Abstract
BACKGROUND
The symptoms of PTSD can be explained, at least in part, as an inability to inhibit learned fear during conditions of safety. Our group has shown that fear inhibition is impaired in both combat and civilian PTSD populations. Based on our earlier findings, we employed an established fear extinction paradigm to further explore fear dysregulation in a civilian traumatized population.
METHODS
Fear-potentiated startle was examined in 127 trauma-exposed individuals with and without PTSD. We used a protocol in which conditioned fear was first acquired through the presentation of one colored shape (reinforced conditioned stimulus, CS+) that was paired with an aversive airblast to the larynx (unconditioned stimulus, US) and a different colored shape that was not paired to the airblast (nonreinforced condition stimulus, CS−). Fear was extinguished 10 minutes later through repeated presentations of the CSs without reinforcement.
RESULTS
Both groups demonstrated successful fear conditioning based on startle and US-expectancy ratings, however, participants with PTSD displayed greater fear-potentiated startle responses to the CS+ and CS− compared to the group without PTSD. During fear extinction, the PTSD group showed elevated fear-potentiated startle responses to the previously reinforced CS+ during the early and middle stages of extinction. During the acquisition and extinction phases, PTSD participants with higher levels of re-experiencing symptoms exhibited greater potentiated startle responses to the CS+ compared to PTSD participants with lower re-experiencing symptoms.
CONCLUSIONS
These results suggest that PTSD is associated with enhanced fear learning and a greater “fear load” to extinguish after conditioned fear is acquired.
Keywords: Fear-potentiated startle, trauma, anxiety disorders, psychophysiology, fear extinction
Introduction
Posttraumatic stress disorder (PTSD) is a debilitating psychiatric illness with neurobiological abnormalities that can develop after exposure to a life-threatening event. PTSD symptoms include persistent re-experiencing of the trauma, avoidance of trauma-related stimuli, and increased arousal(1). These symptoms are believed, in part, to reflect a patients’ inability to inhibit conditioned fear, most notably in symptoms of re-experiencing(2–4). From a fear conditioning perspective, the trauma serves as an unconditioned stimulus (US) that elicits an unconditioned response (UR) that includes intense fear and arousal. The UR is then associated with cues or stimuli in the traumatic environment (context) such as sights or smells that become conditioned stimuli (CSs). Through this CS-US association, these cues later produce conditioned responses such as fear and panic despite the absence of the original US. As a result of these associations, many re-experiencing symptoms can be characterized as persistent conditioned responses(2, 5).
PTSD has been observed in traumatized individuals who have (a) experienced combat situations such as Operations Iraqi Freedom (OIF)(6), (b) been victimized by violent crime(7), (c) exposed to a natural disaster such as the 2004 Florida hurricanes(8), or (d) experienced a terrorist attack such as that of September 11, 2001(9). An effective treatments for PTSD is exposure therapy, a form of extinction learning in which patients are repeatedly exposed to trauma-related stimuli to promote fear inhibition and tolerate the stimuli without suffering extreme fear(10–13).
Our group has examined fear processing in PTSD and its co-morbid disorders through the study of the acoustic startle response (ASR). This is a well-characterized reflexive response to a sudden acoustic stimulus that is mediated by a simple neural circuit. This reflex is modulated under specific conditions as a result of Pavlovian conditioning(14). For example, when a subject is presented with a stimulus (e.g., a colored shape, termed CS) at the same time as an aversive stimulus (e.g., an unpleasant airblast, termed the US), the subject quickly learns that the shape predicts the unpleasant event. Fear-potentiated startle (FPS) is the relative increase in the amplitude of the ASR when a subject sees a CS that predicts the US. Through the use of fear-potentiated startle methods, our group has shown that both combat(15) and civilian(16) PTSD patients are unable to inhibit fear upon presentation of safety signals.
Previous studies on the fear extinction in PTSD patients have suggested that one of the neurobiological mechanisms underlying PTSD symptomatology is impaired inhibition of the amygdala by the prefrontal cortex during extinction learning. For example, Milad and others have reported amygdala hyperactivity during extinction learning and impaired extinction recall or retention, through the use of skin conductance measures, in Vietnam veterans(17) and in a population with diverse trauma histories(18). In addition, an Australian study of civilian trauma victims found a reduction in amygdala activity and an increase in prefrontal cortical activity as PTSD symptoms improved with exposure-based treatment(19).
Recent studies from our group and others(20–22) have shown that African-Americans with low income living in urban environments represent a high-risk population for experiencing a traumatic event and subsequently developing PTSD. Based on these findings and the prior literature, we hypothesized that traumatized individuals with PTSD would display altered fear extinction as compared to traumatized individuals without PTSD in a fear-potentiated startle paradigm.
Methods
Participants
Participants were recruited as part of a larger study investigating the genetic and environmental factors that contribute to PTSD in a primarily African-American, low socioeconomic, inner-city population in Atlanta, GA(22,23). Exclusion criteria included active psychosis and major medical illnesses as assessed by history and physical examinations. Participants were also excluded for urine toxicology that was positive for cocaine and hearing impairment. Prior to their participation, all participants provided written informed consents approved by the Emory University Institutional Review Board.
Psychological Assessment
The following measures were used to index PTSD symptoms, depressive symptoms, childhood trauma history and adult trauma history, respectively: Modified PTSD Symptom Scale (PSS)(24–26), Beck Depression Inventory (BDI)(27), Childhood Trauma Questionnaire (CTQ)(28,29), and the Traumatic Events Inventory (TEI)(26). These measures have all been used previously in our work with this population(23). The categorical definition of PTSD+ vs. PTSD- was determined from responses to the DSM-IV-based PSS questionnaire A-E criteria (A, presence of trauma; B, presence of at least one re-experiencing symptom; C, presence of at least 3 avoidant/numbing symptoms; D, presence of at least 2 hyper-arousal symptoms; E, occurrence for at least one month).
Psychophysiological Assessment
Figure 1A illustrates the experimental session. The fear-potentiated startle protocol consisted of two phases: Fear Acquisition and Fear Extinction. Fear Acquisition began with a habituation phase in which the conditioned stimuli (CSs) were presented without any reinforcement; the conditioning phase consisted of three blocks with four trials of each type (a reinforced conditioned stimulus, CS+; a nonreinforced conditioned stimulus, CS−; and the 40 ms, 108 dB noise probe alone, NA). Figure 1B illustrates the CS trials. Both CSs were colored shapes presented on a computer monitor for 6 sec. The US was a 250-ms, 140-p.s.i airblast directed at the larynx as in our previous studies(16,30). In all phases, the inter-trial intervals were randomized to be 9 to 22 sec.
Figure 1.
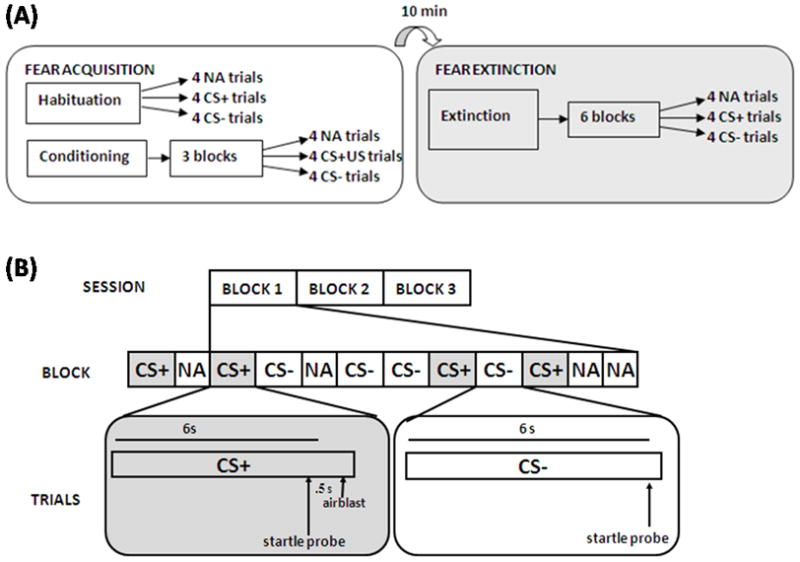
(A) Schematic illustration of the experimental paradigm. (B) Representative breakout diagram of the conditioned stimuli (CS+ and CS−) trial types during the Acquisition Session. Note: The previously reinforced CS+ in the Extinction Session did not include the airblast (US).
Ten minutes after Fear Acquisition, participants underwent the Fear Extinction phase. During these ten minutes the participants engaged in a trauma-neutral task designed to assess attention. Extinction consisted of 6 blocks with four trials of each type (the previously reinforced CS+, CS−, and NA), see Figure 1A. None of the CS presentations during Extinction were reinforced with US.
The startle response data were acquired using the electromyography (EMG) module of the BIOPAC MP150 (Biopac Systems, Inc., Aero Camino, CA) according to our previously published methods(16). The eyeblink component of the acoustic startle response was measured by EMG recordings of the right orbicularis oculi muscle(15,30,31). The startle probe was a 108-dB (A) SPL, 40-ms burst of broadband noise.
A response keypad (SuperLab, Cedrus Corp., San Pedro, CA) was used during each acoustic startle session to record the participants’ expectancy of the US on each CS presentation(32).
Data Analysis
Demographic and clinical data such as age, PTSD symptoms, and childhood and adult trauma history were compared between the PTSD+ and PTSD- groups using a one-way analysis of variance (ANOVA); categorical data, such as sex and race were analyzed using Chi square analyses.
Fear-potentiated startle was calculated using a Difference Score ([startle magnitude in the presence of a CS in each conditioning block] – [startle magnitude to the noise probe alone (NA)]). These variables were analyzed in a mixed ANOVA with the within-subject factor of Block (3 levels for Acquisition; 6 levels for Extinction), trial type (2 levels, CS+ and CS−), and the between-groups factor of Diagnosis (2 levels, PTSD+ or PTSD−). Late Acquisition was defined as block 3 of Acquisition, when discrimination learning was at maximum; Extinction was divided into 3 phases: early (blocks 1 and 2), mid (blocks 3 and 4), and late (blocks 5 and 6) extinction. Significant interactions were followed up by univariate analyses of covariance (ANCOVAs), with depression (BDI), childhood trauma (CTQ), and adult trauma (TEI) used as covariates in all analyses involving diagnostic groups. Baseline startle was measured by comparing average startle magnitude to the noise probe alone between diagnostic groups. Contingency awareness was analyzed by comparing US expectancy ratings of each CS trial with a Repeated Measures ANOVA with diagnostic group as a between-groups factor. All statistical analyses were performed in SPSS 17.0 for Windows (SPSS, Inc., Chicago, IL), with α=0.05.
Results
Participants
One hundred and twenty-seven participants were enrolled; 78 of which did not meet criteria for PTSD (PTSD−) and 49 who met criteria for PTSD (PTSD+). Table 1 illustrates the demographic and clinical information of the PTSD+ and PTSD− participants.
Table 1.
Sample Demographic and Clinical Data
| Demographics | PTSD (n=74) | Non-PTSD (n=123) | |
|---|---|---|---|
| Sex (% female) | 58.9 | 55.3 | ns |
| Race (% AA) | 95.9 | 94.3 | ns |
| Age (M, SD) | 41.82 (11.82) | 41.64 (13.53) | ns |
| Trauma history | |||
| Childhood trauma (M, SD) | 51.43 (22.24) | 38.97 (15.23) | p<0.0001 |
| Z score (CTQ) | 0.56 | −0.35 | p<0.0001 |
| Adult trauma (M, SD) | 4.46 (2.37) | 2.76 (2.19) | p<0.0001 |
| Z score (TEI) | 0.41 | −0.26 | p<0.0001 |
| PTSD symptoms | |||
| Total (M, SD) | 25.15 (9.71) | 6.66 (6.35) | p<0.0001 |
| Re-experiencing (M, SD) | 6.27 (3.81) | 1.38 (2.11) | p<0.0001 |
| Avoidance (M, SD) | 10.58 (4.39) | 2.50 (3.13) | p<0.0001 |
| Hyper-arousal (M, SD) | 8.37 (3.65) | 2.79 (3.31) | p<0.0001 |
Clinical Assessment
As shown in Table 1, PTSD+ participants had higher levels of childhood (F(1,120)=30.64,p<0.001) and adult trauma (F(1,126)=15.26,p<0.001) than PTSD− participants as determined by the CTQ and TEI, respectively. Note that although the total trauma levels were slightly higher in the PTSD+ group, the PTSD− group was also a significantly traumatized cohort. Table 1 also presents the trauma levels as standardized Z scores for group comparison.
As expected, PTSD+ participants had higher total PSS scores (F(1,126)=203.83,p<0.001) and higher symptom cluster sub-scores for re-experiencing (F(1,126)=89.53,p<0.001), avoidance/numbing (F(1,126)=144.80,p<0.001), and hyper-arousal (1,126)=117.28,p<0.001) as compared to the PTSD− group. The PTSD+ group also reported greater depressive symptoms than the PTSD− group as measured by the BDI (F(1,125)=58.09,p<0.001).
Conditioned Fear Acquisition: Fear-potentiated Startle
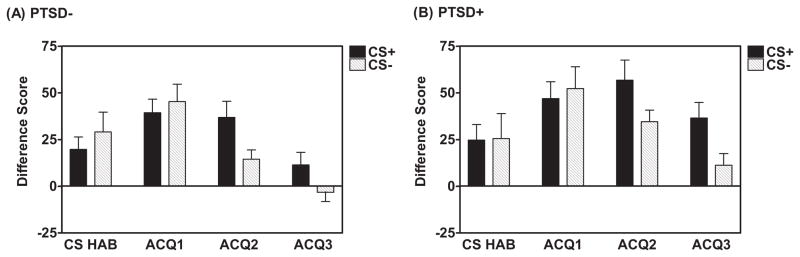
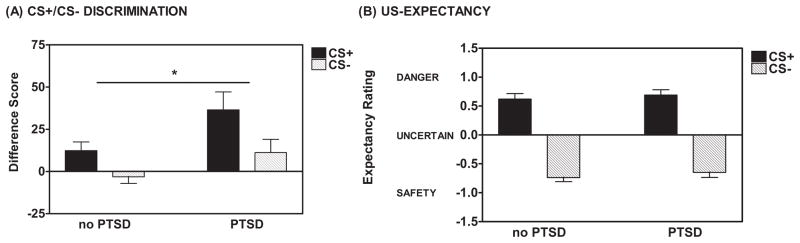
There was no Group difference between the PTSD+ and PTSD− groups with regard to baseline (NA) startle response during Fear Acquisition (F(1,124)=0.33,ns). During late Acquisition, defined as the 3rd block of the Acquisition phase, participants displayed robust fear-potentiated startle to the CS+ as compared to the NA; F(1,124)=41.67,p<0.001) with no Group difference between PTSD+ and PTSD− participants (no Block x Trial Type x Group interaction and no Between-Subjects Effect). In addition, participants demonstrated a significant Block x Trial Type interaction (F(2,248)=8.60,p<0.001) and a clear discrimination between the CS+ and CS−, expressed as a Difference Score, during late Acquisition (F(1,124)=15.09,p<0.001), but no significant Block x Trial Type x Group interaction. Figure 2 (panels A and B) shows the development of the fear discrimination across blocks for the two groups. There was a significant difference between the PTSD+ and PTSD− groups with regard to startle responses to both trial types as expressed as a Difference Score, during late Acquisition (F(1,124)=6.13,p<0.05, see Figure 3A). PTSD+ participants displayed greater responding to both the CS+ and CS− compared to the PTSD− group. Note that both groups showed significant discrimination between the CS+ and CS−. The increased response to the CSs in the PTSD+ group remained evident when co-varying for depressive symptoms (as measured by BDI score) and adult and childhood trauma exposure (as measured by TEI and CTQ, respectively; F(1,114)=5.00,p<0.05). There was no significant Block x Trial Type x Group interaction (F(1,124)=0.90,ns).
Figure 2.
Development of fear – potentiated startle and CS+/CS− discrimination during the Acquisition Session in (A) PTSD− and (B) PTSD+. Difference Score = [Mean startle response to the CS] – [Mean startle response to the noise probe alone (NA)]. Significant Block x Trial Type interaction (F(2,248)=8.60,p<0.001). CS HAB = Habituation phase consisting of 4 presentations each of the noise probe alone and the CS+ (without US pairing) and CS−. ACQ = Acquisition phase
Figure 3.
(A) Using a fear-potentiated startle paradigm, traumatized individuals with PTSD showed greater potentiated startle responses to the reinforced conditioned stimulus (CS+) and the nonreinforced conditioned stimulus (CS−) compared to traumatized individuals without PTSD during late acquisition. Late acquisition was defined as the third block of the Fear Acquisition phase. Difference Score = [Mean startle response to the CS] – [Mean startle response to the noise probe alone (NA)]. * ANOVA, Between-Subjects Effect, F(1,124) =6.13,p<0.05. (B) Based on US-expectancy ratings, the groups with and without PTSD did not significantly differ in their discrimination between the CS+ and CS− during late acquisition. Responses of DANGER indicated that an individual expected the unconditioned stimulus (US) on a CS trial and were scored as +1. Responses of UNCERTAIN were scored as 0 and responses of SAFETY indicated that an individual did not expect the US on a CS trial and were scored as −1.
Fear Acquisition across PTSD symptom sub-clusters
In order to examine whether the group differences in Acquisition were due to a particular PTSD symptom cluster, we categorized all participants into high and low symptom groups, using a median split of the symptoms of each cluster on the PSS, as we have done in our previous work(15,16). Using an ANCOVA with BDI, CTQ, and TEI as covariates, we found that individuals who had high re-experiencing symptoms had greater fear-potentiated startle to the CS+ during late Acquisition (F(1, 114)=8.22,p=0.005) relative to those with low re-experiencing symptoms. High and low avoidance symptom groups did not differ on fear-potentiated startle (F(1, 114)=2.96,ns) while high and low hyper-arousal groups did (F(1, 114)=4.39,p<0.05).
Conditioned Fear Acquisition: US-Expectancy
Participants rated their expectancy of the US on each CS presentation during Acquisition. During late Acquisition, participants correctly identified the CS+ as predicting the US (DANGER) and the CS− as predicting the absence of the US (SAFETY; F(1,87)=198.76,p<0.001, see Figure 3B) with no Group difference between the PTSD+ and PTSD− groups (F(1,87)=0.01,ns. There was no significant Block x Trial Type x Group interaction and no Between-Subjects Effect, F(1,87)=1.26,ns). Note that although the psychophysiological response to fear was markedly increased in PTSD+ subjects, US expectancy was similar between the groups.
Within-session Fear Extinction: Fear-potentiated Startle
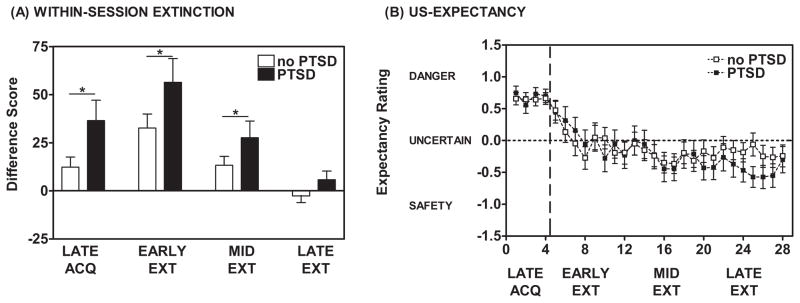
There was no Group difference between PTSD+ and PTSD− participants with regard to baseline (NA) startle during Fear Extinction (F(1,116)=0.12,ns), and no difference in startle habituation during Extinction (F(1,116)=0.12,ns). Participants displayed significant within-session extinction of fear-potentiated startle to the previously reinforced CS+ (F(1,114)=36.35,p<0.001) with a significant Group difference between the PTSD+ and PTSD− groups (Between-Subjects Effect, F(1,114)=4.06,p<0.05 but no significant Block x Group interaction, F(1,114)=1.00,ns, see Figure 4A). The PTSD+ group exhibited a greater degree of fear-potentiated startle to the previously reinforced CS+ during Extinction as compared to the PTSD− group; an effect that remained evident when co-varying for depressive symptoms and adult and childhood trauma (F(1,104)=10.03,p<0.01). When co-varying for depressive symptoms and trauma history, there was a significant Block x Group interaction (F(1,104)=3.97,p< 0.05). We found that the PTSD+ subjects had higher fear-potentiated startle during early (1st 2 blocks) and mid (blocks 3 and 4) Extinction than the PTSD− subjects. By late Extinction (blocks 5 and 6), both groups had low fear potentiation to the extinguished CS+.
Figure 4.
(A) During Early and Mid-Extinction, individuals with PTSD (dark bars) displayed higher fear-potentiated startle responses to the previously reinforced CS+ as compared to those without PTSD (open bars). * ANOVA, significant Between-Subjects Effect, F(1,114) = 4.06, p < 0.05. (B) Based on US-expectancy ratings, the groups with and without PTSD did not significantly differ in their expectancy of the US during the Fear Extinction phase. Both groups exhibited a reduction in their expectancy of the US as the Fear Extinction phase progressed (F(1,47) = 16.30, p < 0.001). Responses of DANGER indicated that an individual expected the unconditioned stimulus (US) on a CS trial and were scored as +1. Responses of UNCERTAIN were scored as 0 and responses of SAFETY indicated that an individual did not expect the US on a CS trial and were scored as −1.
During Extinction, there were no group differences in startle to the CS− (F(1,113)=0.20,ns, no significant Block x Trial Type x Group interaction and no significant Between-Subjects Effect, F(1,113)=1.65,ns).
Within-session Fear Extinction: US Expectancy
As in Acquisition, participants rated their expectancy of the airblast US on each CS presentation. The PTSD+ and PTSD− groups exhibited a significant reduction in ratings of DANGER on CS+ trials (F(1,47)=16.30,p<0.001, see Figure 4B) with no Group difference between PTSD+ and PTSD− groups (Trial x Group interaction, F(1,47)=1.03,ns; Between-Subjects Effect, F(1,47)=0.48,ns). Note that as with the US-expectancy above, in contrast to the different physiological levels of expression of fear, both the PTSD+ and PTSD− groups demonstrated equivalent US-expectancy across Extinction.
Fear Extinction across PTSD symptom sub-clusters
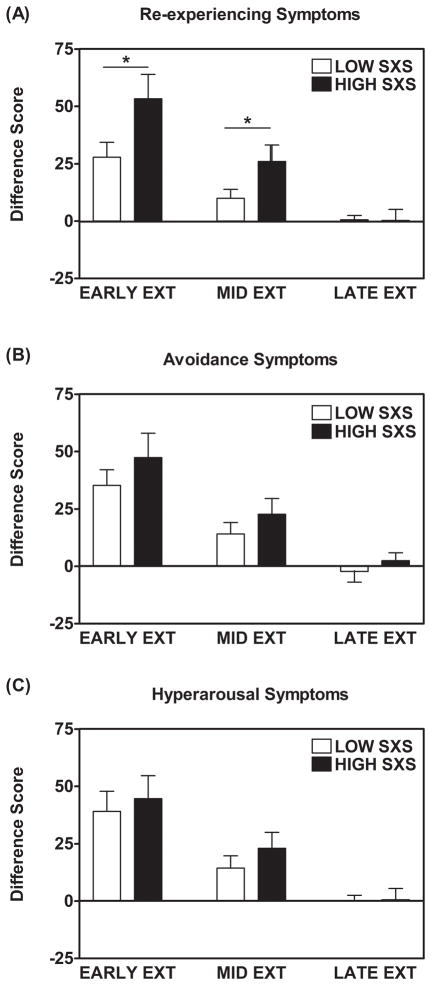
In order to examine whether the group differences in Extinction were due to a particular PTSD symptom cluster, we categorized all participants into high and low symptom groups, using a median split of the symptoms of each cluster on the PSS. Using an ANCOVA with BDI, CTQ, and TEI as covariates, we found that individuals with high re-experiencing symptoms had greater fear potentiation during early Extinction (F(1,104)=5.67,p=0.02) and mid-Extinction (F(1,104)=5.96,p=0.02) relative to low re-experiencing participants, see Figure 5. As with the PTSD group differences, the two groups with different levels of re-experiencing symptom severity had equivalently low levels of fear in late Extinction (F(1,104)=0.07,ns). The high and low symptom groups for avoidance (F(1,104)=2.93,ns) and hyper-arousal (F(1,104)=1.35,ns) symptoms did not differ in any phase of Extinction.
Figure 5.
A median split was performed based on the PTSD symptom cluster scores on the PTSD Symptom Scale (PSS). When comparing individuals with higher re-experiencing symptoms (SXS) to those with lower re-experiencing symptoms, potentiated startle responses to the reinforced CS+ were greater in the high sxs group *ANCOVA with Beck Depression Index, Childhood Trauma Questionnaire, and Traumatic Event Inventory scores as covariates, Early extinction: F(1, 109) = 5.67, p = 0.019) and Mid-extinction F(1, 109) = 5.96, p = 0.016. Early, mid-, and late extinction were defined as the mean difference scores to the CS+ for the first, middle, and last two blocks of the six blocks of extinction.
In order to determine if fear inhibition deficits contributed to the extinction effects observed in the PTSD+ group, we explored the relationship between fear-potentiated startle to the CS− during Acquisition and within-session Extinction to the previously reinforced CS+ during the latter phase. We categorized the PTSD+ participants into high and low responders to the CS− during late Acquisition using a median split of fear-potentiated startle response to the CS− in the 3rd block of Acquisition. High responders were categorized as “poor inhibitors” and low responders were categorized as “good inhibitors.” We then examined within-session extinction in good and poor inhibitors to the previously reinforced CS+ as a means of assessing their ability to inhibit fear to the newly “safe” CS. We found that poor inhibitors did not show a significant decrease in fear-potentiated startle during early, mid, or late Extinction, co-varying for levels of adult and childhood trauma and depressive symptoms, (F(1,16)=1.28,ns). Conversely, the PTSD+ subjects who showed a decreased response to CS− during late Acquisition (i.e., good inhibitors) had a significant decrease in potentiated startle responses to the previously reinforced CS+ during Extinction (F(1,16)=21.37,p<0.001).
In order to assess whether high fear expression and low fear inhibition during late Acquisition independently accounted for deficits in extinction in PTSD+ subjects, we performed hierarchical stepwise regression analyses entering depression in the first step, trauma history in the second step, fear-potentiated startle to the CS+ (DANGER cue) during late Acquisition, and fear-potentiated startle to the CS− (SAFETY cue) during late Acquisition in the final step. We performed separate regression analyses with early and late Extinction entered as a dependent variable (see Table 2). Although the overall model with all four predictors was significant (F(5,39)=10.14,p<0.001) and accounted for 54% of the variance in early Extinction, neither depression nor trauma history had significant contributions. However, fear potentiation to the CS+ during late Acquisition accounted for 46% of the variance in early Extinction beyond depression and trauma history, Fchange(1,35)=36.49,p<0.001. Fear potentiation to the CS− was not associated with early Extinction. On the other hand, the hierarchical regression analyses with late Extinction as the dependent variable, and the same predictors as above entered as independent steps, revealed the opposite association. Potentiated startle in late Extinction was not associated with high fear expression to the danger cue, but rather to high fear potentiation to the safety cue, which alone accounted for 22% of the variance, Fchange(1,34)=9.89,p=0.003.
Table 2.
Hierarchical regression analysis
| Dependent Variable |
||||||
|---|---|---|---|---|---|---|
| Early Extinction FPS | Late Extinction FPS | |||||
| Predictors | Δ R2 | Δ F | p | Δ R2 | Δ F | p |
| 1. Depression | 0.07 | 3.05 | 0.09 | 0.01 | 0.38 | 0.54 |
| 2. Trauma History | 0.03 | 0.53 | 0.59 | 0.02 | 0.32 | 0.73 |
| 3. FPS to Danger Cue | 0.46 | 36.48 | 0.001** | 0.003 | 0.12 | 0.72 |
| 4. FPS to Safety Cue | 0.04 | 3.29 | 0.08 | 0.22 | 9.89 | 0.003* |
Discussion
An inability to inhibit learned fear under conditions of safety can underlie several PTSD symptoms, most notably re-experiencing(3,5). Our group has previously shown fear inhibition deficits in response to safety cues in both civilian(16) and combat PTSD patients(15). In the present study, we expanded this investigation of fear inhibition in PTSD using a fear-potentiated startle extinction paradigm employed in our prior investigations(30,31). The primary findings of the current study are: (a) both PTSD+ participants and traumatized PTSD− individuals displayed robust fear-potentiated startle to the CS+ and significant discrimination between the CS+ and CS− during Acquisition, (b) the PTSD+ group showed increased fear-potentiated startle to both the CS+ and CS− compared to the PTSD− group during late Acquisition, (c) individuals with higher re-experiencing and hyper-arousal symptoms, as measured by the PSS, showed greater levels of fear to the CS+ and CS−, during Acquisition, compared to those with lower re-experiencing and hyper-arousal symptoms, (d) the PTSD+ group showed increased fear-potentiated startle to the previously reinforced CS+ compared to the PTSD− group during early and middle Extinction, (e) individuals with higher re-experiencing symptoms showed increased fear-potentiated startle to the previously reinforced CS+ compared to the low re-experiencing group during Extinction, (f) elevated fear-potentiated startle responses to the CS− (safety) during Acquisition predicted greater fear-potentiated startle to the previously reinforced CS+ during Extinction, and (g) within the PTSD+ group, those who were poor inhibitors to the CS− demonstrated delayed extinction of fear. This final finding suggests that there may be two separate subgroups within the PTSD cohort, one with a primary phenotype of enhanced fear expression, and a second with impaired inhibition of fear and delayed extinction.
Using fear-potentiated startle, we have shown alterations in fear processing in both combat-related PTSD(15) and civilian PTSD(16). In our previous studies using a conditional discrimination paradigm, we have consistently found that PTSD is associated with impaired inhibition of fear(15,16), and a lack of CS+/CS− discrimination when more ambiguous cues are used(16). The present study used a simple discrimination task that reduces the level of ambiguity involved in learning. The result of this simplified task were heightened fear expression to the CS+, which was more than two-fold greater in the PTSD subjects compared to the traumatized controls during late Acquisition. This exaggerated fear expression was also observed in early and mid Extinction, so that fear potentiation to the CS+ continued to be high in the PTSD group after the CS+ was no longer reinforced. Although the sample in the present study was not medication free, the use of psychotropic medication was low and equally distributed across groups; therefore, medication effects did not account for the observed group differences.
Although most fear conditioning studies in PTSD, including our own, have found deficits in safety cue processing, i.e. diagnostic group differences were greater in responses to CS− than group differences to the CS+(16,33), neuroimaging data showing amygdala hyper-activation in response to fear-related cues in PTSD would support the finding of heightened fear expression(34,35). In order to selectively account for responses to danger and safety cues, we then selected PTSD individuals who were also high responders to the CS− during late Acquisition, when CS− potentiation should be low. These individuals, who appeared to be poor inhibitors of fear when learning the safety cue, were also impaired in Extinction. In addition, they did not show decreased startle potentiation to the CS+ in the extinction phase. Given that extinction is thought to be a new inhibitory learning, rather than erasure of the original fear memory(36), similar neural mechanisms may be involved in safety signal processing and extinction of responses to danger signals.
It is important to note that heightened fear expression resulted in an impairment at the onset of extinction, in that PTSD subjects exhibited significantly more fear during early and mid Extinction relative to controls; however, it did not eliminate extinction all together there were no group differences in late Extinction. Therefore, given enough trials, exaggerated fear may be overcome. On the other hand, PTSD participants who showed a deficit in safety cue processing did not show extinction even after 6 blocks. Therefore, it is possible the heightened fear expression accounts for deficits in extinction rate, while impaired fear inhibition accounts for an inability to extinguish danger cues. The observed elevation in fear-potentiated startle to the previously reinforced CS+ during the early and middle stages of the fear extinction session has clinical implications for the treatment of PTSD. Fear extinction is an analog of clinical interventions such as prolonged exposure therapy(4) that rely on the repeated presentation of trauma-related stimuli (CSs) under conditions of safety (ie., devoid of noxious consequences). Recent translational work has suggested that d-cycloserine, or other novel cognitive enhancers, may facilitate exposure-based psychotherapy and reduce the number of exposure sessions required to reduce fear(37–40). The present results suggest the use of this paradigm as a model for the pre-clinical assessment of novel extinction facilitators as treatment options for PTSD especially for those participants in which re-experiencing symptoms produce the greatest degree of impairment.
Notably, the current study sample consisted predominantly of African-American participants, which may potentially reduce generalizability to other populations. To our knowledge, there have been no reports of ethnic/racial differences in the acquisition and extinction of fear-potentiated startle. We have, however, reported differences in baseline startle levels between European-Americans and African-Americans(41). In most of our previous studies (which included Caucasian and African-American participants) individuals who displayed low baseline startle responses consistently showed a robust increase in startle upon introduction of the conditioning paradigm.
An intriguing question raised is the degree to which the PTSD+ and PTSD− participants would recall extinction learning when tested after an elapsed period of time. Prior research has shown that extinction recall is impaired in PTSD(17,18). The sensitivity in detecting extinction recall deficits using paradigms such as this remains unclear given that there are currently conflicting results. For example, we have previously observed spontaneous recovery in psychiatrically healthy volunteers using the same paradigm as that which was administered in the current study(30) whereas other groups (e.g., Milad et al., 2009) have not reported this effect; however, the latter group employed skin conductance and not fear-potentiated startle. Extinction recall studies are currently underway in the urban traumatized population described herein.
These results suggest that extinction deficits may be due to two independent mechanisms: high fear load may account for exaggerated fear at the onset of extinction, while low fear inhibition may account for deficits in fully extinguishing fear responses. In relation to PTSD symptoms, these two mechanisms may be related to differential symptom presentation, so that higher fear load may be associated with re-experiencing, while lower fear inhibition may be associated with hyper-arousal(16). Furthermore, it is possible that these two mechanisms have different, albeit interconnected, neural underpinnings. High fear load, and re-experiencing may be associated with hyperactivity in the amygdala(42), while impaired fear inhibition, both during fear conditioning and extinction, may reflect altered prefrontal control of the amygdala(43). Although the re-experiencing symptoms appear to be most strongly related to fear load, the high correlation between symptom sub-clusters and total PTSD symptoms makes it difficult to parse out the unique contribution of re-experiencing vs. overall symptom severity.
The current findings suggest that PTSD participants experience a greater “fear load” following the acquisition of conditioned fear and that this elevated level of fear may persist after acquisition. The observed increase in fear-potentiated startle to the CS+ following acquisition and during extinction is consistent with previous neurobiological data showing amygdalar hyperactivity and prefrontal cortical hypoactivity in PTSD. In addition, elevated fear levels in PTSD participants are most pronounced during early stages of extinction suggesting that enhancement of extinction learning (e.g., via pharmacological interventions such as d-cycloserine) may prove beneficial in treating PTSD. The extinction paradigm employed here, which demonstrates robust effect sizes supporting smaller study designs, may serve as an effective psychophysiological tool for assessing pre-clinical candidate drug efficacy as well as an objective measure of clinical outcome in PTSD treatment groups using extinction-based exposure therapies.
Supplementary Material
Acknowledgments
We thank A.J. McCarthy, Asher Siegelman, Justine Phifer, Asante Kamkwalala, and Allen W. Graham for their assistance in the preparation of this manuscript.
Footnotes
Disclosure/Conflict of Interest
Dr. Norrholm has research support from the National Alliance for Research on Schizophrenia and Depression (NARSAD), the Department of Defense (DOD)/Congressionally Directed Medical Research Program (CDMRP, Award # W81XWH-08-2-0170), the Emory University Research Committee, and PHS Grant (UL1 RR025008) from the Clinical and Translational Science Award program, National Institutes of Health, National Center for Research Resources. Dr. Jovanovic has research support from NIMH (F32 MH070129). Dr. Bradley has research support from the American Foundation for Suicide Prevention. Dr. Ressler has research support from NIMH (MH071537), National Centers for Research Resources (M01 RR00039), NARSAD, Burroughs Wellcome Foundation, and is co-founder of Extinction Pharmaceuticals for the development of NMDA-based therapeutics to enhance extinction. All other authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.APA. Diagnostic and statistical manual of mental disorders (DSM-IV) 4. Washington, D.C: American Psychiatric Association; 1994. [Google Scholar]
- 2.Amstadter AB, Nugent NR, Koenen KC. Genetics of PTSD: Fear conditioning as a model for future research. Psychiatric Annals. 2009;39(6):358–67. doi: 10.3928/00485713-20090526-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Norrholm SD, Jovanovic T. Tailoring therapeutic strategies for treating posttraumatic stress disorder symptom clusters. Neuropsychiatric Disease and Treatment. 2010 doi: 10.2147/NDT.S10951. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rothbaum BO, Davis M. Applying learning principles to the treatment of post-trauma reactions. Ann N Y Acad Sci. 2003;1008:112–21. doi: 10.1196/annals.1301.012. [DOI] [PubMed] [Google Scholar]
- 5.Friedman M. The latest assessment and treatment strategies. 4. Kansas City, MO: Dean Psych Press Corporation; 2006. Post-traumatic and acute stress disorders. [Google Scholar]
- 6.Hoge CW, Castro CA, Messer SC, McGurk D, Cotting DI, Koffman RL. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med. 2004 Jul 1;351(1):13–22. doi: 10.1056/NEJMoa040603. [DOI] [PubMed] [Google Scholar]
- 7.Rothbaum BO, Kozak MJ, Foa EB, Whitaker DJ. Posttraumatic stress disorder in rape victims: autonomic habituation to auditory stimuli. J Trauma Stress. 2001 Apr;14(2):283–93. doi: 10.1023/A:1011160800958. [DOI] [PubMed] [Google Scholar]
- 8.Koenen K, Aiello AE, Bakshis E, Amstadter AB, Ruggiero KJ, Acierno R, Kilpatrick DG, Gelernter J, Galea S. Modification of the association between serotonin transporter genotype and risk of posttraumatic stress disorder in adults by county-level social environment. Am J Epidemiol. 2009;169(6):704–11. doi: 10.1093/aje/kwn397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Difede J, Cukor J, Jayasinghe N, Patt I, Jedel S, Spielman L, Giosan C, Hoffman HG. Virtual reality exposure therapy for the treatment of posttraumatic stress disorder following September 11, 2001. J Clin Psychiatry. 2007 Nov;68(11):1639–47. [PubMed] [Google Scholar]
- 10.Rothbaum BO, Astin MC, Marsteller F. Prolonged Exposure Versus Eye Movement Desensitization and Reprocessing (EMDR) for PTSD Rape Victims. J Trauma Stress. 2005;18:607–16. doi: 10.1002/jts.20069. [DOI] [PubMed] [Google Scholar]
- 11.Rothbaum BO, Hodges L, Alarcon R, Ready D, Shahar F, Graap K, Pair J, Hebert P, Gotz D, Wills B, Baltzell D. Virtual reality exposure therapy for PTSD Vietnam Veterans: a case study. J Trauma Stress. 1999 Apr;12(2):263–71. doi: 10.1023/A:1024772308758. [DOI] [PubMed] [Google Scholar]
- 12.Rothbaum BO, Meadows EA, Resick P, Foy DW. Cognitive-Behavioral Therapy. In: Foa EB, Friedman MI, Keane T, editors. Effective treatments for posttraumatic stress disorder: practice guidelines from the International Society for Traumatic Stress Studies. Guilford Press; 2000. pp. 60–83. [Google Scholar]
- 13.Foa EB, Hembree E, Rothbaum BO. Prolonged Exposure Therapy for PTSD: Emotional Processing of Traumatic Experiences, Therapist Guide. New York, NY: Oxford University Press; 2007. [Google Scholar]
- 14.Davis M. The Neurophysiological Basis of Acoustic Startle Modulation: Research on Fear Motivation and Sensory Gating. In: Lang PJ, Simons RF, Balaban MT, editors. Attention and Orienting: Sensory and Motivational Processes. Mahwah, NJ: Lawrence Erlbaum Associates; 1997. pp. 69–96. [Google Scholar]
- 15.Jovanovic T, Norrholm SD, Fennell JE, Keyes M, Fiallos A, Myers KM, Davis M, Duncan EJ. Posttraumatic stress disorder may be associated with impaired fear inhibition: relation to symptom severity. Psychiatry Research. 2009;167(1–2):151–60. doi: 10.1016/j.psychres.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jovanovic T, Norrholm SD, Blanding NQ, Davis M, Duncan E, Bradley B, Ressler KJ. Impaired fear inhibition is a biomarker of PTSD but not depression. Depress Anxiety. 2010;27(3):244–51. doi: 10.1002/da.20663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Milad MR, Orr SP, Lasko NB, Chang Y, Rauch SL, Pitman RK. Presence and acquired origin of reduced recall for fear extinction in PTSD: Results of a twin study. J Psychiatr Res. 2008;42(7):515–20. doi: 10.1016/j.jpsychires.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, Zeidan MA, Handwerger K, Orr SP, Rauch SL. Neurobiological Basis of Failure to Recall Extinction Memory in Posttraumatic Stress Disorder. Biol Psychiatry. 2009;66(12):1075–82. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Felmingham K, Kemp A, Williams L, Das P, Hughes G, Peduto A, Bryant R. Changes in anterior cingulate and amygdala after cognitive behavior therapy of posttraumatic stress disorder. Psychological Science. 2007;18(2):127–9. doi: 10.1111/j.1467-9280.2007.01860.x. [DOI] [PubMed] [Google Scholar]
- 20.Alim TN, Graves E, Mellman TA, Aigbogun N, Gray E, Lawson W, Charney DS. Trauma Exposure, Posttraumatic Stress Disorder and Depression in an African-American Primary Care Population. Journal of the National Medical Association. 2006 Oct;98(10):1630–6. [PMC free article] [PubMed] [Google Scholar]
- 21.Breslau N, Peterson EL, Poisson LM, Schultz LR, Lucia VC. Estimating post-traumatic stress disorder in the community: lifetime perspective and the impact of typical traumatic events. Psychol Med. 2004 Jul;34(5):889–98. doi: 10.1017/s0033291703001612. [DOI] [PubMed] [Google Scholar]
- 22.Gillespie CF, Bradley RG, Mercer K, Smith AK, Conneely K, Gapen M, Weiss T, Schwartz AC, Cubells JF, Ressler KJ. Trauma Exposure and Stress-Related Disorders in Inner City Primary Care Patients. General Hospital Psychiatry. 2009 doi: 10.1016/j.genhosppsych.2009.05.003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, Tang Y, Gillespie CF, Heim CM, Nemeroff CB, Schwartz AC, Cubells JF, Ressler KJ. Association of FKBP5 Polymorphisms and Childhood Abuse With Risk of Posttraumatic Stress Disorder Symptoms in Adults. Jama. 2008 March 19;299(11):1291–305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Falsetti S, Resnick H, Resick P, Kilpatrick D. The Modified PTSD Symptom Scale: A brief self-report measure of posttraumatic stress disorder. The Behavior Therapist. 1993;16:161–2. [Google Scholar]
- 25.Foa EB, Tolin DF. Comparison of the PTSD Symptom Scale-Interview Version with the Clinician Administered PTSD Scale. J Trauma Stress. 2000;13(2):181–91. doi: 10.1023/A:1007781909213. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz AC, Bradley RL, Sexton M, Sherry A, Ressler KJ. Posttraumatic Stress Disorder Among African Americans in an Inner City Mental Health Clinic. Psychiatr Serv. 2005 February 1;56(2):212–5. doi: 10.1176/appi.ps.56.2.212. [DOI] [PubMed] [Google Scholar]
- 27.Beck AT, Ward CH, Mendelsohn M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 28.Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, Stokes J, Handelsman L, Medrano M, Desmond D, Zule W. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl. 2003 Feb;27(2):169–90. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- 29.Bernstein DP, Fink L. Childhood Trauma Questionnaire A retrospective self-report manual. San Antonio, TX: The Psychological Corporation; 1998. [Google Scholar]
- 30.Norrholm SD, Vervliet B, Jovanovic T, Boshoven W, Myers KM, Davis M, Rothbaum BO, Duncan EJ. Timing of extinction relative to acquisition: a parametric analysis of fear extinction in humans. Behavioral Neuroscience. 2008;122(5):1016–30. doi: 10.1037/a0012604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Norrholm SD, Jovanovic T, Vervliet B, Myers KM, Davis M, Rothbaum BO, Duncan EJ. Conditioned fear extinction and reinstatement in a human fear-potentiated startle paradigm. Learn Mem. 2006;13(6):681–5. doi: 10.1101/lm.393906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jovanovic T, Norrholm SD, Keyes M, Fiallos A, Jovanovic S, Myers KM, Davis M, Duncan EJ. Contingency awareness and fear inhibition in a human fear-potentiated startle paradigm. Behav Neurosci. 2006;120(5):995–1004. doi: 10.1037/0735-7044.120.5.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lissek S, Powers AS, McClure EB, Phelps EA, Woldehawariat G, Grillon C, Pine DS. Classical fear conditioning in the anxiety disorders: a meta-analysis. Behaviour Research & Therapy. 2005 Nov;43(11):1391–424. doi: 10.1016/j.brat.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 34.Shin LM, Wright CI, et al. A Functional Magnetic Resonance Imaging Study of Amygdala and Medial Prefrontal Cortex Responses to Overtly Presented Fearful Faces in Posttraumatic Stress Disorder. Arch Gen Psychiatry. 2005;62(3):273–81. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- 35.Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, Orr SP, Pitman RK. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biological Psychiatry. 2000;47(9):769–76. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- 36.Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Current Opinions in Neurobiology. 2006;16(6):723–7. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 37.Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, Hodges L, Davis M. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry. 2004 Nov;61(11):1136–44. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
- 38.Davis M. Role of NMDA receptors and MAP kinase in the amygdala in extinction of fear: clinical implications for exposure therapy. Eur J Neurosci. 2002 Aug;16(3):395–8. doi: 10.1046/j.1460-9568.2002.02138.x. [DOI] [PubMed] [Google Scholar]
- 39.Davis M, Ressler K, Rothbaum BO, Richardson R. Effects of d-cycloserine on extinction: translation from preclinical to clinical work. Biol Psychiatry. 2006;60(4):369–75. doi: 10.1016/j.biopsych.2006.03.084. [DOI] [PubMed] [Google Scholar]
- 40.Hofmann SG, Meuret AE, Smits JAJ, Simon NM, Pollack MH, Eisenmenger K, Shiekh M, Otto MW. Augmentation of exposure therapy with d-cycloserine for social anxiety disorder. Arch Gen Psychiatry. 2006;63:298–304. doi: 10.1001/archpsyc.63.3.298. [DOI] [PubMed] [Google Scholar]
- 41.Hasenkamp W, Norrholm SD, Green A, Lewison B, Boshoven W, Keyes M, Duncan E. Differences in startle reflex prepulse in European-American and African-Americans. Psychophysiology. 2008;45(5):876–82. doi: 10.1111/j.1469-8986.2008.00680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shin LM, Rauch SL, Pitman RK. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann N Y Acad Sci. 2006 Jul;1071:67–79. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- 43.Milad MR, Quirk GJ, Pitman RK, Orr SP, Fischl B, Rauch SL. A role for the human dorsal anterior cingulate cortex in fear expression. Biol Psychiatry. 2007 Nov 15;62(10):1191–4. doi: 10.1016/j.biopsych.2007.04.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.