Abstract
Background
Inflammatory bowel disease (IBD) is characterized by chronic inflammation of the gastrointestinal tract. Epstein-Barr virus (EBV) infection is associated with increased disease severity in therapeutically immunosuppressed IBD patients. The role of EBV infection in patients with IBD who are unresponsive to medical therapy is unclear. Anti-viral strategies may be a viable treatment option if severity of EBV infection, reflected in peripheral blood, contributes to IBD progression.
Objectives
We investigated the role of EBV in IBD patients unresponsive to medical therapy by examining EBV reactivation and B-cell proliferation in colonic mucosa.
Study Design
EBV DNA copy numbers were measured by real-time PCR in peripheral blood mononuclear cells (PBMC) of 84 patients with IBD and 115 non-IBD controls in a retrospective cross-sectional study. EBV-infected cells in colonic mucosa were identified by immunohistochemistry.
Results
EBV load in PBMC was higher in patients with IBD than in non-IBD controls, especially in patients not responding to medication. Inflamed colonic mucosa of these patients had high levels of expression of lytic and latent EBV genes that localized to proliferating B-lymphocytes, which was not seen in patients responding to therapy.
Conclusions
EBV replication was associated with severe IBD and mucosal inflammation. Increased proliferation and EBV infection of B-lymphocytes in inflamed colonic mucosa highlight the potential role of EBV in mucosal inflammation. The immunomodulatory effects of EBV could delay the resolution of the IBD associated inflammation, thus contributing to disease progression. These results indicate that anti-viral therapeutic strategies for the resolution of IBD may be useful.
Keywords: Inflammatory Bowel Disease, Epstein Barr Virus, Mucosa, Viral Load, Refractory Disease
1. Background
IBD is a complex polygenetic disease manifested by chronic inflammation of the intestinal mucosa.1–6 Patients with severe disease that cannot be managed by diet or other means receive intensive therapy consisting of immunomodulators and biologics, individually or in combination.7–11 Anti-tumor necrosis factor (TNF)α therapy (Infliximab) is effective in producing disease remission. 8, 12, 13 More than 95% of the general population is infected by EBV, which is a herpes virus. EBV can persist for the lifetime of a host and cause lymphoproliferative disorders such as lymphomas.14, 15,16 EBV infection has been implicated in the etiology of IBD. Reactivation and replication of EBV during immunosuppressive therapy may be important for the increased incidence of lymphoma in IBD patients.17
EBV is harbored in resting memory B-cells and induces B-cell proliferation.18 The immune system of an immunocompetent host can identify and eliminate EBV-infected cells, but immunosuppressed individuals are more susceptible to EBV-induced immunoblastic proliferation.16, 19 The increased magnitude of EBV-induced reactivation in B cells raises the risk for the development of lymphoma.19–22 Herpes virus infections (eg, cytomegalovirus) during anti-TNFα therapy are a major cause of IBD disease progression and are refractory to medical management.23–25 A high EBV load is a significant risk factor for developing post-transplant lymphoproliferative disorder and progression of IBD.19–22 The role of EBV in IBD that is refractory to conventional therapy has not been completely defined and warrants further investigation.16, 19 We hypothesize that EBV replication following reactivation in colonic mucosa could be reflected in peripheral blood and may be associated with the exacerbation of IBD.
2. Objectives
The goal of this retrospective cross-sectional study was to examine the level of EBV in the peripheral blood mononuclear cells (PBMC) and in inflamed colonic mucosal sites in patients with IBD.
3. Study Design
3.1 Patients and samples
Patients with IBD (n=84) and individuals with no prior history of IBD (n=115) were enrolled in the study at the University of California, Davis. The IBD patient group (consisting of 69 patients on medical management and 15 requiring surgical management) were identified based on their clinical, endoscopic and histological evaluations (Table 1). Of the controls, 75 individuals had no known health concerns and 40 had non-IBD inflammatory conditions (4 with rheumatoid arthritis (RA) and 36 with primary biliary cirrhosis (PBC)). Peripheral blood samples from all participants were obtained at a single time-point. Concurrent peripheral blood and perioperative gut resection or colonic mucosal biopsy samples from IBD patients were obtained. DNA was extracted from PBMC (Ficoll-Hypaque density gradient centrifugation) using the Qiagen DNeasy extraction kit (Qiagen, Valencia).26 Mucosal samples were stored in CryoPrep (American Master Tech Scientific, Lodi). This study protocol was approved by the institutional review board at the University of California, Davis.
Table 1.
Patient characteristics
| Group | IBD [n (%)] | RA/PBC [n (%)] | NC [n (%)] | |
|---|---|---|---|---|
| Total Subjects | 84 | 40 | 75 | |
| Sex | Male | 33(39.3%) | 16 (40%) | 30 (40%) |
| Female | 51 (60.7%) | 24 (60%) | 45 (60%) | |
| Disease | Crohn's Disease | 76 (90.5%) | N/A | N/A |
| Ulcerative Colitis | 8 (9.5%) | N/A | N/A | |
| Medications | Prednisone | 15 (17.9%) | N/A | N/A |
| Mesalamines | 17 (20.2%) | N/A | N/A | |
| Methotrxate | 3 (3.6%) | N/A | N/A | |
| AZA /6-MP /6-TG | 38 (45.2%) | N/A | N/A | |
| Infliximab | 44 (52.4%) | N/A | N/A | |
| Disease Activity | Perioperative Period | 15 (17.9%) | N/A | N/A |
NOTE: n = Total Subjects. Within Parenthesis: Percentage of patients
Abbreviations: IBD (Inflammatory Bowel Disease), RA (Rheumatoid Arthritis), PBC (Primary Biliary Cirrhosis), NC (Normal Controls)
N/A: (Not Applicable)
3.2 Quantitative EBV real-time PCR
PCR primers and a fluorogenic probe were generated based on the conserved BALF5 region of the EBV genome as previosly described.27 DNA isolated from a known number of cells was analyzed by real-time PCR assay using an ABI Prism 7900 sequence detector (Applied Biosystems) 27; this assay has been previously validated.28 Cell numbers were confirmed by a standard curve constructed using the CCR5 single copy gene and a known number of cells.
3.3 Detection of tissue EBV, CD19, and Ki67
Tissue sections were obtained from colon biopsies of five patients who were responsive to medical management and from colonic resections of three patients who were refractory to medical therapy. Sections were immunostained as previously described.29 Antibodies were used to detect EBV nuclear antigen (EBNA1, present in latent and lytic cycle), EBV viral capsid antigen (VCA, present in lytic cycle), Ki67, a cell proliferation marker, and CD19, a B cell specific cell surface marker (Santa Cruz Biotech. Inc., Santa Cruz). Tissue sections with no primary antibody were used as negative controls. Images were captured by confocal laser microscopy using LSM 5 and PASCAL software (Zeiss, New York). Cell counts were performed as previously published.26
3.4 Statistical analysis
The EBV viral loads were analyzed using unpaired t test, chi-square analysis and one-way analysis of variance (ANOVA) with appropriate post-tests. Statistical software included GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego) and JMP (JMP Genomics, Cary).
4. Results
4.1 Patient characteristics and pathological findings
The IBD patient group (n=84) consisted of 39.3% male and 60.7% female, ranged in age from 20 to 91 years (mean, 46.5 years; Table 1). Most patients had Crohn’s Disease (CD) (90.5%, n=76), and the remaining had ulcerative colitis (UC) (9.5%, n=8). All patients were on combination therapy for IBD, with 52% receiving infliximab, 18% prednisone and other immunosuppressive medications including methotextrate (3.6%); and azathioprine, 6-mercaptopurine, and 6-thioguanine (45%) Sixty-nine patients (82%) responded well to medical management (the “medical group” MG); the other fifteen (18%) had severe colitis that was not responsive to immunosuppressive therapy and required surgical intervention (gut resection) for control of IBD symptoms (the “surgical group” SG). The decision to initiate treatment of IBD was based on clinical parameters, systemic manifestations, and quality of life.30
Pathologic evaluation of colonic resections and biopsies was performed. Three patients who required surgical resection had refractory ulcerative colitis (n=3), and pathologic evaluation of gut resections indicated severe disease. Microscopic examination identified well-defined areas of chronic and focal acute inflammation in colonic mucosal samples with increased inflammatory response and cellular infiltrate in the lamina propria, as well as lymphoid hyperplasia, distorted mucus-depleted crypts, cryptitis and crypt abscesses. Among CD patients (n=12) in the surgical group, disease severity was evident by the appearance of reactive lymphoid hyperplasia in the colonic mucosa with focal cryptitis. No enteric pathogens or neoplastic changes were detected in any cases.
4.2 High levels of EBV positivity in IBD patients
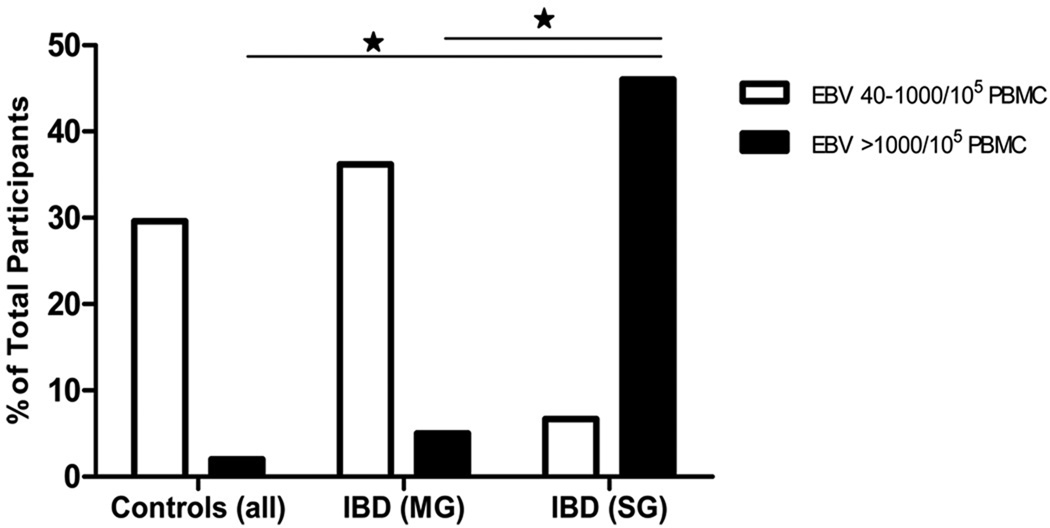
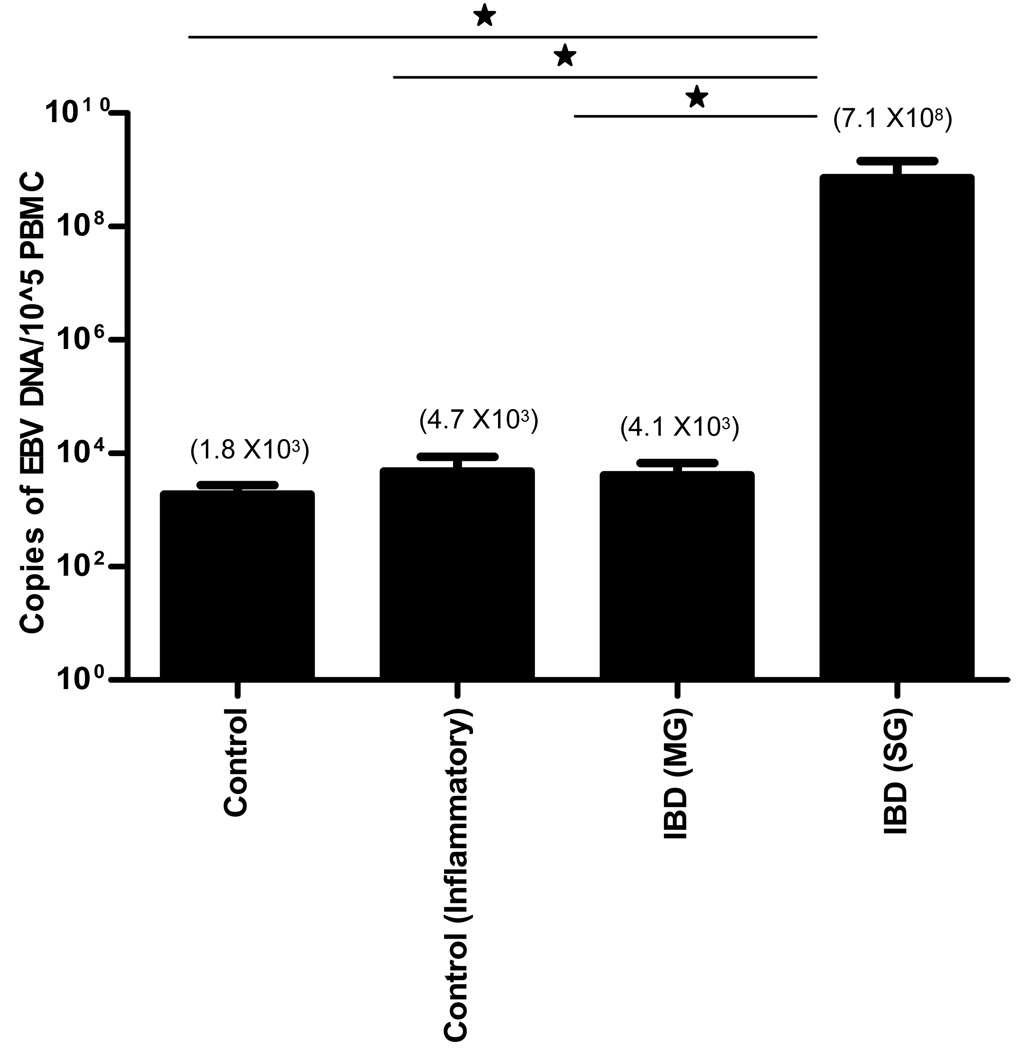
Higher levels of EBV DNA copy numbers were detected in PBMC of IBD patients than in non-IBD controls (Figure 1 and Table 2). Patients were grouped based on the number of viral copies/105 PBMC and categorized as: none detected (below the limit of detection of 40 copies/105 PBMC), low (40–1000 copies/105 PBMC), and high (> 1000 copies/105 PBMC)31. EBV positivity, defined as having a detectible level of EBV DNA, occurred more frequently in IBD patients than in non-IBD controls (44% vs 31%). The patients who failed conventional therapies had the highest occurrence of EBV positivity in peripheral blood (53%). Of IBD patients in the surgical group, 47% had high EBV loads vs only 6% in patients responsive to medical management (Figure 1). Average viral load was 3.5 × 108 DNA copies/105 PBMC in IBD patients vs 3.8 × 102 in the combined control group, and 7.16 × 108 in the surgical group of IBD patients vs 4.1 × 103 in the medical group (Figure 2). These results indicate that EBV loads in peripheral blood may reflect increased viral activity in the colonic mucosa of IBD patients
Figure 1.
EBV positivity in PBMC is higher in IBD patients than in control subjects. Higher overall rates of EBV positivity was observed in IBD patients who were perioperative (SG), while the majority of controls were negative for cell associated virus or had very low levels of EBV. Data is presented for all for categories, as a percentage of total number of patients within each group, with viral loads 40–1000 copies/105 PBMC or viral loads >1000 copies/105 PBMC. (* indicates p<0.05)
Table 2.
Participants groups and % of EBV positivity
| Total Number Participants |
No EBV Detected <40/105 | Viral Load 40-103/ 105 PBMC |
Viral Load >103/ 105 PBMC |
||||
|---|---|---|---|---|---|---|---|
| # | % | # | % | # | % | ||
| Controls | 75 | 49 | 65.3 | 24 | 32.0 | 2 | 2.7 |
| Other (RA/PBC) | 40 | 30 | 75.0 | 10 | 25.0 | 0 | 0.0 |
| IBD Total | 84 | 47 | 56.0 | 26 | 31.0 | 11 | 13.1 |
| IBD (Medical) | 69 | 40 | 58.0 | 25 | 36.2 | 4 | 5.8 |
| IBD (Surgical) | 15 | 7 | 46.7 | 1 | 6.7 | 7 | 46.7 |
Note: IBD (Medical) includes patients responding to medical management (MG).
IBD (Surgical) includes patients not responding to medical management (SG).
# indicates the number of patients in the group
% indicates the percentage of total participants within that category.
EBV limit of detection = 40/105 PBMC
Figure 2.
PBMC viral loads were obtained using real-time PCR. Increased mean viral loads, indicated by elevated levels of EBV DNA/cell, were observed in the IBD positive group when compared to the IBD negative control group. Significant differences were only seen in the perioperative IBD group (p = 0.002 for perioperative patients (SG) compared to controls, p=0.001 for SG compared to medically managed patients (MG), and p=0.0006 for SG compared to inflammatory controls). Patients in the perioperative group had IBD that was refractive to conventional therapies and required surgery. These patients also had very high activity of the disease at time of sample collection. (* indicated p<0.05 by Mann Whitney test for individual comparisons and 1 way ANOVA using the Kruskall-Wallis test and Dunns multiple comparison test).
4.3 Elevated levels of EBV in inflamed colonic mucosa of IBD patients
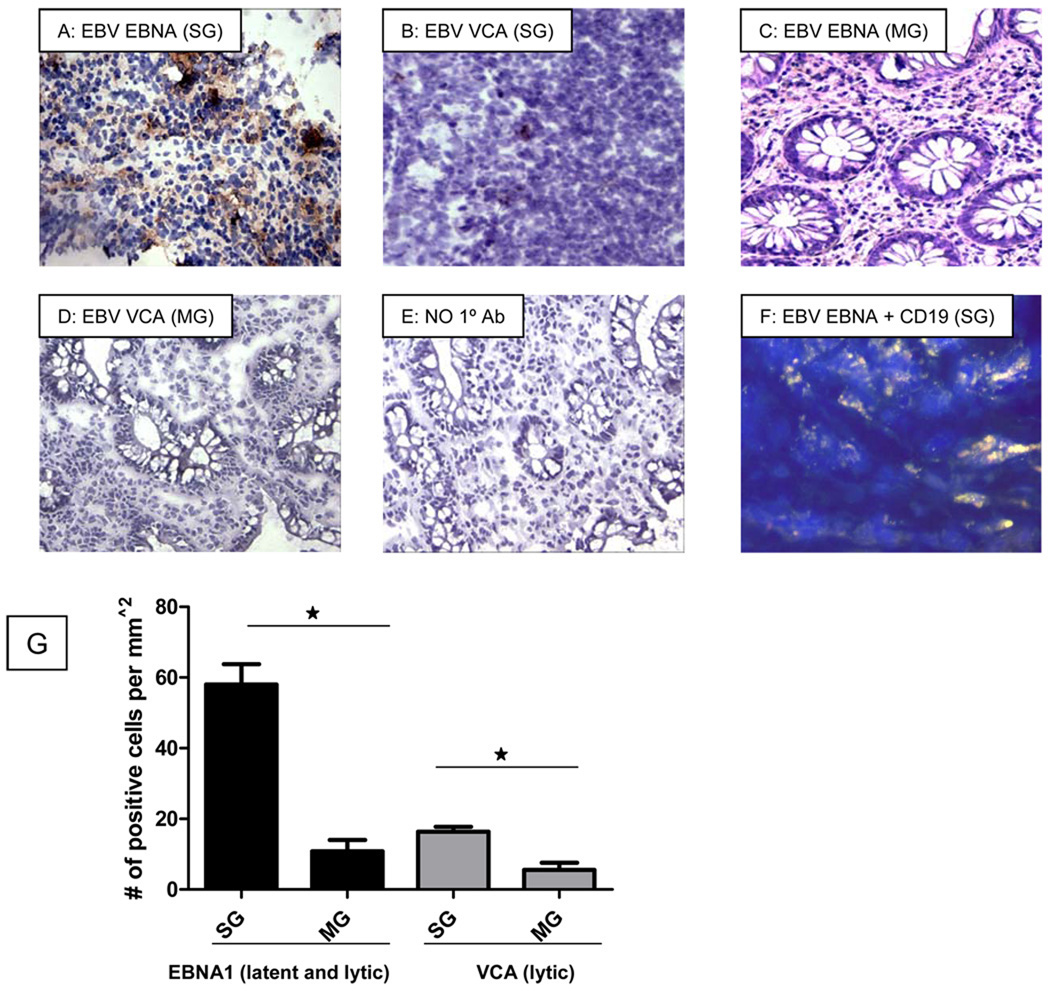
To investigate the association between mucosal EBV replication and inflammation, immunohistochemical analysis was performed to detect the presence of EBV antigens associated with the lytic and latent phases of the EBV life cycle (Figure 3). High levels of EBV-infected cells (> 50/mm2 EBNA1+ and > 15/mm2 VCA+ cells) were detected in colonic samples from patients undergoing surgical resection (Figure 3). The level of latently infected cells, as indicated by the presence of EBV EBNA1 expression, was higher than that of cells positive for the lytic gene EBV VCA (Figure 3). Patients with high EBV viral loads in peripheral blood also had elevated levels of EBV in colonic biopsies. The majority of EBV infection was localized to the CD19+ B lymphocytes, as observed in double immuno-stained tissue sections (Figure 3F). Thus, peripheral blood EBV loads are reflective of mucosal EBV replication in IBD patients. Furthermore, elevated colonic EBV levels are associated with increased disease activity in patients not responding to medical management.
Figure 3.
Immunohistochemical staining of colon resections of SG patients with high EBV viral loads and colonic biopsies of MG patients were performed. Tissues were stained with an antibody against the latent/lytic EBV virus marker EBNA1 (A) and the lytic EBV gene product, VCA (B), then visualized using light microscopy. EBV is stained brown. High levels of EBV EBNA1 and EBV VCA were observed in highly inflamed areas of the colon of SG patients (3A, 3B). A few EBV EBNA1 positive cells (C) and VCA positive cells (D) were observed in biopsies obtained from MG patients. There was no staining observed in the antibody negative control section (E). Double staining for EBV EBNA1 and CD19 was performed (F). In this section, EBV EBNA1+ cells appear teal, B-cells appear pink, and B-cells with EBV appear white (F). Latent EBV appears to be associated with B-cells as well as other unstained cell types in the SG patient. Ennumeration of EBV positive cells also indicated higher levels in SG patients as compared to MG patients (G). (* indicates p<0.05 using the unpaired t-test.; SG indicates patients requiring surgical management; MG indicates patients responding to medical therapy).
4.4 Increased B cell numbers and cellular proliferation in patients with severe IBD
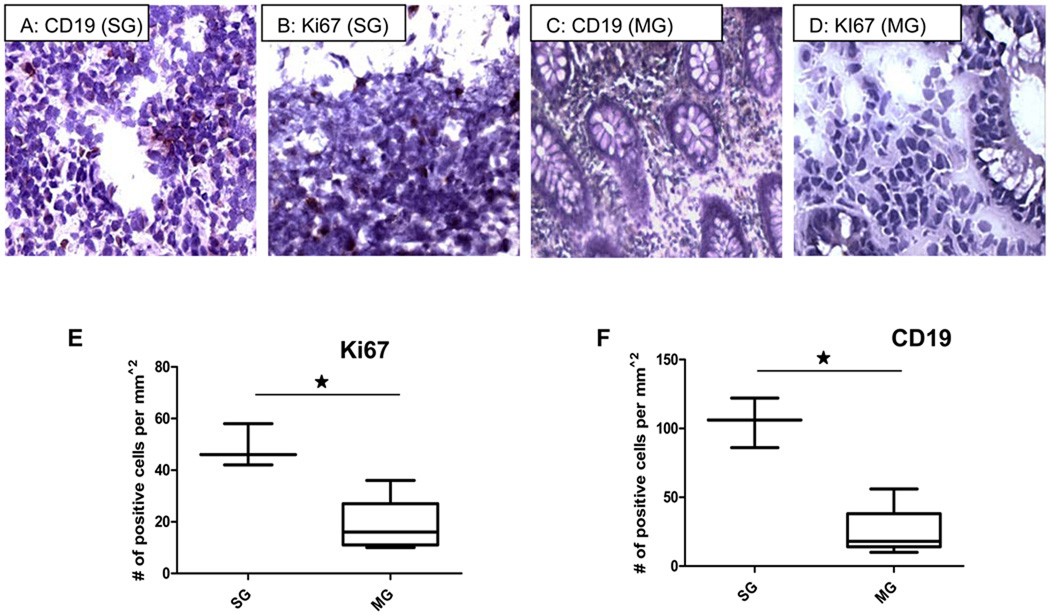
Immunohistochemical analysis demonstrated that mucosal damage with loss of tissue architecture in inflamed colonic mucosa of IBD patients undergoing gut resection was associated with the presence of inflammatory infiltrate including B lymphocytes (Figure 4A and F) compared to patients responding to medical therapy (Figure 4C and F). Elevated B-cell levels in the inflamed region of the colonic biopsies were attributed to increased B-cell proliferation, as demonstrated by the increased expression of the cell proliferation marker, Ki67 (Figure 4B and E).32, 33 In comparison, colonic biopsies from patients with UC and CD without severe disease or with undetectable peripheral viral loads had lower levels of Ki67 expression (Figure 4D and F). Our data suggest that increased disease activity in IBD patients that is not responsive to medical therapy may be associated with elevated EBV levels resulting directly or indirectly from B cell infiltration and inflammation.
Figure 4.
Higher levels of CD19+ cells (B-lymphocytes) (A,C,F) and Ki67 (B,D, E) were seen in highly inflamed bowel in SG patients compared to biopsy samples from MG patients. Lower levels of CD19 positive B-lymphocytes were observed in biopsy samples from patients responding to therapy (MG) with CD (4C). No staining was observed in the tissue sections not treated with primary antibody. (* indicates p<.05 using the unpaired t-test; SG indicates patients requiring surgical management; MG indicates patients responding to medical therapy).
5. Discussion
Patients with IBD have a poor quality of life due to persistent inflammation, diarrhea, bleeding, surgery, and extra-intestinal complications (abscesses, strictures, and neoplastic transformation).34 IBD is treated with disease-modifying agents that down-regulate the immune response. Treatment suppresses inflammation but may also induce loss of immune surveillance.8–11 Increased B-cell proliferation may result in worsening of IBD symptoms, suggesting that this process may be critical for inducing lymphoproliferative disease associated with IBD.35
The importance of EBV proliferation in patients with unresponsive IBD may be an integral component in its clinical management. Following EBV infection, severity of IBD may increase with or without immunosuppressive therapy.36 In this study, EBV replication, as measured by viral loads in PBMC, was higher in patients with IBD than in non-IBD controls. Increased levels of viral replication in the gut were associated with B-cell infiltration and proliferation in inflamed colonic mucosa of patients with severe IBD. Therefore, active EBV replication may be one of the factors increasing mucosal inflammation in the colonic microenvironment, which in turn could delay the resolution of inflammation in the tissue and exacerbate IBD pathology. Furthermore, EBV may drive its own replication through de novo infection of proliferating B cells in the gut mucosa in the absence of immune surveillance due to immunosuppressive therapy.19, 37 Although our retrospective pilot study did not have sufficient statistical power to identify the effects of gender and other variables, the findings suggest that EBV may play a role in mucosal inflammation in IBD and warrant a longitudinal assessment of mucosal samples. The role of the cytokine milieu, contribution of different cell types and their activation status, and the effects of immunosuppressive therapy could also contribute to disease progression.
Our findings highlight the potential role of reactivation of EBV infection in patients with progressive IBD. Inflammation plays an important role in the etiology of IBD as well as in increasing EBV loads in the gut mucosa and in peripheral blood. Our data suggest that EBV infection may contribute to further elevate B-cell proliferation, thus increasing viral load.38 Proactive viral surveillance and intervention with antiviral agents may improve patient response to therapy, but this needs to be investigated prospectively. Additionally, the magnitude of immunosuppression in patients need to considered during the clinical management. In summary, the cycle of EBV replication and B-cell proliferation in the presence of immunosuppression may augment the persistence of chronic inflammation in the colonic mucosa and reduce the responsiveness of the patient to immunosuppressive therapy.
Acknowledgements
We thank the patients for their participation in the study, the nursing staff at the UCDMC for their support and the Lucy Whittier Molecular Core Facility. We would also like to thank Dr M D George for his input in manuscript preparation. This study was supported by National Institutes of Health grants DK61297 and AI43274 and the California HIV AIDS research program grant CH05-D-606. Dr Sankaran is supported by a Building Interdisciplinary Research Careers in Women’s Health award (K12 HD051958) funded by the NICHD, ORWH, ODS, and the NIA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors have no conflict of interest in this study.
This material has not been previously presented or published
References
- 1.Himmel ME, Hardenberg G, Piccirillo CA, Steiner TS, Levings MK. The role of T-regulatory cells and Toll-like receptors in the pathogenesis of human inflammatory bowel disease. Immunology. 2008 Oct;125(2):145–153. doi: 10.1111/j.1365-2567.2008.02939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Macfarlane S, Steed H, Macfarlane GT. Intestinal bacteria and inflammatory bowel disease. Crit Rev Clin Lab Sci. 2009;46(1):25–54. doi: 10.1080/10408360802485792. [DOI] [PubMed] [Google Scholar]
- 3.Niess JH. Role of mucosal dendritic cells in inflammatory bowel disease. World J Gastroenterol. 2008 Sep 7;14(33):5138–5148. doi: 10.3748/wjg.14.5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shih DQ, Targan SR, McGovern D. Recent advances in IBD pathogenesis: genetics and immunobiology. Curr Gastroenterol Rep. 2008 Dec;10(6):568–575. doi: 10.1007/s11894-008-0104-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prindiville TP, Sheikh RA, Cohen SH, Tang YJ, Cantrell MC, Silva J., Jr Bacteroides fragilis enterotoxin gene sequences in patients with inflammatory bowel disease. Emerg Infect Dis. 2000 Mar–Apr;6(2):171–174. doi: 10.3201/eid0602.000210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anton PA. Stress and mind-body impact on the course of inflammatory bowel diseases. Semin Gastrointest Dis. 1999 Jan;10(1):14–19. [PubMed] [Google Scholar]
- 7.Mutlu EA, Farhadi A, Keshavarzian A. New developments in the treatment of inflammatory bowel disease. Expert Opin Investig Drugs. 2002 Mar;11(3):365–385. doi: 10.1517/13543784.11.3.365. [DOI] [PubMed] [Google Scholar]
- 8.Caviglia R, Boskoski I, Cicala M. Long-term treatment with infliximab in inflammatory bowel disease: safety and tolerability issues. Expert Opin Drug Saf. 2008 Sep;7(5):617–632. doi: 10.1517/14740338.7.5.617. [DOI] [PubMed] [Google Scholar]
- 9.Louis E, Belaiche J, Reenaers C. Are we giving biologics too much time? When should we stop treatment? World J Gastroenterol. 2008 Sep 28;14(36):5528–5531. doi: 10.3748/wjg.14.5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sandborn WJ. Current directions in IBD therapy: what goals are feasible with biological modifiers? Gastroenterology. 2008 Nov;135(5):1442–1447. doi: 10.1053/j.gastro.2008.09.053. [DOI] [PubMed] [Google Scholar]
- 11.Shteyer E, Wilschanski M. Novel therapeutic modalities in pediatric inflammatory bowel disease. Isr Med Assoc J. 2008 Nov;10(11):816–820. [PubMed] [Google Scholar]
- 12.Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006 May 17;295(19):2275–2285. doi: 10.1001/jama.295.19.2275. [DOI] [PubMed] [Google Scholar]
- 13.Shepela C. The safety of biologic agents in the treatment of inflammatory bowel disease. Minn Med. 2008 Jun;91(6):42–45. [PubMed] [Google Scholar]
- 14.Decker LL, Klaman LD, Thorley-Lawson DA. Detection of the latent form of Epstein-Barr virus DNA in the peripheral blood of healthy individuals. J Virol. 1996 May;70(5):3286–3289. doi: 10.1128/jvi.70.5.3286-3289.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller G. The switch between EBV latency and replication. Yale J Biol Med. 1989 Mar–Apr;62(2):205–213. [PMC free article] [PubMed] [Google Scholar]
- 16.Lidar M, Langevitz P, Shoenfeld Y. The role of infection in inflammatory bowel disease: initiation, exacerbation and protection. Isr Med Assoc J. 2009 Sep;11(9):558–563. [PubMed] [Google Scholar]
- 17.Yanai H, Shimizu N, Nagasaki S, Mitani N, Okita K. Epstein-Barr virus infection of the colon with inflammatory bowel disease. Am J Gastroenterol. 1999 Jun;94(6):1582–1586. doi: 10.1111/j.1572-0241.1999.01148.x. [DOI] [PubMed] [Google Scholar]
- 18.Babcock GJ, Decker LL, Volk M, Thorley-Lawson DA. EBV persistence in memory B cells in vivo. Immunity. 1998 Sep;9(3):395–404. doi: 10.1016/s1074-7613(00)80622-6. [DOI] [PubMed] [Google Scholar]
- 19.Spieker T, Herbst H. Distribution and phenotype of Epstein-Barr virus-infected cells in inflammatory bowel disease. Am J Pathol. 2000 Jul;157(1):51–57. doi: 10.1016/S0002-9440(10)64516-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gartner B, Preiksaitis JK. EBV viral load detection in clinical virology. J Clin Virol. Jun;48(2):82–90. doi: 10.1016/j.jcv.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 21.Weinstock DM, Ambrossi GG, Brennan C, Kiehn TE, Jakubowski A. Preemptive diagnosis and treatment of Epstein-Barr virus-associated post transplant lymphoproliferative disorder after hematopoietic stem cell transplant: an approach in development. Bone Marrow Transplant. 2006 Mar;37(6):539–546. doi: 10.1038/sj.bmt.1705289. [DOI] [PubMed] [Google Scholar]
- 22.Green M, Bueno J, Rowe D, et al. Predictive negative value of persistent low Epstein-Barr virus viral load after intestinal transplantation in children. Transplantation. 2000 Aug 27;70(4):593–596. doi: 10.1097/00007890-200008270-00010. [DOI] [PubMed] [Google Scholar]
- 23.Baecklund E, Iliadou A, Askling J, et al. Association of chronic inflammation, not its treatment, with increased lymphoma risk in rheumatoid arthritis. Arthritis Rheum. 2006 Mar;54(3):692–701. doi: 10.1002/art.21675. [DOI] [PubMed] [Google Scholar]
- 24.Fishman JA, Emery V, Freeman R, et al. Cytomegalovirus in transplantation - challenging the status quo. Clin Transplant. 2007 Mar–Apr;21(2):149–158. doi: 10.1111/j.1399-0012.2006.00618.x. [DOI] [PubMed] [Google Scholar]
- 25.D'Ovidio V, Vernia P, Gentile G, et al. Cytomegalovirus infection in inflammatory bowel disease patients undergoing anti-TNFalpha therapy. J Clin Virol. 2008 Oct;43(2):180–183. doi: 10.1016/j.jcv.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 26.Guadalupe M, Reay E, Sankaran S, et al. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J Virol. 2003 Nov;77(21):11708–11717. doi: 10.1128/JVI.77.21.11708-11717.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kimura H, Morita M, Yabuta Y, et al. Quantitative analysis of Epstein-Barr virus load by using a real-time PCR assay. J Clin Microbiol. 1999 Jan;37(1):132–136. doi: 10.1128/jcm.37.1.132-136.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aalto SM, Juvonen E, Tarkkanen J, et al. Lymphoproliferative disease after allogeneic stem cell transplantation--pre-emptive diagnosis by quantification of Epstein-Barr virus DNA in serum. J Clin Virol. 2003 Dec;28(3):275–283. doi: 10.1016/s1386-6532(03)00022-2. [DOI] [PubMed] [Google Scholar]
- 29.Sankaran S, Guadalupe M, Reay E, et al. Gut mucosal T cell responses and gene expression correlate with protection against disease in long-term HIV-1-infected nonprogressors. Proc Natl Acad Sci U S A. 2005 Jul 12;102(28):9860–9865. doi: 10.1073/pnas.0503463102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lichtenstein GR, Hanauer SB, Sandborn WJ. Management of Crohn's disease in adults. Am J Gastroenterol. 2009 Feb;104(2):465–483. doi: 10.1038/ajg.2008.168. quiz 464, 484. [DOI] [PubMed] [Google Scholar]
- 31.Lavagna A, Bergallo M, Daperno M, et al. Infliximab and the risk of latent viruses reactivation in active Crohn's disease. Inflamm Bowel Dis. 2007 Jul;13(7):896–902. doi: 10.1002/ibd.20131. [DOI] [PubMed] [Google Scholar]
- 32.Santisteban M, Reynolds C, Barr Fritcher EG, et al. Ki67: a time-varying biomarker of risk of breast cancer in atypical hyperplasia. Breast Cancer Res Treat. Jun;121(2):431–437. doi: 10.1007/s10549-009-0534-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.D'Errico A, Garbisa S, Liotta LA, Castronovo V, Stetler-Stevenson WG, Grigioni WF. Augmentation of type IV collagenase, laminin receptor, and Ki67 proliferation antigen associated with human colon, gastric, and breast carcinoma progression. Mod Pathol. 1991 Mar;4(2):239–246. [PubMed] [Google Scholar]
- 34.Hwang JM, Varma MG. Surgery for inflammatory bowel disease. World J Gastroenterol. 2008 May 7;14(17):2678–2690. doi: 10.3748/wjg.14.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taoka K, Nannya Y, Yamamoto G, et al. Progressive transition of Epstein-Barr virus associated lymphoproliferative disease subtypes with the development of lung cancer. Am J Hematol. 2009 Sep;84(9):600–603. doi: 10.1002/ajh.21479. [DOI] [PubMed] [Google Scholar]
- 36.Pietersma F, Piriou E, van Baarle D. Immune surveillance of EBV-infected B cells and the development of non-Hodgkin lymphomas in immunocompromised patients. Leuk Lymphoma. 2008 Jun;49(6):1028–1041. doi: 10.1080/10428190801911662. [DOI] [PubMed] [Google Scholar]
- 37.Cruchley AT, Williams DM, Niedobitek G, Young LS. Epstein-Barr virus: biology and disease. Oral Dis. 1997 May;3 Suppl 1:S156–S163. doi: 10.1111/j.1601-0825.1997.tb00351.x. [DOI] [PubMed] [Google Scholar]
- 38.Tanner JE, Alfieri C. The Epstein-Barr virus and post-transplant lymphoproliferative disease: interplay of immunosuppression, EBV, and the immune system in disease pathogenesis. Transpl Infect Dis. 2001 Jun;3(2):60–69. doi: 10.1034/j.1399-3062.2001.003002060.x. [DOI] [PubMed] [Google Scholar]