Synopsis
Converging and replicated evidence indicate that psychological stress can modulate wound healing processes. This article reviews the methods and findings of experimental models of wound healing. Psychological stress can have a substantial and clinically relevant impact on wound repair. Physiological stress responses can directly influence wound healing processes. Furthermore, psychological stress can indirectly modulate the repair process by promoting the adoption of health-damaging behaviors. Translational work is needed to develop innovative treatments able to attenuate stress-induced delays in wound healing.
Keywords: wound healing, stress, cytokine, cortisol, psychoneuroimmunology, oxytocin
Wound healing is a critical process involved in the recovery from injury and surgical procedures. Poor healing increases the risk for wound infections or complications, lengthens hospital stays, magnifies patient discomfort, and slows return to activities of daily living. Converging evidence from different research paradigms suggest that psychological stress and other behavioral factors can affect wound healing. A meta-analytic study using diverse wound healing models and outcomes found that across studies there was an average correlation of −.42 between psychological stress and wound healing [1]. This suggests that the relationship between stress and wound repair is not only statistically significant, but also clinically relevant. This review presents data and methods from observational, experimental, and interventional studies corroborating the impact of stress on wound healing. Potential behavioral and physiological mechanisms explaining the association between stress and impaired wound healing are also discussed.
Observational studies
Prospective studies examining wound healing-related complications following surgery provide evidence for the impact of stress on wound repair. Greater fear or distress prior to surgery has been associated with poorer outcomes including longer hospital stays, more postoperative complications, and higher rates of rehospitalization [2,3]. For example, among 111 patients undergoing gallstone removal surgery, those who reported more stress on the third postoperative day had a longer hospital stay, compared to less anxious individuals[4]. Among 309 consenting consecutive patients who underwent an elective coronary artery bypass graft surgery, patients who were more optimistic were less likely to be re-hospitalized than less optimistic individuals. Conversely, patients who experienced more depressive symptoms were more likely to require rehospitalization for infection-related complications than individuals reporting less distress [5]. This result was replicated in a study of 72 patients undergoing coronary artery bypass surgery. Patient who had more depressive symptoms at discharge had more infections and poorer wound healing in the following 6 weeks after surgery, compared to participants who reported less distress [6].
Psychological factors can also modulate healing of chronic wounds. Fifty-three older adults with chronic lower leg wounds were followed longitudinally to assess speed of wound repair. Patients who experienced the highest levels of depression and anxiety (based on a median split of the Hospital Anxiety and Depression Scale) were 4 times more likely to be categorized in the delayed healing group, compared to individuals who reported less distress [7]. Importantly, in these observational studies, distress predicted wound healing outcomes over and above differences in sociodemographic variables and medical status. Psychological distress thus appears to influence recovery from medical procedures and healing of chronic wounds in clinical settings.
Experimental studies
Animal and human studies in which standard wounds are created experimentally and healing is closely monitored over time provide the strongest evidence of the impact of stress on wound repair. Three main wounding methodologies have been used to study the effect of stress on wound healing.
Punch biopsy model
Punch biopsies are used to create standard full-thickness dermal wounds as well as mucosal wounds. Daily pictures of the wound allow for a quantification of changes in wound size over time.
The first human experimental study examining the impact of stress on wound healing involved family dementia caregivers. Caregivers have to deal daily with the loss of memory, inappropriate emotions, and wandering and restless behavior of their loved ones. Caregiving stress has been associated with heightened anxiety and depression, immune dysregulation, increased risk for cardiovascular disorders, and even death [8]. Family dementia caregiving thus represents an excellent model of chronic stress in humans. A 3.5 mm punch biopsy wound was created on the nondominant forearm of 13 women caregivers and 13 sociodemographically-similar noncaregiving controls. Caregivers took 24% longer to heal the small, standardized dermal wound than matched controls, providing initial evidence that chronic stress can delay wound repair [9].
Stress can also impede healing of a punch biopsy wound among younger people who experienced less intense stress. Twenty-four healthy young men were followed for 21 days after a standard 4 mm punch biopsy was performed on their forearm. In that study, wound healing was assessed using ultrasound biomicroscopy. Stress levels were measured using a self-report questionnaire, the Perceived Stress Scale. Higher perceived stress on the day of the biopsy was associated with slower wound healing [10]. A substantial correlation of −.59 was found between perceived stress and healing progress between the days 7 and 21 after the biopsy [10].
Pain, a physical and psychological stressor, can also influence wound healing. A 2 mm full thickness wound was placed on the back of one upper arm of obese women prior to receiving elective gastric bypass surgery. Greater acute pain immediately after surgery and persistent pain in the 4 weeks following surgery were associated with slower healing of the experimental wound [11]. Pain generates psychological distress, and, when compounded by the presence of other stressors, can put a person at increased risk for delayed wound repair [12].
Well-controlled animal studies corroborate the impact of stress on wound healing observed in humans. Mice subjected to restraint stress healed a standardized 3.5 mm full-thickness punch biopsy wound on average of 27% more slowly than control mice who were not exposed to the stressor [13]. Restraint stress was also associated with delayed wound healing in a reptilian species, the Urosaurus ornatus (tree lizard) [14]. Social stressors can also impair wound healing. Monogamous California mice, Peromyscus californicus, healed a punch biopsy wound more slowly when stressed by the separation from their conspecifics, compared to when they were continuously housed with their conspecifics [15].
Like cutaneous wounds, mucosal wound healing is also responsive to psychological stress, as demonstrated by a study with academic examination stress. Using a within-subject design, 11 dental students had a biopsy performed on their hard palate during their summer vacation and again 3 days before a major examination. Mucosal wounds placed before the examination healed on average 40% more slowly than identical wounds made during summer vacation. Importantly, the differences in the rate of healing were very consistent: no student healed as rapidly during examinations as during vacation [16].
The impact of negative emotions on mucosal wound healing was replicated in a larger study. Among 193 healthy undergraduate students who received a 3.5 mm wound on the hard palate, individuals reporting high levels of depressive symptoms were almost 3.6 times more likely to be classified as slow healers, compared to less dysphoric students [17].
Blister wounds model
The blister wounds model is another experimental paradigm to study the impact of psychological factors on wound healing. Blisters wounds are produced by the application of a vacuum pump on the forearm. A gentle suction creates a separation of the epidermis from the dermis over the course of one hour. One of the strengths of this method is that it allows for the collection of data on cytokine production at the wound site, as described below. In this model, wound healing is assessed via measurement of the rate of transepidermal water loss (TEWL). One of the main functions of the skin is to limit movement of water in and out of the body. The permeability of the epidermis increases after the blister wound, but decreases as healing process unfolds. A computerized evaporimetry instrument can measure vapor pressure gradient in the air layers close to the skin surface. TEWL measurement is a noninvasive method to monitor changes in the stratum corneum barrier function of the skin that provides an excellent objective method for evaluation of wound healing.
Using a blister wounds paradigm, the discussion of a marital disagreement, a commonplace stressor, delayed wound repair. Married couples were invited for two 24-hour admissions at a hospital research unit. During both visits, eight 8 mm suction blisters were created on the participants' nondominant forearm. Wound healing was monitored for 14 days using TEWL measurements. During the first admission, couples participated in a structured social support interaction task. During the second visit, couples were asked to discuss marital disagreements during a 30-minute period. After both interaction tasks, couples remained in the research unit until the next morning to allow for cytokine measurements and to minimize external influences on wound healing [18].
Couples' blister wounds healed more slowly following the marital conflict visit than after the social support visit, suggesting that the stress induced by the discussion of marital disagreements interfered with wound repair. Furthermore, the quality of the discussion also influenced the rate of healing. Couples who had more hostile and negative interactions across both the support and the conflict discussions healed wounds more slowly than couples whose interactions were less negative. The overall differences related to hostility were substantial. The blister wounds in high hostile couples healed at only 60% of the rate of low hostile couples [18].
In a different subset of participants from the same study, positive behaviors during the social support task were also related to wound repair. Individuals who displayed more self-disclosure, acceptance of their partner, relationship-enhancing statements, and humour during the interaction task healed the blister wounds faster than participants who exhibited less positive behaviors during the marital interaction task [19].
Difficulties in managing one's anger has also been associated with impaired wound healing. Blister wounds were created on the forearm of 98 community-dwelling participants who were followed for 14 days to monitor healing speed. Anger management styles were assessed via a self-report questionnaire, the Spielberger Anger Expression Scale. Participants who had difficulty controlling the expression of their anger were 4.2 times more likely to be classified as slow healers than individuals who reported better anger control. Furthermore, individuals with anger management issues secreted more cortisol in response to the blistering procedure. The increased glucocorticoid production was in turn related to delayed healing [20].
Tape stripping to disrupt skin barrier function
Another wound healing model consists of the repeated application of cellophane tape to remove a layer of epidermis cells, causing a disruption of the stratum corneum barrier function of the skin. This procedure impacts epidermal permeability. Wound healing is assessed by measuring the rate of recovery of the skin barrier function using TEWL measurements.
Acute laboratory stressors can delay the recovery of skin barrier function following its disruption by tape stripping. Twenty-five women participated in the Trier Social Stress Test (TSST), a psychosical stressor [21]. The TSST, a standardized laboratory stressor with a mock job interview and a mental arithmetic task, induces reliable changes in heart rate, cortisol and cytokine production, and subjective anxiety responses [22,23]. Skin barrier repair was delayed in women after the TSST, compared to a stress-free period [21].
This result was replicated in a larger study of 85 healthy young men and women. Individuals who participated in the TSST had a slower recovery of skin barrier function than participants who engaged in a reading control task [24]. Furthermore, positive affect had a protective effect on stress-induced delays in skin barrier recovery. Stressed individuals reporting more positive affect recovered faster from the tape stripping procedure than stressed participants who had low trait positive affect [25].
Academic examination stress impacts skin barrier recovery. Twenty-seven professional and medical students underwent a tape stripping procedure at 3 occasions: right after their winter and spring vacations, and during their winter final examination week. Skin barrier recovery was significantly delayed at 3, 6, and 24 hours after tape stripping during the examination period, compared to the two vacation periods. Furthermore, students reporting the most stress during the examination period had slower recovery in skin barrier function, compared to participants who experienced less examination-induced stress [26].
The interpersonal stress associated with the dissolution of a committed marital relationship can impede recovery of the stratum corneum barrier function of the skin. Twenty-eight women who were going through a divorce or a separation and 27 women who reported high levels of marital satisfaction underwent a tape stripping procedure on both facial cheeks. Socially stressed women had delayed skin barrier recovery at 3 and 24 hours following the tape stripping procedure, compared to less stressed women [27].
In animal models, different types of stressors can also impair skin barrier recovery. Three days of immobilization stress delayed skin barrier function recovery even for 7 days, compared to control rats not exposed to the stressor [28]. Social reorganization stress associated with cage transfer also impaired the restoration of skin barrier function in rats [29]. These results converge with human data indicating that psychological stress can disrupt skin barrier recovery.
Intervention studies
Intervention studies that improve healing outcomes by reducing psychological stress provide further evidence of the impact of psychological and behavioral factors in wound repair. Meta-analyses of clinical studies show that behavioral stress management interventions before surgery have been associated with improved post-operative outcomes, including fewer medical complications and shorter hospital stays [30,31].
Written emotional disclosure interventions can decrease psychological distress, improve self-reported health, enhance aspects of cellular immunity, and decrease health care utilization [32]. Men were randomized to a written emotional disclosure intervention or a non-intervention control group, and received a punch biopsy on the nondominant forearm. Healing was assessed using ultrasound biomicropsy at 3 occasions during a 21-day period. Men who participated in the emotional disclosure intervention had smaller wounds at 14 and 21 days, compared to control participants [33].
Physical exercise can reduce psychological distress in addition to improving cardiovascular function [34]. Older adults were randomized to an exercise intervention (one-hour aerobic exercise session, 3 times per week) or a non-intervention control group. One month after the beginning of the intervention, participants received a 3.5 mm punch biopsy on the back of their nondominant upper arm. Older adults who exercised healed their wounds faster than those in the control group [35]. In accord with these human data, older mice randomized to a 30-minute daily exercise period during 8 days healed a punch biopsy wound faster than sedentary control mice [36].
Social support is associated with better health outcomes [37]. In animal studies, monogamous rodents who were housed in pairs healed a standard punch biopsy wound faster than rodents housed alone [38]. Pair housing also buffered the impact of restraint stress on wound healing. Immobilization stress impaired cutaneous wound healing in Siberian hamsters housed alone, but not in hamsters housed in pairs [39]. These data indicate that the presence of a familial conspecific improves wound healing outcomes in monogamous rodents.
A pharmacological agent commonly used in the treatment of mood and anxiety disorders, fluoxetine [40]. In a study using alternating isolation and crowding stress, stressed Wistar rats who received fluoxetine healed at a similar pace as their non-stress counterparts, and faster than stressed animals who received only a vehicle injection [41]. These results indicate that pharmacological stress reduction may also improve wound healing.
In summary, a wide array of acute and chronic stressors can disrupt the healing process. Furthermore, the impact of stress on wound repair has been observed across different methodologies and with different healing outcomes and most results have replicated in at least two independent laboratories. Collectively, results from observation, experimental, and intervention studies provide strong evidence that psychological stress can influence wound healing.
Biology of wound healing
A brief review of the biology of wound healing is presented to highlight the pathways by which psychological stress can impede the repair process. Wound healing progresses through several overlapping stages [42]. In the initial inflammatory stage, vasoconstriction and blood coagulation are followed by platelet activation and the release of platelet-derived growth factors (PDGFs) as well as chemoattractant factors released by injured parenchymal cells. Cytokines and chemokines, such as IL-1α, IL-1β, transforming growth factor-β (TGF-β), vascular endothelial growth factor (VEGF), tumor necrosis factor alpha (TNF-α), and IL-8 play important roles in the early stage of wound healing. These factors act as chemoattractants for the migration of phagocytes and other cells to the site, starting the proliferative phase which involves the recruitment and replication of cells necessary for tissue regeneration and capillary regrowth. The final step, wound remodeling, may continue for weeks or months. Thus, the healing process is a cascade, and success in the later stages of wound repair is highly dependent on initial events [43].
Inflammation plays a key role early in this cascade, and proinflammatory cytokines are essential to this effort; they help to protect against infection and prepare injured tissue for repair by enhancing the recruitment and activation of phagocytes [44]. Furthermore, cytokines released by recruited cells regulate the ability of fibroblasts and epithelial cells to remodel the damaged tissue [44]. IL-1 produced early after tissue injury can regulate the production, release, and activation of metalloproteinases that are important in the destruction and remodeling of the wound; IL-1 also regulates fibroblast chemotaxis and the production of collagen [44]. Moreover, IL-1 stimulates the production of other cytokines that are important for wound healing, including IL-2, IL-6, and IL-8 [44]. Confirming the importance of proinflammatory cytokines in the healing process, IL-6 knock-out mice healed a standard wound 3 times more slowly than wild-type mice [45]. Accordingly, deficits early in the wound repair cascade can have adverse downstream consequences.
Physiological pathways of the stress-induced wound healing impairment
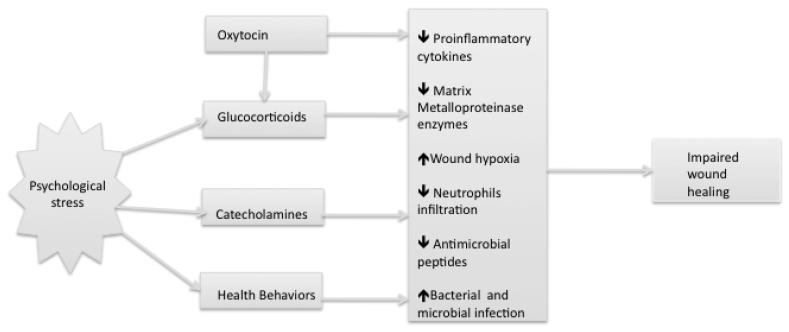
Psychological stress leads to the activation of the hypothalamic-pituitary-adrenal and the sympathetic-adrenal-medullary axes [46]. Enhanced glucocorticoids and catecholamines production can directly influence several components of the healing process. Substantial evidence from animal and humans studies indicate that physiological stress responses can retard the initial inflammatory phase of wound healing [47]. Figure 1 presents a schematic representation of the behavioral and physiological pathways linking stress and wound healing.
Figure 1.
Behavioral and physiological pathways linking psychological stress and wound healing.
Glucocorticoids
Stress-induced glucocorticoid production has been associated with delayed wound healing. In humans, greater awakening cortisol secretion the day following a punch biopsy was associated with greater perceived stress and delayed wound healing [10]. In animal studies, restraint stress led to a four-fold elevation in corticosterone levels [13]. Blocking glucocorticoid function with a glucocorticoid receptor antagonist, RU40555, eliminated the stress-induced delay in wound healing in stressed animals [13,39]. Preventing glucocorticoid production via adrenalectomy also reduced the effects of restraint stress on wound healing [39]. Furthermore, exogenous administration of glucocorticoid slowed wound healing, compared to a vehicle injection [13].
Catecholamines
Increased catecholamine production also appears to play a role in stress-induced impairment in wound healing. Administration of an α-adrenergic receptor antagonist attenuated the restraint stress-induced impairment of wound healing in mice [48]. In a study using a rotation stress model, administration of a β-adrenergic receptor antagonist, propanolol hydrochloride, attenuated the stress-induced impairment in wound healing in mice [49]. In a burn wound model, mice injected with a β-adrenergic receptor antagonist exhibited improved re-epitheliazation of burn wounds, compared to mice who received a vehicle injection [50]. Furthermore, injection of norepinephrenine can reduce keratinocyte motility and migration in vitro [50]. These data provide evidence of a role for catecholamines in stress-induced impairment in wound repair.
Oxytocin and Vasopressin
The two hypothalamic peptides, oxytocin and vasopressin, modulated physiological stress responses and social bonding processes in animal and human work. In a couples study using the blister wounds model, individuals who had more positive interactions with their partner during a social support task had higher plasma oxytocin levels. Higher circulating oxytocin levels were in turn associated with faster healing of the standard blister wounds. Furthermore, in women, but not in men, greater plasma vasopressin levels were related to faster healing [19].
Well-controlled animal studies corroborate the role of oxytocin in mediating the beneficial effects of social relationships on wound healing. Exogenous oxytocin administration attenuated the stress-induced corticosterone production and impairment in wound healing [39,51]. Furthermore, administration of an oxytocin receptor antagonist eliminated the beneficial impact of pair housing on wound healing [39]. Collectively, these results suggest that in addition to modulating stress responses, oxytocin may have a direct influence on the healing process.
Local Cytokine Production
Diminished expression of proinflammatory cytokines at the wound site is another pathway by which stress can delay the initial phase of wound healing. The suction blister model provides a method to monitor in vivo cytokine expression at the wound site in humans. After raising several blisters and removing their roofs (the epidermis), plastic templates with wells containing a salt solution and autologous serum are placed over the lesions to monitor protein expression at the wound site. The autologous serum-buffer solution is aspirated from the wells with a syringe at different time intervals, allowing for cell phenotyping and cytokine measurement as the local immune response evolves.
Using this approach, women who reported more perceived stress produced significantly lower IL-1α and IL-8 levels at the wound site, 5 and 24 hours after the blistering procedure [52]. Marital disagreement also influenced local cytokine production [18]. Production of three proinflammatory cytokines at the wound site, IL-1β, IL-6, and TNF-α, were lower after the discussion of a marital disagreement than after a social support discussion, paralleling the impact of marital conflict on wound healing [18]. In these two studies, local cytokine production was not significantly associated with serum levels of the same cytokines, underscoring the different biological significance of local and systemic production of these molecules.
In a clinical study, patients undergoing an hernia removal surgery who reported greater preoperative stress had a lower concentration of IL-1β in the wound drain fluid, 20 hours after the operation, compared to patients who experienced less preoperative distress [53]. Furthermore, two stressors that can impair cutaneous and mucosal wound healing, family dementia caregiving and academic examinations, were also associated with poorer stimulated production of IL-1β after treatment with lipopolysaccharide [9,16]. Corroborating human data, mice subjected to restraint stress had lower levels of IL-β mRNA at the wound site, compared to control mice [54,55].
Stress-induced glucocorticoid production might effectively decrease cytokine production at the wound site. Exogenous administration of glucocorticoid diminished IL-1α, IL-1β, and TNF-α expression at the site after wounding in mice [42]. Similarly, animal and human studies have also demonstrated that stress-induced elevations in glucocorticoids can transiently suppress IL-1β, TNF-α, and PDGF production [52,55] . Accordingly, dysregulation of glucocorticoid secretion provides one obvious neuroendocrine pathway through which stress alters the initial inflammatory phase of wound healing.
Matrix Metalloproteinase
Matrix metalloproteinase (MMP) enzymes are involved in the degradation of collagen and other extracellular matrix molecules. Degradation of the basement membrane of the wound promotes cellular invasion and migration, an essential component of the early phase of wound healing. Among patients undergoing inguinal hernia surgery, those who reported greater worry about the operation had lower levels of MMP-9 in the wound drain fluid 20 hours after the surgery [53]. In a human study using the blister wounds model, there was a negative correlation between plasma cortisol levels and MMP-2 protein levels at the wound site [56]. Furthermore, in an animal study using a rotation stress model, mice subjected to the stressor had fewer activated MMP-2 and MMP-9 seven days after wounding, compared to control mice [49]. These data indicate that stress can down-regulate MMP production at the wound site.
Wound cellularity
Psychological stress may reduce cell infiltration at the wound site. In a study using a restraint stress paradigm, cellularity of the wound and wound margin areas were analyzed in cross-sections of dermal and epidermal layers. Mice subjected to restraint stress had less leukocyte infiltration to the wound sites at one and three days after wounding, compared to control mice [13].
Increased susceptibility to infection
Stress can also increase susceptibility to wound infection. Mice exposed to restraint stress had a 2-5 log increase in opportunistic bacteria such as Staphylococcus aureus, compared to control mice not exposed to the stressor. Furthermore, 7 days after wounding, 85.4% of restraint-stress mice had bacterial counts predictive of infection, vs. 27.4 of controls [57].
The increased susceptibility to infection appears to be mediated in part by a decreased epidermal antimicrobial peptides production. Mice exposed to insomnia and crowding stress had lower epidermis levels of cathelin-related antimicrobial peptides and exhibited more severe infection following an intradermal injection of group A Streptococcus pyogenes [58]. This effect appears to be glucocorticoid dependent; administration of a glucocorticoid receptor antagonist, eliminated the impact of stress on epidermis antimicrobial peptide production and administration of exogenous glucocorticoid mimicked the effects of stress on antimicrobial production [58].
Wound hypoxia
Oxygen homeostasis is critical to all phases of wound healing. Damage created to blood vessels during wounding decreases oxygen availability. Simultaneously, neutrophils' oxidative burst increases oxygen demand at the wound site. Restraint stress can further promote wound hypoxia [59]. Compared to controls, restraint stressed mice had higher levels of inducible nitric oxide synthase levels, an indicator of wound hypoxia at the wound site [59]. Furthermore, hyperbaric oxygen therapy normalized inducible nitric oxide synthase levels and attenuated stress-induced impairments in wound healing [59].
Behavioral mechanisms linking stress and wound healing
In addition to directly modulating physiological responses to skin damage, stress can also indirectly influence wound repair by promoting the adoption of health-damaging behaviors. Individuals who experience greater levels of stress are more likely to increase their alcohol and tobacco use, decrease their participation in physical activity, experience sleep disturbances, and make poorer diet choices, compared to individuals reporting less distress [60,61]. These negative health behavior practices can then compound the detrimental impact of stress on physiological healing processes [2].
Heavy alcohol use has been associated with delays in cell migration and collagen deposition at the wound site, which in turn can impede the healing process [62]. Smoking has also been related to slowed healing of naturally occurring and surgery wounds [63]. Sleep disruption delays skin barrier recovery after tape stripping and diminishes growth hormone production [21,64]. Lack of regular physical activity can slow wound healing rate [36]. Furthermore, deficient intake of glucose, polyunsaturated, proteins, and certain vitamins can impede the healing process [65-67].
Conclusion
The goal of this review was to present clinical and experimental models of the impact of stress on wound repair. Converging and replicated evidence from experimental and clinical models of wound healing indicate that psychological stress leads to clinically relevant delays in wound healing. New mechanistic data suggest ways to elucidate the multiple physiological pathways by which stress alters wound repair processes. Translational work should focus on identifying conditions in which behavioral and pharmacological treatments are the most effective and on developing new treatments able to attenuate stress-induced delays in wound healing.
Acknowledgments
Work on this chapter was supported by a doctoral research training award from the Fonds de la Recherche en Santé du Québec and NIH grants AG029562, CA126857, CA131029, AT003912, Ohio State Comprehensive Cancer Center Core Grant CA16058, and NCRR Grant UL1RR025755.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Walburn J, Vedhara K, Hankins M, et al. Psychological stress and wound healing in humans: a systematic review and meta-analysis. J Psychosom Res. 2009;67(3):253–71. doi: 10.1016/j.jpsychores.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 2.Kiecolt-Glaser JK, Page GG, Marucha PT, et al. Psychological influences on surgical recovery: Perspectives from psychoneuroimmunology. Am Psychol. 1998;53:1209–18. doi: 10.1037//0003-066x.53.11.1209. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberger PH, Jokl P, Ickovics J. Psychosocial factors and surgical outcomes: an evidence-based literature review. J Am Acad Orthop Surg. 2006;14(7):397–405. doi: 10.5435/00124635-200607000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Boeke S, Duivenvoorden HJ, Verhage F, et al. Prediction of postoperative pain and duration of hospitalization using two anxiety measures. Pain. 1991;45(3):293–7. doi: 10.1016/0304-3959(91)90053-Z. [DOI] [PubMed] [Google Scholar]
- 5.Scheier MF, Matthews KA, Owens JF, et al. Optimism and rehospitalization after coronary artery bypass graft surgery. Arch Intern Med. 1999;159:829–35. doi: 10.1001/archinte.159.8.829. [DOI] [PubMed] [Google Scholar]
- 6.Doering LV, Moser DK, Lemankiewicz W, et al. Depression, healing, and recovery from coronary artery bypass surgery. Am J Crit Care. 2005;14(4):316–24. [PubMed] [Google Scholar]
- 7.Cole-King A, Harding KG. Psychological factors and delayed healing in chronic wounds. Psychosom Med. 2001;63:216–20. doi: 10.1097/00006842-200103000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Gouin JP, Hantsoo L, Kiecolt-Glaser JK. Immune dysregulation and chronic stress among older adults: a review. Neuroimmunomodulation. 2008;15(4-6):251–9. doi: 10.1159/000156468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kiecolt-Glaser JK, Marucha PT, Malarkey WB, Mercado AM, Glaser R. Slowing of wound healing by psychological stress. Lancet. 1995;346:1194–96. doi: 10.1016/s0140-6736(95)92899-5. [DOI] [PubMed] [Google Scholar]
- 10.Ebrecht M, Hextall J, Kirtley LG, et al. Perceived stress and cortisol levels predict speed of wound heating in healthy male adults. Psychoneuroendocrinology. 2004;29(6):798–809. doi: 10.1016/S0306-4530(03)00144-6. [DOI] [PubMed] [Google Scholar]
- 11.McGuire L, Heffner K, Glaser R, et al. Pain and wound healing in surgical patients. Ann Behav Med. 2006;31(2):165–72. doi: 10.1207/s15324796abm3102_8. [DOI] [PubMed] [Google Scholar]
- 12.Graham JE, Robles TF, Kiecolt-Glaser JK, et al. Hostility and pain are related to inflammation in older adults. Brain Behav Immun. 2006;20(4):389–400. doi: 10.1016/j.bbi.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Padgett DA, Marucha PT, Sheridan JF. Restraint stress slows cutaneous wound healing in mice. Brain Behav Immun. 1998;12:64–73. doi: 10.1006/brbi.1997.0512. [DOI] [PubMed] [Google Scholar]
- 14.French SS, Matt KS, Moore MC. The effects of stress on wound healing in male tree lizards (Urosaurus ornatus) Gen Comp Endocrinol. 2006;145(2):128–32. doi: 10.1016/j.ygcen.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Martin LB, 2nd, Glasper ER, Nelson RJ, et al. Prolonged separation delays wound healing in monogamous California mice, Peromyscus californicus, but not in polygynous white-footed mice, P. leucopus. Physiol Behav. 2006;87(5):837–41. doi: 10.1016/j.physbeh.2006.01.035. [DOI] [PubMed] [Google Scholar]
- 16.Marucha PT, Kiecolt-Glaser JK, Favagehi M. Mucosal wound healing is impaired by examination stress. Psychosom Med. 1998;60:362–65. doi: 10.1097/00006842-199805000-00025. [DOI] [PubMed] [Google Scholar]
- 17.Bosch JA, Engeland CG, Cacioppo JT, et al. Depressive symptoms predict mucosal wound healing. Psychosom Med. 2007;69(7):597–605. doi: 10.1097/PSY.0b013e318148c682. [DOI] [PubMed] [Google Scholar]
- 18.Kiecolt-Glaser JK, Loving TJ, Stowell JR, et al. Hostile marital interactions, proinflammatory cytokine production, and wound healing. Arch Gen Psychiatry. 2005;62:1377–84. doi: 10.1001/archpsyc.62.12.1377. [DOI] [PubMed] [Google Scholar]
- 19.Gouin JP, Carter CS, Pournajafi-Nazarloo H, et al. Marital behavior, oxytocin, vasopressin, and wound healing. Psychoneuroendocrinology. doi: 10.1016/j.psyneuen.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gouin JP, Kiecolt-Glaser JK, Malarkey WB, et al. The influence of anger expression on wound healing. Brain Behav Immun. 2008;22(5):699–708. doi: 10.1016/j.bbi.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Altemus M, Rao B, Dhabhar FS, et al. Stress-induced changes in skin barrier function in healthy women. J Invest Dermatol. 2001;117:309–17. doi: 10.1046/j.1523-1747.2001.01373.x. [DOI] [PubMed] [Google Scholar]
- 22.Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130(3):355–91. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- 23.Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’- A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- 24.Robles TF. Stress, social support, and delayed skin barrier recovery. Psychosom Med. 2007;69(8):807–15. doi: 10.1097/PSY.0b013e318157b12e. [DOI] [PubMed] [Google Scholar]
- 25.Robles TF, Brooks KP, Pressman SD. Trait positive affect buffers the effects of acute stress on skin barrier recovery. Health Psychol. 2009;28(3):373–8. doi: 10.1037/a0014662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garg A, Chren MM, Sands LP, et al. Psychological stress perturbs epidermal permeability barrier homeostasis: Implications for the pathogenesis of stress-associated skin disorders. Arch Dermatol. 2000;137:53–59. doi: 10.1001/archderm.137.1.53. [DOI] [PubMed] [Google Scholar]
- 27.Muizzuddin N, Matsui MS, Marenus KD, et al. Impact of stress of marital dissolution on skin barrier recovery:Tape stripping and measurement of trans-epidermal water loss (TEWL) Skin Research and Technology. 2003;9:34–38. doi: 10.1034/j.1600-0846.2003.00354.x. [DOI] [PubMed] [Google Scholar]
- 28.Denda M, Tsuchiya T, Hosoi J, et al. Immobilization-induced and crowded environment-induced strss delay barrier recovery in murine skin. Br J Dermatol. 1998;138:780–85. doi: 10.1046/j.1365-2133.1998.02213.x. [DOI] [PubMed] [Google Scholar]
- 29.Denda M, Tsuchiya T, Elias PM, et al. Stress alters cutaneous permeability barrier homeostasis. American Journal of Physiology: Regulatory, Integrative, and Comparative Physiology. 2000;278:R367–R72. doi: 10.1152/ajpregu.2000.278.2.R367. [DOI] [PubMed] [Google Scholar]
- 30.Johnston M, Vogele C. Benefits of psychological preparation for surgery: A meta-analysis. Ann Behav Med. 1993;15:245–56. [Google Scholar]
- 31.Montgomery GH, David D, Winkel G, et al. The effectiveness of adjunctive hypnosis with surgical patients: a meta-analysis. Anesth Analg. 2002;94(6):1639–45. doi: 10.1097/00000539-200206000-00052. table of contents. [DOI] [PubMed] [Google Scholar]
- 32.Esterling BA, L'Abate L, Murray EJ, et al. Empirical foundations for writing in prevention and psychotherapy: mental and physical health outcomes. Clin Psychol Rev. 1999;19(1):79–96. doi: 10.1016/s0272-7358(98)00015-4. [DOI] [PubMed] [Google Scholar]
- 33.Weinman J, Ebrecht M, Scott S, et al. Enhanced wound healing after emotional disclosure intervention. Br J Health Psychol. 2008;13(Pt 1):95–102. doi: 10.1348/135910707X251207. [DOI] [PubMed] [Google Scholar]
- 34.Emery CF, Blumenthal JA. Effects of physical exercise on psychological and cognitive functioning of older adults. Ann Behav Med. 1991;13:99–107. [Google Scholar]
- 35.Emery CF, Kiecolt-Glaser JK, Glaser R, et al. Exercise accelerates wound healing among healthy older adults: A preliminary investigation. Journals of Gerontology Series a-Biological Sciences and Medical Sciences. 2005;60(11):1432–36. doi: 10.1093/gerona/60.11.1432. [DOI] [PubMed] [Google Scholar]
- 36.Keylock KT, Vieira VJ, Wallig MA, et al. Exercise accelerates cutaneous wound healing and decreases wound inflammation in aged mice. Am J Physiol Regul Integr Comp Physiol. 2008;294(1):R179–84. doi: 10.1152/ajpregu.00177.2007. [DOI] [PubMed] [Google Scholar]
- 37.House JS, Landis KR, Umberson D. Social relationships and health. Science. 1988;241:540–45. doi: 10.1126/science.3399889. [DOI] [PubMed] [Google Scholar]
- 38.Glasper ER, Devries AC. Social structure influences effects of pair-housing on wound healing. Brain Behav Immun. 2005;19(1):61–8. doi: 10.1016/j.bbi.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 39.Detillion CE, Craft TK, Glasper ER, et al. Social facilitation of wound healing. Psychoneuroendocrinology. 2004;29(8):1004–11. doi: 10.1016/j.psyneuen.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 40.Rossi A, Barraco A, Donda P. Fluoxetine: a review on evidence based medicine. Ann Gen Hosp Psychiatry. 2004;3(1):2. doi: 10.1186/1475-2832-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Farahani RM, Sadr K, Rad JS, et al. Fluoxetine enhances cutaneous wound healing in chronically stressed Wistar rats. Adv Skin Wound Care. 2007;20(3):157–65. doi: 10.1097/01.ASW.0000262710.59293.6b. [DOI] [PubMed] [Google Scholar]
- 42.Hubner G, Brauchle M, Smola H, et al. Differential regulation of pro-inflammatory cytokines during wound healing in normal glucocorticoid-treated mice. Cytokine. 1996;8(7):548–56. doi: 10.1006/cyto.1996.0074. [DOI] [PubMed] [Google Scholar]
- 43.Hübner G, Brauchle M, Smola H, et al. Differential regulation of pro-inflammatory cytokines during wound healing in normal and glucocorticoid-treated mice. Cytokine. 1996;8:548–56. doi: 10.1006/cyto.1996.0074. [DOI] [PubMed] [Google Scholar]
- 44.Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83(3):835–70. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- 45.Gallucci RM, Simeonova PP, Matheson JM, et al. Impaired cutaneous wound healing in interleukin-6-deficient and immunosuppressed mice. FASEB J. 2000;14:2525–31. doi: 10.1096/fj.00-0073com. [DOI] [PubMed] [Google Scholar]
- 46.Padgett DA, Glaser R. How stress influences the immune response. Trends in Immunology. 2003;24(8):444–48. doi: 10.1016/s1471-4906(03)00173-x. [DOI] [PubMed] [Google Scholar]
- 47.Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: Implications for health. Nature Reviews Immunology. 2005;5:243–51. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- 48.Eijkelkamp N, Engeland CG, Gajendrareddy PK, et al. Restraint stress impairs early wound healing in mice via alpha-adrenergic but not beta-adrenergic receptors. Brain Behav Immun. 2007;21(4):409–12. doi: 10.1016/j.bbi.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 49.Romana-Souza B, Otranto M, Vieira AM, et al. Rotational stress-induced increase in epinephrine levels delays cutaneous wound healing in mice. Brain Behav Immun. 24(3):427–37. doi: 10.1016/j.bbi.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 50.Sivamani RK, Pullar CE, Manabat-Hidalgo CG, et al. Stress-mediated increases in systemic and local epinephrine impair skin wound healing: potential new indication for beta blockers. PLoS Med. 2009;6(1):e12. doi: 10.1371/journal.pmed.1000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vitalo A, Fricchione J, Casali M, et al. Nest making and oxytocin comparably promote wound healing in isolation reared rats. PLoS ONE. 2009;4(5):e5523. doi: 10.1371/journal.pone.0005523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Glaser R, Kiecolt-Glaser JK, Marucha PT, et al. Stress-related changes in proinflammatory cytokine production in wounds. Arch Gen Psychiatry. 1999;56:450–56. doi: 10.1001/archpsyc.56.5.450. [DOI] [PubMed] [Google Scholar]
- 53.Broadbent E, Petrie KJ, Alley PG, et al. Psychological stress impairs early wound repair following surgery. Psychosom Med. 2003;65(5):865–69. doi: 10.1097/01.psy.0000088589.92699.30. [DOI] [PubMed] [Google Scholar]
- 54.Mercado AM, Padgett DA, Sheridan JF, et al. Altered kinetics of IL-1 alpha, IL-1 beta, and KGF-1 gene expression in early wounds of restrained mice. Brain Behav Immun. 2002;16(2):150–62. doi: 10.1006/brbi.2001.0623. [DOI] [PubMed] [Google Scholar]
- 55.Head CC, Farrow MJ, Sheridan JF, et al. Androstenediol reduces the anti-inflammatory effects of restraint stress during wound healing. Brain Behav Immun. 2006;20(6):590–6. doi: 10.1016/j.bbi.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 56.Yang EV, Bane CM, MacCallum RC, et al. Stress-related modulation of matrix metalloproteinase expression. J Neuroimmunol. 2002;133(1-2):144–50. doi: 10.1016/s0165-5728(02)00270-9. [DOI] [PubMed] [Google Scholar]
- 57.Rojas I, Padgett DA, Sheridan JF, et al. Stress-induced susceptibility to bacterial infection during cutaneous wound healing. Brain Behav Immun. 2002;16:74–84. doi: 10.1006/brbi.2000.0619. [DOI] [PubMed] [Google Scholar]
- 58.Aberg KM, Radek KA, Choi EH, et al. Psychological stress downregulates epidermal antimicrobial peptide expression and increases severity of cutaneous infections in mice. J Clin Invest. 2007;117(11):3339–49. doi: 10.1172/JCI31726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gajendrareddy PK, Sen CK, Horan MP, et al. Hyperbaric oxygen therapy ameliorates stress-impaired dermal wound healing. Brain Behav Immun. 2005;19(3):217–22. doi: 10.1016/j.bbi.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 60.Steptoe A, Wardle J, Pollard TM, et al. Stress, social support and health-related behavior: A study of smoking, alcohol consumption and physical exercise. J Psychosom Res. 1996;41:171–80. doi: 10.1016/0022-3999(96)00095-5. [DOI] [PubMed] [Google Scholar]
- 61.Vitaliano PP, Scanlan JM, Zhang J, et al. A path model of chronic stress, the metabolic syndrome, and coronary heart disease. Psychosom Med. 2002;64:418–35. doi: 10.1097/00006842-200205000-00006. [DOI] [PubMed] [Google Scholar]
- 62.Benveniste K, Thut P. The effect of chronic alcoholism on wound healing. Proceedings of the Society for Experiental Biology and Medicine. 1981;166:568–75. doi: 10.3181/00379727-166-41110. [DOI] [PubMed] [Google Scholar]
- 63.Silverstein P. Smoking and wound healing. Am J Med. 1992;93:22S–24S. doi: 10.1016/0002-9343(92)90623-j. [DOI] [PubMed] [Google Scholar]
- 64.Veldhuis JD, Iranmanesch A. Physiological regulation of the human growth hormone (GH)-insulin-like growth factor type I (IGF-I) axis: Predominant impact of age, obesity, gonadal function, and sleep. Sleep. 1996;19:S221–24. doi: 10.1093/sleep/19.suppl_10.s221. [DOI] [PubMed] [Google Scholar]
- 65.Russell L. The importance of patients' nutritional status in wound healing. Br J Nurs. 2001;10(6 Suppl):S44–9. doi: 10.12968/bjon.2001.10.Sup1.5336. S42. [DOI] [PubMed] [Google Scholar]
- 66.Posthauer ME. The role of nutrition in wound care. Adv Skin Wound Care. 2006;19(1):43–52. doi: 10.1097/00129334-200601000-00015. quiz 53-4. [DOI] [PubMed] [Google Scholar]
- 67.McDaniel JC, Belury M, Ahijevych K, et al. Omega-3 fatty acids effect on wound healing. Wound Repair Regen. 2008;16(3):337–45. doi: 10.1111/j.1524-475X.2008.00388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]