Abstract
Prenodal lymph is generated from the interstitial fluid that surrounds organs, and thus contains products of organ metabolism and catabolism. New proteomic analyses have identified in lymph proteins and peptides that are derived from capillary extravasation and tissue-specific proteins. Many of these peptides are detected at nanomolar concentrations in the lymph prior to passage through a regional lymph node. Before entering the node and once inside proteins and processed peptides are filtered from the lymph by circulating immature DC or non-activated nodal APC (macrophages, B cells and immature DC). Here, we suggest that this process ensures organ-specific self-antigens are displayed to circulating and nodal antigen-presenting cells, thus contributing to the maintenance of peripheral tolerance.
The lymphatic system: a historical perspective
Historically, the first mention of the lymphatic system is found in a medical treaty written by the Greek physician Hippocrates in the V century B.C. whereas Galen (II century A.D.) reported the first dissection of the mesenteric lymph nodes. It was not until the seventeenth century that the lymphatic vessels were described as a separate system that carry aqueous fluid distinct from the blood. Until the early 1900s it was thought that the lymph was a cell-free, protein-free ultrafiltrate of the plasma composed only of electrolytes. In 1925 it was discovered that the prenodal lymph has a cellular component and contains high levels of lipids and proteins [1]. Even though it is generally regarded that the analysis of the pre-nodal lymph could provide a molecular signature of organ-specific “omics” (proteomic, lipidomic and metabolomic) transported to the regional lymph node, the difficulty in collecting primary material has hampered a broader development of the field [2]. Recently, a few studies have been performed on human bovine and goat lymph, collected at different sites, which have provided the first glimpse into the composition of the lymphatic fluid.
In this review we will focus on current knowledge of the proteomic and peptidomic composition of the lymph under physiological conditions, with particular emphasis on its immunological role. Data on the self-antigen composition of the lymph will be discussed with regard to their implication for the maintenance of peripheral tolerance. The cellular composition of the lymph and analysis of the primary secondary and tertiary lymphatic organs is beyond the scope of the review.
Lymph Production and Circulation
Up to seventy percent of the plasma entering the arterial end of a capillary bed will elapse into the tissue space through a filtration process driven by the hydrostatic arterial pressure. A negligible fraction of this extracellular fluid returns to the venule end of the capillary as a result of the intravascular osmotic pressure. The majority gives rise to the lymphatic interstitial fluid, which bathes cells in each parenchymal organ and collects products derived from organ metabolism and catabolism [3–11]. The interstitial lymphatic fluid is then collected into open ended lymphatic capillaries that form a mesh-like network throughout the tissue spaces. By flowing into progressively larger lymphatic vessels, the pre-nodal lymph is transported to the (~ 500) lymph nodes disseminated throughout the human body. Each node receives lymph from a defined region of the body and all lymph passes through at least one, but often more, lymph nodes [2, 12].
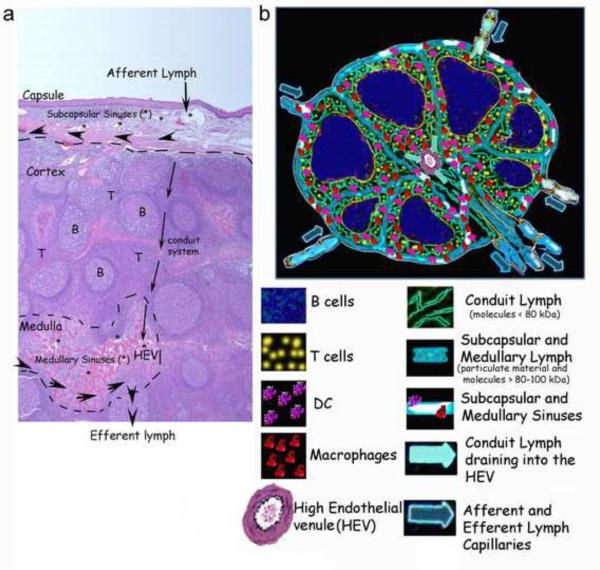
Subcutaneous injection of fluorochrome-labeled particles or proteins indicates that peripherally injected substances are transported to the draining lymph node in a matter of minutes [13–15]. The afferent lymph enters the lymph node from the many lymphatic vessels that perforate the nodal capsule into the subcapsular sinuses (Figure 1). From the sinuses, soluble lymph-carried antigens proceed along two different routes according to size. Particulate material and high molecular weight molecules travel peripherally in the nodal subcapsular and medullary sinuses before entering the efferent lymphatic vessel, thus avoiding the cortical area of the lymph node [15–17] (Figure 1). Their size exclusion from the cortical area is probably due to the presence of pores (0.1 to 1 mm diameter) between the subcapsular and medullary areas and the cortex. Even though the presence of an anatomical filter is still controversial, it is widely accepted that bacteria and particulate material are excluded from the cortical areas [18–19]. In contrast, smaller molecules (<80 kDa) percolate from the subcapsular spaces through a reticular network formed by collagens (I and IV), extracellular matrix proteins (laminins, fibronectin, Perlecan) and fibroblastic reticular cells [15–18]. This three-dimensional network is formed by several conduits, each of 100–200 nm diameters, organized within the nodal T cell areas (Figure 1), which physically connects the subcapsular cortical and paracortical areas with the medullary space into the walls of the high endothelial venule (HEV) (Figure 1). Molecules and pathogens that, due to their size, shortcut the conduit system are phagocytosed by macrophages and dendritic cells (DCs) associated with the subcapsular and medullary sinuses [19]. In contrast, molecules that enter the conduit network encounter DCs that are scattered throughout the T cell areas of the nodes, whose dendrites directly fish into the conduit system (15, 16). In both cases, the lymph, before flowing out of the node either through the efferent lymphatic or through the high endothelial venule, is `filtered` by nodal antigen presenting cells (APC). By percolating through the lymph node a representation of organ-specific self-antigens, as well as non self-antigens, are displayed to cortical and medullary macrophages, DC and B cells to ensure constant immunosurveillance. On the other hand, bacterial and viral pathogens derived from local tissue infections, due to their size, shortcut the cortical area and are either phagocytosed by subcapsular and medullary macrophages and DC or exit the node through the efferent lymphatic without entering the HEV [19].
Figure 1. Anatomical structure of a lymph node and circulation of lymph.
a) H & E staining of a human lymph node. The capsule, cortex and medulla are indicated. * designates subcapsular and medullary sinuses. T and B cell areas are indicated as T and B respectively. The large arrowheads point to the lymphatic circulation of particulate material and high molecular weight proteins (>80 kDa), which bypass the nodal cortex, and will exit the node through efferent lymphatics. The smaller arrows illustrate the lymph flux into the conduit system and its exit through the high endothelial venule (HEV). b) A schematic drawing of a lymph node and nodal lymph circulation.
Self Antigens Carried by the Lymph
Proteins
Interest in the lymph proteome stems from the notion that this fluid is in direct contact with each of the cells forming the parenchymal organs; thus, the lymph collects a true read-out of the metabolic and catabolic intercellular exchanges, as well as exchanges that occur between cells and the surrounding extracellular matrix. In fact, in contrast from what was previously thought, the proteomic between the plasma and the lymph is similar but not overlapping [5]; Proteins that are similarly represented in plasma and lymph result from capillary ultrafiltration; classical examples of which are the major classes of plasma proteins (albumin, α1, α2 and β globulin, immunoglobulins) which are all present in the lymph, albeit at lower concentration than in the plasma. On the other hand proteins uniquely found in the lymph are the one actively secreted or produced by the metabolic/catabolic exchanges of parenchymal cells bathed by the extracellular milieu; these proteins constitute an important source of tissue-specific self-antigen [5, 6, 9–11, 20, 21].
Proteomic analysis combined with 2D gel technology has identified some of these proteins that are uniquely present in the lymph, such as glial proteins, skeletal muscle proteins and catabolic products from parenchymal organs (histones, mitochondrial and ribosomal proteins) [5, 11, 20, 21]. Even though the number of reported studies examining lymph composition is too small to convey the full repertoire of lymph proteins, ongoing efforts will in time provide a full characterization of the proteins uniquely carried by the lymph, as a reflection of the tissue proteome at the site of collection. Such analyses could prove a valuable technique to identify tissue-specific biomarkers of specific physiological and pathological conditions.
We recently produced a lymph-plasma comparative proteomic analysis with scaffolding statistical analysis for data comparison [11]. Instead of focusing our analysis on proteins that are uniquely represented between the two samples, we analyzed the data as an overall representation of the cellular and sub-cellular components present in the lymph and in the plasma. Interesting differences were observed between the samples. Consistent with the notion that the lymph carries apoptotic cells and products of organ and cellular catabolism, proteins deriving from intracellular sources (endosomes, Golgi, ER, mitochondria and cytoplasm) were more abundant in the lymph than in the plasma. Fragments of extracellular matrix proteins (such as collagens, mucins, laminins) derived from organ remodeling as well as proteins derived from surface receptor editing and cytokine and chemokine processing, were also more represented in the lymph compared with the plasma. Altogether the data indicate that a major difference between lymph and plasma is the overwhelming catabolic proteome present in the former [11].
Peptides
A striking major difference between the prenodal lymph and the plasma is the amount of processed protein fragments and peptides found in the lymph compared with plasma [11]. As expected, analysis of the lymph peptidome indicated that processed peptides were derived from the same cellular and extracellular sources as the proteome. Thus the identified peptides fell into three main classes. A large number of the sequenced peptides were generated by processing of endogenous intracellular proteins such as enzymes, transcription factors and nuclear, endosomal, cytosolic, ER and mitochondria proteins. These peptides are likely released by damaged and apoptotic cells, which are not all phagocytosed in parenchymal organs by local APC, but are known to be present in the prenodal lymph as well [22, 23]. These peptides could derive from processing by the proteasome, endosomal proteases, as well as caspases [23, 24]. The sequenced peptides were also generated by processing of extracellular proteins, collagens and extracellular matrix proteins, which are the most abundant proteins in the human body. These matrix proteins are constantly remodeled to accommodate organ growth and cellular migration and movement inside parenchymal organs [25, 26]. Matrix peptides are likely generated by matrix metalloproteinases (MMP), a large family of plasma membrane-bound and secreted proteases known to be the primary processing enzymes involved in collagen and laminin degradation [27]. The third category of sequenced peptides was derived from processing of plasma membrane-associated proteins, soluble receptors, cadherins and surface proteins involved in cell adhesion [28–29]. These proteins are likely to be processed by several families of enzymes that are active at the plasma membrane (such as ADAM, CD13, CD26, MMPs) and function in the regulated proteolysis of membrane receptors, cytokines and growth factors [30–32].
Processing and MHC class I and class II Loading of Lymph-carried Antigens
Lymph-carried self-proteins could either be phagocytosed by tissue-migrating circulating DC [34–36] or upon entering the node could be taken-up by sub-capsular or cortical APC. Thus, in addition to phagocytosis of self-proteins by tissue APC, or expression of tissue-specific self-antigens by AIRE expressing nodal cells [33, 37], lymphatic circulation of self-antigens is an additional mechanism by which extracellular self-antigens can be processed and presented, or cross-presented in an MHC-restricted manner [4–6, 10, 11, 33–37]. Considering the cell number, T cell density and architecture of the lymph node, which supports lymphocyte and APC encounters, having self-antigens directly transported to the nodes is probably an efficient mechanism for T cell immunosurveillance, compared with having T cells patrol each peripheral organ directly [12]. Since lymph-carried proteins are phagocytosed by circulating DC, or by nodal macrophages and nodal DC, the resulting MHC-restricted self peptidome will be produced mostly by endosomal proteases and the proteasome (for cross-presenting antigens) [24, 38].
A large amount of short linear peptides (from 8 to 20 aa in length) have been sequenced from the human lymph [11]. These short lymph-carried peptides could derive from a heterogeneous variety of processing pathways and might not be restricted by endosomal processing. For example: (i) apoptotic cells, that circulate in the human lymph, can release a series of peptides generated by endosomal proteases as well as caspases [23,24]; (ii) peptides derived from the ongoing physiological tissue remodeling, which would produce an extracellular matrix peptidome restricted by MMP processing [25, 27]; (iii) peptides derived from the regulated cell surface proteolysis aimed at receptor editing and processing of cytokines and growth factors, which would produce an extracellular peptidome mostly restricted by MMPs and ADAMs [29–32].
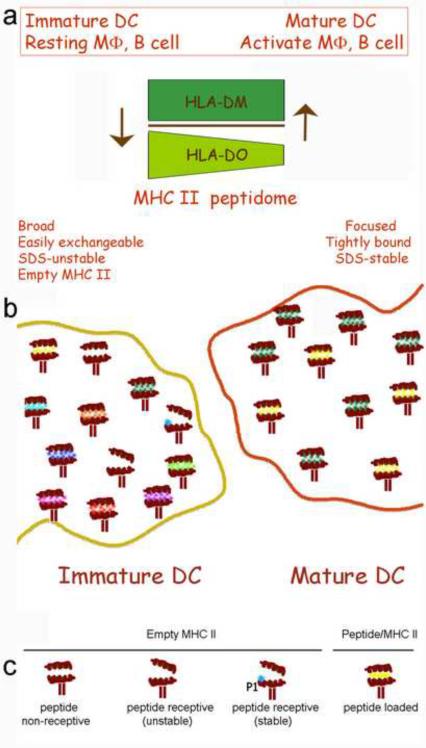
MHC class I molecules (MHC I) are expressed on every cell in the body, and short peptides could directly bind to MHC I on endothelial cells, fibroblasts, T cells, B cells as well as professional APC. This could occur for lymph-derived peptides on empty MHC I molecules, or through exchange with previously loaded peptides [40, 43]. In contrast, under non-inflammatory conditions, MHC class II (MHC II) molecules are restricted to professional APC, and thus short peptides can either be loaded on surface MHC II molecules expressed on parenchymal tissue DC and macrophages, migrating DC or, after entering the node through afferent lymphatics, on the surface MHC II expressed on nodal DC, B cells and macrophages [36, 39, 41–42, 45–49]. Immature DC and non activated APCs are particularly competent for surface MHC II loading compared to mature or activated APCs [42]. In fact, it has been proposed that, since DO expression is generally down regulated upon APC activation, the inhibitory function of DO on DM catalytic activity, favors a broader, less stable and more easily exchanged MHC II/peptide repertoire as well as the formation of empty MHC II complexes (Figure 2) [50, 51].
Figure 2. Conformational MHC class II variations and differential MHC class II peptidome on the surface of immature and mature DCs.
a) Immature DCs and non-activated APCs are particularly competent for surface MHC II loading compared with mature or activated APCs [42]. Becasue DO expression is higher on immature DCs and non-activated APC the inhibitory function of DO on DM catalytic activity, favors a broader, less stable and more easily exchanged MHC II/peptide repertoire [50,51]. b) Immature DCs (left) present a broader peptide repertoire as well as a less stable and empty (peptide non-receptive and receptive) MHC class II complexes. Mature DCs express a more focused peptidome and more stable MHC class II complexes [40–47, 53–56]. c) Schematic showing surface peptide loading events on MHC class II molecules in immature DCs. Five possibleMHC class II molecule conformations are shown: peptide non-receptive (P1 pocket collapsed), peptide-receptive (P1 pocket open or filled) and peptide-loaded (binding groove occupied by the loaded peptide).
With regard to surface loading, exposure of fixed cells to antigen demonstrates that MHC molecules can be loaded directly on the cell surface [42, 43]. `Empty' MHC molecules can be detected on any MHC II expressing cell and are particularly abundant on immature DC [41, 53]. While empty MHC molecules rapidly inactivate by acquiring a peptide `non-receptive' conformation [43, 44], this is a reversible process. It has been known for some time that inactive MHC I can be reloaded in the presence of an excess of β2-microglobulin [44]. More recently, evidence has emerged that non-receptive MHC II molecules can be rescued in an HLA-DM-like fashion by small molecules that are able to fill the P1-pocket to stabilize the peptide-receptive state through defined interactions with the MHC molecule [54–56]. These small molecules include a number of organic compounds [54–55], but short peptide fragments [56] can also act directly as `MHC-loading enhancers and can catalyze ligand-exchange and binding of extracellular peptides. Surface-loading of MHC II molecules may therefore represent an alternative pathway to the default intracellular processing pathway. Since ligand selection is not affected by the specific uptake and processing mechanisms of the endosomal pathway, it widens the range of peptides that can be displayed on the cell surface. Thus, lymph carried peptides may therefore have particular relevance for the induction and maintenance of peripheral tolerance to non-endosomal processed peptides.
Lymph as a Source of Self-Antigens Involved in Peripheral Tolerance
In the last decade, several animal studies as well as clinical trials have been using peptide therapy with the ultimate goal of inducing tolerance or vaccination [58, 59]. All these studies indicate that a key factor in determining the efficacy of peptide therapy is the context in which peptides are presented to the immune system. Peptide vaccination aimed at achieving a long-lasting immunity, such as during cancer immunotherapy, requires peptide injection associated with toll-like receptor agonists or CD40 ligand [59]. On the other hand, peptides injected alone or even with a mild adjuvant are tolerogenic [58]. These peptides are presented by non-professional APC or immature DC. Peptide-driven immunosuppressive therapy has been found to be effective in reducing unwanted immune responses to allergens and self-antigens [58]. A down-regulation in pro-inflammatory cytokines and T cell proliferative responses has been observed following peptide therapy in different diseases such as asthma, rheumatoid arthritis, type 1 diabetes, and cat and bee allergies. Several mechanisms can account for peptide-driven immune tolerance including; (i) depletion of autoreactive T cells, particularly at high peptide regimens, (ii) induction of regulatory T cells, and/or (iii) induction of IL-10 and other immunosuppressive cytokines. Taken together, peptide therapy is a promising antigen-specific approach to the treatment of autoimmune diseases and allergy [60–63].
The effective immunosuppressive peptide dose in animal models of autoimmune disease ranges between a few micrograms to milligrams. Likewise, human clinical trials report the effectiveness of a few micrograms of subcutaneously injected peptides in the treatment of asthmatic individuals [58]. Recently it has been shown in diabetes clinical trials that tolerance is induced upon injection of sub-immunogenic doses of soluble antigens and therapeutic efficacy was demonstrated even with sub-nanomolar doses of antigens [58,60,61].
From an immunological standpoint, for lymph-carried peptides to be tolerogenic their amount should be sufficient for antigen presentation. There is only one study, performed in our laboratory, which has attempted to quantify the amount of some lymph-carried peptides. For this, two different quantitative approaches were performed: amino-acid analysis of peptides eluted from a 2D gel and, MS/MS analysis performed on biological fractions of lymph spiked with synthesized labeled (N14/N15) standard peptides [11]. Peptides visualized and eluted from a 2D gel were estimated to be in the high-nanomolar concentration. Similarly, N14/N15 quantification data indicated that up to micromolar concentrations of self-peptides are transported in the lymph, similar to the low range of concentrations known to be effective in peptide immunotherapy [11, 58]. It should be noted, however that in mouse studies, as well as in protocols for human peptide therapy, one peptide dose is administered weekly or even monthly either subcutaneously or intradermally and no data are available on the actual peptide concentration reaching the blood and the lymph [58]. In contrast, lymph-carried peptides are directly and constantly available for loading on non-professional APC and immature DC and the amount of peptide in the human lymph appears to be in the dose-range for effective tolerization [11].
Concluding remarks
By further characterizing the human lymph proteome, derived from a generalized population it will be possible to focus future studies in order to: (i) quantify self-antigens carried by the lymph, as a read-out of the organ specific proteins that are available to nodal APC for the maintenance of peripheral tolerance; (ii) determine the enzymatic pathways (e.g. caspases, MMPs, ADAMs) involved in processing lymph-carried peptides, particularly in forming peptides that would be “lost” by endosomal processing; (iii) determine the mechanism by which peptides found in nanomolar or micromolar concentrations induce peripheral tolerance; and (iv) characterize the tolerogenic response following surface MHC peptide loading on professional and non professional APC. Altogether, these studies would provide a deeper insight into the mechanism of peripheral tolerance, in relationship to tissue specific catabolism, alternative processing pathways and surface MHC loading. In addition, similar analyses of lymph derived from patients with autoimmune disorders might provide a unique window into possible differences in the spectrum of antigens present in these individuals that could provide new insights into the pathogenesis of these disorders.
Box 1: Characterization and Immunological Function of the Lymph-carried Proteome and Peptidome.
Lymph production
Interstitial fluids from every parenchymal organ
Proteomic of self-antigens carried by the lymph
Proteins derive from: (i) capillary extravasation and (ii) parenchymal metabolic exchanges
Peptidomic of self-antigens carried by the lymph
Peptides derive from processing of: (i) intracellular proteins (proteasome, endosomal proteases, and caspases); (ii) extracellular matrix proteins (MMPs); (iii) membrane regulated proteolysis of receptors, cytokines and growth factors (ADAMs, CD13, CD26, MMPs).
Phagocytosis of lymph-carried proteins
Lymph-circulating and nodal immature DC and non activated MΦ (phagocytosis is down regulated upon DC maturation and macrophage activation)
Surface MHC loading of lymph-carried peptides
Lymph-circulating and nodal immature DC and non activated MΦ and B cells (surface loading is more efficient in immature DC and non activated APC [42, 50,51])
Immunological outcome: Tolerance
(i) Low level or no co-stimulatory molecules on immature DC and non activated APC (ii) Display of MHC I or MHC II peptide complexes without TLR activation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Heim WJ. On the Chemical Composition of lymph from subcutaneous vessels. Harvard University Press; Cambridge, Mass.: 1932. pp. 553–558. [Google Scholar]
- 2.Yoffey J, et al. Lymphatics, Lymph, and Lymphoid tissue. Harvard University Press; Cambridge, Mass.: 1956. [Google Scholar]
- 3.Levick JR, Michel CC. Microvascular fluid exchange and the revised Starling principle. C. ardiovasc. Res. 2010;87(2):198–210. doi: 10.1093/cvr/cvq062. [DOI] [PubMed] [Google Scholar]
- 4.Aukland K, et al. Protein concentration of lymph and interstitial fluid in the rat tail. Am. J. Physiol. 1984;247(1):4–9. doi: 10.1152/ajpheart.1984.247.1.H74. [DOI] [PubMed] [Google Scholar]
- 5.Leak LV, et al. Proteomic analysis of lymph. Proteomics. 2004;4:753–765. doi: 10.1002/pmic.200300573. [DOI] [PubMed] [Google Scholar]
- 6.Interewicz B, et al. Profiling of normal human leg lymph proteins using the 2-D electrophoresis and SELDI-TOF mass spectrophotometry approach. Lymphology. 2004;37:65–72. [PubMed] [Google Scholar]
- 7.Nanjee MN, et al. Lipid and apolipoprotein concentrations in prenodal leg lymph of fasted humans. Associations with plasma concentrations in normal subjects, lipoprotein lipase deficiency, and LCAT deficiency. J. Lipid Res. 2000;41:1317–1327. [PubMed] [Google Scholar]
- 8.Laurent GJ. Dynamic state of collagen: pathways of collagen degradation in vivo and their possible role in regulation of collagen mass. Am. J. Physiol. 1987;252:C1–9. doi: 10.1152/ajpcell.1987.252.1.C1. [DOI] [PubMed] [Google Scholar]
- 9.Reed RK, et al. Lymphatic hyaluronan flux from skin increases during increased lymph flow induced by intravenous saline loading. Int. J. Microcirc. Clin. Exp. 1994;14:56–61. doi: 10.1159/000178207. [DOI] [PubMed] [Google Scholar]
- 10.Olszewski WL, et al. Lymph draining from foot joints in rheumatoid arthritis provides insight into local cytokine and chemokine production and transport to lymph nodes. Arthritis & Rheumatism. 2001;44(3):541–549. doi: 10.1002/1529-0131(200103)44:3<541::AID-ANR102>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 11.Clement CC, et al. An expanded self-antigen peptidome is carried by the human lymph as compared to the plasma. PLoS ONE. 2010;5(3):1–10. doi: 10.1371/journal.pone.0009863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lammermann T, Sixt M. The microanatomy of T-cell responses. Immunol. Rev. 2008;221:26–43. doi: 10.1111/j.1600-065X.2008.00592.x. [DOI] [PubMed] [Google Scholar]
- 13.Ingulli E, et al. In situ analysis reveals physical interactions between CD11b+ dendritic cells and antigen-specific CD4 T cells after subcutaneous injection of antigen. J. Immunol. 2002;169(5):2247–2252. doi: 10.4049/jimmunol.169.5.2247. [DOI] [PubMed] [Google Scholar]
- 14.Itano AA, Jenkins MK. Antigen presentation to naive CD4 T cells in the lymph node. Nat. Immunol. 2003;8:733–739. doi: 10.1038/ni957. [DOI] [PubMed] [Google Scholar]
- 15.Sixt M, et al. The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node. Immunity. 2005;22:19–29. doi: 10.1016/j.immuni.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 16.Gretz JE, et al. Cords, channels, corridors and conduits: critical architectural elements facilitating cell interactions in the lymph node cortex. Immunol. Rev. 1997;156:11–24. doi: 10.1111/j.1600-065x.1997.tb00955.x. [DOI] [PubMed] [Google Scholar]
- 17.Kaldjian EP, et al. Spatial and molecular organization of lymph node T cell cortex: a labyrinthine cavity bounded by an epithelium-like monolayer of fibroblastic reticular cells anchored to basement membrane-like extracellular matrix. Int. Immunol. 2001;13:1243–1253. doi: 10.1093/intimm/13.10.1243. [DOI] [PubMed] [Google Scholar]
- 18.Roozendaal R, et al. Conduits mediate transport of low-molecular-weight antigen to lymph node follicles. Immunity. 2009;30:264–276. doi: 10.1016/j.immuni.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iannacone, et al. Subcapsular sinus macrophages prevent CNS invasion on peripheral infection with a neurotropic virus. Nature. 2010;465(7301):1079–1083. doi: 10.1038/nature09118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldfinch GM, et al. The proteome of gastric lymph in normal and nematode infected sheep. Proteomics. 2008;8:1909–1918. doi: 10.1002/pmic.200700531. [DOI] [PubMed] [Google Scholar]
- 21.Mittal A, et al. The Proteome of Mesenteric Lymph During Acute Pancreatitis and Implications for Treatment. JOP. J Pancreas (Online) 2009;10(2):130–142. [PubMed] [Google Scholar]
- 22.Olszewski WL. Human afferent lymph contains apoptotic cells and “free” apoptotic DNA fragments. Lymphology. 2001;34:179–183. [PubMed] [Google Scholar]
- 23.Pang B, et al. Direct antigen presentation and gap junction mediated cross-presentation during apoptosis. J. Immunol. 2009;183(2):1083–1090. doi: 10.4049/jimmunol.0900861. [DOI] [PubMed] [Google Scholar]
- 24.Honey K, Rudensky AY. Lysosomal cysteine proteases regulate antigen presentation. Nat. Rev. Immunol. 2003;3(6):472–482. doi: 10.1038/nri1110. [DOI] [PubMed] [Google Scholar]
- 25.Badylak SF, et al. Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomater. 2009;5:1–13. doi: 10.1016/j.actbio.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 26.Dustin ML, de Fougerolles AR. Reprogramming T cells: the role of extracellular matrix in coordination of T cell activation and migration. Curr. Opin. Immunol. 2001;3:286–290. doi: 10.1016/s0952-7915(00)00217-x. [DOI] [PubMed] [Google Scholar]
- 27.Cauwe B, et al. The biochemical, biological, and pathological kaleidoscope of cell surface substrates processed by matrix metalloproteinases. Crit. Rev in Biochem. Mol. Biol. 2009;42:113–185. doi: 10.1080/10409230701340019. [DOI] [PubMed] [Google Scholar]
- 28.Reiss K, Saftig P. The “A Disintegrin And Metalloprotease” (ADAM) family of sheddases: Physiological and cellular functions. Seminars in Cell & Developmental Biology. 2009;20:126–137. doi: 10.1016/j.semcdb.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Kataoka H. EGFR ligands and their signaling scissors, ADAMs, as new molecular targets for anticancer treatments. J. Dermatol. Sci. 2009;56(3):148–153. doi: 10.1016/j.jdermsci.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Lai ZW, et al. Membrane proteomics: the development of diagnostics based on protein shedding. Curr. Opin. Mol. Ther. 2009;11(6):623–631. [PubMed] [Google Scholar]
- 31.Murphy G. Regulation of the proteolytic disintegrin metalloproteinases, the `Sheddases'. Semin. Cell. Dev. Biol. 2009;20(2):138–145. doi: 10.1016/j.semcdb.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 32.Heinrich J, Wiegert T. Regulated intramembrane proteolysis in the control of extracytoplasmic function sigma factors. Res. Microbiol. 2009;160(9):696–703. doi: 10.1016/j.resmic.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 33.Savina A, Amigorena S. Phagocytosis and antigen presentation in dendritic cells. Immunol. Rev. 2007;219:143–156. doi: 10.1111/j.1600-065X.2007.00552.x. [DOI] [PubMed] [Google Scholar]
- 34.Vermaelen KY, et al. Specific migratory dendritic cells rapidly transport antigen from the airways to the thoracic lymph nodes. J. Exp. Med. 2001;193:51–60. doi: 10.1084/jem.193.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cavanagh LL, Von Andrian UH. Travellers in many guises: the origins and destinations of dendritic cells. Immunol. Cell. Biol. 2002;80:448–462. doi: 10.1046/j.1440-1711.2002.01119.x. [DOI] [PubMed] [Google Scholar]
- 36.Forster R, et al. CCR7 and its ligands: balancing immunity and tolerance. Nat. Rev. Immunol. 2008;8(5):362–371. doi: 10.1038/nri2297. [DOI] [PubMed] [Google Scholar]
- 37.Mathis D, Benoist C. Ann. Rev. Immunol. 2009;27:287–312. doi: 10.1146/annurev.immunol.25.022106.141532. [DOI] [PubMed] [Google Scholar]
- 38.Rock KL, Shen L. Cross-presentation: underlying mechanisms and role in immune surveillance. Immunol. Rev. 2005;207:166–183. doi: 10.1111/j.0105-2896.2005.00301.x. [DOI] [PubMed] [Google Scholar]
- 39.Belizaire R, Unanue ER. Targeting proteins to distinct subcellular compartments reveals unique requirements for MHC class I and II presentation. Proc Natl Acad Sci USA. 2009;106:17463–17468. doi: 10.1073/pnas.0908583106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Bruijn ML, et al. Peptide loading of empty major histocompatibility complex molecules on RMA-S cells allows the induction of primary cytotoxic T lymphocyte responses. Eur. J. Immunol. 1991;12:2963–2970. doi: 10.1002/eji.1830211210. [DOI] [PubMed] [Google Scholar]
- 41.Santambrogio L, et al. Abundant Empty Class II MHC Molecules on the Surface of Immature Dendritic Cells. Proc.Natl.Acad.Sci. USA. 1999;96:15050–15055. doi: 10.1073/pnas.96.26.15050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Venkatraman P, et al. Fluorogenic probes for monitoring peptide binding to class II MHC proteins in living cells. Nat. Chem. Biol. 2007;3(4):222–228. doi: 10.1038/nchembio868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rabinowitz JD, et al. Formation of a highly peptide-receptive state of class II MHC. Immunity. 1998;9:699–709. doi: 10.1016/s1074-7613(00)80667-6. [DOI] [PubMed] [Google Scholar]
- 44.Rock KL, et al. Dissociation of beta 2-microglobulin leads to the accumulation of a substantial pool of inactive class I MHC heavy chains on the cell surface. Cell. 1991;65:611–620. doi: 10.1016/0092-8674(91)90093-e. [DOI] [PubMed] [Google Scholar]
- 45.Pinet V, et al. Two processing pathways for the MHC class II-restricted presentation of exogenous influenza virus antigen. J. Immunol. 1994;152:4852–4860. [PubMed] [Google Scholar]
- 46.Potolicchio I, et al. Conformational variation of surface class II MHC proteins during myeloid dendritic cell differentiation accompanies structural changes in lysosomal MIIC. J. Immunol. 2005;175:4935–4947. doi: 10.4049/jimmunol.175.8.4935. [DOI] [PubMed] [Google Scholar]
- 47.Natarajan SK, et al. Stable peptide binding to MHC class II molecule is rapid and is determined by a receptive conformation shaped by prior association with low affinity peptides. J. Immunol. 1999;162:4030–4036. [PubMed] [Google Scholar]
- 48.Reich Z, et al. Stability of empty and peptide-loaded class II major histocompatibility complex molecules at neutral and endosomal pH: comparison to class I proteins. Proc. Natl. Acad. Sci. USA. 1997;94:2495–2500. doi: 10.1073/pnas.94.6.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Runnels HA, et al. Intact proteins can bind to class II histocompatibility molecules with high affinity. Mol. Immunol. 1997;34:471–480. doi: 10.1016/s0161-5890(97)00043-6. [DOI] [PubMed] [Google Scholar]
- 50.Denzin LK, et al. Right place, right time, right peptide: DO keep DM focused. Immunol. Rev. 2005;207:279–292. doi: 10.1111/j.0105-2896.2005.00302.x. [DOI] [PubMed] [Google Scholar]
- 51.Jensen PE. Antigen processing: HLA-DO--a hitchhiking inhibitor of HLA-DM. Curr Biol. 1998;8(4):128–131. doi: 10.1016/s0960-9822(98)70988-1. [DOI] [PubMed] [Google Scholar]
- 52.Busch R, et al. Achieving stability through editing and chaperoning: regulation of MHC class II peptide binding and expression. Immunol. Rev. 2005;207:242–260. doi: 10.1111/j.0105-2896.2005.00306.x. [DOI] [PubMed] [Google Scholar]
- 53.Santambrogio L, et al. Extracellular antigen processing and presentation by immature dendritic cells. Proc. Natl. Acad. Sci. USA. 1999;96:15056–15061. doi: 10.1073/pnas.96.26.15056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hopner S, et al. Small organic compounds enhance antigen loading of class II major histocompatibility complex proteins by targeting the polymorphic P1 pocket. J. Biol. Chem. 2006;281:38535–38542. doi: 10.1074/jbc.M606437200. [DOI] [PubMed] [Google Scholar]
- 55.Falk K, et al. Ligand exchange of major histocompatability complex class II proteins is triggered by H-bond donor groups of small molecules. J. Biol. Chem. 2002;277:2709–2715. doi: 10.1074/jbc.M109098200. [DOI] [PubMed] [Google Scholar]
- 56.Chou CL, et al. Short peptide sequences mimic HLA-DM functions. Mol. Immunol. 2008;45(7):1935–1943. doi: 10.1016/j.molimm.2007.10.033. [DOI] [PubMed] [Google Scholar]
- 57.Marin-Esteban V, et al. Chemical analogues of HLA-DM can induce a peptide-receptive state in HLA-DR molecules. J. Biol. Chem. 2004;279:50684–50690. doi: 10.1074/jbc.M407598200. [DOI] [PubMed] [Google Scholar]
- 58.Larche M, Wraith DC. Peptide-based therapeutic vaccines for allergic and autoimmune diseases. Nat. Med. 2005;11:S69–76. doi: 10.1038/nm1226. [DOI] [PubMed] [Google Scholar]
- 59.Melief CJ, van der Burg SH. Immunotherapy of established (pre)malignant disease by synthetic long peptide vaccines. Nat. Rev. Cancer. 2008;5:351–360. doi: 10.1038/nrc2373. [DOI] [PubMed] [Google Scholar]
- 60.Ali FR, Larche M. Peptide-based immunotherapy: a novel strategy for allergic disease. Expert. Rev. Vaccines. 2005;4:881–889. doi: 10.1586/14760584.4.6.881. [DOI] [PubMed] [Google Scholar]
- 61.Santamaria P. The long and winding road to understanding and conquering type 1 diabetes. Immunity. 2010;32:437–441. doi: 10.1016/j.immuni.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 62.Campbell JD, et al. Peptide immunotherapy in allergic asthma generates IL-10-dependent immunological tolerance associated with linked epitope suppression. J. Exp. Med. 2009;206:1535–1547. doi: 10.1084/jem.20082901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hochweller, et al. Immunological tolerance using synthetic peptides--basic mechanisms and clinical application. Curr. Mol. Med. 2006;6:631–643. doi: 10.2174/156652406778194982. [DOI] [PubMed] [Google Scholar]