Introduction
Preclinical studies of gestational cocaine exposure (GCE) are replete with evidence of changes in brain function at the anatomical, physiological, and behavioral levels, to include effects on developing dopaminergic systems [42,56,83,90,131]. In contrast, human studies of child outcomes have produced less consistent results, with many investigators finding only subtle impairments particularly in tasks of attention, language and memory [1,9,11,47,101,110].
Some of the variability in outcome associated with GCE in humans is undoubtedly attributable to the more complex context in which children develop, compared to the highly controlled environments in animal models. Further it is nearly impossible to control for the myriad of factors that correlate with maternal cocaine use. These factors include SES, concurrent use of other drugs, family mental health history, caregiver characteristics, child exposure to violence as witness or victim, homelessness, and parental separation and loss [19,47,86,91,96,118]. Other reasons for these varied findings may be related both to the measures chosen to assess outcomes in children and the timing of these assessments [6,37].
Studies of the effects of GCE on language development have shown mixed results. Our group found no GCE effects on language functioning assessed using the Preschool Language Scale at age 2.5 years and the Battelle Developmental Inventory Communication Subscale at ages 3 and 5 years [70,71]. Morrow et al (2004) reported differences of 2 to 3 points on language measures after controlling for important covariates, concluding that their lower standard scores put cocaine exposed children at increased need for clinical interventions [98]. In a trajectories analysis of language performance of children assessed at ages 1, 2, 4, and 6 years, Lewis et al (2007) reported that cocaine exposed children had lower language scores at each age. Average differences between groups in Receptive, Expressive, and Total Language z-scores after controlling for confounding variables were: 0.14, 0.15, and 0.18 [85] which corresponds to 2.1, 2.25, and 2.7 standard score points, respectively. Environmental variables including HOME scores, maternal cognitive functioning and cigarette exposure also were associated with poorer performance. Similarly, Beeghly et al (2006) found that their cohort of low-income, urban children with GCE had lower receptive language scores than unexposed children at age 6 years but not at age 9.5 years [17]. This age difference in GCE effects on language may be due to the contrast between the increasing influence of cumulative environmental factors and the increasingly distal influence of gestational and natal factors.
Because cocaine exposure is associated with alterations in dopamine-rich prefrontal neural systems, functional processes that have been localized to these areas have been examined in exposed children. Prenatal cocaine exposure has been linked to lower levels of inhibitory control [20], a function localized to the anterior cingulate area of the prefrontal cortex (PFC) [5,39,106,112]. Rose-Jacobs et al (2009) identified effects on cocaine-exposed children's ability to inhibit prepotent responses as assessed using the Stroop at ages 5 and 7 years, but in this same cohort scores on a more general measure of executive function showed no cocaine effects [110]. Noland et al (2005) reported effects of prenatal cocaine, tobacco, marijuana on selective attention indicated by higher errors of commission on a picture deletion task administered at age 4 years [101]. Reports from another longitudinal study showed that cocaine-exposed children scored lower than controls on a summary estimate of attention assessed using three measures across ages 3, 5 and 7 years [1,9] and had slightly elevated risk for ADHD according to results of a diagnostic interview [97]. Ackerman et al (2008) reported effects of prenatal cocaine and/or heroin exposure on sustained visual attention assessed using the Connors Continuous Performance test at age 7 years [3]. In the cohort included in the current report, cocaine-exposed subjects made more commission errors on the Distractibility subtest of the Gordon Diagnostic System but had better Delay Task Efficiency Ratios than controls at age 10 years [114]. No GCE effects were observed for each of the 6 remaining measures of attention and impulsivity assessed. These studies suggest that GCE is linked to poorer inhibitory control and sustained attention, yet differences reported often are for only one of many tests administered as found by Savage et al, 2005 [114]and/or effect sizes are relatively small as reported by Morrow et al, 2009[97].
Imaging studies have shown differences between cocaine-exposed and control subjects in neural characteristics of brain areas that are linked to inhibitory control or attention. Cocaine exposure was related to differences in adjusted volumes in the caudate nucleus [8], more diffuse patterns of activation while performing a task requiring inhibitory control [93], and differences in activation while completing a response inhibition task [116]. In most of these studies no performance differences were observed at the time of evaluation, suggesting that compensatory pathways were developed to ameliorate effects of cocaine exposure on these neural systems.
In studies of more general measures of functioning such as IQ and academic achievement findings are also mixed [22,68,98,118]. In 7 of the 8 studies meeting criteria for inclusion in a recent review article by Ackerman, no cocaine effects were observed but robust effects of environment were reported [4]. The eighth study showed cocaine exposure effects in the WISC-IV composite for Perceptual Reasoning however no effects were observed for the Verbal Comprehension, Working Memory or Processing Speed Composites or for scores on the Wechsler Individual Achievement Test [118].
It now is well established that GCE has not produced the profound deficits anticipated in the 1980 s and 1990 s, with children described variably as joyless, microcephalic, or unmanageable [15,24,31,52,53,75,92,135]. While welcome, the fact that GCE is not as devastating as predicted in no way negates the need for evaluation for more subtle deficits such as impairments in attention, memory, and language. As is clear in the literature, “subtle deficits” may also have far reaching effects [80,98]. For example, attention deficits that are accepted among family and close friends may be more problematic in the classroom or work place. In the classroom, children with attention deficits have difficulty learning with resultant negative effects on academic performance as they approach adolescence [13]. In the workplace, adults with attention deficits are more likely to be fired, change jobs more often, and earn lower work performance ratings [13]. Since children prenatally exposed to cocaine may exhibit multiple subtle deficits, investigations in maturing youth are merited. Until functional outcomes (i.e., successful transition to adulthood) associated with GCE are evaluated, the clinical significance of these subtle but statistically significant effects remains unknown.
Human brain development is far from complete by late childhood and early adolescence, the oldest age at which children with GCE so far have been studied. There are robust data, to include structural and functional neuroimaging, that show the prefrontal cortex, in particular, undergoes substantial maturational change through adulthood [21,55,107,111,115,123,132]. This is a significant factor in investigations of the effects of GCE given that prefrontal cortex is one of the brain regions most likely to be affected [42,62,84,90]. As such, conclusions regarding the effects of exposure on the human brain cannot be drawn with any finality pending the investigation for such effects on the mature brain. Thus, it is possible that GCE has latent effects on NC outcome that do not manifest until adolescence or young adulthood.
Motivated by the incomplete brain maturation of the oldest subjects so far tested, the possibility of latent or “sleeper” effects as suggested by animal and human research [6,40,54,78,79,81,96,128], as well as effects reported so far by others [2,7,9,17,20,63,76,93,101,109,110,114,117], this report includes assessment of our cohort as they progress through adolescence at ages 12, 14.5 and 17 years. We elected to take a cognitive neuroscience approach to measure NC function in our cohort. Localization of NC systems has been demonstrated in cognitive neuroscience studies that show associations between task performance and activation patterns in imaging or damaged areas in patients with brain lesions. The set of tasks used in this report: 1) evaluated both frontal and nonfrontal function[12]; 2) demonstrated sensitivity to age and IQ[65]; 3) discriminated between middle and low SES subjects[49,99]; and 4) revealed specific relationships between aspects of early childhood experience and the development of specific NC systems[48]. Given this we have confidence that these tasks are psychometrically sensitive for detection of potential differences that may emerge between GCE participants and Controls during later stages of maturation. Data regarding NC functioning of GCE adolescents may identify vulnerabilities in these individuals in the classroom or workplace as they transition into adulthood.
2.0 Methods
2.1. Participants
Our participants were 120, primarily African American youth, approximately half with GCE and half without (Controls). All were recruited at birth from a single inner-city hospital, were born at or near term (≤ 34 weeks), and had 5-minute Apgar scores of at least 5. The children were of low SES as defined by maternal receipt of public medical assistance. None of the children had Fetal Alcohol Syndrome or any syndrome known to be associated with developmental delay. As previously reported [66,67], mothers were native English speakers and had no past or present indication of major psychiatric illness as determined by medical chart review at time of enrollment. Maternal use of cocaine, as well as amphetamines, opiates, barbiturates, and benzodiazepines, was ascertained by interview, chart review at time of infant s birth, and by maternal and infant urine drug screens. While use of cigarettes, alcohol, and marijuana during pregnancy was also documented by interview and chart review, specific information on frequency and amount of use of these substances was not. Mothers estimated days of use of cocaine was also ascertained; only babies whose mothers reported cocaine use in at least two trimesters of pregnancy were included. Mothers who reported use of drugs other than tobacco, alcohol, marijuana, and cocaine were excluded from enrollment [66,67]. Since enrollment, the children have been evaluated semi-annually for measurements of growth, development, language, cognitive, and social-emotional outcomes [66,69,70,72,73]. Participant birth and childhood characteristics are shown in Table 1. The original cohort consisted of 224 participants (105 GCE participants and 119 Controls) enrolled at birth. During the ensuing years, five subjects died (1 GCE participant and 4 Controls), with an additional attrition of approximately 42% of GCE participants and 40% of Controls over the course of 12 years. Since this early attrition (largely related to inadequate number of study personnel secondary to fiscal constraints), cohort number has been stable for the past 8 years or more, with 120 participants (55% retention over 20 years) comprising the sample for the current report of NC function. GCE children lost to follow-up did not differ on any birth or maternal characteristics from those GCE children retained, including level of prenatal care (p=0.74), maternal drug screen positive for cocaine (p=0.96), birth weight (p=0.45), head circumference (p=0.82), exposure to alcohol, cigarettes or marijuana, admission to NICU, and discharge to biological mother (p> 0.20). Further, those GCE children lost to follow-up did not differ from those retained in number of days of maternal cocaine use during pregnancy (95 vs. 99, respectively). Participants retained had longer length of neonatal stay in the hospital than those lost to follow-up, 8.7 versus 5.6, respectively (p=0.031). In the Control group, more males (60%) were lost to follow up than females (40%) (p= 0.042). We expanded our examination of attrition by comparing scores on early cognitive outcomes for those lost to follow-up after age 12 months and those included in this report. One hundred and eighty two infants completed the 12 month assessment. Within this subset of participants, scores on the Bayley Scales of Infant Development for those lost to follow-up were similar to those retained at age 12 years (p=.99), with no interaction between cocaine exposure group and follow-up status.
Table 1.
Participant Characteristics by GCE Status
| GCE (n = 55) | Control (n = 65) | p-value | |
|---|---|---|---|
| At Time of Birth | |||
| Gender, Female (n (%))a | 31 (56%) | 37 (57%) | 1.00 |
| Gestational Age (GA), weeks (Mean ± SD) | 37.5 ± 2.1 | 39.1 ± 2.0 | <0.0005 |
| Birth Weight, kg (Mean ± SD) | 2.6 ± 0.5 | 3.1 ± 0.6 | <0.0005 |
| Birth Weight <10th % ile for GA | 4 (7%) | 2 (3%) | 0.41 |
| Head Circumference, cm (Mean ± SD) | 32.1 ± 1.7 | 33.5 ± 1.6 | <0.0005 |
| Head Circumference <10th % ile for GA | 9/54b (17%) | 3 (5%) | 0.036 |
| Race, African American | 52 (94%) | 64 (98%) | 0.33 |
| Other Gestational Exposures | |||
| Alcohol (n (%)) | 30 (54%) | 5 (8%) | <0.0005 |
| Marijuana (n (%)) | 25 (45%) | 2 (3%) | <0.0005 |
| Tobacco (n (%)) | 53 (96%) | 14 (21%) | <0.0005 |
| Gestational Cocaine Exposure, days (Median) | 99 (7, 273) | 0 (0, 0) | <0.0005 |
| Maternal Age, years (Mean ± SD) | 27.2 ± 4.3 | 22.4 ± 5.4 | <0.0005 |
| During Childhood | |||
| Age at Assessment 1, years (Mean ± SD)a | 12.3 ± 1.3 | 11.9 ± 1.2 | 0.074 |
| Age at Assessment 2, years (Mean ± SD) | 14.7 ± 0.9 | 14.5 ± 0.8 | 0.19 |
| Age at Assessment 3, years (Mean ± SD) | 17.5 ± 0.9 | 17.0 ± 0.9 | 0.01 |
| Wechsler Full Scale IQ at age 6 (Mean ± SD) | 82.3 ± 13.6 | 82.6 ± 12.3 | 0.91 |
| Any Foster Care Placement (n (%)) | 26 (47%) | 8 (12%) | <0.0005 |
| HOME Score at age 4 | 40.4 ± 7.6 | 44.2 ± 5.5 | 0.003 |
| HOME Score at age 8 | 47.6 ± 5.0 | 49.5 ± 3.4 | 0.028 |
| HOME Composite Scores | |||
| Parental Nurturance (mean of age 4 and 8)a | −0.09 ± 0.77 | 0.11 ± 0.46 | 0.11 |
| Environmental Stimulation (mean of age 4 and 8)a | −0.17 ± 0.78 | 0.16 ± 0.52 | 0.008 |
| Caregiver BDI-II Depression Scorea | 10.0 ± 8.9 | 8.8 ± 9.3 | 0.46 |
| Caregiver Current Cocaine Use (n (%)) | 30 (54%) | 5 (8%) | <0.0005 |
Candidate Covariates used in GEE models;
Missing data from one subject.
Informed consent was obtained from participant caregivers and assent was obtained from all participants. The project was approved by the Institutional Review Board of the Children's Hospital of Philadelphia.
2.2. NC Assessments
Participants were assessed using a set of pencil and paper and computerized tasks designed to tax frontally and nonfrontally mediated NC systems defined by anatomical and functional criteria. While we understand that the whole brain is working during performance of each task, our strategy was to select tasks that disproportionately tax particular systems as evidenced in the cognitive neuroscience literature cited below. Brief descriptions of NC systems assessed and tasks are listed below, with details available in other published reports [48,49]. The current report evaluates performance on a subset of 4 of the 7 systems presented previously [48,49]. The other three systems were either discontinued after assessment 2 or included tasks that were at ceiling in assessment 1 or 2, necessitating alternate tasks in subsequent assessments. The current report includes only tasks repeated across all three assessments. Systems evaluated include two frontal: Inhibitory Control, also referred to as Cognitive Control, and Working Memory, and two nonfrontal: Receptive Language and Incidental Memory. By examining performance of participants on a varied set of NC tasks we hope to better identify specific differences between GCE participants and Controls.
Executive Systems (Frontal)
The Inhibitory Control system was targeted to assess cognitive control processes and is closely linked to the anterior cingulate cortex in imaging and lesion studies. This system plays a crucial role in monitoring for conflict between the individual s automatic response and the correct response [25,30,95,106,113]. In addition, this system has been linked to the ability to summon additional attention needed to regulate responses. Task: Number Stroop [34,38] This computerized adaptation of the Counting Stroop requires participants to sort cards according to one of two sorting conditions. In the congruent condition, participants are timed as they sort the cards, as quickly as possible, according to the number (digit) shown on the card (e.g., the card with three “4 s” goes into the 4 pile). In the incongruent (conflict) condition, participants are timed as they do the same on the basis of the number of digits (e.g., three “4 s” goes into the 3 pile). The Stroop Effect is the reaction time difference in msec between the congruent and incongruent conditions. This task has the advantage over the classical Stroop Color and Word test in that it does not depend on skilled automatic reading (since poor readers will do paradoxically better on the classic Stroop). Functional neuroimaging studies have shown that for both Color and Counting Stroop the incongruent condition activates the ACC relative to the congruent condition [34].
Working Memory system, closely linked to the dorsolateral prefrontal cortex in imaging and lesion studies, allows an individual to hold the present context, rules, or goals in mind as a complex task is performed. Task: Spatial Working Memory [35]. This self-directed computerized task requires the subject search for hidden tokens one at a time within sets of four to eight randomly positioned boxes. Tokens are hidden only once in each box. Working memory skills are tapped as the subject, while searching, must hold in working memory the locations already checked and, as tokens are found, must remember and update the information about the locations of the found tokens [45]. In functional imaging studies, this specific task reliably activates dorsolateral prefrontal cortex [103–105].
Nonfrontal Systems
The Language system is shown in imaging and lesion studies to be localized to the left perisylvian region of the brain. Task: Peabody Picture Vocabulary Test (PPVT) [44]. The neuropsychological disorders which produce impairments in this task involve damage to left perisylvian system. The PPVT is a standardized receptive vocabulary test for children and adults between the ages of 2.5 through 90. On each trial, the subject hears a word and must select the corresponding picture from among four choices. This tests the lexical-semantic aspect of language. [58,94] Similar word-picture matching tasks used in functional neuroimaging studies also implicate the left perisylvian region [125,126].
The Memory system, shown in imaging research to map onto the medial temporal cortical area [89,124], is required for incidental or one-trial learning. Most standard memory tests are sensitive to both medial temporal and prefrontal function because performance is influenced by the subject s ability to organize the material to be learned and apply mnemonic strategies [127]. In order to obtain a relatively pure measure of learning ability, we used incidental learning tests, in which the subject does not know that a memory test is to be administered when the to-be-remembered stimuli are presented [87]. Tasks: Incidental Word Memory. Patients with medial temporal damage do poorly on incidental word learning. In this picture word memory task, the subject views pairs of pictures [119,120], presented one pair per page, and is instructed to point to the member of the pair that cost less than $20. Sometime later, during the test phase, the subject listens to a list of 80 words and decides which items were viewed earlier. For this task the total number of correct responses is recorded (total correct rejections + total correct hits) [89]. Incidental Face Memory. Medial temporal damage also impairs incidental learning of visual materials, including faces [88,89]. This task is analogous to the task with words, except that the learning set stimuli are photographs of faces, and the exposure to the initial set occurs as the participants judge whether the faces are likely to be a movie star or school teacher. During the test phase the child is presented with a series of 50 photographs and decides which faces were viewed earlier. For this task the total number of correct responses is recorded (total correct rejections + total correct hits). [23]
All tasks were carefully administered according to methods identified in the manuals and cognitive neuroscience literature to minimize errors due to methodological variance. All instructions were presented on the computer screen and the examiner read these aloud to the subject to ensure standardized administration across subjects. To eliminate administration and data processing errors we used computerized tasks for all but the standardized language measure. These careful data processing procedures were followed to ensure that results were truly representative of participant NC performance.
2.3. Other Measures
Other Exposures
At time of enrollment, mothers were asked about use of alcohol, cigarettes, and marijuana during pregnancy. Information was recorded as only “yes” or “no without detailed tabulation of amount and frequency of use.
Measure of Childhood Intelligence
Children's Full Scale Intelligence Quotient (FSIQ) was assessed at age 6 years (6.1± 0.1) using the Wechsler Preschool and Primary Scale of Intelligence Revised Edition (WPPSI-R) [130].
Measure of Childhood Experience
Children's home environments were evaluated at age 4 (4.1 ± 0.2) and 8 years (8.4 ± 0.5) using the HOME Inventory. [27] The HOME is a structured 1-hour, in-home, parental interview and observational checklist with 8 subscales measuring specific aspects of the child s home life. In-home assessments were completed by a trained member of the research team masked to child exposure status. Previous studies have shown that environmental characteristics of parental nurturance and environmental stimulation are related to cognitive outcomes [32,48,51,60]. To examine the influence of these different aspects of the home environment we grouped HOME items to form two composite scores, Environmental Stimulation and Parental Nurturance [32,43,48,77]. The Environmental Stimulation composite incorporated HOME subscales most analogous to experiential factors that vary with environmental enrichment in animal studies. The Parental Nurturance composite incorporates HOME subscales that measured the warmth and availability of parental care. Composite scores were calculated by averaging the Z scores of relevant subscales for each age. Further details are described in Farah al (2008) [48] which also shows these composites to be related to both general and specific aspects of cognitive function. In the current report, Environmental Stimulation and Parental Nurturance composites were computed by averaging the mean composites from the 4 and 8 year evaluations.
Foster Care Placement
At each study visit between birth and time of NC testing, the child s primary caregiver was recorded. We found 4 types of primary caregivers in our cohort: biological mother, biological father, kinship foster care, and non-kinship foster care. This information was then collapsed into the two categories, ever in foster care or never in foster care, used in analyses.
Measure of Maternal Depression
Children's primary caregivers were administered the Beck Depression Inventory-Second Edition (BDI-II) [16]at a study visit when the children were 11 years old. The BDI-II is an instrument designed to assess depressive symptoms in adults.
2.4. Testing Procedure
The NC assessments and all other evaluations included in this report were administered individually by a licensed clinical psychologist unaware of child exposure status. Each NC evaluation was completed in one visit to the research center and lasted an average of 2.5 hours, with 1 or 2 short break periods scheduled within the task administration. For the Stroop Task the difference between the congruent (text) and incongruent (number) conditions was computed and reversed to represent the Stroop Effect with higher scores indicating a better performance. For the Spatial Working Memory task we used the between search errors score which also was reversed so that higher scores indicated better performance. The aged referenced standard score for the PPVT was used in analysis of receptive language and the Total Correct scores for recall condition were used in the analysis of the two Incidental Memory tasks. All data for computerized tasks was processed using computer syntax files designed to transfer data from the testing software directly into the SPSS databases for statistical analysis. Preliminary analyses were performed to identify data irregularities, invalid subject response patterns, and outliers.
2.5 Power Analysis
We assumed that the intraclass correlation coefficient between the repeated measures of the outcome was 0.5. The effective sample size (based on independent observations) for this study with N=120 and three repeated measures is 180 [64]. Therefore, this study had statistical precision equivalent to a study in which 180 participants completed the study and in which no repeated measure is performed. A sample size of 180 participants will be sufficient to detect an effect size of 0.4 standard deviations with 80% power and type I error rate of 0.05. For example, using an estimated standard deviation for Stroop Effect score of 16.7 msec, the study is able to detect a Stroop score difference of 6.7 msec between the GCE and the control groups. Similarly, the differences that can be detected for Spatial Working Memory, PPVT, Incidental Word Memory, and Incidental Face Memory are 7.2 errors, 6.1 standard score points, 3.3 total correct responses and 1.7 total correct responses, respectively.
2.6 Statistical Analyses
To reduce the effect of outliers, data from each NC task were winsorized, that is, the two most extreme values at each end of the distribution of all children's scores were replaced with the third most extreme value at each end. We examined the effect of GCE on the outcomes and their change over time using the method of generalized estimating equations (GEE) [133,134]. Model I was an unadjusted model which included only cocaine exposure status, assessment number and their interaction. The three assessments were coded as −1, 0 and 1 respectively so that the coefficient of cocaine exposure status can be interpreted as its effort on the average of the outcome. In Model II, we then adjusted for potential confounding variables that are measured at baseline only including age at the first assessment and gender. Finally, in Model III, we included the variables that are measured after GCE including environmental stimulation, parental nurturance and caregiver depression. Because of the concerns that the effect of GCE on outcomes may be attenuated by adjusting for the intermediate variables between GCE and outcomes, variables (e.g., birth weight, gestational age) that are potentially on the biological pathways between GCE and outcome were not included in the multivariable adjustment [14]. All analyses were done in SPSS. A p-value less than 0.05 was considered statistically significant.
3.0 Results
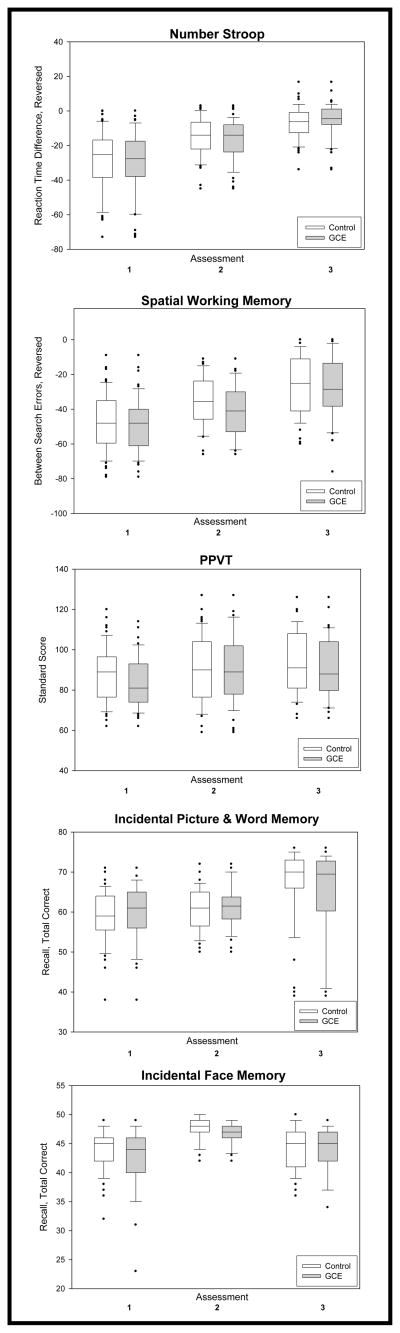
The figure shows box plots of task scores for each assessment for both GCE participants and Controls. These plots show increased scores for both groups across assessments for all tasks except the Incidental Face Memory. Of note, each of the PPVT median standard scores shown in the figure was below the average of the test standardization group of 100. We examined the relationship between tasks scores and 6-year FSIQ. All task scores from assessments 1 through 3 were related to 6-year FSIQ (p< 0.02), except for Incidental Word Memory at assessment 1 (p=0.15), Incidental Face Memory at assessment 2 (p=0.062) and Number Stroop at assessment 3 (p=0.73). (Data not shown.)
Figure 1.
Box plots of neurocognitive task scores for Control and GCE groups at Assessments 1, 2 and 3. Controls are open plots, GCE are shaded plots. The box represents the 25th and 75th percentiles of the distributions with the median marked in the middle. Whiskers are the 10th and 90th percentiles. Outliers are dots above and below the box plot.
Table 2 shows the results of Models I and II for each of the five NC tasks. Model I, containing only GCE, Assessment Number and an interaction term, found no effect of either GCE or GCE x Assessment (p≤0.11). Except for Incidental Face Memory (p=0.054) all tasks showed increases over assessments (p<0.001). Similar results are seen for Model II, which included Gender and Age at First Assessment.
Table 2.
Model I and Model II for Analyses of GCE Effects on Outcomes Using Generalized Estimating Equations
| Predictor | Number Stroop | Spatial Working Memory | PPVT | Incidental Word Memory | Incidental Face Memory | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Model I | ||||||||||
| Intercept | −16.82 (1.12)a | <0.001b | −36.28 (1.64) | <0.001 | 90.28 (1.81) | <0.001 | 62.49 (0.57) | <0.001 | 45.12 (0.29) | <0.001 |
| GCE | −0.19 (1.72) | 0.91 | −3.13 (2.52) | 0.21 | −2.11 (2.56) | 0.41 | −0.69 (1.05) | 0.51 | −0.80 (0.49) | 0.11 |
| Assessment No. | 10.81 (1.25) | <0.001 | 10.06 (1.23) | <0.001 | 2.37 (0.74) | <0.001 | 4.09 (0.80) | <0.001 | 0.18 (0.30) | 0.054 |
| GCE x Assessment No. | 1.52 (1.75) | 0.39 | 0.18 (1.78) | 0.92 | 1.74 (1.09) | 0.11 | −1.76 (1.12) | 0.12 | 0.70 (0.55) | 0.21 |
| Model II | ||||||||||
| Intercept | −33.25 (7.76) | <0.001 | −60.18 (11.26) | <0.001 | 101.89 (11.99) | <0.001 | 63.72 (4.18) | <0.001 | 41.82 (2.30) | <0.001 |
| GCE | −0.64 (1.71) | 0.71 | −3.83 (2.48) | 0.12 | −1.79 (2.54) | 0.48 | −0.63 (1.04) | 0.55 | −0.89 (0.49) | 0.070 |
| Assessment No. | 10.80 (1.26) | <0.001 | 10.08 (1.22) | <0.001 | 2.37 (0.75) | <0.001 | 4.08 (0.80) | <0.001 | 0.18 (0.30) | 0.051 |
| GCE x Assessment No. | 1.50 (1.75) | 0.39 | 0.32 (1.77) | 0.86 | 1.74 (1.09) | 0.11 | −1.77 (1.12) | 0.12 | 0.71 (0.55) | 0.20 |
| Age at first Assessment | 1.22 (0.65) | 0.062 | 2.11 (0.92) | 0.022 | −0.84 (1.00) | 0.40 | −0.15 (0.34) | 0.67 | 0.28 (0.19) | 0.13 |
| Gender (Female) | 3.52 (1.70) | 0.039 | −2.01 (2.33) | 0.41 | −2.88 (2.58) | 0.26 | 0.88 (1.02) | 0.38 | −0.12 (0.49) | 0.81 |
Coefficient (SE);
p-value
Table 3 shows the final GEE model for each task. On the two frontal tasks, Stroop and Spatial Working Memory, we find no GCE effect and no association with any other covariates except Assessment Number (both p<0.001) indicating that there is significant improvement in performance over ages 12–17 years. This association with age is consistent with the current developmental reports of continued prefrontal development through adolescence. [36,121,122]
Table 3.
Model III for Analyses of GCE Effects on Outcomes Using Generalized Estimating Equations
| Predictor | Number Stroop | Spatial Working Memory | PPVT | Incidental Word Memory | Incidental Face Memory | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intercept | −35.38 (10.07)a | 0.001b | −61.63 (14.42) | <0.001 | 97.70 (14.32) | <0.001 | 60.26 (5.82) | <0.001 | 40.88 (2.88) | <0.001 |
| GCE | 1.67 (1.85) | 0.37 | −3.60 (2.66) | 0.18 | −2.89 (2.58) | 0.26 | −0.24 (1.14) | 0.83 | −1.02 (0.53) | 0.055 |
| Assessment No. | 11.14 (1.43) | <0.001 | 10.16 (1.41) | <0.001 | 2.72 (0.80) | <0.001 | 4.20 (0.94) | <0.001 | 0.42 (0.30) | 0.025 |
| GCE x Assessment No. | 1.71 (1.98) | 0.39 | 0.32 (2.01) | 0.87 | 0.58 (1.16) | 0.51 | −2.69 (1.24) | 0.031 | 0.55 (0.61) | 0.37 |
| Age at 1st Assessment | 1.37 (0.83) | 0.098 | 2.02 (1.16) | 0.081 | −0.36 (1.17) | 0.76 | 0.14 (0.47) | 0.77 | 0.35 (0.24) | 0.14 |
| Gender (Female) | 3.11 (1.79) | 0.083 | −2.41 (2.48) | 0.33 | −4.93 (2.60) | 0.058 | 0.89 (1.10) | 0.42 | −0.42 (0.50) | 0.40 |
| Parental Nurturance | 2.10 (1.91) | 0.27 | 1.72 (2.71) | 0.53 | −0.31 (2.25) | 0.89 | 0.49 (1.12) | 0.66 | 0.77 (0.50) | 0.12 |
| Environmental Stimulation | −0.05 (1.70) | 0.98 | 0.65 (2.31) | 0.78 | 5.91 (2.86) | 0.039 | 1.24 (1.08) | 0.25 | 0.84 (0.54) | 0.12 |
| Caregover BDI-II Depression Score | 0.07 (0.10) | 0.50 | 0.14 (0.14) | 0.33 | 0.03 (0.13) | 0.84 | −0.04 (0.07) | 0.62 | 0.03 (0.02) | 0.16 |
Coefficient (SE);
p-value
Standard scores for the PPVT showed no relation to GCE, Age at First Assessment, Parental Nurturance and Maternal Depression (all p≤0.26). Males performed slightly better than males (p<0.058) and performance on the task improved over time (Assessment Number: p < 0.001). As reported in previous analyses [48,65], PPVT scores were related to the Environmental Stimulation composite from the HOME Inventory (p=0.039). (See Table 3)
For the Incidental Word Memory task no overall CGE effect was observed, however, there was a statistically significant effect of GCE by Assessment Number (p=0.031), showing that scores for the GCE group increased at a slower rate than for Controls. The Incidental Faces Memory task showed GCE effects of borderline statistical significance (p=0.055) and scores on this task increased significantly with Assessment Number (p=0.025). No other covariates were related to this outcome including HOME composites, which had been shown previously to be associated with incidental memory performance [65]. (See Table 3)
Table 4 summarizes the GCE effects from Models I, II, and III. In repeated measures analyses such as GEE, group differences are tested in two ways, the difference between overall group means, represented by model coefficients for group alone, and the difference between change over time, represented by the coefficients for the interaction between group and assessment number. In the table these are shown as mean difference and slope difference, respectively.
Table 4.
Summary of GCE Effects from Models I, II, and III
| Outcome | GCE vs. Control | Model I | Model II | Model III | |||
|---|---|---|---|---|---|---|---|
| Coeff | p-value | Coeff | p-value | Coeff | p-value | ||
| Number Stroop | Mean difference | −0.19 | 0.91 | −0.64 | 0.71 | 1.67 | 0.37 |
| Slope difference | 1.52 | 0.39 | 1.50 | 0.39 | 1.71 | 0.39 | |
| Spatial Working Memory | Mean difference | −3.13 | 0.21 | −3.83 | 0.12 | −3.60 | 0.18 |
| Slope difference | 0.18 | 0.92 | 0.32 | 0.86 | 0.32 | 0.87 | |
| PPVT | Mean difference | −2.11 | 0.41 | −1.79 | 0.48 | −2.89 | 0.26 |
| Slope difference | 1.74 | 0.11 | 1.74 | 0.11 | 0.58 | 0.51 | |
| Incidental Word Memory | Mean difference | −0.69 | 0.51 | −0.63 | 0.55 | −0.24 | 0.83 |
| Slope difference | −1.76 | 0.12 | −1.77 | 0.12 | −2.69 | 0.031 | |
| Incidental Face Memory | Mean difference | −0.80 | 0.11 | −0.89 | 0.070 | −1.02 | 0.055 |
| Slope difference | 0.70 | 0.21 | 0.71 | 0.20 | 0.55 | 0.37 | |
Model I: GCE, assessment No and GCE by assessment number interaction
Model II: model I plus age at first assessment and gender
Model III: model II plus parental nurturance, environmental stimulation and Caregiver depression
4.0 Discussion
In this report we found no difference between GCE and Control adolescents on specific NC outcomes: inhibitory control, working memory, or receptive language. GCE effects of borderline statistical significance were observed on the Incidental Face Memory task, and a statistically significant GCE by Assessment Number interaction effect was seen on the Incidental Word Memory task. Determination of the exact mechanism causing this effect is challenging as many variables are included in the multivariable model. The reason that the association between GEC and memory is stronger in Model III than in Models I and II, may not just be due to the addition of in the environmental variables since the coefficients of other variables change as well. Nevertheless, by adding the environmental variables to the model, the relationship between GCE and the outcome changes which indicates that these additional variables act a confounder. Further investigation of these relationships is needed to clarify this interesting finding.
Participant performance on inhibitory control, working memory, and receptive language tasks improved over time. The longitudinal study design used in the current report consisted of multiple evaluations of the same NC constructs at ages 12, 14.5 and 17 years to allow for careful tracking of the behavioral correlates of developing brain systems thought to be affected by GCE [83,90]. This targeted study design revealed little evidence of latent effects of GCE in this sample of low SES adolescents. This finding is comparable to previous reports of similar levels of cognitive functioning in GCE participants and Controls from this low SES inner-city cohort [65,66,69–71].
We found that receptive language scores on the PPVT and both PFC system tasks, Stroop and Spatial Working Memory, increased with age. Scores on the incidental memory tasks did not show as robust a relation to age. These relations between age and NC performance are consistent with cross-sectional and longitudinal imaging studies of late childhood and adolescence showing that non frontal brain regions mature first, followed by frontal regions associated with working memory and inhibitory control [36,121,122].
Effects of Age, Gender and Environmental Stimulation were seen on the PPVT standard score. Scores increased an average of about 2 points across assessments at ages 12, 14.5 and 17 years. This change in age-adjusted standard scores reflects a small increase in language skills between early and late adolescence in this disadvantaged cohort. This small but statistically significant increase may be due to the participants increased access to technology and information during high school years. Males in this cohort performed better (+ 4.93 points higher) than females on average across the three assessments. Studies examining gender differences in verbal abilities have shown better performance in males[74], even though females are commonly thought to have better verbal skills [33,129]. A larger effect (5.91 standard score points) was seen for HOME Environmental Stimulation composite score. Equal to about a half a standard deviation difference, this is a clinically meaningful effect adds to the evidence that early cognitively stimulating experiences in the home environment are critical for later learning [28,29,32,59,60].
We included the HOME composites in our analyses because we previously found that language showed a strong relationship to HOME Environmental Stimulation composite and Incidental Memory scores showed a strong relation to the HOME Parental Nurturance composite [48]. In the current report as noted above, we find a strong relationship between language and environmental stimulation similar to that found in Farah et al (2008) [48]. We did not, however, find a relationship between incidental memory tasks and parental nurturance. We considered why we did not find this effect in this longitudinal analysis. First, the current report examines performance across 3 time points up to age 17 years, unlike the previous report which examined performance at 1 time point only at age 12 years. Second, as children get older it is possible that the relation between childhood experience and later cognitive functioning is mitigated more by intervening variables associated with adolescence such as puberty and changes in social-emotional functioning and experience.
Of note, in Rao et al (2009) [108], we reported that hippocampal volume in adolescence was associated with parental nurturance experienced at age 4. This finding is consistent with animal studies reporting comparable relationships between early experience of maternal care and hippocampal measures. In the current report we chose to use the mean of the age 4 and 8 year Parental Nurturance score as used by Farah et al (2008) [48]. It may be that if we examined the relation between age 4 parental nurturance and incidental memory outcomes, we would have found a difference in the hippocampal memory function assessed in this report. In addition, the development of the hippocampus proceeds in an inverted u-shaped course with increasing volume early in adolescence until age 15 after which subsequent age-related changes are associated with decreased volume in these areas [57]. This developmental course is an example of the complex restructuring that occurs across adolescence. More in-depth analyses of these relations are needed to sort out the interesting patterns found in our cohort to date.
Despite the relatively robust findings in animal studies that GCE has a significant effect on cognitive outcomes, data from our cohort show no effects on three of five tasks assessed at three ages. GCE was substantial with median days of exposure equal to 99 days. Two methodological differences are apparent across the animal and human studies. First, variability in outcome associated with GCE in humans is undoubtedly attributable to the more complex context in which children develop, compared to experimental conditions in animal studies. Despite efforts to be comprehensive in their analyses of all factors associated with outcomes, researchers are unable to control for every risk factor that correlates with maternal cocaine use [18,19,40,41,86,90,91,96], whereas in animal studies these correlates are not present. Second, discrepancies between animal and human studies of GCE are likely related to the differences in how constructs are operationalized. Measures that are selected to evaluate outcome in children and to the timing of these assessments may be limited in comparability to those used in animal studies [6,37].
In our study we did control for a number of factors thought to be associated with outcomes including those listed in Table 1. The baseline model showed no cocaine effects, making covariate meditational analyses unjustified. Even when important covariates were added to the model we were not able to detect statistically significant effects. Tasks used in our study tapped components of important NC domains and have been used in other studies to examine NC function [46,49,50,65,100]. As noted above, each NC system was indexed using one task that was repeated across three ages (12, 14.5 and 17 years). These tasks typically take about 5 to 10 minutes to complete, and are embedded within a larger battery of tasks. The use of computerized tasks (Number Stroop, Spatial Working Memory, and Incidental Memory tasks) allows for more precise stimulus presentation and more accurate recording of subject responses. This methodology reduces the variance in scores that might be attributed to subtle differences in test administration or recording of subject responses by the examiner. Use of the PPVT allowed for standardized assessment of receptive language. This approach allowed for comparison of performance on the same exact task across three ages. While the tasks used were 1) based firmly in the cognitive neuroscience literature, 2) showed expected relations with age, and 3) were related to 6 year IQ scores, they tapped a restricted range of functioning within each NC system. For example, while receptive language is assessed, expressive language was not. With an expanded set of tasks, a more thorough examination of functioning for each NC system would be possible. In a previous report that included a more comprehensive set of tasks but at only one time point, no GCE effects were observed [65].
More extensive evaluation of these NC domains must be completed as these tasks only tap a single component of these very complex NC constructs. Expansion of our longitudinal analysis is needed to include all tasks administered to participants despite changes in NC tasks across assessments. For example, Bandstra et al (2002) [10] in a longitudinal study of the effects of GCE on language functioning used two different language tests but using language performance z-scores at each age, was able to track changes in the language system between ages 3, 5 and 7 years. Applying a similar methodology to the performance data from our cohort may provide a more reliable and comprehensive index of these complex NC systems over time and expansion of our analysis of the GCE effects on the four systems. Understanding of the effects of GCE on performance on an expanded set of tasks and with repeated assessments may have yielded different findings.
There is accumulating evidence for the existence of subtle effects of GCE and their impact on the individual and society may be significant [80]. Small differences in cognitive outcomes may be linked to high risk problem behaviors and poor academic outcomes [81]. Some of the studies that report differences in language and attention have larger sample size and therefore are able to detect small but statistically significant cocaine effects. With a larger number of participants it is likely that the statistical significance level may have increased for some of our comparisons, highlighting the areas of functioning that are negatively affected by gestational cocaine exposure. The existence of small clusters of subtle effects may place children at increased risk for poorer outcome [82,98]. In this regard, we recently examined participant functioning using the following broad functional outcomes: teenage pregnancy, school failure, drug use, and adjudication [61]. We found no GCE effect on rates of these outcomes in our cohort. Instead, these outcomes were related to more proximal factors such as exposure to violence and characteristics of the home environment. Continued monitoring of long-term outcome is needed to determine if GCE leads to compromised child outcomes through adolescence and into adulthood.
While we were able to longitudinally evaluate NC functioning through adolescence this report has several limitations. First, sample size impacts our statistical power. It may be with a larger sample size we would have been able to detect effects GCE smaller than 0.04 standard deviation units for each outcome. Also, if statistically significant effects were detected in our cohort, tests of mediation would have been possible. In mediation models, first the relation between the independent and dependent variable must be statistically significant and this was not the case for our cohort [14]. Furthermore, as listed in Table 1, gestational exposures to cigarettes, alcohol, and marijuana were collinear with GCE. For example, 53 of the 55 GCE children were also exposed to prenatal tobacco leaving only 2 subjects in the GCE group who were not exposed to cigarettes. With this limited amount of variance in prenatal exposure to tobacco in the GCE group, controlling for this variable is not possible. Similarly, variance in foster care placement and participant race was limited. With more participants we may have been able to separate effects of these other important variables. Second, our subjects were all low SES which limits the generalizability of our findings to this demographic group. Using data from early evaluations of the home environment as well as caregiver variables assessed across childhood, we show that these factors are more salient to cognitive development than prenatal exposures. These results also add to the existing literature showing the importance of early childhood environmental factors on developmental outcomes [26,28,29,32,59]. With more variability in SES we may have been able to identify effects of GCE in children who have access to more enriched environments associated with families that have access to more financial and emotional resources [60].
It may be possible that some women who used cocaine were misclassified as controls at study entry. Our study was designed to minimize this possibility, as we required negative history and negative urine screens on both mother and baby for inclusion. Further, maternal profiles of the two groups were quite different. Nevertheless, we cannot entirely exclude the possibility that some women were misclassified. We also considered that we might have enrolled women who were not heavy cocaine users. We doubt this as 88% of our cocaine-using mothers had urines positive for cocaine metabolites at delivery (data not shown) [67], a marker suggested by Zuckerman et al [136] to identify frequent users. Further, we enrolled only women who used in two or more trimesters of pregnancy, with 99% of our mother using in all three trimesters; the top quartile of self-reported days of use in pregnancy in our cohort was 195 days or more.
We examined the possibility results are affected by attrition in our cohort. It is not likely that we would have found a difference in those we retained because levels of GCE were similar to those lost to follow up. While there were more males lost to follow up and because some gender effects are reported by others [102], it may be that with lower rates of attrition of males, we could have observed an interaction between cocaine exposure and gender. As noted above, we had sufficient power to detect differences with effect sizes of at least 0.40. In this regard, with a lower overall attrition rate, our study would have had increased power to detect smaller differences between groups which in turn would allow for determination of the clinical significance of the smaller GCE effects over time.
In summary, this carefully conducted study spanning adolescence shows no effects of GCE on inhibitory control, working memory or receptive language. While cocaine exposure was not related to Incidental Memory in baseline models, addition of environmental covariates to these models revealed that on the Incidental Word Memory task scores for the GCE group increased at a slower rate than for controls and on the Incidental Face Memory task control participants scored higher than GCE participants. Our results provide additional evidence that environmental variables such as those assessed in the HOME Inventory influence cognitive outcomes. As shown here and elsewhere the cognitive performance of our cohort is below average, suggesting that overall effects of poverty are placing GCE and Control children at a clinically significant disadvantage compared to other children their age. An important priority for those who care for GCE children and all children born into high risk environments is to address the problems that contribute to their disadvantage, including not only maternal drug use but also limited access to resources needed to provide cognitively and emotionally stimulating experiences early in life.
Table 5.
NC task scores for Control and GCE groups at Assessments 1, 2 and 3
| GCE (n = 55) | Control (n = 65) | |
|---|---|---|
| Number Stroop (Reaction Time Difference, reversed) | ||
| Assessment 1 | −29.7 ± 18.0a | −28.3 ± 17.7 |
| Assessment 2 | −16.2 ± 11.9 | −15.3 ± 11.8 |
| Assessment 3 | −5.1 ± 9.7 | −6.8 ± 10.1 |
| Spatial Working Memory (Between Search Errors, reversed) | ||
| Assessment 1 | −48.8 ± 15.4 | −46.7 ± 17.3 |
| Assessment 2 | −41.5 ± 15.0 | −35.5 ± 14.1 |
| Assessment 3 | −28.2 ± 19.6 | −26.6 ± 16.8 |
| PPVT (Standard Score) | ||
| Assessment 1 | 83.7 ± 12.4 | 87.8 ± 13.8 |
| Assessment 2 | 89.5 ± 16.9 | 90.7 ± 16.7 |
| Assessment 3 | 91.5 ± 15.2 | 92.8 ± 15.1 |
| Incidental Word Memory (Recognition Total Correct) | ||
| Assessment 1 | 59.6 ± 7.6 | 59.1 ± 6.6 |
| Assessment 2 | 61.4 ± 5.4 | 60.9 ± 5.8 |
| Assessment 3 | 64.3 ± 11.3 | 67.4 ± 9.1 |
| Incidental Face Memory (Recognition Total Correct) | ||
| Assessment 1 | 42.2 ± 6.1 | 44.0 ± 3.5 |
| Assessment 2 | 47.5 ± 2.2 | 47.4 ± 2.0 |
| Assessment 3 | 43.8 ± 4.1 | 44.4 ± 3.7 |
Acknowledgments
The project described was supported by NIDA RO1DA014129 and Grant Number UL1RR024134 from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Tribute to Vince Smeriglio PhD
The authors thank Vince Smeriglio for his steadfast and tireless support, boundless enthusiasm, superb guidance, and enduring friendship, without which this work could not have been conducted. Moreover, Vince, through his support of this and numerous other investigations, has contributed more to the understanding of substance use than any single group. While his name may not be on manuscripts, he is the foundation on which they are built. On behalf of mothers, babies, and our research group, thank you, Vince.
Abbreviations
- BDI-II
Beck Depression Inventory - II
- FSIQ
Full Scale Intelligence Quotient
- GCE
Gestational Cocaine Exposure
- GEE
Generalized Estimating Equations
- HOME
Home Observation for Measurement of the Environment
- NC
Neurocognitive
- PPVT
Peabody Picture Vocabulary Test
- PFC
Prefrontal Cortex
- SES
Socioeconomic Status
- WPPSI-R
Wechsler Preschool and Primary Scale of Intelligence – Revised Edition
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Accornero VH, Amado AJ, Morrow CE, Xue L, Anthony JC, Bandstra ES. Impact of prenatal cocaine exposure on attention and response inhibition as assessed by continuous performance tests. J Dev Behav Pediatr. 2007;28:195–205. doi: 10.1097/01.DBP.0000268560.72580.f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Accornero VH, Morrow CE, Bandstra ES, Johnson AL, Anthony JC. Behavioral outcome of preschoolers exposed prenatally to cocaine: role of maternal behavioral health. J Pediatr Psychol. 2002;27:259–69. doi: 10.1093/jpepsy/27.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ackerman JP, Llorente AM, Black MM, Ackerman CS, Mayes LA, Nair P. The effect of prenatal drug exposure and caregiving context on children's performance on a task of sustained visual attention. J Dev Behav Pediatr. 2008;29:467–74. doi: 10.1097/DBP.0b013e3181903168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ackerman JP, Riggins T, Black MM. A review of the effects of prenatal cocaine exposure among school-aged children. Pediatrics. 2010;125:554–65. doi: 10.1542/peds.2009-0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adleman NE, Menon V, Blasey CM, White CD, Warsofsky IS, Glover GH, Reiss AL. A developmental fMRI study of the Stroop color-word task. NeuroImage. 2002;16:61–75. doi: 10.1006/nimg.2001.1046. [DOI] [PubMed] [Google Scholar]
- 6.Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- 7.Arendt RE, Short EJ, Singer LT, Minnes S, Hewitt J, Flynn S, Carlson L, Min MO, Klein N, Flannery D. Children prenatally exposed to cocaine: developmental outcomes and environmental risks at seven years of age. J Dev Behav Pediatr. 2004;25:83–90. doi: 10.1097/00004703-200404000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Avants BB, Hurt H, Giannetta JM, Epstein CL, Shera DM, Rao H, Wang J, Gee JC. Effects of heavy in utero cocaine exposure on adolescent caudate morphology. Pediatr Neurol. 2007;37:275–9. doi: 10.1016/j.pediatrneurol.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 9.Bandstra ES, Morrow CE, Anthony JC, Accornero VH, Fried PA. Longitudinal investigation of task persistence and sustained attention in children with prenatal cocaine exposure. Neurotoxicol Teratol. 2001;23:545–59. doi: 10.1016/s0892-0362(01)00181-7. [DOI] [PubMed] [Google Scholar]
- 10.Bandstra ES, Morrow CE, Vogel AL, Fifer RC, Ofir AY, Dausa AT, Xue L, Anthony JC. Longitudinal influence of prenatal cocaine exposure on child language functioning. Neurotoxicol Teratol. 2002;24:297–308. doi: 10.1016/s0892-0362(02)00192-7. [DOI] [PubMed] [Google Scholar]
- 11.Bandstra ES, Vogel AL, Morrow CE, Xue L, Anthony JC. Severity of prenatal cocaine exposure and child language functioning through age seven years: a longitudinal latent growth curve analysis. Subst Use Misuse. 2004;39:25–59. doi: 10.1081/JA-120027765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banich MT. Cognitive Neuroscience and Neuropsychology. Houghton Mifflin Company; Boston, MA: 2004. Editoin Edition. [Google Scholar]
- 13.Barkley RA. ADHD in Children and Adolescents. Lancaster, PA: 2002. ADHD in children and adolescents: Theory, diagnosis, and management. [Google Scholar]
- 14.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–82. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 15.Barone D. Myths About "Crack Babies". Educational Leadership. 1994;52 [Google Scholar]
- 16.Beck AT, Steer RA, Brown GK. Beck Depression Inventory II Manual. The Psychological Corporation; San Antonio, TX: 1996. Editoin Edition. [Google Scholar]
- 17.Beeghly M, Martin B, Rose-Jacobs R, Cabral H, Heeren T, Augustyn M, Bellinger D, Frank DA. Prenatal cocaine exposure and children's language functioning at 6 and 9.5 years: moderating effects of child age, birth weight, and gender. J Pediatr Psychol. 2006;31:98–115. doi: 10.1093/jpepsy/jsj028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Behnke M, Eyler FD, Warner TD, Garvan CW, Hou W, Wobie K. Outcome from a prospective, longitudinal study of prenatal cocaine use: preschool development at 3 years of age. J Pediatr Psychol. 2006;31:41–9. doi: 10.1093/jpepsy/jsj027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bendersky M, Bennett D, Lewis M. Aggression at age 5 as a function of prenatal exposure to cocaine, gender, and environmental risk. J Pediatr Psychol. 2006;31:71–84. doi: 10.1093/jpepsy/jsj025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bendersky M, Gambini G, Lastella A, Bennett DS, Lewis M. Inhibitory motor control at five years as a function of prenatal cocaine exposure. J Dev Behav Pediatr. 2003;24:345–51. doi: 10.1097/00004703-200310000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bennett CM, Baird AA. Anatomical changes in the emerging adult brain: A voxel-based morphometry study. Hum Brain Mapp. 2005 doi: 10.1002/hbm.20218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bennett DS, Bendersky M, Lewis M. Children's cognitive ability from 4 to 9 years old as a function of prenatal cocaine exposure, environmental risk, and maternal verbal intelligence. Dev Psychol. 2008;44:919–28. doi: 10.1037/0012-1649.44.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bernstein LJ, Beig S, Siegenthaler AL, Grady CL. The effect of encoding strategy on the neural correlates of memory for faces. Neuropsychologia. 2002;40:86–98. doi: 10.1016/s0028-3932(01)00070-7. [DOI] [PubMed] [Google Scholar]
- 24.Blakeslee S. Crack's toll among babies: A joyless view, even of toys. New York Times. 1989;17:26. [Google Scholar]
- 25.Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108:624–52. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- 26.Bradley RH. Children's home environments, health, behavior, and intervention efforts: a review using the HOME Inventory as a marker measure. Genetic, Social, and General Psychology Monographs. 1993;119:437–490. [PubMed] [Google Scholar]
- 27.Bradley RH. The HOME Inventory: Review and reflections. In: Reese HW, editor. Advances in Child Development and Behavior. Academic Press, Inc; San Diego: 1994. pp. 241–288. [DOI] [PubMed] [Google Scholar]
- 28.Bradley RH, Caldwell BM. The relation of infants' home environments to acheivement test performance in first grade: a follow-up study. Child Dev. 1984;55:803–809. doi: 10.1111/j.1467-8624.1984.tb03817.x. [DOI] [PubMed] [Google Scholar]
- 29.Bradley RH, Convyn RF, Burchinal M, McAdoo HP, Coll CG. The home environments of children in the United States part II: relations with behavioral development through age thirteen. Child Dev. 2001;72:1868–86. doi: 10.1111/1467-8624.t01-1-00383. [DOI] [PubMed] [Google Scholar]
- 30.Braver TS, Barch DM, Gray JR, Molfese DL, Snyder A. Anterior cingulate cortex and response conflict: Effects of frequency, inhibition and errors. Cerebral Cortex. 2001;11:825–836. doi: 10.1093/cercor/11.9.825. [DOI] [PubMed] [Google Scholar]
- 31.Brodkin A. Are Crack Babies Doomed to School Failure? Instructor. 1992;101:16–17. [Google Scholar]
- 32.Brooks-Gunn J, Klebanov PK, Duncan GJ. Ethnic differences in children's intelligence test scores: role of economic deprivation, home environment, and maternal characteristics. Child Dev. 1996;67:396–408. [PubMed] [Google Scholar]
- 33.Burman DD, Bitan T, Booth JR. Sex differences in neural processing of language among children. Neuropsychologia. 2008;46:1349–62. doi: 10.1016/j.neuropsychologia.2007.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bush G, Whalen PJ, Rosen BR, Jenike MA, McInerney SC, Rauch SL. The Counting Stroop: an interference task specialized for functional neuroimaging-validation study with functional MRI. Human Brain Mapping. 1998;6:270–82. doi: 10.1002/(SICI)1097-0193(1998)6:4<270::AID-HBM6>3.0.CO;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.L. Cambridge Cognition. Cambridge Neuropsychological Test Automated Battery (CANTAB) Cambridge, UK: 1999. [Google Scholar]
- 36.Casey BJ, Getz S, Galvan A. The adolescent brain. Developmental Review. 2008;28:62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Casey BJ, Tottenham N, Liston C, Durston S. Imaging the developing brain: what have we learned about cognitive development? Trends in Cognitive Sciences. 2005;9:104–10. doi: 10.1016/j.tics.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 38.Casey BJ, Trainor JL, Schubert AB, Nystrom LE, Giedd JN, Castellanos FX, Haxby JV, Noll DC, Cohen JD, Forman SD, et al. A developmental functional MRI study of prefrontal activation during performance of a go-no-go task. Journal of Cognitive Neuroscience. 1997;9:835–847. doi: 10.1162/jocn.1997.9.6.835. [DOI] [PubMed] [Google Scholar]
- 39.Casey BJ, Trainor R, Giedd J, Vauss Y, Vaituzis CK, Hamburger S, Kozuch P, Rapoport JL. The role of the anterior cingulate in automatic and controlled processes: a developmental neuroanatomical study. Dev Psychobiol. 1997;30:61–9. [PubMed] [Google Scholar]
- 40.Coles CD, Black MM. Introduction to the special issue: Impact of prenatal substance exposure on children's health, development, school performance, and risk behavior. Journal of Pediatric Psychology. 2006;31:1–4. doi: 10.1093/jpepsy/jsj036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cone-Wesson B. Prenatal alcohol and cocaine exposure: influences on cognition, speech, language, and hearing. J Commun Disord. 2005;38:279–302. doi: 10.1016/j.jcomdis.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 42.Dow-Edwards D, Mayes L, Spear L, Hurd Y. Cocaine and development: clinical, behavioral, and neurobiological perspectives - A symposium report. Neurotoxicol Teratol. 1999;21:481–90. doi: 10.1016/s0892-0362(99)00008-2. [DOI] [PubMed] [Google Scholar]
- 43.Duncan GJ, Brooks-Gunn J, Klebanov PK. Economic deprivation and early childhood development. Child Dev. 1994;65:296–318. [PubMed] [Google Scholar]
- 44.Dunn LM, Dunn LM. Examiner's Manuel for the Peabody Picture Vocabulary Test-III. American Guidance Service, Inc; Circle Pines, MN: 1997. Editoin Edition. [Google Scholar]
- 45.Elliott R, Sahakian BJ, Matthews K, Bannerjea A, Rimmer J, Robbins TW. Effects of methylphenidate on spatial working memory and planning in healthy young adults. Psychopharmacology. 1997;131:196–206. doi: 10.1007/s002130050284. [DOI] [PubMed] [Google Scholar]
- 46.Evans GW, Schamberg MA. Childhood poverty, chronic stress, and adult working memory. Proc Natl Acad Sci U S A. 2009;106:6545–9. doi: 10.1073/pnas.0811910106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eyler FD, Warner TD, Behnke M, Hou W, Wobie K, Garvan CW. Executive functioning at ages 5 and 7 years in children with prenatal cocaine exposure. Dev Neurosci. 2009;31:121–36. doi: 10.1159/000207500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Farah M, Betancourt L, Shera D, Savage J, Giannetta J, Brodsky N, Malmud EK, Hurt H. Environmental Stimulation, Parental Nurturance and Cognitive Development in Humans. Dev Sci. 2008;11:793–801. doi: 10.1111/j.1467-7687.2008.00688.x. [DOI] [PubMed] [Google Scholar]
- 49.Farah MJ, Shera DM, Savage JH, Betancourt L, Giannetta JM, Brodsky NL, Malmud EK, Hurt H. Childhood poverty: specific associations with neurocognitive development. Brain Res. 2006;1110:166–74. doi: 10.1016/j.brainres.2006.06.072. [DOI] [PubMed] [Google Scholar]
- 50.Ford S, Farah MS, Shera DM, Hurt H. Neurocognitive correlates of problem behavior in environmentally at-risk adolescents. J Dev Behav Pediatr. 2007;28:376–85. doi: 10.1097/DBP.0b013e31811430db. [DOI] [PubMed] [Google Scholar]
- 51.Francis D, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999;286:1155–8. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- 52.Frank DA. Letter to the Editor. New York Times; Nov 28, 2003. "Crack Baby" Syndrome? p. A42. [Google Scholar]
- 53.Frank DA, Augustyn M, Knight WG, Pell T, Zuckerman B. Growth, development, and behavior in early childhood following prenatal cocaine exposure: a systematic review. JAMA. 2001;285:1613–25. doi: 10.1001/jama.285.12.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fried PA, Smith AM. A literature review of the consequences of prenatal marihuana exposure. An emerging theme of a deficiency in aspects of executive function. Neurotoxicol Teratol. 2001;23:1–11. doi: 10.1016/s0892-0362(00)00119-7. [DOI] [PubMed] [Google Scholar]
- 55.Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–3. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 56.Glatt SJ, Bolanos CA, Trksak GH, Jackson D. Effects of prenatal cocaine exposure on dopamine system development: a meta-analysis. Neurotoxicol Teratol. 2000;22:617–29. doi: 10.1016/s0892-0362(00)00088-x. [DOI] [PubMed] [Google Scholar]
- 57.Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd, Herman DH, Clasen LS, Toga AW, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–9. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goodglass H, Kaplan E. The Assessment of Aphasia and Related Disorders. Lea and Febiger; Philadelphia: 1982. Editoin Edition. [Google Scholar]
- 59.Gottfried AE, Fleming JS, Gottfried AW. Role of cognitively stimulating home environment in children's academic intrinsic motivation: a longitudinal study. Child Dev. 1998;69:1448–60. [PubMed] [Google Scholar]
- 60.Guo G, Harris KM. The mechanisms mediating the effects of poverty on children's intellectual development. Demography. 2000;37:431–47. doi: 10.1353/dem.2000.0005. [DOI] [PubMed] [Google Scholar]
- 61.Handzel J, Brodsky N, Betancourt L, Hurt H. What happens to inner-city youth between ages 8–19: Perceptions and intentions vs. reality. Platform Presentation. 2009 Eastern Society for Pediatric Research; Philadelphia, Pennsylvania. 2009. p. 751788. [Google Scholar]
- 62.Harvey JA. Cocaine effects on the developing brain: current status. Neurosci Biobehav Rev. 2004;27:751–64. doi: 10.1016/j.neubiorev.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 63.Heffelfinger AK, Craft S, White DA, Shyken J. Visual attention in preschool children prenatally exposed to cocaine: implications for behavioral regulation. J Int Neuropsychol Soc. 2002;8:12–21. [PubMed] [Google Scholar]
- 64.Hennessy S, Leonard CE, Yang W, Kimmel SE, Townsend RR, Wasserstein AG, Ten Have TR, Bilker WB. Effectiveness of a two-part educational intervention to improve hypertension control: a cluster-randomized trial. Pharmacotherapy. 2006;26:1342–7. doi: 10.1592/phco.26.9.1342. [DOI] [PubMed] [Google Scholar]
- 65.Hurt H, Betancourt LM, Malmud EK, Shera DM, Giannetta JM, Brodsky NL, Farah MJ. Children with and without gestational cocaine exposure: A neurocognitive systems analysis. Neurotoxicol Teratol. 2009 doi: 10.1016/j.ntt.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hurt H, Brodsky NL, Betancourt L, Braitman LE, Malmud E, Giannetta J. Cocaine-exposed children: follow-up through 30 months. J Dev Behav Pediatr. 1995;16:29–35. [PubMed] [Google Scholar]
- 67.Hurt H, Brodsky NL, Braitman LE, Giannetta J. Natal status of infants of cocaine users and control subjects: a prospective comparison. J Perinatol. 1995;15:297–304. [PubMed] [Google Scholar]
- 68.Hurt H, Brodsky NL, Roth H, Malmud E, Giannetta JM. School performance of children with gestational cocaine exposure. Neurotoxicol Teratol. 2005;27:203–11. doi: 10.1016/j.ntt.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 69.Hurt H, Malmud E, Betancourt L, Braitman LE, Brodsky NL, Giannetta J. Children with in utero cocaine exposure do not differ from control subjects on intelligence testing. Arch Pediatr Adolesc Med. 1997;151:1237–41. doi: 10.1001/archpedi.1997.02170490063011. [DOI] [PubMed] [Google Scholar]
- 70.Hurt H, Malmud E, Betancourt L, Brodsky NL, Giannetta J. A prospective evaluation of early language development in children with in utero cocaine exposure and in control subjects. J Pediatr. 1997;130:310–2. doi: 10.1016/s0022-3476(97)70361-5. [DOI] [PubMed] [Google Scholar]
- 71.Hurt H, Malmud E, Betancourt L, Brodsky NL, Giannetta JM. A prospective comparison of developmental outcome of children with in utero cocaine exposure and controls using the Battelle Developmental Inventory. J Dev Behav Pediatr. 2001;22:27–34. doi: 10.1097/00004703-200102000-00005. [DOI] [PubMed] [Google Scholar]
- 72.Hurt H, Malmud EK, Betancourt L, Braitman LE, Brodsky N, Giannetta J. Inner-city children perform poorly on intelligence testing regardless of in utero cocaine exposure. Archives of Pediatric and Adolescent Medicine. 1997;151 doi: 10.1001/archpedi.1997.02170490063011. [DOI] [PubMed] [Google Scholar]
- 73.Hurt H, Malmud EK, Betancourt L, Brodsky N, Giannetta J. A prospective comparison of children with in utero cocaine exposure (COC) and controls (CON) on the Battelle Developmental Inventory. Pediatr Res. 1997;41 doi: 10.1097/00004703-200102000-00005. [DOI] [PubMed] [Google Scholar]
- 74.Hyde JS, Linn MC. Gender differences in verbal ability: a meta analysis. Psychol Bull. 1988;104:53–69. [Google Scholar]
- 75.Jabez A. Crack babies. Nurs Times. 1990;86:18–9. [PubMed] [Google Scholar]
- 76.Jacobson SW, Jacobson JL, Sokol RJ, Martier SS, Chiodo LM. New evidence for neurobehavioral effects of in utero cocaine exposure. J Pediatr. 1996;129:581–90. doi: 10.1016/s0022-3476(96)70124-5. [DOI] [PubMed] [Google Scholar]
- 77.Klebanov PK, Brooks-Gunn J, McCarton C, McCormick MC. The contribution of neighborhood and family income to developmental test scores over the first three years of life. Child Dev. 1998;69(5):1420–1436. [PubMed] [Google Scholar]
- 78.Kosofsky BE. Cocaine induced alterations in neuro-development. Seminars in Speech and Language. 1998;19:109–121. doi: 10.1055/s-2008-1064040. [DOI] [PubMed] [Google Scholar]
- 79.Lassen K, Oei TP. Effects of maternal cigarette smoking during pregnancy on long-term physical and cognitive parameters of child development. Addict Behav. 1998;23:635–53. doi: 10.1016/s0306-4603(98)00022-7. [DOI] [PubMed] [Google Scholar]
- 80.Lester BM, LaGasse LL, Seifer R. Cocaine exposure and children: the meaning of subtle effects [see comments] Science. 1998;282:633–4. doi: 10.1126/science.282.5389.633. [DOI] [PubMed] [Google Scholar]
- 81.Lester BM, Padbury JF. Third pathophysiology of prenatal cocaine exposure. Dev Neurosci. 2009;31:23–35. doi: 10.1159/000207491. [DOI] [PubMed] [Google Scholar]
- 82.Levine TP, Liu J, Das A, Lester B, Lagasse L, Shankaran S, Bada HS, Bauer CR, Higgins R. Effects of prenatal cocaine exposure on special education in school-aged children. Pediatrics. 2008;122:e83–91. doi: 10.1542/peds.2007-2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Levitt P. Prenatal effects of drugs of abuse on brain development. Drug Alcohol Dependence. 1998;51:109–25. doi: 10.1016/s0376-8716(98)00070-2. [DOI] [PubMed] [Google Scholar]
- 84.Levitt P, Harvey JA, Friedman E, Simansky K, Murphy EH. New evidence for neurotransmitter influences on brain development. Trends in Neuroscience. 1997;20:269–274. doi: 10.1016/s0166-2236(96)01028-4. [DOI] [PubMed] [Google Scholar]
- 85.Lewis BA, Kirchner HL, Short EJ, Minnes S, Weishampel P, Satayathum S, Singer LT. Prenatal cocaine and tobacco effects on children's language trajectories. Pediatrics. 2007;120:e78–85. doi: 10.1542/peds.2006-2563. [DOI] [PubMed] [Google Scholar]
- 86.Linares TJ, Singer LT, Kirchner HL, Short EJ, Min MO, Hussey P, Minnes S. Mental health outcomes of cocaine-exposed children at 6 years of age. J Pediatr Psychol. 2006;31:85–97. doi: 10.1093/jpepsy/jsj020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mandzia JL, McAndrews MP, Grady CL, Graham SJ, Black SE. Neural correlates of incidental memory in mild cognitive impairment: an fMRI study. Neurobiol Aging. 2009;30:717–30. doi: 10.1016/j.neurobiolaging.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 88.Mayes AR, Meudell PR, Neary D. Do amnesiacs adopt inefficient encoding strategies with faces and random shapes? Neuropsychologia. 1980;18:527–541. doi: 10.1016/0028-3932(80)90154-2. [DOI] [PubMed] [Google Scholar]
- 89.Mayes AR, Meudell PR, Neary D. Must amnesia be caused by either encoding or retrieval disorders? In: Grueneberg MM, Morris E, Sykes RN, editors. Practical Aspects of Memory. Academic Press; London: 1978. [Google Scholar]
- 90.Mayes LC. Developing brain and in utero cocaine exposure: effects on neural ontogeny. Dev Psychopathol. 1999;11:685–714. doi: 10.1017/s0954579499002278. [DOI] [PubMed] [Google Scholar]
- 91.Mayes LC, Fahy T. Prenatal Drug Exposure and Cognitive Development. In: Sternberg RJ, Grigorenko EL, editors. Environmental Effects on Cognitive Abilities. Lawrence Erlbaum Associates; Mahwah, New Jersey: 2001. pp. 189–219. [Google Scholar]
- 92.Mayes LC, Granger RH, Bornstein MH, Zuckerman B. The problem of prenatal cocaine exposure. A rush to judgment. JAMA. 1992;267:406–408. [PubMed] [Google Scholar]
- 93.Mayes LC, Molfese DL, Key AP, Hunter NC. Event-related potentials in cocaine-exposed children during a Stroop task. Neurotoxicol Teratol. 2005;27:797–813. doi: 10.1016/j.ntt.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 94.McCarthy RA, Warrington EK. Cognitive Neuropsychology: A Clinical Introduction. New York: 1990. Editoin Edition. [Google Scholar]
- 95.Menon V, Adleman NE, White CD, Glover GH, Reiss AL. Error-related brain activation during a Go/NoGo response inhibition task. Hum Brain Mapp. 2001;12:131–43. doi: 10.1002/1097-0193(200103)12:3<131::AID-HBM1010>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Moe V, Slinning K. Prenatal drug exposure and the conceptualization of long-term effects. Scand J Psychol. 2002;43:41–7. doi: 10.1111/1467-9450.00267. [DOI] [PubMed] [Google Scholar]
- 97.Morrow CE, Accornero VH, Xue LH, Manjunath S, Culbertson JL, Anthony JC, Bandstra ES. Estimated Risk of Developing Selected DSM-IV Disorders Among 5-Year-Old Children with Prenatal Cocaine Exposure. J Child Fam Stud. 2009;18:356–364. doi: 10.1007/s10826-008-9238-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Morrow CE, Culbertson JL, Accornero VH, Xue L, Anthony JC, Bandstra ES. Learning disabilities and intellectual functioning in school-aged children with prenatal cocaine exposure. Developmental neuropsychology. 2006;30:905–31. doi: 10.1207/s15326942dn3003_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Noble KG, Norma MF, Farah MJ. The neurocognitive correlates of socioeconomic status. Presented; Cognitive Neuroscience Society Conference; New York. 2003. [Google Scholar]
- 100.Noble KG, Norman MF, Farah MJ. Neurocognitive correlates of socioeconomic status in kindergarten children. Dev Sci. 2005;8:74–87. doi: 10.1111/j.1467-7687.2005.00394.x. [DOI] [PubMed] [Google Scholar]
- 101.Noland JS, Singer LT, Short EJ, Minnes S, Arendt RE, Kirchner HL, Bearer C. Prenatal drug exposure and selective attention in preschoolers. Neurotoxicol Teratol. 2005;27:429–38. doi: 10.1016/j.ntt.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 102.Nordstrom Bailey B, Sood BG, Sokol RJ, Ager J, Janisse J, Hannigan JH, Covington C, Delaney-Black V. Gender and alcohol moderate prenatal cocaine effects on teacher-report of child behavior. Neurotoxicol Teratol. 2005;27:181–9. doi: 10.1016/j.ntt.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 103.Owen AM. Cognitive planning in humans: neuropsychological, neuroanatomical and neuropharmacological perspectives. Progress in Neurobiology. 1997;53:431–50. doi: 10.1016/s0301-0082(97)00042-7. [DOI] [PubMed] [Google Scholar]
- 104.Owen AM. The functional organization of working memory processes within human lateral frontal cortex: the contribution of functional neuroimaging. European Journal of Neuroscience. 1997;9:1329–39. doi: 10.1111/j.1460-9568.1997.tb01487.x. [DOI] [PubMed] [Google Scholar]
- 105.Owen AM, Doyon J, Petrides M, Evans AC. Planning and spatial working memory: a positron emission tomography study in humans. European Journal of Neuroscience. 1996;8:353–64. doi: 10.1111/j.1460-9568.1996.tb01219.x. [DOI] [PubMed] [Google Scholar]
- 106.Pardo JV, Pardo PJ, Janer KW, Raichle ME. The anterior cingulate cortex mediates processing selection in the Stroop attentional conflict paradigm. Proc Natl Acad Sci U S A. 1990;87:256–9. doi: 10.1073/pnas.87.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, Rapoport JL, Evans AC. Structural maturation of neural pathways in children and adolescents: in vivo study. Science. 1999;283:1908–11. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- 108.Rao H, Betancourt L, Giannetta JM, Brodsky NL, Korczykowski M, Avants BB, Gee JC, Wang J, Hurt H, Detre JA, et al. Early parental care is important for hippocampal maturation: Evidence from brain morphology in humans. NeuroImage. 2009 doi: 10.1016/j.neuroimage.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Richardson GA, Conroy ML, Day NL. Prenatal cocaine exposure: effects on the development of school-age children. Neurotoxicol Teratol. 1996;18:627–34. doi: 10.1016/s0892-0362(96)00121-3. [DOI] [PubMed] [Google Scholar]
- 110.Rose-Jacobs R, Waber D, Beeghly M, Cabral H, Appugleise D, Heeren T, Marani J, Frank DA. Intrauterine cocaine exposure and executive functioning in middle childhood. Neurotoxicol Teratol. 2009;31:159–68. doi: 10.1016/j.ntt.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SC, Simmons A, Andrew C, Bullmore ET. Functional frontalisation with age: mapping neurodevelopmental trajectories with fMRI. Neurosci Biobehav Rev. 2000;24:13–9. doi: 10.1016/s0149-7634(99)00055-x. [DOI] [PubMed] [Google Scholar]
- 112.Rubia K, Russell T, Overmeyer S, Brammer MJ, Bullmore ET, Sharma T, Simmons A, Williams SC, Giampietro V, Andrew CM, et al. Mapping motor inhibition: conjunctive brain activations across different versions of go/no-go and stop tasks. NeuroImage. 2001;13:250–61. doi: 10.1006/nimg.2000.0685. [DOI] [PubMed] [Google Scholar]
- 113.Salgado-Pineda P, Vendrell P, Bargallo N, Falcon C, Junque C. Functional magnetic resonance in the evaluation of the activity of the anterior cingulate cortex using Stroop's paradigm. Rev Neurol. 2002;34:607–11. [PubMed] [Google Scholar]
- 114.Savage J, Brodsky NL, Malmud E, Giannetta JM, Hurt H. Attentional functioning and impulse control in cocaine-exposed and control children at age ten years. J Dev Behav Pediatr. 2005;26:42–7. [PubMed] [Google Scholar]
- 115.Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, Evans A, Rapoport J, Giedd J. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440:676–9. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- 116.Sheinkopf SJ, Lester BM, Sanes JN, Eliassen JC, Hutchison ER, Seifer R, Lagasse LL, Durston S, Casey BJ. Functional MRI and response inhibition in children exposed to cocaine in utero. Preliminary findings. Dev Neurosci. 2009;31:159–66. doi: 10.1159/000207503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Singer LT, Arendt R, Minnes S, Farkas K, Salvator A. Neurobehavioral outcomes of cocaine-exposed infants. Neurotoxicol Teratol. 2000;22:653–66. doi: 10.1016/s0892-0362(00)00092-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Singer LT, Nelson S, Short E, Min MO, Lewis B, Russ S, Minnes S. Prenatal cocaine exposure: drug and environmental effects at 9 years. J Pediatr. 2008;153:105–11. doi: 10.1016/j.jpeds.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Snodgrass JG, Corwin J. Pragmatics of measuring recognition memory: applications to dementia and amnesia. J Exp Psychol Gen. 1988;117:34–50. doi: 10.1037//0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
- 120.Snodgrass JG, Vanderwart M. A standardized set of 260 pictures: Norms for name agreement, image agreement, familiarity, and visual complexity. Journal of Experimental Psychology: Human Learning and Memory. 1980;6:174–215. doi: 10.1037//0278-7393.6.2.174. [DOI] [PubMed] [Google Scholar]
- 121.Sowell ER, Delis D, Stiles J, Jernigan TL. Improved memory functioning and frontal lobe maturation between childhood and adolescence: a structural MRI study. J Int Neuropsychol Soc. 2001;7:312–22. doi: 10.1017/s135561770173305x. [DOI] [PubMed] [Google Scholar]
- 122.Sowell ER, Peterson BS, Thompson PM. Mapping cortical change across the human lifespan. Nature Neuroscience. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- 123.Sowell ER, Thompson PM, Tessner KD, Toga AW. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: Inverse relationships during postadolescent brain maturation. J Neurosci. 2001;21:8819–29. doi: 10.1523/JNEUROSCI.21-22-08819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Stark CE, Okado Y. Making memories without trying: medial temporal lobe activity associated with incidental memory formation during recognition. J Neurosci. 2003;23:6748–53. doi: 10.1523/JNEUROSCI.23-17-06748.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Thompson-Schill S, D'Esposito M, Aguirre GK, Farah MJ. The role of left prefrontal cortex in semantic retrieval: A re-evaluation. Proceedings of the National Academy of Sciences. 1997;94:14792–14797. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Thompson-Schill SL, Swick D, Farah MJ, D'Esposito M, Kan IP, Knight RT. Verb generation in patients with focal frontal lesions: a neuropsychological test of neuroimaging findings. Proc Natl Acad Sci U S A. 1998;95:15855–15860. doi: 10.1073/pnas.95.26.15855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Vingerhoets G, Vermeule E, Santens P. Impaired intentional content learning but spared incidental retention of contextual information in non-demented patients with Parkinson's disease. Neuropsychologia. 2005;43:675–81. doi: 10.1016/j.neuropsychologia.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 128.Wakschlag LS, Pickett KE, Cook E, Jr, Benowitz NL, Leventhal BL. Maternal smoking during pregnancy and severe antisocial behavior in offspring: a review. Am J Public Health. 2002;92:966–74. doi: 10.2105/ajph.92.6.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wallentin M. Putative sex differences in verbal abilities and language cortex: a critical review. Brain Lang. 2009;108:175–83. doi: 10.1016/j.bandl.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 130.Wechsler D. The Wechsler Preschool and Primary Scale of Intelligence- Revised. The Psychological Corp; San Antonio, TX: 1989. [Google Scholar]
- 131.Weese-Mayer DE, Silvestri JM, Lin D, Buhrfiend CM, Lo ES, Carvey PM. Effect of cocaine in early gestation on striatal dopamine and neurotrophic activity. Pediatr Res. 1993;34:389–92. doi: 10.1203/00006450-199309000-00029. [DOI] [PubMed] [Google Scholar]
- 132.Yakovlev PI, Lecours AR. The myelogenic cycles of regional maturation of the brain. Blackwell; Oxford: 1967. Editoin Edition. [Google Scholar]
- 133.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–30. [PubMed] [Google Scholar]
- 134.Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988;44:1049–60. [PubMed] [Google Scholar]
- 135.Zuckerman B, Frank DA. "Crack kids": not broken. Pediatrics. 1992;89:337–9. [PubMed] [Google Scholar]
- 136.Zuckerman B, Frank DA, Hingson R, Amaro H, Levenson SM, Kayne H, Parker S, Vinci R, Aboagye K, Fried LE, et al. Effects of maternal marijuana and cocaine use on fetal growth. N Engl J Med. 1989;320:762–8. doi: 10.1056/NEJM198903233201203. [DOI] [PubMed] [Google Scholar]