μ- ([D-Ala(2),N-Me-Phe(4),Gly(5)-ol]-enkephalin, DAMGO) and ∂-(Tyr-D-Ala-Phe-Glu-Val-Val-Gly amide) but not κ-opioid receptor ((trans)-3,4-dichloro-N-methyl-N-[2-(1-pyrrolidinyl)-cyclohexyl] benzeneacetamide methanesulfonate hydrate) agonists when applied intrathecally139. Similarly, intrathecal tertiapin, a selective GIRK channel inhibitor123, reduced the efficiency of DAMGO to increase the latency for withdrawal in the immersion tail-flick test. Therefore, while further studies are needed to clarify the role of GIRK channels in opioid-induced analgesia, there is general agreement that GIRK channels modulate systemic opioid (or Gio)-mediated analgesia through postsynaptic inhibition.
The periaqueductal grey (PAG), a small nucleus in the brain stem, has a crucial role in central analgesia; direct application of opioids into the PAG inhibits GABA neurons through both GIRK-independent (presynaptic) and GIRK-dependent (postsynaptic) mechanisms, which is sufficient to induce analgesia140,141. The disinhibition of principal neurons of PAG that project to nuclei of the caudal brain stem and modulate ascending pain pathways likely plays a significant role in the analgesic effects of opioids.
GIRK channels have also been implicated in opioid tolerance and dependence due to adaptive changes in chronic exposure to morphine. However, opioid receptor agonist-induced desensitization of GIRK currents seems to involve additional mechanisms. In locus coeruleus neurons, inhibiting both G protein receptor kinase 2 (GRK2) and extracellular signal-regulated kinase (ERK) signaling significantly reduces the met-enkephalin induced GIRK channel desensitization that normally occurs within minutes of drug application142, an adrenergic nucleus in the pons that lowers pain threshold when activated. However, GIRK channel desensitization may not occur when μ-opioid receptors are activated by morphine. In fact, morphine elicits much less GIRK channel desensitization in vitro and in vivo143 compared to met-enkephalin. This response is surprising because met-enkephalin induces much less drug tolerance and dependence, than morphine. A recent report even suggests that the potency of morphine to reduce pain (i.e. the effect at a submaximal concentration) may actually increase during the development of tolerance (i.e. reduction of maximal effect) 144. This is paradoxical but could be explained if tolerance and dependence are mediated by effectors of opioid receptors other than GIRK channels.
An alternate role of GIRK channels in opioid dependence is suggested by the following observations. Mice lacking GIRK2 and GIRK3 have strongly reduced withdrawal signs normally induced in wild-type controls by the opioid receptor antagonist naloxone after chronic exposure to morphine. In the mutant mice, the withdrawal syndrome can be rescued if the output from locus coeruleus neurons is inhibited during the exposure to morphine30. This suggests that GIRK channels ‘gate’ the induction of opioid dependence by silencing the neurons in the locus coeruleus every time opioids are injected.
Taken together these data suggest that GIRKs have a role in pain perception under specific conditions, and that they contribute to opioid-analgesia. However, the literature remains controversial on the role of the PAG and the LC as two key regions in the regulation of opioid-analgesia. Further insight may come from mouse lines in which GIRK subunits are mutated in a cell type-specific manner (e.g., only GIRK-expressing neurons in the locus coeruleus) or where GIRK channels are coupled to light-activated GPCRs.
Altered excitability and dying neurons: GIRK channels in disease
GIRK channels are implicated in the pathophysiology of several diseases, including epilepsy, addiction, Down’s syndrome, ataxia and Parkinson’s disease. Two broad principles have been distinguished. First, loss of GIRK function can lead to excessive excitability leading, such as in epilepsy, while a gain of GIRK function can significantly reduce neural activity, as postulated in Down’s syndrome. Second, loss of selectivity can cause ion fluxes across GIRK channels, such as excessive K+ efflux that triggers cell death, exemplified in a model of Parkinson’s disease.
Changes in excitability
The involvement of GIRK channels in epilepsy has been inferred from the phenotype of mice where a GIRK subunit has been deleted. GIRK2 knockout mice develop spontaneous convulsions and show a propensity for generalized seizures when injected with a pro-convulsive GABAA receptor antagonist145. Drugs such as desimipramine, fluoxetine, haloperidol, thioridazine, pimozide and clozapine146 (Supplemental Table S1) that are used for the treatment of various neurological disorders, may inhibit GIRK channels and hence cause seizures, a known side effect of these drugs. On the other hand, electroconvulsive shock leads to increased expression of GIRK channels147, which may serve as a possible neuroprotection for inappropriate activity. In support of this idea, stimulation of galanin type 2 receptors that activate GIRK channels prevents kindled epileptogenesis in rats148, highlighting GIRK channels as a novel target for anticonvulsants.
Down’s syndrome is a genetic disorder caused by duplication of human chromosome 21, which contains the Girk2 (Kcnj6) gene. To investigate the contribution of a third copy of the Girk2 gene to the phenotype of Down’s syndrome, two mouse models have been generated that carry either a partial or full segment duplication of the mouse chromosome 16, the ortholog to human chromosome 21149,150. In both lines, GIRK2 protein is upregulated, resulting in a larger slow IPSC mediated by GABAB receptors151. Moreover, hippocampal LTP is reduced while LTD is enhanced in both lines and therefore synaptic plasticity of glutamatergic transmission is altered. Since the duplicated chromosomal segment also carries other genes, additional studies are needed to specifically test the role of GIRK channels in the malfunction of the nervous system in Down’s syndrome.
Addiction
GIRK channels are thought to have a role in the acute rewarding effects and/or the adaptation that occurs with chronic exposure to addictive drugs that work through GPCRs, such as opioids, the club-drug GHB, and cannabinoids. Addictive drugs are known to strongly increase dopamine levels in the mesocorticolimbic system. Based on in vivo and in vitro experiments, distinct cellular mechanisms have been proposed for different classes of drugs152. Opioids and the club drug γ-hydroxbutyrate (GHB) activate GIRK channels leading to disinhibition of DA neurons. Morphine stimulates μ-opioid receptors that are selectively expressed on interneurons of the VTA and activate GIRK channels, reducing the firing of these cells, eventually leading to disinhibition of DA neurons153. The effect of GHB is more complex, as GABAB receptors are expressed on both GABA and DA neurons. However, DA neurons in the VTA are an order magnitude less sensitive (higher EC50) to GABAB receptor agonists than GABA interneurons43 most likely due to the selective expression of GIRK2c and GIRK3 and the lack of GIRK1 subunit (see discussion above). Consequently, low concentrations of GHB only activate GIRK currents in GABA neurons (containing GIRK1 subunits), leading to disinhibition of DA neurons. In addition, DA neurons in the VTA also selectively express RGS2, which contributes to the lower GABABR-GIRK sensitivity26. Interestingly, chronic exposure to morphine or GHB enhances the coupling efficiency (EC50) for GABAB receptors and GIRK channels by lowering levels of RGS2 in DA neurons26. Under these circumstances the concentration window for disinhibition is much narrower and now low concentrations of drug are sufficient to inhibit DA neurons and become behaviorally aversive26. Thus, the combination of GIRK subunits expressed in a neuron can determine the signaling properties of neuronal circuits. In the case of cannabinoids, the disinhibition of DA neurons seems to occur primarily through presynaptic, GIRK-independent mechanisms154 (but see 124,125).
GIRK channels may also be involved in the response to psychostimulants, such as cocaine and amphetamines. SNX27, a psychostimulant-inducible protein155, specifically regulates surface expression of GIRK2c and GIRK340. GIRK2 and GIRK3 knockout mice exhibit dramatically reduced intravenous self-administration of cocaine relative to wild-type mice35, while surprisingly, the GIRK2/3 double knockout self-administer more cocaine than wild type mice suggesting compensation in the single subunit knockouts that is no longer possible in the double knockout. Elucidating the changes in GIRK signaling with psychostimulants is an exciting area for future research and may lead to further insight of the role of GIRK channels in addiction.
Because ethanol directly binds and activates GIRK channels46-48,156, they may mediate some ethanol-related behaviors. In fact, ethanol has been shown to enhance GABAB-activated GIRK currents in neurons of the VTA156, which would decrease the firing frequency of DA VTA neurons in rodents. As discussed above, GIRK channels likely have a role in the analgesic effects of ethanol138,157, but whether they also contribute to the reinforcing effects of ethanol is less clear. GIRK2 knockout mice exhibit reduced conditioned taste aversion, compared to wild-type mice suggesting that GIRK activation could be involved in the motivational response of ethanol158,. However, these differences were observed with moderate but not high doses of ethanoll158. Using a different approach, involving identification of a quantitative trait locus (QTL) with large effects on predisposition to physical dependence and associated withdrawal from sedative hypnotics such as ethanol, a 0.44 Mb region of mouse chromosome 1 was mapped for this QTL that contains Girk3 (Kcnj9)34. In a behavioral assay designed to measure withdrawal, GIRK3 −/− mice show less severe withdrawal symptoms from ethanol and barbituarates use34.
Based on these results, new approaches for addiction therapies have emerged and more may come. For example, pharmacological activation of GIRK channels by administration of the GABAB receptor agonist baclofen reverses behavioral sensitization to morphine in rats159 and clinical Phase 2 & 3 trials are underway examining the effect of baclofen for treating ethanol and cocaine addiction.
Cell death due to GIRK signaling
Parkinson’s disease (PD) is a debilitating motor coordination disorder caused by degeneration of dopamine neurons in the substantia nigra (SN). The involvement of GIRK channels in Parkinson’s disease was first inferred from the weaver mouse132, where constitutively active GIRK2wv channels produce chronic depolarization and cell death in a subset of neurons in the brain22,133,134 mimicking the neuronal degeneration observed in Parkinson’s disease160. The gain-of-function phenotype in DA neurons of weaver mice is of clinical interest due to the progressive degeneration of DA neurons in the substantia nigra, while DA neurons in the VTA are spared in the early stages of the disease161. Interestingly, DA neurons of the SN express only GIRK2, which results in a gain of function, while DA neurons in the VTA coexpress GIRK2/GIRK3, which may produce a loss of function due to co-assembly with GIRK322,133. Other mechanisms of DA cell death in the weaver mouse have been proposed, however. One study has suggested that constitutively active GIRK2wv channels trigger activation of K-ATP (Kir6.2) channels in response to mild mitochondrial uncoupling in SNc DA neurons but not VTA DA neurons162 while other studies suggest a general susceptibility to oxidative stress (due to reduced thiol redox state) in DA neurons163. Weaver mice have additional developmental abnormalities that result in a complex phenotype including learning deficits, epileptic seizures, and motor disturbances (e.g. ataxia).
Another line of evidence implicating GIRK channels comes from a recent study investigating nerve growth factor (NGF) mediated programmed cell death in dorsal root ganglion neurons, an important step in the proper normal development of the nervous system. This study shows that activation of the neurotrophin receptor p75 NTR increases plasma membrane levels of PIP2, activating GIRK2 channels and creating a sustained K+ efflux that stimulates programmed cell death164.
Taken together, GIRK channels may contribute to the pathophysiology of a variety of human diseases, either by altering the excitability of specific neurons, changing the slow IPSCs, or promoting cell death. It is important to note that the suggested roles for GIRK channels in brain diseases are based on observations in animal models. Future genetic studies may provide direct evidence for altered GIRK function in human diseases.
Conclusions
Research over the last twenty years has elucidated the molecular and structural determinants of GIRK channel activation and modulation by G proteins (Gα andGβγ, with the emerging concept of the existence of macromolecular signaling complexes. Knockout mice for all four mammalian subunits greatly contributed to the understanding of GIRK functions, namely to regulate the excitability of neurons in a cell autonomous fashion, in-between neighboring neurons by regulating synaptic transmission and also regulating the activity of large-scale networks. While we only begin to understand the role of GIRK channels with different subunit compositions, their respective expression pattern in the brain will give further functional insight. For example, DA neurons of the VTA express a unique GIRK2/3 heteromeric channel that has a particularly low affinity for the Gβγ dimer. More research is needed to link such cellular observations to function and behavior. The development of novel tools, such as light activation of GPCRs (optoXRs)165 that couple to GIRK channels as well as conditional and cell-specific knock-out mouse lines, will be important to further advance our understanding of GIRK function. These tools will also be crucial for the investigation of GIRK channels in disease and may help to design specific drugs, selectively opening or closing GIRK channels composed of different subunit compositions, to treat addiction, epilepsy, Down’s syndrome, or Parkinson’s disease.
Supplementary Material
Acknowledgements
We thank Dr. Prafulla Aryal for the 3D structural figures and Arnaud Lalive d’Epinay for compiling the Supplemental Tables. Our work was made possible by financial support from McKnight Endowment Fund for Neuroscience (PAS), the Human Frontiers Science Foundation (PAS, CL), the NARSAD (PAS), the Swiss National Science Foundation (CL) and the National Institute of Neurological Disorders and Stroke (R01 NS37682; PAS), and National Institute on Drug Abuse (R01 DA019022; PAS, CL). We apologize to all our colleagues whose works, due to limitation in the number of references, we have not been able to include in the review.
References
- 1.Trevelyan AJ, Watkinson O. Does inhibition balance excitation in neocortex? Progress in Biophysics and Molecular Biology. 2005;87:109–143. doi: 10.1016/j.pbiomolbio.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 2.Lujan R, Maylie J, Adelman JP. New sites of action for GIRK and SK channels. Nat Rev Neurosci. 2009;10:475–480. doi: 10.1038/nrn2668. [DOI] [PubMed] [Google Scholar]
- 3.Yamada M, Inanobe A, Kurachi Y. G protein regulation of potassium ion channels. Pharmacol Rev. 1998;50:723–757. [PubMed] [Google Scholar]
- 4.Pfaffinger PJ, Martin JM, Hunter DD, Nathanson NM, Hille B. GTP-binding proteins couple cardiac muscarinic receptors to a K channel. Nature. 1985;317:536–538. doi: 10.1038/317536a0. [DOI] [PubMed] [Google Scholar]
- 5.Logothetis DE, Kurachi Y, Galper J, Neer EJ, Clapham DE. The βγ subunits of GTP-binding proteins activate the muscarinic K+ channel in heart. Nature. 1987;325:321–326. doi: 10.1038/325321a0. [DOI] [PubMed] [Google Scholar]
- 6.Wickman KD, Iniguez-Lluhl JA, Davenport PA, Taussig R, Krapivinsky GB, Linder ME, Gilman AG, Clapham DE. Recombinant G-protein βγ-subunits activate the muscarinic-gated atrial potassium channel. Nature. 1994;368:255–257. doi: 10.1038/368255a0. [DOI] [PubMed] [Google Scholar]
- 7.Reuveny E, Slesinger PA, Inglese J, Morales JM, Iniguez-Lluhi JA, Lefkowitz RJ, Bourne HR, Jan YN, Jan LY. Activation of the cloned muscarinic potassium channel by G protein βγ subunits. Nature. 1994;370:143–146. doi: 10.1038/370143a0. [DOI] [PubMed] [Google Scholar]
- 8.Inanobe A, Morishige K-I, Takahashi N, Ito H, Yamada M, Takumi T, Nishina H, Takahashi K, Kanaho Y, Katada T, Kurachi Y. Gβγ directly binds to the carboxyl terminus of the G protein-gated muscarinic K+ channel, GIRK1. Biochem Biophys Res Commun. 1995;212:1022–1028. doi: 10.1006/bbrc.1995.2072. [DOI] [PubMed] [Google Scholar]
- 9.Huang CL, Slesinger PA, Casey PJ, Jan YN, Jan LY. Evidence that direct binding of Gβγ to the GIRK1 G protein-gated inwardly rectifying K+ channel is important for channel activation. Neuron. 1995;15:1133–1143. doi: 10.1016/0896-6273(95)90101-9. [DOI] [PubMed] [Google Scholar]
- 10.Wickman K, Karschin C, Karschin A, Picciotto MR, Clapham DE. Brain localization and behavioral impact of the G-protein-gated K+ channel subunit GIRK4. J Neurosci. 2000;20:5608–5615. doi: 10.1523/JNEUROSCI.20-15-05608.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lesage F, Guillemare E, Fink M, Fabrice D, Heurteaux C, Fosset M, Romey G, Barhanin J, Lazdunski M. Molecular properties of neuronal G-protein-activated inwardly rectifying K+ channels. J Biol Chem. 1995;270:28660–28667. doi: 10.1074/jbc.270.48.28660. [DOI] [PubMed] [Google Scholar]
- 12.Isomoto S, Kondo C, Takahashi N, Matsumoto S, Yamada M, Takumi T, Horio Y, Kurachi Y. A novel ubiquitously distributed isoform of GIRK2 (GIRK2B) enhances GIRK1 expression of the G-protein-gated K+ current in Xenopus oocytes. Biochem Biophys Res Commun. 1996;218:286–291. doi: 10.1006/bbrc.1996.0050. [DOI] [PubMed] [Google Scholar]
- 13.Inanobe A, Horio Y, Fujita A, Tanemoto M, Hibino H, Inageda K, Kurachi Y. Molecular cloning and characterization of a novel splicing variant of the Kir3.2 subunit predominantly expressed in mouse testis. J Physiol. 1999;521(Pt 1):19–30. doi: 10.1111/j.1469-7793.1999.00019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lesage F, Duprat F, Fink M, Guillemare E, Coppola T, Lazdunski M, Hugnot JP. Cloning provides evidence for a family of inward rectifier and G-protein coupled K+ channels in the brain. FEBS Lett. 1994;353:37–42. doi: 10.1016/0014-5793(94)01007-2. [DOI] [PubMed] [Google Scholar]
- 15.Wei J, Hodes ME, Piva R, Feng Y, Wang Y, Ghetti B, Dlouhy SR. Characterization of murine Girk2 transcript isoforms: Structure and differential expression. Genomics. 1998;51:379–390. doi: 10.1006/geno.1998.5369. [DOI] [PubMed] [Google Scholar]
- 16.Stoffel M, Tokuyama Y, Trabb JB, German MS, Tsaar ML, Jan LY, Polonsky KS, Bell GI. Cloning of rat KATP-2 channel and decreased expression in pancreatic islets of male Zucker diabetic fatty rats. Biochem Biophys Res Commun. 1995;212:894–899. doi: 10.1006/bbrc.1995.2053. [DOI] [PubMed] [Google Scholar]
- 17.Bond CT, Ämmälä C, Ashfield R, Blair TA, Gribble F, Khan RN, Lee K, Proks P, Rowe ICM, Sakura H, Ashford MJ, Adelman JB, Ashcroft FM. Cloning and functional expression of the cDNA encoding an inwardly-rectifying potassium channel expressed in pancreatic β-cells and in the brain. FEBS Lett. 1995;367:61–66. doi: 10.1016/0014-5793(95)00497-w. [DOI] [PubMed] [Google Scholar]
- 18.Nelson CS, Marino JL, Allen CN. Cloning and characterization of Kir3.1 (GIRK1) C-terminal alternative splice variants. Mol Br Res. 1997;46:185–196. doi: 10.1016/s0169-328x(96)00301-4. [DOI] [PubMed] [Google Scholar]
- 19.Liao YJ, Jan YN, Jan LY. Heteromultimerization of G-protein-gated inwardly rectifying K+ channel proteins GIRK1 and GIRK2 and their altered expression in weaver brain. J Neurosci. 1996;16:7137–7150. doi: 10.1523/JNEUROSCI.16-22-07137.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jelacic TM, Kennedy ME, Wickman K, Clapham DE. Functional and biochemical evidence for G-protein-gated inwardly rectifying K+ (GIRK) channels composed of GIRK2 and GIRK3. J Biol Chem. 2000;275:36211–36216. doi: 10.1074/jbc.M007087200. [DOI] [PubMed] [Google Scholar]
- 21.Tucker SJ, Pessia M, Adelman JP. Muscarine-gated K+ channel: subunit stoichiometry and structural domains essential for G protein stimulation. Amer J Physiol. 1996;271:H379–H385. doi: 10.1152/ajpheart.1996.271.1.H379. [DOI] [PubMed] [Google Scholar]
- 22.Slesinger PA, Patil N, Liao YJ, Jan YN, Jan LY, Cox DR. Functional effects of the mouse weaver mutation on G protein-gated inwardly rectifying K+ channels. Neuron. 1996;16:321–331. doi: 10.1016/s0896-6273(00)80050-1. [DOI] [PubMed] [Google Scholar]
- 23.Kofuji P, Davidson N, Lester HA. Evidence that neuronal G-protein-gated inwardly rectifying K+ channels are activated by Gβγ subunits and function as heteromultimers. Proc Natl Acad Sci USA. 1995;92:6542–6546. doi: 10.1073/pnas.92.14.6542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krapivinsky G, Gordon EA, Wickman K, Velimirovic B, Krapivinsky L, Clapham DE. The G-protein-gated atrial K+ channel IKACh is a heteromultimer of two inwardly rectifying K+-channel proteins. Nature. 1995;374:135–141. doi: 10.1038/374135a0. [DOI] [PubMed] [Google Scholar]
- 25.Jelacic TM, Sims SM, Clapham DE. Functional expression and characterization of G-protein-gated inwardly rectifying K+ channels containing GIRK3. J Membr Biol. 1999;169:123–129. doi: 10.1007/s002329900524. [DOI] [PubMed] [Google Scholar]
- 26.Labouèbe G, Lomazzi M, Cruz H, Creton C, Luján R, Li M, Yanagawa Y, Obata K, Watanabe M, Wickman K, Boyer S, Slesinger P, Lüscher C. RGS2 modulates coupling between GABAB receptors and GIRK channels in dopamine neurons of the ventral tegmental area. Nat Neurosci. 2007;12:1559–1568. doi: 10.1038/nn2006. [DOI] [PubMed] [Google Scholar]
- 27.Lüscher C, Jan LY, Stoffel M, Malenka RC, Nicoll RA. G-protein-coupled inwardly rectifying K+ channels (GIRKs) mediate postsynaptic, but not presynaptic transmitter actions in hippocampal neurons. Neuron. 1997;19:687–695. doi: 10.1016/s0896-6273(00)80381-5. [DOI] [PubMed] [Google Scholar]
- 28.Koyrakh L, Lujan R, Colon J, Karschin C, Kurachi Y, Karschin A, Wickman K. Molecular and Cellular Diversity of Neuronal G-Protein-Gated Potassium Channels. J Neurosci. 2005;25:11468–11478. doi: 10.1523/JNEUROSCI.3484-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Torrecilla M, Marker CL, Cintora SC, Stoffel M, Williams JT, Wickman K. G-protein-gated potassium channels containing Kir3.2 and Kir3.3 subunits mediate the acute inhibitory effects of opioids on locus ceruleus neurons. J Neurosci. 2002;22:4328–4334. doi: 10.1523/JNEUROSCI.22-11-04328.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cruz HG, Berton F, Sollini M, Blanchet C, Pravetoni M, Wickman K, Luscher C. Absence and rescue of morphine withdrawal in GIRK/Kir3 knock-out mice. J Neurosci. 2008;28:4069–4077. doi: 10.1523/JNEUROSCI.0267-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marker CL, Lujan R, Colon J, Wickman K. Distinct populations of spinal cord lamina II interneurons expressing G-protein-gated potassium channels. J Neurosci. 2006;26:12251–12259. doi: 10.1523/JNEUROSCI.3693-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Slesinger PA, Stoffel M, Jan YN, Jan LY. Defective γ-aminobutyric acid type B receptor-activated inwardly rectifying K+ currents in cerebellar granule cells isolated from weaver and Girk2 null mutant mice. Proc Natl Acad Sci USA. 1997;94:12210–12217. doi: 10.1073/pnas.94.22.12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pravetoni M, Wickman K. Behavioral characterization of mice lacking GIRK/Kir3 channel subunits. Genes, Brain and Behavior. 2008;7:523–531. doi: 10.1111/j.1601-183X.2008.00388.x. [DOI] [PubMed] [Google Scholar]
- 34.Kozell LB, Walter NA, Milner LC, Wickman K, Buck KJ. Mapping a barbiturate withdrawal locus to a 0.44 Mb interval and analysis of a novel null mutant identify a role for Kcnj9 (GIRK3) in withdrawal from pentobarbital, zolpidem, and ethanol. J Neurosci. 2009;29:11662–11673. doi: 10.1523/JNEUROSCI.1413-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morgan AD, Carroll ME, Loth AK, Stoffel M, Wickman K. Decreased cocaine self-administration in Kir3 potassium channel subunit knockout mice. Neuropsychopharm. 2003;28:932–938. doi: 10.1038/sj.npp.1300100. [DOI] [PubMed] [Google Scholar]
- 36.Slesinger PA, Reuveny E, Jan YN, Jan LY. Identification of structural elements involved in G protein gating of the GIRK1 potassium channel. Neuron. 1995;15:1145–1156. doi: 10.1016/0896-6273(95)90102-7. [DOI] [PubMed] [Google Scholar]
- 37.Nemec J, Wickman K, Clapham DE. Gβγ binding increases the open time of IKACh: kinetic evidence for multiple Gβγ binding sites. Biophys J. 1999;76:246–252. doi: 10.1016/S0006-3495(99)77193-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ivanova-Nikolova TT, Breitwieser GE. Effector contributions to Gβγ-mediated signaling as revealed by muscarinic potassium channel gating. J Gen Physiol. 1997;109:245–253. doi: 10.1085/jgp.109.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma D, Zerangue N, Raab-Graham K, Fried SR, Jan YN, Jan LY. Diverse trafficking patterns due to multiple traffic motifs in G protein-activated inwardly rectifying potassium channels from brain and heart. Neuron. 2002;33:715–729. doi: 10.1016/s0896-6273(02)00614-1. [DOI] [PubMed] [Google Scholar]
- 40.Lunn M-L, Nassirpour R, Arrabit C, Tan J, McLeod I, Arias CM, Sawchenko PE, Yates JR, Slesinger PA. A unique sorting nexin regulates trafficking of potassium channels via a PDZ domain interaction. Nat Neurosci. 2007;10:1249–1259. doi: 10.1038/nn1953. [DOI] [PubMed] [Google Scholar]
- 41.Inanobe A, Yoshimoto Y, Horio Y, Morishige K-I, Hibino H, Matsumoto S, Tokunaga Y, Maeda T, Hata Y, Takai Y, Kurachi Y. Characterization of G-protein-gated K+ channels composed of Kir3.2 subunits in dopaminergic neurons of the substantia nigra. J Neurosci. 1999;19:1006–1017. doi: 10.1523/JNEUROSCI.19-03-01006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nehring RB, Wischmeyer E, Döring F, Veh RW, Sheng M, Karschin A. Neuronal inwardly rectifying K+ channels differentially couple to PDZ proteins of the PSD-95/SAP90 Family. J Neurosci. 2000;20:156–162. doi: 10.1523/JNEUROSCI.20-01-00156.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cruz HG, Ivanova T, Lunn ML, Stoffel M, Slesinger PA, Luscher C. Bi-directional effects of GABAB receptor agonists on the mesolimbic dopamine system. Nat Neurosci. 2004;7:153–159. doi: 10.1038/nn1181. [DOI] [PubMed] [Google Scholar]
- 44.Sui JL, Chan KW, Logothetis DE. Na+ activation of the muscarinic K+ channel by a G-protein-independent mechanism. J Gen Physiol. 1996;108:381–391. doi: 10.1085/jgp.108.5.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ho IH, Murrell-Lagnado RD. Molecular determinants for sodium-dependent activation of G protein-gated K+ channels. J Biol Chem. 1999;274:8639–8648. doi: 10.1074/jbc.274.13.8639. [DOI] [PubMed] [Google Scholar]
- 46.Aryal P, Dvir H, Choe S, Slesinger PA. A discrete alcohol pocket involved in GIRK channel activation. Nat Neurosci. 2009;12:988–995. doi: 10.1038/nn.2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kobayashi T, Ikeda K, Kojima H, Niki H, Yano R, Yoshiola T, Kumanishi T. Ethanol opens G-protein-activated inwardly rectifying K+ channels. Nat Neurosci. 1999;2:1091–1097. doi: 10.1038/16019. [DOI] [PubMed] [Google Scholar]
- 48.Lewohl JM, Wilson WR, Mayfield RD, Brozowski SJ, Morrisett RA, Harris RA. G-protein-coupled inwardly rectifying potassium channels are targets of alcohol action. Nat Neurosci. 1999;2:1084–1090. doi: 10.1038/16012. [DOI] [PubMed] [Google Scholar]
- 49.Mullner C, Vorobiov D, Bera AK, Uezono Y, Yakubovich D, Frohnwieser-Steinecker B, Dascal N, Schreibmayer W. Heterologous facilitation of G protein-activated K+ channels by β-adrenergic stimulation via cAMP-dependent protein kinase. J Gen Physiol. 2000;115:547–558. doi: 10.1085/jgp.115.5.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Medina I, Krapivinsky G, Arnold S, Kovoor P, Krapivinsky L, Clapham DE. A switch mechanism for Gβγ activation of IKACh. J Biol Chem. 2000;275:29709–29716. doi: 10.1074/jbc.M004989200. [DOI] [PubMed] [Google Scholar]
- 51.Sharon D, Vorobiov D, Dascal N. Positive and negative coupling of the metabotropic glutamate receptors to a G protein-activated K+ channel, GIRK, in Xenopus oocytes. J Gen Physiol. 1997;109:477–490. doi: 10.1085/jgp.109.4.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stevens EB, Shah BS, Pinnock RD, Lee K. Bombesin receptors inhibit G protein-coupled inwardly rectifying K+ channels expressed in Xenopus oocytes through a protein kinase C-dependent pathway. Mol Pharmacol. 1999;55:1020–1027. [PubMed] [Google Scholar]
- 53.Leaney JL, Dekker LV, Tinker A. Regulation of a G protein-gated inwardly rectifying K+ channel by a Ca2+-independent protein kinase C. J Physiol. 2001;534:367–379. doi: 10.1111/j.1469-7793.2001.00367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mao J, Wang X, Chen F, Wang R, Rojas A, Shi Y, Piao H, Jiang C. Molecular basis for the inhibition of G protein-coupled inward rectifier K+ channels by protein kinase C. Proc Natl Acad Sci USA. 2004;101:1087–1092. doi: 10.1073/pnas.0304827101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lei Q, Talley EM, Bayliss DA. Receptor-mediated inhibition of G protein-coupled inwardly rectifying potassium channels involves Gαq family subunits, phospholipase C, and a readily diffusable messenger. J Biol Chem. 2001;276:16720–16730. doi: 10.1074/jbc.M100207200. [DOI] [PubMed] [Google Scholar]
- 56.Sohn JW, Lee D, Cho H, Lim W, Shin HS, Lee SH, Ho WK. Receptor-specific inhibition of GABAB-activated K+ currents by muscarinic and metabotropic glutamate receptors in immature rat hippocampus. J Physiol. 2007;580:411–422. doi: 10.1113/jphysiol.2006.125914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Witkowski G, Szulczyk B, Rola R, Szulczyk P. D1 dopaminergic control of G protein-dependent inward rectifier K+ (GIRK)-like channel current in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2008;155:53–63. doi: 10.1016/j.neuroscience.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 58.Lopes CM, Zhang H, Rohacs T, Jin T, Yang J, Logothetis DE. Alterations in conserved Kir channel-PIP2 interactions underlie channelopathies. Neuron. 2002;34:933–944. doi: 10.1016/s0896-6273(02)00725-0. [DOI] [PubMed] [Google Scholar]
- 59.Huang C-L, Feng S, Hilgemann DW. Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by Gβγ. Nature. 1998;391:803–806. doi: 10.1038/35882. [DOI] [PubMed] [Google Scholar]
- 60.Zhang H, He C, Yan X, Mirshahi T, Logothetis DE. Activation of inwardly rectifying K+ channels by distinct Ptdlns(4,5)P2 interactions. Nat Cell Biol. 1999;1:183–188. doi: 10.1038/11103. [DOI] [PubMed] [Google Scholar]
- 61.Kobrinsky E, Mirshahi T, Zhang H, Jin T, Logothetis DE. Receptor-mediated hydrolysis of plasma membrane messenger PIP2 leads to K+-current desensitization. Nature Cell Biol. 2000;2:507–514. doi: 10.1038/35019544. [DOI] [PubMed] [Google Scholar]
- 62.Cho H, Nam G-B, Lee SH, Earm YE, Ho W-K. Phosphatidylinositol 4,5-bisphosphate is acting as a signal molecule in α1-adrenergic pathway via the modulation of acetylcholine-activated K+ channels in mouse atrial myocytes. J Biol Chem. 2001;276:159–164. doi: 10.1074/jbc.M004826200. [DOI] [PubMed] [Google Scholar]
- 63.Meyer T, Wellner-Kienitz M-C, Biewald A, Bender K, Eickel A, Pott L. Depletion of phosphatidylinositol 4,5-bisphosphate by activation of phospholipase C-coupled receptors causes slow iInhibition but not desensitization of G protein-gated inward rectifier K+ current in atrial myocytes. J Biol Chem. 2001;276:5650–5658. doi: 10.1074/jbc.M009179200. [DOI] [PubMed] [Google Scholar]
- 64.Nishida M, MacKinnon R. Structural basis of inward rectification. Cytoplasmic pore of the G protein-gated inward rectifier GIRK1 at 1.8 Å resolution. Cell. 2002;111:957–965. doi: 10.1016/s0092-8674(02)01227-8. [DOI] [PubMed] [Google Scholar]
- 65.Pegan S, Arrabit C, Zhou W, Kwiatkowski W, Collins A, Slesinger PA, Choe S. Cytoplasmic domain structures of Kir2.1 and Kir3.1 show sites for modulating gating and rectification. Nat Neurosci. 2005;8:279–287. doi: 10.1038/nn1411. [DOI] [PubMed] [Google Scholar]
- 66.Inanobe A, Matsuura T, Nakagawa A, Kurachi Y. Structural diversity in the cytoplasmic region of G protein-gated inward rectifier K+ channels. Channels. 2007;1:39–45. [PubMed] [Google Scholar]
- 67.Chang HK, Marton LJ, Liang KK, Shieh RC. K+ binding in the G-loop and water cavity facilitates Ba2+ movement in the Kir2.1 channel. Biochim Biophys Acta. 2009;1788:500–506. doi: 10.1016/j.bbamem.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 68.Ma D, Tang XD, Rogers TB, Welling PA. An andersen-Tawil syndrome mutation in Kir2.1 (V302M) alters the G-loop cytoplasmic K+ conduction pathway. J Biol Chem. 2007;282:5781–5789. doi: 10.1074/jbc.M608776200. [DOI] [PubMed] [Google Scholar]
- 69.Tao X, Avalos JL, Chen J, MacKinnon R. Crystal structure of the eukaryotic strong inward-rectifier K+ channel Kir2.2 at 3.1 Å resolution. Science. 2009;326:1668–1674. doi: 10.1126/science.1180310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xu Y, Shin H-G, Szep S, Lu Z. Physical determinants of strong voltage sensitivity of K+ channel block. Nat Struct Mol Biol. 2009;16:1252–1258. doi: 10.1038/nsmb.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pegan S, Arrabit C, Slesinger PA, Choe S. Andersen’s syndrome mutation effects on the structure and assembly of the cytoplasmic domains of Kir2.1. Biochemistry. 2006;45:8599–8606. doi: 10.1021/bi060653d. [DOI] [PubMed] [Google Scholar]
- 72.Nishida M, Cadene M, Chait BT, MacKinnon R. Crystal structure of a Kir3.1-prokaryotic Kir channel chimera. Embo J. 2007;26:4005–4015. doi: 10.1038/sj.emboj.7601828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kuo A, Gulbis JM, Antcliff JF, Rahman T, Lowe ED, Zimmer J, Cuthbertson J, Ashcroft FM, Ezaki T, Doyle DA. Crystal structure of the potassium channel KirBac1.1 in the closed state. Science. 2003;300:1922–1926. doi: 10.1126/science.1085028. [DOI] [PubMed] [Google Scholar]
- 74.Osawa M, Yokogawa M, Muramatsu T, Kimura T, Mase Y, Shimada I. Evidence for the direct interaction of spermine with the inwardly rectifying potassium channel. J Biol Chem. 2009;284:26117–26126. doi: 10.1074/jbc.M109.029355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kunkel MT, Peralta EG. Identification of domains conferring G protein regulation on inward rectifier potassium channels. Cell. 1995;83:443–449. doi: 10.1016/0092-8674(95)90122-1. [DOI] [PubMed] [Google Scholar]
- 76.Huang CL, Jan YN, Jan LY. Binding of the G protein βγ subunit to multiple regions of G protein-gated inward-rectifying K+ channels. FEBS Lett. 1997;405:291–298. doi: 10.1016/s0014-5793(97)00197-x. [DOI] [PubMed] [Google Scholar]
- 77.He C, Zhang H, Mirshahi T, Logothetis DE. Identification of a potassium channel site that interacts with G protein βγ subunits to mediate agonist-induced signaling. J Biol Chem. 1999;274:12517–12524. doi: 10.1074/jbc.274.18.12517. [DOI] [PubMed] [Google Scholar]
- 78.He C, Yan X, Zhang H, Mirshahi T, Jin T, Huang A, Logothetis DE. Identification of critical residues controlling G protein-gated inwardly rectifying K+ channel activity through interactions with the βγ subunits of G proteins. J Biol Chem. 2002;277:6088–6096. doi: 10.1074/jbc.M104851200. [DOI] [PubMed] [Google Scholar]
- 79.Krapivinsky G, Kennedy ME, Nemec J, Medina I, Krapivinsky L, Clapham DE. Gβγ binding to GIRK4 subunit is critical for G protein-gated K+ channel activation. J Biol Chem. 1998;273:16946–16952. doi: 10.1074/jbc.273.27.16946. [DOI] [PubMed] [Google Scholar]
- 80.Ivanina T, Rishal I, Varon D, Mullner C, Frohnwieser-Steinecke B, Schreibmayer W, Dessauer CW, Dascal N. Mapping the Gβγ-binding sites in GIRK1 and GIRK2 subunits of the G protein-activated K+ channel. J Biol Chem. 2003;278:29174–29183. doi: 10.1074/jbc.M304518200. [DOI] [PubMed] [Google Scholar]
- 81.Finley M, Arrabit C, Fowler C, Suen KF, Slesinger PA. βL-βM loop in the C-terminal domain of GIRK channels is important for Gβγ activation. J Physiol. 2004;555:643–657. doi: 10.1113/jphysiol.2003.056101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rubinstein M, Peleg S, Berlin S, Brass D, Keren-Raifman T, Dessauer CW, Ivanina T, Dascal N. Divergent regulation of GIRK1 and GIRK2 subunits of the neuronal G protein gated K+ channel by GαiGDP and Gβγ. J Physiol. 2009;587:3473–3491. doi: 10.1113/jphysiol.2009.173229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kawano T, Zhao P, Floreani CV, Nakajima Y, Kozasa T, Nakajima S. Interaction of Galphaq and Kir3, G protein-coupled inwardly rectifying potassium channels. Mol Pharmacol. 2007;71:1179–1184. doi: 10.1124/mol.106.032508. [DOI] [PubMed] [Google Scholar]
- 84.Clancy S, Fowler C, Finley M, Arrabit C, Suen K-F, Berton F, Kozasa T, Casey P, Slesinger PA. Pertussis-toxin-sensitive Gα subunits selectively bind to C-terminal domain of neuronal GIRK channels: evidence for a heterotrimeric G-protein-channel complex. Mol Cell Neurosci. 2005;28:375–389. doi: 10.1016/j.mcn.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 85.Peleg S, Varon D, Ivanina T, Dessauer CW, Dascal N. Gαi controls the gating of the G protein-activated K+ channel, GIRK. Neuron. 2002;33:87–99. doi: 10.1016/s0896-6273(01)00567-0. [DOI] [PubMed] [Google Scholar]
- 86.Sarac R, Hou P, Hurley KM, Hriciste D, Cohen NA, Nelson DJ. Mutation of critical GIRK subunit residues disrupts N- and C-termini association and channel function. J Neurosci. 2005;25:1836–1846. doi: 10.1523/JNEUROSCI.4783-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Riven I, Kalmanzon E, Segev L, Reuveny E. Conformational rearrangements associated with the gating of the G protein-coupled potassium channel revealed by FRET microscopy. Neuron. 2003;38:225–235. doi: 10.1016/s0896-6273(03)00193-4. [DOI] [PubMed] [Google Scholar]
- 88.Yi BA, Lin Y, Jan YN, Jan LY. Yeast screen for constitutively active mutant G protein-activated potassium channels. Neuron. 2001;29:657–667. doi: 10.1016/s0896-6273(01)00241-0. [DOI] [PubMed] [Google Scholar]
- 89.Sadja R, Smadja K, Alagem N, Reuveny E. Coupling Gβγ-dependent activation to channel opening via pore elements in inwardly rectifying potassium channels. Neuron. 2001;29:669–680. doi: 10.1016/s0896-6273(01)00242-2. [DOI] [PubMed] [Google Scholar]
- 90.Jin T, Peng L, Mirshahi T, Rohacs T, Chan KW, Sanchez R, Logothetis DE. The βγ subunits of G proteins gate a K+ channel by pivoted bending of a transmembrane segment. Mol Cell. 2002;10:469–481. doi: 10.1016/s1097-2765(02)00659-7. [DOI] [PubMed] [Google Scholar]
- 91.Rosenhouse-Dantsker A, Sui JL, Zhao Q, Rusinova R, Rodriguez-Menchaca AA, Zhang Z, Logothetis DE. A sodium-mediated structural switch that controls the sensitivity of Kir channels to PtdIns(4,5)P(2) Nat Chem Biol. 2008;4:624–631. doi: 10.1038/nchembio.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Riven I, Iwanir S, Reuveny E. GIRK channel activation involves a local rearrangement of a preformed G protein channel complex. Neuron. 2006;51:561–573. doi: 10.1016/j.neuron.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 93.David M, Richer M, Mamarbachi AM, Villeneuve LR, Dupre DJ, Hebert TE. Interactions between GABA-B1 receptors and Kir3 inwardly rectifying potassium channels. Cell Signal. 2006;18:2172–2181. doi: 10.1016/j.cellsig.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 94.Nobles M, Benians A, Tinker A. Heterotrimeric G proteins precouple with G protein-coupled receptors in living cells. Proc Natl Acad Sci USA. 2005;102:18706–18711. doi: 10.1073/pnas.0504778102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nikolov EN, Ivanova-Nikolova TT. Coordination of Membrane Excitability through a GIRK1 Signaling Complex in the Atria. J Biol Chem. 2004;279:23630–23636. doi: 10.1074/jbc.M312861200. [DOI] [PubMed] [Google Scholar]
- 96.Lavine N, Ethier N, Oak JN, Pei L, Liu F, Trieu P, Rebois RV, Bouvier M, Hebert TE, Van Tol HH. G protein-coupled receptors form stable complexes with inwardly rectifying potassium channels and adenylyl cyclase. J Biol Chem. 2002;277:46010–46019. doi: 10.1074/jbc.M205035200. [DOI] [PubMed] [Google Scholar]
- 97.Lober RM, Pereira MA, Lambert NA. Rapid activation of inwardly rectifying potassium channels by immobile G-protein-coupled receptors. J Neurosci. 2006;26:12602–12608. doi: 10.1523/JNEUROSCI.4020-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schreibmayer W, Dessauer CW, Vorobiov D, Gilman AG, Lester HA, Davidson N, Dascal N. Inhibition of an inwardly rectifying K+ channel by G-protein α-subunits. Nature. 1996;380:624–627. doi: 10.1038/380624a0. [DOI] [PubMed] [Google Scholar]
- 99.Ivanina T, Varon D, Peleg S, Rishal I, Porozov Y, Dessauer CW, Keren-Raifman T, Dascal N. Gαi1 and Gαi3 differentially interact with, and regulate, the G protein-activated K+ channel. J Biol Chem. 2004;279:17260–17268. doi: 10.1074/jbc.M313425200. [DOI] [PubMed] [Google Scholar]
- 100.Geng X, Du XN, Rusinova R, Liu BY, Li F, Zhang X, Chen XJ, Logothetis DE, Zhang HL. Specificity of Gβγ signaling depends on Gα subunit coupling with G-protein-sensitive K channels. Pharmacology. 2009;84:82–90. doi: 10.1159/000227772. [DOI] [PubMed] [Google Scholar]
- 101.Gales C, Rebois RV, Hogue M, Trieu P, Breit A, Hebert TE, Bouvier M. Real-time monitoring of receptor and G-protein interactions in living cells. Nat Methods. 2005;2:177–184. doi: 10.1038/nmeth743. [DOI] [PubMed] [Google Scholar]
- 102.Fowler CE, Aryal P, Suen KF, Slesinger PA. Evidence for association of GABAB receptors with Kir3 channels and RGS4 proteins. J Physiol. 2007;580:51–65. doi: 10.1113/jphysiol.2006.123216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bünemann M, Frank M, Lohse MJ. Gi protein activation in intact cells involves subunit rearrangement rather than dissociation. Proc Natl Acad Sci USA. 2003;100:16077–16082. doi: 10.1073/pnas.2536719100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Frank M, Thumer L, Lohse MJ, Bunemann M. G Protein activation without subunit dissociation depends on a Gαi-specific region. J Biol Chem. 2005;280:24584–24590. doi: 10.1074/jbc.M414630200. [DOI] [PubMed] [Google Scholar]
- 105.Digby GJ, Sethi PR, Lambert NA. Differential dissociation of G protein heterotrimers. The Journal of Physiology. 2008;586:3325–3335. doi: 10.1113/jphysiol.2008.153965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Digby GJ, Lober RM, Sethi PR, Lambert NA. Some G protein heterotrimers physically dissociate in living cells. Proc Natl Acad Sci USA. 2006;103:17789–17794. doi: 10.1073/pnas.0607116103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gibson SK, Gilman AG. Giα and Gβ subunits both define selectivity of G protein activation by α2-adrenergic receptors. Proc Natl Acad Sci U S A. 2006;103:212–217. doi: 10.1073/pnas.0509763102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Clancy SM, Boyer SB, Slesinger PA. Coregulation of Natively Expressed Pertussis Toxin-Sensitive Muscarinic Receptors with G-Protein-Activated Potassium Channels. J Neurosci. 2007;27:6388–6399. doi: 10.1523/JNEUROSCI.1190-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Boyer S, Clancy S, Terunuma M, Revilla-Sanchez R, Thomas S, Moss S, Slesinger P. Direct interaction of GABAB receptors with M2 muscarinic receptors enhances muscarinic signaling. J Neurosci. 2009;29:15796–15809. doi: 10.1523/JNEUROSCI.4103-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kulik A, Vida I, Fukazawa Y, Guetg N, Kasugai Y, Marker CL, Rigato F, Bettler B, Wickman K, Frotscher M, Shigemoto R. Compartment-dependent colocalization of Kir3.2-containing K+ channels and GABAB receptors in hippocampal pyramidal vells. J Neurosci. 2006;26:4289–4297. doi: 10.1523/JNEUROSCI.4178-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen X, Johnston D. Constitutively active G-protein-gated inwardly rectifying K+ channels in dendrites of hippocampal CA1 pyramidal neurons. J Neurosci. 2005;25:3787–3792. doi: 10.1523/JNEUROSCI.5312-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fernandez-Alacid L, Aguado C, Ciruela F, Martin R, Colon J, Cabanero MJ, Gassmann M, Watanabe M, Shigemoto R, Wickman K, Bettler B, Sanchez-Prieto J, Lujan R. Subcellular compartment-specific molecular diversity of pre- and post-synaptic GABA-activated GIRK channels in Purkinje cells. J Neurochem. 2009;110:1363–1376. doi: 10.1111/j.1471-4159.2009.06229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bacci A, Huguenard JR, Prince DA. Long-lasting self-inhibition of neocortical interneurons mediated by endocannabinoids. Nature. 2004;431:312–316. doi: 10.1038/nature02913. [DOI] [PubMed] [Google Scholar]
- 114.Lacey MG, Mercuri NB, North RA. Dopamine acts on D2 receptors to increase potassium conductance in neurones of the rat substantia nigra zona compacta. J Physiol. 1987;392:397–416. doi: 10.1113/jphysiol.1987.sp016787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Newberry NR, Nicoll RA. Comparison of the action of baclofen with gamma-aminobutyric acid on rat hippocampal pyramidal cells in vitro. J Physiol. 1985;360:161–185. doi: 10.1113/jphysiol.1985.sp015610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Beckstead MJ, Williams JT. Long-term depression of a dopamine IPSC. J Neurosci. 2007;27:2074–2080. doi: 10.1523/JNEUROSCI.3251-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dutar P, Vu HM, Perkel DJ. Pharmacological characterization of an unusual mGluR-evoked neuronal hyperpolarization mediated by activation of GIRK channels. Neuropharm. 1999;38:467–475. doi: 10.1016/s0028-3908(98)00206-8. [DOI] [PubMed] [Google Scholar]
- 118.Nicoll RA. My close encounter with GABAB receptors. Biochem Pharmacol. 2004;68:1667–1674. doi: 10.1016/j.bcp.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 119.Scanziani M. GABA spillover activates postsynaptic GABAB receptors to control rhythmic hippocampal activity. Neuron. 2000;25:673–681. doi: 10.1016/s0896-6273(00)81069-7. [DOI] [PubMed] [Google Scholar]
- 120.Isaacson JS, Solis JM, Nicoll RA. Local and diffuse synaptic actions of GABA in the hippocampus. Neuron. 1993;10:165–175. doi: 10.1016/0896-6273(93)90308-e. [DOI] [PubMed] [Google Scholar]
- 121.Luscher B, Keller CA. Regulation of GABAA receptor trafficking, channel activity, and functional plasticity of inhibitory synapses. Pharmacology & Therapeutics. 2004;102:195–221. doi: 10.1016/j.pharmthera.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 122.Beckstead MJ, Grandy DK, Wickman K, Williams JT. Vesicular dopamine release elicits an inhibitory postsynaptic current in midbrain dopamine neurons. Neuron. 2004;42:939–946. doi: 10.1016/j.neuron.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 123.Jin W, Lu Z. A novel high-affinity inhibitor for inward-rectifyier K+ channels. Biochem. 1998;37:13291–13299. doi: 10.1021/bi981178p. [DOI] [PubMed] [Google Scholar]
- 124.Michaeli A, Yaka R. Dopamine inhibits GABAA currents in ventral tegmental area dopamine neurons via activation of presynaptic G-protein coupled inwardly-rectifying potassium channels. Neuroscience. 2009 doi: 10.1016/j.neuroscience.2009.11.045. [DOI] [PubMed] [Google Scholar]
- 125.Ladera C, del Carmen Godino M, Cabaòero MJ, Torres M, Watanabe M, Lujan R, Sanchez-Prieto J. Pre-synaptic GABAB receptors inhibit glutamate release through GIRK channels in rat cerebral cortex. J Neurochem. 2008;107:1506–1517. doi: 10.1111/j.1471-4159.2008.05712.x. [DOI] [PubMed] [Google Scholar]
- 126.Sun QQ, Huguenard JR, Prince DA. Somatostatin inhibits thalamic network oscillations in vitro: actions on the GABAergic neurons of the reticular nucleus. J Neurosci. 2002;22:5374–5386. doi: 10.1523/JNEUROSCI.22-13-05374.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pietersen AN, Lancaster DM, Patel N, Hamilton JB, Vreugdenhil M. Modulation of gamma oscillations by endogenous adenosine through A1 and A2A receptors in the mouse hippocampus. Neuropharm. 2009;56:481–492. doi: 10.1016/j.neuropharm.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 128.Rice ME, Cragg SJ. Dopamine spillover after quantal release: rethinking dopamine transmission in the nigrostriatal pathway. Brain Res Rev. 2008;58:303–313. doi: 10.1016/j.brainresrev.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Huang CS, Shi S-H, Ule J, Ruggiu M, Barker LA, Darnell RB, Jan YN, Jan LY. Common molecular pathways mediate long-term potentiation of synaptic excitation and slow synaptic inhibition. Cell. 2005;123:105–118. doi: 10.1016/j.cell.2005.07.033. [DOI] [PubMed] [Google Scholar]
- 130.Chung HJ, Qian X, Ehlers M, Jan YN, Jan LY. Neuronal activity regulates phosphorylation-dependent surface delivery of G protein-activated inwardly rectifying potassium channels. Proc Natl Acad Sci USA. 2009;106:629–634. doi: 10.1073/pnas.0811615106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chung HJ, Ge WP, Qian X, Wiser O, Jan YN, Jan LY. G protein-activated inwardly rectifying potassium channels mediate depotentiation of long-term potentiation. Proc Natl Acad Sci USA. 2009;106:635–640. doi: 10.1073/pnas.0811685106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Patil N, Cox DR, Bhat D, Faham M, Myers RM, Peterson AS. A potassium channel mutation in weaver mice implicates membrane excitability in granule cell differentiation. Nature Genet. 1995;11:126–129. doi: 10.1038/ng1095-126. [DOI] [PubMed] [Google Scholar]
- 133.Kofuji P, Hofer M, Millen KJ, Millonig JH, Davidson N, Lester HA, Hatten ME. Functional analysis of the weaver mutant GIRK2 K+ channel and rescue of weaver granule cells. Neuron. 1996;16:941–952. doi: 10.1016/s0896-6273(00)80117-8. [DOI] [PubMed] [Google Scholar]
- 134.Navarro B, Kennedy ME, Velimirovic B, Bhat D, Peterson AS, Clapham DE. Nonselective and Gβγ-insensitive weaver K+ channels. Science. 1996;272:1950–1953. doi: 10.1126/science.272.5270.1950. [DOI] [PubMed] [Google Scholar]
- 135.Ikeda K, Kobayashi T, Kumanishi T, Niki H, Yano R. Involvement of G-protein-activated inwardly rectifying K (GIRK) channels in opioid-induced analgesia. Neurosci Res. 2000;38:113–116. doi: 10.1016/s0168-0102(00)00144-9. [DOI] [PubMed] [Google Scholar]
- 136.Roffler-Tarlov S, Martin B, Graybiel AM, Kauer JS. Cell death in the midbrain of the murine mutation weaver. J Neurosci. 1996;16:1819–1826. doi: 10.1523/JNEUROSCI.16-05-01819.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mitrovic I, Margeta-Mitrovic M, Bader S, Stoffel M, Jan LY, Basbaum AI. Contribution of GIRK2-mediated postsynaptic signaling to opiate and α2-adrenergic analgesia and analgesic sex differences. PNAS. 2003;100:271–276. doi: 10.1073/pnas.0136822100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Blednov YA, Stoffel M, Alva H, Harris RA. A pervasive mechanism for analgesia: Activation of GIRK2 channels. Proc Natl Acad Sci USA. 2003;100:277–282. doi: 10.1073/pnas.012682399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Marker CL, Lujan R, Loh HH, Wickman K. Spinal G-protein-gated potassium channels contribute in a dose-dependent manner to the analgesic effect of μ- and δ- but not κ-opioids. J Neurosci. 2005;25:3551–3559. doi: 10.1523/JNEUROSCI.4899-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Moreau JL, Fields HL. Evidence for GABA involvement in midbrain control of medullary neurons that modulate nociceptive transmission. Brain Res. 1986;397:37–46. doi: 10.1016/0006-8993(86)91367-3. [DOI] [PubMed] [Google Scholar]
- 141.Depaulis A, Morgan MM, Liebeskind JC. GABAergic modulation of the analgesic effects of morphine microinjected in the ventral periaqueductal gray matter of the rat. Brain Res. 1987;436:223–228. doi: 10.1016/0006-8993(87)91665-9. [DOI] [PubMed] [Google Scholar]
- 142.Dang VC, Napier IA, Christie MJ. Two distinct mechanisms mediate acute mu-opioid receptor desensitization in native neurons. J Neurosci. 2009;29:3322–3327. doi: 10.1523/JNEUROSCI.4749-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Whistler JL, Chuang H.-h., Chu P, Jan LY, von Zastrow M. Functional dissociation of μ opioid receptor signaling and endocytosis: Implications for the biology of opiate tolerance and addiction. Neuron. 1999;23:737–746. doi: 10.1016/s0896-6273(01)80032-5. [DOI] [PubMed] [Google Scholar]
- 144.Ingram SL, Macey TA, Fossum EN, Morgan MM. Tolerance to repeated morphine administration is associated with increased potency of opioid agonists. Neuropsychopharm. 2007;33:2494–2504. doi: 10.1038/sj.npp.1301634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Signorini S, Liao YJ, Duncan SA, Jan LY, Stoffel M. Normal cerebellar development but susceptibility to seizures in mice lacking G protein-coupled, inwardly rectifying K+ channel GIRK2. Proc Natl Acad Sci USA. 1997;94:923–927. doi: 10.1073/pnas.94.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kobayashi T, Washiyama K, Ikeda K. Modulators of G protein-activated inwardly rectifying K+ channels: potentially therapeutic agents for addictive drug users. Ann N Y Acad Sci. 2004;1025:590–594. doi: 10.1196/annals.1316.073. [DOI] [PubMed] [Google Scholar]
- 147.Pei Q, Lewis L, Grahame-Smith DG, Zetterstrom TSC. Alteration in expression of G-protein-activated inward rectifier K+-channel subunits GIRK 1 and GIRK 2 in the rat brain following electroconvulsive shock. Neurosci. 1999;90:621–627. doi: 10.1016/s0306-4522(98)00453-9. [DOI] [PubMed] [Google Scholar]
- 148.Mazarati A, Lundstrom L, Sollenberg U, Shin D, Langel U, Sankar R. Regulation of kindling epileptogenesis by hippocampal galanin type 1 and type 2 receptors: The effects of subtype-selective agonists and the role of G-protein-mediated signaling. J Pharmacol Exp Ther. 2006;318:700–708. doi: 10.1124/jpet.106.104703. [DOI] [PubMed] [Google Scholar]
- 149.Reeves RH, Irving NG, Moran TH, Wohn A, Kitt C, Sisodia SS, Schmidt C, Bronson RT, Davisson MT. A mouse model for Down syndrome exhibits learning and behaviour deficits. Nat Genet. 1995;11:177–184. doi: 10.1038/ng1095-177. [DOI] [PubMed] [Google Scholar]
- 150.Sago H, Carlson EJ, Smith DJ, Kilbridge J, Rubin EM, Mobley WC, Epstein CJ, Huang TT. Ts1Cje, a partial trisomy 16 mouse model for Down syndrome, exhibits learning and behavioral abnormalities. Proc Natl Acad Sci USA. 1998;95:6256–6261. doi: 10.1073/pnas.95.11.6256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Siarey RJ, Carlson EJ, Epstein CJ, Balbo A, Rapoport SI, Galdzicki Z. Increased synaptic depression in the Ts65Dn mouse, a model for mental retardation in Down syndrome. Neuropharm. 1999;38:1917–1920. doi: 10.1016/s0028-3908(99)00083-0. [DOI] [PubMed] [Google Scholar]
- 152.Luscher C, Ungless MA. The mechanistic classification of addictive drugs. PLoS Med. 2006;3:e437. doi: 10.1371/journal.pmed.0030437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Johnson SW, North RA. Two types of neurone in the rat ventral tegmental area and their synaptic inputs. J Physiol. 1992;450:455–468. doi: 10.1113/jphysiol.1992.sp019136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Szabo B, Siemes S, Wallmichrath I. SHORT COMMUNICATION Inhibition of GABAergic neurotransmission in the ventral tegmental area by cannabinoids. Eur J Neurosci. 2002;15:2057–2061. doi: 10.1046/j.1460-9568.2002.02041.x. [DOI] [PubMed] [Google Scholar]
- 155.Kajii Y, Muraoka S, Hiraoka S, Fujiyama K, Umino A, Nishikawa T. A developmentally regulated and psychostimulant-inducible novel rat gene mrt1 encoding PDZ-PX proteins isolated in the neocortex. Mol Psychiatry. 2003;8:434–444. doi: 10.1038/sj.mp.4001258. [DOI] [PubMed] [Google Scholar]
- 156.Federici M, Nistico R, Giustizieri M, Bernardi G, Mercuri NB. Ethanol enhances GABAB-mediated inhibitory postsynaptic transmission on rat midbrain dopaminergic neurons by facilitating GIRK currents. Eur J Neurosci. 2009;29:1369–1377. doi: 10.1111/j.1460-9568.2009.06700.x. [DOI] [PubMed] [Google Scholar]
- 157.Ikeda K, Kobayashi T, Kumanishi T, Yano R, Sora I, Niki H. Molecular mechanisms of analgesia induced by opioids and ethanol: is the GIRK channel one of the keys? Neuroscience Research. 2002;44:121–131. doi: 10.1016/s0168-0102(02)00094-9. [DOI] [PubMed] [Google Scholar]
- 158.Hill KG, Alva H, Blednov YA, Cunningham CL. Reduced ethanol-induced conditioned taste aversion and conditioned place preference in GIRK2 null mutant mice. Psychopharmacology (Berl) 2003;169:108–114. doi: 10.1007/s00213-003-1472-4. [DOI] [PubMed] [Google Scholar]
- 159.Bartoletti M, Ricci F, Gaiardi M. A GABAB agonist reverses the behavioral sensitization to morphine in rats. Psychoparm. 2007;192:79–85. doi: 10.1007/s00213-006-0693-8. [DOI] [PubMed] [Google Scholar]
- 160.Schein JC, Wang JK, Roffler-Tarlov SK. The effect of GIRK2(wv) on neurite growth, protein expression, and viability in the CNS-derived neuronal cell line, CATH.A-differentiated. Neuroscience. 2005;134:21–32. doi: 10.1016/j.neuroscience.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 161.Harkins AB, Fox AP. Cell death in weaver mouse cerebellum. Cerebellum. 2002;1:201–206. doi: 10.1080/14734220260418420. [DOI] [PubMed] [Google Scholar]
- 162.Liss B, Haeckel O, Wildmann J, Miki T, Seino S, Roeper J. K-ATP channels promote the differential degeneration of dopaminergic midbrain neurons. Nat Neurosci. 2005;8:1742–1751. doi: 10.1038/nn1570. [DOI] [PubMed] [Google Scholar]
- 163.Patsoukis N, Papapostolou I, Zervoudakis G, Georgiou CD, Matsokis NA, Panagopoulos NT. Thiol redox state and oxidative stress in midbrain and striatum of weaver mutant mice, a genetic model of nigrostriatal dopamine deficiency. Neurosci Lett. 2005;376:24–28. doi: 10.1016/j.neulet.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 164.Coulson EJ, May LM, Osborne SL, Reid K, Underwood CK, Meunier FA, Bartlett PF, Sah P. p75 neurotrophin receptor mediates neuronal cell death by activating GIRK channels through phosphatidylinositol 4,5-bisphosphate. J Neurosci. 2008;28:315–324. doi: 10.1523/JNEUROSCI.2699-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Airan RD, Thompson KR, Fenno LE, Bernstein H, Deisseroth K. Temporally precise in vivo control of intracellular signalling. Nature. 2009;458:1025–1029. doi: 10.1038/nature07926. [DOI] [PubMed] [Google Scholar]
- 166.Soejima M, Noma A. Mode of regulation of the ACh-sensitive K-channel by the muscarinic receptor in rabbit atrial cells. Pflügers Arch. 1984;400:424–431. doi: 10.1007/BF00587544. [DOI] [PubMed] [Google Scholar]
- 167.Yatani A, Mattera R, Codina J, Graf R, Okabe K, Padrell E, Iyengar R, Brown AM, Birnbaumer L. The G protein-gated atrial K+ channel is stimulated by three distinct Giα-subunits. Nature. 1988;336:680–682. doi: 10.1038/336680a0. [DOI] [PubMed] [Google Scholar]
- 168.Codina J, Yatani A, Grenet D, Brown AM, Birnbaumer L. The α subunit of the GTP binding protein Gk opens atrial potassium channels. Science. 1987;236:442–445. doi: 10.1126/science.2436299. [DOI] [PubMed] [Google Scholar]
- 169.Leaney JL, Tinker A. The role of members of the pertussis toxin-sensitive family of G proteins in coupling receptors to the activation of the G protein-gated inwardly rectifying potassium channel. Proc Natl Acad Sci USA. 2000;97:5651–5656. doi: 10.1073/pnas.080572297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hein P, Frank M, Hoffmann C, Lohse MJ, Bunemann M. Dynamics of receptor/G protein coupling in living cells. Embo J. 2005;24:4106–4114. doi: 10.1038/sj.emboj.7600870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ippolito DL, Xu M, Bruchas MR, Wickman K, Chavkin C. Tyrosine phosphorylation of K(ir)3.1 in spinal cord is induced by acute inflammation, chronic neuropathic pain, and behavioral stress. J Biol Chem. 2005;280:41683–41693. doi: 10.1074/jbc.M507069200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bettahi I, Marker CL, Roman MI, Wickman K. Contribution of the Kir3.1 subunit to the muscarinic-gated atrial potassium channel IKACh. J Biol Chem. 2002;277:48282–48288. doi: 10.1074/jbc.M209599200. [DOI] [PubMed] [Google Scholar]
- 173.Blednov YA, Stoffel M, Chang SR, Harris RA. GIRK2 deficient mice. Evidence for hyperactivity and reduced anxiety. Physiol Behav. 2001;74:109–117. doi: 10.1016/s0031-9384(01)00555-8. [DOI] [PubMed] [Google Scholar]
- 174.Blednov YA, Stoffel M, Chang SR, Harris RA. Potassium channels as targets for ethanol: studies of G-protein-coupled inwardly rectifying potassium channel 2 (GIRK2) null mutant mice. J Pharmacol Exp Ther. 2001;298:521–530. [PubMed] [Google Scholar]
- 175.Wickman K, Nemec J, Gendler SJ, Clapham DE. Abnormal heart rate regulation in GIRK4 knockout mice. Neuron. 1998;20:103–114. doi: 10.1016/s0896-6273(00)80438-9. [DOI] [PubMed] [Google Scholar]
- 176.VanDongen AM, Codina J, Olate J, Mattera R, Joho R, Birnbaumer L, Brown AM. Newly identified brain potassium channels gated by the guanine nucleotide binding protein Go. Science. 1988;242:1433–1437. doi: 10.1126/science.3144040. [DOI] [PubMed] [Google Scholar]
- 177.Leaney JL. Contribution of Kir3.1, Kir3.2A and Kir3.2C subunits to native G protein-gated inwardly rectifying potassium currents in cultured hippocampal neurons. Eur J Neurosci. 2003;18:2110–2118. doi: 10.1046/j.1460-9568.2003.02933.x. [DOI] [PubMed] [Google Scholar]
- 178.Uchida S, Akaike N, Nabekura J. Dopamine activates inward rectifier K+ channel in acutely dissociated rat substantia nigra neurones. Neuropharm. 2000;39:191–201. doi: 10.1016/s0028-3908(99)00111-2. [DOI] [PubMed] [Google Scholar]
- 179.Grigg JJ, Kozasa T, Nakajima Y, Nakajima S. Single-channel properties of a G-protein-coupled inward rectifier potassium channel in brain neurones. J Neurophysiol. 1996;75:318–328. doi: 10.1152/jn.1996.75.1.318. [DOI] [PubMed] [Google Scholar]
- 180.Miyake M, Christie MJ, North RA. Single potassium channels opened by opioids in rat locus coeruleus neurons. Proc Natl Acad Sci USA. 1989;86:3419–3422. doi: 10.1073/pnas.86.9.3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Bajic D, Koike M, Albsoul-Younes AM, Nakajima S, Nakajima Y. Two different inward rectifier K+ channels are effectors for transmitter-induced slow excitation in brain neurons. PNAS. 2002;99:14494–14499. doi: 10.1073/pnas.222379999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kawano T, Zhao P, Nakajima S, Nakajima Y. Single-cell RT-PCR analysis of GIRK channels expressed in rat locus coeruleus and nucleus basalis neurons. Neurosci Let. 2004;358:63–67. doi: 10.1016/j.neulet.2003.12.104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


