Abstract
We present a new centralized randomization method for multicenter emergency treatment clinical trials. With this step-forward method, treatment randomization for the next subject is performed immediately after the enrollment of the current subject. This design ensures the readiness of the treatment assignment for each subject at the point of study enrollment, and it simultaneously provides effective control on treatment assignments balance and distributions of covariates. We discuss procedures of the step-forward randomization method along with its implementation for two NINDS-funded multicenter acute stroke trials, one double-blinded and one open-labeled. Advantages and limitations are presented based on experiences gained in these two trials.
Keywords: Step-forward, Randomization, Multicenter Clinical Trials, Emergency Treatment
1. Background
Acute stroke and other emergency treatment clinical trials demand rapid processing of subject enrollment and randomization, because both safety and efficacy depend heavily on the time to treatment.1, 2 The subject enrollment process may be shortened by simplifying the eligibility criteria; however, the subject randomization procedure may cause a delay in treatment administration.3 For this reason, local randomization has been widely used in emergency treatment trials.4-6 Since local randomization only uses information available to the local study team, there is no information exchange between the local center and the central study database. Therefore, there is no risk of time delay for treatment administration due to the randomization procedure. However, randomized controlled multicenter trials require balancing of treatment assignment and baseline covariate distributions not only within each center, but across all centers. Treatment imbalance is measured by the actual ratio of subject numbers among treatment arms and the ratio defined in the study protocol. In cases where equal treatment group size is demanded, treatment imbalance can be measured by the difference in subject numbers between the two treatment arms. Covariate distribution imbalance is defined by the discrepancy of the covariate distributions between the two treatment arms. If the covariate is categorized, covariate imbalance can be defined by the treatment imbalances within each covariate category. To evaluate these imbalances, data exchange between clinical centers and the central study database for information on the treatment assignments and baseline covariate values of previously randomized subjects is necessary.7 When emergency treatment clinical trials are conducted in multiple clinical centers, investigators face the difficult task of deciding between local randomization and traditional central randomization, and neither method is ideal.
The purpose of this paper is to describe a new randomization procedure for multi-center emergency treatment trials, the step-forward approach. This new method ensures the ready availability of treatment assignment at each clinical center, like local randomization, and it provides an effective control on overall treatment assignment and covariate distribution imbalances across all centers, similar to traditional central randomization.
The paper is organized as follows. The advantages and limitations of local randomization and traditional central randomization are explored in Section 2. A new randomization method, the step-forward randomization, is proposed in Section 3 as an alternative method which combines advantages from both local randomization and traditional central randomization and minimizes their limitations. Implementations of the step-forward randomization in two National Institutes of Neurological Disorders and Stroke (NINDS) funded multicenter acute ischemic stroke treatment trials are presented in Section 4, followed by results presented in Section 5. A discussion of the advantages and limitations of the step-forward randomization method is included in Section 6.
2. Local randomization versus traditional central randomization
For blinded, concurrently-controlled multicenter clinical trials, the subject treatment allocation procedures can be divided into two types: local randomization and central randomization. Both use randomization codes to prevent possible selection biases and protect treatment blinding. A randomization code is unique non-sequential number (usually at least 4-digit) associated with a specific study treatment kit, drug, or device. A central pharmacy uses the unblinded randomization code list for packaging the study treatment material. The link between the randomization code and the corresponding treatment remains blinded for the remaining study team members. During the process of randomization, the subject is assigned to a randomization code, and will be treated with the treatment package with that code.
Local randomization
For local randomization, a pre-generated center-specific randomization code list is used at each clinical center. Permuted block randomization is the most commonly used method for the creation of the randomization list.6-8 It ensures treatment assignment balance within each complete block. The randomization for each new eligible subject is implemented by simply picking the next available randomization code from the list. The subject will be given the study treatment corresponding to the selected code. The readiness of randomization code at the clinical center is the most important advantage of local randomization, particularly in emergency treatment trials. This and the ease of implementation explain the common use of local randomization for trials like these. Local randomization becomes problematic when the number of clinical centers is large or when the stratification by important baseline covariates is desired4, 7
Local randomization in a multicenter trial is a form of stratified randomization. The entire study sample is stratified by clinical center first. If a covariate is included in the randomization process, subjects within each center will be further stratified by the level of that covariate. If the covariate is a continuous or ordinal variable, it is usually categorized into a number of levels before stratification. The number of strata for each center will be equal to the product of the number of levels for each covariate, and when a local randomization is stratified, one randomization code list will be needed for each stratum. For example, if gender (male vs. female), age (young vs. old) and baseline disease severity (moderate vs. severe) are being considered for balancing, there will be 2x2x2=8 randomization code lists for each clinical center. This will add to the complexity of the trial operation and increase the risk of randomization errors. In a local stratified design, as the number of strata increases, the average number of subjects in each stratum decreases for a particular samples size. When the stratum size is close to the permuted block size, the overall treatment assignment balance and covariate distribution balance may not be ensured due to the possibility of incomplete blocks.7 If a smaller block size was used in order to reduce the impact of incomplete blocks, the predictability of treatment assignments and the possibility of selective bias will increase.
Central randomization
In traditional central randomization, a computerized system is setup at the study coordination center. To randomize a new eligible subject, the clinical center will access the central randomization system with the help of a certain type of technology (e.g., internet or telephone), provide subject information to the system, and obtain the randomization code. The subject will be treated with the study treatment kit labeled with the same code. The central randomization algorithm uses not only data on the current subject, but also data on all previously randomized subjects across all centers. The primary purpose of central randomization is to balance treatment assignment and baseline covariates between treatment arms across all centers.4, 8 A variety of randomization methods can be used to achieve this goal, including stratification with the permuted block method, the biased coin method, the urn method, and minimization.9-12
Using permuted block in traditional central randomization, the next available code on the randomization list is assigned to each new subject. Permuted block method has the advantage of ensuring that the overall treatment assignments are balanced throughout the trial. With the biased coin design, a biased probability, such as 2/3 suggested by Efron,14 toward reducing treatment imbalance will be used for new subject randomization if the current imbalance exceeds a pre-specified threshold, otherwise, simple randomization will be applied.9 The urn method starts from an equal number of balls represent each treatment arm. When a new subject randomization is requested, a ball is randomly picked from the urn and treatment arm is assigned accordingly. After that, the picked ball is returned back to the urn, and one ball represent the opposite treatment arm is added to the urn, so the next subject will have a smaller chance to be assigned to the same treatment arm.10 All these three common randomization methods can be combined to stratification in order to balance the distribution of important covariates, including clinical center, demographic category, and baseline disease category.
In case where number of clinical centers and covariate strata are too big for stratification, a more effective treatment and baseline covariate balancing goal may be achieved by using the minimization method.11 In this more sophisticated randomization method, a target function containing components for all imbalance items was programmed in the central randomization algorithm. When a new subject randomization is requested, this target function is evaluated for all possible treatment assignments, and the treatment arm associated with the minimum target function value will be assigned to the subject with a biased probability, for example, 0.75.7
All these methods used in central randomization require information exchange between the clinical center and the central study database. Traditionally, the information exchange in central randomization depends on the technology, like web-based randomization systems and interactive voice response systems (IVRS).13-17
Choices for multicenter emergency treatment trials
While the technologies involved in central randomization are reasonably reliable for many clinical trials, they face special challenges in emergency trials, such as acute stroke trials, where the time between a subject's arrival to the emergency department (ED) and study treatment administration has significant impact on the safety and efficacy of the treatment.18, 19 Efforts have been made to avoid treatment delay in clinical trials, and examples include simplified or minimal inclusion/exclusion criteria. Time delay and risks associated with technology-dependent central randomization remains an issue for emergency treatment trials. As a result, local randomization using pre-generated randomization code lists remains attractive for emergency treatment trials, but it sacrifices control in overall treatment and covariate balance between treatment arms.
3. Step-forward Randomization
When separate randomization code lists are used for local randomization, treatment assignments for all subjects are pre-specified before the enrollment of the first subject. In traditional central randomization, none of the treatment assignments are specified prior to the subject's enrollment. A logical middle ground is a procedure that specifies the treatment assignment only for the next subject at each center, based on the information of previously randomized subjects from all centers, while keeping the treatment assignment for remaining subjects unspecified. In other words, we will specify the treatment assignment for the first subject at each center before the study begins using a constrained randomization method that ensures a balanced start point for overall treatment distribution. After enrolling a subject at a center, we perform central randomization for the next eligible subject at that center, but who has not yet arrived at the center, based on the information on treatment assignments and covariates of all subjects currently enrolled in the study across all centers. In this way, the treatment randomization procedure is always one step ahead of the subject enrollment. Thus, we name the new procedure the step-forward randomization method. The following outline describes the basic procedure of the step-forward design:
A randomization code list is generated and stored in the central study database. The list contains a sufficient number of codes for the whole study period. All randomization codes are globally unique and listed in random order. Each randomization code is randomly assigned to one treatment arm based on the treatment arm ratio specified in the study protocol. Unlike the randomization code list used in permuted block randomization, where the code list order represents the sequence of subject randomization, the code list used in the step-forward randomization only links the code to a treatment arm. The listing order does not reflect the randomization time sequence. Codes will be selected based on the treatment arm required and the availability of the study drug inventory at the clinical center.
For blinded, concurrently controlled trials, the randomization code list will be sent to the central pharmacy where study drug kits are packaged. The randomization code will be printed on the study drug kit label. The central pharmacy will ship a certain number of study drug kits to each clinical center before it is released for subject enrollment. Clinical centers are required to confirm that all drug kits received are in good condition. When study drug kits are used for subject treatment, damaged or expired, resupply of study drug kits will be shipped to clinical centers to maintain a minimal drug inventory, so that at least one drug kit is available at each center for each treatment arm.
For open label trials, a sealed envelope with a treatment assignment card enclosed will be created for each randomization code. Each center will receive randomization code envelopes sufficient for the whole study period.
Before the study begins, a constrained randomization will be performed to assign the randomization code for the first subject at each clinical center. In trials with two treatment arms, half of the centers will have their first subject assigned to one treatment arm, and the other half will have their first subject assigned to the other treatment arm. Each center will put a “USE NEXT” label on the study drug kit (or envelope) with the assigned randomization code.
The first eligible subject at the center is then treated with the drug kit labeled as “USE NEXT”. The actual process of this step may vary based on the study treatment type and the local hospital's study drug management settings. In cases where the study drug is managed by pharmacy personnel, the study coordinator at the Emergency Department (ED) will need to contact the pharmacy to get the “Use Next” drug kit. This will require 24/7 availability of the study pharmacy, which should be the standard operation. If the study treatments are procedures or devices used in standard patient care, there will be no pharmacy involved.
Within the protocol-specified time window (e.g., 8 hours from treatment initiation and prior to the treatment of the next subject.), the study coordinator will log on the study website and enter the subject's information (including the values of stratification covariates if the randomization is stratified) into the central database. At this stage, only information relevant to subject randomization is required. Therefore, this step will not take a long time. If another subject arrives immediately after the current subject, the study coordinator will enter the first subject's information in to the website and get the “Use Next” randomization code as soon as technology will allow. This can be done while the eligibility exam is conducted for the second subject. If technical problems prohibit this procedure, the center study coordinator will follow the protocol specified contingency procedures (e.g., pick the drug kit with the smallest randomization code).
In the central randomization system, the randomization algorithm will determine the treatment arm assignment for the next subject at that center, based on data from all enrolled subjects and “USE NEXT” assignments across all centers.
The system will randomly pick one drug kit from the center inventory in the specified treatment arm, and send the corresponding randomization code of that drug kit to the center through the randomization confirmation page of the study website.
At the clinical center, the “USE NEXT” label will be placed on the drug kit with the randomization code provided by the central database, and the kit will be ready for the next subject.
Repeat steps (5-9) until the end of the study.
If the balance of a covariate among treatment arms is important to the study, stratification on that covariate can be used together with the step-forward randomization method. In this case, there will be one “USE NEXT” randomization code for each stratum at each center. Unlike the stratified permuted block design for local randomization, where study subjects are stratified by the combination of clinical center and covariate levels, the step-forward approach stratifies subjects by the covariate only and performs a central randomization algorithm within a stratum of the covariate across all clinical centers. Clinical center does not necessary to be a stratification factor. This strategy allows direct balancing of marginal distributions within each covariate stratum, each clinical center, and overall treatment assignments by using minimization methods.11, 12 For example, if treatment allocation is stratified by age groups (e.g., young versus old), the minimization method can use an objective function that accounts for overall imbalances, imbalances within each clinical center for both age groups, and imbalances within each age group for all clinical sites with weights based on priority levels. Including stratification in the step-forward randomization increases the complexity of trial operation. We suggest that stratification be used only when number of strata is small (2 or 3) in order to maintain a manageable operation procedure at clinical centers.
4. Implementation in Two Acute Ischemic Stroke Trials
The step-forward design has been implemented by the Data Coordination Unit (DCU) at the Medical University of South Carolina in the randomization for two large multicenter acute ischemic stroke trials funded by the National Institute of Neurological Disorders and Stroke (NINDS).
Step-forward Randomization in ALIAS Trial Part 1
Albumin in Acute Stroke (ALIAS, ClinicalTrials.gov identifier: NCT00235495) Part 1 was an NINDS-funded phase III randomized, double- blinded, placebo-controlled multicenter clinical trial of high-dose human albumin (ALB) therapy in patients with acute ischemic stroke. The trial was conducted in two groups of subjects: those who were eligible and received the standard of care treatment with intravenous tissue plasminogen activator (tPA) (hereafter referred to as the thrombolysis cohort), and those who did not receive tPA (non-thrombolysis cohort). Sixty-two centers from the United States and Canada enrolled 434 subjects into the study. Within each cohort, subjects were randomized in a 1:1 ratio to the ALB or control arm. The goal of the randomization scheme was to control within each cohort the treatment assignment imbalance across all centers, as well as within each individual center.
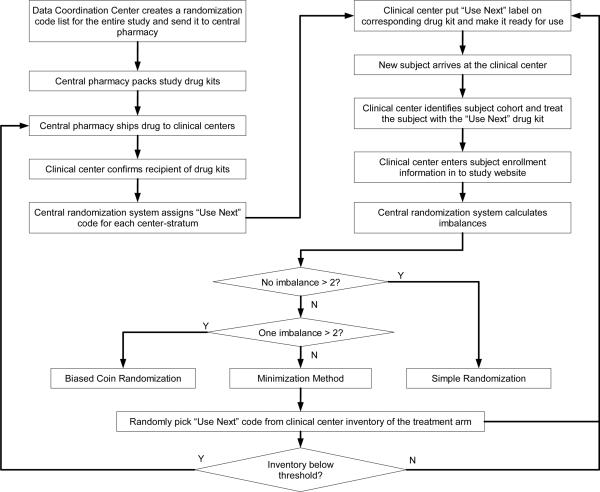
A drug kit label contained a 4-digit randomization code, the drug lot number, expiration date, and the treatment name covered by scratchable materials for emergency unblinding purposes. When a center was released to enroll subjects, an initial batch of study drug kits were shipped from the central pharmacy to the center, together with 2 sets of color coded “USE NEXT” labels. Blue labels were designed for the thrombolysis cohort and yellow labels for the non-thrombolysis cohort. After receiving the drug kits, the center personnel log into the study website to confirm the receipt (in good condition) of all drug kits. The system then assigned one drug kit for each cohort as the “USE NEXT” kit for the first subject at that center. A constrained randomization was used for the assignment of the first subject for each center-cohort, so treatment assignments for all “USE NEXT” kits were balanced before the trial started. A combination of minimization,11 biased coin randomization,14 and simple randomization was used for all following treatment assignments. Figure 1 outlines the randomization plan used for the ALIAS Part 1 trial.
Figure 1.
Randomization scheme for ALIAS Part 1 Trial
The imbalance tolerance limit was set to 2 for both overall imbalance and within-center imbalance. After each subject randomization, if none of the imbalances exceeded this limit, simple randomization was used. If only one imbalance exceeded the limit, a biased probability of 0.8 was used in favor of reducing that imbalance. If both imbalances exceeded the limit, a minimization method is used based on the objective function with a heavier weight (w1 = 1.55) on overall imbalance than within center imbalance (w2 = 1.00). When the treatment arm for the next subject was obtained, the “USE NEXT” randomization code was randomly picked from all available randomization codes of that treatment arm at the center. Upon receiving the randomization code, the study personnel placed the color-coded cohort-specific “USE NEXT” label on the drug kit with the assigned randomization code. During the study period, each center had two “USE NEXT” drug kits placed in a convenient but secured location, waiting for the next subjects in the two cohorts to be enrolled. When an eligible patient arrived at the ED and completed eligibility checking and informed consent procedure, the subject was treated with the drug in the study kit labeled as “USE NEXT” for the appropriate cohort. Within 8 hours after treating the subject and no later than the randomization of the next subject, the study personnel entered the subject enrollment information into the study website. A new “USE NEXT” randomization code for the cohort was selected based on the result of the randomization process.
Step-forward Randomization in IMS III Trial
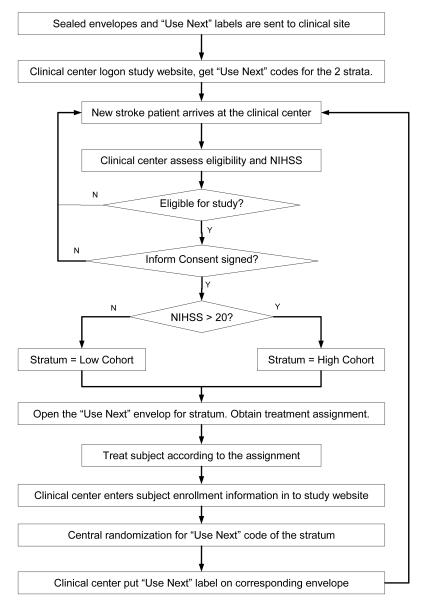
The Interventional Management of Stroke (IMS III, ClinicalTrials.gov identifier: NCT00359424) Trial is a randomized, open-label multi-center study that compares intravenous only tPA (IV) to a combined intravenous and intra-arterial thrombolysis (IV+IA) treatment approach to restoring blood flow to the brain to the current standard FDA approved treatment approach of giving IV alone. Currently, a projected 900 subjects with moderate-to-large (NIHSS ≥10) ischemic strokes between ages 18-80 are being enrolled at more than 50 centers in the United States, Canada and Australia. Subjects are designated into one of the two strata based on baseline NIHSS score (≤19 or ≥20) and are randomized in a 2:1 ratio with more subjects enrolled in the combined IV+IA group. Treatment imbalance is defined as , where NIV+IA is the number of subjects in the (IV+IA) treatment arm and NIV is the number of subjects in the IV only treatment arm. We have implemented the step-forward randomization design in order to ensure that all eligible subjects receive study treatment as quickly as possible, while overall treatment imbalance and within center imbalances for both strata are controlled. Since IV treatment is standard of care and the IA devices are stocked at each clinical center by the participating suppliers, the study drug/device kits are not centrally pre-packaged and distributed to the centers.
In this open label trial, 60 sealed envelopes with treatment assignment sheets are mailed to each clinical center before it begins subject enrollment. A label with a unique randomization code is placed on each envelope, and a “USE NEXT” label is placed on one envelope for each stratum at each center. When an eligible subject arrives at the ER, center personnel open the “USE NEXT” envelope for the appropriate stratum, and treat the subject according to the treatment assignment provided on a label inside the envelope. After that, the subject's enrollment information is entered into the study website and a new “USE NEXT” randomization code is assigned based on the central randomization algorithm.
5. Results
As a new randomization approach being used in emergency treatment clinical trials, the step-forward method has been accepted by investigators and study coordinators from both ALAIS Part 1 trial and IMS III trial.
ALIAS Part 1 trial result
A total of 434 subjects were enrolled in ALIAS part 1 trial. The overall treatment distribution in the thrombolysis cohort was (173:176). Among the 62 clinical centers, the mean imbalance (measured by absolute value of treatment group size difference) was 1.08, with a 95% confidence interval of (0.87–1.30). In the non-thrombolysis cohort, the overall treatment distribution was (42:43). The mean imbalance of treatment within clinical centers was 1.07, with a 95% confidence interval of (0.79–1.27). Over the entire course of 18-month recruitment period, there were 16 subject randomization related abnormal events reported. Among them, 5 subjects were enrolled with protocol violation in eligibility assessment, and were identified after the study kits were opened and before treatment started. Four subjects used the drug kit with the lowest randomization code, as instructed by the randomization contingency plan, because the clinical center had an insufficient study drug inventory for randomization. Four subjects' enrollment information data entry was delayed because of technical problems in study website access. Three subjects were treated with wrong study kit because of site operation mistakes. Information on subject ED arrival time and study treatment start time was collected and monitored. No treatment delay was reported because of subject randomization.
IMS III trial result
IMS III trial is still on going. To date, 320 subjects have been enrolled from 54 clinical centers. A total of 428 randomization codes have been assigned by the central randomization program, including 320 codes used by enrolled subjects, 54 “Use Next” codes for the low NIHSS stratum, and 54 “Use Next” codes for the high NIHSS stratum. The treatment allocations for these 428 randomization codes between the two treatment arms are (191:93) and (95:49) for the low and high strata, respectively. Both are very close to the prefect balanced ratio (191:95.5) and (95:47.5) specified in the study protocol. Since study started, there are 7 subject randomization related abnormal events reported. Four subjects were used a wrong “Use Next” envelope for treatment assignment because of site operation mistakes. Technical problems in study website accessing caused delay in one subject enrollment information data entry. Two subjects were randomized using the lowest randomization code envelope because of insufficient training. No treatment delay caused by subject randomization has been reported so far.
6. Comparison of Randomization Approaches
Advantages
Advantages of the step-forward randomization for multi-center emergency treatment trials is that it provides both the readiness of study treatment, like local randomization, and the capacity of balancing overall treatment assignment distribution across all clinical centers, similar to traditional central randomization. Unlike the permuted block method commonly used in local randomization, which assigns all randomization codes at once prior to study initiation, the step-forward randomization method assigns only one randomization code at a time. The progressive randomization procedure enables the implementation of central randomization to control overall imbalances. This feature is especially important when the emergency treatment trial is conducted in a large number of clinical centers. In both ALIAS part 1 trial and IMS III trial, operation mistakes in subject randomization were been reported. Similar mistakes could occur in trials using other randomization methods. Step-forward randomization provides the capacity of adapting such operation mistakes and maintaining adequate control on imbalances. Unlike traditional central randomization, which is done immediately before subject treatment, the step-forward method performs the randomization for the next eligible patient after the current subject is treated. This switch in the randomization procedure sequence saves valuable time, enabling clinical center personnel to treat the subjects with emergency conditions immediately and deal with data management after treatment. This property also reduces the technology dependency burden associated with traditional central randomization. Technology dependent central randomization is vulnerable to technical problems with the central database server, web server, or internet connection,. With the step-forward approach, the clinical center can treat the current subject with the “Use Next” kit and then resolve any technical problems after the subject has been treated.
Limitations
Based on our experiences in ALIAS Part 1and IMS III trials, we have noted that the step-forward randomization has some limitations and disadvantages. First, for double blinded controlled trials involving study drug kits, the step-forward randomization increases the complexity level of trial operation for clinical center personnel, especially when randomization is stratified. As a new approach, investigators and study coordinators are not familiar with the concept and procedure. Extra user training is required. Second, although the step-forward randomization allows study personnel time to work on the study website in order to obtain a new “USE NEXT” kit, this savings in time is meaningful only when the next eligible subject has not yet arrived. When two consecutive subjects arrive at the ED within a short time, the information of the first subject must be entered in the study website in order to obtain the randomization code for the second. Contingency plans must be pre-specified to cover such scenarios when technical problems with study website access happen at the same time. Third, the step-forward randomization applies to emergency treatment clinical trials only when the treatment is performed at the place where the sole “Use Next” drug kit for the clinical center (or stratum) is accessible. Since the one “Use Next” drug kit cannot be placed at multiple and unpredictable locations, step-forward randomization will not work when the emergency treatment is conducted at a pre-hospital setting, like emergency medical services (EMS) trucks. Finally, balanced treatment arms and covariate distribution are desired to ensure the compatibility of the treatment arms. However, a balanced arm does not necessary maximize the power of the trial or minimize the sample size needed. The step-forward approach does not yield more balancing effects compared to traditional central randomization.
7. Conclusion
With all the advantages and limitations discussed above, we would recommend consideration of the step-forward randomization for emergency treatment trials with a large number of clinical centers and no more than 2 strata. Despite its limitations, the step-forward approach is a valuable method of randomization for multicenter emergency clinical trials to control treatment and baseline covariate imbalances as well as to minimize time between eligibility assessment and treatment initiation of the study drug.
Figure 2.
Subject randomization procedure for IMSIII Trial
Acknowledgements
This work was supported by the National Institute of Neurological Diseases and Stroke (NINDS) grants U01 NS054630, U01 NS040406, and U01 NS052220. The manuscript was reviewed by Dr. Ginsberg (PI, ALIAS Trial), Dr. Michael D. Hill (co-PI, ALIAS Trial), Dr. Broderick (PI, IMS III Trial), and Dr. Thomas Tomsick (co-PI, IMS III Trial), as well as by the NINDS Project Officers of the two trials (Drs. Claudia Moy and Scott Janis).
References
- 1.Frey JL. Recombinant tissue plasminogen activator (rtpa) for stroke: The perspective at 8 years. The Neurologist. 2005;11:123–133. doi: 10.1097/01.nrl.0000156205.66116.84. [DOI] [PubMed] [Google Scholar]
- 2.Foex B. The problem of informed consent in emergency medicine research. Emergency Medicine Journal. 2001;18:198–204. doi: 10.1136/emj.18.3.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wichman A, Sandler AL. Research involving critically ill subjects in emergency circumstances: New regulations, new challenges. Neurology. 1997;48:1151–1177. doi: 10.1212/wnl.48.5.1151. [DOI] [PubMed] [Google Scholar]
- 4.Friedman LM, Furberg CD, DeMets DL. Fundamentals of clinical trials. Springer Science + Business Media, LLC; New York: 1998. [Google Scholar]
- 5.Wood KA, Lewis L, Von Harz B, Kollef MH. The use on noninvasive positive pressure ventilation in the emergency department: Results of a randomized clinical trial. Chest. 1998;113:1339–1346. doi: 10.1378/chest.113.5.1339. [DOI] [PubMed] [Google Scholar]
- 6.Wright DW, Kellermann AL, Hertzberg VS, Clark PL, Frankel M, Goldstein FC, et al. Protect: A randomized clinical trial of progesterone for acute traumatic brain injury. Annals of Emergency Medicine. 2007;49:391–402. doi: 10.1016/j.annemergmed.2006.07.932. [DOI] [PubMed] [Google Scholar]
- 7.McEntegart DJ. The pursuit of balance using stratified and dynamic randomization techniques: An overview. Drug Information Journal. 2005;37:293–308. [Google Scholar]
- 8.Rosenberger WF, Lachin JM. Randomization in clinical trials: Theory and practice. Wiley Interscience; New York: 2002. [Google Scholar]
- 9.Wei LJ. The adaptive biased coin design for sequential experiments. The Annals of Statistics. 1978;6:92–100. [Google Scholar]
- 10.Wei LJ. An application of an urn model to the design of sequential controlled clinical trials. Journal of the American Statistical Association. 1978;73:559–563. [Google Scholar]
- 11.Taves D. Minimization: A new methods of assigning patients to treatment and control groups. Clinical Pharmacology and Therapeutics. 1974;15:443–453. doi: 10.1002/cpt1974155443. [DOI] [PubMed] [Google Scholar]
- 12.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31:103–115. [PubMed] [Google Scholar]
- 13.Kobak KA, Greist JH, Jefferson JW, Katselnick DJ, Mundt JC. New technologies to improve clinical trials. The Journal of clinical Psychopharmacology. 2001;21:255–256. doi: 10.1097/00004714-200106000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Efron B. Forcing a sequential experiment to be balanced. Biometrika. 1971;58:403–417. [Google Scholar]
- 15.Buster JE, Koltun WD, Pascual MLG, Day WW, Peterson C. Low-dose estradiol spray to treat vasomotor sytmptoms: A randomized controlled trial. Obstetrics and Gynecology. 2008;111:1343–1351. doi: 10.1097/AOG.0b013e318175d162. [DOI] [PubMed] [Google Scholar]
- 16.Zeiher BG, Steingrub J, Laterre P-F, Dmitrienko A, Fukiishi Y, Abraham E. Ly315920na/s-5920, a selective inhibitor of group iia secretory phospholipase a2, fails to improve clinical outcome for patients with severe sepsis. Critical Care Medicine. 2005;33:1741–1748. doi: 10.1097/01.ccm.0000171540.54520.69. [DOI] [PubMed] [Google Scholar]
- 17.Lees K, Barer D, Ford G, Hacke W, Kostulas V, Sharma A, Odergren T. Tolerability of nxy-059 at higher target concentrations in patients with acute stroke. Stroke. 2003;34:482–487. doi: 10.1161/01.str.0000053032.14223.81. [DOI] [PubMed] [Google Scholar]
- 18.Tissue plasminogen activator for acute ischemic stroke. The national institute of neurological disorders and stroke rt-pa stroke study group. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 19.Marler JR. Ninds clinical trials in stroke: Lessons learned and future directions. Stroke. 2007;38:3301–3307. doi: 10.1161/STROKEAHA.107.485144. [DOI] [PubMed] [Google Scholar]