Abstract
Purpose
To compare axial length measurements by contact and immersion technique in pediatric cataractous eyes.
Design
Prospective comparative case series.
Participants
In this prospective study, fifty cataractous eyes of fifty children were enrolled. In bilateral cataract, only one eye was selected to avoid correlation effect in statistical analysis.
Methods
Axial length was measured by both contact and immersion techniques for all eyes, randomized as to which to do first to avoid measurement bias.
Main outcome measures
Axial length measured by contact and immersion technique and the difference between contact and immersion axial length measurements.
Results
Age at cataract surgery and at axial length measurement was 3.87 ±3.72 years. Axial length measurement by contact technique was significantly shorter as compared with immersion technique (21.36 ±3.04 and 21.63 ± 3.09 mm, respectively; P < .001). Axial length measurements using the contact technique were on an average 0.27 mm shorter than those taken using the immersion technique. Forty-two eyes (84%) had shorter axial length when measured using the contact technique as compared with the immersion technique. Lens thickness measurement by contact technique was not significantly different from that of immersion technique (3.61 ±0.74 and 3.60 ±0.67 mm, respectively; P = .673). Anterior chamber depth measurement was significantly more shallow with contact technique (3.39 ±0.59 and 3.69 ± 0.54 mm, respectively; P < .001). Intraocular lens power needed for emmetropia was significantly different (28.68 vs. 27.63 diopter; P < .001).
Conclusion
Contact A-scan measurements yielded shorter axial length than immersion A-scan. This difference was mainly due to the anterior chamber depth rather than the lens thickness value. During intraocular lens (IOL) power calculation, if axial length measured by contact technique is used, it will result in the use of average 1 diopter stronger IOL power than is actually required. This can lead to induced myopia in the postoperative refraction.
Intraocular lens (IOL) implantation in eyes of select children has become a common practice during pediatric cataract surgery.1 In the past few years, there have been several improvements in pediatric cataract removal techniques. However, IOL power calculation accuracy has not improved at the same pace.2 Accurate determination of IOL power remains one of the major challenges for the management of pediatric cataract patients. After pediatric cataract surgery, the postoperative refraction is commonly different from what had been predicted or aimed for by the surgeon.3-13 While many of the late refractive surprises are attributed to a myopic shift in refraction from axial eye growth, early refractive surprises can be attributed to inaccuracy in IOL power calculation.
Errors in axial length are the most significant errors in IOL power calculation. The ultrasound axial length of the eye is commonly measured using either contact or immersion techniques. In the contact method, the probe touches the cornea and may result in corneal compression and a shorter axial length. Immersion A-scan eliminates corneal compression and has been shown to be superior to contact biometry in adults. It is accepted as a gold-standard technique for axial length measurment.14-18 Ben-Zion and colleagues19 compared prediction errors of 138 pediatric eyes measured by contact A-scan with a later group of 65 children measured with the immersion technique. However, to the best of our knowledge, no study has compared the accuracy of IOL power calculation by prospectively measuring axial length in pediatric eyes using both immersion and contact A-scan techniques. The present study compares the contact technique with the immersion technique in terms of the magnitude of axial length measurements in pediatric cataractous eyes. A subsequent paper will compare prediction errors in these eyes as measured postoperatively.
Method
In this prospective study, globe axial length was measured using immersion and contact A-scan techniques in children undergoing cataract surgery. This study complied with the Health Insurance Portability and Accountability Act. Institutional Review Board approval was obtained from the Medical University of South Carolina. Informed consent was obtained from the parents of all the patients enrolled in the study. A computer generated randomized code was used to decide which technique to perform first: 1) contact measurement, followed by immersion measurement; or 2) immersion measurement followed by contact measurement. This was done to minimize observation bias. Data collection for both the groups included age at the time of surgery, axial length, anterior chamber depth, lens thickness, IOL power for emmetropia (using Holladay 1 formula for SN60WF IOL) and corneal thickness measurements. The difference between the values obtained for both the groups is defined as contact minus immersion measurements (for axial length, lens thickness, anterior chamber depth and IOL power for emmetropia). In bilateral cataract, only one eye was selected to avoid a correlation effect in statistical analysis. Fifty eyes of fifty subjects were enrolled.
The axial length of each eye was measured under general anesthesia at the time of cataract surgery using an I3 (Innovative Imaging Inc., Sacramento, CA) ophthalmic A-scan ultrasound.20 A Prager scleral immersion shell (OPSM Instruments) was used to support the probe, and normal saline was used as the coupling fluid. The chamber was filled using a syringe connected to a silicone tube. The experienced ultrasonographer looked for the proper combination of peaks in the scan tracing and froze the frame accordingly. Copies of all the A-scan tracings were stored. The differences in measurements obtained using the two methods were assessed using the paired t test. A P value less than 0.05 was considered statistically significant.
Results
Age at the time of measurement was 3.87 ±3.72 years (22 days – 15.4 years). Thirty of 50 subjects (60%) were male. Twenty-one (42%) were white, twenty-one (42%) were African-American and eight (16%) fell into the ‘other’ racial category. Table 1 shows axial length measurements, lens thickness, anterior chamber depth and IOL power for emmetropia obtained using each technique. Table 2 shows the difference of measurements between contact and immersion techniques.
Table 1.
Biometry measurements using both contact and immersion techniques
| Contact | Immersion | P value | |
|---|---|---|---|
| Axial length (mm) | 21.36 ± 3.04 (15.31 – 27.57) | 21.63 ± 3.09 (15.89 to 27.58) | <0.001 |
| Lens thickness*(mm) | 3.61 ± 0.74 (2.56 – 6.31) | 3.60 ± 0.67 (2.21 to 6.46) | .673 |
| ACD*(mm) | 3.39 ± 0.59 (2.06 – 5.07) | 3.69 ± 0.54 (2.44 to 5.41) | <0.001 |
| IOL power for emm (D) | 28.68 ±11.10 (6.79 – 52.51) | 27.63 ±11.02 (6.76 to 50.30) | <0.001 |
N = 37
IOL = intraocular lens, mm = millimeter, ACD = anterior chamber depth, D = diopter, emm = emmetropia
Table 2.
Difference between contact and immersion techniques (defined as contact minus immersion measurements)
| Difference in axial length (mm) | −0.27 ±0.3 (−0.96 to 0.33) |
| Difference in lens thickness (mm) | 0.02 ±0.24 (−0.61 to 0.77) |
| Difference in anterior chamber depth (mm) | −0.30 ±0.34 (−1.11 to 0.24) |
| Difference in Intraocular lens power for emmetropia (D) | 1.06 ±1.33 (−2.22 to 4.45) |
mm = millimeter, D = diopter
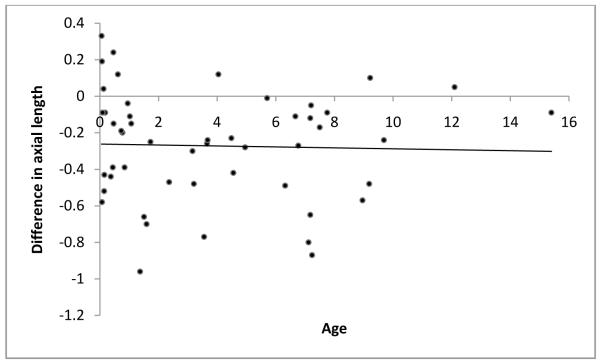
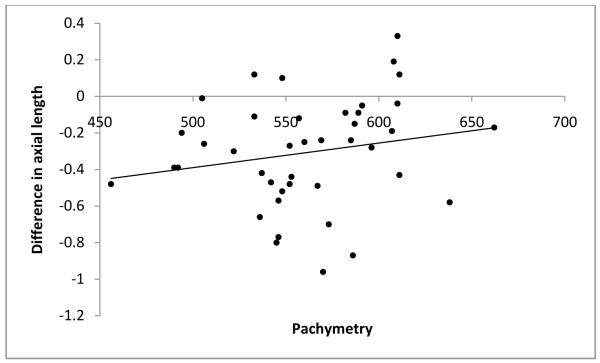
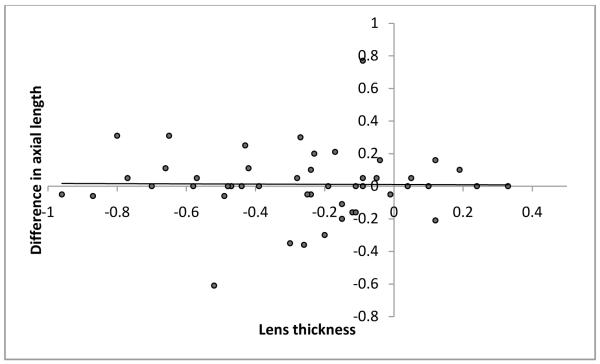
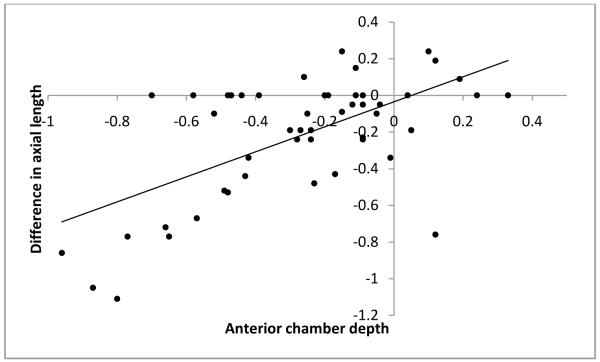
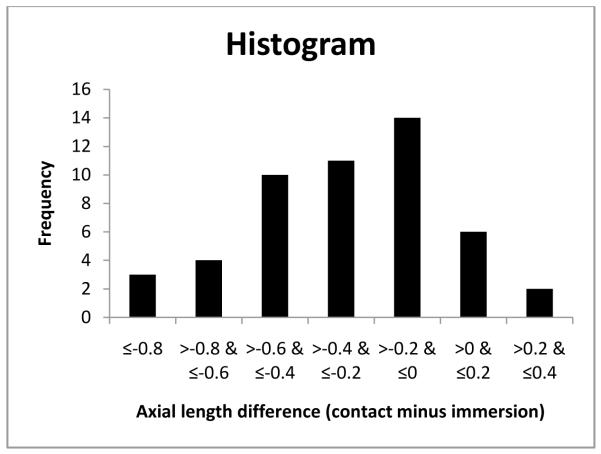
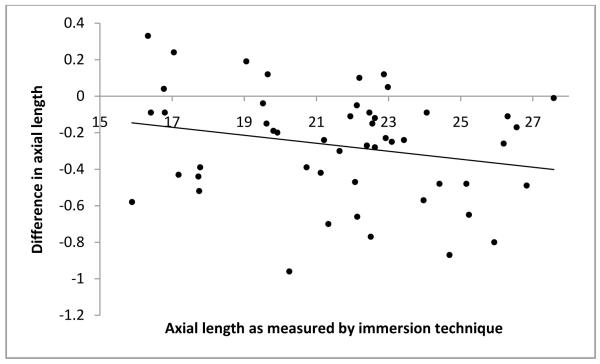
As mentioned before, to avoid examiner bias, we randomly chose the immersion technique as the first technique for half the subjects. The order in which the techniques were performed did not affect the axial length measurements obtained. The technique differences between axial length measurements were −0.27 ±0.31 if the contact method was used first and −0.27 ±0.29 if the immersion method was used first. Additionally, age of the subjects at the time of measurement, preoperative immersion axial length and central corneal thickness were each found to have no significant influence on the differences in axial length measurements obtained using the two methods (P=0.823, P=0.222 and P=0.110 respectively; Figure 1-3). Differences in axial length measurements were due to differences in anterior chamber depth (ACD) rather than lens thickness (LT) values (P=<0.001 and 0.951, Figure 4-5). Forty-two eyes (84%) had shorter axial length when measured using the contact technique as compared with the immersion technique (Figure 6). Five of 16 eyes (31.2%) with preoperative axial length measurements ≤20 mm had greater axial length when measured using the contact technique. These 16 eyes with axial length ≤20 mm were operated before their first birthday. Three of 34 eyes (8.8%) with preoperative axial length measurements >20 mm had greater axial length when measured using the contact technique. Table 3 illustrates the absolute difference in axial length measurements obtained using the two techniques.
Figure 1.
Scatterplot of age versus difference in axial length value
Figure 3.
Scatterplot of pachymetry versus difference in axial length value
Figure 4.
Scatterplot of lens thickness versus difference in axial length value
Figure 5.
Scatterplot of anterior chamber depth versus difference in axial length value
Figure 6.
Histogram showing axial length at difference and frequency
Table 3.
Absolute difference between contact and immersion technique (defined as absolute value of contact minus immersion measurements)
| Absolute difference in millimeters | N (%) |
|---|---|
| ≤0.1 | 10 (20) |
| >1, ≤0.2 | 10 (20) |
| >2, ≤0.3 | 9 (18) |
| >3, ≤0.4 | 4 (8) |
| >0.4 | 17 (34) |
| 50 (100) |
Discussion
Pediatric cataract surgeons use the contact technique more frequently when measuring the axial length of pediatric eyes at the time of cataract surgery. This statement is based on our informal, unpublished, 2009 email survey send to all members of Listserve of members of The American Association for Pediatric Ophthalmology and Strabismus in which 173 (82.4%) surgeons reported using contact A-scan compared to 37 (17.6%) who reported using the immersion technique. Because of a lack of cooperation in the clinic setting, axial length measurements in young children must often be done in the operating room under general anesthesia. In the operating room setting, an experienced ultrasonographer may not be available. Contact A-scan measurements are easier for the surgeon or an operating room technician to perform. Immersion A-scan requires more experience and practice and is best performed by an experienced ultrasonographer. The risk of axial length measurement errors from corneal compression when using contact A-scan ultrasound is well known. However, for those surgeons using contact A-scan techniques, is there a compelling reason to change to immersion A-scan with the increased technical expertise that switch would require? This is the question this study sought to answer. We used a certified ophthalmic technician and ultrasonographer who had years of experience in both A-scan techniques to avoid mistakes that might be related to the learning curve of a new technique.
In the current study, we measured axial length by contact and immersion technique in 50 subjects. The axial length of the eyes measured was significantly different when measured by contact compared to immersion A-scan ultrasound (21.36 mm and 21.63 mm respectively, P<0.001). Ben-Zion and colleagues compared immersion and contact axial length measurement in children. As stated before, this study used different ultrasound machines to measure the first 138 eyes (contact group) versus the subsequent 65 (immersion group). Axial length was 21.20 mm in contact group versus 22.26 mm in immersion group (P = 0.0015).
In adult eyes, it has been reported that axial length measurements taken using the contact technique were, on an average, 0.24 to 0.32 mm less than measurements taken using the immersion technique.14-18 The present study confirms these findings in children. Axial length measurements using the contact technique were on an average 0.27 mm shorter than those taken using the immersion technique. The difference of contact and immersion axial length measurement was −0.27 ±0.3 mm. With mean contact and immersion axial length difference of 0.27 mm, standard deviation of 0.3, Type I error of 0.05, and power of 0.9, we will need 15 children to be able to reject the null hypothesis.
As the contact method involves the probe touching the cornea, corneal compression is likely to occur. It can be argued that this can be corrected by introducing a correction factor. For example, axial length measurements using contact technique were on an average 0.27 mm shorter than that using the immersion technique. Can we add correction factor of 0.27 mm to axial length obtained by contact technique? The amount of corneal compression will vary depending on the pressure the examiner exerts on the cornea, as well as the pressure of the eye. This is not a systemic error. Even if the same technician takes each biometry measurement, it is still likely that he/she will not always apply the same amount of probe pressure with every reading. In our data, the differences in axial length measurements ranged from −0.96 to 0.33 mm. Based on the results of our study, we believe that using correction factor will not avoid error in IOL power calculation. Similarly, use of personalized lens constant will not be useful in this setting.
Our results suggest that lens thickness values were not different when measured using these two techniques. However, anterior chamber depth was on an average 0.3 mm shallower in the contact group (3.39 versus 3.69 mm, <.001). In adults’ eyes, Giers and Epple21 reported similar findings. The mean anterior chamber depth in their study was 0.3 mm less when measured by the contact method compared to measurements obtained using the immersion technique.
During IOL power calculation, if a lower axial length value is entered, it will result in the use of a stronger IOL power than is actually required. This can lead to induced myopia in the postoperative refraction. IOL power for emmetropia was significantly different when measured using the contact and immersion techniques (28.68 versus 27.63 D respectively, P<0.001). We routinely use the immersion technique for axial length measurement. However, if IOL power values obtained using the contact technique had been chosen and if the IOL power was aimed at emmetropia, the average implanted power would have been 1 diopter (D) greater (IOL power calculated using the Holladay 1 formula). Note that a difference of one D in IOL power results in an approximate difference of 0.75 D refraction at the spectacle plane. We entered average immersion A-scan data and average contact A-scan data of this study with average keratometry value of 44 D at 4 year age22 (average age of this cohort) in to Holladay consultant software. The required IOL power for emmetropia was 27 D if immersion technique was used and 28 D if contact technique was used. If child was implanted using contact biometry data (28 D IOL), predicted postoperative refraction using Holladay 1 formula was −0.77 D. This suggests that we may be able minimize early myopic refraction and long term myopic shift of refraction (approximately 0.75 D) by routinely using immersion A-scan for children undergoing cataract surgery.
Age at the time of measurement, preoperative immersion axial length or central corneal thickness did not cause any difference in axial length measurements. (P=0.823, P=0.222 and P=0.110 respectively; Figure 1-3). We hypothesized that a thick cornea might have less indentation and less error. However, the results of our study do not support this hypothesis.
Although contact A-scan yields shorter eyes as compared to immersion A-scan, it is interesting to note that 5 of 16 eyes (31.2%) with a preoperative axial length of ≤20 mm had a greater axial length when measured by the contact technique. In each of these 16 eyes, age at the time of surgery was less than one year. Only 3 of 34 (8.8%) eyes with an axial length >20 mm had a greater axial length measured by contact as compared to immersion. These data emphasize that IOL power calculation is another major obstacle when implanting IOLs in children before their first birthday. In terms of absolute difference in axial length, differences of <=0.1, <=0.2, <=0.3, <=0.4 and >0.4 were noted in 20%, 20%, 18%, 8% and 34% of eyes. Similar values in adult eyes are reported as 8 %, 43%, 33%, 13% and 3%.18
Our study is limited by the fact that the axial length measurement was done under anesthesia without the benefit of visual fixation on the red light target within the A-scan ultrasound machine. However, this is the only available option to measure axial length in young children. Another limitation of our study is that we have not evaluated the reliability of our findings by taking repeated measurements at same time by different observers or at different time using the same observer. As axial length in children needs to be measured under anesthesia, it would not have been ethical to do so.
The use of contact A-scan can at least partially explain the error in IOL power calculation and more specifically myopic refraction. However, further postoperative follow-up of these subjects will help us to understand how this difference influences postoperative refraction.
Figure 2.
Scatterplot of immersion axial length versus difference in axial length value
Acknowledgments
Supported in part by Grady Lyman Fund, ASCRS foundation grant, NIH grant EY-14793, and an unrestricted grant to MUSC-SEI from Research to Prevent Blindness, Inc., New York, NY. Presented in part as a poster at the American Academy of Ophthalmology, Atlanta, GA, 2008.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no financial or proprietary interest in any product mentioned herein.
References
- 1.Wilson ME, Jr, Bartholomew LR, Trivedi RH. Pediatric cataract surgery and intraocular lens implantation: practice styles and preferences of the 2001 ASCRS and AAPOS memberships. J Cataract Refract Surg. 2003;29:1811–20. doi: 10.1016/s0886-3350(03)00220-7. [DOI] [PubMed] [Google Scholar]
- 2.Trivedi RH, Peterseim MM, Wilson ME., Jr New techniques and technologies for pediatric cataract surgery. Curr Opin Ophthalmol. 2005;16:289–93. doi: 10.1097/01.icu.0000177415.17149.b5. [DOI] [PubMed] [Google Scholar]
- 3.Moore DB, Ben Zion I, Neely DE, et al. Accuracy of biometry in pediatric cataract extraction with primary intraocular lens implantation. J Cataract Refract Surg. 2008;34:1940–7. doi: 10.1016/j.jcrs.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 4.Crouch ER, Crouch ER, Jr, Pressman SH. Prospective analysis of pediatric pseudophakia: myopic shift and postoperative outcomes. J AAPOS. 2002;6:277–82. doi: 10.1067/mpa.2002.126492. [DOI] [PubMed] [Google Scholar]
- 5.Plager DA, Lipsky SN, Snyder SK, et al. Capsular management and refractive error in pediatric intraocular lenses. Ophthalmology. 1997;104:600–7. doi: 10.1016/s0161-6420(97)30264-4. [DOI] [PubMed] [Google Scholar]
- 6.Weakley DR, Birch E, McClatchey SK, et al. The association between myopic shift and visual acuity outcome in pediatric aphakia. J AAPOS. 2003;7:86–90. doi: 10.1016/mpa.2003.S1091853103000090. [DOI] [PubMed] [Google Scholar]
- 7.McClatchey SK. Intraocular lens calculator for childhood cataract. J Cataract Refract Surg. 1998;24:1125–9. doi: 10.1016/s0886-3350(98)80108-9. [DOI] [PubMed] [Google Scholar]
- 8.McClatchey SK, Dahan E, Maselli E, et al. A comparison of the rate of refractive growth in pediatric aphakic and pseudophakic eyes. Ophthalmology. 2000;107:118–22. doi: 10.1016/s0161-6420(99)00033-0. [DOI] [PubMed] [Google Scholar]
- 9.McClatchey SK, Hofmeister EM. Intraocular lens power calculation for children. In: Wilson ME Jr, Trivedi RH, Pandey SK, editors. Pediatric Cataract Surgery: Techniques, Complications, and Management. Lippincott Williams &Wilkins; Philadelphia, PA: 2005. pp. 30–8. [Google Scholar]
- 10.Eibschitz-Tsimhoni M, Archer SM, Del Monte MA. Intraocular lens power calculation in children. Surv Ophthalmol. 2007;52:474–82. doi: 10.1016/j.survophthal.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 11.Fan DS, Rao SK, Yu CB, et al. Changes in refraction and ocular dimensions after cataract surgery and primary intraocular lens implantation in infants. J Cataract Refract Surg. 2006;32:1104–8. doi: 10.1016/j.jcrs.2006.01.097. [DOI] [PubMed] [Google Scholar]
- 12.Astle WF, Ingram AD, Isaza GM, Echeverri P. Paediatric pseudophakia: analysis of intraocular lens power and myopic shift. Clin Experiment Ophthalmol. 2007;35:244–51. doi: 10.1111/j.1442-9071.2006.01446.x. [DOI] [PubMed] [Google Scholar]
- 13.Barry JS, Ewings P, Gibbon C, Quinn AG. Refractive outcomes after cataract surgery with primary lens implantation in infants. Br J Ophthalmol. 2006;90:1386–9. doi: 10.1136/bjo.2006.097469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hennessy MP, Chan DG. Contact versus immersion biometry of axial length before cataract surgery. J Cataract Refract Surg. 2003;29:2195–8. doi: 10.1016/s0886-3350(03)00224-4. [DOI] [PubMed] [Google Scholar]
- 15.Watson A, Armstrong R. Contact or immersion technique for axial length measurement? Aust N Z J Ophthalmol. 1999;27:49–51. doi: 10.1046/j.1440-1606.1999.00162.x. [DOI] [PubMed] [Google Scholar]
- 16.Schelenz J, Kammann J. Comparison of contact and immersion techniques for axial length measurement and implant power calculation. J Cataract Refract Surg. 1989;15:425–8. doi: 10.1016/s0886-3350(89)80062-8. [DOI] [PubMed] [Google Scholar]
- 17.Olsen T, Nielsen PJ. Immersion versus contact technique in the measurement of axial length by ultrasound. Acta Ophthalmol (Copenh) 1989;67:101–2. doi: 10.1111/j.1755-3768.1989.tb00732.x. [DOI] [PubMed] [Google Scholar]
- 18.Shammas HJ. A comparison of immersion and contact techniques for axial length measurement. J Am Intraocul Implant Soc. 1984;10:444–7. doi: 10.1016/s0146-2776(84)80044-0. [DOI] [PubMed] [Google Scholar]
- 19.Ben-Zion I, Neely DE, Plager DA, et al. Accuracy of IOL calculations in children: a comparison of immersion versus contact A-scan biometry. J AAPOS. 2008;12:440–4. doi: 10.1016/j.jaapos.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 20.Trivedi RH, Wilson ME. Biometry data from Caucasian and African-American cataractous pediatric eyes. Invest Ophthalmol Vis Sci. 2007;48:4671–8. doi: 10.1167/iovs.07-0267. [DOI] [PubMed] [Google Scholar]
- 21.Giers U, Epple C. Comparison of A-scan device accuracy. J Cataract Refract Surg. 1990;16:235–42. doi: 10.1016/s0886-3350(13)80737-7. [DOI] [PubMed] [Google Scholar]
- 22.Trivedi RH, Wilson ME. Keratometry in pediatric eyes with cataract. Arch Ophthalmol. 2008;126:38–42. doi: 10.1001/archophthalmol.2007.22. [DOI] [PubMed] [Google Scholar]