1. Introduction
Marijuana is the most commonly used illegal substance during pregnancy. In the 1999 National Survey on Drug Use and Health [65], almost 3% of pregnant women reported marijuana use in the past month. In the Maternal Health Practices and Child Development (MHPCD) Study, this rate was much higher: 30% of a sequential cohort of 1360 women from a hospital-based prenatal clinic reported using marijuana in their first trimester. Thus, although the rates vary by demographic characteristics such as race, maternal age, and socioeconomic status, use of marijuana during pregnancy is prevalent [12]. In spite of this, little is known about the long-term effects of prenatal marijuana exposure (PME).
Studies in laboratory animals have demonstrated the importance of the endocannabinoid system (ECS) in the developing fetal brain. Endocannabinoid receptors and are found in the fetal brain as early as the 14th week of gestation [9]. During fetal development, the ECS plays an important role in the development of neuronal connectivity [24,34] and intercellular signaling [54]. The concern with PME lies in the fact that the endocannabinoid receptors interact with exogenous substances such as Δ9-tetrahydrocannabinol (THC), a component of cannabis/marijuana [39]. Prenatal exposure to THC can lead to changes in the ECS, resulting in effects on the fetus such as disruption of the position, postsynaptic target selectivity, and differentiation of the developing axons [6,7]. Over the long-term, these changes result in deficits in physical, cognitive, emotional, social, and motor functioning in the offspring that last into adulthood [18,33,53,69].
Changes in CNS functioning are seen in human studies as well. The Maternal Health Practices and Child Development Study (MHPCD) is a longitudinal cohort study of PME. In the MHPCD cohort, PME affected sleep continuity and organization at birth [62] and at age 3. We found an association with lower efficiency and maintenance, more awake time, and a higher number of arousals [14], which are indications of early subtle differences in brain development. At 3 years [15], we found effects of PME on the composite score, and the short-term memory and verbal reasoning subscales of the Stanford Binet Intelligence Scale [67]. At the same age, Griffith et al. [31] also found a relation between PME and abstract/visual reasoning in a cohort of poly-substance users. In the Ottawa Prenatal Prospective Study (OPPS), PME was associated with significantly lower scores on the verbal and memory domains of the McCarthy Scales of Children's Abilities at 4 years [25]. At 6 years in the MHPCD study, there was a significant nonlinear relationship between marijuana exposure and child intelligence. Use of one or more joints of marijuana per day was associated with lower composite, short-term memory, quantitative, and verbal reasoning scores, depending on the trimester of exposure [29]. In the OPPS, there were no significant effects of PME on IQ at 5-6 or 9-12 years of age [27,56].
In the pre-teen and adolescent phases of the MHPCD study, we measured development using neuropsychological assessments. At 6 years, PME predicted more errors of commission on a Continuous Performance Test (CPT), a measure of impulsivity [40]. At age 10, there were significant associations between PME and attention, hyperactivity, and impulsivity [28] and memory deficits [61] on the design memory and the screen score of the WRAML [63]. At age 10 in the MHPCD study, exposed offspring also had significantly higher rates of depression and anxiety [42,30]. At age 14 [73], adolescents with PME did less well on the coding, block design, and mazes tests on the WISC-II [70]. The OPPS also found deficits in cognitive development [27], attention [26], and executive functioning [25] that were associated with PME as the offspring matured.
These deficits on the neuropsychological assessments are indicators of subtle changes in CNS functioning. They are also risk factors for other consequences, including problem behaviors. In this analysis, we will focus on the association between PME and delinquent behaviors. We will also extend this analysis to identify which of the neuropsychological outcomes may mediate the subsequent development of problem behaviors.
In an earlier analysis [28], we demonstrated an association between PME and delinquent behaviors at age 10, using the Child Behavior Checklist (CBCL) [1], a maternal report. We dichotomized PME, defining heavy exposure as exposure to an average of 1 or more joints/day. Twenty-six percent of the offspring with this level of exposure in the first trimester scored above the clinical cut-point of the CBCL compared to 13% who had no PME and 15% among those with exposures of less than 1 joint/day. The relative risk for delinquency, given heavy PME, was 2.4 (CI: 1.3-4.5; p<.01). PME was not related to any of the other subscales on the CBCL. In this same analysis, we also showed that attention, as measured by the SNAP [57], was significantly predicted by first trimester PME and was significantly correlated with delinquency. Using structural equation modeling, we tested the effect of adding attention to the model on the significant association between PME and delinquency. When attention was added, the association between PME and delinquency were no longer significant. In the OPPS cohort O'Connell & Fried [56] also found a significant association between PME and conduct problems at 6 to 9 years.
In another analysis of MHPCD data [41], we used configural frequency analyses, and found that there were three pathways to delinquency: 1) 3-year temperament predicted 10-year temperament, which predicted delinquency; 2) 3-year IQ predicted 10-year IQ, which predicted psychological problems and subsequently delinquency, and 3) behavior problems at age 3 predicted behavior problems at 10 years, which predicted, in turn, peer use, offspring substance use, and then delinquency. This analysis also identified the correlates of delinquency at age 10 including race, gender, anxiety, substance use, perceived substance use of peers, IQ, and shyness. PME was correlated with delinquency, prenatal tobacco and alcohol use were not. In a related analysis, we demonstrated that deficits in mental flexibility, the ability to switch from one task to another, and attention measured at 10 years of age predicted delinquent behavior in offspring at age 16 [43].
In other research, measures of memory [46] and executive function [4,72] have been identified as correlates or predictors of delinquency, as have attention deficits [50], hyperactivity when combined with disruptive behaviors [51], impulsivity [47], aggression [23,44,57,58], and intelligence [47]. A study by Ellickson et al. [21] found that violent teenagers were more likely to have mental health problems than nonviolent youth. Others have reported higher levels of depression and anxiety in delinquent versus non-delinquent ninth graders [71]. Multiple social and psychological factors also predict the occurrence of delinquency among early adolescents including social class, race, gender, abuse and neglect, and parental factors including education, socioeconomic status, and mental illness [9,17,20,30,42,57,61].
Thus, we have previously demonstrated an association between PME and delinquency in childhood that was mediated by attention. In addition, we have identified correlates of delinquency and pathways to delinquency. These analyses only addressed part of the picture, however. The Goldschmidt et al. paper [28] addressed the effects of 10-year attention on 10-year delinquency and the Leech et al. paper [41] only included PME as a covariate in the analyses.
In this and other research, however, we have found the PME predicts multiple outcomes, using many different levels of measurement. The outcomes can be defined as those that are more proximal to brain functioning, including neuropsychological assessments, mood, attention, impulsivity, and activity, and those that are more distal from brain functioning, including problem behaviors. Our over-arching hypothesis is that the proximal effects of PME will mediate the more distal outcomes.
This analysis is an exploration of the relations between PME, neurocognitive deficits, and delinquent behaviors. We hypothesized that: 1) PME will predict each of the proposed mediators, 2) PME will predict a higher rate of delinquent behavior, 3) Each of the proposed mediators will predict delinquency, and 4) The proposed mediators will attenuate the association between PME and delinquency. The neurocognitive domains that we have found to be associated with PME by age 10 included depressive symptoms, attention, activity, impulsivity, learning and memory, and IQ.
The Human Subjects Review Board at the University of Pittsburgh approved the protocols for each study phase.
2. Material and Methods
2.1 Study Design
The MHPCD Project is a longitudinal study of the long-term effects of exposure to marijuana or alcohol during gestation. Woman who were at least 18 years of age were recruited from a prenatal clinic. A sequential sample of 1360 women who were in their fourth gestational month was interviewed. The refusal rate was 15%. Two study cohorts were selected from the original cohort of 1360 women. First, all women who used marijuana at least twice a month in the first trimester, and the next woman interviewed who used less than that amount or none, were selected for a study of the effects of prenatal marijuana exposure. Second, all women who drank three or more drinks a week, and the next woman interviewed who used less than that amount or none, were selected for a study of the effects of gestational alcohol exposure. The two cohorts overlap and were combined for this report (n=829).
Women selected for the study cohort were interviewed again in their seventh prenatal month. At delivery, 8, and 18 months, and 3, 6, 10, 14, 16, 22 years postpartum, these women and their children were evaluated. At each phase, a standardized protocol assessed maternal psychological, social, and environmental factors, demographic status, and substance use, and the children's cognitive, behavioral, psychological, and physical development.
At birth, there were 763 live-born singleton infants. Only mothers and children who were assessed at birth were included in the follow-up. At 10 years, 636 children and their mothers were interviewed. This was 83% of the birth cohort. A total of 127 pairs were lost between birth and 10 years because subjects had moved out of the Pittsburgh area (n= 44), refused participation (n= 44), or were lost to follow-up (n=25). Five children had died and 9 were adopted or in foster care. Women who were interviewed at 10 years did not differ from the birth cohort in marital status, education, income, prenatal alcohol, marijuana, or tobacco use. At the 14-year phase, 580 adolescents and their mothers were interviewed, which was 76% of the birth cohort. Between birth and the 14-year phase, 49 subjects had moved out of the Pittsburgh area, 52 refused participation, 69 pairs were lost to follow-up, and 7 children were adopted or in foster care. In the interval between 10 and 14 years, 1 child died. Similar to the 10-year results, women interviewed at the 14-year phase did not differ from the birth cohort in marital status, education, income, prenatal alcohol or tobacco use. There were more African-Americans participating at 14 years than Caucasians and those who participated had used more marijuana in the third trimester than the women who did not participate. Eleven percent of the adolescents were not with their biological mothers at 14 years and their current caregiver was interviewed. For simplicity, we refer to the caregivers as mothers. Only mother-child dyads who were present at birth, 10, and 14 years were included in these analyses, resulting in an analysis sample of 525.
2.2 Measures
2.2.1. Outcome variables
The Self-Report Delinquency Scale (SRD) was developed by the Pittsburgh Youth Survey (PYS) [23] and is based on the National Youth Survey delinquency questionnaire [45]. Comparisons of prevalence rates on the SRD to those ascertained in the Denver Youth Survey and the Rochester Youth Development Study showed high agreement in the prevalence of delinquency, confirming the validity of the measure [20]. The instrument has 33 items that assess antisocial behaviors such as purposely breaking or damaging things, stealing, cheating, hitting, and running away from home. These items are combined into four subscales; damage, theft, violence, and status offenses. Adolescents who scored 2 or more on the theft, violence, or status scales, or 1 or more on the damage scale were defined as delinquent and given a score of 1. These cut-points were selected because they represented the 20th percentile for the respective scales. All other adolescents received a score of 0.
A maternal report, the Child Behavior Checklist (CBCL) [1], was also used to assess delinquency. The CBCL delinquency subscale is composed of 13 items that assess lying, stealing, running away, and using alcohol and drugs over the last six months. The test-retest reliability correlation for the delinquency subscale was 0.86. The delinquency subscale was dichotomized to high (≥67) versus low (<67) scores, based on the borderline clinical cut-point. A summary variable that included both the SRD and the CBCL delinquency subscale was created by assigning a code of one to those who were identified as delinquent on either the SRD or CBCL and a code of zero to all others.
2.2.2. Mediators
The mediators were selected from the 10-year assessment. This was the first phase at which we were able to use a self-report instrument for psychological symptoms and to assess neuropsychological performance. Offspring depressive symptoms were measured at age 10 with the Children's Depression Inventory (CDI) [37], a self-report assessment adapted from the Beck Depression Inventory with adequate internal consistency and test-retest reliability. Activity, attention, and impulsivity were assessed using the SNAP [59], a 23-item rating scale completed by the mothers. The Wide Range Assessment of Memory and Learning (WRAML) [63] screen score was used as a summary measure of memory and learning. Child IQ was measured using the composite score of the Stanford-Binet Intelligence Scale, 4th edition [67].
2.2.3. Prenatal substance exposure
Prenatal marijuana use was based on the usual, minimum, and maximum quantity and frequency of marijuana, hashish, and sinsemilla used in each trimester of pregnancy. Because the concentration of THC, differs in these substances, the quantities of hashish and sinsemilla were transformed into 3 and 2 joints of marijuana, respectively, based on the amount of THC, and combined with the reports of marijuana [32,36]. Marijuana use was calculated as the average number of joints per day (ADJ).
The measures of prenatal alcohol exposure (PAE) were combined into average daily volume (ADV), the number of drinks per day for each trimester. Prenatal tobacco exposure (PTE) was expressed as the number of cigarettes smoked per day in each trimester. The development of the MHPCD substance use questionnaire, ascertainment of patterns, duration and quantity of use, and methods to minimize recall error and maximize accuracy, are described elsewhere [16,17]. First trimester PAE and PTE were used in the analyses to parallel the use of first trimester PME. Both were included as continuous variables. There is overlap among PME and PTE, and PME and PAE, but only a small portion of the first trimester heavy marijuana users used high amounts of either tobacco or alcohol. Among the heavy first trimester marijuana users, 67% used any tobacco and 16% smoked more than one pack per day; 85% of the women drank any alcohol and only 29% drank more than one drink/day. We also created a measure of maternal substance use that would represent heavier use in the environment of the offspring in the postpartum. Maternal alcohol use at 10 years was dichotomized at an ADV of two or more drinks. Marijuana and other illicit drug use were each recoded into none vs. any use. The three substances were combined and coded as 0, 1, and 2 or more.
2.2.4. Covariates
The Home Observation for Measurement of the Environment-Short Form (HOME) [2] is a maternal report on the levels of cognitive stimulation and emotional support in the home. The HOME was adapted into an interview format by the authors and the reliability coefficient is .92 [2,3]. We also included a dichotomous variable that measured whether there was an adult male in the household.
Maternal cognitive ability was measured by the WAIS-R vocabulary and block design subtests version [10]. Maternal depressive symptoms were assessed using the Center for Epidemiological Studies-Depression Scale (CES-D) [60], and maternal hostility was assessed with the State-Trait Anxiety Inventory [65]. The life events measure was adapted from the Human Population Laboratory instrument [8] by the addition of parallel items on pregnancies and pregnancy outcomes. Other environmental and demographic variables included household income, maternal education, number of people living in the household, and child in maternal custody (yes/no). Medications for depression and ADHD were collected. However, we had very few children (5 for depression; 12 at age 10 and 8 at age 14 for ADHD) who had been prescribed medications. Medication use was not related to delinquency or PME and, therefore, was not included in the analyses.
2.3. Data Analyses
To address Hypotheses 1-3, we identified the covariates of PME, delinquency, and each of the hypothesized mediators in bivariate analyses using T-tests and Chi-square tests. We will control for these significant covariates in the multivariate analyses of the association between PME and delinquency that are addressed in Hypothesis 4. In the final model, we only include variables that are associated with both PME and delinquency.
Hypothesis 4 was analyzed using logistic regression. First trimester PAE and PTE, child's race, and gender were included in all multivariate models. Variables that were identified in Hypotheses 1-3 as significantly associated with the dependent or independent variables or each separate mediator, were included in the appropriate multivariate models. Identification of outliers and influential points was done using the Mahalanobis distance (D2). No influential points or outliers were removed.
A variable is defined as a mediator if it “accounts for the relation between the predictor and the criterion” [5]. A mediational model is based on the hypothesis of a pathway and must meet the requirement of temporal order, the independent variable must precede the hypothesized mediator and the independent variable must be significantly related to the mediator [5,38]. We hypothesized that the neurocognitive variables that were significantly predicted by PME at 10 years would mediate the association between PME and delinquency at age 14.
Following the Baron and Kenny Model [51], we will demonstrate mediation if the significant relation between PME and delinquency becomes non-significant after we enter the mediator into the model. In addition, mediation was estimated using the products of the coefficients [48]. The effect of PME on the mediator was also estimated by an ordinary linear regression and the effect of the mediator on the outcome was estimated by using a probit regression model. The statistical package Mplus [52] was used to estimate the indirect effect size. We have further imposed a requirement that the mediator precede the outcome.
Because we had differential sample loss by race and by third trimester PME, we repeated the analyses with weights. Weights were constructed using the inverse of the probability of response for each racial group and for each exposure group in the third trimester. The results did not differ from the unweighted results and we have presented the unweighted data.
3. Results
This study began in 1982. At the first trimester, 73% of the women had completed high school, 62% earned less than $400 per month. Their median age was 22 years (range 18-42), 30% of the women were primigravidous, 54% were African American, and 68% were not married. On average, marijuana use was 0.4 joints/day (range 0-9), alcohol use was 0.6 drinks/day (range 0-8.5), and the number of cigarettes was 7.8/day (range 0-50). Eleven percent of the women reported illicit drug use other than marijuana, including 3.0% who reported cocaine use. At birth, 50% of the infants were male, 8% were premature (<37 weeks gestation), and 10% were small-for-gestational age (SGA: birth weight <10th percentile for gestational age). The average birth weight in the sample was 3210 grams (7.1 lbs) (range 1150-4990 gm). Few infants had major anomalies, 7% had two or more minor physical anomalies.
At the 14-year phase, the average age of the offspring was 14.8 years (range 13.9-16.5). Forty-six percent of the adolescents had ever smoked a cigarette, 35% had drunk alcohol, and 30% had tried marijuana. The average education of the mothers was 12.4 years and the mean household monthly income was $1903. Forty-three percent of the mothers were married, 75% worked outside the home, and there were, on average, four people living in the household.
3.1. Hypothesis 1
PME will predict each of the hypothesized mediators. We chose the mediators to represent measures of neurocognitive functioning that were significantly associated with PME in the MHPCD. Child depressive symptoms, attention, learning and memory, and the child's composite IQ score at 10 years were significantly associated with PME at the bivariate level. Activity and impulsivity were not associated with PME at 10 years (Table 1). We initially analyzed these associations using continuous variables. However, the association between PME and three of the proposed mediators, child depressive symptoms, learning and memory, and child IQ had a threshold association with PME at ADJ ≥ .89. For this reason, we used PME as a dichotomized variable for all remaining analyses. The cut-point of 0.89 is equal to one joint per day (7 joints/week × 4 weeks/month)/31 days/month = .89).
Table 1.
Characteristics of Mother-Child Dyads with Heavy Prenatal Marijuana Exposure Compared to All Others.
| Mean or % | Prenatal Marijuana Exposure | ||
|---|---|---|---|
| Not Heavy1 n=450 |
Heavy2 n=75 |
p-value | |
| Delinquency at 14 years | |||
| Any delinquent behavior | 44 | 61 | <.01 |
| Prenatal Measures | |||
| Prenatal alcohol use (av. drinks/day) | .51 | .92 | <.03 |
| Prenatal tobacco use (av. cig./day) | 7.52 | 9.50 | ns |
| Maternal Age | 23.1 | 23.1 | ns |
| Maternal Education | 11.8 | 11.7 | ns |
| Work/Attend School (%) | 27.8 | 16.0 | <.05 |
| Marital Status (% unmarried) | 34.7 | 18.7 | <.01 |
| Household Income (%<$400/mo) | 60 | 72 | ns |
| Gravidity | 1.4 | 1.5 | ns |
| Mediators at 10 years | |||
| Child depression (av. score, CDI) | 7. 0 | 10.2 | <.001 |
| SNAP-activity (av. score) | 9.7 | 10.3 | ns |
| SNAP-attention (av. score) | 8. 8 | 9.8 | <.01 |
| SNAP-impulsivity (av. score) | 10.1 | 10.6 | ns |
| WRAML screen (av. score) | 88.7 | 83.8 | <.01 |
| Child IQ (av. score, SBIS) | 91.9 | 88.0 | <.01 |
| Covariates at 10 years | |||
| Child | |||
| Gender (% male) | 50 | 51 | ns |
| Race (% African American) | 50 | 79 | <.001 |
| Maternal | |||
| Maternal depression (av. score, CES-D) | 38.2 | 38.4 | ns |
| Maternal hostility (av. score, STAI) | 16.2 | 16.0 | ns |
| Substance use3 () | .38 | .84 | <.001 |
| Maternal education (yrs.) | 12.2 | 12.1 | ns |
| Maternal IQ (av. score; WAIS-R) | 88.7 | 84.0 | <.001 |
| Environmental | |||
| HOME Score (av. score) | 12.7 | 12.0 | <.05 |
| Life events (av. number) | 3.3 | 3.4 | ns |
| Household Income ($/mo) | 1526 | 1212 | <.05 |
| Male in household (%) | 56 | 47 | ns |
| People in household (av. number) | 4.5 | 4.3 | ns |
| Child custody (% not) | 5 | 12 | <.05 |
≤.89 joints/day
>.89 joints/day
Average score on the measure of heavier substance use.
3.2. Hypothesis 2
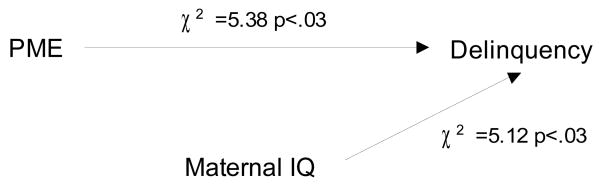
PME will predict a higher rate of delinquent behaviors. Delinquency was positively and significantly associated with PME (Table 1). This association was significant when PME was used as a continuous variable or as a dichotomized variable. The rates of delinquency for non-exposed, light to moderate, and heavy exposure were 41.1%, 50.4%, and 61.1%, respectively. However, because of the relations between PME and the mediators noted above, we elected to use PME as a dichotomized variable for the rest of the analyses. The significant correlates of PME in the first trimester included PAE, mother's work/study, and marital status. At 10 years, PME was significantly associated with current maternal substance use, household income, home environment, and maternal IQ, and child's race (Table 1). After controlling for these significant covariates in the multivariate analyses, the association between first trimester PME and delinquency at age 14 remained significant (Figure 1a). The adjusted odds ratio for delinquency among those offspring who were exposed to heavy marijuana use in the first trimester compared to all other offspring was 1.84 (χ2=5.38, CI 1.05-2.96; p<.03). In addition to PME, maternal IQ predicted delinquent behavior in adolescence.
Figure 1.
Figure 1a. Prenatal marijuana exposure (heavy vs. all others) predicts delinquency at age 14
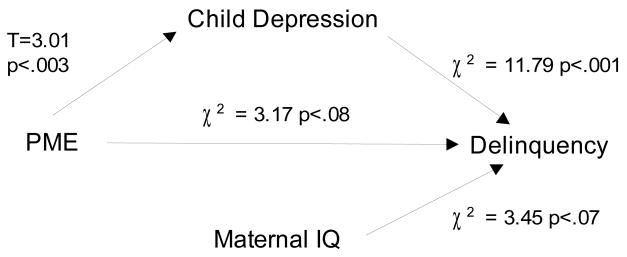
Figure 1b. Depression mediates the association between prenatal marijuana exposure (heavy vs. all others) and delinquency
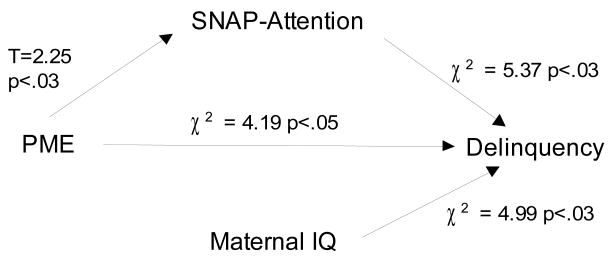
Figure 1c. Attention partially mediates the association between prenatal marijuana exposure (heavy vs. all others) and delinquency
In the multivariate analyses, the associations between PME and child's depressive symptoms and PME and attention remained significant. Learning and memory and child IQ were not significantly associated with PME after including the significant covariates in the model and were dropped from further analyses. Correlates of child's depression included maternal depression and the home environment. Gender, race, maternal depression and hostility, first trimester tobacco exposure, and the HOME score were significantly associated with child attention at age 10.
3.3. Hypothesis 3
The hypothesized mediators will be associated with delinquency. In the bivariate analyses, child depressive symptoms, activity, attention, and impulsivity at age 10 were significantly related to higher rates of delinquent behavior at age 14 (Table 2), while learning and memory, and IQ were not. Because child depressive symptoms and attention problems were the only mediators significantly related to PME, only these two measures were evaluated multivariately. Maternal IQ was the only other significant predictor.
Table 2.
Mediators and Covariates by Delinquency Outcomes
| Delinquent Behaviors | |||
|---|---|---|---|
| None N=282 |
Any1 N=243 |
p | |
| Prenatal Measures | |||
| Prenatal marijuana use (av. joints/day) | .30 | .56 | <.01 |
| Prenatal marijuana use (%Heavy Use) | 10.1 | 18.4 | <0.01 |
| Prenatal alcohol use (av. drinks/day) | .51 | .64 | ns |
| Prenatal tobacco use (av. cig./day) | 7.6 | 8.1 | ns |
| Mediators at 10 years | |||
| Child depression (av. score CDI) | 6.8 | 8.8 | <.001 |
| SNAP-Activity (av. score) | 9.5 | 10.1 | <.05 |
| SNAP-Attention (av. score) | 8.6 | 9.3 | <.01 |
| SNAP-Impulsivity (av. score) | 9.9 | 10.6 | <.01 |
| WRAML average screen score | 88.8 | 87.0 | ns |
| Child IQ (av. score, SBIS) | 91.7 | 90.0 | ns |
| Covariates at 10 years | |||
| Child | |||
| Gender (% male) | 47 | 52 | ns |
| Race (% African American) | 47 | 44 | ns |
| Maternal | |||
| Depression (av. score, CES-D) | 38.0 | 38.5 | ns |
| Hostility(av. score, STAI) | 16.0 | 16.2 | ns |
| Substance use2 | .40 | .49 | ns |
| Education (Years) | 12.3 | 12.1 | ns |
| IQ (av. SBIS score) | 89.2 | 86.6 | <.01 |
| Environmental Characteristics | |||
| Household Income ($/mo) | 1576 | 1372 | <.05 |
| HOME score (av. score) | 12.9 | 12.3 | <.01 |
| Life events (av. number) | 3.2 | 3.4 | ns |
| People in household (av. number | 4.5 | 4.5 | ns |
| Male in household (%) | 57 | 52 | ns |
| Not in maternal custody (%) | 6.4 | 4.9 | ns |
Above the 20th percentile on the SRD and/or ≥67 on the CBCL delinquency scale.
Average score on the measure of heavier substance use.
3.4 Hypothesis 4
The hypothesized mediators will attenuate the association between PME and delinquency. Depression significantly mediated the association between PME and delinquency. The direct effect of PME on delinquency became marginally significant (χ2=3.17; p<.08) after taking into account the effect of depression on delinquency (Figure 1b). Using the product of the coefficients, the estimated mediation was significant for depression (coefficient of indirect effect = .09, p<.01). Attention problems at age 10 only partially mediated the association between PME and delinquency. The direct effect of PME on delinquency remained significant after inclusion of attention problems to the model, but the significance level of PME was reduced (χ2=4.19, p<.05) (Figure 1c). The estimated mediation based on the product of coefficients was marginally significant (indirect effect = .04, p<.08).
4. Discussion
In this paper, we report a significant association between PME and delinquency in exposed adolescents. The odds ratio for delinquency among those who are exposed to one or more joints per day during the first trimester was 1.76, which means that the odds of a heavily-exposed adolescent being delinquent are nearly double those of adolescents who were not exposed or who were exposed to lesser amounts.
While this association is important in itself, we felt that it would be more informative to explore the potential pathways to this result. We have previously reported that PME predicts increased rates of depressive symptoms [30], attention deficits [73], increases in activity and impulsivity [40,29], difficulties on tests of learning and memory [61], and lower IQ scores [13,29]. We hypothesized that these measures of neurocognitive functioning would mediate the association between PME and delinquency.
There was a mediated association between marijuana exposure during gestation, depressive symptoms at age 10, and delinquency at age 14. There was also a partially significant mediated pathway from PME to attention to delinquency. These results replicate our findings at age 10 of a significant association between PME and delinquency. This was the first true test of mediation in these data, as earlier reports used a mediator and outcome from the same phase. We have confirmed the earlier report of attention as a mediation and also identified child depression as a significant mediator of the association between PME and delinquency. It is, of course, necessary to replicate this finding in other studies with populations that differ from the lower SES group studied in the MHPCD.
There are multiple pathways to delinquency, and the effects of PME only explain a portion of the delinquency rate in the adolescent population. However, it is a significant proportion and it is a preventable proportion. These analyses controlled for major predictors of adolescent delinquency including demographic factors, other prenatal exposures, household factors such as family composition and household income, characteristics such as maternal social and psychological status, substance use, education, and occupation.
There are limitations in this study. The sample is lower-income and may not represent the experience of a general population. This is the population, however, that is most likely to use marijuana during pregnancy, and the offspring who are most at risk. It is significant that our findings on the consequences of PME are very similar to results reported from the OPPS, which was a middle class sample of women obtaining prenatal care through private physicians. This suggests that these findings are generalizable. Moreover, the correlates of PME, child depressive symptoms, and delinquency that were identified in this analysis agree with other reports in the literature.
To ensure accurate maternal reporting during pregnancy, we initially used a bogus pipeline technique [35], to convince the women that we had a method to check on the reliability of their substance use reports. We found that for marijuana, the reporting did not change when we compared reports gained with the bogus pipeline to those interviews where it was not used, leading us to conclude that the women felt comfortable with our promise of confidentiality, the fact that the project had a Certificate of Confidentiality, and the professionalism of our interviewers. There is also a possibility that the adolescents lied about their delinquent behaviors, but we have maternal reports on the CBCL that serve as a partial check on their accuracy. The teenagers knew our staff and were comfortable with our study; reports on substance use, for example, have been accurate when compared to laboratory results. This analysis used depressive symptoms rather than a clinical diagnosis because Major Depressive Disorder is uncommon at this age [13]. Symptoms are important measures in themselves, however. A higher rate of depressive symptoms significantly predicted dysfunctional behavior.
There are alternative hypotheses that could explain these findings. It could be that environmental factors determined both the mothers' use of marijuana during pregnancy and the subsequent problem behaviors of the offspring. We identified the covariates of both of these characteristics and included the significant covariates in the multivariate models. It is also possible that we have missed a significant confounder that is associated with both with PME and adolescent delinquent behaviors. For example, mothers and their offspring could share an underlying genetic propensity to engage in risky behaviors such as substance use and delinquency. However, the lifetime rates of Conduct Disorder, Anti-Social Personality Disorder, and Major Depressive Disorder did not differ between the women who used marijuana at the rate of one or more joints/day during the first trimester and those who did not, making this unlikely.
5. Conclusion
In summary, we have demonstrated that there is a significant effect of PME on the rate of delinquency in adolescence. The relation between PME and delinquent behavior is mediated by the effects of marijuana on depressive symptoms and partially mediated by attention deficits in the exposed offspring. The pathway from PME to delinquency, which is mediated by depressive symptoms and attention deficits, provides several potential points for intervention. Intervention to stop marijuana use during pregnancy would decrease the rates of delinquency in the offspring, as would early treatment of children with high levels of depressive symptoms or attention deficits. The need for intervention has become even more important in recent years. The prenatal data in this study were collected between 1982 and 1985, and the marijuana that was available during that time period was not as potent as the marijuana that is currently available, making the risk of the harmful effects of PME higher today for each joint, bowl, or blunt that is used during pregnancy [49].
Acknowledgments
We would like to acknowledge Dr. Vincent Smeriglio who was the Project Officer for our research grants for many years. Dr. Smeriglio was always available for questions or advice. He was helpful when things didn't go well, and supportive and excited when they did. Thank you Vince for your kindness, your wisdom, and your wonderful sense of humor!
This research was supported by a grant from the National Institute on Drug Abuse (DA03874), N. Day, P.I.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Nancy L. Day, Email: nday@pitt.edu.
Sharon L. Leech, Email: slleech@pitt.edu.
Lidush Goldschmidt, Email: lidush@pitt.edu.
References
- 1.Achenbach T. Manual for the Child Behavior Checklist/4-18 and 1991 Profile. University of Vermont Department of Psychiatry; Burlington, VT: 1991. [Google Scholar]
- 2.Baker P, Mott F. National Longitudinal Study of Youth - Child Handbook. State University Center for Human Resource Research; Columbus, OH: 1989. [Google Scholar]
- 3.Baker PC, Mott FL. Tabulations and summary discussion. State University Center for Human Resource Research; Columbus, OH: 1991. Children of the National Longitudinal Study of Youth. [Google Scholar]
- 4.Barker ED, Seguin JR, White HR, Bates ME, Lacourse E, Carbonneau R, Tremblay RE. Developmental trajectories of male physical violence and theft. Arch Gen Psychiat. 2007;64:592–599. doi: 10.1001/archpsyc.64.5.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 6.Berghuis P, Rajnicek AM, Morozov AM, Ross RA, Mulder J, Urban GM, Monory K, Marsicano G, Matteoli M, Canty A, Irving AJ, Katona I, Yanagawa Y, Rakic P, Lutz B, Mackie K, Harkany T. Hardwiring the Brain: Endocannabinoids shape neuronal connectivity. Science. 2007;316:1212–1216. doi: 10.1126/science.1137406. [DOI] [PubMed] [Google Scholar]
- 7.Berghuis P, Dobszay MB, Wang X, Spano S, Ledda F, Sousa KM, Schulte G, Ernfors P, Mackie K, Paratch G, Hurd YL, Harkany T. Endocannabinoids regulate interneuron migration and morphogenesis by transactivating the TrkB receptor. Proc Natl Acad Sci. 2005;102:19115–19120. doi: 10.1073/pnas.0509494102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berkman L, Syme L. Social networks, host resistance, and mortality: a nine year follow-up study of Alameda County residents. Am J Epidemiol. 1979;109:186–204. doi: 10.1093/oxfordjournals.aje.a112674. [DOI] [PubMed] [Google Scholar]
- 9.Biegon A, Kerman HA. Autoradiographic study of pre- and postnatal distribution of cannabinoid receptors in human brain. Neuroimage. 2001;14:1463–1468. doi: 10.1006/nimg.2001.0939. [DOI] [PubMed] [Google Scholar]
- 10.Brooker B, Cyr J. Tables for clinicians to use to convert WAIS-R short forms. J Clin Psychol. 1986;42:982–986. [Google Scholar]
- 11.Caspi A, Henry B, McGee RO, Moffit TE, Silva PA. Temperamental origins of child and adolescent behavior problems: From age three to age fifteen. Child Dev. 1995;66:55–68. doi: 10.1111/j.1467-8624.1995.tb00855.x. [DOI] [PubMed] [Google Scholar]
- 12.Cornelius MD, Day NL, Richardson GA, Taylor PM. Epidemiology of substance abuse during pregnancy. In: Ott PJ, Tarter RE, editors. Sourcebook on Substance Abuse: Etiology, Epidemiology, Assessment and Treatment. Allyn & Bacon, Inc.: Needham Heights, MA; 1999. pp. 1–13. [Google Scholar]
- 13.Costello EJ, Pine DS, Hammen C, March JS, Plotsky PM, Weissman MM, Biederman J, Goldsmith HH, Kaufman J, Lewinsohn PM, Hellander M, Hoagwood K, Koretz DS, Nelson CA, Leckman JF. Development and natural history of mood disorders. Biol Psych. 2002;52:529–542. doi: 10.1016/s0006-3223(02)01372-0. [DOI] [PubMed] [Google Scholar]
- 14.Dahl RE, Scher MS, Williamson DE, Robles N, Day N. A longitudinal study of prenatal marijuana use: Effects on sleep and arousal at age 3 years. Arch Pediat Adol Med. 1995;149:145–150. doi: 10.1001/archpedi.1995.02170140027004. [DOI] [PubMed] [Google Scholar]
- 15.Day NL, Richardson GA, Goldschmidt L, Robles N, Taylor P, Stoffer D, Cornelius M, Geva D. The effect of prenatal marijuana exposure on cognitive development at age three. Neurotoxicol Teratol. 1994;16:169–175. doi: 10.1016/0892-0362(94)90114-7. [DOI] [PubMed] [Google Scholar]
- 16.Day N, Robles N. Methodological issues in the measurement of substance use. Ann NY Acad Sci. 1989;562:8–13. doi: 10.1111/j.1749-6632.1989.tb21002.x. [DOI] [PubMed] [Google Scholar]
- 17.Day N, Wagener D, Taylor P. Measurement of substance use during pregnancy: Methodologic issues. In: Pinkert T, editor. Prenatal Drug Exposure and Consequences of Maternal Drug Use, NIDA Res Monogr. Vol. 59. 1985. pp. 36–40. [PubMed] [Google Scholar]
- 18.Del Arco I, Munoz R, Rodriguez De Fonseca F, Escudero L, Martín-Calderón JL, Navarro M, Villanúa MA. Maternal exposure to the synthetic cannabinoid HU-210: effects on the endocrine and immune systems of the adult male offspring. Neuroimmunomodulation. 2000;7:16–26. doi: 10.1159/000026416. [DOI] [PubMed] [Google Scholar]
- 19.Elliott DS. Serious violent offenders: Onset, developmental course, and termination - Presidential Address, The American Society of Criminology. Criminology. 1993;32:1–21. [Google Scholar]
- 20.Elliot DS, Huizinga D, Ageton SS. Explaining Delinquency and Drug Use. Sage Publications; Beverly Hills, CA: 1985. [Google Scholar]
- 21.Ellickson P, Saner H, McGuigan KA. Profiles of violent youth: Substance use and other concurrent problems. Am J Pub Health. 1997;87:985–991. doi: 10.2105/ajph.87.6.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farrington DP. Childhood aggression and adult violence: Early precursors and later life outcomes. In: Pepler DJ, Rubin KH, editors. The Development and Treatment of Childhood Aggression. Erlbaum; Hillsdale, NJ: 1991. pp. 5–29. [Google Scholar]
- 23.Farrington DP, Loeber R, Stouthamer-Loeber M, Van Kammen WB, Schmidt L. Self-reported delinquency and a combined delinquency seriousness scale based on boys, mother, and teachers: Concurrent and predictive validity for African-Americans and Caucasians. Criminology. 1996;34:493–514. [Google Scholar]
- 24.Fride E, Mechoulam R. Developmental aspects of anandamide; ontogeny of response and prenatal exposure. Psychoneuroendocrinol. 1996;21:157–172. doi: 10.1016/0306-4530(95)00039-9. [DOI] [PubMed] [Google Scholar]
- 25.Fried PA, Watkinson B. 36- and 48-month neurobehavioral follow-up of children prenatally exposed to marihuana, cigarettes and alcohol. J Dev Behav Pediatr. 1990;11:49–58. [PubMed] [Google Scholar]
- 26.Fried PA, Watkinson B. Differential effects on facets of attention in adolescents prenatally exposed to cigarettes and marihuana. Neurotoxicol Teratol. 2001;55:421–430. doi: 10.1016/s0892-0362(01)00160-x. [DOI] [PubMed] [Google Scholar]
- 27.Fried PA, Watkinson B, Gray R. Differential effects on cognitive functioning in 9- to 12-year-olds prenatally exposed to cigarettes and marihuana. Neurotoxicol Teratol. 1998;20:293–306. doi: 10.1016/s0892-0362(97)00091-3. [DOI] [PubMed] [Google Scholar]
- 28.Goldschmidt L, Day NL, Richardson GA. Effects of prenatal marijuana exposure on child behavior problems at age 10. Neurotoxicol Teratol. 2000;22:325–336. doi: 10.1016/s0892-0362(00)00066-0. [DOI] [PubMed] [Google Scholar]
- 29.Goldschmidt L, Richardson GA, Willford JA, Day NL. Prenatal marijuana exposure and intelligence test performance at age six. J Am Acad Child Psy. 2008;47:254–263. doi: 10.1097/CHI.0b013e318160b3f0. [DOI] [PubMed] [Google Scholar]
- 30.Gray KA, Day NL, Leech SL, Richardson GA. Prenatal marijuana exposure: Effect on child depressive symptoms at ten years of age. Neurotoxicol Teratol. 2005;27:439–448. doi: 10.1016/j.ntt.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 31.Griffith DR, Azuma SD, Chasnoff IJ. Three-year outcome of children exposed prenatally to drugs. J Am Acad Child Psy. 1994;33:20–27. doi: 10.1097/00004583-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Hawks R, Chiang C. Examples of specific drug assays. NIDA Res Monogr. 1986;73:84–112. [PubMed] [Google Scholar]
- 33.Harkany T, Guzman M, Galve-Roperh I, Berghuis P, Devi LA, Mackie K. The emerging functions of endocannabinoid signaling during CNS development. Trends Pharmacol Sci. 2007;28:83–92. doi: 10.1016/j.tips.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 34.Harkany T, Mackie K, Doherty P. Wiring and firing neuronal networks: endocannabinoids take center stage. Curr Opin Neurobiol. 2008;18:338–345. doi: 10.1016/j.conb.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones E, Sigall H. The bogus pipeline: A new paradigm for measuring affect and attitude. Psychol Bull. 1979;76:349–364. [Google Scholar]
- 36.Julien RM. A Concise Nontechnical Guide to the Actions, Uses, and Side Effects of Psychoactive Drugs. eighth. W.H. Freeman; New York: 1997. A Primer of Drug Action. [Google Scholar]
- 37.Kovacs M. The Children's Depression Inventory. Multi-Health Systems, Inc.; North Tonawanda, NY: 1992. [Google Scholar]
- 38.Kraemer HC, Kiernan M, Essex M, Kupfer DJ. How and why criteria defining moderators and mediators differ between the Baron & Kenny and Macarthur approaches. Health Psychol. 2008;27:S101–S108. doi: 10.1037/0278-6133.27.2(Suppl.).S101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumar RN, Chambers WA, Pertwee RG. Pharmacological actions and therapeutic uses of cannabis and cannabinoids. Anesthesia. 2001;56:1059–1068. doi: 10.1046/j.1365-2044.2001.02269.x. [DOI] [PubMed] [Google Scholar]
- 40.Leech S, Richardson G, Goldschmidt L, Day N. Prenatal substance exposure: Effects on attention and impulsivity of six-year-olds. Neurotoxicol Teratol. 1999;21:109–118. doi: 10.1016/s0892-0362(98)00042-7. [DOI] [PubMed] [Google Scholar]
- 41.Leech SL, Day NL, Richardson GA, Goldschmidt L. Predictors of self-reported delinquent behavior in a sample of young adolescents. J Early Adoles. 2003;23:78–106. [Google Scholar]
- 42.Leech SL, Larkby CA, Day R, Day NL. Predictors of high levels of depression and anxiety symptoms among children at age 10. J Am Acad Child Psy. 2006;45:223–230. doi: 10.1097/01.chi.0000184930.18552.4d. [DOI] [PubMed] [Google Scholar]
- 43.Leech SL, DeGenna N, Day NL. Poster presented at the 12th Biennial Meeting of the Society for Research on Adolescence. Chicago, IL: Mar, 2008. Do neuropsychological deficits at 10 years of age predict delinquent behaviors at 16? [Google Scholar]
- 44.Loeber R, Dishion T. Early predictors of male delinquency: A review. Psychol Bull. 1983;94:68–99. [PubMed] [Google Scholar]
- 45.Loeber R, Stouthamer-Loeber M, Van Kammen W, Farrington D. Development of a new measure of self-reported antisocial behavior for young children: Prevalence and reliability. In: Klein MW, editor. Cross-National Research and Self-Reported Crime and Delinquency. Kluwer-Nijhoff; Dordrecht, Netherlands: 1989. pp. 203–225. [Google Scholar]
- 46.Loeber R, Farrington DP, Stouthamer-Loeber M, Van Kammen WB. Antisocial Behavior and Mental Health Problems: Explanatory Factors in Childhood and Adolescence. Lawrence Erlbaum Associates; Matwah, NY: 1998. [Google Scholar]
- 47.Loeber R, Farrington DP. Young children who commit crime: Epidemiology, developmental origins, risk factors, early interventions, and policy implications. Dev Psychopath. 2000;12:838–762. doi: 10.1017/s0954579400004107. [DOI] [PubMed] [Google Scholar]
- 48.MacKinnon D. Introduction to Statistical Mediation Analysis, Lawrence Erlbaum Associates. Taylor and Francis Group, LLC.; New York: 2008. [Google Scholar]
- 49.McLaren J, Swift W, Dillon P, Allsop S. Cannabis potency and contamination: a review of he literature. Addiction. 2008;103:1100–1109. doi: 10.1111/j.1360-0443.2008.02230.x. [DOI] [PubMed] [Google Scholar]
- 50.Moffitt TE, Silva PA. Neuropsychological deficit and self-reported delinquency in an unselected birth cohort. J Am Acad Child Psy. 1988;27:233–240. doi: 10.1097/00004583-198803000-00017. [DOI] [PubMed] [Google Scholar]
- 51.Moffitt TE, Caspi A. Childhood predictors differentiate life-course persistent and adolescence-limited antisocial pathways among males and females. Dev Psychopathol. 2001;3:355–375. doi: 10.1017/s0954579401002097. [DOI] [PubMed] [Google Scholar]
- 52.Muthén LK, Muthén BO. User's Guide. 5th. Muthén & Muthén; Los Angeles, CA: 2007. Mplus: Statistical Ananlysis with Latent Variables. [Google Scholar]
- 53.Navarro M, Rodriguez-de-Fonseca F, Hernandez ML, Ramos JA, Fernandez-Ruiz JJ. Motor behavior and nigrostriatal dopaminergic activity in adult rats perinatally exposed to cannabinoids. Pharmacol Biochem Beh. 1994;47:47–58. doi: 10.1016/0091-3057(94)90110-4. [DOI] [PubMed] [Google Scholar]
- 54.Navarrete M, Araquje A. Endocannabinoids mediate neuron-astrocyte communication. Neuron. 2008;57:883–893. doi: 10.1016/j.neuron.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 55.Navarro M, Rubio P. Sex-dimorphic psychomotor activation after perinatal exposure to delta (9)-tetrahydrocannabinol. An ontogenetic study in Wistar rats. Psychopharmacol Berl. 1994;116:414–422. doi: 10.1007/BF02247471. [DOI] [PubMed] [Google Scholar]
- 56.O'Connell CM, Fried PA. Prenatal exposure to cannabis: A preliminary report of postnatal consequences in school-age children. Neurotoxicol Teratol. 1991;13:631–639. doi: 10.1016/0892-0362(91)90047-z. [DOI] [PubMed] [Google Scholar]
- 57.Pelham W, Bender M. Peer relationships in hyperactive children. Description and treatment. Adv Learn Beh Dis. 1982;1:365–436. [Google Scholar]
- 58.O'Donnell J, Hawkins JD, Abbott RS. Predicting serious delinquency and substance use among aggressive boys. J Consult Clin Psychol. 1995;63:529–537. doi: 10.1037//0022-006x.63.4.529. [DOI] [PubMed] [Google Scholar]
- 59.Oyserman D, Saltz E. Competence, delinquency, and attempts to attain possible selves. J Pers Soc Psychol. 1993;65:360–374. doi: 10.1037//0022-3514.65.2.360. [DOI] [PubMed] [Google Scholar]
- 60.Radloff L. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1996;1:385–401. [Google Scholar]
- 61.Richardson GA, Ryan C, Willford JA, Day NL, Goldschmidt L. Prenatal alcohol and marijuana exposure: Effects on neuropsychological outcomes at 10 years. Neurotoxicol Teratol. 2002;24:309–320. doi: 10.1016/s0892-0362(02)00193-9. [DOI] [PubMed] [Google Scholar]
- 62.Scher M, Richardson G, Coble P, Day N, Stoffer D. The effects of prenatal alcohol and marijuana exposure: Disturbances in neonatal sleep cycling and arousal. Pediat Res. 1988;24:101–105. doi: 10.1203/00006450-198807000-00023. [DOI] [PubMed] [Google Scholar]
- 63.Sheslow D, Adams W. Manual for the Wide Range Assessment of Memory and Learning. Jastak Associates; Wilmington, DE: 1990. [Google Scholar]
- 64.Snyder HN, Sickmund M. Juvenile Offenders and Victims: 1999 National Report. National Center for Juvenile Justice; Washington DC: 1999. [Google Scholar]
- 65.Spielberger C, Gorsuch R, Lushene R. Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press; Palo Alto, CA: 1970. [Google Scholar]
- 66.Substance Abuse and Mental Health Services Administration. Summary of Findings from the 1999 National Household Survey on Drug Abuse. DHHS Publication; Rockville, MD: 2000. (H-12). [Google Scholar]
- 67.Thorndike R, Hagen E, Sattler J. The Stanford-Binet Intelligence Scale. third. Riverside Publishing Co.; Chicago, IL: 1986. [Google Scholar]
- 68.Tremblay RE, Masse LC, Vitaro F, Dobkin PL. The impact of friends' deviant behavior on early onset of delinquency: Longitudinal data from 6 to 13 years of age. Dev Psychopath. 1995;7:649–667. [Google Scholar]
- 69.Trezza V, Campolongo P, Cassano T, Macheda T, Dipasquale P, Carratu MR, Gaetani S, Cuomo V. Effects of perinatal exposure to delta-9-tetrahydrocannabinol on the emotional reactivity of the offspring: a longitudinal behavioral study in Wistar rats. Psychopharmacol. 2008;98:529–537. doi: 10.1007/s00213-008-1162-3. [DOI] [PubMed] [Google Scholar]
- 70.Wechsler D. Wechsler Intelligence Scale for Children. third. Psychological Corporation; San Antonio, TX: 1991. [Google Scholar]
- 71.Weist MD, Paskewitz DA, Jackson CY, Jones D. Self-reported delinquent behavior and psychosocial functioning in inner-city teenagers: A brief report. Child Psychiat Hum D. 1998;28:241–248. doi: 10.1023/a:1022684031414. [DOI] [PubMed] [Google Scholar]
- 72.White JL, Moffitt TE, Earls F, Robins L, Silva PA. How early can we tell? Predictors of childhood conduct disorder and adolescent delinquency. Criminology. 1990;28:507–528. [Google Scholar]
- 73.Willford JA, Richardson GA, Leech SL, Day NL. Prenatal alcohol or marijuana exposure differentially affects executive function in adolescents. Neurotoxicol Teratol. 2001;23:286. [Google Scholar]