Abstract
Background
Chronic methamphetamine abusers exhibit deficits in tasks requiring intact prefrontal cortex (PFC) function, and PFC dysfunction has been implicated in the loss of control over drug use. The current study used a combination of behavioral and electrophysiological assessments in rats with a history of long access methamphetamine self-administration to determine methamphetamine-induced changes in PFC-dependent attentional set-shifting performance, drug-seeking, and PFC neuronal activity.
Methods
Male Long-Evans rats self-administered methamphetamine (0.02 mg/infusion, i.v.) or received yoked saline infusions for 6 hours/day for 14 days. Cognitive flexibility was assessed using an attentional set-shifting task prior to 2 weeks of self-administration and one day after self-administration. Animals then underwent 11 days of abstinence, followed by three subsequent tests for context-induced drug-seeking. Finally, animals were anesthetized and single-unit in vivo extracellular recordings were performed in the dorsomedial PFC.
Results
Methamphetamine-experienced rats showed escalated drug intake and context-induced drug-seeking following abstinence. During the extra-dimensional set-shift component, meth-experienced rats showed selective impairments that were identical to deficits produced by excitotoxic lesions of the PFC. Rats with a history of chronic methamphetamine intake also exhibited higher basal firing frequency and a significantly greater proportion of burst-firing cells in the PFC as compared to yoked-saline controls.
Conclusions
PFC-specific alterations in neuronal function may play a key role in methamphetamine-induced attentional deficits and drug-seeking. These data support the possibility that targeting PFC pathology may improve treatment outcome in methamphetamine addiction.
Keywords: attentional set-shift, electrophysiology, methamphetamine, prefrontal cortex, relapse, self-administration
Cognitive performance deficits and abnormal frontal lobe structure and function have been reported in chronic methamphetamine (meth) addiction (1–3). These neurocognitive changes are common and likely contribute to the impaired inhibitory control associated with excessive meth use and subsequent relapse. Repeated exposure to psychostimulants, such as meth, causes supraphysiological changes in neurotransmitter activity involved in normal learning, reward, and executive function (4–6) and has been shown to alter neuroplasticity (7, 8) and electrophysiological activity (9–11) in brain regions that mediate cognitive and motivational functions. In animal models of chronic psychostimulant addiction and relapse, corticostriatal pathways, including the dorsomedial PFC (12, 13) and nucleus accumbens (14, 15), have been found to underlie motivated drug-seeking during relapse. However, less is known about the relationship between the impact of chronic psychostimulants on PFC neurophysiology and PFC-dependent attentional performance in the context of relapse to renewed drug use.
Human post mortem and fMRI studies (16–19) have shown chronic meth-induced alterations in frontal gray and white matter and dopamine transporter (DAT) function in regions including the anterior cingulate and dorsolateral PFC, structures analogous to the dorsomedial prefrontal cortex (dmPFC) in the rat (20). These changes may be behaviorally manifested in the cognitive deficits exhibited by psychostimulant addicts in verbal memory (3, 21), decision making (22, 23), adaptive cognitive control (24), and performance in strategy set-shifting (25). Analogous cognitive and memory deficits have been found in rodent tasks measuring object recognition (26, 27), spatial working memory (28), and reversal learning (29) following binge meth injection regimens. These systemic meth treatments can also result in neurotoxic changes in the cortex and striatum including decreases in dopamine, tyrosine hydroxylase, and DAT levels (30, 31). Such changes, particularly decreased DAT, have been observed in human meth addicts (32–34). However, while noncontingent meth administration regimens produce neurotoxic effects, the use of meth self-administration (SA) paradigms in animals allows for motivated drug-taking, and are thus more akin to human meth addiction. In parallel with human addicts, more prolonged intake of meth in rats (i.e., longer daily use sessions) leads to escalation of meth-taking (35, 36) and progressively greater neuronal deficits in both cortical and striatal regions (37–39). However, the relationship between extended access meth SA and PFC-dependent cognitive function, neurophysiology, and behavioral flexibility has not previously been assessed in SA models.
The Wisconsin Card Sort Task and similar attentional set-shifting tasks identify specific deficits in attention and cognitive flexibility that relate to PFC function in humans. Several studies have reported that chronic meth addicts exhibit deficits in these tasks that correlate with changes in prefrontal white and gray matter (40, 41), similar to the poor performance found in non-addicted subjects with PFC damage (42), or disorders associated with frontal cortex pathology, such as schizophrenia (43). Importantly, comparable deficits can be assessed in rodents using the attentional set-shifting task (ASST) (44). The ASST is analogous to the Wisconsin Card Sort Task and similar set-shifting task variations and is composed of a series of learning tasks, each assessing selective PFC functions. Within the ASST, reversal performance (i.e., working memory), intradimensional set shift (i.e., procedural memory), and extradimensional set shift (i.e., flexibility in performance strategy) can each be measured separately during a single test session. Inactivation or lesioning of the PFC, particularly the medial PFC (44, 45), produces a specific performance deficit in extradimensional set-shifting while leaving performance in the rest of the task components intact. Interestingly, lesion or inactivation of the dorsal, but not the ventral mPFC, also blocks drug-primed and cue-induced reinstatement of drug-seeking in rats with a history of chronic cocaine (13, 15) or meth (46) SA, suggesting that dmPFC function is critical for, and likely compromised by, chronic psychostimulant abuse. These converging findings suggest that neuronal dysfunction in the dmPFC underlies both cognitive deficits and maladaptive drug-seeking behavior in an interrelated manner.
To assess whether chronic meth SA causes changes in multiple dimensions of PFC function, we utilized an animal model in rats that self-administered i.v. meth in a daily access regimen previously shown to produce escalated intake of large amounts of meth (35, 36, 38). This chronic access SA model provides greater homology to the binge patterns of human meth addiction than the commonly used approach of noncontigent, single trial, high dose meth injections. We determined performance on the ASST before and directly following the last day of meth SA. Responding on a previously meth-paired lever was used to measure drug-seeking when rats were returned to the SA context after a period of abstinence. Finally, we used single unit in vivo extracellular electrophysiological recordings of the dmPFC to assess changes in individual neuron activity within subregions of the dmPFC after chronic meth SA, abstinence, and testing of drug-seeking. We hypothesized that rats with a history of escalated chronic meth intake would have selective performance deficits during the extradimensional component of the ASST, enhanced meth-seeking, and dysregulated dmPFC neuronal activity, as compared with yoked-saline control animals.
Methods and Materials
Subjects
Male Long-Evans rats (Charles-River; 275–300 g) were individually housed on a 12 hour reversed light-dark cycle (lights off 06:00 to 18:00). Animals were provided water ad libitum and maintained on 25 g/day of rat chow (Harlan, Indianapolis, IN) when not undergoing cognitive testing. Housing and care of the rats were carried out in accordance with the Guide for the Care and Use of Laboratory Rats (Institute of Laboratory Animal Resources on Life Sciences, National Research Council, 1996). Procedures were approved by the Institutional Animal Care and Use Committee of the Medical University of South Carolina.
Surgical Procedures
Rats were anesthetized (i.p.) with ketamine hydrochloride (66 mg/kg), xylazine (1.3 mg/kg), and equithesin (0.5 ml/kg). Catheters were constructed with Silastic tubing (12 cm; ID = 0.64 mm; OD = 1.19 mm; Dow Corning Corporation, Midland, MI). One end of the catheter was inserted into the right jugular vein; the other end exited via a small incision on the back and was attached to an external harness (Instech, Plymouth Meeting, PA). An antibiotic solution of cefazolin (10 mg/0.1 ml, i.v.; Schein Pharmaceuticals, Florham Park, NJ) was infused after surgery and catheters were flushed once daily for 4 days after surgery with 0.1 ml each of cefazolin (100 mg/ml) dissolved in heparinized saline (70 U/ml; Elkins-Sinn, Cherry Hill, NJ) and 0.1 ml of heparinized saline. For the duration of the experiment, each subject received 0.1 ml of heparinized saline (10 U/ml) prior to each SA session, and cefazolin and 70 U/ml heparinized saline after the session. To verify catheter patency, rats occasionally received a 0.12 ml infusion of methohexital sodium (10.0 mg/ml i.v.; Eli Lilly and Co., Indianapolis, IN), a short-acting barbiturate that produces a rapid loss of muscle tone.
Methamphetamine Self-Administration and Context-induced Drug-seeking
Meth SA was conducted in chambers (30 × 20 × 20 cm, Med Associates) linked to a computerized data collection program (MED PC). Each chamber was contained within a sound-attenuating cubicle and was equipped with two retractable levers, two stimulus lights, a speaker for tone delivery, and a house light. Infusion tubing was enclosed in a wire coil and connected to the harness on the rat’s back. A weighted swivel apparatus suspended above the box allowed for free movement within the chamber. Methamphetamine hydrochloride (Sigma-Aldrich Co., St. Louis, MO) was mixed in sterile saline, filtered, and self-administered (0.02 mg/50 ul infusion) along a fixed ratio 1 (FR1) schedule of reinforcement. We selected this regimen based on previous studies using meth SA in rats (47–49). Each meth infusion (2 sec) was paired with a 5 sec tone (78 dB, 4.5 kHz) and a white stimulus light over the active lever, followed by a 20-sec time-out to prevent overdose. Responding during the time-out or on the inactive lever was recorded, but had no programmed consequences.
During the short access phase, rats self-administered meth in daily 1 hour sessions for 7 days, or they received yoked-saline infusions. The yoked procedures were identical to those used for meth SA; however, the yoked subjects received 50 ul infusions of 0.9% sterile saline whenever the matched SA subject received a meth infusion. After 7 days, rats were switched to long access daily sessions (6 hours/day) for meth or yoked-saline for 14 days. At the end of SA, rats underwent 11 days of abstinence, which consisted of daily placement in a Plexiglas box in an alternate environment room to control for handling and transportation. Following abstinence, rats underwent three tests of context-induced drug-seeking on three consecutive days. Rats were reintroduced to the SA chambers for 1 hour with the house light illuminated and levers presented, but lever pressing no longer resulted in programmed consequences.
Attentional Set-Shifting Task Procedure
All ASST procedures were based on those described previously (44, 45). Briefly, rats underwent seven consecutive test sessions in the same order: Simple Discrimination (SD), Complex Discrimination (CD), reversal, Intradimensional Shift (ID), reversal, Extradimensional shift (ED), and reversal. Each session increased in difficulty and measured different domains, including reversal learning, adaptability to a rule change, and strategy perseveration. Once a criterion of 6 correct consecutive responses was achieved, the subject moved on to the subsequent session until all sessions and reversals were complete. Rats were then returned to their home cage and given a handful of cheerios and a full food ration as a reward. Detailed methods for the ASST are provided in the supplement (Supplement 1). Rats were first tested prior to SA (baseline) and then one day after the last SA session. We chose this timepoint in order to be close to the period of active meth use, but at a time of no systemic meth levels, based on the pharmacokinetic profile of i.v. meth in rats (t½ of ~1 hour)(50).
For comparison with previous evidence of PFC lesion-induced ED deficits (44), a separate group of age-matched rats with no catheters received either bilateral excitotoxic (n=5) or sham (n=4) lesions of the mPFC two days prior to ASST testing. Lesions were made with bilateral ibotenic acid infusions (0.06 M in sterile phosphate buffer; 0.3 µl per site over 2 min) at 2 sites with a 30-gauge needle as described in Tait et al. (51). Lesion site coordinates (in mm) were: 1) AP +3.5, ML ±0.6, DV −5.2, and 2) AP +2.5, ML ±0.6, DV −5.0 according to the atlas of Paxinos and Watson (52). Following testing, rats were sacrificed, tissue stained with cresyl violet, and the extent of lesions verified to be within the medial PFC (Figure S1 in Supplement 1).
Single Unit Recordings and Electrophysiological Analysis
We used single-unit electrophysiological recordings to sample the dorsal-to-ventral, as well as rostral-to-caudal prelimbic cortex. Recordings of putative pyramidal cells were performed the day following the last context-induced drug-seeking test. All animals were anesthetized with urethane (1.5 g/kg, i.p.) and mounted in a stereotactic device. Body temperature was constantly maintained at 36–38°C using a thermostat-controlled electric heating pad. A hole was drilled over the site of recording and the dura removed under a microscope. Single-barrel electrodes (~10 MOhms resistance measured at 1000 Hz) containing 2% Pontamine sky blue dye in 2M NaCl were constructed using a vertical microelectrode puller (PE-2; Narishige). Electrodes were slowly lowered with a hydraulic micromanipulator to the three appropriate coordinates: AP: +3.7, +3.2, and +2.7; L: 0.3; V: 2.4 to 3.6, 2.5 to 3.7, and 2.7 to 3.9. The tracks passed through the anterior cingulate and prelimbic regions of the medial PFC.
Action potential shape (biphasic or triphasic) and coordinates of the recording electrode were noted at the time of recording for further reference. The electrode signal was sampled at 10 kHz, amplified (200×), filtered (100–1000 Hz) using a P511 Grass Instruments high performance AC amplifier (Astro-Med, West Warwick, RI). Putative pyramidal cells were differentiated from interneurons based on: 1) firing frequency (FF < 10 Hz), 2) waveform, and 3) action potential duration (> 2.5 msec). Cells exhibiting 3 consecutive spikes with inter-spike intervals < 45 msec were classified as burst-firing cells. Neuron classification was modified from spike analysis as previously reported (53, 54) and performed using custom-made modifications of Axograph software. At the end of the all recording, rats were deeply anesthetized and rapidly decapitated. The brains were preserved in 4% formaldehyde and switched to 20% sucrose solution before placement histology was assessed.
Data Analysis
Data were analyzed using analysis of variance (ANOVA) followed by the Bonferroni method for post hoc comparisons, or independent two-tailed t-tests. Meth intake (mg/kg/day) was analyzed using one-way repeated measures ANOVA, followed by comparison between single SA sessions. Lever responding for context-induced drug-seeking was analyzed using two-way repeated measures ANOVA, followed by Bonferroni posttests based on a significant interaction. ASST data were analyzed with two-way ANOVA. If we found a significant interaction, we compared meth and saline groups for trials to criterion on specific components of the ASST (e.g., ED shift). Independent two-tailed t-tests were used to compare sessions before and after SA within each group (i.e., meth or saline) for specific components of the ASST. Finally, t tests were used to assess differences in the number of recorded cells, cell firing frequency, burst-firing activity, and inter-spike interval between meth and saline. Statistical significance was conducted with the alpha set at 0.05 and analyses were carried out using Prism 5 for Mac OS X (Graphpad Software, La Jolla, CA). All data are reported as mean±SEM.
Results
Meth Self-administration
Rats self-administered an average of 1.07 ± 0.07 mg/kg/day of meth across the one week short access phase. During subsequent 6 hour daily access sessions, rats self-administered meth in an escalating manner from an average of 3.05 ± 0.76 mg/kg/day on day 1 to 6.75 ± 1.04 mg/kg/day by day 14 (Figure 1). The escalation in total meth intake was highly significant [F(13,117) = 25.07, p < .0001], with differences found between day 1 and days 5–14 of long access [ps < .05 to .001], as was the escalation in meth intake during the first hour of each long access session [F(13, 117) = 137.6, p < .0001], with significant differences seen between day 1 and days 12–14 [p < .01].
Figure 1.
Meth SA during short access (1 hour/day) and long access (6 hours/day) sessions. Stable meth intake (average = 1.07 mg/kg/day) was seen during the short access phase (○), but robust escalation of meth intake occurred during the long-access phase (day 1 = 3.05±0.76 mg/kg/day; day 14 = 6.75±0.50 mg/kg/day). Meth intake is shown for the total 6 hour session (■) and for the first hour of the session (●). Significant differences are noted between the first day and subsequent days of long access sessions (*p < .05; **p < .01, ***p < .001).
Attentional Set-Shifting Performance
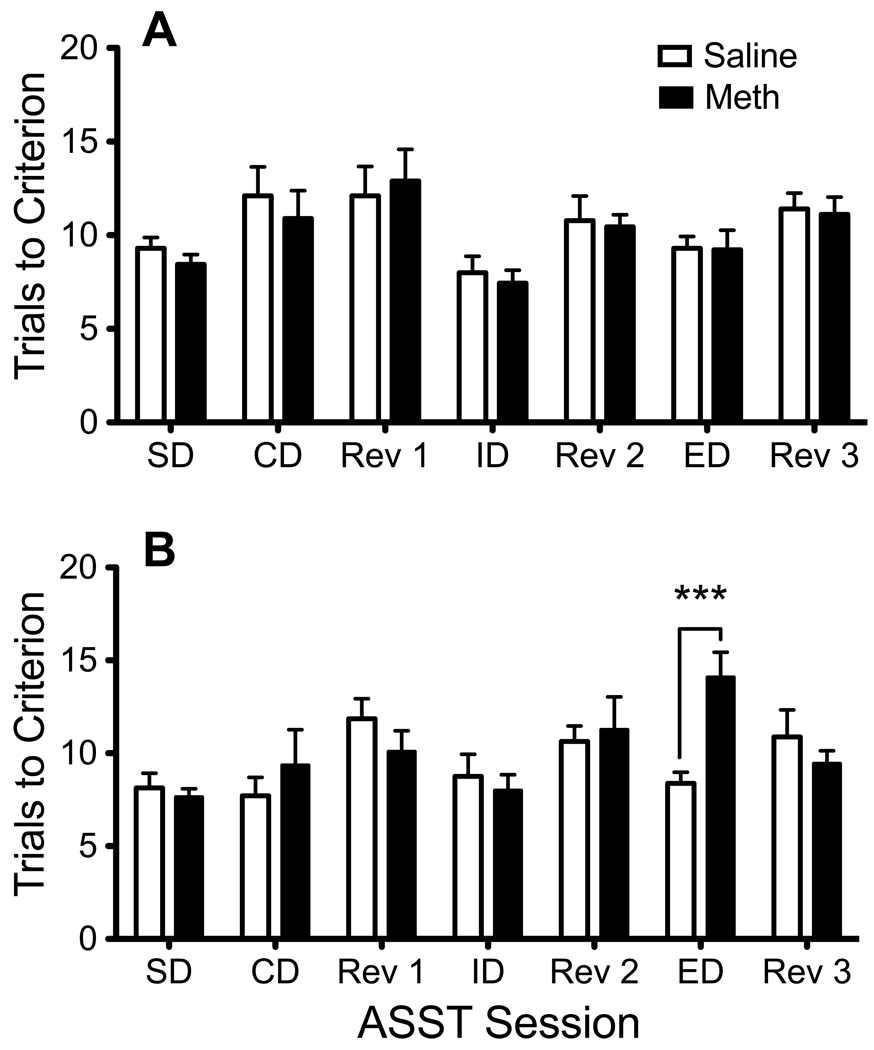
Rats assigned to meth or saline conditions showed no baseline differences in ASST performance [Figure 2A; Interaction F(6,102) = 1.09, p = .38]. One day following chronic meth SA, rats with a history of meth differed significantly from yoked-saline rats [Figure 2B; Interaction F(6,102) = 4.62, p < .001] only during the extradimensional shift [p < .001], but not for any other task phase of the ASST. Further analysis showed that the number of trials-to-criterion during the ED shift between baseline and post-SA performance significantly increased (reflecting a decline in performance) between post-meth and baseline extradimensional shift scores in meth rats [t(8) = 4.80, p < .005], but not yoked-saline rats. In a separate experiment (Figure 3A), rats with excitotoxic lesions of the medial PFC showed selective deficits [Interaction F(6,42) = 3.78, p < .005] only during the ED shift [p < .001]. When directly compared with the meth-experienced rats, the ED deficits for the PFC lesioned and meth-experienced rats looked almost identical (Figure 3B).
Figure 2.
Attentional Set-Shifting Task (ASST) performance before and after chronic meth SA. (A) At baseline, the number of trials to reach the six consecutive correct responses (trials-to-criterion) were the same in both groups, regardless of session. (B) Meth-experienced rats showed a specific deficit during the extradimensional (ED) shift subsequent to the last day of meth SA (***p < .001). SD, Simple Discrimination; CD, Complex Discrimination; Rev 1, Reversal 1; ID, Intradimensional shift; Rev 2, Reversal 2; ED, Extradimensional shift; Rev 3, Reversal 3.
Figure 3.
Excitotoxic lesions of the medial PFC produce a selective deficit in extradimensional set-shifting. (A) Rats with bilateral excitotoxic lesions of the medial PFC showed poorer ED performance relative to sham-lesioned animals (***p < .001). (B) Direct comparison of PFC-lesioned rats with chronic meth-experienced rats shows a striking parallel in the ED deficit (significantly different from respective control group; ***p < .001). SD, Simple Discrimination; CD, Complex Discrimination; Rev 1, Reversal 1; ID, Intradimensional shift; Rev 2, Reversal 2; ED, Extradimensional shift; Rev 3, Reversal 3.
Context-induced Drug-seeking
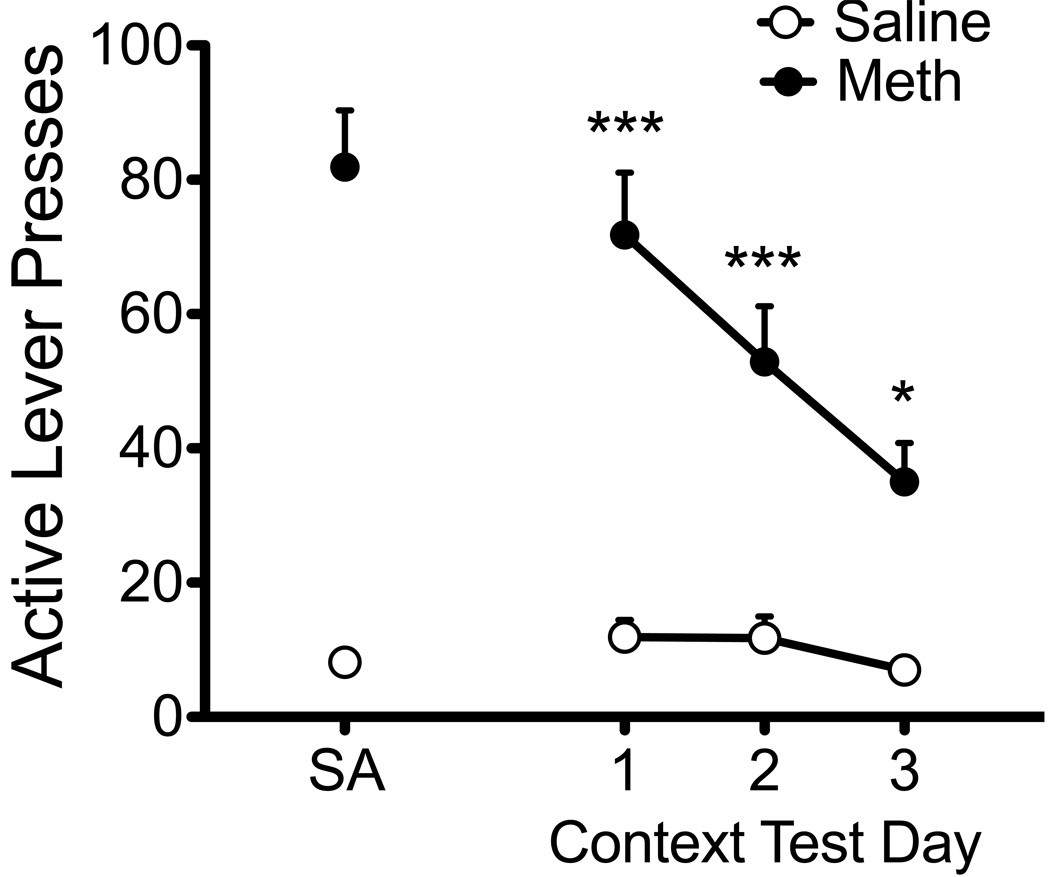
Animals were returned to the SA chambers for three daily context-induced drug-seeking tests at 12–14 days after SA. Rats showed robust meth-seeking, as evidenced by high rates of lever responding on the previously meth-paired lever (Figure 4). A two-way repeated measures ANOVA of lever responses across the three context tests revealed a significant interaction of drug history (meth vs. yoked saline) × time (day of testing) [F(2,16) = 4.08, p < .05]. While meth rats showed significant drug-seeking on all three test days [ps < .05 to .001], lever responding significantly decreased between test days one and three [t(9) = 4.31, p < .01], indicative of extinction learning.
Figure 4.
Context-induced meth-seeking after abstinence from SA. Lever pressing during the context tests resulted in no drug reinforcement or cue presentation. Active lever responding remained elevated for the three daily test trials (post-SA days 12, 13, and 14) in meth-experienced animals as compared with yoked-saline subjects (*p < .05; ***p < .001), but showed an extinction pattern between days 1 and 3 (p < .05).
PFC Neuronal Activity
A total of 126 pyramidal neurons were recorded in the dmPFC (meth=55 neurons; saline=71 neurons). Histological assessment showed that tracks extended from the CG1 region down 2 mm to the dorsal prelimbic border. Since only 5 cells were classified as interneurons, they were not analyzed or included in the results. We found no significant differences in the distribution of recorded cells when putative pyramidal cells were compared by drug history (Figure 5A). However, meth-experienced rats exhibited a significantly higher basal firing frequency (1.43 ± 0.18 Hz) than yoked-saline rats (0.94 ± 0.14 Hz), as seen in Figure 5B [t(124) = 2.15, p < .05]. Additionally, a significantly greater proportion of burst-firing neurons were observed in meth animals (22/55), when compared to yoked-saline controls (11/71) [Figure 5C, p < .005, Fishers exact test]. Further analyses showed no differences between groups for the half-width of tonic- and burst-firing neurons (Figure 5D) or the average firing frequency of tonic- or burst-firing neurons (Figure 5E).
Figure 5.
Electrophysiological characteristics of putative pyramidal cells in the medial PFC in chronic meth and yoked-saline animals at two weeks after SA. (A) Distribution of recorded cells showed no differences between meth and yoked-saline in the number of cells recorded or the location of recording sites. (B) Animals with a history of chronic meth showed faster average firing frequency than yoked-saline controls (*p < .05). (C) Animals with a history of chronic meth showed a higher percentage of burst-firing cells than yoked-saline controls (**p < .005). (D) Half-width of tonic- or burst-firing neurons showed no differences between groups. (E) Average spike firing frequency of tonic- or burst-firing neurons showed no differences between groups.
Discussion
We have shown here that rats with a history of escalated meth SA during daily long access exhibit a selective deficit in extradimensional set-shifting, a medial PFC-dependent task, as well as significant drug-seeking after a period of abstinence. Of note, ASST performance in meth-experienced animals was strikingly similar to rats with lesions of the medial PFC, as seen in the current study and as previously reported (44, 45); lesions resulted in selective deficits in ED set-shifting. Furthermore, meth-experienced animals showed altered neuronal firing states in the dmPFC, reflected specifically in higher basal firing frequency and a higher proportion of burst-firing neurons, relative to yoked-saline control rats. These data suggest that chronic daily meth SA may modify both attention processing and motivated drug-seeking through changes in pyramidal cell firing activity within the dmPFC.
While others have demonstrated cognitive deficits following systemic meth regimens (29, 55), we used a long access regimen of self-administered meth since this approach provides a model with greater face and construct validity than noncontingent meth administered in single sessions, which is commonly done in the preclinical literature (33). Consistent with our previous findings (36, 38) and others (35, 48), rats given daily long access to meth showed significantly greater drug intake during the latter days of meth SA than during the initial days for both the full 6 hour session or the first hour of responding. Subsequent to meth SA and abstinence, rats with a history of escalated meth demonstrated robust meth-seeking, as measured by their responding on the previously meth-paired lever. Furthermore, the degree of meth-seeking was substantially higher following 11 days of abstinence than that seen on the first day of extinction immediately after a similar long access period (36, 38), likely reflecting an “incubation” period, similar to that reported after chronic cocaine SA (56–58). While not tested in the current study, we previously found that a long access meth regimen enhanced meth-primed reinstatement (36) and reduced DAT levels in PFC (38) when compared to a short access meth regimen, further indicating that prolonged meth leads to greater neuroadaptations.
Psychostimulant addicts have been reported to exhibit various cognitive performance deficits in PFC-dependent tasks, any of which may contribute to a loss of self-control and relapse (1). Neuroimaging studies have shown that meth addicts exhibit less PFC activation while performing decision-making tasks (59), as well as the Stroop task (3). An obvious advantage of the animal model of meth addiction is the ability to compare pre-drug to post-drug performance, thus identifying differences that do not precede a drug history, but are clearly due to chronic meth intake. Our data indicate that chronic meth SA does not result in global deficits in the ASST (e.g., reversal performance), but shows selectivity to a specific domain within the ASST. In contrast to these results, a recent report indicated that non-contingent, single day high dose meth treatment produced deficits during reversals on the ASST in rats (29). It is possible that extended practice with the ASST during the baseline assessment could have overcome subsequent meth-induced reversal deficits in our paradigm. However, clear differences in the experimental paradigms exist that also likely account for differences in ASST performance, particularly the contingent drug delivery and prolonged daily use that occurs with escalating meth SA, an approach that is more akin to the pattern of meth abuse in humans. It is important to note that the current results do not preclude other cognitive deficits that can result from escalated chronic meth SA. Indeed, we have previously found that escalated meth intake decreased performance on a novel object memory task (36). It will be critical in the future to use the long access meth SA model to further examine the degree of attentional processing deficits at multiple timepoints and to use other behavioral tasks (60) that may uncover alterations in set-shifting.
Single-unit electrophysiological recordings two weeks after meth SA showed that rats with a history of chronic meth intake had faster average firing frequency of spontaneously active dmPFC pyramidal neurons compared to yoked-saline rats. Using anesthetized single-unit extracellular recordings allowed us to specifically investigate neuronal firing properties of individual neurons, as well as collect data from a greater number of cells per animal, facilitating comparisons by drug treatment condition. The general increase in firing frequency in the dmPFC may be explained by a shift in the cell firing mode, with an increase in the proportion of burst-firing to tonic-firing cells in animals with a history of meth intake. As no differences were found in the firing properties when comparing within firing mode, we conclude that the increased firing frequency indicates a general shift in the firing mode of these PFC neurons.
Since burst-firing entails a greater signal to noise ratio, which decreases susceptibility to background or alternate signals, this shift in firing mode may precipitate the cognitive inflexibility observed during the ED shift. Moreover, altered burst-firing patterns of pyramidal PFC neurons can serve as indicators of dysregulated pyramidal cell function due to changes in the integration of multiple neuronal inputs (61, 62), which may underlie both cognitive and motivational deficits. Sun and Rebec (10) reported that repeated cocaine SA increased burst-related firing in the medial PFC, which they interpreted as enhanced processing of cocaine-related information relevant to cocaine-seeking. As such, extended access meth SA could act in a similar manner by disrupting these PFC neuronal networks, known to mediate attention and executive function, to promote inflexible performance and drug-seeking. In support of this, it has been reported that repeated systemic administration of psychostimulants, including amphetamines, generally dampens mPFC physiology, leading to behavioral disinhibition and perseverative performance in cognitive tasks (61, 63). While we cannot directly draw a connection between the increases in dmPFC pyramidal cell burst firing to changes in ED shift or drug-seeking, future studies are warranted to directly examine this connection.
Together, these data suggest that disruption of mPFC pyramidal cell function impairs the capacity to modify existing knowledge or to inhibit inappropriate responses, specifically set-shifting (64). Thus, chronic meth may lead to lasting changes in pyramidal efferent cell firing in the mPFC that produce attentional processing deficits (65), as well as promote uncontrolled drug-seeking (10). As such, interventions that enhance cognition and restore PFC function may serve as effective treatments for addiction. For example, the cognitive enhancing agent, modafinil, attenuates cue and drug-primed reinstatement in rats (66), perhaps via regulation of cortical GLU receptors, such as mGluR2/3 (67). Also, the selective mGluR5 receptor allosteric modulator, CDPPB, has been shown to attenuate set-shifting deficits and restore mPFC pyramidal cell function impaired by systemic administration of the NMDA receptor antagonist, MK-801 (64, 68). The current results on meth-induced changes in pyramidal cell firing support the future exploration of cortical glutamatergic neuroadaptations after escalated meth SA.
In conclusion, our findings extend upon the growing evidence for chronic psychostimulant-induced changes in PFC function in both behavioral and neuronal domains. The identification of neurobiological substrates for cognitive and motivational deficits in meth addiction will be critical for the development of effective somatic and behavioral based therapies. The long access SA and relapse model, coupled with additional measures of both cognitive and neurophysiological determinations (e.g., in vivo recordings in animals concurrently performing set-shifting tasks), will enable findings that promise to improve addiction treatment.
Supplementary Material
Acknowledgements
The authors thank Dr. David Moorman for consultation on the electrophysiology, Dr. Joyce Nicholas for assistance with statistical analysis, and Dr. Timothy Whitfield for his useful discussions. This research was supported by NIDA Grants DA022658 (RES), T32 D007288 (AP), DA014698 (AL), and NIH grant C06 RR015455.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures: All authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Baicy K, London ED. Corticolimbic dysregulation and chronic methamphetamine abuse. Addiction. 2007;102:5–15. doi: 10.1111/j.1360-0443.2006.01777.x. [DOI] [PubMed] [Google Scholar]
- 2.Homer BD, Solomon TM, Moeller RW, Mascia A, DeRaleau L, Halkitis PN. Methamphetamine abuse and impairment of social functioning: a review of the underlying neurophysiological causes and behavioral implications. Psychol Bull. 2008;134:301–310. doi: 10.1037/0033-2909.134.2.301. [DOI] [PubMed] [Google Scholar]
- 3.Salo R, Nordahl TE, Galloway GP, Moore CD, Waters C, Leamon MH. Drug abstinence and cognitive control in methamphetamine-dependent individuals. J Subst Abuse Treat. 2009;37:292–297. doi: 10.1016/j.jsat.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bamford NS, Zhang H, Joyce JA, Scarlis CA, Hanan W, Wu N-P, et al. Repeated exposure to methamphetamine causes long-lasting presynaptic corticostriatal depression that is renormalized with drug readministration. Neuron. 2008;58:89–103. doi: 10.1016/j.neuron.2008.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feltenstein MW, See RE. The neurocircuitry of addiction: an overview. Br J Pharmacol. 2008;154:261–274. doi: 10.1038/bjp.2008.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas MJ, Kalivas PW, Shaham Y. Neuroplasticity in the mesolimbic dopamine system and cocaine addiction. Br J Pharmacol. 2008;154:327–342. doi: 10.1038/bjp.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- 8.Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47 Suppl 1:33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 9.Nogueira L, Kalivas PW, Lavin A. Long-term neuroadaptations produced by withdrawal from repeated cocaine treatment: role of dopaminergic receptors in modulating cortical excitability. J Neurosci. 2006;26:12308–12313. doi: 10.1523/JNEUROSCI.3206-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun W, Rebec GV. Repeated cocaine self-administration alters processing of cocaine-related information in rat prefrontal cortex. J Neurosci. 2006;26:8004–8008. doi: 10.1523/JNEUROSCI.1413-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trantham H, Szumlinski KK, McFarland K, Kalivas PW, Lavin A. Repeated cocaine administration alters the electrophysiological properties of prefrontal cortical neurons. Neuroscience. 2002;113:749–753. doi: 10.1016/s0306-4522(02)00246-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuchs RA, Eaddy JL, Su ZI, Bell GH. Interactions of the basolateral amygdala with the dorsal hippocampus and dorsomedial prefrontal cortex regulate drug context-induced reinstatement of cocaine-seeking in rats. Eur J Neurosci. 2007;26:487–498. doi: 10.1111/j.1460-9568.2007.05674.x. [DOI] [PubMed] [Google Scholar]
- 13.McLaughlin J, See RE. Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2003;168:57–65. doi: 10.1007/s00213-002-1196-x. [DOI] [PubMed] [Google Scholar]
- 14.Fuchs RA, Evans KA, Parker MC, See RE. Differential involvement of the core and shell subregions of the nucleus accumbens in conditioned cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2004;176:459–465. doi: 10.1007/s00213-004-1895-6. [DOI] [PubMed] [Google Scholar]
- 15.McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nordahl TE, Salo R, Natsuaki Y, Galloway GP, Waters C, Moore CD, et al. Methamphetamine users in sustained abstinence: a proton magnetic resonance spectroscopy study. Arch Gen Psychiatry. 2005;62:444–452. doi: 10.1001/archpsyc.62.4.444. [DOI] [PubMed] [Google Scholar]
- 17.Sekine Y, Minabe Y, Ouchi Y, Takei N, Iyo M, Nakamura K, et al. Association of dopamine transporter loss in the orbitofrontal and dorsolateral prefrontal cortices with methamphetamine-related psychiatric symptoms. Am J Psychiatry. 2003;160:1699–1701. doi: 10.1176/appi.ajp.160.9.1699. [DOI] [PubMed] [Google Scholar]
- 18.Thompson PM, Hayashi KM, Simon SL, Geaga JA, Hong MS, Sui Y, et al. Structural abnormalities in the brains of human subjects who use methamphetamine. J Neurosci. 2004;24:6028–6036. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang L, Alicata D, Ernst T, Volkow N. Structural and metabolic brain changes in the striatum associated with methamphetamine abuse. Addiction. 2007;102 Suppl 1:16–32. doi: 10.1111/j.1360-0443.2006.01782.x. [DOI] [PubMed] [Google Scholar]
- 20.Uylings HB, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Behav Brain Res. 2003;146:3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 21.Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D, et al. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychiatry. 2001;158:377–382. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- 22.Grant S, Contoreggi C, London ED. Drug abusers show impaired performance in a laboratory test of decision making. Neuropsychologia. 2000;38:1180–1187. doi: 10.1016/s0028-3932(99)00158-x. [DOI] [PubMed] [Google Scholar]
- 23.McCann UD, Kuwabara H, Kumar A, Palermo M, Abbey R, Brasic J, et al. Persistent cognitive and dopamine transporter deficits in abstinent methamphetamine users. Synapse. 2008;62:91–100. doi: 10.1002/syn.20471. [DOI] [PubMed] [Google Scholar]
- 24.Salo R, Ursu S, Buonocore MH, Leamon MH, Carter C. Impaired prefrontal cortical function and disrupted adaptive cognitive control in methamphetamine abusers: a functional magnetic resonance imaging study. Biol Psychiatry. 2009;65:706–709. doi: 10.1016/j.biopsych.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ornstein TJ, Iddon JL, Baldacchino AM, Sahakian BJ, London M, Everitt BJ, et al. Profiles of cognitive dysfunction in chronic amphetamine and heroin abusers. Neuropsychopharmacology. 2000;23:113–126. doi: 10.1016/S0893-133X(00)00097-X. [DOI] [PubMed] [Google Scholar]
- 26.Belcher AM, O'Dell SJ, Marshall JF. Impaired object recognition memory following methamphetamine, but not p-chloroamphetamine- or d-amphetamine-induced neurotoxicity. Neuropsychopharmacology. 2005;30:2026–2034. doi: 10.1038/sj.npp.1300771. [DOI] [PubMed] [Google Scholar]
- 27.Bisagno V, Ferguson D, Luine VN. Short toxic methamphetamine schedule impairs object recognition task in male rats. Brain Res. 2002;940:95–101. doi: 10.1016/s0006-8993(02)02599-4. [DOI] [PubMed] [Google Scholar]
- 28.Nagai T, Takuma K, Dohniwa M, Ibi D, Mizoguchi H, Kamei H, et al. Repeated methamphetamine treatment impairs spatial working memory in rats: reversal by clozapine but not haloperidol. Psychopharmacology (Berl) 2007;194:21–32. doi: 10.1007/s00213-007-0820-1. [DOI] [PubMed] [Google Scholar]
- 29.Izquierdo A, Belcher A, Scott L, Cazares V, Chen J, O'dell S, et al. Reversal-specific learning impairments after a binge regimen of methamphetamine in rats: possible involvement of striatal dopamine. Neuropsychopharmacology. 2009;35:505–514. doi: 10.1038/npp.2009.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hotchkiss AJ, Morgan ME, Gibb JW. The long-term effects of multiple doses of methamphetamine on neostriatal tryptophan hydroxylase, tyrosine hydroxylase, choline acetyltransferase and glutamate decarboxylase activities. Life Sci. 1979;25:1373–1378. doi: 10.1016/0024-3205(79)90414-4. [DOI] [PubMed] [Google Scholar]
- 31.Wagner GC, Ricaurte GA, Seiden LS, Schuster CR, Miller RJ, Westley J. Long-lasting depletions of striatal dopamine and loss of dopamine uptake sites following repeated administration of methamphetamine. Brain Res. 1980;181:151–160. doi: 10.1016/0006-8993(80)91265-2. [DOI] [PubMed] [Google Scholar]
- 32.Krasnova I, Cadet J. Methamphetamine toxicity and messengers of death. Brain Res Rev. 2009;60:379–407. doi: 10.1016/j.brainresrev.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Volz TJ, Fleckenstein AE, Hanson GR. Methamphetamine-induced alterations in monoamine transport: implications for neurotoxicity, neuroprotection and treatment. Addiction. 2007;102 Suppl 1:44–48. doi: 10.1111/j.1360-0443.2007.01771.x. [DOI] [PubMed] [Google Scholar]
- 34.Wilson JM, Kalasinsky KS, Levey AI, Bergeron C, Reiber G, Anthony RM, et al. Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat Med. 1996;2:699–703. doi: 10.1038/nm0696-699. [DOI] [PubMed] [Google Scholar]
- 35.Kitamura O, Wee S, Specio SE, Koob GF, Pulvirenti L. Escalation of methamphetamine self-administration in rats: a dose-effect function. Psychopharmacology (Berl) 2006;186:48–53. doi: 10.1007/s00213-006-0353-z. [DOI] [PubMed] [Google Scholar]
- 36.Rogers JL, De Santis S, See RE. Extended methamphetamine self-administration enhances reinstatement of drug seeking and impairs novel object recognition in rats. Psychopharmacology (Berl) 2008;199:615–624. doi: 10.1007/s00213-008-1187-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krasnova IN, Justinova Z, Ladenheim B, Jayanthi S, McCoy MT, Barnes C, et al. Methamphetamine self-administration is associated with persistent biochemical alterations in striatal and cortical dopaminergic terminals in the rat. PLoS ONE. 2010;5:e8790. doi: 10.1371/journal.pone.0008790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwendt M, Rocha A, See RE, Pacchioni AM, McGinty JF, Kalivas PW. Extended methamphetamine self-administration in rats results in a selective reduction of dopamine transporter levels in the prefrontal cortex and dorsal striatum not accompanied by marked monoaminergic depletion. J Pharmacol Exp Ther. 2009;331:555–562. doi: 10.1124/jpet.109.155770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wee S, Wang Z, Woolverton WL, Pulvirenti L, Koob GF. Effect of aripiprazole, a partial dopamine D2 receptor agonist, on increased rate of methamphetamine self-administration in rats with prolonged session duration. Neuropsychopharmacology. 2007;32:2238–2247. doi: 10.1038/sj.npp.1301353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chung A, Lyoo IK, Kim SJ, Hwang J, Bae SC, Sung YH, et al. Decreased frontal white-matter integrity in abstinent methamphetamine abusers. Int J Neuropsychopharmacol. 2007;10:765–775. doi: 10.1017/S1461145706007395. [DOI] [PubMed] [Google Scholar]
- 41.Kim SJ, Lyoo IK, Hwang J, Chung A, Hoon Sung Y, Kim J, et al. Prefrontal grey-matter changes in short-term and long-term abstinent methamphetamine abusers. Int J Neuropsychopharmacol. 2006;9:221–228. doi: 10.1017/S1461145705005699. [DOI] [PubMed] [Google Scholar]
- 42.Demakis GJ. A meta-analytic review of the sensitivity of the Wisconsin Card Sorting Test to frontal and lateralized frontal brain damage. Neuropsychology. 2003;17:255–264. doi: 10.1037/0894-4105.17.2.255. [DOI] [PubMed] [Google Scholar]
- 43.Shad MU, Tamminga CA, Cullum M, Haas GL, Keshavan MS. Insight and frontal cortical function in schizophrenia: a review. Schizophr Res. 2006;86:54–70. doi: 10.1016/j.schres.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 44.Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ng C-W, Noblejas MI, Rodefer JS, Smith CB, Poremba A. Double dissociation of attentional resources: prefrontal versus cingulate cortices. J Neurosci. 2007;27:12123–12131. doi: 10.1523/JNEUROSCI.2745-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rocha A, Kalivas PW. Role of the prefrontal cortex and nucleus accumbens in reinstating methamphetamine seeking. Eur J Neurosci. 2010;31:903–909. doi: 10.1111/j.1460-9568.2010.07134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anggadiredja K, Sakimura K, Hiranita T, Yamamoto T. Naltrexone attenuates cue-but not drug-induced methamphetamine seeking: a possible mechanism for the dissociation of primary and secondary reward. Brain Res. 2004;1021:272–276. doi: 10.1016/j.brainres.2004.06.051. [DOI] [PubMed] [Google Scholar]
- 48.Mandyam CD, Wee S, Eisch AJ, Richardson HN, Koob GF. Methamphetamine self-administration and voluntary exercise have opposing effects on medial prefrontal cortex gliogenesis. J Neurosci. 2007;27:11442–11450. doi: 10.1523/JNEUROSCI.2505-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roth ME, Carroll ME. Sex differences in the acquisition of IV methamphetamine self-administration and subsequent maintenance under a progressive ratio schedule in rats. Psychopharmacology (Berl) 2004;172:443–449. doi: 10.1007/s00213-003-1670-0. [DOI] [PubMed] [Google Scholar]
- 50.Riviere GJ, Byrnes KA, Gentry WB, Owens SM. Spontaneous locomotor activity and pharmacokinetics of intravenous methamphetamine and its metabolite amphetamine in the rat. J Pharmacol Exp Ther. 1999;291:1220–1226. [PubMed] [Google Scholar]
- 51.Tait D, Marston H, Shahid M, Brown V. Asenapine restores cognitive flexibility in rats with medial prefrontal cortex lesions. Psychopharmacology (Berl) 2008 doi: 10.1007/s00213-008-1364-8. [DOI] [PubMed] [Google Scholar]
- 52.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 3rd ed. New York: Academic Press; 1997. [DOI] [PubMed] [Google Scholar]
- 53.Connors BW, Gutnick MJ. Intrinsic firing patterns of diverse neocortical neurons. Trends Neurosci. 1990;13:99–104. doi: 10.1016/0166-2236(90)90185-d. [DOI] [PubMed] [Google Scholar]
- 54.Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: burst firing. J Neurosci. 1984;4:2877–2890. doi: 10.1523/JNEUROSCI.04-11-02877.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Belcher AM, Feinstein EM, O'Dell SJ, Marshall JF. Methamphetamine influences on recognition memory: comparison of escalating and single-day dosing regimens. Neuropsychopharmacology. 2008;33:1453–1463. doi: 10.1038/sj.npp.1301510. [DOI] [PubMed] [Google Scholar]
- 56.Fuchs RA, Branham RK, See RE. Different neural substrates mediate cocaine seeking after abstinence versus extinction training: a critical role for the dorsolateral caudate-putamen. J Neurosci. 2006;26:3584–3588. doi: 10.1523/JNEUROSCI.5146-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci. 2000;20:798–805. doi: 10.1523/JNEUROSCI.20-02-00798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Paulus MP, Hozack NE, Zauscher BE, Frank L, Brown GG, Braff DL, et al. Behavioral and functional neuroimaging evidence for prefrontal dysfunction in methamphetamine-dependent subjects. Neuropsychopharmacology. 2002;26:53–63. doi: 10.1016/S0893-133X(01)00334-7. [DOI] [PubMed] [Google Scholar]
- 60.Floresco SB, Block AE, Tse MT. Inactivation of the medial prefrontal cortex of the rat impairs strategy set-shifting, but not reversal learning, using a novel, automated procedure. Behav Brain Res. 2008;190:85–96. doi: 10.1016/j.bbr.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 61.Homayoun H, Moghaddam B. Progression of cellular adaptations in medial prefrontal and orbitofrontal cortex in response to repeated amphetamine. J Neurosci. 2006;26:8025–8039. doi: 10.1523/JNEUROSCI.0842-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lapish CC, Kroener S, Durstewitz D, Lavin A, Seamans JK. The ability of the mesocortical dopamine system to operate in distinct temporal modes. Psychopharmacology (Berl) 2007;191:609–625. doi: 10.1007/s00213-006-0527-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moghaddam B, Homayoun H. Divergent plasticity of prefrontal cortex networks. Neuropsychopharmacology. 2008;33:42–55. doi: 10.1038/sj.npp.1301554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stefani MR, Groth K, Moghaddam B. Glutamate receptors in the rat medial prefrontal cortex regulate set-shifting ability. Behav Neurosci. 2003;117:728–737. doi: 10.1037/0735-7044.117.4.728. [DOI] [PubMed] [Google Scholar]
- 65.Jackson ME, Homayoun H, Moghaddam B. NMDA receptor hypofunction produces concomitant firing rate potentiation and burst activity reduction in the prefrontal cortex. Proc Natl Acad Sci U S A. 2004;101:8467–8472. doi: 10.1073/pnas.0308455101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reichel CM, See RE. Modafinil effects on reinstatement of methamphetamine seeking in a rat model of relapse. Psychopharmacology (Berl) 2010;210:337–346. doi: 10.1007/s00213-010-1828-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tahsili-Fahadan P, Carr GV, Harris GC, Aston-Jones G. Modafinil blocks reinstatement of extinguished opiate-seeking in rats: mediation by a glutamate mechanism. Neuropsychopharmacology. 2010 doi: 10.1038/npp.2010.94. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Darrah JM, Stefani MR, Moghaddam B. Interaction of N-methyl-D-aspartate and group 5 metabotropic glutamate receptors on behavioral flexibility using a novel operant set-shift paradigm. Behav Pharmacol. 2008;19:225–234. doi: 10.1097/FBP.0b013e3282feb0ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.