Abstract
Background & Aims
Pegylated-Interferon-α2a (peg-IFN), a first line therapy for Hepatitis C virus (HCV) patients, also impacts recurrence of hepatocellular carcinoma (HCC). Wnt pathway activation due to β-catenin gene mutations contributes to the development of a significant subset of HCC. Here, we explored the effect of peg-IFN on Wnt/β-catenin signaling in vitro and in vivo.
Methods
Multiple human hepatoma cell lines were treated with Peg-IFN to assess its effect on Wnt pathway and address mechanism. Transgenic (TG) mice expressing stable β-catenin mutant in the liver were exposed to diethylnitrosamine (DEN) and treated with peg-IFN.
Results
In vitro, peg-IFN decreased transcriptional activity of β-catenin/Tcf and did so independent of JAK/Stat signaling. Peg-IFN treatment led to increased mRNA and protein expression of β-catenin nuclear export factor RanBP3 in all hepatoma cells. Coprecipitation studies showed increased association between RanBP3 and β-catenin after peg-IFN treatment. siRNA-mediated RanBP3 knockdown abrogated Peg-IFN-induced decrease in TOPFlash reporter activity. In vivo, Peg-IFN treatment led to increased nuclear RanBP3, decreased nuclear β-catenin and cyclin D1, and decreased cytoplasmic glutamine synthetase. Increased association of RanBP3 and β-catenin was also observed in vivo in response to Peg-IFN that led to decreased hepatocyte proliferation.
Conclusions
Peg-IFN inhibits β-catenin signaling through upregulation of RanBP3, which may be a contributory mechanism of delayed HCC and improved survival in treated HCV patients. This observation might have chemo preventive or chemotherapeutic implications in tumor with aberrant Wnt pathway activation.
Keywords: Wnt, Liver cancer, Interferon, Proliferation, Hepatocellular cancer, Ran binding protein-3 (RanBP3), treatment
INTRODUCTION
Interferons (IFN) are a family of glycoprotein cytokines that were first described for their antiviral activity [15]. Indeed, IFNs are currently a first line therapy for reducing viral load in patients infected with hepatitis C virus (HCV). Studies have also identified the role of these cytokines in controlling cell proliferation and differentiation [9, 12]. In fact, IFNs have proven useful in inhibiting proliferation of tumor cells in animals [36]. Such anti-proliferative activity makes IFN an intriguing option for chemoprevention and chemotherapy.
One of the long-term consequences of HCV infection is the development of hepatocellular carcinoma (HCC). Various studies have reported range of effects of IFN therapy on HCC in HCV patients ranging from effectively preventing the development of HCC to only reducing the late recurrence of HCC in HCV-infected patients [20, 28, 32]. Interestingly, even patients who were classified as not having a response to therapy showed a decrease in development of HCC. Furthermore, several case reports have reported successful treatment of HCC with IFN-α as part of the chemotherapeutic regimen [16, 17, 23, 25]. Thus, it is conceivable that IFN-α may have anti-tumor effects beyond decreasing the viral load in these patients. In fact, IFN-α has also shown to promote apoptosis in human hepatoma cells in the absence of HCV infection [30].
A molecular mechanism for IFN-α’s anti-tumor effects is yet to be elucidated. It was previously reported that β-catenin mutations occur in approximately 40% of HCV patients who develop HCC [13]. Additionally, HCV nonstructural NS5A protein activity ultimately leads to phosphorylation and inactivation of GSK3β, one of the key players in the degradation of β-catenin [33]. Such activity leads to accumulation of β-catenin in the cytoplasm and nucleus with subsequent transcriptional activation of Wnt pathway targets. These findings implicate the Wnt/β-catenin pathway as a culprit in HCV-associated hepatocarcinogenesis.
Given the likely role of β-catenin in HCV-associated HCC and the anti-tumor effects of pegylated interferon-α2a (peg-IFN), we investigated the hypothesis that peg-IFN could negatively regulate Wnt/β-catenin signaling. We report here evidence from studies on several human hepatoma cell lines, as well from in vivo experiments with serine-45 mutated-β-catenin transgenic mice [26], that Peg-IFN can effectively target the Wnt pathway. Intriguingly, this effect appears to be mediated by upregulation of a nuclear export factor, RanBP3. Ultimately, the results presented support the efficacy of utilizing peg-IFN to inhibit the Wnt/β-catenin pathway in hepatocellular carcinoma.
MATERIALS AND METHODS
Cell Culture and Reagents
Human HCC cell lines, Hep3B, HepG2, Huh-7 and Snu-449, were obtained from American Tissue Type Culture (Manassas, VA). Cells were cultured in Eagle’s minimum essential medium (EMEM, Cambrex, Walkersville, MD) supplemented with either 2 or 10% fetal bovine serum and incubated in 5% CO2 at 37ºC. Treatments were begun when cells were approximately 30–50% confluent. Peg-IFN was utilized at a concentration of 100 U/ml. Ribavirin was utilized at a concentration of 5, 50, or 200 μM. Cells were treated and media changed every 48 hr for 1–4 doses for various cells as indicated in results, at which time the cells were harvested for analysis.
β-Catenin/Tcf Transcription Reporter Assay and siRNA Transfection
HepG2 cells (treated 3 or 4 times every 48 hours with Peg-IFN) or Hep3B cells (treated once with Peg-IFN), plated in six-well plates, were transiently transfected with 0.8 μg of TOPFlash and FOPFlash plasmids (Upstate Biotechnology, Lake Placid, NY) with FuGene HD reagent (Roche, Indianapolis, IN). To normalize transfection efficiency, cells were co-transfected with 0.1 μg of internal control reporter Renilla reniformis luciferase driven under the TK promoter (pRL-TK; Promega, Madison, WI). Luciferase assay was performed using the Dual Luciferase Assay System kit according to the manufacturer’s protocols (Promega). Relative luciferase activity was reported as a ratio of firefly/renilla luciferase activity. Experiments were performed in triplicates.
To study the effect of Peg-IFN on exogenous Wnt/β-catenin activation, Hep3B cells were treated with Lithium chloride (25mM) followed one hour later by TOPFlash/FOPFlash transfection followed 5 hours later by Peg-IFN. Cells were harvested at 18 hours for analysis of reporter activity.
For siRNA studies, Hep3B cells were cultured in 6-well plates in 2ml of EMEM medium complemented with 10% FBS. Following serum starvation for 16 hours, the cells at 30–50% confluency were transfected with pre-validated human RanBP3 siRNA (Ambion, Inc, Cat No. 4392420) or control (scrambled) siRNA (Ambion, Inc, Cat No. 4390846) at a final concentration of 20nM using Lipofectamine (Invitrogen, Carlsbad, CA). Transfected cells were harvested 48 hours after transfections for whole cell lysate (see below) for western blots.
For examining the functional role of RanBP3 in Peg-IFN-mediated decrease in β-catenin activity, serum was reintroduced to Hep3B cultures after 6 hours of control of RanBP3 or control siRNA transfection. TOPFlash transfection was done 24 hours after siRNA transfection followed 6 hours later by a single treatment of Peg-IFN (100 U/ml). Cells were harvested 18 hours later to assay for reporter activity. All experiments were performed in triplicates.
Diethylnitrosamine Induction and Peg-IFN treatment
Transgenic (TG) animals overexpressing serine-45 mutant β-catenin in the liver were recently reported [26]. At 14 days of age, TG mice were given a single i.p. injection of diethylnitrosamine (DEN) (Sigma) at a dose of 5 mg/kg of body weight. At 2.5 months following DEN exposure, mice received subcutaneous injection of 0.9% saline or Peg-IFN (n=4). Mice received six weekly injections of 7000U or 70,000U of Peg-IFN and sacrificed. Livers were harvested and processed for various analyses. All animal studies were performed as per the institutional guidelines at the University of Pittsburgh and NIH.
Protein Extraction, Coprecipitation and Western Blot Analysis
Western blot analysis was performed as previously described [22]. Nuclear and cytoplasmic extraction was performed using NE-PER nuclear and cytoplasmic extraction reagents according to the manufacturer’s protocol (Pierce). The following primary antibodies were-mouse monoclonal anti-β-catenin (1:500) and mouse monoclonal anti-RanBP3 (1:500) (BD Biosciences); rabbit anti-cyclin D1 (1:500, Neomarkers), rabbit anti-Lamin B1 (Santa Cruz, 1:300), mouse monoclonal anti-actin (Santa Cruz, 1:5000). HRP-conjugated secondary antibodies (Chemicon International Inc., Temecula, CA) were used at concentrations of 1:10,000 to 1:50,000. Blots were visualized with Western Lightning™ chemiluminescence kit (PerkinElmer Life Sciences, Boston, MA). Whole-cell lysates were used to immunoprecipitate β-catenin to assess association with RanBP3 as described previously [34]. Densitometry on autoradiographs was performed by NIH Imager.
Real-time PCR
mRNA isolation and cDNA synthesis has been described elsewhere [22]. Real-time PCR was performed for Dkk-1 (sense: 5’-ctc ggt tct caa ttc caa cg-3’; antisense: 5’-gca ctc ctc gtc ctc tg-3’) and RanBP3 (sense: 5’-tga aga gga agc ctg tga gaa-3’; antisense: 5’-gtg tcc agc att ctc cat gtc-3’) on ABI Prism 7000 sequence detection system (Applied biosystems). Three samples were pooled for each condition and run in duplicate. Treated samples were compared to control using the ΔΔCt method and plotted as fold change. Actin was used as normalization control.
Immunohistochemistry
Cell proliferation was assessed by immunohistochemistry for PCNA as described elsewhere [22]. Slides were viewed on Axioskop 40 microscope (Zeiss) and digital images obtained by Nikon Coolpix camera. PCNA-positive hepatocytes were counted in 10 fields (200x), averaged and statistically assessed by student t-test.
RESULTS
Peg-IFN decreases β-catenin/Tcf-mediated transcriptional activity in human hepatoma cells
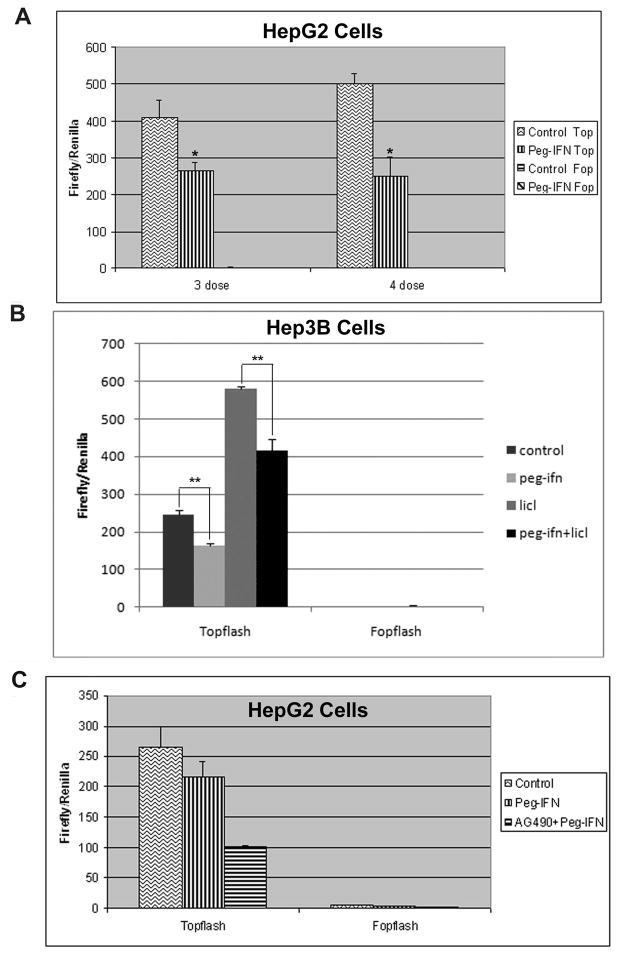
To examine the effect of Peg-IFN on the Wnt pathway, we utilized the TOPFlash luciferase reporter assay that specifically measures β-catenin/Tcf-dependent transcriptional activation. We initiated the Peg-IFN treatment on the HepG2 cells that harbor a deletion in exon-3 of the β-catenin gene rendering it stable and constitutively active, and thus exhibit high baseline β-catenin/Tcf-mediated transcriptional activity [6]. Treatment with 3 or 4 doses of Peg-IFN resulted in 37% and >50% decrease in TOPFlash reporter activity respectively (p<0.05) (Fig. 1A). We next tested the effect of Peg-IFN treatment on Hep3B cells that carry wild-type β-catenin gene. Peg-IFN treatment led to a significant decrease in TOPFlash reporter activity (p<0.005) (single dose shown in Fig. 1B). Treatment with Peg-IFN also decreased TOPFlash reporter activity that was stimulated by inclusion of Lithium chloride, which induces Wnt signaling through GSK3β inhibition (Fig. 1B). Thus, through use of a highly specific and sensitive reporter assay, we demonstrate an anti-β-catenin effect of Peg-IFN [1].
Figure 1. Peg-IFN decreases β-catenin activity in vitro and does so independent of JAK/STAT signaling.
A. Relative luciferase activity following transfection with either TOPFlash or FOPFlash reporter plasmid and treatment with three or four doses of Peg-IFN in HepG2 cells. Experiments were done in triplicate and averaged. (*p<0.05)
B. Relative luciferase activity following transfection with either TOPFlash or FOPFlash reporter plasmid and one treatment with Peg-IFN or with one treatment with lithium followed by one dose treatment of Peg-IFN in Hep3Bcells. Experiments were done in triplicate and averaged. (**p<0.005)
C. Inhibition of JAK/Stat pathway with AG490 does not reverse the effect of Peg-IFN on Topflash indicating that this effect is independent of the JAK/Stat pathway.
Peg-IFN effect on Wnt Pathway independent of Jak/Stat signaling
Given that interferon cytokines typically signal via the Jak/Stat pathway [21], we wanted to explore the possibility that the anti-β-catenin effect of Peg-IFN’s maybe dependent on these downstream effectors. To investigate, we simultaneously treated HepG2 cells with Peg-IFN and a chemical inhibitor of JAK, AG490, with the expectation that transcriptional activity would return to normal levels if the anti-β-catenin effect of Peg-IFN were JAK/Stat-dependent. On the contrary, we observed that inhibition of the JAK/Stat pathway led to an enhanced anti- β - catenin activity following Peg-IFN treatment (Fig. 1C). This observation suggests that Peg-IFN may inhibit Wnt signaling in JAK/Stat-independent manner.
Peg-IFN increases transcription of Dkk-1 and RanBP3
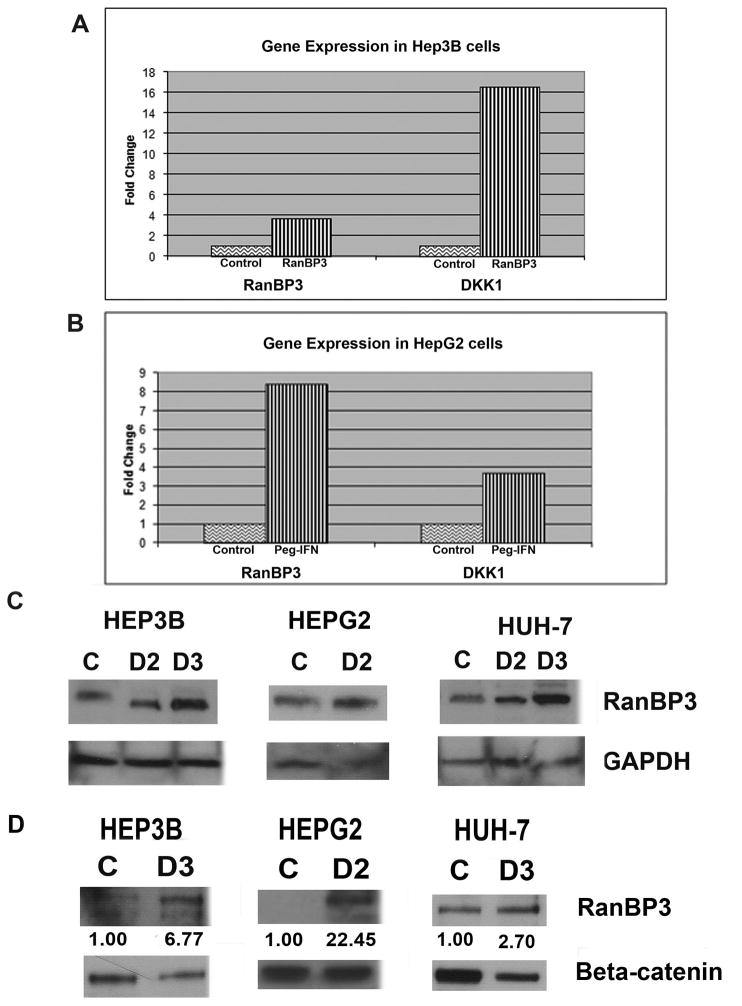
To determine the possible mechanisms through which Peg-IFN may be affecting the Wnt/β-catenin signaling, we explored its effect on some well-known negative regulators of the Wnt pathway. One well-characterized inhibitor of the Wnt signaling is Dikkopf-1 (Dkk-1), which was previously shown to be upregulated in cells treated with IFN- α [8, 29]. We observed an increase in the expression of Dkk-1 in both Hep3B (16-fold) and HepG2 cells (4-fold) that were treated with Peg-IFN (Fig. 2A&B). Since, Dkk1 is known to downregulate the Wnt signaling by preventing Wnt-Frizzled-LRP5/6 complex formation, it is likely to be relevant in inhibiting β-canonical Wnt signaling in cell lines such as Hep3B cells that contain wild-type β-catenin [19]. Hence Dkk1 upregulation would likely be ineffective in tumor cells that have downstream defects resulting in Wnt activation such as in HepG2 cells, which harbor β-catenin gene with exon-3 deletion. Given this, we explored any changes in another known mediator of Wnt signaling, the Ran Binding Protein 3 (RanBP3). It was previously reported that RanBP3 is involved in the nuclear export of β-catenin and, importantly, is capable of acting on both wild-type and mutant forms of β-catenin [11]. Interestingly, we observed a several-fold increase in the gene expression of RanBP3 in Hep3B, HepG2, (Fig. 2A, B) and Snu-449 (data not shown) cells after peg-IFN treatment. These findings indicate that RanBP3 is a target of the IFN-α signaling and could potentially explain the negative effect on the Wnt pathway exhibited by Peg-IFN therapy.
Figure 2. Peg-IFN increases Dkk-1 and RanBP3 expression in multiple hepatoma cell lines, and increases β-catenin-RanBP3 association.
A. Real-time PCR showing increase in expression of Dkk-1 and RanBP3 in Hep3B cells after Peg-IFN. Values normalized to control and presented as fold-change.
B. Real-time PCR showing increase in expression of Dkk-1 and RanBP3 in HepG2 cells after Peg-IFN. Values normalized to control and presented as fold-change.
C. Increased total RanBP3 protein is evident after dose 1 (D1) and D2 in Hep3B, D2 in HepG2 and D2 and D3 in Huh-7 cells by western blots.
D. Immunoprecipitation studies demonstrate an increase in RanBP3-β-catenin association following Peg-IFN doses. Densitometric values representing an increase in β-catenin-RanBP3 association following Peg-IFN treatment relative to saline-treated controls are indicated.
Peg-IFN increases levels of RanBP3 protein along with its association to β-catenin in hepatoma cells
To determine if an increase in gene expression of RanBP3 coincided with its protein, we performed western blot analysis on whole cell lysates from Peg-IFN treated human hepatoma cells. Indeed, a dose-dependent increase in levels of RanBP3 occurred after Peg-IFN treatment (Fig. 2C; shown two and three doses in Hep3B cells, two doses in HepG2 cells and two and three doses of peg-IFN in Huh-7 cells).
Since RanBP3 binds to β-catenin to induce its nuclear export (30), we next determined their association after Peg-IFN treatment. A dramatic and multi-fold increase in association between RanBP3 and β-catenin in Hep3B, HepG2 cells and Huh-7 cells, was induced by Peg-IFN treatment (Fig. 2D). Taken together these results suggest that Peg-IFN induces RanBP3 expression and interaction with β-catenin to induce a decrease in Wnt/β-catenin activity in hepatoma cells.
RanBP3 acts downstream of Peg-IFN to negatively regulate Wnt pathway
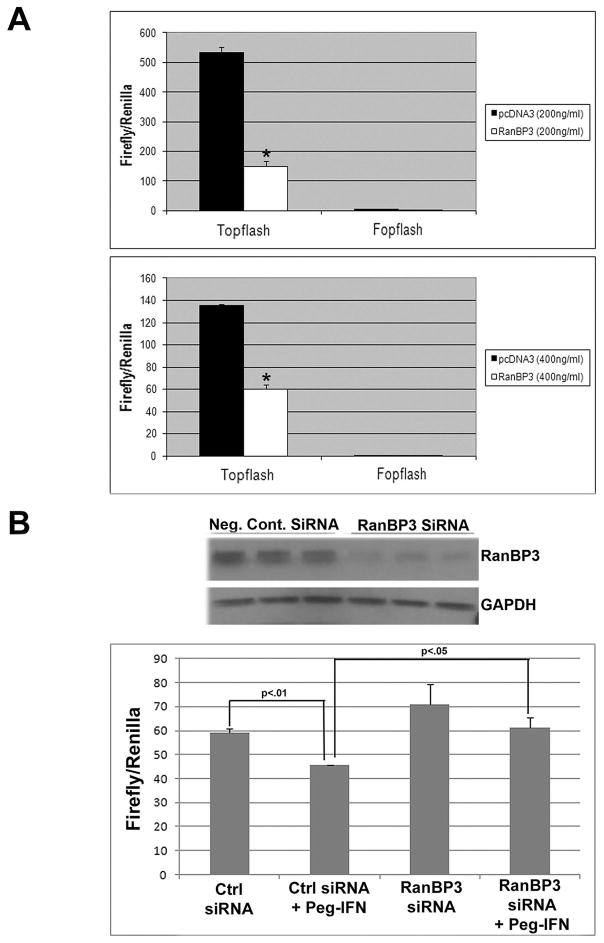
To verify the negative regulatory effect of RanBP3 on the canonical Wnt signaling in human hepatoma cells, we overexpressed RanBP3 in HepG2 cells and measured the effect on pathway activity by the TOPFlash assay. Indeed, HepG2 cells transfected with a RanBP3 overexpression vector exhibited a significant decrease in reporter activity much like Peg-IFN treatment (Fig. 3A).
Figure 3. Negative effect on Wnt pathway after Peg-IFN treatment is via upregulation of RanBP3.
A. TOPFlash luciferase assay showing a decrease in reporter activity after overexpression of RanBP3 in HepG2 cells.
B. Topflash luciferase reporter assay shows a significant decrease in β-catenin activity after Peg-IFN treatment in control siRNA transfected Hep3B cells (p<0.01). Transfection of RanBPB3 siRNA that leads to a dramatic suppression of RanBP3 protein expression after 48 hours (upper panel) led to a significant abrogation of Peg-IFN-induced reduction of TOPFlash reporter activity (p<0.05).
However, to more conclusively address the role of RanBP3 in mediating Peg-IFN’s negative effect on the Wnt pathway, we sought to examine the impact of Peg-IFN treatment following siRNA knockdown of RanBP3. Transfection of RanBP3 siRNA led to a dramatic decrease in its total protein after 48 hours when compared to control demonstrating the efficacy of siRNA (Fig. 3B). As described in the methods, sequential transfection of RanBP3 or control siRNA, of TOPFlash followed by Peg-IFN treatment was next performed in Hep3B cells. Peg-IFN treatment led to a significant suppression of β-catenin activity (p<0.01) in the presence of control siRNA (Fig. 3B). However, presence of RanBP3 siRNA significantly abrogated the decrease in TOPFlash reporter activity (p<0.05) after Peg-IFN treatment when compared to control siRNA (Fig. 3B). As expected, suppression of RanBP3 expression did lead to a basal increase in β-catenin/Tcf mediated transcriptional activity. The above observations signify the importance of RanBP3 in Peg-IFN-mediated suppression of Wnt/β-catenin signaling.
Peg-IFN decreases β-catenin activity and proliferation in vivo
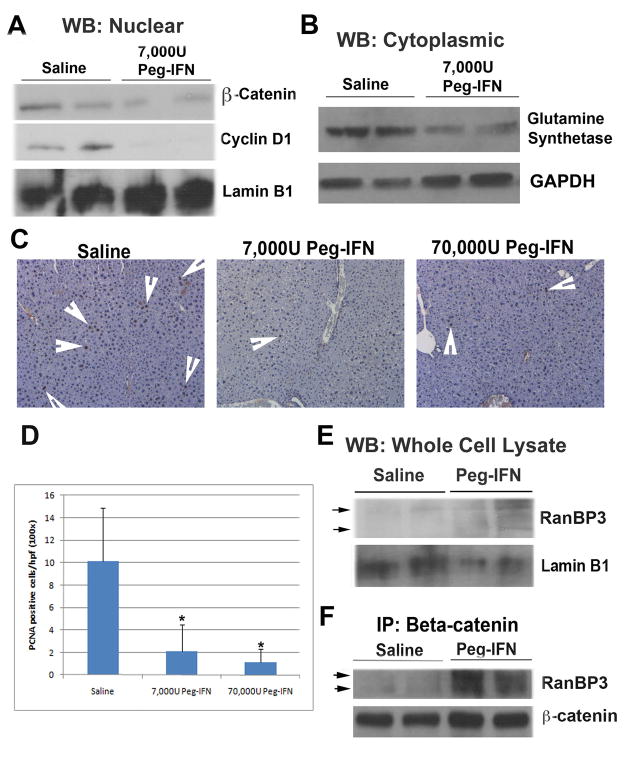
To examine the effect of Peg-IFN on the Wnt signaling pathway in vivo, we utilized recently characterized liver-specific serine-45 mutant β-catenin transgenic mice (TG). While the TG mice do not show an increase in spontaneous HCC, these animals demonstrate accelerated tumorigenesis in response to diethylnitrosamine (DEN) [26]. For the current study, TG mice were administered a single IP injection of DEN at day 15 after birth and 10 weeks later randomly received 6 weekly saline and 7000U or 70,000U Peg-IFN (n=4) [14, 35]. Peg-IFN treatment at both doses led to decreased nuclear levels of β-catenin as observed by western blot analysis (shown for lower dose in Fig. 4A). Furthermore, cyclin-D1, a target of the Wnt pathway and an indicator of activation of this pathway, was dramatically reduced in the same nuclear extracts (Fig. 4A). Glutamine synthetase, a specific target of the Wnt pathway, was decreased in cytoplasmic extracts after the treatment with Peg-IFN as well (Fig. 4B).
Figure 4. Peg-IFN decreases β-catenin activity in vivo.
A. Immunoblotting shows decreased levels of nuclear β-catenin and cyclin D1 after 6 weekly treatments with 7000U of Peg-IFN.
B. Immunoblotting shows decreased cytoplasmic levels of glutamine synthetase after 6 weekly treatments with 7000U of Peg-IFN.
C. IHC for PCNA in liver of animals treated with saline or Peg-IFN. Arrows indicate representative PCNA positive hepatocytes.
D. Significant difference in the numbers of PCNA positive hepatocytes per high power field (200x) in the liver of animals treated with saline or Peg-IFN at either dose (*p<0.05).
E. An increase in total RanBP3 is observed in whole cell liver lysates from mice that underwent treatment with 6 weekly doses of Peg-IFN at 7000U.
F. Coprecipitation studies identify a dramatic increase in RanBP3-β-catenin association in whole liver cell lysates of mice treated with 6 weekly Peg-IFN injections of 7000U each as compared to saline treated animals.
To assess any biological response to Peg-IFN treatment, hepatocyte proliferation was measured by immunohistochemistry for PCNA (Fig. 4C). A significant decrease in the number of PCNA positive hepatocytes was evident after Peg-IFN treatment (p<0.05) (Fig. 4D). To determine if these in vivo observations could be due to RanBP3 as determined in vitro, we assayed whole cell liver lysates for RanBP3 protein in control and experimental group. Indeed an increase in total RanBP3 was observed in Peg-IFN treated DEN-exposed TG livers as compared to saline controls (Fig. 4E). To further strengthen this observation, we assessed if RanBP3 was associating with β-catenin following Peg-IFN treatment. Indeed such a treatment led to a dramatic increase in association between these two proteins after Peg-IFN treatment clearly implicating RanBP3 as a chief mechanism of inhibition of β-catenin through its nuclear export (Fig. 4F).
DISCUSSION
While an important player in liver development and growth, the Wnt/β-catenin pathway is also known for its oncogenicity in HCC [24]. Our laboratory has performed proof-of-principle studies demonstrating decreased survival and proliferation of human liver tumor cells secondary to β-catenin suppression [2, 37]. Successful targeting of the Wnt/β-catenin pathway via drug therapy as a treatment strategy for HCC patients is thus significanct [18, 24]. This is also true for many cases of HCC that are observed in the setting of HCV infection, which show high rates of β-catenin gene mutations [13]. In the current study, we show that Peg-IFN, a first line therapy for the treatment of HCV infection, is capable of decreasing the transcriptional activity of β-catenin in liver tumor cells and does so by a lesser recognized but highly relevant mechanism that entails an upregulation of the β-catenin nuclear export factor RanBP3 and enhanced association between β-catenin and RanBP3. Peg-IFN affected TOPFlash luciferase reporter assay, a specific and sensitive measure of β-catenin/Tcf mediated transcription decrease in multiple hepatoma cells and during exogenous activation of β-catenin signaling [1]. The role of RanBP3 in Peg-IFN-mediated downregulation of the Wnt/β-catenin activity was further verified when siRNA-mediated suppression of RanBP3 led to reversal of β-catenin activity after Peg-IFN treatment of hepatoma cells. Eventually, the in vivo significance of this observation was realized in DEN-exposed β-catenin TG mice [26], where Peg-IFN treatment led to increased RanBP3 and its association to β-catenin and decreased nuclear β-catenin, cyclin-D1 and cell proliferation. Whether this decrease in hepatocyte proliferation would eventually impact the accelerated tumorigenesis reported in the TG mice at 6 months, remains under investigation in the laboratory.
Multiple negative regulators of the Wnt pathway have been identified over the last 20 years. One well-known negative regulator that is upregulated in HepG2 cells after recombinant IFN-α treatment is Dkk-1 [29]. We also identified increased expression of Dkk-1 by Peg-IFN treatment. Dkk-1 acts to inhibit Wnt pathway signaling by binding to LRP6 and preventing its interaction with frizzled that is essential for canonical Wnt signaling [19, 31]. While an increase in Dkk-1 expression maybe effective in reducing Wnt signaling when β-catenin gene is not mutated as in Hep3B cells and Huh-7 cells, it is unlikely to be meaningful in tumors such as HepG2 cells that harbor stabilizing mutations or deletions in β-catenin gene. Given this, we sought to explore additional negative regulators of the Wnt pathway, which would be more realistic and significant candidates.
One candidate that is proven to be effective against wild-type and mutated β-catenin alike is the nuclear export factor RanBP3 [11]. We report here that RanBP3 is a novel downstream target of Peg-IFN and mediates its negative impact on Wnt signaling. Overexpression of RanBP3 in HepG2 cells mimicked the effect of Peg-IFN and inhibited Wnt-mediated transcriptional activation. Qu et al had previously reported an increase in RanBP5 but not RanBP3 in response to interferon-α treatment of the HepG2 cells [29]. RanBP3 was first identified as a chromosome region maintainence-1 (CRM1)-dependent factor involved in protein nuclear export [7]. Interestingly, RanBP3 appears to act on β-catenin independent of CRM1 [11]. Several other mechanisms for nuclear export of β-catenin have been previously reported including direct binding of β-catenin to the nuclear pore complex and CRM1 dependent export involving APC (reviewed in [10]).
Patients studies have previously shown Peg-IFN to exhibit anti-HCC properties HCC [16, 17, 23, 25, 27, 32]. We believe this effect maybe in part due to suppression of β-catenin signaling through upregulation of RanBP3. Thus, Peg-IFN therapy may have broader applications in treatment of HCC that exhibit Wnt activation. To circumvent some of the untoward side effects of this agent, inclusion of Peg-IFN in loco-regional therapies for HCC such as in transarterial chemo-embolization may be efficacious, after Wnt activation is confirmed through assessment of β-catenin, GS or others by various diagnostic modalities [3–5, 38].
Acknowledgments
Grant Support: This study was funded by NIH grants 1R01DK62277 and 1R01CA124414 to SPSM, 1F30DK083235 to MDT, and by Rango’s Fund for the Enhancement of Pathology Research. This project was also partly sponsored by Roche Pharmaceuticals, grant number PEG157 to SPSM.
We would like to thank Dr. Maarten Fornerod for generously providing the constructs for overexpression of RanBP3.
Abbreviations
- Peg-IFN
Pegylated Interferon-α2a
- HCC
hepatocellular cancer
- RANBP3
Ran binding protein 3
- GSK
glycogen synthase kinase
- CRM1
chromosome region maintainence-1
- HCV
Hepatitis C virus
- TG
transgenic
- APC
adenomatous polyposis coli gene
- DKK
dickkopf
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barker N, Clevers H. Mining the Wnt pathway for cancer therapeutics. Nat Rev Drug Discov. 2006 Dec;5(12):997–1014. doi: 10.1038/nrd2154. [DOI] [PubMed] [Google Scholar]
- 2.Behari J, Zeng G, Otruba W, Thompson MD, Muller P, Micsenyi A, et al. R-Etodolac decreases beta-catenin levels along with survival and proliferation of hepatoma cells. Journal of hepatology. 2007 May;46(5):849–857. doi: 10.1016/j.jhep.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cadoret A, Ovejero C, Terris B, Souil E, Levy L, Lamers WH, et al. New targets of beta-catenin signaling in the liver are involved in the glutamine metabolism. Oncogene. 2002 Nov 28;21(54):8293–8301. doi: 10.1038/sj.onc.1206118. [DOI] [PubMed] [Google Scholar]
- 4.Cieply B, Zeng G, Proverbs-Singh T, Geller DA, Monga SP. Unique phenotype of hepatocellular cancers with exon-3 mutations in beta-catenin gene. Hepatology. 2009 Mar;49(3):821–831. doi: 10.1002/hep.22695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colnot S, Decaens T, Niwa-Kawakita M, Godard C, Hamard G, Kahn A, et al. Liver-targeted disruption of Apc in mice activates beta-catenin signaling and leads to hepatocellular carcinomas. Proceedings of the National Academy of Sciences of the United States of America. 2004 Dec 7;101(49):17216–17221. doi: 10.1073/pnas.0404761101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de La Coste A, Romagnolo B, Billuart P, Renard CA, Buendia MA, Soubrane O, et al. Somatic mutations of the beta-catenin gene are frequent in mouse and human hepatocellular carcinomas. Proceedings of the National Academy of Sciences of the United States of America. 1998 Jul 21;95(15):8847–8851. doi: 10.1073/pnas.95.15.8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Englmeier L, Fornerod M, Bischoff FR, Petosa C, Mattaj IW, Kutay U. RanBP3 influences interactions between CRM1 and its nuclear protein export substrates. EMBO reports. 2001 Oct;2(10):926–932. doi: 10.1093/embo-reports/kve200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gollob JA, Sciambi CJ, Huang Z, Dressman HK. Gene expression changes and signaling events associated with the direct antimelanoma effect of IFN-gamma. Cancer research. 2005 Oct 1;65(19):8869–8877. doi: 10.1158/0008-5472.CAN-05-1387. [DOI] [PubMed] [Google Scholar]
- 9.Gutterman JU. Cytokine therapeutics: lessons from interferon alpha. Proceedings of the National Academy of Sciences of the United States of America. 1994 Feb 15;91(4):1198–1205. doi: 10.1073/pnas.91.4.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henderson BR, Fagotto F. The ins and outs of APC and beta-catenin nuclear transport. EMBO reports. 2002 Sep;3(9):834–839. doi: 10.1093/embo-reports/kvf181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hendriksen J, Fagotto F, van der Velde H, van Schie M, Noordermeer J, Fornerod M. RanBP3 enhances nuclear export of active (beta)-catenin independently of CRM1. The Journal of cell biology. 2005 Dec 5;171(5):785–797. doi: 10.1083/jcb.200502141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hertzog PJ, Hwang SY, Kola I. Role of interferons in the regulation of cell proliferation, differentiation, and development. Molecular reproduction and development. 1994 Oct;39(2):226–232. doi: 10.1002/mrd.1080390216. [DOI] [PubMed] [Google Scholar]
- 13.Huang H, Fujii H, Sankila A, Mahler-Araujo BM, Matsuda M, Cathomas G, et al. Beta-catenin mutations are frequent in human hepatocellular carcinomas associated with hepatitis C virus infection. The American journal of pathology. 1999 Dec;155(6):1795–1801. doi: 10.1016/s0002-9440(10)65496-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang SF, Kim SJ, Lee AT, Karashima T, Bucana C, Kedar D, et al. Inhibition of growth and metastasis of orthotopic human prostate cancer in athymic mice by combination therapy with pegylated interferon-alpha-2b and docetaxel. Cancer research. 2002 Oct 15;62(20):5720–5726. [PubMed] [Google Scholar]
- 15.Isaacs A, Lindenmann J. Virus interference. I. The interferon. Proceedings of the Royal Society of London Series B, Containing papers of a Biological character. 1957 Sep 12;147(927):258–267. doi: 10.1098/rspb.1957.0048. [DOI] [PubMed] [Google Scholar]
- 16.Katsuramaki T, Furuhata T, Kimura Y, Yamaguchi K, Ohmura T, Hata F, et al. A case of recurrent hepatocellular carcinoma successfully treated with a combination therapy of interferon-alpha and intravenous continuous infusion of 5-fluorouracil. Gan to kagaku ryoho. 2002 Oct;29(10):1801–1804. [PubMed] [Google Scholar]
- 17.Kurokohchi K, Takaguchi K, Kita K, Masaki T, Kuriyama S. Successful treatment of advanced hepatocellular carcinoma by combined administration of 5-fluorouracil and pegylated interferon-alpha. World J Gastroenterol. 2005 Sep 14;11(34):5401–5403. doi: 10.3748/wjg.v11.i34.5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Llovet JM, Bruix J. Molecular targeted therapies in hepatocellular carcinoma. Hepatology. 2008 Oct;48(4):1312–1327. doi: 10.1002/hep.22506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mao B, Wu W, Li Y, Hoppe D, Stannek P, Glinka A, et al. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature. 2001 May 17;411(6835):321–325. doi: 10.1038/35077108. [DOI] [PubMed] [Google Scholar]
- 20.Mazzaferro V, Romito R, Schiavo M, Mariani L, Camerini T, Bhoori S, et al. Prevention of hepatocellular carcinoma recurrence with alpha-interferon after liver resection in HCV cirrhosis. Hepatology. 2006 Dec;44(6):1543–1554. doi: 10.1002/hep.21415. [DOI] [PubMed] [Google Scholar]
- 21.Melen K, Keskinen P, Lehtonen A, Julkunen I. Interferon-induced gene expression and signaling in human hepatoma cell lines. Journal of hepatology. 2000 Nov;33(5):764–772. doi: 10.1016/s0168-8278(00)80308-6. [DOI] [PubMed] [Google Scholar]
- 22.Micsenyi A, Tan X, Sneddon T, Luo JH, Michalopoulos GK, Monga SP. Beta-catenin is temporally regulated during normal liver development. Gastroenterology. 2004 Apr;126(4):1134–1146. doi: 10.1053/j.gastro.2003.12.047. [DOI] [PubMed] [Google Scholar]
- 23.Miyamoto A, Umeshita K, Sakon M, Nagano H, Eguchi H, Kishimoto S, et al. Advanced hepatocellular carcinoma with distant metastases, successfully treated by a combination therapy of alpha-interferon and oral tegafur/uracil. Journal of gastroenterology and hepatology. 2000 Dec;15(12):1447–1451. doi: 10.1046/j.1440-1746.2000.02289.x. [DOI] [PubMed] [Google Scholar]
- 24.Monga SP. Role of Wnt/beta-catenin signaling in liver metabolism and cancer. Int J Biochem Cell Biol. 2009 Sep 9; doi: 10.1016/j.biocel.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakamura M, Nagano H, Wada H, Noda T, Ota H, Damdinsuren B, et al. A case of hepatocellular carcinoma with multiple lung, spleen, and remnant liver metastasis successfully treated by combination chemotherapy with the novel oral DPD-inhibiting chemotherapeutic drug S-1 and interferon-alpha. Journal of gastroenterology. 2006 Nov;41(11):1120–1125. doi: 10.1007/s00535-006-1907-x. [DOI] [PubMed] [Google Scholar]
- 26.Nejak-Bowen KN, Thompson MD, Singh S, Bowen WC, Jr, Dar MJ, Khillan J, et al. Accelerated liver regeneration and hepatocarcinogenesis in mice overexpressing serine-45 mutant beta-catenin. Hepatology. 2010 May;51(5):1603–1613. doi: 10.1002/hep.23538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishiguchi S, Shiomi S, Nakatani S, Takeda T, Fukuda K, Tamori A, et al. Prevention of hepatocellular carcinoma in patients with chronic active hepatitis C and cirrhosis. Lancet. 2001 Jan 20;357(9251):196–197. doi: 10.1016/S0140-6736(00)03595-9. [DOI] [PubMed] [Google Scholar]
- 28.Omata M, Yoshida H, Shiratori Y. Prevention of hepatocellular carcinoma and its recurrence in chronic hepatitis C patients by interferon therapy. Clin Gastroenterol Hepatol. 2005 Oct;3(10 Suppl 2):S141–143. doi: 10.1016/s1542-3565(05)00713-5. [DOI] [PubMed] [Google Scholar]
- 29.Qu JH, Cheng J, Zhang LX, Zhang LY, Zhong YW, Liu Y, et al. Identification of genes upregulated by recombinant interferon-alpha in HepG2 cells by suppressive subtractive hybridization analysis. Hepatobiliary Pancreat Dis Int. 2007 Jun;6(3):290–293. [PubMed] [Google Scholar]
- 30.Schlosser SF, Schuler M, Berg CP, Lauber K, Schulze-Osthoff K, Schmahl FW, et al. Ribavirin and alpha interferon enhance death receptor-mediated apoptosis and caspase activation in human hepatoma cells. Antimicrobial agents and chemotherapy. 2003 Jun;47(6):1912–1921. doi: 10.1128/AAC.47.6.1912-1921.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Semenov MV, Tamai K, Brott BK, Kuhl M, Sokol S, He X. Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr Biol. 2001 Jun 26;11(12):951–961. doi: 10.1016/s0960-9822(01)00290-1. [DOI] [PubMed] [Google Scholar]
- 32.Soga K, Shibasaki K, Aoyagi Y. Effect of interferon on incidence of hepatocellular carcinoma in patients with chronic hepatitis C. Hepato-gastroenterology. 2005 Jul–Aug;52(64):1154–1158. [PubMed] [Google Scholar]
- 33.Street A, Macdonald A, McCormick C, Harris M. Hepatitis C virus NS5A-mediated activation of phosphoinositide 3-kinase results in stabilization of cellular beta-catenin and stimulation of beta-catenin-responsive transcription. Journal of virology. 2005 Apr;79(8):5006–5016. doi: 10.1128/JVI.79.8.5006-5016.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tan X, Apte U, Micsenyi A, Kotsagrelos E, Luo JH, Ranganathan S, et al. Epidermal growth factor receptor: a novel target of the Wnt/beta-catenin pathway in liver. Gastroenterology. 2005 Jul;129(1):285–302. doi: 10.1053/j.gastro.2005.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tedjarati S, Baker CH, Apte S, Huang S, Wolf JK, Killion JJ, et al. Synergistic therapy of human ovarian carcinoma implanted orthotopically in nude mice by optimal biological dose of pegylated interferon alpha combined with paclitaxel. Clin Cancer Res. 2002 Jul;8(7):2413–2422. [PubMed] [Google Scholar]
- 36.Thomas H, Balkwill FR. Effects of interferons and other cytokines on tumors in animals: a review. Pharmacology & therapeutics. 1991 Dec;52(3):307–330. doi: 10.1016/0163-7258(91)90030-p. [DOI] [PubMed] [Google Scholar]
- 37.Zeng G, Apte U, Cieply B, Singh S, Monga SP. siRNA-mediated beta-catenin knockdown in human hepatoma cells results in decreased growth and survival. Neoplasia. 2007 Nov;9(11):951–959. doi: 10.1593/neo.07469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zucman-Rossi J, Benhamouche S, Godard C, Boyault S, Grimber G, Balabaud C, et al. Differential effects of inactivated Axin1 and activated beta-catenin mutations in human hepatocellular carcinomas. Oncogene. 2007 Feb 1;26(5):774–780. doi: 10.1038/sj.onc.1209824. [DOI] [PubMed] [Google Scholar]