Abstract
Given our limited ability to predict analgesic efficacy, further research is needed to understand factors influencing analgesic response patterns. The aim of this study was to better understand the relationship between morphine and butorphanol analgesic efficacy tested against multiple pain modalities within the same individuals. Participants included healthy men (n=72) and women (n=67) who underwent thermal, pressure, and ischemic experimental pain testing prior to and following the double-blind administration of morphine and butorphanol during separate testing sessions. Factor analysis revealed six factors with analgesic effects grouped primarily by pain modality and specific to either morphine or butorphanol. Hierarchical cluster analysis of individual factor scores led to four distinct drug response profiles. Three groups displayed exceptional analgesic efficacy produced by one type of opioid on one pain stimulus modality, while the fourth drug response profile was characterized by average analgesic efficacy across all pain modalities for both opioids. These findings suggest that opioids with varying efficacy at the mu and kappa receptors produce independent effects on unique pain mechanisms and that individual responsiveness for some is dependent on pain mechanism and opioid type, although a subset of the population is moderately responsive to opioids regardless of efficacy of receptor binding or predominant pain mechanism being activated.
Keywords: Experimental pain, Opioid, Psychophysics, Factor analysis, Cluster analysis, Pain sensitivity
Introduction
Although opioids are the most widely used medication for the management of moderate to severe pain, ability to predict which opioid is best suited to treat a particular individual or pain condition remains limited. Research investigating person-related factors has not been entirely consistent39 but has revealed that gender16, psychological attributes41, age11 and genetic profile28 likely play a role in opioid response. Previous studies have also shown that analgesic efficacy is influenced by an individual’s sensitivity to pain stimulation, opioid sensitivity of a particular pain mechanism, and opioid efficacy at particular receptor subtypes. In reality, it is likely that opioid efficacy is dependent on a complex interaction among all of these factors.
Considerable preclinical and clinical evidence demonstrates that opioid efficacy varies across experimental pain modality.26,29,32,48 Given that different experimental pain stimuli activate distinct neural substrates1, these findings may indicate that opioids display varying efficacy depending on the mechanism of pain being targeted. Preclinical studies have revealed different levels of analgesic efficacy depending on the nociceptive assay being studied, indicating a modality-specific drug response. This finding has been demonstrated in opioid32 and non-opioid analgesics.4 Clinical studies have documented similar findings in that not all pain relieving medications are equally effective in all experimental pain models. Again, this is independent of type of analgesia29,36 or route of administration.26
While the dependence of analgesic efficacy on pain modality has been clearly demonstrated in preclinical research4, less is known about how different analgesics compare to one another in response to different experimental pain stimuli among humans. Neural circuits associated with distinct physiological responses are differentially populated by opioid receptor subtypes.16 Drug affinity for particular molecular binding sites in combination with stimulation of a particular circuit could play a role in determining analgesic efficacy. However, recent preclinical research reported a high correlation among mouse strains on analgesic sensitivity to five centrally acting drugs54, indicating a common analgesic response pattern to varying drugs. This high correlation among analgesic sensitivity was consistent across two distinct nociceptive assays. The authors propose the possibility of a “master set” of antinociception genes such that individuals might have a common analgesic response across disparate analgesics. Translation of this proposal to the human population could indicate the existence of subgroups characterized by high, low or average analgesic response profiles independent of drug tested. Determination of whether such analgesic patterns varied by experimental pain modality would be of importance in understanding analgesic response profiles to different types of pain.
The goal of the current investigation was to better understand opioid response patterns to multiple experimental pain modalities and the influence that opioid type and individual characteristics had on these responses. This was accomplished by assessing the analgesic effects of morphine and butorphanol in response to thermal, pressure and ischemic pain tasks in healthy adults. This investigation was embedded in a larger project studying sex differences in opioid analgesia. Previous work21,22 has suggested that kappa agonist-antagonists produce significantly less analgesic efficacy in men as compared to women. For the parent project, morphine and butorphanol were used to determine if sex differences in opioid analgesia might be affected by opioid subtype. Both opioids exhibit binding at the mu and kappa opioid receptors with varying efficacy.14 Morphine displays high efficacy at the mu receptor and low efficacy at the kappa receptor55 while butorphanol exhibits weak efficacy at both.52 For the current project we used factor and cluster analysis to better understand opioid response patterns and determine the effect of pain mechanism, individual factors and type of opioid on analgesic efficacy using factor and cluster analysis. This work was presented at the 2010 American Pain Society Annual Scientific Meeting. 24
Methods
Subjects
Seventy two males and 67 females were recruited through IRB approved posted advertisements. The sample contained only healthy non-smoking individuals between the ages of 18 and 45 without clinical pain, psychiatric disturbance, substance use disorder or use of centrally acting medications assessed by self-report health history. Twenty nine percent of women were taking oral contraceptives. Women were scheduled between days four and 20 after the onset of menses to avoid testing perimenstrually, as this time has been associated with heightened pain sensitivity.17 Prior to participation in each experimental session, it was confirmed that subjects had refrained from taking over-the-counter medications within the past 24 hours, caffeine in the past two hours, and reported no significant health changes. Participants were paid $25 an hour for their involvement in the study.
General Experimental Procedures
The study was conducted at the General Clinical Research Center at the University of Florida. Subjects participated in four sessions; the first was an introduction to the study protocol and the following three involved the administration of morphine, butorphanol and saline in a double blind, randomized order. All subjects provided verbal and written informed consent and completed a number of psychological and health related questionnaires prior to participation in the research protocol. Following the introductory session, the three remaining experimental sessions were identical in format with the exception of drug (saline, morphine or butorphanol) administration.
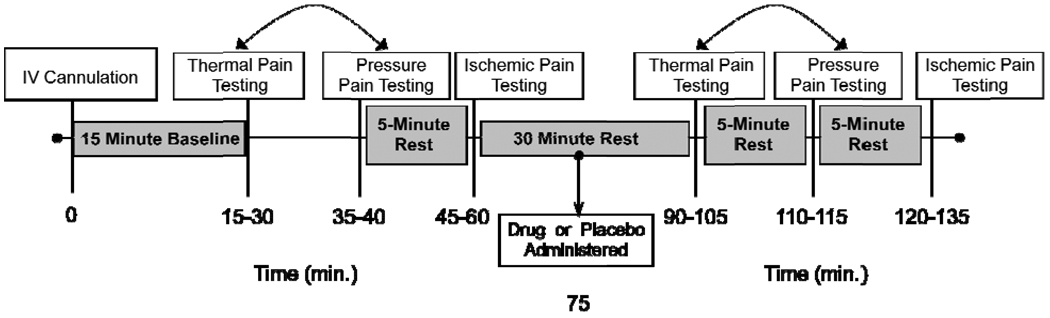
Experimental procedures used in this study followed the general protocol implemented successfully in our previous studies.18,19 Specifically, two experimenters and a registered nurse conducted the experimental sessions with one experimenter responsible for the sensory testing component by the bedside and the other experimenter operating the equipment and recording the data. The gender of the bedside experimenter remained consistent for each of the experimental sessions. The clinical nurse was responsible for monitoring vital signs, administering the placebo/analgesic drug and completing blood draws. Subjects maintained a semi-recumbent position in a hospital bed during all study procedures. An intravenous (IV) cannula was inserted at the beginning of each experimental session followed by a ten minute rest period. Six minutes into the rest period, vital signs were taken including blood pressure, heart rate, respiratory rate, mean arterial pressure, carbon dioxide level, and oxygen (O2) saturation. Ten minutes following IV placement, the pre-drug sensory testing protocol was completed, including thermal pain, pressure pain, and ischemic pain measures (described below). The order of thermal and pressure pain was randomly determined for each subject and maintained for all sessions. The ischemic pain procedure was always completed last to reduce carry over effects. Following pre-drug sensory testing, a 15-minute rest period was observed followed by the double blind IV administration of 0.08mg/kg of morphine, 0.016mg/kg butorphanol, or saline in randomized order given over five minutes. These doses approximate a low to moderate clinical dose with estimated equianalgesia.21 Fifteen minutes following drug administration post-drug sensory testing was repeated in a manner identical to the pre-drug testing. Following the conclusion of the sensory testing procedures, subjects completed questionnaires assessing somatic, cognitive, and affective side effects. A timeline of the experimental session is presented in Figure 1. All clinically significant adverse effects (either reported by subjects or observed by the experimenters) were documented, reported to the Institutional Review Board, and included in the data analyses. The protocol and all procedures were approved by the University of Florida’s Institutional Review Board.
Figure 1.
Timeline of procedures during experimental drug sessions. The boxed text represents the procedures (white) and rest breaks (gray) implemented during the study session. The bidirectional arrows between thermal pain and pressure pain indicate that these two procedures were conducted in counterbalanced order. Time estimates for each testing procedure are documented below the timeline.
Adapted from: Fillingim RB, Ness TJ, Glover TL, Campbell CM, Hastie BA, Price DD, Staud R: Morphine responses and experimental pain: Sex differences in side effects and cardiovascular responses but not analgesia. Journal of Pain 6:116-124, 2005.
Pain Testing Procedures
Similar to our previous studies18,19, experimental pain procedures were conducted once during the introductory session to reduce novelty effects. Digitally recorded instructions were provided to the subjects during this session for each experimental pain procedure. During the three experimental sessions, these same procedures were conducted prior to and following drug administration with verbal instructions reiterated before beginning each procedure.
Pressure Pain Threshold
Pressure pain threshold (PPT) was assessed with a handheld algometer (Pain Diagnostics and Therapeutics, Great Neck, NY). Mechanical pressure was applied with a 1-cm2 probe and increased at a rate of 1kg per second, which helps reduce artifact related to reaction time. Subjects were instructed to report (verbally or by raising their hand) their first feeling of pain as a result of the pressure. Three sites were used to assess PPTs on the right side of the body: the center of the upper trapezius (posterior to the clavicle), the upper masseter (approximately midway between the ear opening and the corner of the mouth) and the ulna (dorsal forearm, approximately 8 cm distal to the elbow). The site order was randomly counterbalanced and a minimum of three trials (with readings within 1 kg) were recorded at each position. The average of the three assessments for each site was calculated and used in subsequent analysis.
Thermal Pain Procedures
Heat pain threshold and tolerance
The first thermal procedure involved assessment of heat pain threshold and tolerance. Contact heat stimuli were delivered using a computer-controlled Medoc Thermal Sensory Analyzer (Pathway Pain & Sensory evaluation System, Ramat Yishai, Israel), which includes a Peltier-element-based stimulator. Temperature levels were monitored by a contactor-contained thermistor, and returned to a preset baseline of 32°C by active cooling at a rate of 10°C/s. The 3 cm × 3 cm contact probe was applied to the right ventral forearm. In separate series of trials, heat pain threshold (HPTh) and heat pain tolerance (HPTo) were assessed using an ascending method of limits. From a baseline of 32°C, probe temperature increased at a rate of 0.5°C/s until the subject responded by pressing a button to indicate when they first felt warmth (WTh), pain (HPTh), and when no longer able to tolerate the pain (HPTo). This slow rise-time was selected as a test of pain evoked mainly by stimulation of C-nociceptive afferents, as has been previously demonstrated.56, 57 Four trials of HPTh and HPTo were presented to each subject. The position of the thermode was altered slightly between trials (though it remained on the ventral forearm) in order to avoid either sensitization or response suppression of cutaneous heat nociceptors. For each measure, the average of all four trials was computed for use in subsequent analyses.
Temporal summation of thermal pain
The second thermal procedure involved administration of brief, repetitive, suprathreshold heat pulses to assess temporal summation of heat pain.39 Three series of ten repetitive pulses were applied to the right dorsal forearm using the Contact Heat Evoked Potential Stimulator (CHEPS), which combines heat-foil technology with a Peltier element, thereby achieving heating and cooling rates of at least 40°C /sec. One series of ten stimuli was applied at each of three different target temperatures (46°C, 48°C and 50°C). For each series, the baseline temperature was 35°C, the target temperature was delivered for 700 msec, and the inter-stimulus interval (at the baseline temperature) was 2.5 seconds. Subjects rated the peak pain for each of the ten heat pulses using a numerical measure (0 represented no sensation and 100 represented the most intense pain imaginable). The average rating across all 10 trials for each temperature was used in subsequent analyses.
Modified Submaximal Tourniquet Procedure
Following completion of the pressure and thermal pain procedures, a rest period of 5-minutes was implemented prior to beginning the tourniquet procedure.30,35 Blood flow to the right arm was decreased by elevating it above heart level for 30 seconds, after which the arm was occluded with a standard blood pressure cuff positioned proximal to the elbow and inflated to 240 mm Hg using a Hokanson E20 Rapid Cuff Inflator (D.E. Hokanson, Bellevue, WA, USA). Subjects then performed 20 hand grip exercises of two second duration at four second intervals at 50% of their maximum grip strength. Subjects were instructed to report when they first felt pain (ischemic pain threshold) then to continue until the pain became intolerable (ischemic pain tolerance), at which point the procedure was stopped. The ischemic pain threshold and tolerance time points were recorded. Thirty three subjects reached the uninformed time limit of 15 minutes during pre-drug testing, which creates a ceiling effect for computing analgesic response. For this reason, ischemic pain tolerance was not included in subsequent analyses. Every 30 seconds during the procedure, subjects were prompted to alternately rate either the intensity or unpleasantness of their pain using joint numerical (0–20) and verbal descriptor box scales.49 These scales, with their verbal descriptors, were designed to help participants conceptualize the difference between ratings of pain intensity and unpleasantness and have been previously validated to distinguish between these two pain dimensions. 5 Two total pain scores were created by summing these ratings: ischemic pain intensity and ischemic pain unpleasantness. Additionally, cardiovascular measures (systolic, diastolic, and mean arterial blood pressure, and heart rate) were recorded every 60 seconds.
Opioid side effects
To assess side effects in response to morphine and butorphanol, subjects completed the Somatic Side Effects Questionnaire (SSE) and Cognitive and Affective Side Effects Questionnaire (CASE) 60 to 70 minutes following the administration of each opioid. The SSE is a standardized instrument with 28 questions that assess a range of somatic side effects commonly produced by opioids. Symptoms such as sleepiness, feeling flushed, tingling/numbness, balance and nausea/vomiting are rated on a 5-point force choice scale where 1 = Not at all; 2 = A little bit; 3 = Somewhat; 4 = Quite a bit; 5 = Extremely.9 For purposes of analysis, the 28 symptoms were collapsed into seven symptom dimensions as determined by previous confirmatory factor analysis.43 These side effect dimensions included sedation, thermoregulatory symptoms, tingling, dry mouth, dizziness, nausea and motor symptoms. The CASE consists of 44 items that assess a range of common cognitive and affective side effects associated with the use of opioid medications. Items are rated on a 5 point scale with the following response choices: 1 = Not at all; 2 = A little bit; 3 = Somewhat; 4 = Quite a bit; 5 = Extremely.43 For analysis purposes, the 44 side effects were collapsed into six symptom dimensions as determined by previous confirmatory factor analysis.43 These side effect dimensions included mental dulling, relaxation, psychological symptoms, feeling in control, confusion and euphoria.
Psychological measures
Participants completed several psychological measures to evaluate associations between psychological constructs, pain and analgesia response.
Kohn Reactivity Scale (KRS)
This measure consists of 24 items that assess an individual’s level of reactivity or central nervous system arousability.25 Subjects are asked to indicate the extent to which they agree with particular statements (e.g., I could never bath or shower in ice cold water or I’ve often had motion sickness) on a five point scale (1=disagree strongly; 5=agree strongly). This measure has been shown to correlate negatively with pain tolerance9 and has been used to measure the construct of hypervigilance.29 The KRS has demonstrated adequate reliability and validity.25
Pennebaker Inventory of Limbic Languidness (PILL)
The PILL is a checklist that assesses the frequency of 54 common physical symptoms and sensations and has been related to the construct of somatization or the tendency to endorse physical symptoms.38 Participants are asked to indicate how often they experience each symptom on a five point scale (1 = have never or almost never experienced this symptom; 5 = experience this symptom more than once every week). The PILL has demonstrated high internal consistency and adequate test retest reliability.
Pain Catastrophizing Scale (PCS)
The PCS50 was used to measure pain catastrophizing in the study subjects. This tool is a 13 item scale that assesses catastrophic cognitive (I keep thinking about how much it hurts) and affective (I worry all the time about when the pain will end) responses to pain. The participant is asked to recall the extent in which they have had specific catastrophic thoughts and feelings in response to a past occurrence of pain. Ratings (1=not at all; 4=always) are summed for a score range of 0 to 52 with higher scores indicating greater frequency of cognitions related to helplessness, magnification, and/or rumination. A recent study established the psychometric soundness of the PCS for use in both men and women.7
Positive and Negative Affect Scale (PANAS)
This 20 item scale assesses the frequency with which participants generally experience ten positive (e.g., excited, inspired) and ten negative (e.g., nervous, irritable) feelings.53 Each item is rated on a 5-point scale (1 = very slightly or not at all; 5 = extremely). The PANAS yields two scores, one for positive affect and one for negative affect. This measure has demonstrated adequate reliability and validity, assessing mood states which appear to be relatively stable over time.53
Data analysis
To determine drug effects for each experimental pain measure, a change score was calculated by determining the difference between the pre-drug and post-drug scores on each pain task. Therefore each subject had a morphine change score and a butorphanol change score for each experimental pain task, yielding 22 variables.
Nine exploratory factor analyses were performed to determine the underlying factor structure of morphine and butorphanol’s effects on the different experimental pain modalities. We used Principal Components Analysis and two Common Factor Analysis (Maximum Likelihood, Principal Axis Factoring) methods for factor extraction. Since the goal of this project was to evaluate the associations among the 22 variables versus strict data reduction15, we chose to use two common factor analysis methods. Both Maximum Likelihood and Principal Axis Factoring were used to determine the best fit given our distribution of variables. Although some have argued that Principal Components Analysis is not a true factor analysis method15, we chose to use it for comparison given its common usage in this type of research. We paired each of the three factor extraction methods with two oblique (Oblimin and Promax) and one orthogonal (Varimax) factor rotational techniques for a total of nine analyses. Both oblique and orthogonal rotations were used to compare solutions achieved with and without correlation among factors. Results of the nine exploratory factor analyses were compared to determine agreement in one factor structure and primary factor loadings for individual items. The Scree test was used to determine the appropriate number of factors to retain. Criteria used to establish a unifying factor structure included: 1) individual items loaded above .30, 2) lack of crossloading above .40 and 3) items loaded on the same factor in the majority of analyses.
Factor scores were determined such that each subject had a standardized (z) score for each factor. Factor scores reflected the average change in pain sensitivity produced by either morphine or butorphanol on each pain task in that factor. Standardizing the factor scores set the mean analgesic response for each factor to zero and allowed for comparison of individual and group responses to the average for the entire sample. Therefore the value of the standardized factor score indicated the number of standard deviations away from the overall sample mean. A positive factor score indicated enhanced analgesia compared to the mean during the experimental pain modality while a negative factor score reflected a lower analgesic effect as compared to the mean level of analgesia experienced by the group as a whole. Hierarchical cluster analysis of these factor scores was used to identify groups with specific patterns of analgesic response to multiple experimental pain modalities. Subgroups characterized by particular drug response profiles were formed using Ward’s method with squared Euclidean distances as these methods minimize within cluster variance, create smaller, more distinct cluster solutions and are sensitive to inter-subject profile shape.15 Agglomeration coefficients were inspected and the percentage change between successive cluster profiles was calculated. The point at which the percentage change was largest between clusters was used as a stopping rule to reveal our final number of clusters.
Finally, to assess differences between cluster groups we used ANOVA for continuous variables and χ2 for categorical variables. To determine group differences for significant ANOVA findings, a Tukey post hoc analysis was used when population variances among the groups were equal on the dependent variable. Dunnett’s C post-hoc analysis was used when variances were unequal. These analyses were conducted to determine group differences in baseline pain sensitivity and opioid related side effects. Scores on baseline pain sensitivity were calculated by averaging pre-drug scores on each pain modality across the three experimental sessions. Finally, the groups were compared on psychological variables including the Kohn Reactivity Scale, Pennebaker Inventory of Limbic Languidness, Positive and Negative Affect Scale and Pain Catastrophizing Scale.
Results
Subjects
Out of the 139 subjects participating in this study, 18 subjects were not included in analyses reported below. Eight subjects received only morphine or butorphanol, not both, and ten had missing data on one or more pain modality. Therefore 121 subjects were included in the analyses. The sample included 56 women and 65 men with an average age of 23 years (SD=4.5 years). The ethnic distribution of the sample consisted of 7% (n=9) African American, 2% (n=3) Asian, 12% (n=15) Hispanic, 77% (n=93) white and less than 1% (n=1) reported being of another race.
Factor Structure for Opioid Effect on Experimental Pain
Based on interpretation of the scree test, all nine factor analyses revealed a six factor model. Factor extraction with Maximum Likelihood and Principal Axis Factoring led to a structure with all items loading on one of the six factors and no crossloadings above .40. These six factors included: 1) butorphanol ischemic pain (consisting of butorphanol ischemic threshold, intensity and tolerance); 2) morphine ischemic pain (consisting of morphine ischemic threshold, intensity and tolerance); 3) butorphanol temporal summation (consisting of butorphanol temporal summation at 46, 48 and 50°C); 4) morphine thermal pain (consisting of morphine thermal threshold, tolerance and temporal summation at 46, 48 and 50°C); 5) morphine pressure pain (consisting of morphine pressure pain threshold at the ulna, trapezius and masseter); 6) butorphanol thermal/pressure pain (including butorphanol thermal tolerance, threshold and PPT at the ulna, trapezius and masseter). Extraction with Principal Components Analysis led to cross loadings of morphine heat threshold and tolerance onto factors 4 (morphine thermal) and 6 (butorphanol thermal/pressure pain). Since the Principal Components Analyses did not fit with the goal of this project (associations among variables) and led to crossloadings, we decided to utilize the factor structure and loadings achieved with Maximum Likelihood and Principal Axis Factoring. Eigen values ranged from 3.63 to 1.196 while this factor structure explained 59.84% of the variance. The Maximum Likelihood Goodness of Fit estimation (χ2 = 172.41, p <.001) indicated that the predicted values from the model differed from the observed values. It has been suggested that the χ2 goodness of fit index should be interpreted with caution.15 This finding could be influenced by the number of variables tested (22 variables) since a significant finding can occur even with good fit when using a large number of variables. Table 1 displays the factor matrix using Maximum Likelihood with a Varimax rotation which came closest to a simple structure.
Table 1.
Maximum Likelihood loadings of morphine and butorphanol change scores on 22 experimental pain modalities.
| Variable | Factor 1 BU Ischemic |
Factor 2 MS Ischemic |
Factor 3 BU TS |
Factor 4 MS Thermal |
Factor 5 MS PPT |
Factor 6 BU Thermal and PPT |
|---|---|---|---|---|---|---|
| BU ischemic unpleasantness |
.927 | |||||
| BU ischemic intensity |
.843 | |||||
| BU ischemic threshold |
.627 | |||||
| MS ischemic intensity |
.883 | |||||
| MS ischemic unpleasantness |
.862 | |||||
| MS ischemic threshold | .643 | |||||
| BU TS at 48°C | .861 | |||||
| BU TS at 46°C | .668 | |||||
| BU TS at 50°C | .586 | |||||
| MS heat threshold | .677 | |||||
| MS heat tolerance | .594 | |||||
| MS TS at 50°C | .503 | |||||
| MS TS at 48°C | .439 | |||||
| MS TS at 46°C | .362 | |||||
| MS PPT ulna | .988 | |||||
| MS PPT trapezius | .496 | |||||
| MS PPT masseter | .396 | |||||
| BU heat tolerance | .536 | |||||
| BU PPT ulna | .490 | |||||
| BU PPT trapezius | .449 | |||||
| BU PPT masseter | .376 | |||||
| BU heat threshold | .309 |
BU: butorphanol; MS: morphine; TS: temporal summation; PPT: pressure pain threshold
Cluster analysis of subjects on factor scores
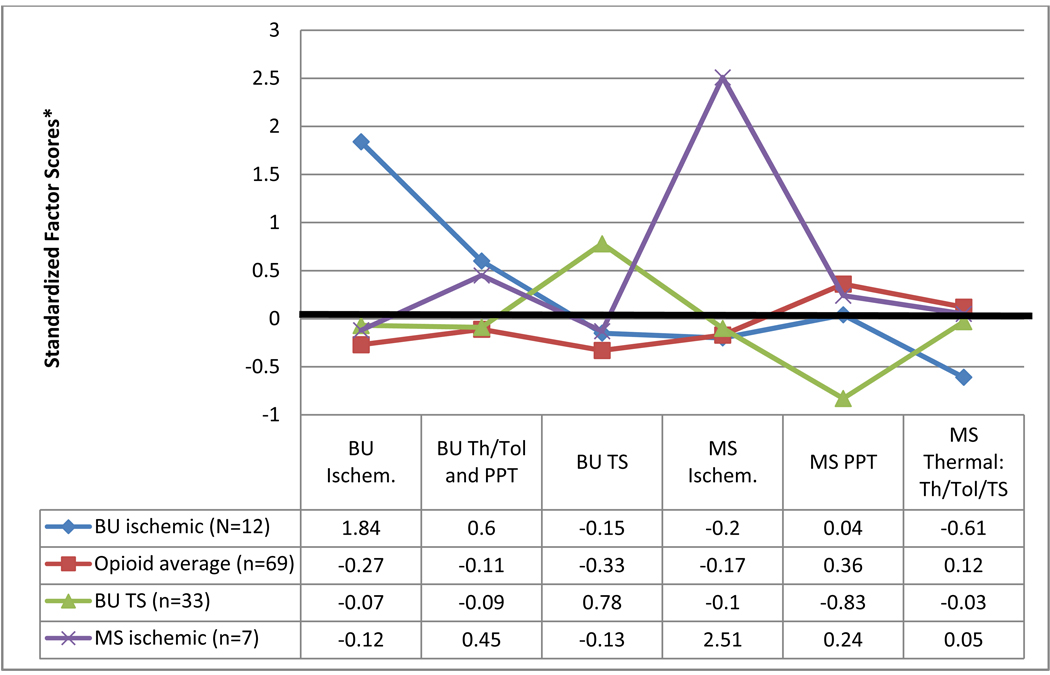
Clustering subjects using their factor scores on the six factors identified using Maximum Likelihood revealed four distinct clusters, or drug response profiles. Cluster one (butorphanol ischemic responders) consisted of twelve subjects who displayed above average analgesic efficacy from butorphanol on ischemic pain testing. This group of subjects exhibited slightly increased analgesia on the butorphanol thermal /pressure pain factor, average analgesia on butorphanol temporal summation and morphine ischemic and pressure pain but decreased analgesia on the morphine thermal factor. Cluster two (average opioid responders) contained 69 subjects who showed average analgesic effects from both butorphanol and morphine on all pain modalities. Cluster three (butorphanol temporal summation responders) consisted of 33 subjects who had above average analgesic efficacy for butorphanol on temporal summation and decreased analgesic efficacy for morphine on pressure pain. This group exhibited average analgesia on all other factors. Cluster four (morphine ischemic responders) contained seven subjects who demonstrated greater than average morphine analgesia during the ischemic pain task. This cluster of individuals displayed average levels of analgesia on all other modalities. Figure 2 profiles each cluster across the six factors based on the group’s standardized factor scores. These standardized scores represent the number of standard deviations that the cluster’s analgesic response differed from the overall sample mean. Therefore a score of zero is equivalent to the sample mean whereas a score of +1.0 represents one standard deviation above the sample mean, and −1.0 is one standard deviation below the sample mean. Table 2 displays the raw scores for each cluster on response to morphine and butorphanol. Average change scores (the difference between the pre-drug and post-drug scores) for each cluster are documented across pain tasks.
Figure 2. Cluster profile across standardized factor scores.
* Values reported in this figure are standardized factor scores, consequently a value of 0 is equivalent to the group mean, a value of +1.0 represent 1 (SD) above the mean, and −1.0 represents 1 SD below the mean.
BU: butorphanol; MS: morphine; TS: temporal summation; PPT: pressure pain threshold; Th: threshold; Tol: tolerance; ischem.: ischemic
Table 2.
Average morphine and butorphanol change scores for each pain modality by cluster.
| Variable | Cluster 1 BU schemic responders (n=12) |
Cluster 2 Average responders (n=69) |
Cluster 3 BU TS responders (n=33) |
Cluster 4 Morphine ischemic responders (n=7) |
|---|---|---|---|---|
|
Average morphine change score mean (SD) |
||||
| Ischemic threshold (seconds) |
30.25 (52.19) | 23.23 (49.84) | 12.49 (42.68) | 168.86 (66.43) |
| Ischemic intensity (summed ratings) |
21.42 (13.54) | 23.73 (22.52) | 22.06 (28.48) | 95.29 (11.93) |
| Ischemic unpleasantness (summed ratings) |
25.67 (19.01) | 23.36 (23.63) | 24.06 (26.82) | 95.14 (22.34) |
| Heat pain threshold (°C) |
−0.35 (1.80) | 0.89 (1.70) | 0.35 (1.94) | 0.33 (0.93) |
| Heat pain tolerance (°C) |
−0.11 (0.95) | 0.48 (0.99) | 0.52 (0.94) | 0.96 (0.79) |
| TS at 46°C (mean pain rating) |
−2.27 (6.21) | 1.07 (10.33) | 6.31 (16.61) | 5.43 (11.72) |
| TS at 48°C (mean pain rating) |
−2.78 (6.21) | 2.64 (12.31) | 4.02 (9.70) | 3.33 (4.89) |
| TS at 50°C (mean pain rating) |
0.32 (6.10) | 4.73 (11.47) | 0.33 (8.97) | 2.79 (7.20) |
| PPT masseter (kg) | 0.24 (0.39) | 0.26 (0.46) | 0.01 (0.43) | 0.42 (0.37) |
| PPT ulna (kg) | 0.34 (0.49) | 0.70 (0.83) | −0.40 (1.08) | 0.37 (1.09) |
| PPT trapezius (kg) | 0.24 (0.68) | 0.47 (0.91) | −0.13 (0.93) | 0.82 (0.98) |
|
Average butorphanol change score mean (SD) |
||||
| Ischemic threshold (seconds) |
173.50 (174.95) | 21.88 (46.95) | 35.12 (95.19) | 42.00 (53.17) |
| Ischemic intensity (summed ratings) |
125.00 (39.34) | 24.28 (34.73) | 31.10 (59.42) | 39.00 (47.36) |
| Ischemic unpleasantness (summed ratings) |
134.83 (44.72) | 23.59 (29.09) | 39.79 (43.32) | 48.71 (39.36) |
| Heat pain threshold (°C) |
0.43 (1.48) | −0.09 (1.67) | 0.21 (2.48) | 1.50 (2.46) |
| Heat pain tolerance (°C) |
1.18 (1.18) | 0.18 (1.14) | 0.30 (1.19) | 1.16 (1.11) |
| TS at 46°C (mean pain rating) |
4.05 (9.83) | 1.50 (10.28) | 10.02 (13.46) | 0.19 (9.97) |
| TS at 48°C (mean pain rating) |
1.83 (16.59) | 1.12 (9.38) | 17.00(12.02) | 2.34 (7.32) |
| TS at 50°C (mean pain rating) |
9.48 (8.25) | 2.15 (7.36) | 11.66 (11.97) | 10.48 (10.63) |
| PPT masseter(kg) | 0.54 (0.57) | 0.29 (0.54) | 0.19 (0.42) | 0.32 (0.36) |
| PPT ulna (kg) | 1.00 (1.50) | 0.50 (1.08) | 0.34 (0.78) | 1.21 (1.00) |
| PPT trapezius (kg) | 1.46 (1.35) | 0.53 (1.18) | 0.62 (1.14) | 0.65 (1.19) |
BU: butorphanol; TS: temporal summation; PPT: pressure pain threshold
Change scores were calculated by determining the difference between the pre-drug and post-drug score on each pain task. Positive changes scores indicate a reduction in pain, while negative change scores indicate an increase in pain.
Cluster differences on demographics, baseline pain sensitivity, opioid side effects and psychological measures
Analysis with ANOVA and χ2 revealed that the clusters did not differ on sex, age, race, baseline pain responses or psychological measures (Tables 3 and 4). The clusters experienced significantly different levels of sedation from butorphanol (F(3, 105)=3.047, p=.032), such that the butorphanol ischemic responders experienced significantly less sedation than the average opioid responders. The groups also differed on the amount of dry mouth experienced in response to butorphanol (F(3, 105)=2.786, p=.044) with the butorphanol ischemic responders again reporting significantly less dry mouth as compared to the average opioid responders. The groups also differed in tingling side effects in response to morphine (F(3, 106)=5.903, p=.001). The butorphanol ischemic responders experienced significantly more tingling as compared to all other groups. These results are presented in Table 4.
Table 3.
Demographics across cluster membership
| Variable | Cluster 1 BU ischemic responders (n=12) |
Cluster 2 Average responders (n=69) |
Cluster 3 BU TS responders (n=33) |
Cluster 4 Morphine ischemic responders (n=7) |
F or χ2 value | P value |
|---|---|---|---|---|---|---|
| Age | 25.3 (SD=8.7) | 22.8 (SD=4.1) | 22.8 (SD=3.1) | 22.0 (3.3) | F(3,117)=1.187 | .318 |
| Sex (% female) |
25.0% (n=3) | 50.7% (n=35) | 42.4% (n=14) | 57.1% (n=4) | χ2=3.264 (df=3) | .353 |
| Race | χ2=1.273 (df=3) | .735 | ||||
| Non-white | 33.3% (n=4) | 23.2% (n=16) | 18.2% (n=6) | 28.6% (n=2) | ||
| White | 66.7% (n=8) | 76.8% (n=53) | 81.8% (n=27) | 71.4% (n=5) |
BU: butorphanol; TS: temporal summation
Table 4.
Baseline pain, side effects, and psychological measures across cluster membership
| Variable | Cluster 1 BU ischemic responders (n=12) |
Cluster 2 Average responders (n=69) |
Cluster 3 BU TS responders (n=33) |
Cluster 4 Morphine ischemic responders (n=7) |
F value | P value |
|---|---|---|---|---|---|---|
| Baseline pain response: mean (SD) |
||||||
| Ischemic threshold (seconds) |
135.8 (60.6) |
109.6 (67.6) |
107.3 (65.1) |
106.1 (29.5) |
0.644 | .588 |
| Ischemic intensity (summed ratings) |
191.9 (56.4) |
213.5 (56.3) |
223.4 (50.0) |
198.1 (52.3) |
1.181 | .320 |
| Ischemic unpleasantness (summed ratings) |
205.8 (56.8) |
223.9 (55.7) |
227.7 (46.1) |
202.4 (46.8) |
0.851 | .469 |
| Heat pain threshold (°C) |
43.0 (1.7) |
41.8 (2.4) |
41.6 (2.2) |
41.8 (1.8) |
1.118 | .345 |
| Heat pain tolerance (°C) |
48.0 (1.8) |
46.7 (2.4) |
46.9 (2.2) |
47.5 (1.7) |
1.249 | .295 |
| TS at 46°C (mean pain rating) |
31.9 (21.9) |
38.1 (27.0) |
44.1 (22.6) |
40.8 (31.5) |
0.773 | .512 |
| TS at 48°C (mean pain rating) |
49.0 (26.3) |
55.1 (28.7) |
61.5 (22.6) |
57.4 (28.9) |
0.774 | .528 |
| TS at 50°C (mean pain rating) |
65.9 (26.3) |
65.7 (27.1) |
76.9 (18.2) |
70.1 (24.9) |
1.481 | .224 |
| PPT masseter (kg) | 2.7 (0.9) | 2.6 (0.8) | 2.4 (0.9) | 2.4 (0.7) | 0.783 | .506 |
| PPT ulna (kg) | 4.7 (1.7) | 4.3 (1.8) | 4.0 (1.7) | 3.9 (1.1) | 0.715 | .545 |
| PPT trapezius (kg) | 4.7 (1.9) | 4.3 (1.7) | 3.6 (1.3) | 4.2 (1.4) | 1.988 | .120 |
| Butorphanol side effects: mean (SD) |
||||||
| SSE sedaton | 2.33 (1.26) | 3.41 (1.07) | 3.21 (1.35) | 2.95 (1.39) | 3.047 | 0.032 |
| SSE thermoregulation | 1.39 (0.63) | 2.02 (0.98) | 1.83 (0.93) | 1.62 (1.35) | 1.522 | 0.213 |
| SSE tingling | 1.61 (0.84) | 1.53 (0.65) | 1.49 (0.68) | 1.24 (0.66) | 0.481 | 0.696 |
| SSE dry mouth | 1.21 (0.40) | 1.72 (0.76) | 1.44 (0.48) | 1.43 (0.53) | 2.786 | 0.044 |
| SSE dizziness | 1.91 (0.98) | 2.28 (0.85) | 2.21 (0.81) | 1.81 (0.77) | 1.115 | 0.347 |
| SSE nausea | 1.14 (0.26) | 1.40 (0.70) | 1.27 (0.42) | 1.25 (0.56) | 0.838 | 0.476 |
| SSE motor symptoms | 1.27 (0.36) | 1.34 (0.49) | 1.21 (0.35) | 1.33 (0.51) | 0.640 | 0.591 |
| Morphine side effects: mean (SD) |
||||||
| SSE sedation | 2.73 (1.25) | 2.28 (0.96) | 2.65 (1.06) | 2.14 (1.15) | 1.379 | 0.253 |
| SSE thermoregulation | 1.33 (0.47) | 1.44 (0.57) | 1.34 (0.38) | 1.24 (0.37) | 0.533 | 0.660 |
| SSE tingling | 2.24 (0.86) | 1.48 (0.62) | 1.41 (0.48) | 1.33 (0.47) | 5.903 | 0.001 |
| SSE dry mouth | 1.48 (0.85) | 1.38 (0.59) | 1.30 (0.43) | 1.38 (0.73) | 0.295 | 0.829 |
| SSE dizzy | 1.76 (0.68) | 1.56 (0.59) | 1.74 (0.70) | 1.57 (0.79) | 0.738 | 0.532 |
| SSE nausea | 1.21 (0.60) | 1.21 (0.39) | 1.18 (0.39) | 1.29 (0.76) | 0.111 | 0.954 |
| SSE motor symptoms | 1.48 (0.90) | 1.22 (0.40) | 1.16 (0.29) | 1.29 (0.49) | 1.516 | 0.215 |
| Psychological measures : mean (SD) |
||||||
| Kohn Reactivity Scale | 61.25 (9.18) |
67.39 (11.49) |
65.15 (12.13) |
66.71 (11.29) |
1.089 | 0.357 |
| PILL | 94.83 (18.87) |
94.51 (19.08) |
96.79 (18.13) |
93.86 (14.62) |
0.125 | 0.945 |
| Pain Catastrophizing Scale |
2.17 (0.93) | 3.03 (2.10) | 2.62 (2.14) | 2.75 (1.33) | 0.791 | 0.501 |
| PANAS (positive affect) | 3.70 (0.32) | 3.59 (0.58) | 3.68 (0.46) | 3.47 (0.59 | 0.522 | 0.668 |
| PANAS (negative affect) | 1.66 (0.62) | 1.79 (0.60) | 1.63 (0.45) | 1.73 (0.49 | 0.659 | 0.579 |
Bu: butorphanol; TS: temporal summation; PPT: pressure pain threshold; SSE: Somatic Side Effect Questionnaire; PILL: Pennebaker Inventory of Limbic Languidness; PANAS: Positive and Negative Affect Scale
Discussion
The aim of this study was to better understand the relationship between morphine and butorphanol analgesic efficacy tested against multiple pain modalities within the same individuals. We discovered that analgesic effects grouped primarily by pain modality and were specific to either morphine or butorphanol. Categorizing participants based on their response to these six opioid and modality specific factors led to the identification of four subgroups. Each group had unique analgesic response profiles; three of which were distinguished by substantial analgesic efficacy in response to one opioid on a specific pain modality. Group characteristics across drug response profiles were similar in regards to demographic factors, baseline pain sensitivity and psychological profile with some variation in side effect profiles. These findings indicate that analgesic efficacy in a given individual could be opioid and pain mechanism specific.
That analgesic response patterns fell into distinct groupings according to opioid and pain modality could indicate that specific pain mechanisms respond differently to opioids. The pain modalities used in this study were chosen to capture a broad range of pain characteristics and have been proposed to activate different pan mechanisms.1,48 Previous studies have demonstrated that analgesic responses to opioids vary across pain induction methods. In previous studies morphine has demonstrated efficacy in pressure and electrical pain47 as well as ischemic pain18, produced mixed results in heat pain40, 51, and demonstrated a lack of efficacy in visceral pain.47 Findings from the current study support the notion that opioids produce varying analgesic effects depending on the pain mechanism stimulated.
This study also demonstrated that morphine and butorphanol, opioids with differing efficacies at the mu and kappa opioid receptors, produce independent effects on different pain mechanisms. Had the analgesic effects of morphine and butorphanol on ischemic pain been mediated by similar underlying mechanisms, one ischemic analgesic factor would have emerged regardless of opioid. Instead morphine and butorphanol led to distinct ischemic factors, indicating that different mechanisms underlie responses to these two opioids on this modality. This finding is similar to preclinical work demonstrating that mu and kappa opioids do not produce equal analgesic effects on different pain models in rats.8,20 While the antinociceptive effects of morphine and butorphanol predominately differ in their efficacy at the mu opioid receptor, butorphanol’s full agonist effect at the kappa receptor (albeit with low efficacy) does lead to some pharmacological differences between these two opioids.6 Neural circuits involved in different aspects of pain processing are differentially populated by mu and kappa opioid receptors.3,16 Given that each pain induction method stimulates unique pain mechanisms and neural circuits, opioids with differing activity at the mu and kappa receptors would have differential effects on the various pain modalities. The fact that each opioid did not produce consistent results on certain pain modalities (ie. butorphanol was not consistently most effective for ischemic pain or morphine for thermal pain) indicates that individual differences in opioid metabolism and efficacy at the mu and kappa receptors interact with characteristics inherent to each neural circuit to modulate opioid efficacy.
Grouping participants on the opioid and pain modality specific factors revealed four distinct drug response profiles. Three of the profiles stood out due to their exceptional analgesic efficacy produced by one type of opioid on one pain stimulus modality. That the “butorphanol ischemic responders”, for instance, did not consistently respond with such intensity to all butorphanol measures or, conversely, to the morphine ischemic factor, indicates that opioid efficacy might reflect a unique combination of individual variability to both opioid receptor and pain mechanism. With the exception of the “average opioid responders”, none of the other drug response profiles exhibited consistent efficacy to one opioid across pain modalities or to one type of pain stimuli across opioids. This finding contradicts the notion that one type of opioid is consistently best for one type of pain across populations or that a person will respond equally well to one opioid for all types of pain. However, the largest profile group, the “average opioid responders”, consisted of individuals who received average analgesic efficacy across all pain modalities for both morphine and butorphanol. This could indicate that a portion of the population is moderately responsive to opioids, regardless of binding affinity to a particular opioid receptor or the predominant pain mechanism being activated.
The four groups characterized by different drug response profiles did not differ on demographic factors (sex, age, or race), psychological profile, or baseline pain sensitivity. That the clusters identified in the current study did not differ according to baseline sensitivity could indicate that opioid responsiveness is relatively unrelated to a person’s inherent level of pain sensitivity. Preclinical findings have demonstrated an inverse association between basal nociceptive sensitivity and analgesic efficacy2,13,33; therefore, one might have hypothesized greater baseline pain responses would be associated with reduced opioid analgesia in this population. However, our findings indicate a relative lack of correlation between pain sensitivity and opioid efficacy to particular painful stimuli in healthy individuals.
The only area in which the group profiles differed was in terms of opioid induced side effects. The “butorphanol ischemic responders” demonstrated increased tingling to morphine as compared to all other clusters and decreased sedation and dry mouth in response to butorphanol as compared to the “average opioid responders”. These findings indicate that individuals who received high levels of analgesia from butorphanol had a better side effect profile to this drug as compared to morphine. While these minor differences cannot lead to broad generalizations, these findings could provide an indication that some individuals are characterized by a broader therapeutic window for certain opioids compared to others.
The lack of group differences in demographic, pain sensitivity or psychological factors indicates that analgesic response patterns are likely driven by variables not analyzed in this study. Recent research indicates that individual pain variability is, in part, genetically controlled 37 and emerging research demonstrates that the same is likely true for analgesic efficacy.28 An individual’s genetic profile can affect genes coding for a drug’s molecular binding site, genes that regulate drug transport and metabolism, or genes that control enzymes responsible for the drug’s pharmacokinetic properties.27 Several genetic polymorphisms have demonstrated associations with analgesic efficacy, including variants of the mu opioid receptor gene (OPRM1) and catechol-O-methyltransferase (COMT)42, melanocortin 1 receptor (MC1R)34, and the cytochrome P450 isoenzyme 2D6 (CYP2D6)46 among others. It is likely that such genetic factors, not assessed in this investigation, explain at least part of the variability seen among the four drug response profiles.
While the current study sought to provide a foundation for understanding patterns of opioid efficacy in varying types of pain, clinical validity cannot be established without producing these results in a clinical population. The current study was carried out in healthy, young adults using experimentally induced pain. This overcomes many of the complexities inherent in clinical pain that likely affects analgesic efficacy, including but not limited to neuroplastic changes effecting both opioid receptor modulation and pain related neural circuits. There is currently a lack of evidence indicating whether analgesic response patterns assessed against experimental pain are predictive of analgesic response to clinical pain. This study utilized a variety of pain modalities thought to approximate different aspects of clinical pain.1,12,44 Our findings suggest that not all pain models are equally responsive to opioids of differing receptor affinity, perhaps indicating that not all clinical pain syndromes will be equally responsive. While we cannot be sure that analgesic response in these experimental pain models directly translates to opioid efficacy in clinical pain, our findings suggest that opioid response patterns are more complex than originally thought and that there exists substantial individual variability in determining which opioid might work best for differing pain mechanisms. Our findings, particularly those comparing drug response profiles, were limited by small subject numbers in two of the subgroups. Despite the fact that our sample was substantially larger than has been previously reported in studies of this nature, inadequate power could have led to a lack of group differences when comparing the drug response profile groups. Finally, using only one dose of each opioid limited our ability to investigate how a dose response relationship might have affected drug response profiles. While testing multiple doses would have expanded our understanding of opioid response patterns, this would have limited our sample size given the need to add experimental testing sessions to test each drug at different doses.
These limitations notwithstanding, this investigation provides a preliminary analysis of morphine and butorphanol analgesic response patterns to a variety of pain modalities. The findings demonstrated that these compounds elicit differing analgesic effects according to the pain mechanism stimulated. Interestingly, grouping individuals according to their opioid response profiles established that slightly less than half of the individuals demonstrated substantial opioid and pain modality specific analgesic effects. The remaining individuals showed average analgesic efficacy across opioids and pain modalities. That the groups did not differ on demographic, baseline pain response or psychological profile could point toward a genetic influence on opioid efficacy. This study adds evidence to the varying effects of opioids depending on individual variability and pain mechanism, reiterating the need for further investigation.
Perspective
This investigation provides a foundation for understanding patterns of opioid efficacy in varying types of pain. Our findings suggest that opioid response patterns are more complex than originally thought with about half of individuals exhibiting opioid and pain modality specific analgesic response profiles.
Acknowledgements
This work was supported by NIH/NINDS grant NS41670, NINDS training grant NS045551, and CTSA grant RR029890.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arendt-Nielsen L, Yarnitsky D. Experimental and clinical applications of quantitative sensory testing applied to skin, muscles and viscera. J Pain. 2009;10:556–572. doi: 10.1016/j.jpain.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Belknap JK, Noordewier B, Lame M. Genetic dissociaiton of multiple morphine effects among C57BL/6J, DBA/2J and C3H–HEJ inbred mouse strains. Physiol Behav. 1989;46:69–74. doi: 10.1016/0031-9384(89)90324-7. [DOI] [PubMed] [Google Scholar]
- 3.Bellgowan PSF, Helmstetter FJ. The role of mu and kappa opioid receptors within the periaqueductal gray in the expression of conditional hypoalgesia. Brain Res. 1998;791:83–89. doi: 10.1016/s0006-8993(98)00057-2. [DOI] [PubMed] [Google Scholar]
- 4.Chesler EJ, Ritchie J, Kokayeff A, Lariviere WR, Wilson SG, Mogil JS. Genotype-dependence of gabapentin and pregabalin sensitivity: the pharmacogenetic mediation of analgesia is specific to the type of pain being inhibited. Pain. 2003;106:325–335. doi: 10.1016/S0304-3959(03)00330-0. [DOI] [PubMed] [Google Scholar]
- 5.Coghill RC, Gracely RH. Validation of combined numerical-analog descriptor scales for rating pain intensity and pain unpleasantness. Proc Am Pain Soc. 1996;15:86. [Google Scholar]
- 6.Commiskey S, Fan LW, Ho IK, Rockhold RW, Commiskey S, Fan L-W, Ho IK, Rockhold RW. Butorphanol: Effects of a prototypical agonist-antagonist analgesic on kappa-opioid receptors. J Pharmacol Sci. 2005;98:109–116. doi: 10.1254/jphs.crj05001x. [DOI] [PubMed] [Google Scholar]
- 7.D'Eon JL, Harris CA, Ellis JA. Testing factorial validity and gender invariance of the pain catastrophizing scale. J Behav Med. 2004;27:361–372. doi: 10.1023/b:jobm.0000042410.34535.64. [DOI] [PubMed] [Google Scholar]
- 8.Danzebrink RM, Green SA, Gebhart GF. Spinal mu and delta, but not kappa, opioid-receptor agonists attenuate responses to noxious colorectal distension in the rat. Pain. 1995;63:39–47. doi: 10.1016/0304-3959(94)00275-J. [DOI] [PubMed] [Google Scholar]
- 9.Davies PS, Roth-Roemer S, Coda B. Somatic side effects of morphine and hydromorphone during sustained equianalgesia infusions. Proc Am Pain Soc. 1997;16:120. [Google Scholar]
- 10.Dubreuil D, Kohn PM. Reactivity and response to pain. Personal Individ Diff. 1986;7:907–909. [Google Scholar]
- 11.Edwards RR, Fillingim RB, Ness TJ. Age-related differences in endogenous pain modulation: A comparison of diffuse noxious inhibitory controls in healthy older and younger adults. Pain. 2003;101:155–165. doi: 10.1016/s0304-3959(02)00324-x. [DOI] [PubMed] [Google Scholar]
- 12.Edwards RR, Sarlani E, Wesselmann U, Fillingim RB. Quantitative assessment of experimental pain perception: Multiple domains of clinical relevance. Pain. 2005;114:315–319. doi: 10.1016/j.pain.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 13.Elmer GI, Pieper JO, Negus SS, Woods JH. Genetic variance in nociception and its relationship to the potency of morphine-induced analgesia in thermal and chemical tests. Pain. 1998;75:129–140. doi: 10.1016/S0304-3959(97)00215-7. [DOI] [PubMed] [Google Scholar]
- 14.Emmerson PJ, Clark MJ, Mansour A, Akil H, Woods JH, Medzihradsky F. Characterization of opioid agonist efficacy in a C6 glioma cell line expressing the mu opioid receptor. J Pharmacol Exp Ther. 1996;278:1121–1127. [PubMed] [Google Scholar]
- 15.Fabrigar LR, Wegener DT, MacCallum RC, Strahan EJ. Evaluating the use of exploratory factor analysis in psychological research. Psychol Methods. 1999;4:272–299. [Google Scholar]
- 16.Fillingim RB, Gear RW. Sex differences in opioid analgesia: Clinical and experimental findings. Eur J Pain. 2004;8:413–425. doi: 10.1016/j.ejpain.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL., III Sex, gender, and pain: A review of recent clinical and experimental findings. J Pain. 2009;10:447–485. doi: 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fillingim RB, Ness TJ, Glover TL, Campbell CM, Hastie BA, Price DD, Staud R. Morphine responses and experimental pain: Sex differences in side effects and cardiovascular responses but not analgesia. J Pain. 2005;6:116–124. doi: 10.1016/j.jpain.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 19.Fillingim RB, Ness TJ, Glover TL, Campbell CM, Price DD, Staud R. Experimental pain models reveal no sex differences in pentazocine analgesia in humans. Anesthesiology. 2004;100:1263–1270. doi: 10.1097/00000542-200405000-00031. [DOI] [PubMed] [Google Scholar]
- 20.Gallantine EL, Meert TF. Antinociceptive and adverse effects of mu and kappa opioid receptor agonists: A comparison of morphine and U50488-H. Basic Clin Pharmacol Toxicol. 2008;103:419–427. doi: 10.1111/j.1742-7843.2008.00306.x. [DOI] [PubMed] [Google Scholar]
- 21.Gear RW, Miaskowski C, Gordon NC, Paul SM, Heller PH, Levine JD. Kappa-opioids produce significantly greater analgesia in women than in men. Nat Med. 1996;2:1248–1250. doi: 10.1038/nm1196-1248. [DOI] [PubMed] [Google Scholar]
- 22.Gear RW, Miaskowski C, Gordon NC, Paul SM, Heller PH, Levine JD. The kappa opioid nalbuphine produces gender- and dose-dependent analgesia and antianalgesia in patients with postoperative pain. Pain. 1999;83:339–345. doi: 10.1016/s0304-3959(99)00119-0. [DOI] [PubMed] [Google Scholar]
- 23.Gutstein HB, Akil H. Opioid Analgesics. In: Brunton LL, Lazo J, Parker K, editors. Goodman and Gilmans' The Pharmacologic Basis of Therapeutics. ed 11. New York: McGraw Hill; 2006. pp. 547–590. [Google Scholar]
- 24.Kindler LL, Riley JL, III, Sibille K, Fillingim R. Analgesic responses for morphine and butorphanol are independent and pain modalitiy specific. J Pain. 2010;11 Suppl 4:S44. [Google Scholar]
- 25.Kohn PM. Sensation-seeking, augmenting-reducing, and the strength of the nervous system. In: Spence JT, Izard CE, editors. Motivation, emotion, and personality. Amsterdam: Elsevier; 1985. pp. 167–173. [Google Scholar]
- 26.Koltzenburg M, Pokorny R, Gasser UE, Richarz U. Differential sensitivity of three experimental pain models in detecting the analgesic effects of transdermal fentanyl and buprenorphine. Pain. 2006;126:165–174. doi: 10.1016/j.pain.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 27.LaCroix-Fralish ML, Mogil JS. Progress in genetic studies of pain and analgesia. Annu Rev Pharmacol Toxicol. 2009;49:97–121. doi: 10.1146/annurev-pharmtox-061008-103222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lotsch J, Geisslinger G, Tegeder I. Genetic modulation of the pharmacological treatment of pain. Pharmacol Therap. 2009;124:168–184. doi: 10.1016/j.pharmthera.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 29.Luginbuhl M, Schnider TW, Petersen-Felix S, Arendt-Nielsen L, Zbinden AM. Comparison of five experimental pain tests to measure analgesic effects of alfentanil. Anesthesiology. 2001;95:22–29. doi: 10.1097/00000542-200107000-00009. [DOI] [PubMed] [Google Scholar]
- 30.Maixner W, Gracely RH, Zuniga JR, Humphrey CB, Bloodworth GR. Cardiovascular and sensory responses to forearm ischemia and dynamic hand exercise. Am J Physiol. 1990;259:R1156–R1163. doi: 10.1152/ajpregu.1990.259.6.R1156. [DOI] [PubMed] [Google Scholar]
- 31.McDermid AJ, Rollman GB, McCain GA. Generalized hypervigilance in fibromyalgia: Evidence of perceptual amplification. Pain. 1996;66:133–144. doi: 10.1016/0304-3959(96)03059-x. [DOI] [PubMed] [Google Scholar]
- 32.Mogil JS, Kest B, Sadowski B, Belknap JK. Differential genetic mediation of sensitivity to morphine in genetic models of opiate antinociception: Influence of nociceptive assay. J Pharmacol Exp Ther. 1996;276:532–544. [PubMed] [Google Scholar]
- 33.Mogil JS, Marek P, Flodman P, Spence MA, Sternberg WF, Kest B, Sadowski B, Liebeskind JC. One or two genetic-loci mediate high opiate analgesia in selectively bred mice. Pain. 1995;60:125–135. doi: 10.1016/0304-3959(94)00098-Y. [DOI] [PubMed] [Google Scholar]
- 34.Mogil JS, Wilson SG, Chesler EJ, Rankin AL, Nemmani KV, Lariviere WR, Groce MK, Wallace MR, Kaplan L, Staud R, Ness TJ, Glover TL, Stankova M, Mayorov A, Hruby VJ, Grisel JE, Fillingim RB. The melanocortin-1 receptor gene mediates female-specific mechanisms of analgesia in mice and humans. Proc Nat Acad Sci USA. 2003;100:4867–4872. doi: 10.1073/pnas.0730053100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moore PA, Duncan GH, Scott DS, Gregg JM, Ghia JN. Submaximal effort tourniquet test It's use in evaluating experimental and chronic pain. Pain. 1979;6:375–382. doi: 10.1016/0304-3959(79)90055-1. [DOI] [PubMed] [Google Scholar]
- 36.Naef M, Curatolo M, Petersen-Felix S, Arendt-Nielsen L, Zbinden A, Brenneisen R. The analgesic effect of oral delta-9-tetrahydrocannabinol (THC), morphine, and a THC-morphine combination in healthy subjects under experimental pain conditions. Pain. 2003;105:79–88. doi: 10.1016/s0304-3959(03)00163-5. [DOI] [PubMed] [Google Scholar]
- 37.Nielsen CS, Stubhaug A, Price DD, Vassend O, Czajkowski N, Harris JR. Individual differences in pain sensitivity: Genetic and environmental contributions. Pain. 2008;136:21–29. doi: 10.1016/j.pain.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 38.Pennebaker JW. The Psychology of Physical Symptoms. New York: Springer; 1982. [Google Scholar]
- 39.Price DD, Hu JW, Dubner R, Gracely RH. Peripheral suppression of first pain and central summation of second pain evoked by noxious heat pulses. Pain. 1977;3:57–68. doi: 10.1016/0304-3959(77)90035-5. [DOI] [PubMed] [Google Scholar]
- 40.Price DD, Von der Gruen A, Miller J, Rafii A, Price C. A psychophysical analysis of morphine analgesia. Pain. 1985;22:261–269. doi: 10.1016/0304-3959(85)90026-0. [DOI] [PubMed] [Google Scholar]
- 41.Pud D, Yarnitsky D, Sprecher E, Rogowski Z, Adler R, Eisenberg E. Can personality traits and gender predict the response to morphine? An experimental cold pain study. Eur J Pain. 2006;10:103–112. doi: 10.1016/j.ejpain.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 42.Reyes-Gibb CC, Shete S, Rakvag T, Bhat SV, Skorpen F, Bruera E, Kaasa S, Klepstad P. Exploring joint effects of genes and the clinical efficacy of morphine for cancer pain: OPRM1 and COMT gene. Pain. 2007;130:25–30. doi: 10.1016/j.pain.2006.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Riley JL, Hastie BA, Glover TL, Fillingim RB, Staud R, Campbell CM. Cognitive-affective and somatic side effects of morphine and pentazocine: Side-effect profiles in healthy adults. Pain Med. 2009 doi: 10.1111/j.1526-4637.2009.00680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rollman GB, Lautenbacher S. Sex differences in musculoskeletal pain. Clin J Pain. 2001;17:20–24. doi: 10.1097/00002508-200103000-00004. [DOI] [PubMed] [Google Scholar]
- 45.Roth-Roemer S, Coda B, Davies PS. Cognitive and psychomotor side effects of morphine and hydromorphone during sustained equianalgesic infusions. Proc Am Pain Soc. 1997;16:120. [Google Scholar]
- 46.Sindrup SH, Brosen K. The pharmacogenetics of codeine hypoalgesia. Pharmacogenetics. 1995;5:335–346. doi: 10.1097/00008571-199512000-00001. [DOI] [PubMed] [Google Scholar]
- 47.Staahl C, Christrup LL, Andersen SD, Arendt-Nielsen L, Drewes AM. A comparative study of oxycodone and morphine in a multi-modal, tissue-differentiated experimental pain model. Pain. 2006;123:28–36. doi: 10.1016/j.pain.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 48.Staahl C, Olesen AS, Andresen T, Arendt-Nielsen L, Drewes AM. Assessing analgesic actions of opioids by experimental pain models in healthy volunteers - An updated review. Br J Clin Pharmacol. 2009;68:149–168. doi: 10.1111/j.1365-2125.2009.03456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sternberg WF, Bailin D, Grant M, Gracely RH. Competition alters the perception of noxious stimuli in male and female athletes. Pain. 1998;76:231–238. doi: 10.1016/s0304-3959(98)00050-5. [DOI] [PubMed] [Google Scholar]
- 50.Sullivan MJ, Bishop SR, Pivik J. The Pain Catastrophizing Scale: Development and validation. Psychol Assess. 1995;7:524–532. [Google Scholar]
- 51.Vanderburght M, Rasmussen SE, Arendtnielsen L, Bjerring P. Morphine does not affect laser induced warmth and pin prick pain thresholds. Acta Anaesthesiol Scand. 1994;38:161–164. doi: 10.1111/j.1399-6576.1994.tb03859.x. [DOI] [PubMed] [Google Scholar]
- 52.Walsh SL, Chausmer AE, Strain EC, Bigelow GE. Evaluation of the mu and kappa opioid actions of butorphanol in humans through differential naltrexone blockade. Psychopharmacology. 2008;196:143–155. doi: 10.1007/s00213-007-0948-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 54.Wilson SG, Smith SB, Chesler EJ, Melton KA, Haas JJ, Mitton B, Strasburg K, Hubert L, Rodriguez-Zas SL, Mogil JS. The heritability of antinociception: Common pharmacogenetic mediation of five neurochemically distinct analgesics. J Pharmacol Exp Ther. 2003;304:547–559. doi: 10.1124/jpet.102.041889. [DOI] [PubMed] [Google Scholar]
- 55.Yamada H, Shimoyama N, Sora I, Uhl GR, Fukuda Y, Moriya H, Shimoyama M. Morphine can produce analgesia via spinal kappa opioid receptors in the absence of mu opioid receptors. Brain Res. 2006;1083:61–69. doi: 10.1016/j.brainres.2006.01.095. [DOI] [PubMed] [Google Scholar]
- 56.Yeomans DC, Pirec V, Proudfit HK. Nociceptive responses to high and low rates of noxious cutaneous heating are mediated by different nociceptors in the rat: Behavioral evidence. Pain. 1996;68:133–140. doi: 10.1016/S0304-3959(96)03176-4. [DOI] [PubMed] [Google Scholar]
- 57.Yeomans DC, Proudfit HK. Nociceptive responses to high and low rates of noxious cutaneous heating are mediated by different nociceptors in the rat: Electrophysiological evidence. Pain. 1996;68:141–150. doi: 10.1016/S0304-3959(96)03177-6. [DOI] [PubMed] [Google Scholar]