Abstract
Nicotine and cocaine each stimulate hypothalamic-pituitary-adrenal and -gonadal axis hormones, and there is increasing evidence that the hormonal milieu may modulate the abuse-related effects of these drugs. This review summarizes some clinical studies of the acute effects of cigarette smoking or IV cocaine on plasma drug and hormone levels, and subjective effects ratings. The temporal covariance between these dependent measures was assessed with a rapid (two min) sampling procedure in nicotine-dependent volunteers or current cocaine users. Cigarette smoking and IV cocaine each stimulated a rapid increase in LH and ACTH, followed by gradual increases in cortisol and DHEA. Positive subjective effects ratings increased immediately after initiation of cigarette smoking or IV cocaine administration. However, in contrast to cocaine’s sustained positive effects (< 20 min), ratings of “High” and “Rush” began to decrease within one or two puffs of a high nicotine cigarette while nicotine levels were increasing. Peak nicotine levels increased progressively after each of three successive cigarettes smoked at 60 min intervals, but the magnitude of the subjective effects ratings and peak ACTH and cortisol levels diminished. Only DHEA increased consistently after successive cigarettes. The possible influence of neuroactive hormones on nicotine dependence and cocaine abuse, and implications for treatment of these addictive disorders is discussed.
List of up to 10 keywords: ACTH, cigarette smoking, cocaine, cortisol, DHEA, estradiol, HPA axis, neuroactive hormones, nicotine, progesterone
Introduction
The prevalence of numerous health hazards (lung cancer, stroke, cardiovascular and respiratory disease, osteoporosis) associated with cigarette smoking has been well documented (CDC, 2002a, 2004, 2005; Pollock et al., 2009). There is now little question that cigarette smoking is an addictive disorder, characterized by tolerance and physical dependence (APA, 1994; Benowitz, 1996, 2001; Henningfield et al., 1995; Jaffe and Martin, 1990; Le Foll and Goldberg, 2009). Nicotine appears to be the primary addictive agent, and intravenous nicotine is self-administered by humans and experimental animals under a number of conditions (Corrigall, 1999; Donny et al., 1999; Goldberg et al., 1981; Harvey et al., 2004; Henningfield and Goldberg, 1983a, 1983b; Sofuoglu et al., 2008; Spealman and Goldberg, 1982). Addiction to cigarette smoking is influenced by a myriad of social and contextual factors, as well as the pharmacology of tobacco. Although social sanctions and restrictions on public areas where smoking is permissible, combined with increasing costs for cigarettes, has resulted in decreases in cigarette sales and use estimated at two percent per year (1998 to 2007), the use of alternative tobacco products has increased (Connolly and Alpert, 2008). Nicotine replacement therapies have not been uniformly effective, and despite multimodal treatment efforts, relapse rates remain high (Harvey et al., 2004; Pollock et al., 2009). It is difficult to predict the eventual impact of legislation to regulate the tobacco industry by the Food and Drug Administration on cigarette smoking behavior. The development of more effective medication-based interventions to treat nicotine dependence is urgently needed. An improved understanding of the complex neurobiology underlying nicotine addiction is important for achieving this goal.
The abuse-related effects of nicotine are mediated, in part, by stimulating nicotine acetylcholine receptors on mesolimbic dopamine neurons to release dopamine (Corrigall et al., 1992; Di Chiara, 2000; Fu et al., 2000; Kuhar et al., 1991; Watkins et al., 2000). The importance of dopamine in the reinforcing effects of nicotine is suggested by the fact that dopamine D1–like and D2-like receptor antagonists, as well as nicotine receptor antagonists, reduce nicotine self-administration in preclinical studies (Pierce and Kumaresan, 2006; Watkins et al., 2000). The neuroactive steroid hormones can also directly alter dopamine release under some conditions (Becker et al., 2001; Cabrera et al., 2002; Castner et al., 1993; Pasqualini et al., 1995, 1996; Thilbin et al., 1999) and can modulate the activity of nicotinic receptors (Curtis et al., 2002; Paradiso et al., 2001; Valera et al., 1992). However, relatively little is known about the functional impact of the neuroactive steroids on nicotine’s reinforcing properties. This issue is of interest because an emerging literature suggests that the neuroactive steroid hormones can enhance or diminish the reinforcing effects of another psychostimulant, cocaine (Carroll et al., 2004; Evans, 2007; Lynch et al., 2002; Mello and Mendelson, 2002, 2009a, 2009b in press). Although cocaine and nicotine have different molecular structures, and increase extracellular dopamine levels by different mechanisms, there are many similarities in their hormonal and behavioral effects (Chausmer et al., 2003; Jones et al., 1999).
Evidence that the hypothalamic-pituitary-adrenal (HPA) axis hormones are important in the addictive process has been shown for cocaine. Cocaine consistently increases levels of adrenocorticotropin hormone (ACTH) and cortisol/corticosterone in man, nonhuman primates and rodents (see for review Goeders, 2002b; Marinelli and Piazza, 2002; Mello and Mendelson, 2002, 2009a, 2009b in press). Hypothalamic corticotropin-releasing hormone (CRH) stimulates anterior pituitary corticotrophs to secrete ACTH, which in turn stimulates cortisol or corticosterone release from the adrenal cortex. Moreover, there was a significant temporal co-variance between cocaine’s positive subjective effects and activation of the HPA axis in cocaine abusers (Mendelson et al., 2002). These data were consistent with our early hypothesis that the hormonal milieu may be one important modulator of the reinforcing effects of drugs (Mendelson et al., 1989, 1992). To evaluate the generality of our findings in clinical studies of the hormonal and behavioral effects of cocaine, we have conducted a series of parallel clinical studies of nicotine that are summarized here.
This review describes clinical laboratory studies of the hormonal and subjective effects of cigarette smoking conducted at the Alcohol and Drug Abuse Research Center, McLean Hospital. In each of our studies, subject met DSM-IV criteria for nicotine dependence or current cocaine abuse and provided written informed consent. Each study was approved by the McLean Hospital Institutional Review Board. One goal of these studies was to examine the temporal covariance between changes in hormone levels and subjective responses associated with cigarette smoking and IV cocaine administration. We used a rapid sampling procedure in which measures of each dependent variable were collected at 2 min intervals for 20 to 30 min after drug administration. This sampling frequency enabled us to measure HPA axis hormones, cardiovascular and subjective responses during the ascending limb of the plasma nicotine or cocaine curve. A second goal was to compare the effects of cocaine and nicotine studied under these conditions.
The first section of this review describes the effects of smoking a single cigarette on HPA axis hormones and subjective effects in men. The second section describes the effects of smoking three successive cigarettes studied under the same conditions. In each study, the effects of low and high nicotine cigarettes are compared, and the temporal concordance between plasma nicotine levels, HPA axis hormones and subjective reactions to nicotine is described. The third section describes preliminary data on the subjective effects of smoking a single high nicotine cigarette during the follicular and luteal phase of the menstrual cycle. The fourth section compares the subjective and hormonal effects of cigarette smoking and IV cocaine administration. The concluding section discusses some implications of the several similarities between the hormonal and abuse-related effects of nicotine and cocaine for understanding the neurobiology of these drugs as well as possible approaches to medication-based treatment.
Nicotine-Hormone Interactions
Studies to explore the interactions between HPA axis hormones and the abuse-related effects of cigarette smoking were conducted in ten nicotine-dependent men (average age 25.6) (Mendelson et al., 2005). Men had smoked for at least 5 years and smoked 15 or more cigarettes each day. Abstinence from cigarettes and caffeinated beverages/foods after midnight on the night before the study was verified by CO measures. CO levels were below 4 ppm in all subjects on the study day. A Triage® urine screen and breath alcohol measures indicated that there was no other drug use. Each subject smoked a high nicotine cigarette (Marlboro Red; 15.48 mg nicotine; 16 mg of tar) or a low nicotine cigarette (1.1 mg of nicotine and 2.8 mg of tar) for 12 min under controlled conditions (24 puffs at a rate of one five sec puff every 30 sec). We used a rapid sampling procedure to examine the temporal covariance between plasma nicotine levels, changes in HPA axis hormones, cardiovascular measures, and reports of subjective effects during as well as after cigarette smoking. All dependent variables were measured at 2 min intervals during and for 20 min following cigarette smoking. Then, samples were collected at 25, 30, 40, 50, 60, 80, 100 and 120 min.
Cigarette smoking produced nicotine dose-dependent increases in anterior pituitary and adrenal hormones as well as cardiovascular and subjective responses in nicotine-dependent men. Nicotine plasma levels were significantly higher after smoking a high nicotine cigarette than after smoking a low nicotine cigarette. The low nicotine cigarette did not increase pituitary or adrenal hormones, and although there were increases in heart rate and VAS ratings of positive subjective effects, these were significantly lower than after smoking a high nicotine cigarette. Smoking behavior, defined in terms of duration of cigarette exposure and number and duration of puffs, was equivalent for high and low nicotine cigarettes. Thus, it is likely that the significant differences in hormonal and subjective effects were attributable to the relative dose of nicotine.
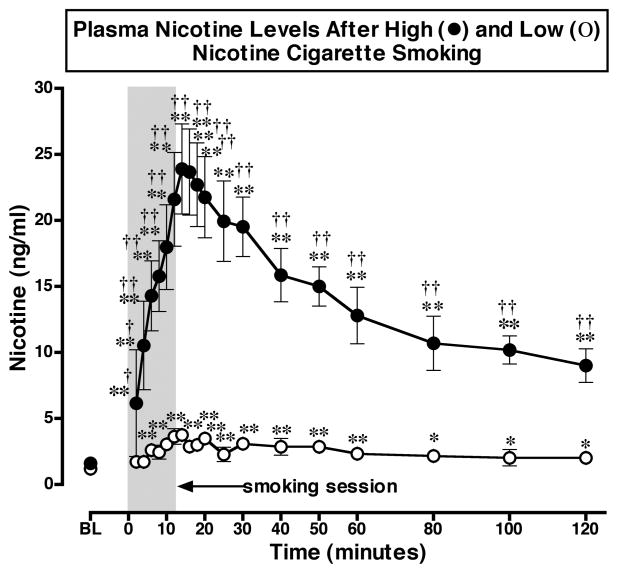
Nicotine Plasma Levels after Smoking a High or Low Nicotine Cigarette (Figure 1)
Figure 1. Plasma Nicotine Levels After Smoking a Low or High Nicotine Cigarette.
Plasma nicotine levels after smoking a high nicotine cigarette (filled circles) and a low nicotine cigarette (open circles) are shown on the left ordinates. Time (min) is shown on the abscissae. Points above BL were collected 10 min before cigarette smoking began at time 0. The 12 min cigarette smoking period is indicated by a grey rectangle. Each data point is the average (± S.E.M.) of 10 subjects. Statistical analyses indicated significant changes in plasma nicotine levels from baseline in both low [df=18, F=6.96, P=.03)], and high [df=18, F=21.8, P<.0001] nicotine cigarette groups. Significant changes from the pre-smoking baseline are indicated by asterisks (* = P < 0.05; ** = P < 0.01). Daggers indicate points at which plasma nicotine levels after smoking a low nicotine cigarette differed from plasma nicotine levels after smoking a high nicotine cigarette († = P < 0.05; †† = P <0.01) [df=1, F= 23.9, P=.0004]. Reprinted with permission from Mendelson, J.H., Sholar, M.B., Goletiani, N., Siegel, A.J. and Mello, N.K.: Effects of low and high nicotine cigarette smoking on mood states and the HPA axis in men. Neuropsychopharmacology 30:1751–1763, 2005.
Figure 1 shows that nicotine plasma levels increased significantly above baseline within 2 min (4 puffs) and reached peak levels within 12 to 14 min after cigarette smoking began. This time course was similar to that reported previously after 10 min of smoking at 1 puff per min (Tmax = 11.9 ± 1.9 min) (Gourlay and Benowitz, 1997). Nicotine plasma levels and pharmacokinetic profiles in the present study also were similar to those we previously reported under similar smoking conditions (Mendelson et al., 2003). Although the low nicotine cigarette produced measurable levels of nicotine (less than 4 ng/ml), these levels were significantly lower than peak plasma levels produced by high nicotine cigarettes (over 20 ng/ml). Moreover, the small but significant increase in plasma nicotine occurred later (after 6 min or 12 puffs) after smoking a low than a high nicotine cigarette and remained at relatively stable, low levels for 120 min.
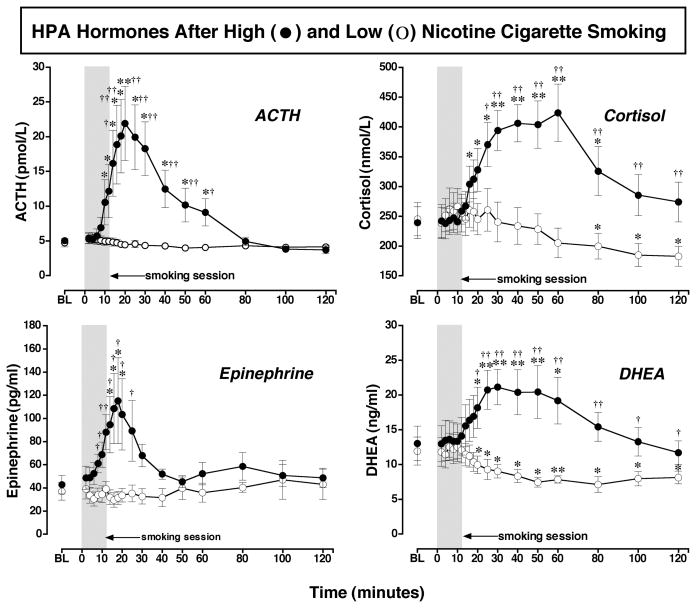
Cigarette Smoking Effects on the HPA Axis (Figure 2)
Figure 2. ACTH, Cortisol, DHEA and Epinephrine Levels after Smoking a Low or High Nicotine Cigarette.
Hormone levels after smoking a high nicotine cigarette (filled circles) and a low nicotine cigarette (open circles) are shown on the left ordinates. Time (min) is shown on the abscissae. Points above BL were collected 10 min before cigarette smoking began at time 0. The 12-min cigarette-smoking period is indicated by a grey rectangle. Each data point is the average (± S.E.M.) of 10 subjects. Statistical analyses indicated significant changes from baseline in ACTH levels [df=18, F= 8.8, P=.005], epinephrine levels [df=18, F=3.9, P=.05], DHEA levels [df=18, F=8.7, P=.0006], and cortisol levels [df=18, F=9.0, P=.002] after high dose nicotine. ACTH (pmol/L) and epinephrine (pg/ml) are shown in the left column; cortisol (nmol/L) and DHEA (ng/ml) are shown in the right column. Asterisks indicate points that were significantly different from baseline (* = P< 0.05; ** = P< 0.01). Daggers indicate points at which hormone levels were significantly different after high nicotine cigarette smoking than after low nicotine cigarette smoking († = P< 0.05; †† = P< 0.01); ACTH [df=1, F=7.0, P=.016], Epinephrine [df=1, F=5.8, P=.026], DHEA [df=1, F=4.8, P=.048], and cortisol [df=1, F=5.5, P=.03]. Reprinted with permission from Mendelson, J.H., Sholar, M.B., Goletiani, N., Siegel, A.J. and Mello, N.K.: Effects of low and high nicotine cigarette smoking on mood states and the HPA axis in men. Neuropsychopharmacology 30:1751–1763, 2005.
ACTH and Epinephrine
Increases in ACTH after cigarette smoking have been reported under conditions where samples for analysis were collected once a day or at relatively infrequent intervals, often without concurrent plasma nicotine measurement (Baron et al., 1995; Coiro and Vescovi, 1999; del Arbol et al., 2000; Pickworth and Fant, 1998; Seyler et al., 1984, 1986). Carefully controlled preclinical studies have consistently shown that nicotine rapidly stimulates the HPA axis (Matta et al., 1993, 1998; Sharp and Beyer, 1986). Figure 2 shows that when samples were collected every 2 min, ACTH and epinephrine began to increase within 6 min after high nicotine cigarette smoking began and reached peak levels within 4 to 6 min after peak nicotine plasma levels were detected (Fig. 1). This time course was similar to our previous report of the temporal covariance between ACTH, epinephrine and plasma cocaine levels, after IV cocaine administration in men (Mendelson et al., 2002). In that study, peak levels of ACTH, epinephrine, “Euphoria” and plasma cocaine were detected within 8 to 10 min after IV cocaine injection. We interpreted those data to suggest that the HPA hormone increases may be significant concomitants of the abuse-related effects of cocaine in humans (Mendelson et al., 2002). Corticotropin-releasing-hormone (CRH) from the hypothalamus stimulates ACTH release from the anterior pituitary, and antagonism of CRH release by a CRH-1 antagonist selectively reduced IV cocaine self-administration by rats (Goeders and Guerin, 2000). The possible influence of HPA axis activation, as indicated by ACTH and cortisol increases, on the abuse-related effects of cocaine has been discussed in several reviews (Goeders, 1997, 2002a, b; Mello and Mendelson, 2002, 2009a). The similar patterns of HPA axis activation and subjective responses after IV cocaine and smoked nicotine are provocative.
Cortisol and DHEA
It is also interesting to consider the possible contribution of cortisol and DHEA to the abuse-related effects of cigarette smoking. Figure 2 shows that cortisol began to increase significantly when ACTH levels were highest, and reached peak levels between 30 and 60 min after smoking began, when ACTH levels were decreasing. This time course is consistent with established feedback relationships between ACTH and cortisol (Yen et al., 1999). The increases in cortisol and DHEA were significantly correlated, and each hormone had a similar half-life after high nicotine cigarette smoking. These data are consistent with previous reports that smokers have higher basal cortisol and DHEA levels than non-smokers (al’Absi et al., 2003; del Arbol et al., 2000; Field et al., 1994; Pomerleau et al., 1992). Cigarette smoking usually induces an increase in plasma cortisol levels (Cryer et al., 1976; Gossain et al., 1986; Seyler et al., 1984; Spohr et al., 1979; Wilkins et al., 1982; Winternitz and Quillen, 1977; see for review Pickworth and Fant, 1998), and cortisol may remain elevated as a consequence of repeated cigarette smoking throughout the day. It has also been suggested that nicotine may inhibit the diurnal decrease in plasma cortisol levels (Pomerleau et al., 1992). The contribution of high cortisol levels to the maintenance of smoking behavior is unclear, but decreases in cortisol on the first day of smoking abstinence were associated with a higher rate of relapse during the first week (al’Absi et al., 2004).
DHEA is an adrenal androgen precursor of testosterone (Yen et al., 1999). DHEA is believed to improve feelings of wellbeing and sexuality in the elderly, although the evidence is conflicting (see for review, Nair et al., 2006; Spark, 2002). Two placebo-controlled clinical trials support the notion that DHEA treatment improves mood and alleviates depression (Morales et al., 1994; Schmidt et al., 2005). In a 6 month, placebo-controlled, cross-over trial, DHEA administration at physiological levels (50 mg, P.O.) consistently increased energy and improved mood and feelings of wellbeing in both men and women (Morales et al., 1994). In a 6 week, placebo-controlled cross-over trial in men and women with major or minor depression, DHEA administration (90 mg/day for 3 weeks; 450 mg/day for 3 weeks) significantly improved mood and depression ratings in comparison to pre-treatment baseline and placebo conditions (Schmidt et al., 2005). In the present study, peak levels of DHEA after smoking a high nicotine cigarette (21.13 ± 2.55 ng/ml or 73.32 ± 8.84 nmol/L or 2113.0 ng/dl) were over four times higher than average DHEA levels achieved after 3 months of DHEA replacement in men (14.72 ± 1.4 nmol/L) (Morales et al., 1994), and over twice as high as average DHEA levels after 6 weeks of high dose DHEA treatment (1047.2 ± 709.1 ng/dl) (Schmidt et al., 2005). It is interesting to speculate that increases in DHEA levels may contribute to the mood elevating effects reported after cigarette smoking, as well as to the alleviation of anxiety and depression (Picciotto et al., 2002).
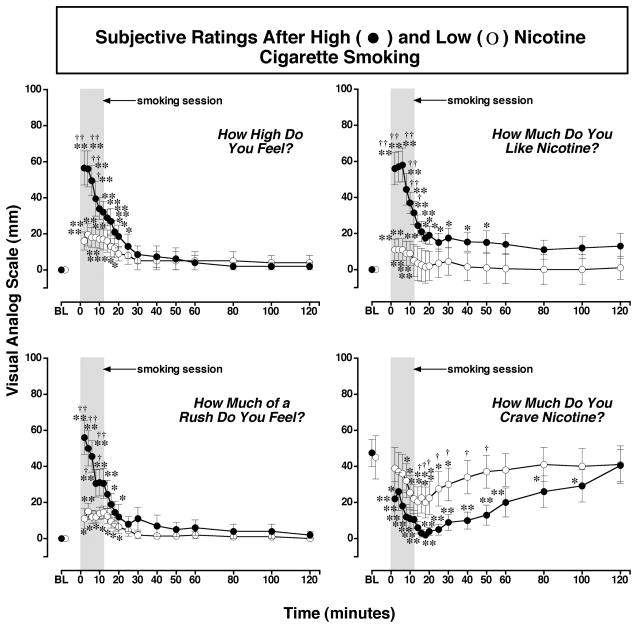
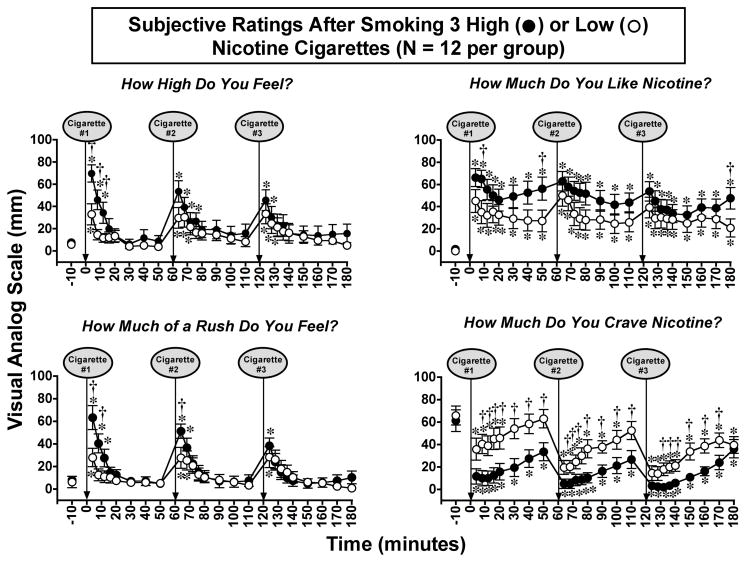
Subjective Responses to Smoking a High or Low Nicotine Cigarette (Figure 3)
Figure 3. Reports of Subjective Effects After Smoking a Low or High Nicotine Cigarette.
Subjective ratings on a Visual Analogue Scale (VAS) (0–100) are shown on the left ordinates and time (min) is shown on the abscissae. Points above BL were collected 10 min before smoking began at time 0. Each data point is the average (± S.E.M.) of 10 subjects. The 12 min cigarette-smoking period is indicated by a grey rectangle. Asterisks indicate points that were significantly different from baseline (* = P< 0.05; ** = P< 0.01). Statistical analyses indicated significant changes from baseline in reports of “high” after high nicotine cigarettes [df=18, F=16.9, P<.0001] and low nicotine cigarettes [df=18, F=5.2, P=.01; reports of “liking” after high nicotine cigarettes [df=18, F=14.1, P<.0001] and low nicotine cigarettes [df=18, F=3.9, P=.05]; reports of “rush” after high nicotine cigarettes [df=18, F=13.2, P<.0001] and low nicotine cigarettes [df=18, F=5.1, P=.009; and reports of “craving“ after high nicotine cigarettes [df=18, F=8.1, P=.0007] and after low nicotine cigarettes [df=18, F=5.6, P=.007]. Daggers indicate points that were significantly different after high nicotine cigarette smoking than after low nicotine cigarette smoking († = P< 0.05; †† = P<0.01) “high “ [df=1, F=4.5 P=.049], “like [df=1, F=6.2, P=.023], “rush” [df=1, F=6.3, P=.02], and “craving” [df=1, F=5.6, P=.04]. Reprinted with permission from Mendelson, J.H., Sholar, M.B., Goletiani, N., Siegel, A.J. and Mello, N.K.: Effects of low and high nicotine cigarette smoking on mood states and the HPA axis in men. Neuropsychopharmacology 30:1751–1763, 2005.
High, Rush and Liking
Significant increases in ratings of positive subjective effects occurred within 4 puffs (over 2 min) after initiation of smoking both a low and a high nicotine cigarette (Figure 3). However, Visual Analog Scale (VAS) ratings were significantly higher during high than low nicotine cigarette smoking, and this suggests that subjects could detect the relative nicotine levels within 4 puffs. Low nicotine cigarettes usually fail to reduce smoking behavior because smokers adjust their smoking topography and smoke more cigarettes to try to compensate for a low nicotine yield (Benowitz, 2001). These data are consistent with an earlier report that nicotine-dependent smokers were able to distinguish a low dose of nicotine nasal spray dose of 3 μg/kg from placebo (Perkins et al., 2001). This dose of nicotine nasal spray produced nicotine plasma levels of 2.6 ng/ml, or slightly lower than the low nicotine cigarette described above. Despite differences in routes of nicotine administration (nasal spray and smoking), these data converge to suggest that experienced smokers can discriminate very low doses of nicotine.
During high nicotine cigarette smoking, the highest positive VAS ratings were reported during the initial ascending limb of the plasma nicotine curve, then gradually diminished during the smoking period as plasma nicotine approached peak levels. During low nicotine cigarette smoking, positive VAS ratings remained relatively stable across the 12 min smoking period. Interestingly, the time course of increases in VAS ratings of positive subjective effects was similar during smoking a high nicotine cigarette and after intravenous nicotine administration. When intravenous nicotine was infused over 10 sec., peak VAS ratings of “High”, “Rush” and “Liking” (Jones et al., 1999) and “Strength of Drug Effect” (Soria et al., 1996) occurred within two min. In the present study, VAS ratings of “High”, “Rush” and “Liking” began to decrease within 4 min after high nicotine cigarette smoking began. A similar rapid decrease in positive subjective reports was also observed after intravenous nicotine administration, although plasma nicotine levels were not measured (Jones et al., 1999; Soria et al., 1996). It is unlikely that the decrease in positive VAS ratings reflected an increase in the aversive properties of smoking, because ratings of “Sick”, “Jittery” and “Bad Feeling” did not change significantly from baseline (Mendelson et al., 2005).
Craving
Ratings of cigarette “Craving” decreased rapidly from high baseline levels during and after smoking both low and high nicotine cigarettes. The greatest reductions in “Craving” ratings occurred within the first 20 min after smoking. Smoking a high nicotine cigarette reduced ratings of craving significantly more than smoking a low nicotine cigarette, and craving scores remained significantly below baseline for much longer (100 min vs 30 min). Decreases in cigarette “Craving” often are reported after smoking low nicotine cigarettes that contain relatively low doses of nicotine (<0.06 to 0.1 mg) (Gross et al., 1997; Pickworth et al., 1999; Robinson et al., 2000; Rose et al., 2000). The salient effects of low nicotine cigarettes on reports of “Craving”, “Liking”, and reduction of acute nicotine withdrawal symptoms are usually interpreted as evidence that complex sensory cues are important in maintaining cigarette smoking behavior (Butschky et al., 1995; Gross et al., 1997; Pickworth et al., 1999; Rose et al., 2000; Shahan et al., 1999).
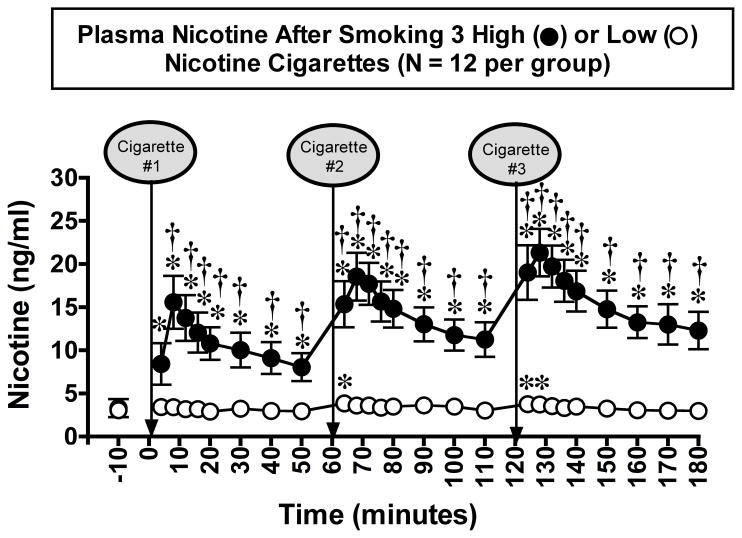
Nicotine Plasma Levels After Smoking Three Successive Cigarettes (Figure 4)
Figure 4. Plasma Nicotine Levels After Smoking Three Low- or High-Nicotine Cigarettes.
Plasma nicotine levels after smoking a high-nicotine cigarette (filled circles) and a low-nicotine cigarette (open circles) are shown on the left ordinates. Time (min) is shown on the abscissae. Points above baseline were collected 10 min before smoking the first cigarette began at time 0. A vertical line and an arrow indicate each 4-min cigarette-smoking period. Each data point is the average (± S.E.M.) of 12 men. Statistical analyses indicated significant changes in plasma nicotine levels from baseline in both low- (df=25, F=2.4, P=0.04) and high- (df=25, F=21.1, P<0.0001) nicotine cigarette groups. Significant changes from the pre-smoking baseline are indicated by asterisks (*) (P=0.05 – < 0.0001). Daggers (†) indicate points at which plasma nicotine levels after smoking a low-nicotine cigarette differed from plasma nicotine levels after smoking a high-nicotine cigarette (df=1, F=4.2 – 43.5, P=0.05 – <0.0001). Reprinted with permission from Mendelson, J.H., Goletiani, N.V., Sholar, M.B., Siegel, A.J. and Mello, N.K.: Effects of smoking successive low- and high-nicotine cigarettes on hypothalamic-pituitary-adrenal axis hormones and mood in men. Neuropsychopharmacology 33:749–760, 2008.
In a subsequent study, we examined the effects of smoking three successive cigarettes on nicotine levels, HPA axis hormones, heart rate and subjective effects measures using rapid (4 min) sampling procedures (Mendelson et al., 2008). Figure 4 shows that plasma nicotine levels increased significantly from baseline after smoking each of three high-nicotine cigarettes at 1 hr intervals. Moreover, nicotine levels were significantly higher after the third cigarette than after the first cigarette. The progressive increase in peak nicotine levels after smoking three successive high-nicotine cigarettes presumably reflects some accumulation of nicotine. A similar pattern of increases in nicotine levels was reported when subjects smoked five cigarettes at 30-min intervals (Isaac and Rand, 1972) and seven cigarettes at 1 hr intervals (Russell, 1985). However, subjective effects and cardiovascular measures were not reported in those studies. Progressive increases in nicotine levels were also reported after successive IV nicotine injections (Rosenberg et al., 1980). In contrast, smoking three low-nicotine cigarettes did not result in cumulative increases in nicotine levels (Fig. 4).
Effects of Smoking Three Successive Cigarette on HPA Axis Hormones (Figure 5)
Figure 5. ACTH, Cortisol and DHEA Levels after Smoking Three Low- or High-Nicotine Cigarettes.
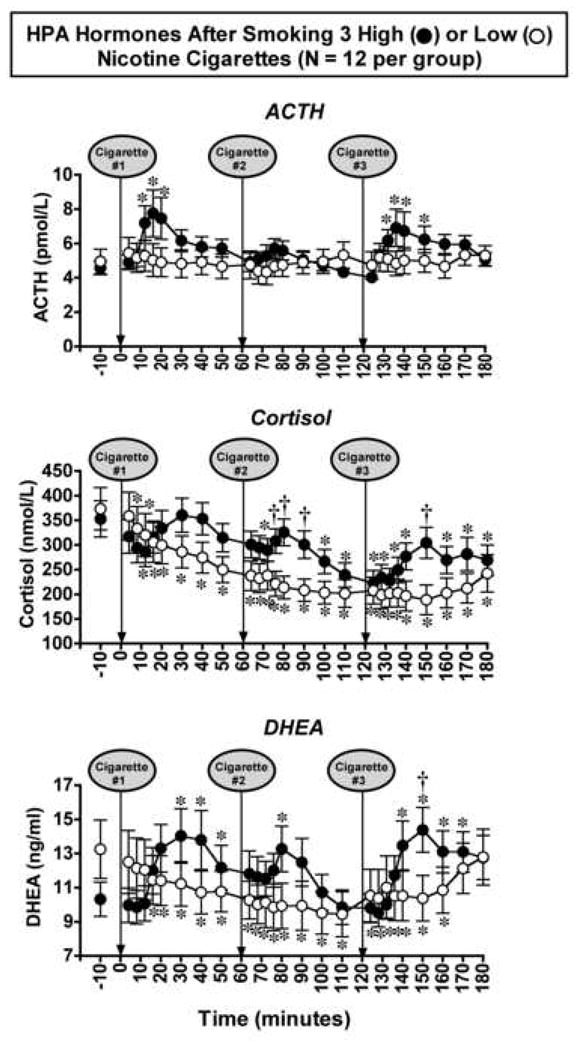
Hormone levels after smoking a high-nicotine cigarette (filled circles) or a low nicotine cigarette (open circles) are shown on the left ordinates. Time (min) is shown on the abscissae. Points above baseline were collected 10 min before cigarette smoking began at time 0. A vertical line and an arrow indicate each 4-min cigarette-smoking period. Each data point is the average (± S.E.M.) of 12 men. Data are shown for ACTH (pmol/L) cortisol (nmol/L) and DHEA (ng/ml). Statistical analyses indicated significant changes from baseline in ACTH levels (df=25, F=2.8, P=0.04), cortisol levels (df=25, F=4.8, P=0.006) and DHEA levels (df=25, F=4.0, P=0.007) after high-nicotine cigarette smoking. Asterisks (*) indicate points that were significantly different from baseline (P=0.05 – <0.0001). Daggers (†) indicate points at which hormone levels were significantly different after high-nicotine cigarette smoking than after low-nicotine cigarette smoking, DHEA (df=1, F=5.7, P=0.026), and cortisol (df=1, F=5.9 – 8.3, P=0.02 – 0.009). Reprinted with permission from Mendelson, J.H., Goletiani, N.V., Sholar, M.B., Siegel, A.J. and Mello, N.K.: Effects of smoking successive low- and high-nicotine cigarettes on hypothalamic-pituitary-adrenal axis hormones and mood in men. Neuropsychopharmacology 33:749–760, 2008.
The cumulative increases in peak nicotine levels shown in Figure 4 were not accompanied by progressive increases in peak levels of ACTH, cortisol and DHEA (Figure 5) or peak positive subjective effects ratings (Figure 6) or in peak heart rate (data not shown). Rather, these measures gradually decreased or remained the same after smoking each successive cigarette. Although the magnitude of changes in VAS ratings and heart rate were nicotine-dose related, the pattern of changes in these measures was similar after low- and high-nicotine cigarette smoking. HPA axis hormone levels increased after smoking high-nicotine cigarettes, but no changes or a significant decrease occurred after smoking low-nicotine cigarettes.
Figure 6. Reports of Subjective Effects After Smoking Three Low- or High-Nicotine Cigarettes.
Subjective ratings on a Visual Analogue Scale (VAS) (0–100) after smoking a high nicotine cigarette (filled circles) or a low-nicotine cigarette (open circles) are shown on the left ordinates. Time (min) is shown on the abscissae. Points above baseline were collected 10 min before smoking began at time 0. Each data point is the average (± S.E.M.) of 12 men. A vertical line and an arrow indicate each 4-min cigarette-smoking period. Asterisks (*) indicate points that were significantly different from baseline (P=0.05 – < 0.0001). Statistical analyses indicated significant changes from baseline in reports of “High” after high-nicotine cigarettes (df=25, F=9.1, P<0.0001) and low-nicotine cigarettes (df=25, F=4.0, P=0.01); reports of “Liking” after high-nicotine cigarettes (df=25, F=5.8, P<0.0001) and low-nicotine cigarettes (df=25, F=3.6, P=0.01); reports of “Rush” after high-nicotine cigarettes (df=25, F=10.3, P<0.0001) and low-nicotine cigarettes (df=25, F=4.0, P=0.02); and reports of “Craving” after high-nicotine cigarettes (df=25, F=7.5, P< 0.0001) and after low-nicotine cigarettes (df=25, F=6.3, P=0.0007). Daggers (†) indicate points that were significantly different after high-nicotine cigarette smoking than after low-nicotine cigarette smoking “High” (df=1, F=4.9 – 9.6, P=0.04 – 0.005), “Liking” (df=1, F=4.1 – 5.4, P=0.05 – 0.03), “Rush” (df=1, F=4.4 – 7.9, P=0.46 – 0.01), and “Craving” (df=1, F=4.2 – 9.2, P=0.05 – 0.006). Reprinted with permission from Mendelson, J.H., Goletiani, N.V., Sholar, M.B., Siegel, A.J. and Mello, N.K.: Effects of smoking successive low- and high-nicotine cigarettes on hypothalamic-pituitary-adrenal axis hormones and mood in men. Neuropsychopharmacology 33:749–760, 2008.
ACTH, Cortisol and DHEA
ACTH increased significantly after smoking the first high-nicotine cigarette, and the time course was similar to that shown earlier in Figure 2 (Mendelson et al., 2005). ACTH levels did not increase significantly after the second cigarette, and this may have reflected negative feedback from the sustained elevation in cortisol at the time of smoking. The plausibility of this explanation is strengthened by the fact that when the third cigarette was smoked, cortisol levels were much lower and ACTH increased significantly. Interpretation of these data is complicated by the feedback relationships between ACTH and cortisol (see for review Yen et al., 1999).
After smoking each low-nicotine cigarette, ACTH did not increase, and both cortisol and DHEA decreased significantly from baseline (Figure 5). The decrease in cortisol and DHEA may have reflected the fact that smoking low-nicotine cigarettes did not stimulate ACTH at any time point, and/or the normal circadian rhythm of cortisol release in the absence of nicotine/ACTH stimulation. Peak levels of cortisol occur between 8 and 9 a.m., then gradually decline to a nadir between 1 and 2 a.m. the next morning (Gianoulakis et al., 2005; Selmaoui and Touitou, 2003). Low levels of cortisol and DHEA have also been observed after smoking cessation (Oncken et al., 2002) and during nicotine withdrawal. Decreases in cortisol on the first day of smoking abstinence and a decrease in the plasma DHEA/cortisol ratio were associated with a higher rate of relapse (al’Absi et al., 2004; Rasmusson et al., 2006). The high craving scores reported by the low-nicotine group suggests that subjects remained in a state of relative nicotine deprivation throughout the study.
As noted earlier, DHEA is believed to enhance feelings of wellbeing and sexuality (Nair et al., 2006; Spark, 2002), and to improve mood and alleviate depression (Morales et al., 1994; Picciotto et al., 2002; Schmidt et al., 2005). After smoking three successive high-nicotine cigarettes, peak levels of DHEA were over three times higher than average DHEA levels achieved after three months of DHEA replacement in men (Morales et al., 1994), and higher than average DHEA levels after six weeks of high dose DHEA treatment (Schmidt et al., 2005). These data are consistent with DHEA levels after smoking a single high nicotine cigarette. Interestingly, IV cocaine also stimulated significant increases in DHEA and cortisol that were similar in time course and magnitude to smoking a high-nicotine cigarette (Mendelson et al., 2002). The significant increases in cortisol and DHEA induced by both IV cocaine and smoked nicotine suggest that these hormones may contribute to the abuse-related effects of both drugs (Mendelson et al., 2002, 2005). DHEA has been suggested as a potential medication for smoking cessation (Marx et al., 2006).
Heart Rate
Heart rate also increased significantly after smoking each high-nicotine cigarette, but the greatest increase occurred after the first high-nicotine cigarette. These data suggest that tolerance to nicotine’s stimulation of heart rate developed, even though peak nicotine levels increased with each successive cigarette. Tolerance to the acute cardiovascular stimulating effects of nicotine has also been observed after repeated injections of the same dose of intravenous nicotine (Benowitz et al., 1990; Rosenberg et al., 1980) and nicotine nasal spray (Perkins et al., 1989, 1995, 1991).
Effects of Smoking Successive Cigarettes on VAS Reports of Subjective Ratings (Figure 6)
“High”, “Rush” and “Liking”
Changes in reports of “High”, “Rush”, “Liking” and “Craving” after smoking three successive high- or low-nicotine cigarettes are summarized in Figure 6. Peak VAS ratings after the first cigarette were similar in time course and magnitude to ratings shown earlier after a single cigarette (Figure 3). Although VAS ratings of positive subjective effects were significantly higher after high- than low-nicotine cigarette smoking, nicotine levels did not appear to be the sole determinant. In contrast to the progressive increases in peak plasma nicotine levels shown in Figure 4, peak ratings of positive subjective effects were progressively lower after smoking each successive cigarette. This decrease in peak VAS ratings was most apparent for reports of “High” and “Rush”. The successive decrease in peak positive VAS ratings after repeated cigarette smoking is consistent with decreases in subjective responses after repeated nicotine nasal spray administration (Perkins et al., 1993, 1995). The decrease in peak positive VAS ratings after successive cigarettes appeared to be unrelated to changes in the aversive properties of smoking. Ratings of “Sick” and “Bad Feeling” did not change significantly from baseline after high-nicotine cigarette smoking, and peak ratings of “Dizzy” and “Jittery” decreased after successive cigarettes. The initial nicotine dose-dependent differences in “High,” “Rush” and “Liking” suggests that subjects could detect relative nicotine levels very rapidly, yet VAS ratings continued to increase significantly after each successive low-nicotine cigarette, when nicotine levels averaged between 2.9 and 3.8 ng/ml. Nicotine-dependent smokers appear to be quite sensitive to relative nicotine levels (Mendelson et al., 2008; Perkins et al., 2001).
Craving
Ratings of cigarette “Craving” decreased rapidly after smoking, and the time course of changes in “Craving” was virtually identical in the low- and high-nicotine groups. ”Craving” ratings decreased most within the first 20 min after smoking began, then increased to peak levels within 50 min. The time course and magnitude of “Craving” reports was almost identical to that observed after smoking a single high- or low-nicotine cigarette (see Figure 3). These findings are also consistent with previous reports that “Craving” often decreases after smoking low-nicotine cigarettes that contain <0.06 to 0.1 mg of nicotine (Gross et al., 1997; Mendelson et al., 2005; Pickworth et al., 1999; Robinson et al., 2000; Rose et al., 2000). The effectiveness of low-nicotine cigarettes in reducing “Craving” may offer a way to facilitate smoking cessation (see for review Rose, 2006). The extent to which increases in “Craving” within 30 min after smoking may prompt the next smoking episode is unclear. However, during several weeks’ residence on a clinical research ward, men usually initiated smoking within 20 to 30 min after the last cigarette (Mello et al., 1985, 1980; Mutschler et al., 2002). In other naturalistic smoking conditions, inter-cigarette intervals of 30 - 35 min are often reported (Hatsukami et al., 1988).
The progressive diminution in peak hormone levels and VAS ratings after smoking successive cigarettes suggests that tolerance may have developed to these effects (Figures 5 and 6). Acute tolerance develops to some effects of nicotine under a number of conditions, and chronic tolerance may persist for weeks or even years after cessation of smoking (Benowitz et al., 1990; Fant et al., 1995; Perkins et al., 1991, 1993, 1994, 1995, 2001; Rosenberg et al., 1980; West and Russell, 1987). There was a clear dissociation between the monotonic increases in peak nicotine levels, and the progressive decreases in peak VAS ratings of positive subjective effects and peak heart rate. The robust subjective, cardiovascular and hormonal response to the first cigarette is consistent with the usual observation that after a period of nicotine abstinence, the first cigarette of the day is the most reinforcing (Fant et al., 1995; Pomerleau and Pomerleau, 1992; West and Russell, 1987). However, it is not clear if the subsequent decreases in peak subjective and cardiovascular effects primarily reflect acute tolerance to these effects of nicotine or relief of nicotine withdrawal symptoms after overnight abstinence from smoking. Because these men had been abstinent from nicotine for at least 12 hours (as evidenced by CO levels below 6 ppm and nicotine levels below 4 ng/ml) and were well matched in years of smoking and nicotine dependence, it is not possible to distinguish between these two alternatives with certainty (cf. Pillitteri et al., 1997; West and Russell, 1987).
It is interesting to compare these effects of repeated cigarette smoking with previous clinical laboratory studies of cocaine, where drug abstinence symptoms were not an issue. As in the cigarette smoking study, repeated administration of cocaine did not result in cumulative increases in ratings of positive subjective effects or heart rate (Dudish et al., 1996; Evans et al., 1999; Fischman and Schuster, 1982; Fischman et al., 1985; Foltin and Fischman, 1991; Sofuoglu et al., 1999; Ward et al., 1997). Only ratings of negative subjective effects increased after repeated doses of IV cocaine (Foltin and Fischman, 1998) whereas, peak ratings of negative effects after cigarette smoking tended to decrease or stay about the same.
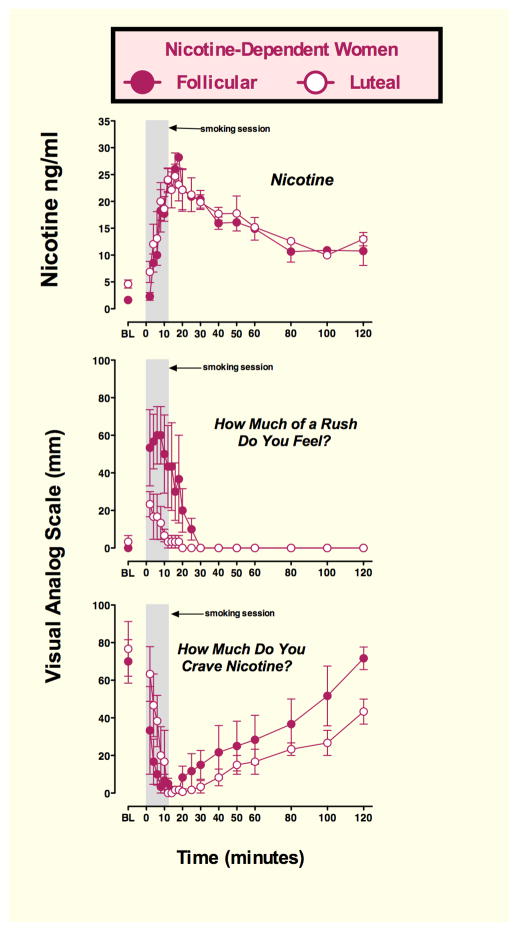
Nicotine and Menstrual Cycle Phase (Figure 7)
Figure 7. Plasma nicotine, and Visual Analog Scale (VAS) ratings in women during the follicular and luteal phase of the menstrual cycle.
Abscissae: time (min); Ordinates: plasma nicotine levels (ng/ml) and subjective ratings of “Rush” and “Craving” on a VAS scale of 0–100. Points above BL were collected 10 min before smoking began at time 0. The 12 min cigarette-smoking period is indicated by a gray rectangle. Each data point is the average of three nicotine-dependent women studied during the follicular (closed circles) and the luteal (open circles) phase of successive menstrual cycles. (Preliminary data from N.K. Mello, ongoing studies).
Both sex and phase of the menstrual cycle appear to influence the biologic effects and subjective responses to cocaine (Evans, 2007; Mello and Mendelson, 2002, 2009a, 2009b in press; Newman and Mello, 2009). In clinical laboratory studies, cocaine produced higher ratings of positive subjective effects in women studied during the mid-follicular phase than during the luteal phase of the menstrual cycle (Evans et al., 2002; Sofuoglu et al., 1999). These findings suggested that high progesterone levels during the luteal phase of an ovulatory menstrual cycle might contribute to the observed attenuation of cocaine’s positive subjective effects. This hypothesis was confirmed in subsequent clinical studies where progesterone, at doses that mimicked luteal phase progesterone levels, was administered during the follicular phase. Under these conditions, women reported lower ratings of positive subjective responses to cocaine in comparison to their responses during a normal follicular phase without progesterone treatment (Evans and Foltin, 2006; Sofuoglu et al., 2002, 2004). In preclinical studies, progesterone treatment reduced the facilitory effect of estradiol on acquisition of cocaine self-administration in ovariectomized rats (Jackson et al., 2006; Yang et al., 2007). Progesterone also decreased cocaine self- administration and shifted the cocaine dose-effect curve to the right in rhesus monkeys (Mello et al., 2007, 2009 in preparation). Similarly, in a cocaine-primed reinstatement paradigm, progesterone treatment attenuated responding during estrus, but not during proestrus or diestrus phases of the estrous cycle in gonadally intact female rats (Feltenstein and See, 2007). Progesterone treatment also reduced cocaine-primed reinstatement in ovariectomized rats (Anker et al., 2007).
There has been considerably less research on the interactions between progesterone and nicotine. In rats, it has been difficult to detect an effect of estrous cycle phase on patterns of nicotine self-administration (Donny et al., 2000). Studies of the effects of menstrual cycle phase on cigarette smoking behavior or responses to acute nicotine administration have often yielded inconsistent results (Perkins et al., 1999). Menstrual cycle phase appears to be more important in modulating nicotine withdrawal symptoms than ongoing patterns of smoking behavior (Allen et al., 1999; Marks et al., 1994; Perkins et al., 2001; Pomerleau et al., 1994). One clinical study reported that progesterone administration during the follicular phase of the menstrual cycle reduced ratings of “good effects” after two puffs on a cigarette, and resulted in a non-significant decrease in choice of cigarettes over tokens worth one dollar (Sofuoglu et al., 2001).
We are currently examining the subjective and hormonal effects of cigarette smoking in nicotine-dependent women during the follicular and luteal phases of the menstrual cycle. The experimental procedures were identical to those described earlier for studies of cigarette smoking in men and presented in Figures 1, 2 and 3. Women were studied during the mid-follicular and mid-luteal phases of successive menstrual cycles. Menstrual cycle phase was verified by analysis of progesterone levels within 24 hours of the study day. Progesterone levels averaged 0.52±0.2 ng/ml during the follicular phase and 8.0±1.9 ng/ml during the luteal phase.
Preliminary data on three women are shown in Figure 7. Peak plasma nicotine levels after smoking a high nicotine cigarette (Marlboro Red, Phillip Morris Brand) were equivalent in the follicular and luteal phase women (28.2±0.0 and 24.7±2.23 ng/ml). Plasma nicotine levels increased rapidly within two minutes, or four puffs on a high nicotine cigarette and peak levels were detected within 12–14 minutes. Nicotine levels gradually decreased over 14 minutes, but remained above 10 ng/ml throughout the 120-minute sampling period. The small number of subjects precludes statistical analysis, but the overall time course and shape of the nicotine curve is similar to that measured in men shown earlier in Figure 1.
Despite comparable plasma nicotine levels, ratings of “Rush” were higher during the follicular phase than during the luteal phase, (Figure 7). “Rush” ratings increased rapidly above baseline within two min after cigarette smoking began, then gradually decreased during and after the 12 min smoking period. Baseline ratings of “Craving” for cigarettes were equivalent in follicular and luteal phase women, and decreased rapidly below baseline levels within two minutes after smoking began. During the 12 min smoking period, “Craving” ratings were higher in luteal phase women than in follicular phase women. After smoking, “Craving” reports were higher in follicular phase women than in luteal phase women. These findings suggest that the higher rating of “Rush” reported by follicular women may have prompted a more rapid resurgence of ”Craving” once the ”Rush” ratings decreased to zero at 30 min. Although these observations of the effects of spontaneous increases in endogenous progesterone levels during the luteal phase is a less powerful experimental design than administration of exogenous progesterone during the follicular phase, these findings on nicotine are consistent with previous clinical reports on the effects of exogenous progesterone administration on subjective reactions to cocaine in women (Evans, 2007; Evans and Foltin, 2006; Sofuoglu et al., 2002, 2004). Thus far, progesterone appears to be less effective in reducing positive responses to cocaine in men (Evans and Foltin, 2006; Sofuoglu et al., 2007).
In our ongoing preclinical studies on the effects of progesterone administration on nicotine self-administration in nonhuman primates, we also found that progesterone decreased nicotine self-administration. Monkeys were trained to self-administer nicotine on a progressive ratio schedule in which the number of responses required for each successive nicotine injection (i.e. the “response cost”) was increased by 0.05 log units. Administration of progesterone (0.3 mg/kg, IM) produced a downward shift in the nicotine self-administration dose-effect curve (0.001–0.1 mg/kg, IV). The decrease in nicotine self-administration did not appear to be related to the sedative effects of progesterone because, at the end of each session, monkeys were alert, took treats, and scored zero on a sedation scale completed by trained observers. These preliminary findings on nicotine self-administration are consistent with clinical and preclinical reports on the effects of progesterone on cocaine’s abuse-related effects, as well as our clinical studies in follicular and luteal phase women described earlier (Figure 7). Taken together, these clinical and preclinical data suggest that synthetic derivatives of the neuroactive gonadal steroid hormones may offer a novel approach to the medication-based treatment of cigarette smoking in women.
Comparison of Cigarette Smoking and IV Cocaine Effects on LH and “High” Ratings
Some similarities between the abuse-related effects of cocaine and nicotine have been noted throughout this review. Clinical studies have shown that the positive subjective and physiological effects of nicotine are very similar to the effects of IV cocaine in cocaine abusers who smoke cigarettes (Chausmer et al., 2003; Jones et al., 1999). Nicotine and cocaine also have similar pharmacokinetic profiles after both inhalation and intravenous administration (Evans et al., 1996; Rose et al., 1999). The rapid distribution of both nicotine and cocaine, combined with the relatively brief duration of positive subjective effects, is characteristic of drugs with high abuse liability (Balster and Schuster, 1973). As described earlier, nicotine, like cocaine, activates the mesolimbic dopamine system, and the resulting increases in extracellular dopamine levels appear to mediate the abuse-related effects of both drugs (Corrigall et al., 1992; Di Chiara, 2000; Fu et al., 2000; Kuhar et al., 1991; Watkins et al., 2000). However, cocaine and nicotine increase extracellular dopamine levels by different mechanisms. Nicotine stimulates dopamine release in the nucleus accumbens by stimulating nicotinic acetylcholine receptors on the cell bodies of mesolimbic dopamine neurons (Watkins et al., 2000), whereas cocaine increases extracellular dopamine levels by blocking dopamine reuptake by the dopamine transporter (Kuhar et al., 1991). Interestingly, microdialysis studies indicate that administration of equipotent doses of nicotine and cocaine, given in combination, produce additive effects on nucleus accumbens dopamine release (Gerasimov et al., 2000; Sziraki et al., 1999).
In addition to dopaminergic effects, cocaine and nicotine both stimulate release of hypothalamic-anterior pituitary-gonadal and-adrenal hormones. Preclinical studies suggest that these rapid hormonal changes may contribute to the abuse-related effects of cocaine (Goeders, 1997, 2002a, b; Mello and Mendelson, 2002, 2009a). Cocaine (0.2 and 0.4 mg/kg, IV) also produced a rapid dose-dependent increase in LH levels in men who currently abuse cocaine (Mendelson et al., 2001). Because stimulation of LH release is a robust effect of cocaine administration in humans and in non-human primates (Mello and Mendelson, 2002), we compared the effects of IV cocaine and cigarette smoking on LH and T in men to determine the generality of these findings (Mendelson et al., 2003). These data are shown in Figures 8, 9, and 10. We found that IV cocaine and high nicotine cigarette smoking each stimulated a significant increase in LH release that was temporally correlated with increases in plasma levels of cocaine and nicotine. Moreover, high nicotine cigarette smoking produced significantly greater stimulation of LH than IV cocaine. Pharmacokinetic analyses showed that the Tmax and Cmax for LH were significantly higher after cigarette smoking than after IV cocaine. However, the T1/2 of LH did not differ significantly after cigarette smoking and IV cocaine. Testosterone levels did not change significantly after cocaine or nicotine administration. Placebo-cocaine and low nicotine cigarettes had no effect on any endocrine or subjective report measures.
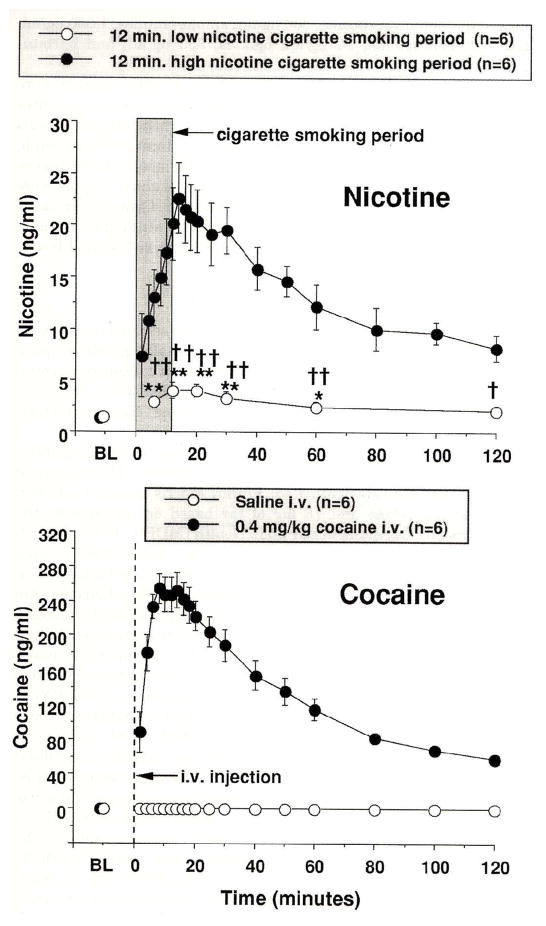
Figure 8. Plasma Nicotine and Plasma Cocaine Levels.
Plasma drug levels (ng/ml) are shown on the left ordinates and time (min) is shown on the abscissae. Points above BL were collected 10 min before drug administration at time 0. Each data point is the average (± S.E.M.) of six subjects. The top panel shows plasma nicotine levels after smoking a high nicotine yield cigarette (filled circles) and a placebo cigarette (open circles). The 12 min cigarette smoking period is indicated by the grey rectangle. The bottom panel shows plasma cocaine levels after administration of 0.4 mg/kg, i.v. cocaine (closed circles). The vertical line indicates when cocaine was administered. Significant changes from the pre-smoking baseline are indicated by asterisks (* = P < 0.05; ** = P < 0.01). Daggers indicate points at which plasma nicotine levels after smoking a low nicotine placebo cigarette differed from plasma nicotine levels after smoking a high nicotine cigarette († = P < 0.05; †† = P < 0.01). Reprinted with permission from Mendelson, J.H., Sholar, M.B., Mutschler, N.H., Jaszyna-Gasior, M., Goletiani, N.V., Siegel, A.J. and Mello, N.K.: Effects of intravenous cocaine and cigarette smoking on luteinizing hormone, testosterone and prolactin in men. J. Pharmacol. Exp. Ther. 307:339–348, 2003.
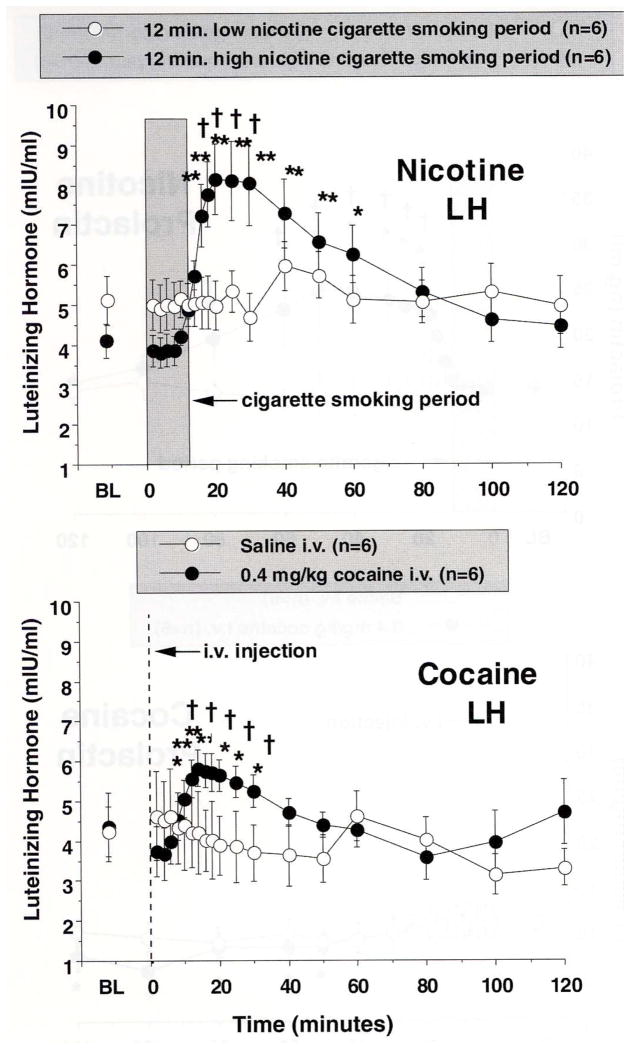
Figure 9. Luteinizing Hormone Levels after Cigarette Smoking or i.v. Cocaine Administration.
Plasma LH levels (mIU/ml) is shown on the abscissae. Points above BL were collected 10 min before drug administration at time 0. Each data point is the average (± S.E.M.) of six subjects. The top panel shows LH levels after smoking a high nicotine yield cigarette (filled circles) and a placebo cigarette (open circles). The 12 min cigarette-smoking period is indicated by the grey rectangle. The bottom panel shows LH levels after administration of 0.4 mg/kg, i.v. cocaine (closed circles) or placebo-cocaine. The vertical line indicates when cocaine or placebo-cocaine was administered. Asterisks indicate points that were significantly different from baseline (* = P< 0.05; ** = P< 0.01). Daggers indicate points at which LH was significantly different after high nicotine yield cigarette smoking than after placebo cigarette smoking, or when LH was significantly different after active cocaine than after placebo-cocaine († = P< 0.05; †† = P< 0.01). Reprinted with permission from Mendelson, J.H., Sholar, M.B., Mutschler, N.H., Jaszyna-Gasior, M., Goletiani, N.V., Siegel, A.J. and Mello, N.K.: Effects of intravenous cocaine and cigarette smoking on luteinizing hormone, testosterone and prolactin in men. J. Pharmacol. Exp. Ther. 307:339–348, 2003.
Nicotine and Cocaine Plasma Levels [Figure 8]
Figure 8 (top panel) shows plasma nicotine levels before, during and after low and high dose nicotine cigarette smoking. Before cigarette smoking, low levels of nicotine that averaged 1.22 ± 0.27 ng/ml and 1.33 ± 0.30 ng/ml were detected in the high and low nicotine cigarette groups, respectively. Baseline CO levels averaged 3.5 ± 1.4 ppm in the high nicotine cigarette group and 4.2 ± 1.1 ppm in the low nicotine cigarette group. Nicotine plasma levels increased significantly within 2 min (or 4 puffs) after smoking a high nicotine cigarette and remained significantly above baseline throughout the 120 min sampling period. Peak plasma nicotine levels of 22.6 ± 3.4 ng/ml were detected within 14 min, and remained above 20 ng/ml between 12 and 20 min after cigarette smoking began. Plasma nicotine levels also increased significantly above pre-smoking baseline levels after smoking the low nicotine cigarette. Peak plasma nicotine levels of 3.90 ± 0.77 ng/ml were detected at 12 min after low nicotine cigarette smoking began, and averaged 2.03 ± 0.49 ng/ml at the end of the sampling period.
Cocaine plasma levels were maximal within 8 min after 0.4 mg/kg IV cocaine administration, and averaged 254 ± 18 ng/ml (Figure 8). Plasma cocaine levels gradually decreased over the remainder of the sampling period and averaged 58.8 ± 3.2 ng/ml at 120 min. The calculated half-life (T1/2) of cocaine in plasma was 47.8 ± 0.5 min. No cocaine was detected after placebo-cocaine injection.
Effects of Cigarette Smoking and IV Cocaine on LH [Figure 9]
Cocaine and high nicotine cigarette smoking each stimulated rapid increases in LH (Figure 9). LH levels increased significantly from baseline within 14 min after high nicotine cigarette smoking and reached peak levels of 8.15 ± 0.89 mIU/ml when plasma nicotine levels averaged 20.3 ± 3.1 ng/ml. The increase in LH was significantly correlated with the increase in plasma nicotine levels shown in Figure 8 (R = 0.642; P = .003). After low nicotine cigarette smoking, LH levels did not change significantly from baseline. After administration of 0.4 mg/kg IV cocaine, LH increased significantly within 20 min in comparison to baseline (Fig. 9, lower panel). LH reached peak levels of 5.8 ± 0.4 mIU/ml when plasma cocaine levels averaged 221.5 ± 16.9 ng/ml. The increase in LH was significantly correlated with the increase in plasma cocaine levels shown in Figure 8. After placebo-cocaine administration, LH levels did not change significantly from baseline.
The time course and duration of LH increases after cocaine are consistent with previous clinical reports (Mendelson et al., 2001; for review see Mello and Mendelson, 2002, 2009a, 2009b in press). Moreover, cigarette smoking produced significantly greater and more sustained increases in LH than IV cocaine. These findings disagree with earlier clinical studies where no effect of cigarette smoking on LH was detected (Seyler et al., 1986; Winternitz and Quillen, 1977), and a number of procedural differences limit meaningful comparisons between those studies and our study (Mendelson et al., 2003). Differences in the frequency and duration of sample collection after cigarette smoking could account for these discrepant findings. For example, no changes in LH were detected when a single sample was collected after each cigarette (Winternitz and Quillen, 1977) or at 5 min intervals for 25 min after smoking (Seyler et al., 1986). In the present study, significant increases in LH were not detected until 18 min after smoking began.
The behavioral implications of rapid increases in LH release after IV cocaine and cigarette smoking are unclear. The significant correlations between increases in LH and plasma nicotine and plasma cocaine levels suggest that LH may contribute to the abuse-related effects of both drugs (for review see Mello and Mendelson, 2002, 2009a). High LH levels are often associated with enhanced sexual feelings. For example, in young men, increases in LH were temporally related to behavioral and physiological measures of sexual arousal in response to sexual stimuli (La Ferla et al., 1978). LH is essential for normal reproductive function (Yen et al., 1999), and high LH levels during the periovulatory phase in females may also be associated with increased sexual receptivity (Mello and Mendelson, 2002).
Effects of Cigarette Smoking and Cocaine on Testosterone
The significant increases in LH after IV cocaine and high nicotine cigarette smoking were not followed by changes in T levels. Previous clinical studies also reported no changes in T in men who smoked 6 cigarettes over 2 hr (Winternitz and Quillen, 1977) or who were given intranasal cocaine (Heesch et al., 1996). However, stimulation of LH is usually followed by stimulation of T, which in turn inhibits LH release (Yen et al., 1999). For example, in rhesus monkeys, when LH was stimulated by an opioid antagonist, nalmefene, T increased significantly within 40 to 50 min after significant increases in LH (Mello et al., 2000). It may be that the magnitude of LH increases in the present study was not sufficient to stimulate T. LH increased by 80 percent from baseline after IV cocaine and by 107 percent after smoking a high nicotine cigarette. Whereas in rhesus monkey, nalmefene stimulated LH increases of 147 and 187 percent from baseline were followed by significant increases in T (Mello et al., 2000). Differences in LH assay procedures, as well as species differences limit these comparisons.
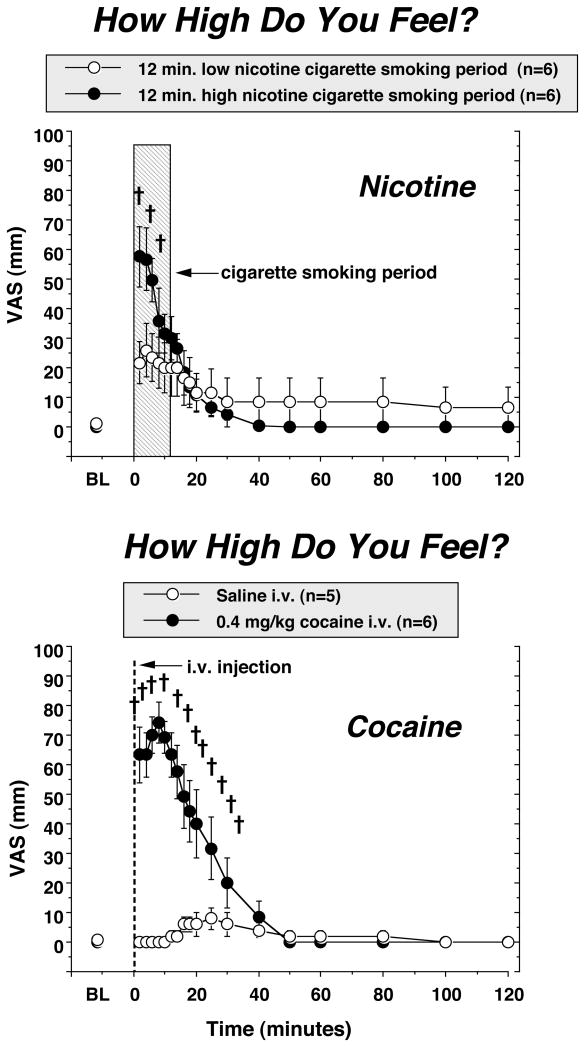
Subjective Effects of Cigarette Smoking and IV Cocaine [Figure 10]
Figure 10. Reports of “high’ after high and low dose nicotine cigarette smoking, and after cocaine and placebo-cocaine administration.
Subjective ratings on a Visual Analogue Scale (0–100) are shown on the left ordinates and time (min) is shown on the abscissae. Points above BL were collected 10 min before drug administration at time 0. Each data point is the average (± S.E.M.) of five or six subjects. All other details are as described in the legend for Figure 8. Reprinted with permission from Mendelson, J.H., Sholar, M.B., Mutschler, N.H., Jaszyna-Gasior, M., Goletiani, N.V., Siegel, A.J. and Mello, N.K.: Effects of intravenous cocaine and cigarette smoking on luteinizing hormone, testosterone and prolactin in men. J. Pharmacol. Exp. Ther. 307:339–348, 2003.
The “High” ratings following IV cocaine administration were significantly greater than the “High” ratings after smoking a high nicotine cigarette (Figure 10). Moreover, cocaine “High” ratings followed the plasma cocaine curve, whereas nicotine “High” ratings decreased as plasma nicotine levels were increasing. Interestingly, the “High” ratings after smoking a low nicotine cigarette were significantly greater than “High” ratings measured after placebo cocaine administration. These data differ from a previous study in which the “High” ratings after the highest dose of IV nicotine (3 mg/70 kg) were greater than after IV cocaine (40 mg/70kg or 0.571 mg/kg) (Jones et al., 1999). Moreover, the highest IV nicotine dose produced higher ratings on most of the subjective effects measures than IV cocaine (Jones et al., 1999). It is possible that IV nicotine has more potent subjective effects than smoked nicotine and is more comparable to IV cocaine. Drug plasma levels were not reported by Jones and co-workers, so nicotine dose levels cannot be directly compared with the present study. It is unlikely that differences in the time course of IV and smoked nicotine can account for these different findings. Smoked nicotine is estimated to reach the brain within 10 to 20 sec and is faster than IV nicotine (Benowitz et al., 2009). One limitation of these cigarette-smoking studies is that nicotine and hormone levels were measured in venous rather than arterial blood. Arterial nicotine levels are 2 to 3 or 6 to 10 fold higher than venous nicotine levels (Benowitz, 1996; Rose et al., 1999). After the first 5 sec puff, nicotine from cigarette smoke reached peak levels in the arterial circulation within 15 to 20 sec and continued to increase after the second and third puff (Rose et al., 1999). We detected significant increases in subjective ratings and plasma nicotine within 2–4 min (Mendelson et al., 2003, 2005, 2008), but we recognize that arterial nicotine, CRH and ACTH levels may have increased significantly after a single puff.
Although the time course of the rapid increases after cocaine and cigarette smoking were similar, but it is not clear if these drugs stimulate LH release by similar mechanisms. Deconvolution analysis of cocaine’s stimulation of LH suggested that the LH increase reflected a burst of LHRH (Mello and Mendelson, 2002). However, LHRH may be stimulated by norepinephrine, epinephrine and neuropeptide y, either stimulated or inhibited by dopamine and gonadal steroid hormones, and inhibited by endogenous opioid peptides (Yen et al., 1999). The relative contribution of these complex and inter-related systems to the acute effects of cocaine and nicotine on LH are unknown.
Summary and Conclusions
Smoking a high-nicotine cigarette significantly increased plasma nicotine levels, HPA axis hormones, heart rate and VAS reports of positive subjective effects (Mendelson et al., 2005). Smoking three successive high nicotine cigarettes at 1-hr intervals resulted in progressive increases in peak nicotine levels that probably reflected an accumulation of nicotine over time. However, peak VAS ratings and heart rate gradually diminished after each successive cigarette suggesting that tolerance developed to these effects of nicotine (Mendelson et al., 2008). It is often postulated that behavioral and biologic indicators of tolerance reflect desensitization of nicotinic acetylcholine receptors (nAChRs), and this concept is supported by a number of preclinical studies of endocrine (Sharp and Beyer, 1986; Sharp and Matta, 1993) and behavioral endpoints (James et al., 1994; Robinson et al., 2006). Speculation about receptor mechanisms of acute nicotine tolerance is beyond the scope of this review.
Peak levels of ACTH and cortisol also were lower after the second and third cigarette than after the first cigarette. These changes probably reflected alterations in the direct effects of nicotine as well as the feedback relationships between ACTH and cortisol. The exception was DHEA. Peak levels of DHEA did not differ significantly across successive cigarettes, and ratings of cigarette “Craving” increased as DHEA and plasma nicotine levels decreased at the end of each smoking interval (Figures 1, 2, 3, 4, 5, 6). It is interesting to speculate that the antidepressant effects of cigarette smoking (Breslau et al., 1993; Glassman et al., 1990; Picciotto et al., 2002) are associated in part with stimulation of DHEA. Controlled clinical trials have shown that DHEA is an effective antidepressant (Morales et al., 1994; Schmidt et al., 2005), and, DHEA has been suggested as a medication for smoking cessation (Marx et al., 2006). It was surprising to find that DHEA levels measured after smoking a single cigarette were over twice as high as levels measured after 6 weeks of high dose DHEA treatment for depression (Mendelson et al., 2005; Schmidt et al., 2005). The sustained increases in DHEA after repeated cigarette smoking are consistent with the hypothesis that DHEA may contribute to the abuse-related effects of cigarette smoking (Mendelson et al., 2005, 2008).
Relatively, little is known about the interactions between DHEA and cocaine. Preclinical studies have shown that DHEA can reduce cocaine self-administration and reinstatement of cocaine seeking in rats (Doron et al., 2006; Maayan et al., 2006), but an outpatient trial reported no decrease in cocaine use (Shoptaw et al., 2004). However, the significant increases in DHEA and cortisol after smoking high nicotine cigarettes (Figs. 2 and 5) were very similar in time course and magnitude to those observed after IV cocaine administration to men who were current cocaine users (Mendelson et al., 2002). Taken together, these clinical findings suggest that neuroactive steroid-based medications that are effective for nicotine treatment might also be effective for cocaine. Unfortunately, no uniformly effective medications to treat either cocaine or nicotine are available as yet, although there are many promising candidates (Henningfield et al., 2005; McCann, 2008; Vocci et al., 2005).
The neuroactive steroid hormones may offer a novel approach to modifying the abuse-related effects of nicotine and cocaine. There is considerable interest in using neuroactive steroid hormones to treat depression, anxiety and panic disorder (Eser et al., 2006; Rupprecht, 2003; Rupprecht and Holsboer, 1999a, 1999b) as well as drug abuse (Gasior et al., 1999; Zinder and Dar, 1999). The potential importance of this approach was illustrated in clinical and preclinical studies of the anxiolytic effectiveness of a compound that binds selectively to a translator protein (18κD) which enhances the biosynthesis of endogenous neurosteroids (Rupprecht et al., 2009). There were no benzodiazepine-like adverse side effects, possibly due to the fact that neuro steroids and benzodiazepines modulate GABAA receptors at different allosteric sites (Rupprecht et al., 2009).
The neuroactive gonadal steroid hormones, progesterone and estradiol, have opposite effects on the abuse-related effects of cocaine (Mello and Mendelson, 2009b in press). Estradiol enhances cocaine’s effects under many, but not all conditions (Caine et al., 2004; Carroll et al., 2004; Lynch et al., 2002; Mello et al., 2008). Progesterone usually decreases the abuse-related effects of cocaine and nicotine in women but not in men (Evans, 2007; Evans and Foltin, 2006; Sofuoglu et al., 2002, 2004, 2007; Mello NK ongoing studies). A better understanding of the interactions between the hormonal milieu and the abuse-related effects of nicotine and cocaine may provide a novel perspective on the neurobiology of these drugs and eventually lead to the development of more effective medication-based treatments.
Acknowledgments
This review summarizes some findings from a program of clinical research on the hormonal and behavioral concomitants of cigarette smoking and IV cocaine led by my late husband, Jack H. Mendelson, M.D. (August 30, 1929 – August 15, 2007). After pioneering research on alcoholism, opioid addiction and marihuana abuse, he became interested in studying cigarette smoking and cocaine abuse during the last decades of his life. This research was supported in part by grants R01-DA15067, R01-DA024642, R01-DA026892, P01-DA14528, K05-DA00064, K05-DA00101, and T32-DA07252 from the National Institute on Drug Abuse, NIH. I am grateful to Murty Pharmaceuticals Inc., Lexington, KY 40509, for generously supplying the low nicotine cigarettes used in these studies. I thank the clinical research team, and especially Drs. A.J. Siegel, N.V. Goletiani and M.B. Sholar for their many contributions to these studies and Alicja Skupny for excellent technical assistance in conducting the hormone and cocaine analyses. I also thank Dr. Peton Jacob III, University of California at San Francisco for conducting the nicotine assays.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- al’Absi M, Hatsukami DK, Davis GL, Wittmers LE. Prospective examination of effects of smoking abstinence on cortisol and withdrawal symptoms as predictors of early smoking relapse. Drug Alc Depend. 2004;73:267–278. doi: 10.1016/j.drugalcdep.2003.10.014. [DOI] [PubMed] [Google Scholar]
- al’Absi M, Wittmers LE, Erickson J, Hatsukami DK, Crouse B. Attenuated adrenocortical and blood pressure responses to psychological stress in ad libitum and abstinent smokers. Pharmacol Biochem Behav. 2003;74:401–410. doi: 10.1016/s0091-3057(02)01011-0. [DOI] [PubMed] [Google Scholar]
- Allen SS, Hatsukami DK, Christianson D, Nelson D. Withdrawal and pre-menstrual symptomatology during the menstrual cycle in short-term smoking abstinence: effects of menstrual cycle on smoking abstinence. Nicotine Tob Res. 1999;1:129–142. doi: 10.1080/14622299050011241. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Larson EB, Gliddon LA, Carroll ME. Effects of progesterone on the reinstatement of cocaine-seeking behavior in female rats. Exp Clin Psychopharmacol. 2007;15:472–480. doi: 10.1037/1064-1297.15.5.472. [DOI] [PubMed] [Google Scholar]
- APA. Diagnostic and Statistical Manual of Mental Disorders. 4. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Balster RL, Schuster CR. Fixed-interval schedule of cocaine reinforcement: Effect of dose and infusion duration. J Exper Anal Behav. 1973;20:119–129. doi: 10.1901/jeab.1973.20-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron JA, Comi RJ, Cryns V, Brinck-Johnsen T, Mercer NG. The effect of cigarette smoking on adrenal cortical hormones. J Pharmacol Exp Ther. 1995;272:151–155. [PubMed] [Google Scholar]
- Becker JB, Molenda H, Hummer DL. Gender differences in the behavioral responses to cocaine and amphetamine. Implications for mechanisms mediating gender differences in drug abuse. Ann NY Acad Sci. 2001;937:172–187. doi: 10.1111/j.1749-6632.2001.tb03564.x. [DOI] [PubMed] [Google Scholar]
- Benowitz NL. Pharmacology of nicotine: Addiction and therapeutics. Annu Rev Pharmcol Toxicol. 1996;36:597–613. doi: 10.1146/annurev.pa.36.040196.003121. [DOI] [PubMed] [Google Scholar]
- Benowitz NL. Risks associated with smoking cigarettes with low machine-measured yields of tar and nicotine Smoking and tobacco control monograph No 13. U.S. Department of Health and Human Services, National Insitutes of Health, National Cancer Institute; Bethesda, MD: 2001. Oct, Compensatory smoking of low-yield cigarettes; pp. 39–63. NIH Pub. No. 02-5074. 2001. [Google Scholar]
- Benowitz NL, Hukkanen J, Jacob PI. Nicotine chemistry, metabolism, kinetics and biomarkers. In: Henningfield JE, London ED, Pogun S, editors. Nicotine Psychopharmacology, Handbook of Experimental Pharmacology. Vol. 192. Springer-Verlag; Heidelberg: 2009. pp. 29–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Porchet H, Jacob P., III . Pharmacokinetics, metabolism, and pharmacodynamics of nicotine. In: Wonnacott S, Russell MAH, Stolerman IP, editors. Nicotine Psychopharmacology: Molecular, Cellular and Behavioural Aspects. Oxford University Press; Oxford: 1990. pp. 112–157. [Google Scholar]
- Breslau N, Kilbey MM, Andreski P. Nicotine dependence and major depression. New evidence from a prospective investigation. Arch Gen Psychiatry. 1993;50:31–35. doi: 10.1001/archpsyc.1993.01820130033006. [DOI] [PubMed] [Google Scholar]
- Butschky MF, Bailey D, Henningfield JE, Pickworth WB. Smoking without nicotine delivery decreases withdrawal in 12-hour abstinent smokers. Pharmacol Biochem Behav. 1995;50:91–96. doi: 10.1016/0091-3057(94)00269-o. [DOI] [PubMed] [Google Scholar]
- Cabrera RJ, Bregonzio D, Laconi M, Mampel A. Allopregnanolone increase in striatal N-methyl-D-aspartic acid evoked [3H] dopamine release is estrogen and progesterone dependent. Cell Mol Neurobiol. 2002;22:445–454. doi: 10.1023/A:1021015705597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine SB, Bowen CA, Yu G, Zuzga D, Negus SS, Mello NK. Effect of gonadectomy and gonadal hormone replacement on cocaine self-administration in female and male rats. Neuropsychopharmacology. 2004;29:929–942. doi: 10.1038/sj.npp.1300387. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lynch WJ, Roth ME, Morgan AD, Cosgrove KP. Sex and estrogen influence drug abuse. Trends Pharmacol Sci. 2004;25:273–279. doi: 10.1016/j.tips.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Castner SA, Xiao L, Becker JB. Sex differnces in striatal dopamine: in vivo microdialysis and behavioral studies. Brain Res. 1993;610:127–134. doi: 10.1016/0006-8993(93)91225-h. [DOI] [PubMed] [Google Scholar]
- CDC. Annual smoking-attributable mortality, years of potential life lost, and economic costs - United States, 1995–1999. Morbidity and Mortality Weekly Report. 2002a;51:300–303. [PubMed] [Google Scholar]
- CDC. U.S. Department of Health and Human Services. The Health Consequences of Smoking: A Report of the Surgeon General. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Atlanta, GA: 2004. [Google Scholar]
- CDC. Annual smoking-attributable mortality, years of potential life lost and productivity losses - United States, 1997–2001. Centers for Disease Control and Prevention. MMWR Morb Mortal Weekly Report. 2005;54:625–628. [PubMed] [Google Scholar]
- Chausmer AL, Smith BJ, Kelly RY, Griffiths RR. Cocaine-like subjective effects of nicotine are not blocked by the D1 selective antagonist ecopipam (SCH 39166) Behav Pharmacol. 2003;14:111–120. doi: 10.1097/00008877-200303000-00002. [DOI] [PubMed] [Google Scholar]
- Coiro V, Vescovi PP. Effect of cigarette smoking on ACTH/cortisol secretion in alcoholics after short- and medium-term abstinence. Alcohol Clin Exp Res. 1999;23:1515–1518. [PubMed] [Google Scholar]
- Connolly GN, Alpert HR. Trends in the use of cigarettes and other tobacco produces: 2000–2007. JAMA. 2008;299:2692–2630. doi: 10.1001/jama.299.22.2629. [DOI] [PubMed] [Google Scholar]
- Corrigall WA. Nicotine self-administration in animals as a dependence model. Nicotine Tob Res. 1999;1:11–20. doi: 10.1080/14622299050011121. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Franklin KBI, Coen KM, Clarke PBS. The mesolimbic dopamine system is implicated in the reinforcing effects of nicotine. Psychopharmacology. 1992;107:285–289. doi: 10.1007/BF02245149. [DOI] [PubMed] [Google Scholar]
- Cryer PE, Haymond MW, Santiago JV, Shah SD. Norepinephrine and epinephrine release and adrenergic mediation of smoking-associated hemodynamic and metabolic events. N Engl J Med. 1976;295:573–577. doi: 10.1056/NEJM197609092951101. [DOI] [PubMed] [Google Scholar]
- Curtis L, Buisson B, Bertrand S, Bertrand D. Potentiation of human alpha4beta2 neuronal nicotinic acetylcholine receptor by estradiol. Mol Pharmacol. 2002;61:127–135. doi: 10.1124/mol.61.1.127. [DOI] [PubMed] [Google Scholar]
- del Arbol JL, Munoz JR, Ojeda L, Cascales AL, Irles JR, Mianda MT, Ruiz Requena ME, Aguirre JC. Plasma concentrations of beta-endophin in smokers who consume different numbers of cigarettes per day. Pharm Biochem Behav. 2000;67:25–28. doi: 10.1016/s0091-3057(00)00291-4. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Role of dopamine in the behavioural actions of nicotine related to addiction. Eur J Pharmacol. 2000;393:295–314. doi: 10.1016/s0014-2999(00)00122-9. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Mielke MM, Booth S, Gharib MA, Hoffman A, Maldovan V, Shupenko C, McCallum SE. Nicotine self-administration in rats on a progressive ratio schedule of reinforcement. Psychopharmacology (Berl) 1999;147:135–142. doi: 10.1007/s002130051153. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Rowell PP, Gharib MA, Maldovan V, Booth S, Mielke MM, Hoffman A, McCallum S. Nicotine self-administration in rats: Estrous cycle effects, sex differences and nicotinic receptor binding. Psychopharmacology (Berl) 2000;15:392–405. doi: 10.1007/s002130000497. [DOI] [PubMed] [Google Scholar]
- Doron R, Fridman L, Gispan-Herman I, Maayan R, Weizman A, Yadid G. DHEA, a neurosteroid, decreases cocaine self-administration and reinstatement of cocaine-seeking behavior in rats. Neuropsychopharmacology. 2006;31:2231–2236. doi: 10.1038/sj.npp.1301013. [DOI] [PubMed] [Google Scholar]
- Dudish SA, Pentel PA, Hatsukami DK. Smoked cocaine self-administration in females. Psychopharmacology. 1996;123:79–87. doi: 10.1007/BF02246284. [DOI] [PubMed] [Google Scholar]
- Eser D, Schule C, Romeo E, Baghai TC, di Michele F, Pasini A, Zwanzger P, Padberg F, Rupprecht R. Neuropsychopharmacological properties of neuroactive steroids in depression and anxiety disorders. Psychopharmacology (Berl) 2006;186:373–387. doi: 10.1007/s00213-005-0188-z. [DOI] [PubMed] [Google Scholar]
- Evans SM. The role of estradiol and progesterone in modulating the subjective effects of stimulants in humans. Exp Clin Psychopharmacol. 2007;15:418–426. doi: 10.1037/1064-1297.15.5.418. [DOI] [PubMed] [Google Scholar]
- Evans SM, Cone EJ, Henningfield JR. Arterial and venous cocaine plasma concentrations in humans: relationship to route of administration, cardiovascular effects and subjective effects. J Pharmacol Exp Ther. 1996;279:1345–1356. [PubMed] [Google Scholar]
- Evans SM, Foltin RW. Exogenous progesterone attenuates the subjective effects of smoked cocaine in women, but not in men. Neuropsychopharmacology. 2006;31:659–674. doi: 10.1038/sj.npp.1300887. [DOI] [PubMed] [Google Scholar]
- Evans SM, Haney M, Fischman MW, Foltin RW. Limited sex differences in response to “binge” smoked cocaine use in humans. Neuropsychopharmacology. 1999;21:445–454. doi: 10.1016/S0893-133X(98)00120-1. [DOI] [PubMed] [Google Scholar]
- Evans SM, Haney M, Foltin RW. The effects of smoked cocaine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology. 2002;159:397–406. doi: 10.1007/s00213-001-0944-7. [DOI] [PubMed] [Google Scholar]
- Fant RV, Schuh KJ, Stitzer ML. Response to smoking as a function of prior smoking amounts. Psychopharmacology. 1995;119:385–390. doi: 10.1007/BF02245853. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, See RW. Plasma progesterone levels and cocaine-seeking in freely cycling female rats across the estrous cycle. Drug Alcohol Depend. 2007;89:183–189. doi: 10.1016/j.drugalcdep.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field AE, Colditz GA, Willett WC, Longcope C, McKinlay JB. The relation of smoking, age, relative weight, and dietary intake to serum adrenal steroids, sex hormones, and sex hormone-binding globulin in middle-aged men. J Clin Endocrinol Metab. 1994;79:1310–1316. doi: 10.1210/jcem.79.5.7962322. [DOI] [PubMed] [Google Scholar]
- Fischman MW, Schuster CR. Cocaine self-administration in humans. Fed Proc. 1982;41:241–246. [PubMed] [Google Scholar]
- Fischman MW, Schuster CR, Javaid J, Hatono Y, Davis J. Acute tolerance development to the cardiovascular and subjective effects of cocaine. J Pharmacol Exp Ther. 1985;235:677–682. [PubMed] [Google Scholar]
- Foltin RW, Fischman MW. Smoked and intravenous cocaine in humans: Acute tolerance, cardiovascular and subjective effects. J Pharmacol Exp Ther. 1991;257:247–261. [PubMed] [Google Scholar]
- Foltin RW, Fischman MW. Effects of “binge” use of intravenous cocaine in methadone-maintained individuals. Addiction. 1998;93:825–836. doi: 10.1046/j.1360-0443.1998.9368254.x. [DOI] [PubMed] [Google Scholar]
- Fu Y, Matta SG, Gao W, Brower VG, Sharp BM. Systemic nicotine stimulates dopamine release in nucleus accumbens: Re-evaluation of the role of N-methyl-D-aspartate receptors in the ventral tegmental area. J Pharmacol Exp Ther. 2000;294:458–465. [PubMed] [Google Scholar]
- Gasior M, Carter RB, Wilkin JM. Neuroactive steroids: potential therapeutic use in neurological and psychiatric disorders. Trends Pharmacol Sci. 1999;20:107–112. doi: 10.1016/s0165-6147(99)01318-8. [DOI] [PubMed] [Google Scholar]
- Gerasimov MR, Franceschi M, Volkow ND, Rice O, Schiffer WK, Dewey SL. Synergistic interactions between nicotine and cocaine or methylphenidate depend on the dose of dopamine transporter inhibitor. Synapse. 2000;38:432–437. doi: 10.1002/1098-2396(20001215)38:4<432::AID-SYN8>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Gianoulakis C, Dai X, Thavundayil J, Brown T. Levels and circadian rhythmicity of plasma ACTH, cortisol, and beta-endorphin as a function of family history of alcoholism. Psychopharmacology (Berl) 2005;181:437–444. doi: 10.1007/s00213-005-0129-x. [DOI] [PubMed] [Google Scholar]
- Glassman AH, Helzer JE, Covey LS, Cottler LB, Stetner F, Tipp JE, Johnson J. Smoking, smoking cessation, and major depression. JAMA. 1990;264:1546–1549. [PubMed] [Google Scholar]
- Goeders NE. A neuroendocrine role in cocaine reinforcement. Psychoneuroendocrinology. 1997;22:237–259. doi: 10.1016/s0306-4530(97)00027-9. [DOI] [PubMed] [Google Scholar]
- Goeders NE. Stress and cocaine addiction. J Pharmacol Exp Ther. 2002a;301:785–789. doi: 10.1124/jpet.301.3.785. [DOI] [PubMed] [Google Scholar]
- Goeders NE. The HPA axis and cocaine reinforcement. Psychoneuroendocrinology. 2002b;27:13–33. doi: 10.1016/s0306-4530(01)00034-8. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Guerin GF. Effects of the CRH receptor antagonist CP-154,526 on intravenous cocaine self-administration in rats. Neuropsychopharmacology. 2000;23:577–586. doi: 10.1016/S0893-133X(00)00148-2. [DOI] [PubMed] [Google Scholar]
- Goldberg SR, Spealman RD, Goldberg DM. Persistent behavior at high rates maintained by intravenous self-administration of nicotine. Scinece. 1981;214:573–575. doi: 10.1126/science.7291998. [DOI] [PubMed] [Google Scholar]
- Gossain VV, Sherma NK, Srivastava L, Michelakis AM, Rovner DR. Hormonal effects of smoking--II: Effects on plasma cortisol, growth hormone, and prolactin. Am J Med Sci. 1986;291:325–327. doi: 10.1097/00000441-198605000-00007. [DOI] [PubMed] [Google Scholar]
- Gourlay SG, Benowitz NL. Arteriovenous differences in plasma concentration of nicotine and catecholamines and related cardiovascular effects after smoking, nicotine nasal spray, and intravenous nicotine. Clin Pharmacol Ther. 1997;62:453–463. doi: 10.1016/S0009-9236(97)90124-7. [DOI] [PubMed] [Google Scholar]
- Gross J, Lee J, Stitzer ML. Nicotine-containing versus de-nicotinized cigarettes: Effects on craving and withdrawal. Pharmacol Biochem Behav. 1997;57:159–165. doi: 10.1016/s0091-3057(96)00309-7. [DOI] [PubMed] [Google Scholar]
- Harvey DM, Yasar S, Heishman SJ, Panlilio LV, Henningfield JE, Goldberg SR. Nicotine serves as an effective reinforcer of intravenous drug-taking behavior in human cigarette smokers. Psychopharmacology (Berl) 2004;175:134–142. doi: 10.1007/s00213-004-1818-6. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Pickens RW, Svikis DS, Hughes JR. Smoking topography and nicotine blood levels. Addict Behav. 1988;13:91–95. doi: 10.1016/0306-4603(88)90031-7. [DOI] [PubMed] [Google Scholar]
- Heesch CM, Negus BH, Bost JE, Keffer JH, Snyder RW, Eichhorn EJ. Effects of cocaine on anterior pituitary and gonadal hormones. J Pharmacol Exp Ther. 1996;278:1195–1200. [PubMed] [Google Scholar]
- Henningfield JE, Fant RV, Buchhalter AR, Stitzer ML. Pharmacotherapy for nicotine dependence. CA Cancer J Clin. 2005;55:281–299. doi: 10.3322/canjclin.55.5.281. quiz 322–283, 325. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Goldberg SR. Control of behavior by intravenous nicotine injections in human subjects. Pharmacol Biochem Behav. 1983a;19:1021–1026. doi: 10.1016/0091-3057(83)90409-4. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Goldberg SR. Nicotine as a reinforcer in human subjects and laboratory animals. Pharmacol Biochem Behav. 1983b;19:989–992. doi: 10.1016/0091-3057(83)90405-7. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Schuh LM, Jarvik ME. Pathophysiology of Tobacco Dependence. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology The Fourth Generation of Progress. Raven Press; New York: 1995. pp. 1715–1729. [Google Scholar]
- Isaac PF, Rand MU. Cigarette smoking and plasma levels of nicotine. Nature. 1972;236:308–310. doi: 10.1038/236308a0. [DOI] [PubMed] [Google Scholar]
- Jackson LR, Robinson TE, Becker JB. Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacology. 2006;31:129–138. doi: 10.1038/sj.npp.1300778. [DOI] [PubMed] [Google Scholar]
- Jaffe JH, Martin WR. Opioid analgesics and antagonists. In: Gilman AG, Rall TW, Nies AS, Taylor P, editors. The Pharmacological Basis of Therapeutics. Pergamon Press; New York: 1990. pp. 485–521. [Google Scholar]
- James JR, Villanueva HF, Johnson JH, Arezo S, Rosecrans JA. Evidence that nicotine can acutely desensitize central nicotinic acetylcholinergic receptors. Psychopharmacology (Berl) 1994;114:456–462. doi: 10.1007/BF02249336. [DOI] [PubMed] [Google Scholar]
- Jones HE, Garrett BE, Griffiths RR. Subjective and physiological effects of intravenous nicotine and cocaine in cigarette smoking cocaine abusers. J Pharmacol Exp Ther. 1999;288:188–197. [PubMed] [Google Scholar]
- Kuhar MJ, Ritz MC, Boja JW. The dopamine hypothesis of the reinforcing properties of cocaine. Trends Nerosci. 1991;14:299–302. doi: 10.1016/0166-2236(91)90141-g. [DOI] [PubMed] [Google Scholar]
- La Ferla J, Anderson DL, Schalch DS. Psychoendocrine response to sexual arousal in human males. Psychosomatic Med. 1978;40:166–172. doi: 10.1097/00006842-197803000-00007. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR. Effects of nicotine in experimental animals and humans: An update on addictive properties. In: Henningfield JE, London ED, Pogun S, editors. Nicotine Psychopharmacology, Handbook of Experimental Pharmacology. Vol. 192. Springer- Verlag; Heidelberg: 2009. pp. 335–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacol. 2002;164:121–137. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- Maayan R, Lotan S, Doron R, Shabat-Simon M, Gispan-Herman I, Weizman A, Yadid G. Dehydroepiandrosterone (DHEA) attenuates cocaine-seeking behavior in the self-administration model in rats. Eur Neuropsychopharmacol. 2006;16:329–339. doi: 10.1016/j.euroneuro.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Marinelli M, Piazza PV. Interaction between glucocorticoid hormones, stress and psychostimulant drugs. Eur J Neurosci. 2002;16:387–394. doi: 10.1046/j.1460-9568.2002.02089.x. [DOI] [PubMed] [Google Scholar]
- Marks JL, Hair CS, Klock SC, Ginsburg BE, Pomerleau CS. Effects of menstrual phase on intake of nicotine, caffeine, and alcohol and nonprescribed drugs in women with late luteal phase dysphoric disorder. J Subst Abuse. 1994;6:235–243. doi: 10.1016/s0899-3289(94)90265-8. [DOI] [PubMed] [Google Scholar]
- Marx CE, Trost WT, Shampine L, Behm FM, Giordano LA, Massing MW, Rose JE. Neuroactive steroids, negative affect, and nicotine dependence severity in male smokers. Psychopharmacology. 2006;186:463–472. doi: 10.1007/s00213-005-0226-x. [DOI] [PubMed] [Google Scholar]
- Matta SG, Foster CA, Sharp BM. Selective administration of nicotine into catecholaminergic regions of rat brainstem stimulates adrencorticotropin secretion. Endocrinology. 1993;133:2935–2942. doi: 10.1210/endo.133.6.8243321. [DOI] [PubMed] [Google Scholar]
- Matta SG, Yitong F, Valentine JD, Sharp BM. Response of the hypothalamo-pituitary-adrenal axis to nicotine. Psychoneuroendocrinology. 1998;23:103–113. doi: 10.1016/s0306-4530(97)00079-6. [DOI] [PubMed] [Google Scholar]
- McCann DJ. Potential of buprenorphine/naltrexone in treating polydrug addiction and co-occurring psychiatric disorders. Clinical pharmacology and therapeutics. 2008;83:627–630. doi: 10.1038/sj.clpt.6100503. [DOI] [PubMed] [Google Scholar]
- Mello NK, Lukas SE, Mendelson JH. Buprenorphine effects on cigarette smoking. Psychopharmacology. 1985;86:417–424. doi: 10.1007/BF00427902. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH. Cocaine, hormones and behavior: Clinical and preclinical studies. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. Academic Press; New York: 2002. pp. 665–745. [Google Scholar]
- Mello NK, Mendelson JH. Cocaine, Hormones and Behavior: Clinical and Preclinical Studies. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. 2. Academic Press; San Diego, CA: 2009a. pp. 3081–3139. [Google Scholar]
- Mello NK, Mendelson JH. Cocaine, Hormones and Behavior: Clinical Studies. In: Rubin RT, Pfaff DW, editors. Hormone/Behavior Relations of Clinical Importance. 2009b. in press. [Google Scholar]
- Mello NK, Mendelson JH, Kelly M. Acute effects of nalmefene on LH, prolactin, and testosterone in male rhesus monkeys. Pharm Biochem Behav. 2000;66:275–284. doi: 10.1016/s0091-3057(00)00190-8. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, Knudson IM, Fivel P, Kelly M. Acute effects of progesterone and testosterone on cocaine self-administration by female nonhuman primates. Presented at the 2007 meeting of the College on Problems of Drug Dependence.2009. in preparation. [Google Scholar]
- Mello NK, Mendelson JH, Knudson IM, Negus SS, Kelly M. Acute effects of progesterone and testosterone on cocaine self-administration by female nonhuman primates. Presented at the 2007 meeting of the College on Problems of Drug Dependence.2007. [Google Scholar]
- Mello NK, Mendelson JH, Sellers ML, Kuehnle JC. Effects of heroin self-administration on cigarette smoking. Psychopharmacology. 1980;67:45–52. doi: 10.1007/BF00427594. [DOI] [PubMed] [Google Scholar]
- Mello NK, Negus SS, Knudson IM, Kelly M, Mendelson JH. Effects of estradiol on cocaine self-administration and cocaine discrimination by female rhesus monkeys. Neuropsychopharmacology. 2008;33:783–795. doi: 10.1038/sj.npp.1301451. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Goletiani NV, Sholar MB, Siegel AJ, Mello NK. Effects of smoking successive low- and high-nicotine cigarettes on hypothalamic-pituitary-adrenal axis hormones and mood in men. Neuropsychopharmacology. 2008;33:749–760. doi: 10.1038/sj.npp.1301455. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Mello NK, Lukas SE, Woods BT, Teoh SK. Office, U.G.P. Promising new biological and behavioral correlates of the reinforcing properties of drugs. In: Fischman MW, Mello NE, editors. Testing for abuse liability of drugs in humans. NIDA Research Monograph; Rockville, MD: 1989. pp. 307–340. [PubMed] [Google Scholar]
- Mendelson JH, Mello NK, Sholar MB, Siegel AJ, Mutschler N, Halpern J. Temporal concordance of cocaine effects on mood states and neuroendocrine hormones. Psychoneuroendocrinology. 2002;27:71–82. doi: 10.1016/s0306-4530(01)00036-1. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Mello NK, Teoh SK, Lukas SE, Phipps W, Ellingboe J, Palmieri SL, Schiff I. Human studies on the biological basis of reinforcement: A neuroendocrine perspective. In: O’Brien CP, Jaffe JH, editors. Addictive States. Raven Press, Ltd; New York: 1992. pp. 131–155. [PubMed] [Google Scholar]
- Mendelson JH, Sholar M, Siegel AJ, Mello NK. Effects of cocaine on luteinizing hormone in women during the follicular and luteal phases of the menstrual cycle and in men. J Pharmacol Exp Ther. 2001;296:972–979. [PubMed] [Google Scholar]
- Mendelson JH, Sholar MB, Goletiani NV, Siegel AJ, Mello NK. Effects of low and high nicotine cigarette smoking on mood states and the HPA axis in men. Neuropsychopharmacology. 2005;30:1751–1763. doi: 10.1038/sj.npp.1300753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson JH, Sholar MB, Mutschler NH, Jaszyna-Gasior M, Goletiani NV, Siegel AJ, Mello NK. Effects of intravenous cocaine and cigarette smoking on luteinizing hormone, testosterone and prolactin in men. J Pharmacol Exp Ther. 2003;307:339–348. doi: 10.1124/jpet.103.052928. [DOI] [PubMed] [Google Scholar]
- Morales AJ, Nolan JJ, Nelson JC, Yen SSC. Effects of replacement dose of dehydroepiandrosterone in men and women of advancing age. J Clin Endo Metab. 1994;78:1360–1367. doi: 10.1210/jcem.78.6.7515387. [DOI] [PubMed] [Google Scholar]
- Mutschler NH, Stephen BJ, Teoh SK, Mendelson JH, Mello NK. An inpatient study of the effects of buprenorphine on cigarette smoking in men concurrently dependent on cocaine and opioids. Nicotine Tob Res. 2002;4:223–228. doi: 10.1080/14622200210124012. [DOI] [PubMed] [Google Scholar]
- Nair KS, Rizza RA, O’Brien P, Khatariya K, Short KR, Nehra A, Vittone JL, Klee GG, Basu A, Basu R, Cobelli C, Toffolo G, Dalla Man C, Tindall DJ, Melton LJ, Smith GE, Khosla S, Jensen MD. DHEA in elderly women and DHEA or testosterone in elderly men. N Engl J Med. 2006;355:1647–1659. doi: 10.1056/NEJMoa054629. [DOI] [PubMed] [Google Scholar]
- Newman JL, Mello NK. Neuroactive gonadal steroid hormones and drug addicton in women. In: Brady KT, Backs SE, Greenfield SF, editors. Women and Addiction: A Comprehensive Textbook. Guilford Press; New York, NY: 2009. pp. 35–64. [Google Scholar]
- Oncken C, Prestwood K, Cooney JL, Unson C, Fall P, Kulldorff M, Raisz LG. Effects of smoking cessation or reduction on hormone profiles and bone turnover in postmenopausal women. Nicotine Tob Res. 2002;4:451–458. doi: 10.1080/1462220021000018399. [DOI] [PubMed] [Google Scholar]
- Paradiso K, Zhang J, Steinbach JH. The C terminus of the human nicotinic alpha4beta2 receptor forms a binding site required for potentiation by an estrogenic steroid. J Neurosci. 2001;21:6561–6568. doi: 10.1523/JNEUROSCI.21-17-06561.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasqualini C, Olivier V, Gluibert B, Frain O, Leviel V. Rapid stimulation of striatal dopamine synthesis by estradiol. Cell Mol Neurobiol. 1996;16:411–415. doi: 10.1007/BF02088105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasqualini C, Olivier V, Guibert B, Frain O, Leviel V. Acute stimulatory effect of estradiol on striatal dopamine synthesis. J Neurochem. 1995;65:1651–1657. doi: 10.1046/j.1471-4159.1995.65041651.x. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Donny E, Caggiula AR. Sex differences in nicotine effects and self-administration: review of human and animal evidence. Nicotine Tob Res. 1999;1:301–315. doi: 10.1080/14622299050011431. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Epstein LH, Stiller RL, Marks BL, Jacob RG. Chronic and acute tolerance to the heart rate effects of nicotine. Psychopharmacology. 1989;97:529–534. doi: 10.1007/BF00439559. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Fonte C, Sanders M, Meeker J, Wilson A. Threshold doses for nicotine discrimination in smokers and non-smokers. Psychopharmacology. 2001;155:163–170. doi: 10.1007/s002130000660. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Grobe JE, Epstein LH, Caggiula A, Stiller RL, Jacob RG. Chronic and acute tolerance to subjective effects of nicotine. Pharmacol Biochem Behav. 1993;45:375–381. doi: 10.1016/0091-3057(93)90254-q. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Grobe JE, Mitchell SL, Goettler J, Caggiula A, Stiller RL, Scierka A. Acute tolerance to nicotine in smokers: Lack of dissipation within 2 hours. Psychopharmacology. 1995;118:164–170. doi: 10.1007/BF02245835. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Sexton JE, Reynolds WA, Grobe J, Fonte C, Stiller RL. Comparison of acute subjective and heart rate effects of nicotine intake via tobacco smoking versus nasal spray. Pharmacol Biochem Behav. 1994;47:295–299. doi: 10.1016/0091-3057(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Stiller RL, Jennings JR. Acute tolerance to the cardiovascular effects of nicotine. Drug Alc Depend. 1991;29:77–85. doi: 10.1016/0376-8716(91)90024-s. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Brunzell DH, Caldarone BJ. Effect of nicotine and nicotinic receptors on anxiety and depression. Neuroreport. 2002;13:1097–1106. doi: 10.1097/00001756-200207020-00006. [DOI] [PubMed] [Google Scholar]
- Pickworth WB, Fant RV. Endocrine effects of nicotine administration, tobacco and other drug withdrawal in humans. Psychoneuroendocrinology. 1998;23:131–141. doi: 10.1016/s0306-4530(97)00075-9. [DOI] [PubMed] [Google Scholar]
- Pickworth WB, Fant RV, Nelson RA, Rohrer MS, Henningfield JE. Pharmacodynamic effects of new de-nicotinized cigarettes. Nicotine Tob Res. 1999;1:357–364. doi: 10.1080/14622299050011491. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kumaresan V. The mesolimbic dopamine system: The final common pathway for the reinforcing effect of drugs of abuse? Neurosc Behav Rev. 2006;30:215–238. doi: 10.1016/j.neubiorev.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Pillitteri JL, Kozlowski LT, Sweeney CT, Heatherton TF. Individual differences in the subjective effects of the first cigarette of the day; a self-report method for studying tolerance. Exp Clin Psychopharmacol. 1997;5:83–90. doi: 10.1037//1064-1297.5.1.83. [DOI] [PubMed] [Google Scholar]
- Pollock JD, Koustova E, Hoffman A, Shurtleff D, Volkow ND. Treatments for nicotine addiction should be a top priority. Lancet. 2009 Apr 24; doi: 10.1016/S0140-6736(09)60352-4. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerleau CS, Cole PA, Lumley MA, Marks JL, Pomerleau OF. Effects of menstrual phase on nicotine, alcohol, and caffeine intake in smokers. J Subst Abuse. 1994;6:227–234. doi: 10.1016/s0899-3289(94)90253-4. [DOI] [PubMed] [Google Scholar]
- Pomerleau CS, Pomerleau OF. Euphoriant effects of nicotine in smokers. Psychopharmacology. 1992;108:460–465. doi: 10.1007/BF02247422. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF, Flessland KA, Pomerleau CS, Hariharan M. Controlled dosing of nicotine via an intranasal nicotine aerosol delivery device (INADD) Psychopharmacology. 1992;108:519–526. doi: 10.1007/BF02247431. [DOI] [PubMed] [Google Scholar]
- Rasmusson AM, Wu R, Paliwal P, Anderson GM, Krishnan-Sarin S. A decrease in the plasma DHEA to cortisol ratio during smoking abstinence may predict relapse: a preliminary study. Psychopharmacology (Berl) 2006;186(3):473–480. doi: 10.1007/s00213-006-0367-6. [DOI] [PubMed] [Google Scholar]
- Robinson ML, Houtsmuller EJ, Moolchan ET, Pickworth WB. Placebo cigarettes in smoking research. Exp Clin Psychopharmacol. 2000;8:326–332. doi: 10.1037//1064-1297.8.3.326. [DOI] [PubMed] [Google Scholar]
- Robinson SE, James JR, Lapp LN, Vann RE, Gross DF, Philibin SD, Rosecrans JA. Evidence of cellular nicotinic receptor desensitization in rats exhibiting nicotine-induced acute tolerance. Psychopharmacology. 2006;184:306–313. doi: 10.1007/s00213-005-0049-9. [DOI] [PubMed] [Google Scholar]
- Rose JE. Nicotine and nonnicotine factors in cigarette addiction. Psychopharmacology. 2006;184:274–285. doi: 10.1007/s00213-005-0250-x. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC, Coleman RE. Arterial nicotine kinetics during cigarette smoking and intravenous nicotine administration: implications for addiction. Drug Alc Depend. 1999;56:99–107. doi: 10.1016/s0376-8716(99)00025-3. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC, Johnson M. Dissociating nicotine and nonnicotine components of cigarette smoking. Pharmacol Biochem Behav. 2000;67:71–81. doi: 10.1016/s0091-3057(00)00301-4. [DOI] [PubMed] [Google Scholar]
- Rosenberg L, Shapiro S, Kaufman DW, Slone D, Miettinen OS, Stolley PD. Cigarette smoking in relation to the risk of myocardial infarction in young women. Modifying influence of age and predisposing factors. Int J Epidemiol. 1980;9:57–63. doi: 10.1093/ije/9.1.57. [DOI] [PubMed] [Google Scholar]
- Rupprecht R. Neuroactive steroids: mechanisms of action and neuropsychopharmacological properties. Psychoneuroendocrinology. 2003;28:139–168. doi: 10.1016/s0306-4530(02)00064-1. [DOI] [PubMed] [Google Scholar]
- Rupprecht R, Holsboer DF. Neuropsychopharmacological properties of neuroactive steroids. Steroids. 1999b;64:83–91. doi: 10.1016/s0039-128x(98)00101-9. [DOI] [PubMed] [Google Scholar]
- Rupprecht R, Holsboer F. Neuroactive steroids: mechanisms of action and neuropsychopharmacological perspectives. Trends Neurosci. 1999a;22:410–416. doi: 10.1016/s0166-2236(99)01399-5. [DOI] [PubMed] [Google Scholar]
- Rupprecht R, Rammes G, Eser D, Baghai TC, Schule C, Nothdurfter C, Troxler T, Gentsch C, Kalkman HO, Chaperon F, Uzunov V, McAllister KH, Bertaina-Anglade V, LaRochelle CD, Tuerck D, Floesser A, Kiese B, Schumacher M, Landgraf R, Holsboer F, Kucher K. Translocator protein (18 kD) as target for anxiolytics without benzodiazepine-like side effects. Science. 2009;325:490–493. doi: 10.1126/science.1175055. [DOI] [PubMed] [Google Scholar]
- Russell MAH. Subjective and behavioural effects of nicotine in humans: some sources of individual variation. In: Nordberg A, Fuxe K, Helmstedt B, Sundwall A, editors. Progress in Brain Research. Elsevier Science Publkishers; 1985. pp. 289–302. [DOI] [PubMed] [Google Scholar]
- Schmidt PJ, Daly RC, Bloch M, Smith MJ, Danaceau MA, St Clair LS, Murphy JH, Haq N, Rubinow DR. Dehydroepiandrosterone monotherapy iin midlife-onset major and minor depression. Arch Gen Psychiatry. 2005;62:154–162. doi: 10.1001/archpsyc.62.2.154. [DOI] [PubMed] [Google Scholar]
- Selmaoui B, Touitou Y. Reproducibility of the circadian rhythms of serum cortisol and melatonin in healthy subjects: a study of three different 24-h cycles over six weeks. Life Sci. 2003;73:3339–3349. doi: 10.1016/j.lfs.2003.05.007. [DOI] [PubMed] [Google Scholar]
- Seyler LE, Jr, Fertig J, Pomerleau O, Hunt D, Parker K. The effects of smoking on ACTH and cortisol secretion. Life Sci. 1984;34:57–65. doi: 10.1016/0024-3205(84)90330-8. [DOI] [PubMed] [Google Scholar]
- Seyler LE, Jr, Pomerleau OF, Fertig JB, Hunt D, Parker K. Pituitary hormone response to cigarette smoking. Pharmacol Biochem Behav. 1986;24:159–162. doi: 10.1016/0091-3057(86)90062-6. [DOI] [PubMed] [Google Scholar]
- Shahan TA, Bickel WK, Madden GJ, Badger GJ. Comparing the reinforcing efficacy of nicotine containing and de-nicotinized cigarettes: A behavioral economic analysis. Psychopharmacology. 1999;147:210–216. doi: 10.1007/s002130051162. [DOI] [PubMed] [Google Scholar]
- Sharp BM, Beyer HS. Rapid desensitization of the acute stimulatory effects of nicotine on rat plasma adrenocorticotropin and prolactin. J Pharmacol Exp Ther. 1986;238:486–491. [PubMed] [Google Scholar]
- Sharp BM, Matta SG. Detection by in vivo microdialysis of nicotine-induced norepinephrine secretion from the hypothalamic paraventricular nucleus of freely moving rats: Dose-dependency and desensitization. Endocrinology. 1993;133:11–19. doi: 10.1210/endo.133.1.8391419. [DOI] [PubMed] [Google Scholar]
- Shoptaw S, Majewska MD, Wilkins J, Twitchell G, Yang X, Ling W. Participants receiving dehydroepiandrosterone during treatment for cocaine dependence show high rates of cocaine use in a placebo-controlled pilot study. Exp Clin Psychopharmacol. 2004;12:126–135. doi: 10.1037/1064-1297.12.2.126. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Babb DA, Hatsukami DK. Progesterone treatment during the early follicular phase of the menstrual cycle: effects on smoking behavior in women. Pharmacol Biochem Behav. 2001;69:299–304. doi: 10.1016/s0091-3057(01)00527-5. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Babb DA, Hatsukami DK. Effects of progesterone treatment on smoked cocaine response in women. Pharmacol Biochem Behav. 2002;72:431–435. doi: 10.1016/s0091-3057(02)00716-5. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Dudish-Poulsen S, Nelson D, Pentel PR, Hatsukami DK. Sex and menstrual cycle differences in the subjective effects from smoked cocaine in humans. Exp Clin Psychopharmacol. 1999;7:274–283. doi: 10.1037//1064-1297.7.3.274. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Mitchell E, Kosten TR. Effects of progesterone treatment on cocaine responses in male and female cocaine users. Pharmacol Biochem Behav. 2004;78:699–705. doi: 10.1016/j.pbb.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Poling J, Gonzalez G, Gonsai K, Oliveto A, Kosten TR. Progesterone effects on cocaine use in male cocaine users maintained on methadone: a randomized, double-blind, pilot study. Exp Clin Psychopharmacol. 2007;15:453–460. doi: 10.1037/1064-1297.15.5.453. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Yoo S, Hill KP, Mooney M. Self-administration of intravenous nicotine in male and female cigarette smokers. Neuropsychopharmacology. 2008;33:715–720. doi: 10.1038/sj.npp.1301460. [DOI] [PubMed] [Google Scholar]
- Soria R, Stapleton JM, Gilson SF, Sampson-Cone A, Henningfield JE, London ED. Subjective and cardiovascular effects of intravenous nicotine in smokers and non-smokers. Psychopharmacology (Berl) 1996;128:221–226. doi: 10.1007/s002130050129. [DOI] [PubMed] [Google Scholar]
- Spark RF. Dehydroepiandrosterone: a springboard hormone for female sexuality. Fertility and Sterility. 2002;77:S19–S25. doi: 10.1016/s0015-0282(02)02987-4. [DOI] [PubMed] [Google Scholar]
- Spealman RD, Goldberg SR. Maintenance of schedule-controlled behavior by intravenous injections of nicotine in squirrel monkeys. J Pharmacol Exp Ther. 1982;223:402–408. [PubMed] [Google Scholar]
- Spohr U, Hofmann K, Steck W, Harenberg J, Walter E, Hengen N, Augustin J, Morl H, Koch A, Horsch A, Weber E. Evaluation of smoking-induced effects on sympathetic, hemodynamic and metabolic variables with respect to plasma nicotine and COHb levels. Atherosclerosis. 1979;33:271–283. doi: 10.1016/0021-9150(79)90179-5. [DOI] [PubMed] [Google Scholar]
- Sziraki I, Sershen H, Benuck M, Hashim A, Laitha A. Differences in receptor system participation between nicotine- and cocaine-induced dopamine overflow in nucleus accumbens. NY Acad Sci. 1999;877:800–802. doi: 10.1111/j.1749-6632.1999.tb09326.x. [DOI] [PubMed] [Google Scholar]
- Thilbin I, Finn A, Ross SB, Stenfors C. Increased dopaminergic and 5-hydroxytryptaminergic activities in male rat brain following long-term treatment with anabolic androgenic steroids. Br J Pharmacol. 1999;126:1301–1306. doi: 10.1038/sj.bjp.0702412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valera S, Vallivet M, Bertrand D. Progesterone modulates a neuronal nicotinic acetylcholinic receptor. Prog Natl Acad Sci USA. 1992;89:9949–9953. doi: 10.1073/pnas.89.20.9949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vocci FJ, Acri J, Elkashef A. Medication development for addictive disorders: The state of the science. Am J Psychiatry. 2005;162:1432–1440. doi: 10.1176/appi.ajp.162.8.1432. [DOI] [PubMed] [Google Scholar]
- Ward AS, Haney M, Fischman MW, Foltin R. Binge cocaine self-administration in humans: Intravenous cocaine. Psychopharmacology. 1997;132:375–381. doi: 10.1007/s002130050358. [DOI] [PubMed] [Google Scholar]
- Watkins SS, Koob GF, Markou A. Neural mechanisms underlying nicotine addiction: acute positive reinforcement and withdrawal. Nicotine Tob Res. 2000;2:19–37. doi: 10.1080/14622200050011277. [DOI] [PubMed] [Google Scholar]
- West RJ, Russell MAH. Cardiovascular and subjective effects of smoking before and after 24 h of abstinence from cigarettes. Psychopharmacology. 1987;98:118–121. doi: 10.1007/BF00215491. [DOI] [PubMed] [Google Scholar]
- Wilkins JN, Carlson HE, Van Vunakis H, Hill MA, Gritz E, Jarvik ME. Nicotine from cigarette smoking increaes circulating levels of cortisol, growth hormone, and prolactin in male chronic smokers. Psychopharmacology (Berl) 1982;78:305–308. doi: 10.1007/BF00433730. [DOI] [PubMed] [Google Scholar]
- Winternitz WW, Quillen D. Acute hormonal response to cigarette smoking. J Clin Pharmacol. 1977;17:389–397. doi: 10.1002/j.1552-4604.1977.tb04621.x. [DOI] [PubMed] [Google Scholar]
- Yang H, Zhao W, Hu M, Becker JB. Interactions among ovarian hormones and time of testing on behavioral sensitization and cocaine self-administration. Behav Brain Res. 2007;184:174–184. doi: 10.1016/j.bbr.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen SSC, Jaffe RB, Barbieri RL. Reproductive Endocrinology: Physiology, Pathophysiology and Clinical Management. 4. W.B. Saunders; Philadelphia: 1999. [Google Scholar]
- Zinder O, Dar DE. Neuroactive steroids: their mechanism of action and their function in the stress response. Acta Physiol Scand. 1999;167:181–188. doi: 10.1046/j.1365-201x.1999.00579.x. [DOI] [PubMed] [Google Scholar]