Abstract
Chronic granulomatous disease (CGD) is a rare primary immunodeficiency with x-linked or autosomal recessive inheritance involving defects in genes encoding phox proteins which are the subunits of the phagocyte NADPH oxidase. This results in failure to produce superoxide anion and downstream antimicrobial oxidant metabolites and to activate antimicrobial proteases. Affected patients are susceptible to severe, life-threatening bacterial and fungal infections and excessive inflammation characterized by granulomatous enteritis resembling Crohn's disease and genitourinary obstruction. Early diagnosis of CGD and rapid treatment of infections are critical. Prophylaxis with antibacterial and mould-active antifungal agents and the administration of interferon-γ has significantly improved the natural history of CGD. Currently, the only cure is allogeneic hematopoietic cell transplant (HCT) although there remains controversy as to which patients with CGD should get a transplant. Allele-based HLA typing of alternative donors, improved supportive care measures and use of reduced toxicity conditioning have resulted in EFS of at least 80% even with an unrelated donor and even better when the patient has no active infections/inflammation. Gene correction of CGD would eliminate the risks of GVHD and the immunoablative chemotherapy required for allogeneic HCT. Based on gene therapy trials in patients with SCID-X1, ADA-SCID and the early experience with CGD, it is clear that at least some degree of myeloablation will be necessary for CGD as there is no inherent selective growth advantage for gene-corrected cells. Current efforts for gene therapy focus on use of lentivector constructs which are thought to be safer from the standpoint of insertional mutagenesis and more efficient in transducing hematopoietic stem cells.
Keywords: Chronic Granulomatous disease, NADPH oxidase, hematopoietic cell transplantation, gene therapy
The Diagnosis, Clinical Presentation and Management of Chronic Granulomatous Disease
A disorder of NADPH oxidase
Chronic granulomatous disease (CGD) is an inherited disorder of the NADPH oxidase characterized by severe bacterial and fungal infections and excessive inflammation. CGD affects approximately 1 in 200,000 persons [1]. CGD was first described in the 1950s as a fatal granulomatous disease of childhood. In the 1960s, classic studies linked CGD with impaired neutrophil bactericidal activity. Neutrophils from CGD patients failed to show an increase in oxygen consumption and hydrogen peroxide formation. This rapid oxygen consumption (“respiratory burst”) was initially attributed to increased mitochondrial respiration, but later linked to the NADPH oxidase. CGD was subsequently identified as a disorder of NADPH oxidase activation.
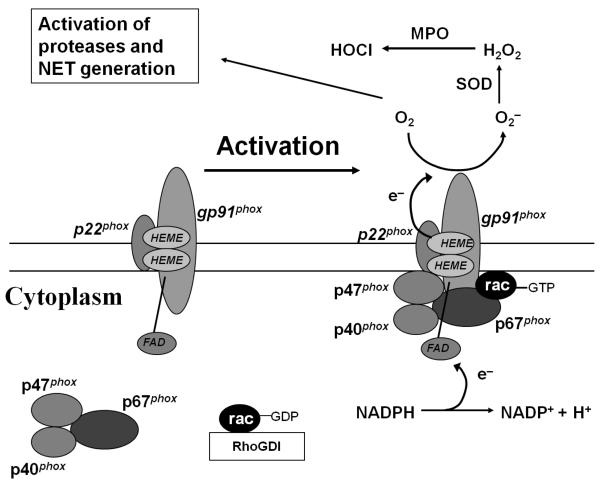
The phagocyte NADPH oxidase functions to rapidly generate superoxide anion by transferring electrons from NADPH to molecular oxygen (Figure 1). The cytochrome of NADPH oxidase, composed of gp91phox (phox, phagocyte oxidase) and p22phox, is embedded in membranes. Upon activation of the oxidase, the cytoplasmic subunits p47phox, p67phox, and p40phox appear to translocate en-bloc to the membrane-bound cytochrome. Activation of Rac, a member of the low molecular weight GTP-binding proteins, and translocation of Rac to the membrane-bound cytochrome are also critical for NADPH oxidase activation. CGD results from disabling mutations in genes encoding phox proteins. Approximately two-thirds of CGD cases are X-linked (gp91phox –deficient) and the remainder is autosomal recessive [1].
Figure 1.
NADPH oxidase activation requires translocation of cytosolic phox proteins and Rac to the membrane-bound flavocytochrome. Upon activation, NADPH is oxidized and electrons are transported to the other side of the membrane where molecular oxygen is converted to superoxide anion, leading to generation of downstream metabolites, such as hydrogen peroxide and hypohalous acid. In addition to the direct microbicidal properties of these reactive oxidants, activation of NADPH oxidase in neutrophils is associated with activation of intracellular proteases (e.g., neutrophil elastase, cathepsin G) and release of neutrophil extracellular traps (NETs) that also mediate antimicrobial host defense. SOD, superoxide dismutase; MPO, myeloperoxidase.
NADPH oxidase-mediated antimicrobial host defense
NADPH oxidase activation results in production of superoxide anion and downstream antimicrobial oxidant metabolites, such as hydrogen peroxide and hypohalous acid. Reeves et al. [2] showed that activation of the NADPH oxidase also leads to the activation of antimicrobial proteases sequestered in the primary (azurophilic) granules of neutrophils. Activation of these granular proteases likely enhances killing of pathogens within phagolysosomes. Neutrophils also release granule proteins and chromatin that co-mingle in the extracellular space and together form neutrophil extracellular traps (NETs). These NETs bind to and kill extracellular bacteria, degrade bacterial virulence factors [3], and target fungi [4]. Release of NETs requires death of neutrophils and breakdown of cell membranes [5]. Neutrophils from CGD patients are deficient in NET formation [4,5]; this deficiency was reversed in neutrophils from a CGD patient following gene therapy [4], supporting the role of NADPH oxidase in NET generation.
CGD: a disease of impaired antimicrobial host defense and excessive inflammation
CGD patients are susceptible to a spectrum of bacterial and fungal infections. Patients with the X-linked CGD appear to be at greater risk for infection and early mortality compared to patients with autosomal recessive forms of CGD [1]. In a U.S. registry of 368 patients with CGD, pneumonia was the most frequent type of infection occurring in 79% of patients, with Aspergillus species being the most common cause [1] (Table 1). Fifty-three percent of patients had suppurative adenitis, 42% had a subcutaneous abscess, and 27% had a liver abscess; Staphylococcus aureus was the most common cause of soft tissue and liver abscesses. Twenty-five percent (25%) had osteomyelitis (Serratia marcescens was the most prevalent cause), and 18% had sepsis (Salmonella species were the most prevalent cause). The most common causes of death were pneumonia and/or sepsis due to Aspergillus species (23 patients) or Burkholderia cepacia (12 patients). A European registry of 429 CGD patients showed that the most frequently cultured pathogens per episode were Staphylococcus aureus (30%) Aspergillus species, (26%) and Salmonella species. (16%); Aspergillus species (111 cases) were the most common cause of pneumonia [6].
Table 1.
Infections in CGD
| Site | Most Common Pathogens | Diagnostic Methods |
|---|---|---|
| Lungs (pneumonia) |
Aspergillus species and other moulds, B. cepacia, S. aureus, Nocardia species |
Sputum culture (least invasive but insensitive for moulds); blood culture (in cases of pneumonia and secondary bacteremia); bronchoalveolar lavage (BAL) and biopsy; percutaneous lung biopsy; and thoracoscopic or open lung biopsy. More than one pathogen can be present. |
| Lymph Nodes (suppurative adenitis) |
S. aureus | Culture |
| Skin (subcutaneous abscesses; infected cysts) |
S. aureus | Culture |
| Liver (abscesses) | S. aureus | Culture |
| Bone (osteomyelitis) | S. marcescens | Culture |
| Blood (sepsis) |
Salmonella species, S. aureus, B. cepacia |
Culture |
In addition to recurrent infections, CGD is also characterized by abnormally exuberant inflammatory responses leading to granuloma formation, such as granulomatous enteritis resembling Crohn's disease [7] and genitourinary obstruction. “Mulch pneumonitis” is a recently described life-threatening hyperinflammatory response to fungal pneumonia in CGD, requiring both antifungal therapy and systemic corticosteroids [8].
Mouse models of CGD support the notion that excessive inflammatory responses are not simply the result of unresolved infection, but, rather, reflect an important role of NADPH oxidase in regulating inflammation. For example, Morgenestern et al. [9] showed that intratracheal administration of heat-killed A. fumigatus hyphae elicited mild self-limited inflammation in wildtype mice, but robust and persistent inflammation in CGD mice. Romani et al. [10] linked impaired antifungal host defense and excessive inflammation in CGD mice to defective activation of tryptophan catabolism and generation of regulatory T-cell responses. Segal et al. [11] showed that CGD mice and peripheral blood mononuclear cells (PBMCs) from CGD patients had impaired activation of Nrf2, a redox-sensitive transcriptional factor that induces oxidant scavenging pathways and functions to limit cellular injury and inflammation. These results support a model in which NADPH oxidase can limit innate and T-cell responses by modulating specific redox-sensitive pathways.
Care of the CGD patient
Diagnosis of CGD
The first component in care of the CGD patient is early diagnosis. CGD should be suspected in patients with recurrent or unusually severe infections, such as a liver abscess caused by S. aureus. In addition, specific opportunistic infections should prompt an evaluation for CGD; these include invasive mould diseases (e.g., aspergillosis), and infections by B. cepacia, S. marcescens, and Nocardia species in the absence of a known immunodeficiency. Inflammatory disorders such as inflammatory bowel disease at an early age and granulomatous cystitis can be manifestations of CGD. A family history of males with severe or unusual infections can be a clue to the diagnosis of X-linked CGD, while consanguineous parents increase the risk for autosomal recessive disorders.
The diagnosis of CGD requires demonstration of defective NADPH oxidase activity in neutrophils. The most common diagnostic assays are the nitroblue tetrazolium dye reduction method (a measure of superoxide anion release) and flow cytometry evaluating dihydrorhodamine 123 (DHR) fluorescence (a measure of intracellular hydrogen peroxide). DHR fluorescence is likely to be the most sensitive method for diagnosis, particularly in cases of variant X-linked and autosomal recessive forms of CGD, where low levels of NADPH oxidase activity may lead to false-positive results with the nitroblue tetrazolium method.
Antibiotic and recombinant interferon-γ prophylaxis
CGD patients should receive antibacterial and mould-active antifungal prophylaxis. Trimethoprim-sulfamethoxazole is generally the recommended agent for antibacterial prophylaxis. It is well-tolerated in CGD patients, and has activity against the majority of bacterial pathogens encountered in CGD patients: S. aureus (including the predominant community-acquired strain of methicillin-resistant S. aureus), B. cepacia, and Nocardia species. If trimethoprim-sulfamethoxazole is not feasible (e.g., due to allergy), an anti-staphylococcal penicillin (e.g., dicloxacillin) is advised. Given the high risk of invasive fungal diseases in CGD, mould-active antifungal prophylaxis is also warranted. Itraconazole was safe and effective in patients with CGD [12]. Extended-spectrum azoles (voriconazole and posaconazole) are alternative agents that can be used as prophylaxis.
In a randomized trial, recombinant interferon-γ significantly reduced the incidence of serious infections in patients with CGD [13]. Interferon-γ was beneficial regardless of age, the use of prophylactic antibiotics, and the type of CGD (X-linked or autosomal recessive), and was well-tolerated. Although prior studies showed that interferon-γ could augment superoxide production in phagocytes from CGD patients, there were no significant changes in the measures of superoxide production by phagocytes in the randomized trial. Thus, the benefit of prophylactic recombinant intereferon-γ likely results from augmentation of oxidant-independent pathways.
Diagnosis and treatment of infections
CGD patients may not manifest typical signs of infection. Fever and leukocytosis may be absent, and an elevated sedimentation rate may be the only abnormal laboratory test. In a review of aspergillosis in CGD patients at the NIH, one third of patients were asymptomatic at diagnosis and only ~20% were febrile [14]. In many of these patients, a pulmonary infiltrate on routine screening chest x-ray or CT scan was the first indication of an infection. The white blood cell count was ≤10,000/μl in 13/23 cases and the sedimentation rate was ≤40 mm/hr in 9/20 cases.
When infections are suspected, it is important to establish a culture diagnosis when feasible prior to initiating antimicrobial therapy. Chest CT scans are useful to detect early pneumonia. If non-invasive testing (e.g., blood and sputum cultures) are non-diagnostic, an invasive procedure should be considered. Serum galactomannan (a diagnostic marker for invasive aspergillosis) appears to be insensitive in CGD patients; this may be related to the fact that hyphal vascular invasion, a common feature of invasive aspergillosis in neutropenic patients, is generally not observed in invasive aspergillosis in CGD. A percutaneous lung biopsy is probably the most useful approach for peripheral lung lesions. Biopsy material should be submitted for pathology as well as bacterial and fungal culture, including Nocardia. Frequent radiographic evaluation (e.g., chest radiographs during routine clinic visits and CT scans in patients with fever or focal signs) is critical to making early diagnoses.
Debridement or resection of infected tissue may be required. Infections that involve bone or deep soft tissue are generally most effectively treated with antibiotics and surgery. Aspergillus nidulans is associated with severe infections in CGD patients, frequently manifesting with extension to the chest wall and vertebrae, and requiring prolonged therapy in combination with debridements [14].
Adjunctive granulocyte transfusions have been used for severe or refractory infections in CGD patients. Use of granulocyte transfusions in CGD is supported by the principle that a small proportion of normal phagocytes may be able to complement the oxidative defect in CGD phagocytes. Hydrogen peroxide generated by normal neutrophils can diffuse into CGD neutrophils and provide the necessary reagent to generate hypohalous acid and hydroxyl anion in vitro. Transfused granulocytes retain respiratory burst activity and appear to traffic normally based on their recovery from sites of infection. Granulocyte transfusions are generally well tolerated, but adverse effects include fevers, development of leukoagglutinins leading to rapid loss of transfused granulocytes, and rarely, pulmonary leukostasis. The likelihood of pulmonary leukostasis may be increased if amphotericin B and granulocytes are administered concomitantly; therefore granulocyte transfusions and amphotericin B should be administered several hours apart. Granulocyte transfusions can predispose to alloimmunization, which is of concern for patients under consideration for hematopoietic cell transplantation.
Hematopoietic Cell Transplantation (HCT) for Chronic Granulomatous Disease
HCT provides curative therapy for patients with CGD, although controversy exists over the requirement for HCT in all patients, and the optimal timing for any HCT procedure. While uncomplicated CGD is not necessarily an indication for transplantation, HCT should be considered for CGD patients whose clinical history demonstrates significant morbidity (recurrent life-threatening infections, an ongoing infection refractory to treatment, progressive granulomatous restrictive lung disease, and/or high-dose steroid-dependent or refractory severe granulomatous colitis). In patients with these morbidity indicators, non-availability of specialist medical care or non-compliance with long term antimicrobial prophylaxis may additionally influence the decision to transplant [15].
In the largest reported series, twenty-seven patients underwent HCT for CGD complicated as above in 14 cooperating European centers between 1985 and 2000 [16] (Table 2). Most transplants were in children (n=25), received a myeloablative busulfan-based regimen (n=23), and had unmodified marrow allografts (n=23) from HLA-identical sibling donors (MSD). Twenty-three of 27 (85%) survive with 22/23 survivors cured of CGD. Pre-existing infections and chronic inflammatory lesions cleared in all engrafted survivors, even children with severe lung restriction profited, slowly normalizing decreased oxygen saturation and reversing clubbing of fingers and toes. Survival was especially good in patients without infection at the time of HCT (18/18), and one could argue that with the availability of a geno-identical donor, HCT should be performed in all patients early in life, at a time when HCT is more easily tolerated, and prior to the development of complications which cannot be predicted by laboratory parameters. With the introduction of in vivo T-cell depletion using Alemtuzumab excellent outcomes have also been reported using matched unrelated donors (MUD) in 9/10 CGD patients undergoing largely myeloablative HCT [17]; as a consequence, the same arguments for performing HCT from a sibling donor could equally be applied to CGD patients with a closely matched unrelated donor.
Table 2.
Summary of HCT studies for CGD.
| Study | N | Regimen1 | Donor2 | OS3 | EFS3 |
|---|---|---|---|---|---|
| Segar et al [16] | 25 | MA | MSD | 85% | 81% |
| Güngör et al [18, 19] | 8 | RIC | MSD/MUD | 88% | 88% |
| Veys (Personal Communication) |
5 | RIC | MUD/MMUD | 100% | 100% |
| Horwitz et al. [23] | 10 | NMA | MSD | 70% | 70% |
| Kang (Personal Communicaiton) |
11 | NMA | MSD/MUD | 91% | 82% |
MA= myeloablative; RIC = reduced intensity condition; NMA = nonmyeloablative
MSD = matched sibling donor; MUD = matched unrelated donor; MMUD = 1 Antigen mismatched unrelated donor
OS = overall survival; EFS = event free survival
In contrast to HCT in the uncomplicated child, HCT during active infection (eg aspergillosis) or inflammation (eg colitis) is sometimes complicated by severe inflammatory reactions at the site of infection / inflammation [16]. Ideally, infections and inflammatory lesions should be brought under control prior to HCT but in chronically infected patients or patients with ongoing inflammation, morbidity may be reduced by employing less toxic conditioning regimens and including serotherapy with ATG or Alemtuzumab. HCT using reduced intensity conditioning (RIC) combining busulfan 8 -10 mg/kg (adjusted with busulfan kinetics in pediatric patients), fludarabine 180 mg/m2 and ATG 40 mg/kg and matched donors (MSD=5, MUD=3) was performed in 8 high-risk CGD patients. With this approach 90 -100% donor chimerism was achieved in all cases at a median follow-up of 26 months [18, 19]; this was despite the use of bone marrow rather than mobilized peripheral blood stem cells (PBSC) in 7/8 cases. Seven patients are alive and well and all active inflammatory and infectious foci are resolved. One adult patient who had received PBSC from a CMV negative MUD died on day +150 of CMV pneumonitis. An alternative RIC regimen (4 Gy of total body irradiation, cyclophosphamide 50 mg/kg and fludarabine 200 mg/m2) followed by two mismatched unrelated cord blood units in a single adult McLeod (K0 red cell) phenotype CGD patient with invasive aspergillosis also resulted in full donor engraftment and cure [20]. However, all 5 CGD patients who received Alemtuzumab and fludarabine in combination with melphalan 140mg/m2 [21] survived but sustained donor engraftment was achieved in only 2/5 [Gungor T 2008 - personal communication], suggesting that the risk of graft rejection may be increased in CGD patients with this protocol in comparison to other phagocytic disorders (Table 2). Busulfan may also be substituted with treosulfan [22], and RIC HCT employing Alemtuzumab, treosulfan 42g/m2 and fludarabine 150mg/m2 resulted in full donor chimerism and cure in 5/5 CGD patients undergoing matched or mismatched (single HLA antigen) unrelated donor HCT with mobilized peripheral blood stem cells. [Veys 2010 – personal communication] (Table 2). One of these children undergoing mismatched unrelated donor HCT had severe CGD complications prior to HCT including lung and cerebral aspergillosis, which has resolved completely post HCT.
Horwitz and colleagues [23] reported 10 patients with CGD who underwent HCT with minimal intensity conditioning comprising cyclophosphamide (120 mg/kg), fludarabine (125 mg/m2) and ATG (160 mg/kg), followed by transplant of CD34+-selected PBSCs from matched sibling donors (Table 2). Delayed donor lymphocyte infusions were given at intervals of 30 or more days to increase the level of donor chimerism. After a median follow-up of 17 months donor myeloid chimerism in 8/10 patients ranged from 33 to 100%, a level that could be expected to provide normal host defense. In 2 patients graft rejection occurred. Significant aGHVD developed in three of four adult patients with engraftment, one of whom subsequently had extensive cGVHD. Seven patients were reported to have survived from 16 to 26 months. Two patients died of transplant-related complications.
In an ongoing transplant study initiated in late 2007 at the NIH (Kang EM and colleagues, personal communication), 11 patients with CGD have been transplanted using a non-myeloablative regimen of busulfan plus Campath or ATG, along with low dose radiation for those receiving an unrelated donor product (Table 2). High dose sirolimus is used as GVHD prophylaxis. Two have had HLA matched sibling donors; 8 have received matched unrelated PBSC grafts; and one has had an unrelated cord blood. Of particular note is that 9 of the 11 patients had ongoing refractory infections, all of which are improving or have resolved following successful transplant. There has been one death (pre-existing renal failure unrelated to the transplant), only one graft failure (the cord blood), and only one Grade 2 or higher GVHD (Grade 2, skin only).
In the absence of a matched sibling or closely matched unrelated donor (\m=ge\ 7/8 HLA antigens), haploidentical or mismatched cord (<5/6 HLA antigens) HCT have been attempted but are probably too risky. In at least one reported case of a high risk CGD patient the family resorted to pre-implantation genetic diagnosis and HLA typing to create a “savior sibling” for successful HCT of a brother suffering from X-CGD. [24]. Unfortunately, at least in the U.S., this approach is not typically covered by 3rd party payers and thus, is not readily available to the general population. An alternative approach would be to pursue stem cell gene therapy (see below).
In conclusion, myeloablative HCT using a closely matched related or unrelated donor is a valid therapeutic option for children with CGD if performed early in life or at the first signs of a severe course of the disease. The use of RIC HCT awaits further evaluation, but combinations of ATG/busulfan/ fludarabine or Alemtuzuab/treosulfan/fludarabine appear to be particularly suitable for high-risk patients with CGD, and may eventually be utilized in more standard-risk patients if long-term donor myeloid engraftment can be secured.
Development of Gene Therapy for Chronic Granulomatous Disease (CGD)
Among the first uses of gene therapy for monogenic disorders that demonstrated unequivocal substantial long term clinical benefit were for two forms of severe combined immune deficiency, SCID-X1 (caused by mutations in the IL2RG gene) and ADA-SCID (caused by mutations in the gene encoding adenosine deaminase). The approaches taken to achieve successful gene therapy for SCID-X1 and ADA-SCID, and the problems encountered have had a substantial impact on informing the past and ongoing initiatives to develop clinically beneficial gene therapy for CGD.
The first clinical trial of gene therapy for CGD occurred in 1995 and was directed toward young adult CGD patients with the p47phox deficient autosomal recessive form of CGD [25]. The MFGS gene transfer vector (derived from murine Moloney leukemia virus) encoding p47phox cDNA was used to transduce peripheral blood mobilized autologous CD34+ cytokine-mobilized peripheral blood stem cells (PBSC) in a 4 day culture. Five patients were treated with a single cycle of gene therapy without any chemotherapy conditioning. Most received >0.5 × 106 transduced autologous CD34+ cells/kg. Based on the dihydrorhodamine flow cytometry (DHR) assay to measure reactive oxygen species (ROS) in individual neutrophils [26], all five patients had the appearance of <1:2000 oxidase normal neutrophils in the peripheral blood. Trace numbers of oxidase normal neutrophils persisted for only a few months, and no clinical benefit was demonstrated. This trial pioneered the first clinical use of a closed system of gas permeable flexible plastic bags for culture and transduction of CD34+ hematopoietic stem cells (HSC) [27].
In 1998 a clinical trial of similar design was initiated at the National Institutes of Health (NIH), also without conditioning, using the same MFGS retrovirus vector backbone encoding gp91phox and autologous CD34+ PBSC to treat 5 older teenage or young adult patients with X-linked CGD (X-CGD)[28]. The much higher titer of vector and the application of Retronectin® (fibronectin fragment) coating of the inner surface of the culture bags resulted in ex vivo transduction efficiencies of >60%. Furthermore, patients received 1-4 cycles of treatment of >10 × 106 transduced autologous CD34+ cells/kg/cycle. Most patients had transient appearance of up to 1:900 oxidase normal neutrophils, which persisted in the circulation for only a few months with equivocal clinical benefit. Despite extraordinarily high bulk transduction rates and the large number of transduced autologous CD34+ PBSC infused, gene marking never exceeded 0.2%. However, the individual gene marked neutrophils in the circulation appeared to produce normal levels of ROS. Thus, the critical problem appeared to be that in the absence of bone marrow conditioning, circulating gene-corrected neutrophils were very low and transient.
Concordant with this second CGD clinical trial, investigators in France had begun to treat infants with SCID-X1 using a very similar MFG vector backbone encoding the IL2RG gene cDNA to transduce bone marrow CD34+ HSC [29]. Despite the facts that the culture and transduction conditions for the SCID-X1 trial were similar to those in the two CGD trials, that rates of bulk ex vivo transduction averaged only 40%, that the number of transduced HSC infused were less than in the second CGD trial, that the SCID-X1 infants received only a single cycle of treatment, and that no conditioning was given, the clinical results were spectacularly better. The great majority of treated SCID-X1 infants developed normal numbers of functionally corrected T lymphocytes. There was some production of functionally normal B lymphocytes and even detection of some NK cells for a period of time. Investigators in London confirmed these results in a second trial [30]. The general consensus for why there was such a successful outcome with SCID-X1 is that the substantially “empty” T lymphocyte compartment in SCID-X1 provides a setting where gene corrected T lymphocytes, with no competition from host T lymphocytes, have no barrier to peripheral growth and can expand to fill this empty hematologic niche. For B lymphocytes the hematologic niche occupied by this cell type is not “empty”; as it is occupied by B cells that cannot mature to produce antibody, suggesting why the correction of B cell immunity in SCID-X1 is substantially less than for T cell immunity. Even this cannot be the full explanation because these investigators found a persistent modest level of gene marking in the myeloid compartment of the infants where there should be no selective growth advantage. One can speculate that infants likely have a high marrow turnover rate that additionally facilitates substantially more efficient engraftment of gene marked autologous HSC than older children or adults.
Of the 20 patients with SCID-X1 achieving substantial immune reconstitution in the Paris and London gene therapy studies, 5 have had vector insertional mutagenesis-associated clonal lymphocytic leukemia [31,32]. This has raised concerns about the safety of the genome insertion pattern of murine retrovirus vectors and in particular about the potential activation of nearby proto-oncogenes from this type of vector's long terminal repeat (LTR).
One of the earliest series of clinical trials of ex vivo gene therapy beginning in the early 1990's was the attempted treatment of children with ADA-SCID [33,34]. While there was prolonged gene marking, possibly some improvement of immune function, and possible clinical benefit, none of the patients achieved a profound outgrowth of gene corrected T lymphocytes, substantial improvements in immune function, or significant clinical benefit. In 2002 investigators from Italy reported the results of a clinical trial of gene therapy for ADA-SCID that did achieve significant immune reconstitution and clinical benefit by using non-myeloablative conditioning with low dose busulfan (4 mg/kg total) prior to infusion of gene corrected autologous CD34+ HSC [35]. This was a critical conceptual breakthrough demonstrating that in settings where selective growth advantage is less evident, marrow conditioning to enhance engraftment of gene corrected HSC can substantially improve the clinical outcome. These investigators also withheld enzyme replacement therapy with PEG-conjugated ADA in order to further enhance the growth advantage of gene corrected cells [36]. These results were confirmed by studies in London and the U.S. [37]. To date, no vector insertion mutagenesis related adverse events have been seen in the nearly 20 ADA-SCID patients treated with murine retrovirus gene therapy.
Gene correction for CGD should not provide any growth advantage either within the stem cell or myeloid compartments affected by the immune defect. Borrowing the lesson about conditioning from the ADA-SCID experience, investigators in Germany initiated a clinical trial of ex vivo gene therapy for X-CGD that incorporated non-myeloablative conditioning with a higher dose of busulfan (8 mg/kg total) to treat two adults [38]. They used retrovirus vector derived from murine spleen focus forming virus (SFFV vector) encoding the human gp91phox cDNA to transduced autologous CD34+ PBSC, achieving about 40% transduction efficiency. Initially, there was 20-30% gene marking with significant levels of ROS activity in circulating neutrophils. Unexpectedly, the level of gene corrected neutrophils increased over six months until gene marked neutrophils comprised more than half of the circulating neutrophils. However, this temporal increase in gene marking was associated with oligoclonal expansion. The predominant clone in both patients had the vector inserted into MDS1/EVI1, where activation of this gene was likely responsible for the expansion. Initially, this benefited these patients in that their infections cleared. However, over the next 2 1/2 years, both patients developed myelodysplasia with monosomy 7 and loss of oxidase function in the gene marked myeloid cells (silencing), though with persistence of activity of the SFFV enhancer sequence [39]. One patient died of severe sepsis likely related to the myelodysplasia, while the other was successfully treated with an allogeneic HSC transplant. Subsequently, a child with X-CGD achieved cure of a severe infection following gene therapy with the same SFFV vector, but without oligoclonal or clonal expansion [4]. Nonetheless, concern for the safety of the SFFV vector has been raised.
In 2006 the NIH group used the same MFGS-gp91phox vector to treat X-CGD as in their 1998-2001 study, but used conditioning with non-myeloablative busulfan (10 mg/kg total) before infusing the transduced autologous CD34+ HSC [40]. Interestingly, one patient in this trial previously had been treated in the 1999 trial with the same vector but without conditioning, affording an opportunity to evaluate the efficacy of busulfan conditioning. In 1999 this patient achieved gene marking of ~1:1500 (~0.75%) circulating neutrophils and the marking became undetectable after a few months. In 2006 this patient achieved gene marking of 24% of all circulating neutrophils at 3 weeks post infusion. This decreased to ~10% by three months and to ~1% at eight months after gene therapy. Strikingly, about 0.8% of circulating neutrophils in this patient are marked at almost 4 years after gene therapy (Figure 2). Of particular note is that the individual gene marked neutrophils still demonstrate normal production of ROS without any evidence for silencing. An important conclusion from this study and the CGD study from Germany is that busulfan 8-10 mg/kg is likely adequate to achieve the necessary level of engraftment of gene-transduced HSC to correct a disorder such as CGD where there is no selective growth advantage in gene-corrected cells. The critical need is to find a vector with a high safety profile and improved transduction efficiency of HSC.
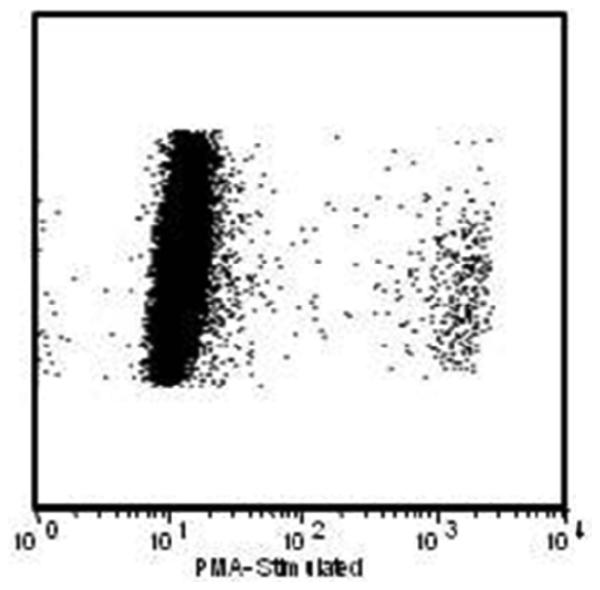
Figure 2.
Dihydrorhodamine flow cytometry dot plot analysis of oxidase activity in phorbol ester stimulated circulating blood neutrophils of a patient in the NIH study with X-CGD who had busulfan conditioning followed by infusion of autologous CD34+ HSC transduced with MFGS-gp91phox vector 3 years and 9 months prior to this analysis. The analysis is gated to show only neutrophils where Y-axis is side-scatter and X-axis is fluorescence intensity. Note that the 0.8% of neutrophils producing ROS has intensity of fluorescence equal to that seen in normal neutrophils.
Most of the current gene therapy research focus for CGD is on lentivirus vectors because they may have a safer pattern of preferred insertion sites than murine retrovirus vectors. More important, lentivectors are more easily designed to incorporate 3′ LTR deletions resulting in self-inactivation of the LTR at the 5′ end following insertion into the genome of the target cell [41]. This self-inactivating structure of the current generation of lentivectors may be intrinsically safer with respect to insertional mutagenesis mediated adverse events than the standard murine retrovirus vectors. However, more clinical experience with self-inactivating lentivirus vectors is necessary.
The recent report of clinically beneficial functional correction of X-linked adrenoleukodystrophy with high level persistent gene marking of myeloid cells using a lentivirus vector provides compelling evidence that they may result in intrinsically higher levels of gene transfer into HSC [42]. Furthermore, in the adrenoleukodystrophy study, myeloablative conditioning was used and some have concluded that for future gene therapy trials for disorders with no intrinsic selective growth advantage conferred by gene correction, e.g. CGD, that not only should lentivectors be used, but that myeloablative conditioning is necessary. The counter-argument is that CGD patients who are candidates for gene therapy likely have ongoing infections, making use of ablative conditioning an unacceptable risk; and that previous trials of gene therapy for CGD suggest that sub-ablative conditioning may be sufficient. In addition, the use of myeloablative chemotherapy and resulting increase in early and late side effects reduces at least some of the attraction of the gene theapy approach compared to allogeneic HCT.
Self-inactivating lentivectors require the construct to have an internal promoter to drive production of the therapeutic transgene. The strongest internal promoters are virus promoters, but for safety reasons an adequately functioning mammalian gene promoter is preferable for CGD. This is problematic in designing lentivectors for X-CGD because most mammalian internal promoters to date are not sufficiently active to drive adequate production of gp91phox from the transgene to achieve functional correction of gene marked X-CGD neutrophils. Codon optimization of the gp91phox cDNA seems to help [43], but this alone is insufficient. Current effort in lentivector development for X-CGD is focused on finding the best internal promoter to achieve functional correction of the gene marked neutrophil. An example of a candidate lentivector under development to treat X-CGD is shown in Figure 3.
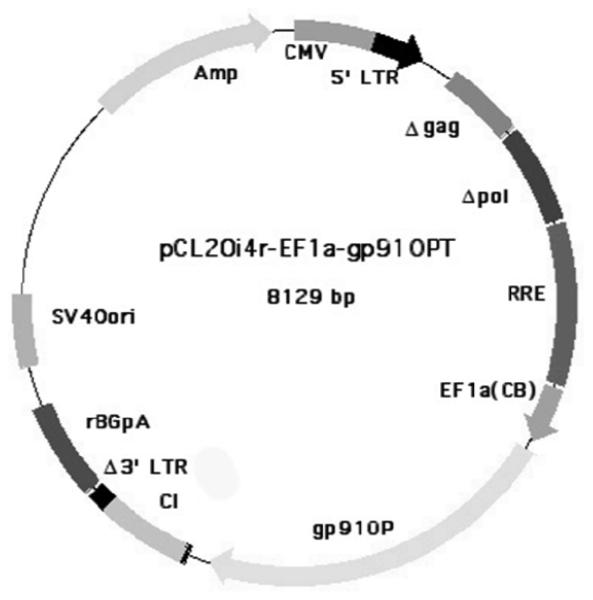
Figure 3.
Candidate clinical insulated self-inactivating lentivirus vector plasmid pCL20i4r-EF1a-gp91OPT under development for treatment of X-CGD (unpublished data: HL Malech, EM Kang, U Choi, SS De Ravin, BP Sorrentino, RE Throm, and JT Gray). Shown is the vector plasmid indicating that gag and pol and the 3′ LTR all have inactivating deletions; tat, rev, env and other elements have been removed; 400 bp portion of the chicken H4 globin insulator has been added (CI); a short version of the human Elongation factor 1 alpha has been added as the internal promoter; and the gp91phox cDNA has been codon optimized (gp91OPT). The structure is based upon the vector described by Zhou et al. [44]. A producer cell line is also being developed based upon what is described in Throm et al [45].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Winkelstein JA, Marino MC, Johnston RB, Jr, Boyle J, Curnutte J, et al. Chronic granulomatous disease: report on a national registry of 368 patients. Medicine (Baltimore) 2000;79:155–169. doi: 10.1097/00005792-200005000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Reeves EP, Lu H, Jacobs HL, Messina CG, Bolsover S, et al. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature. 2002;416:291–297. doi: 10.1038/416291a. [DOI] [PubMed] [Google Scholar]
- 3.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 4.Bianchi M, Hakkim A, Brinkmann V, Siler U, Seger RA, et al. Restoration of NET formation by gene therapy in CGD controls aspergillosis. Blood. 2009;114:2619–2622. doi: 10.1182/blood-2009-05-221606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, et al. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van den Berg JM, van Koppen E, Ahlin A, Belohradsky BH, Bernatowska E, et al. Chronic granulomatous disease: the European experience. PLoS ONE. 2009;4:e5234. doi: 10.1371/journal.pone.0005234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marciano BE, Rosenzweig SD, Kleiner DE, Anderson VL, Darnell DN, et al. Gastrointestinal involvement in chronic granulomatous disease. Pediatrics. 2004;114:462–468. doi: 10.1542/peds.114.2.462. [DOI] [PubMed] [Google Scholar]
- 8.Siddiqui S, Anderson VL, Hilligoss DM, Abinun M, Kuijpers TW, et al. Fulminant mulch pneumonitis: an emergency presentation of chronic granulomatous disease. Clin Infect Dis. 2007;45:673–681. doi: 10.1086/520985. [DOI] [PubMed] [Google Scholar]
- 9.Morgenstern DE, Gifford MA, Li LL, Doerschuk CM, Dinauer MC. Absence of respiratory burst in X-linked chronic granulomatous disease mice leads to abnormalities in both host defense and inflammatory response to Aspergillus fumigatus. J Exp Med. 1997;185:207–218. doi: 10.1084/jem.185.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romani L, Fallarino F, De Luca A, Montagnoli C, D'Angelo C, et al. Defective tryptophan catabolism underlies inflammation in mouse chronic granulomatous disease. Nature. 2008;451:211–215. doi: 10.1038/nature06471. [DOI] [PubMed] [Google Scholar]
- 11.Segal BH, Han W, Bushey JJ, Joo M, Bhatti Z, et al. NADPH oxidase limits innate immune responses in the lungs in mice. PLoS ONE. 2010;5:e9631. doi: 10.1371/journal.pone.0009631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallin JI, Alling DW, Malech HL, Wesley R, Koziol D, Marciano B, Eisenstein EM, Turner ML, DeCarlo ES, Starling JM, Holland SM. Itraconazole to prevent fungal infections in chronic granulomatous disease. N Engl J Med. 2003 Jun 12;348(24):2416–22. doi: 10.1056/NEJMoa021931. [DOI] [PubMed] [Google Scholar]
- 13.The International Chronic Granulomatous Disease Cooperative Study Group A Controlled Trial of Interferon Gamma to Prevent Infection in Chronic Granulomatous Disease. N Engl J Med. 1991;324:509–516. doi: 10.1056/NEJM199102213240801. [DOI] [PubMed] [Google Scholar]
- 14.Segal BH, DeCarlo ES, Kwon-Chung KJ, Malech HL, Gallin JI, et al. Aspergillus nidulans infection in chronic granulomatous disease. Medicine (Baltimore) 1998;77:345–354. doi: 10.1097/00005792-199809000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Seger RA. Modern management of chronic granulomatous disease. British Journal of Haematology. 2008;140(3):255–266. doi: 10.1111/j.1365-2141.2007.06880.x. [DOI] [PubMed] [Google Scholar]
- 16.Seger RA, Gungor T, Belohradsky BH, Blanche S, Bordigoni P, Di Bartolomeo P, Flood T, Landais P, Müller S, Ozsahin H, Passwell JH, Porta F, Slavin S, Wulffraat N, Zintl F, Nagler A, Cant A, Fischer A. Treatment of chronic granulomatous disease with myeloablative conditioning and an unmodified hemopoietic allograft: a survey of the European experience, 1985-2000. Blood. 2002 Dec 15;100(13):4344–50. doi: 10.1182/blood-2002-02-0583. [DOI] [PubMed] [Google Scholar]
- 17.Soncini E, Slatter MA, Jones LB, Hughes S, Hodges S, Flood TJ, Barge D, Spickett GP, Jackson GH, Collin MP, Abinun M, Cant AJ, Gennery AR. Unrelated donor and HLA-identical sibling haematopoietic stem cell transplantation cure chronic granulomatous disease with good long-term outcome and growth. Br J Haematol. 2009 Apr;145(1):73–83. doi: 10.1111/j.1365-2141.2009.07614.x. Epub 2009 Feb 16. [DOI] [PubMed] [Google Scholar]
- 18.Güngör T, Halter J, Klink A, et al. Successful low toxicity hematopoietic stem cell transplantation for high-risk adult chronic granulomatous disease patients. Transplantation. 2005;79(11):1596–606. doi: 10.1097/01.tp.0000163466.73485.5e. [DOI] [PubMed] [Google Scholar]
- 19.Güngör T, Halter J, Stussi G, Scherer F, Schanz U, Seger R. Successful busulphan-based reduced intensity conditioning in high-risk paediatric and adult chronic granulomatous disease – The Swiss experience. Bone Marrow Transplant. 2009;43(suppl 1):S75. abstract. [Google Scholar]
- 20.Suzuki N, Hatakeyama N, Yamamoto M, Mizue N, Kuroiwa Y, Yoda M, Takahashi J, Tani Y, Tsutsumi H. Treatment of McLeod phenotype chronic granulomatous disease with reduced-intensity conditioning and unrelated-donor umbilical cord blood transplantation. Int J Hematol. 2007;85(1):70–2. doi: 10.1532/IJH9706129. [DOI] [PubMed] [Google Scholar]
- 21.Rao K, Amrolia PJ, Jones A, Cale CM, Naik P, King D, Davies GE, Gaspar HB, Veys PA. Improved survival after unrelated donor bone marrow transplant in children with primary immunodeficiency using a reduced intensity conditioning regimen. Blood. 2005;105:879–85. doi: 10.1182/blood-2004-03-0960. [DOI] [PubMed] [Google Scholar]
- 22.Greystoke B, Bonanomi S, Carr TF, Gharib M, Khalid T, Coussons M, Jagani M, Naik P, Rao K, Goulden N, Amrolia P, Wynn RF, Veys PA. Treosulfan-containing regimens achieve high rates of engraftment associated with low transplant morbidity and mortality in children with non-malignant disease and significant co-morbidities. Br J Haematol. 2008 Jun;142(2):257–62. doi: 10.1111/j.1365-2141.2008.07064.x. [DOI] [PubMed] [Google Scholar]
- 23.Horwitz ME, Barrett AJ, Brown MR, et al. Treatment of chronic granulomatous disease with nonmyeloablative conditioning and a T-cell-depleted hematopoietic allograft. N Engl J Med. 2001;344(12):881–8. doi: 10.1056/NEJM200103223441203. [DOI] [PubMed] [Google Scholar]
- 24.Duke K. Belgian loophole allows Swiss parents a “saviour” baby. Lancet. 2006 Jul 29;368(9533):355–6. doi: 10.1016/S0140-6736(06)69089-2. [DOI] [PubMed] [Google Scholar]
- 25.Malech HL, Maples PB, Whiting-Theobald N, Linton GF, Sekhsaria S, Vowells SJ, Li F, Miller JA, DeCarlo E, Holland SM, Leitman SF, Carter CS, Butz RE, Read EJ, Fleisher TA, Schneiderman RD, Van Epps DE, Spratt SK, Maack CA, Rokovich JA, Cohen LK, Gallin JI. Prolonged production of NADPH oxidase-corrected granulocytes after gene therapy of chronic granulomatous disease. Proc Natl Acad Sci U S A. 1997 Oct 28;94(22):12133–8. doi: 10.1073/pnas.94.22.12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vowells SJ, Fleisher TA, Sekhsaria S, Alling DW, Maguire TE, Malech HL. Genotype-dependent variability in flow cytometric evaluation of reduced nicotinamide adenine dinucleotide phosphate oxidase function in patients with chronic granulomatous disease. J Pediatr. 1996 Jan;128(1):104–7. doi: 10.1016/s0022-3476(96)70437-7. [DOI] [PubMed] [Google Scholar]
- 27.Malech HL. Use of serum-free medium with fibronectin fragment enhanced transduction in a system of gas permeable plastic containers to achieve high levels of retrovirus transduction at clinical scale. Stem Cells. 2000;18(2):155–6. doi: 10.1634/stemcells.18-2-155. [DOI] [PubMed] [Google Scholar]
- 28.Malech HL, Choi U, Brenner S. Progress toward effective gene therapy for chronic granulomatous disease. Jpn J Infect Dis. 2004 Oct;57(5):S27–8. [PubMed] [Google Scholar]
- 29.Hacein-Bey-Abina S, Le Deist F, Carlier F, Bouneaud C, Hue C, De Villartay JP, Thrasher AJ, Wulffraat N, Sorensen R, Dupuis-Girod S, Fischer A, Davies EG, Kuis W, Leiva L, Cavazzana-Calvo M. Sustained correction of X-linked severe combined immunodeficiency by ex vivo gene therapy. N Engl J Med. 2002 Apr 18;346(16):1185–93. doi: 10.1056/NEJMoa012616. [DOI] [PubMed] [Google Scholar]
- 30.Gaspar HB, Parsley KL, Howe S, King D, Gilmour KC, Sinclair J, Brouns G, Schmidt M, Von Kalle C, Barington T, Jakobsen MA, Christensen HO, Al Ghonaium A, White HN, Smith JL, Levinsky RJ, Ali RR, Kinnon C, Thrasher AJ. Gene therapy of X-linked severe combined immunodeficiency by use of a pseudotyped gammaretroviral vector. Lancet. 2004 Dec 18-31;364(9452):2181–7. doi: 10.1016/S0140-6736(04)17590-9. [DOI] [PubMed] [Google Scholar]
- 31.Hacein-Bey-Abina S, Garrigue A, Wang GP, Soulier J, Lim A, Morillon E, Clappier E, Caccavelli L, Delabesse E, Beldjord K, Asnafi V, MacIntyre E, Dal Cortivo L, Radford I, Brousse N, Sigaux F, Moshous D, Hauer J, Borkhardt A, Belohradsky BH, Wintergerst U, Velez MC, Leiva L, Sorensen R, Wulffraat N, Blanche S, Bushman FD, Fischer A, Cavazzana-Calvo M. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J Clin Invest. 2008 Sep;118(9):3132–42. doi: 10.1172/JCI35700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Howe SJ, Mansour MR, Schwarzwaelder K, Bartholomae C, Hubank M, Kempski H, Brugman MH, Pike-Overzet K, Chatters SJ, de Ridder D, Gilmour KC, Adams S, Thornhill SI, Parsley KL, Staal FJ, Gale RE, Linch DC, Bayford J, Brown L, Quaye M, Kinnon C, Ancliff P, Webb DK, Schmidt M, von Kalle C, Gaspar HB, Thrasher AJ. Insertional mutagenesis combined with acquired somatic mutations causes leukemogenesis following gene therapy of SCID-X1 patients. J Clin Invest. 2008 Sep;118(9):3143–50. doi: 10.1172/JCI35798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blaese RM, Culver KW, Miller AD, Carter CS, Fleisher T, Clerici M, Shearer G, Chang L, Chiang Y, Tolstoshev P, Greenblatt JJ, Rosenberg SA, Klein H, Berger M, Mullen CA, Ramsey WJ, Muul L, Morgan RA, Anderson WF. T lymphocyte-directed gene therapy for ADA- SCID: initial trial results after 4 years. Science. 1995 Oct 20;270(5235):475–80. doi: 10.1126/science.270.5235.475. [DOI] [PubMed] [Google Scholar]
- 34.Bordignon C, Notarangelo LD, Nobili N, Ferrari G, Casorati G, Panina P, Mazzolari E, Maggioni D, Rossi C, Servida P, Ugazio AG, Mavilio F. Gene therapy in peripheral blood lymphocytes and bone marrow for ADA- immunodeficient patients. Science. 1995 Oct 20;270(5235):470–5. doi: 10.1126/science.270.5235.470. [DOI] [PubMed] [Google Scholar]
- 35.Aiuti A, Slavin S, Aker M, Ficara F, Deola S, Mortellaro A, Morecki S, Andolfi G, Tabucchi A, Carlucci F, Marinello E, Cattaneo F, Vai S, Servida P, Miniero R, Roncarolo MG, Bordignon C. Correction of ADA-SCID by stem cell gene therapy combined with nonmyeloablative conditioning. Science. 2002 Jun 28;296(5577):2410–3. doi: 10.1126/science.1070104. [DOI] [PubMed] [Google Scholar]
- 36.Aiuti A, Cattaneo F, Galimberti S, Benninghoff U, Cassani B, Callegaro L, Scaramuzza S, Andolfi G, Mirolo M, Brigida I, Tabucchi A, Carlucci F, Eibl M, Aker M, Slavin S, Al-Mousa H, Al Ghonaium A, Ferster A, Duppenthaler A, Notarangelo L, Wintergerst U, Buckley RH, Bregni M, Marktel S, Valsecchi MG, Rossi P, Ciceri F, Miniero R, Bordignon C, Roncarolo MG. Gene therapy for immunodeficiency due to adenosine deaminase deficiency. N Engl J Med. 2009 Jan 29;360(5):447–58. doi: 10.1056/NEJMoa0805817. [DOI] [PubMed] [Google Scholar]
- 37.Gaspar HB, Bjorkegren E, Parsley K, Gilmour KC, King D, Sinclair J, Zhang F, Giannakopoulos A, Adams S, Fairbanks LD, Gaspar J, Henderson L, Xu-Bayford JH, Davies EG, Veys PA, Kinnon C, Thrasher AJ. Successful reconstitution of immunity in ADA-SCID by stem cell gene therapy following cessation of PEG-ADA and use of mild preconditioning. Mol Ther. 2006 Oct;14(4):505–13. doi: 10.1016/j.ymthe.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 38.Ott MG, Schmidt M, Schwarzwaelder K, Stein S, Siler U, Koehl U, Glimm H, Kühlcke K, Schilz A, Kunkel H, Naundorf S, Brinkmann A, Deichmann A, Fischer M, Ball C, Pilz I, Dunbar C, Du Y, Jenkins NA, Copeland NG, Lüthi U, Hassan M, Thrasher AJ, Hoelzer D, von Kalle C, Seger R, Grez M. Correction of X-linked chronic granulomatous disease by gene therapy, augmented by insertional activation of MDS1-EVI1, PRDM16 or SETBP1. Nat Med. 2006 Apr;12(4):401–9. doi: 10.1038/nm1393. [DOI] [PubMed] [Google Scholar]
- 39.Stein S, Ott MG, Schultze-Strasser S, Jauch A, Burwinkel B, Kinner A, Schmidt M, Krämer A, Schwäble J, Glimm H, Koehl U, Preiss C, Ball C, Martin H, Göhring G, Schwarzwaelder K, Hofmann WK, Karakaya K, Tchatchou S, Yang R, Reinecke P, Kühlcke K, Schlegelberger B, Thrasher AJ, Hoelzer D, Seger R, von Kalle C, Grez M. Genomic instability and myelodysplasia with monosomy 7 consequent to EVI1 activation after gene therapy for chronic granulomatous disease. Nat Med. 2010 Feb;16(2):198–204. doi: 10.1038/nm.2088. [DOI] [PubMed] [Google Scholar]
- 40.Kang EM, Choi U, Theobald N, Linton G, Long Priel DA, Kuhns D, Malech HL. Retrovirus gene therapy for X-linked chronic granulomatous disease can achieve stable long-term correction of oxidase activity in peripheral blood neutrophils. Blood. 2010 Jan 28;115(4):783–91. doi: 10.1182/blood-2009-05-222760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Montini E, Cesana D, Schmidt M, Sanvito F, Bartholomae CC, Ranzani M, Benedicenti F, Sergi LS, Ambrosi A, Ponzoni M, Doglioni C, Di Serio C, von Kalle C, Naldini L. The genotoxic potential of retroviral vectors is strongly modulated by vector design and integration site selection in a mouse model of HSC gene therapy. J Clin Invest. 2009 Apr;119(4):964–75. doi: 10.1172/JCI37630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cartier N, Hacein-Bey-Abina S, Bartholomae CC, Veres G, Schmidt M, Kutschera I, Vidaud M, Abel U, Dal-Cortivo L, Caccavelli L, Mahlaoui N, Kiermer V, Mittelstaedt D, Bellesme C, Lahlou N, Lefrère F, Blanche S, Audit M, Payen E, Leboulch P, l'Homme B, Bougnères P, Von Kalle C, Fischer A, Cavazzana-Calvo M, Aubourg P. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science. 2009 Nov 6;326(5954):818–23. doi: 10.1126/science.1171242. [DOI] [PubMed] [Google Scholar]
- 43.Moreno-Carranza B, Gentsch M, Stein S, Schambach A, Santilli G, Rudolf E, Ryser MF, Haria S, Thrasher AJ, Baum C, Brenner S, Grez M. Transgene optimization significantly improves SIN vector titers, gp91phox expression and reconstitution of superoxide production in X-CGD cells. Gene Ther. 2009 Jan;16(1):111–8. doi: 10.1038/gt.2008.143. [DOI] [PubMed] [Google Scholar]
- 44.Zhou S, Mody D, DeRavin SS, Hauer J, Lu T, Ma Z, Hacein-Bey Abina S, Gray JT, Greene MR, Cavazzana-Calvo M, Malech HL, Sorrentino BP. A self-inactivating lentiviral vector for SCID-X1 gene therapy that does not activate LMO2 expression in human T cells. Blood. 2010 Aug 12;116(6):900–8. doi: 10.1182/blood-2009-10-250209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Throm RE, Ouma AA, Zhou S, Chandrasekaran A, Lockey T, Greene M, De Ravin SS, Moayeri M, Malech HL, Sorrentino BP, Gray JT. Efficient construction of producer cell lines for a SIN lentiviral vector for SCID-X1 gene therapy by concatemeric array transfection. Blood. 2009 May 21;113(21):5104–10. doi: 10.1182/blood-2008-11-191049. [DOI] [PMC free article] [PubMed] [Google Scholar]