Abstract
Objective
To document the influence of age on step activity patterns in children with cerebral palsy (CP) and typically-developing (TD) children.
Design
Cross-sectional
Setting
All step activity data were collected in free-living environments.
Participants
Children with CP (n=27; 4 –18 years; 22 boys, 5 girls, Gross Motor Function Classification System Levels I and II) and 27 age- and sex-matched TD children were recruited through public advertisements and contacts with local clinicians. CP and TD participants were stratified into younger (< 10 years; n = 14) and older (10–18 years; n = 13) age groups.
Intervention
Daily step activity was monitored using a StepWatch Activity Monitor that was individually programmed to account for the gait characteristics of each participant. Step activity data were collected in 1-min epochs during waking hours on three weekdays and one weekend day. Stored data were analyzed to yield average values of daily step activity, percentage of inactive time (0 steps) over the entire day, and percentage of total daily active time spent in low step activity (1–15 steps/min), medium step activity (16–40 steps/min), and high step activity (> 40 steps/min).
Main Outcome Measures
Daily step activity, percentage of inactive time, and percentage of active time spent in low-, moderate-, and high-intensity step activity.
Results
A significant (p < .05) interaction was observed between age (younger, older) and condition (CP, TD) for daily step activity, percentage of inactive time, and percentage of active time spent in low- and high-intensity step activity. The main effect of age was significant for each physical activity measure except for relative high-intensity step activity and the main effect of condition was significant for all physical activity measures. Followup analyses (p < .025) revealed that older children with CP took fewer daily steps and displayed higher relative levels of inactivity and low-intensity activity and lower relative levels of high-intensity activity compared to older TD. Older children with CP also exhibited lower daily step activity, demonstrated higher relative levels of inactivity and low-intensity activity, and displayed lower relative levels of moderate-intensity activity compared to younger children with CP.
Conclusion
Compared to younger children with CP and age- and sex-matched TD youth, older youth with CP generally displayed step activity patterns typified by lower levels of physical activity and a greater degree of inactivity. These findings highlight the need to provide multiple opportunities for adolescents with CP to engage in a variety of physical activities that are appropriate to the needs, abilities, and preferences of the child and can aid in maintaining functional mobility, health, and quality of life.
Keywords: Ambulation, Cerebral palsy, Difficulty walking, Physical activity, Rehabilitation
Cerebral palsy (CP) is a neurological syndrome characterized by non-progressive, but frequently adapting, abnormal patterns of posture and movement and changes in muscle tone. These abnormal motor patterns result from lesions or anomalies of the brain occurring in the early stages of development.1 On average, children with CP attain about 90% of expected motor function during early childhood, based on their Gross Motor Functional Classification System (GMFCS) level.2 The GMFCS categorizes children with CP into one of five levels based on self-initiated movement, with children classified as Level I demonstrating the highest degree of motor function. Although CP is a non-progressive condition, loss of physical function has been observed over time, resulting in higher GMFCS levels.3 These functional changes, which appear to be related to secondary complications of CP, include diminished motor performance and gait efficiency, as well as reduced participation in activities of daily living.4,5,6
In a longitudinal study of children with CP, researchers found that gross motor function peaked at a mean age of 7 years and 3 months before declining by an average of 6.3 points on the Gross Motor Functional Measure-66 (GMFM-66) scale during adolescence for youth in GMFCS Levels III, IV, and V.3 In addition, young children with CP who are able to walk often lose this ability as they reach the adolescent years. It has been demonstrated, for instance, that 75% of ambulatory children with CP who lose functional locomotion will experience this decrease in mobility by age 25.7 Because of the stable nature of the cerebral lesion in these individuals, reasons for this decline in physical function and mobility are poorly understood. Researchers have suggested that these losses may be attributed to increased postural insecurity related to physical growth, the residual effects of orthopedic surgery, and higher energy demands associated with increased weight and height.5 Consequently, it is vital to better understand the relationship between chronological age and functional ambulation as a first step towards promoting and maintaining greater levels of independent ambulation in youth with CP.
The mobility of children with CP is typically assessed in controlled settings (e.g., clinic, gait laboratory) that may not accurately reflect walking performance in the child’s natural environment.8 In one of the few non-clinical studies available, Bjornson et al9, reported significantly lower community stepping activity in youth with CP compared to a group of typically-developing children. While this study identified differences in stepping activity between children with CP and typically-developing peers, it did not specifically examine the influence of chronological age on locomotor status. Hence, there is a particular need to document age-related differences in the gait performance of children with CP in their natural surroundings and to determine the extent to which basic gait patterns in youth with CP vary from those exhibited by able-bodied children. Against this backdrop, the purpose of this study was to quantify community walking activity in younger and older children with CP and to compare findings with an age- and sex-matched group of typically-developing children.
Methods
Participants
Twenty-seven children with spastic CP (age = 4 to 18 years; 22 boys, 5 girls) and 27 age- and sex-matched typically-developing children were recruited to participate in this study through public advertisements and contacts with local clinicians. Both groups were stratified into younger- (<10 years; n=14) and older- (10 to 18 years; n= 13) aged samples. All participants with CP were independent ambulators and classified at GMFCS Level I (n =21) or II (n =6). A nearly equal number of children at both GMFCS levels were observed in the younger and older samples of youth with CP (younger age group: GMFCS Level I=11; GMFCS Level II= 3; older age group: GMFCS Level I=10; GMFCS Level II= 3). Table 1 provides descriptive information relative to GMFCS level, topographical distribution, and type of motor impairment for children with CP. Following approval from the university Institutional Review Board and prior to data collection, all participants and their parents or guardians provided written assent and consent, respectively.
Table 1.
GMFCS classification, topographical distribution, and type of motor impairment for children with cerebral palsy.
| Younger Children | Older Children | ||||||
|---|---|---|---|---|---|---|---|
| Subject Number |
GMFCS level |
Topographical designation |
Motor Impairment |
Subject Number |
GMFCS level |
Topographical designation |
Motor Impairment |
| 1 | I | Diplegic | Spastic | 1 | I | Diplegic | Dystonic |
| 2 | I | Hemiplegic | Spastic | 2 | I | Diplegic | Spastic |
| 3 | I | Diplegic | Spastic | 3 | II | Quadriplegic | Spastic |
| 4 | I | Diplegic | Spastic | 4 | I | Diplegic | Spastic |
| 5 | II | Diplegic | Spastic | 5 | I | Diplegic | Spastic |
| 6 | I | Diplegic | Spastic | 6 | II | Quadriplegic | Dystonic |
| 7 | I | Diplegic | Spastic | 7 | I | Diplegic | Spastic |
| 8 | I | Diplegic | Spastic | 8 | I | Diplegic | Spastic |
| 9 | I | Diplegic | Spastic | 9 | I | Diplegic | Spastic |
| 10 | II | Diplegic | Spastic | 10 | I | Diplegic | Spastic |
| 11 | II | Quadriplegic | Spastic | 11 | I | Diplegic | Dystonic |
| 12 | I | Hemiplegic | Spastic | 12 | I | Diplegic | Spastic |
| 13 | I | Diplegic | Spastic | 13 | II | Diplegic | Spastic |
| 14 | I | Triplegic | Spastic | ||||
Procedures
Daily step activity was monitored using an Orthocare Step Activity Monitora (SAM) that was programmed individually to account for the gait characteristics of each participant. The SAM has been evaluated and determined to be a valid and reliable measurement tool for assessing time-based ambulatory activity in community environments for children and adolescents.10 Subsequent testing has validated the SAM for use in atypical pediatric populations, including children with muscular dystrophy 11 and obesity 12. Additionally, in a recent study, Bjornson et al9 used the SAM to assess step activity in youth with CP.
Calibration of the SAM occurred during an initial observation session, during which adjustments were made for the expected sensitivity, cadence, threshold, and motion characteristics of each participant. Participants were observed walking overground and on a treadmill at several speeds to assure that all step activity was captured before using the activity monitors in community settings. If step counts were missed or if non-step activity was registered, sensitivity, cadence, threshold, and motion values were readjusted until all valid step activity was recorded. Participants and their primary caregiver(s) were provided with verbal and written instructions on how to properly wear and position the step monitor on the right ankle. Because step activity counts using the SAM reflect step activity of the right leg only, the step activity of both legs can be calculated by doubling the step activity of the right leg.
Step activity data were collected in 1-minute epochs during waking hours, with the exception of time spent showering or bathing, during three randomly-selected weekdays and one weekend day. This sampling schedule exceeded that proposed by Tudor-Locke et al., who found that accumulating at least three days of step count data was adequate to compute average daily step activity in children.13 Additional studies suggest that a minimum of four measurement days can provide reliable accelerometry estimates of daily physical activity in children and adults.14, 15 For each participant, step count data were stored within the SAM and downloaded and screened for accuracy at a later date. Step activity data were analyzed to obtain mean values of daily step activity, percentage of daily inactive time (0 steps), and percentage of daily active time spent in low-intensity (1 to 15 steps/min), moderate-intensity (16 to 40 steps/min), and high-intensity (> 40 steps/min) step activity.16
Statistical analyses were performed using SPSSb (Version 16.0). Five 2×2 factorial analyses of variance (ANOVA) [age (younger, older) by condition (CP, typically-developing)] were conducted to identify potential differences in 5 patterns of physical activity (i.e. daily step activity, percentage of daily inactive time, percentage of active time spent in low-intensity activity, percentage of active time spent in moderate-intensity activity, and percentage of active time spent in high-intensity activity) among children with CP and typically-developing children. Simple effect ANOVA tests were performed to further explore significant interactions.
Results
Descriptive statistics for each analysis are presented in Table 2 and factorial ANOVA results (alpha = .05) are shown in Table 3. Significant interactions were observed between age and condition for daily step activity, percentage of daily inactive time, percentage of active time spent in low-intensity step activity, and percentage of active time spent in high-intensity step activity. The main effect of age was significant for each physical activity measure except for percentage of active time spent in high-intensity activity. The main effect of condition was significant for all five measures of physical activity.
Table 2.
Daily step activity, percentage of daily inactive time, and percentage of active time spent in low-, moderate-, and high-intensity step activity for typically-developing youth and youth with cerebral palsy.
| Active Time by Intensity | |||||||
|---|---|---|---|---|---|---|---|
| Age Group |
Condition1 | Steps2 | Inactive% | Low % | Mod % | High % | |
| Younger Children |
Typical (n = 14) | M | 6630 | 66.2 | 63.9 | 30.3 | 5.9 |
| SD | 1707 | 5.9 | 4.5 | 5.0 | 3.0 | ||
| CP (n = 14) | M | 5801 | 70.1 | 67.1 | 27.7 | 5.2 | |
| SD | 2312 | 6.1 | 8.6 | 6.5 | 2.5 | ||
| Older Children |
Typical (n = 13) | M | 6812 | 67.0 | 63.4 | 27.5 | 9.2 |
| SD | 1983 | 8.0 | 6.1 | 4.3 | 4.5 | ||
| CP (n = 13) | M | 3171 | 80.7 | 75.7 | 20.7 | 3.7 | |
| SD | 1927 | 8.1 | 9.3 | 6.4 | 3.4 | ||
Typical = typically-developing youth, CP = youth with cerebral palsy;
Steps taken by the right leg only.
Table 3.
Factorial ANOVA Results for Each Physical Activity Measure
| Dependent Variable | Factor | F | p | Partial η2 |
|---|---|---|---|---|
| Daily Steps | Age | 5.07 | .029* | .092 |
| Condition | 16.91 | <.001* | .25 | |
| Interaction | 6.69 | .013* | .12 | |
| Inactivity % | Age | 8.73 | .005* | .15 |
| Condition | 21.09 | <.001* | .30 | |
| Interaction | 6.60 | .013* | .12 | |
| Low Activity % | Age | 4.09 | .049* | .076 |
| Condition | 14.80 | <.001* | .23 | |
| Interaction | 5.15 | .028* | .093 | |
| Moderate Activity % | Age | 10.28 | .002* | .17 |
| Condition | 9.19 | .004* | .16 | |
| Interaction | 1.91 | .17 | .037 | |
| High Activity % | Age | 0.98 | .33 | .019 |
| Condition | 10.68 | .002* | .18 | |
| Interaction | 6.79 | .012* | .12 | |
Note. df = (1, 50) for each F test.
p < .05;
Partial η2 = effect size.
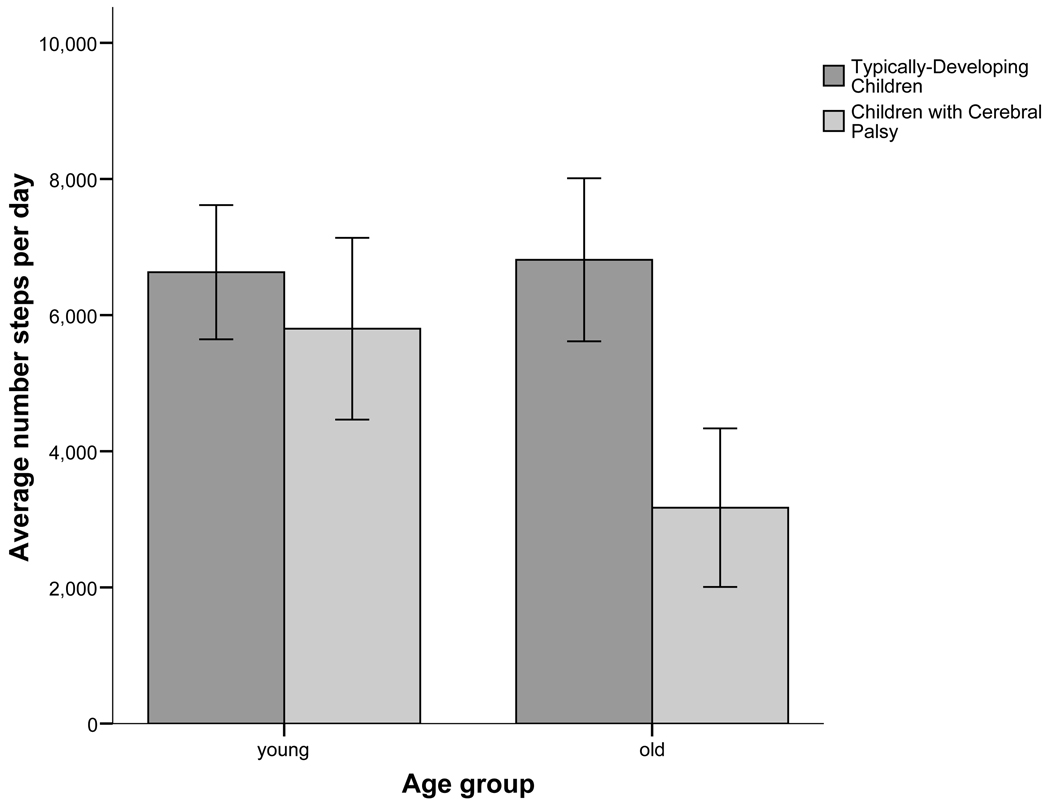
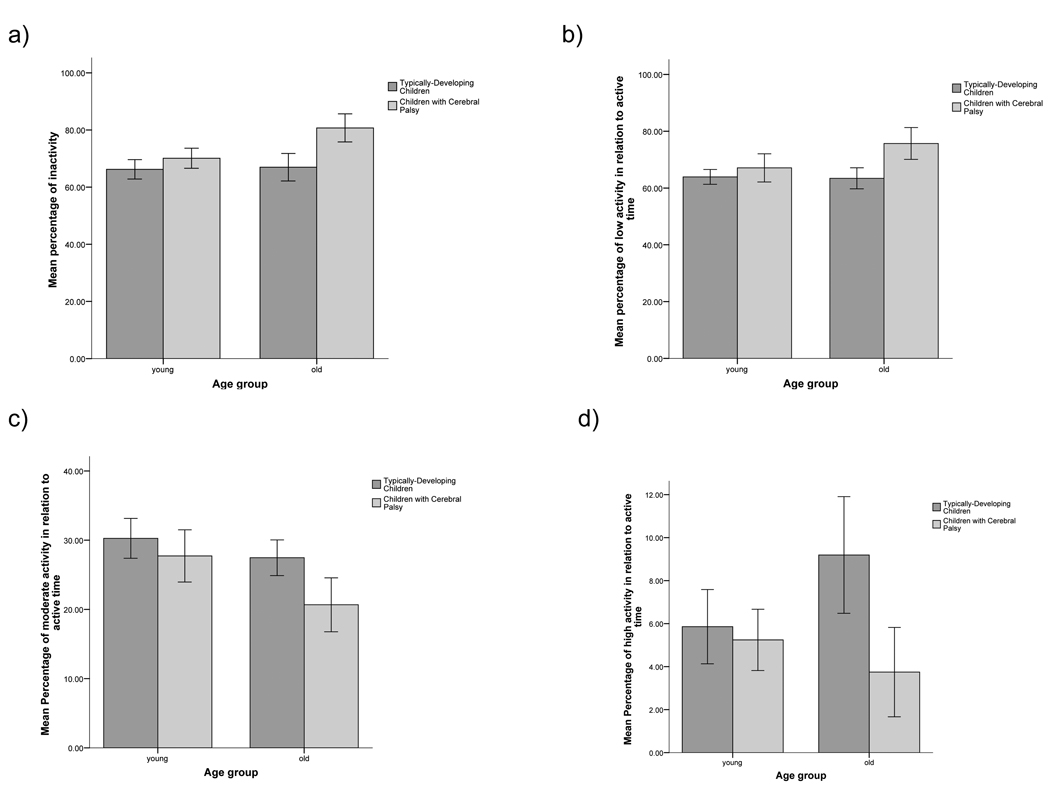
Results from simple effects ANOVA tests (adjusted alpha = .025) revealed no differences in daily step activity between young typically-developing children and young children with CP. However, for the older children, significant differences were noted for each physical activity measure. Specifically, older children with cerebral palsy took fewer daily steps and displayed higher relative levels of inactivity and low- intensity activity and lower relative levels of high-intensity activity compared to typically-developing older children, [F (1, 24) = 22.55, p < .001; F = 18.94, p < .001; F = 15.83, p = .001; and F = 12.06, p = .002, respectively]. Graphic descriptions of these results can be found in Figures 1 and 2.
Figure 1.
Mean daily steps activity for younger and older children with and without cerebral palsy, as measured using the right leg only.
Figure 2.
Mean percentage of (a) time per day spent being inactive, (b) active time spent at low-intensity step activity, (c) active time spent at moderate-intensity step activity, and (d) active time spent at high-intensity step activity for younger and older children with and without cerebral palsy.
No age-related differences in physical activity patterns were observed for typically-developing children. In contrast, among children with CP, age-related differences were present on a number of physical activity measures. Specifically, while relative levels of high-intensity activity were similar for younger and older children with CP, older children with CP exhibited lower daily step activity and demonstrated higher relative levels of inactivity and low-intensity activity and displayed lower relative levels of moderate-intensity activity compared to younger children with CP [F (1, 25) = 10.21, p = .004; F = 14.89, p = .001; F = 6.25, p = .019, F =7.98, p = .009, respectively].
Discussion
This study was designed to explore differences in daily step activity between children with CP and typically-developing children as a function of age. To reflect the typical movement activity of our participants, step activity was recorded in community settings. With regard to younger typically-developing children, the mean daily step count observed was 13,260 steps (6812 steps per day on one leg × 2 legs), which is nearly identical to the average of 13,110 steps per day (SD 2870) reported by Le Masurier et al. for typically-developing 6- to 9-year-old boys.17 Relative to older typically-developing children, the mean daily step count of 13,624 steps (6812 steps per day on one leg × 2 legs) noted in the present study was higher than, but within one standard deviation of, values published by Le Masurier and colleagues17, who reported an average walking activity of 11,082 steps per day (SD 3437) for 12- to 14-year-old males and 10,828 steps per day (SD 3241) for 15- to 18-year-old males. Although speculative, this variation in daily step count activity may reflect a bias towards a younger-aged sample in our investigation (10 to 18 years; mean age 13.6 years) and an older sample (12 to 18 years) in the Le Masurier paper.
Few data exist quantifying the daily step activity of young children with CP. In considering older children with CP, the mean daily step activity observed in the present investigation was 6342 steps (3171 steps per day × 2 legs) a value which is lower than the average of 8444 steps per day (4222 steps per day × 2 legs), reported by Bjornson, et al9 for ambulatory youth with CP (GMFCS Levels I and II). This difference in step count values may also be tied to age disparities between participants in both studies, as children in the Bjornson et al9. study ranged between 10 and 13 years of age (mean = 11.8 years), whereas youth with CP in the current investigation were generally older (mean age = 13.6 years, age range = 10 to 18 years).
The primary finding of our study is that among youth with CP displaying high levels of gross motor function, the quantity and intensity of daily step activity is generally lower in older children with CP compared to younger children with CP. When compared to typically-developing peers, older youth with CP also took fewer steps each day, were more inactive, spent more time engaged in low-intensity step activity and devoted less time participating in high-intensity step activity. This age-related reduction in daily step activity may be linked to modifications in several anatomical and physiological variables. Relative to this point, Johnson, Damiano, and Abel18 suggest that an age-related decrease in dynamic stability in children with CP is associated with a diminished range of motion in the pelvis, knees, and ankle and, a decrease in the popliteal angle. Children with CP also adopt compensatory patterns of motion to accommodate movement restrictions imposed by abnormal muscle tone in the hips and knees.19 Over time, these adaptive strategies, while enabling purposeful ambulation, require increased hip and knee extensor muscle effort to maintain joint stabilization, thus leading to a higher walking energy demand, greater fatigue, and reduced levels of physical activity. 19 In addition, children with CP who display greater muscle weakness tend to exhibit more limitations in motor function. 20 Along these lines, Ross and Engsberg 21 reported that leg strength explained 69% of the variation in GMFC-66 scores, an amount exceeding that accounted for by spasticity alone.21 It has also been demonstrated that hip and knee extensor muscles, which provide major support for the body during locomotion, are weaker in youth with CP. 20 In the face of primary muscle weakness, walking becomes more difficult to accomplish in the older child with CP because body mass and adiposity increase without commensurate gains in lower-extremity strength. Hence, walking in a natural environment may become laborious and energy-expensive. It has been shown that the energy cost of walking increases with advancing age in youth with cerebral palsy22, a trend which runs counter to the age-related reduction in walking energy use observed in able-bodied youth. 23 Because maximal aerobic power values are lower in the child with CP compared to typically-developing youth 24, the older child with cerebral palsy incurs a higher relative energy demand at any given walking speed and experiences a greater level of fatigue.
From an overall health perspective, results from our study illustrate the disparity between recommended levels of health-producing physical activity and the volume and relative intensity of physical activity documented in our sample of older children with CP. In a systematic review of more than 850 articles25, 60 minutes per day of moderate to vigorous physical activity was recommended for school-aged youth to improve measures of aerobic fitness, muscular strength and endurance, and skeletal health. It should be emphasized that this guideline represents minimal levels of physical activity and more health benefits may be realized if greater amounts of physical activity are performed.25 Guidelines established by the Centers for Disease Control (CDC) also indicate that youth should perform 60 minutes per day of aerobic activity (such as brisk walking or running) and that 30 minutes of vigorous aerobic activity should be included at least three times per week.26 When translated from percentages to absolute time measures, younger and older typically-developing children and younger children with CP achieved the CDC duration guidelines when total time spent in moderate and vigorous activity (based on currently existing steps/min criteria16) were considered. However, older children with CP did not attain the CDC activity criterion for combined moderate and vigorous physical activity. Taken together, results from our investigation support the notion that older children with CP should be encouraged to increase daily levels of daily physical activity to avoid negative health consequences related to sedentary living, such as increased weight, decreased cardiovascular health, increased levels of anxiety and depression, loss of muscle strength and endurance, and decreased bone mineral density.25 In cases where the intensity of moderate and vigorous walking may be too exhausting, alternative types of locomotor activity, such as wheelchair sports and exercise, can be performed. Engaging in strengthening activities which address specific deficits causing decreased ambulation, such as lower extremity muscle weakness, may also improve or prolong mobility in youth with CP. In this regard, the use of resistive exercise, has resulted in greater strength and functional improvement in adolescents with CP without adverse effects.27
Physical activity has been described as a buffer in the cycle of disuse which often accompanies physical disability. 28 While it has been suggested that an active lifestyle and improved physical conditioning can improve physical health and function and reduce secondary health consequences in children with CP 28, an inverse relationship has been observed between physical inactivity and quality of life in adolescents and adults with CP.29,30,31 Maher, Olds, Williams, and Lane documented self-reported quality of life in adolescents with CP and found that 67% of participants reported quality of life scores greater than one standard deviation below the mean for children without disabilities.32 Furthermore, quality of life scores were significantly related to gross motor function, such that lower quality of life scores were observed in adolescents displaying more physical limitations32. Although speculative, these limited findings suggest that the accumulation of regular doses of physical activity on a daily basis may enhance both quality of life and overall physical functioning in adolescents with CP.
Study Limitations
As a final comment, it is important to emphasize the descriptive nature of this investigation, as two distinct cohorts of younger and older children classified as either typically-developing or diagnosed with CP were studied. Consequently, variation in physical activity levels between younger and older groups of typically-developing participants or participants with CP may be partially attributable to factors which are independent of age. Given this limitation, future research should be conducted to track activity patterns in youth with CP during childhood and adolescence.
Conclusions
In conclusion, when compared to younger counterparts and to age- and sex-matched typically-developing peers, older youth with CP generally displayed step activity patterns typified by lower levels of physical activity and a greater degree of inactivity. Viewed collectively, these findings highlight the need to provide multiple opportunities for adolescents with CP to engage in a variety of physical activities that are appropriate to the needs, abilities, and preferences of the child and can aid in maintaining functional mobility, health, and quality of life.
Acknowledgments
Supported by a grant from the National Institutes of Health (grant no. HD48742).
List of Abbreviations
- ANOVA
analyses of variance
- CDC
Centers for Disease Control
- CP
cerebral palsy
- GMFCS
Gross Motor Functional Classification System
- GMFM-66
Gross Motor Functional Measure-66
- SAM
Step Activity Monitor
- TD
typically-developing
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented to the American Academy for Cerebral Palsy and Developmental Medicine, September 17–20, 2008, Atlanta, GA.
No commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit on the authors or on any organization with which the authors are associated.
Suppliers
Orthocare Step Activity Monitor. 6405 218th St SW, Suite 100 Mountlake Terrace, WA 98043-2180
SPSS Inc, 233 S Wacker Dr, 11th floor, Chicago, IL 60606
References
- 1.Gupta R, Appleton RE. Cerebral palsy: not always what it seems. Arch Dis Child. 2001;85:356–361. doi: 10.1136/adc.85.5.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenbaum PL, Walter SD, Hanna SE, et al. Prognosis for gross motor function in cerebral palsy: creation of motor development curves. JAMA. 2002;288:1357–1363. doi: 10.1001/jama.288.11.1357. [DOI] [PubMed] [Google Scholar]
- 3.Hanna S, Rosenbaum P, Bartlett D, et al. Stability and decline in gross motor function among children youth with cerebral palsy aged 2 to 21 years. Dev Med Child Neurol. 2009;51:295–302. doi: 10.1111/j.1469-8749.2008.03196.x. [DOI] [PubMed] [Google Scholar]
- 4.Novacheck TF, Gage JR. Orthopedic management of spasticity in cerebral palsy. Childs Nerv Syst. 2007;23:1015–1032. doi: 10.1007/s00381-007-0378-6. [DOI] [PubMed] [Google Scholar]
- 5.Bottos M, Gericke C. Ambulatory capacity in cerebral palsy: prognostic criteria and consequences for intervention. Dev Med Child Neurol. 2003;45:786–790. doi: 10.1017/s0012162203001452. [DOI] [PubMed] [Google Scholar]
- 6.Maher CA, Williams MT, Olds T, Lane AE. Physical and sedentary activity in adolescents with cerebral palsy. Dev Med Child Neurol. 2007;49:450–457. doi: 10.1111/j.1469-8749.2007.00450.x. [DOI] [PubMed] [Google Scholar]
- 7.Murphy KP, Molnar GE, Lankasky K. Medical and functional status of adults with cerebral palsy. Dev Med Child Neurol. 1995;37:1075–1084. doi: 10.1111/j.1469-8749.1995.tb11968.x. [DOI] [PubMed] [Google Scholar]
- 8.Tieman BL, Palisano RJ, Gracely EJ, Rosenbaum PL. Gross motor capability and performance of mobility in children with cerebral palsy: a comparison across home, school, and outdoors/community settings. Phys Ther. 2004;84:419–429. [PubMed] [Google Scholar]
- 9.Bjornson KF, Belza B, Kartin D, Logsdon R, McLaughlin JF. Ambulatory physical activity performance in youth with cerebral palsy and youth who are developing typically. Phys Ther. 2007;87:248–257. doi: 10.2522/ptj.20060157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McDonald CM, Widman LM, Abresch RT, Walsh SA, Walsh DD. Utility of a step activity monitor for the measurement of daily ambulatory activity in children. Arch Phys Med Rehabil. 2005;86:793–801. doi: 10.1016/j.apmr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 11.McDonald CM, Widman LM, Walsh DD, Walsh SA, Abresch RT. Use of a step activity monitoring for continuous physical activity assessment in boys with Duchenne muscular dystrophy. Arch Phys Med Rehabil. 2005;86:802–808. doi: 10.1016/j.apmr.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 12.McDonald CM, Walsh D, Widman LM, Walsh SA. Use of a step activity monitoring for continuous objective physical activity assessment in children with obesity. Dev Med Child Neurol. 2000;42:22–23. [Google Scholar]
- 13.Tudor-Locke C, Ainsworth BE, Thompson RW, Matthews CE. Comparison of pedometer and accelerometer measures of free-living physical activity. Med Sci Sports Exerc. 2002;34:2945–2951. doi: 10.1097/00005768-200212000-00027. [DOI] [PubMed] [Google Scholar]
- 14.Berlin JE, Storti KL, Brach JS. Using activity monitors to measure physical activity in free-living conditions. Phys Ther. 2006;8:1137–1145. [PubMed] [Google Scholar]
- 15.Trost SG, Pate R, Freedson PS, et al. Using objective physical activity measures with youth: how many days of monitoring are needed? Med Sci Sports Exerc. 2000;32:426–431. doi: 10.1097/00005768-200002000-00025. [DOI] [PubMed] [Google Scholar]
- 16.StepWatch™ 3.1 User’s Manual. Activity Level Definitions. Mountlake Terrace, WA: Cyma Corporation; 2006. [Google Scholar]
- 17.Le Masurier G, Beighle A, Corbin C, Darst P, Morgan C, Pangrazi R, et al. Pedometer-detemined physical activity levels of youth. J Phys Act Health. 2005;2:159–168. [Google Scholar]
- 18.Johnson DC, Damiano DL, Abel MF. The evolution of gait in childhood and adolescent cerebral palsy. J Pediatr Orthop. 1997;17:392–396. [PubMed] [Google Scholar]
- 19.Waters R, Mulroy S. The energy expenditure of normal and pathological gait. Gait Posture. 1999;9:207–231. doi: 10.1016/s0966-6362(99)00009-0. [DOI] [PubMed] [Google Scholar]
- 20.Damiano D, Abel M. Functional outcomes of strength training in spastic cerebral palsy. Arch Phys Med Rehabil. 1998;79:119–125. doi: 10.1016/s0003-9993(98)90287-8. [DOI] [PubMed] [Google Scholar]
- 21.Ross SA, Engsberg JR. Relationship between spasticity, strength, gait, and the GMFC-66 in persons with spastic diplegia cerebral palsy. Arch Phys Med Rehabil. 2007;88:1114–1120. doi: 10.1016/j.apmr.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 22.Campbell J, Ball J. Energetics of walking in cerebral palsy. Orthop Clin N Am. 1978;9:374–377. [PubMed] [Google Scholar]
- 23.Morgan DW, Tseh W, Caputo JL, Keefer DK, Craig IS, Griffith KB, Akins MB, Griffith GE, Martin PE. Longitudinal profiles of oxygen uptake during treadmill walking in able-bodied children: The locomotion energy and growth study. Gait Posture. 2001;15:230–235. doi: 10.1016/s0966-6362(01)00160-6. [DOI] [PubMed] [Google Scholar]
- 24.Hoofwijk M, Unnithan V, Bar Or O. Maximal treadmill performance of children with cerbral palsy. Pediatr Exerc Sci. 1995;7:305–313. [Google Scholar]
- 25.Strong WB, et al. Evidence-based physical activity for school-aged youth. J Pediatr. 2005;146:732–737. doi: 10.1016/j.jpeds.2005.01.055. [DOI] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention. Physical activity for everyone: Guidelines: Children. [retrieved 7/04/10]; http://www.cdc.gov/physicalactivity/everyone/guidelines/children.html.
- 27.Dodd KJ, Taylor NF, Damiano DL. A systematic review of the effectiveness of strength-training programs for people with cerebral palsy. Arch Phys Med Rehabil. 2002;83:1157–1164. doi: 10.1053/apmr.2002.34286. [DOI] [PubMed] [Google Scholar]
- 28.Damiano DL. Activity, activity, activity: rethinking our physical therapy approach to cerebral palsy. Phys Ther. 2006;86:1534–1540. doi: 10.2522/ptj.20050397. [DOI] [PubMed] [Google Scholar]
- 29.Varni J, Burwinkle T, Sherman S, et al. Health-related quality of life of children and adolescents with cerebral palsy: hearing the voices of children. Dev Med Child Neurol. 2005;47(9):592–597. [PubMed] [Google Scholar]
- 30.Svien L, Berg P, Stephenson C. Issues in aging with cerebral palsy. Top Geriatr Rehabil. 2008;24:26–40. [Google Scholar]
- 31.Shelly A, Davis E, Waters E, et al. The relationship between quality of life and functioning for children with cerebral palsy. Dev Med Child Neurol. 2008;50(3):199–203. doi: 10.1111/j.1469-8749.2008.02031.x. [DOI] [PubMed] [Google Scholar]
- 32.Maher C, Olds T, Williams M, Lane A. Self-reported quality of life in adolescents with cerebral palsy. Phys Occup Ther Pediatr. 2008;28:41–57. doi: 10.1300/j006v28n01_04. [DOI] [PubMed] [Google Scholar]