Abstract
Inorganic nanoparticles including semiconductor quantum dots, iron oxide nanoparticles, and gold nanoparticles have been developed as contrast agents for diagnostics by molecular imaging. Compared to traditional contrast agents, nanoparticles offer several advantages: their optical and magnetic properties can be tailored by engineering the composition, structure, size, and shape; their surfaces can be modified with ligands to target specific biomarkers of disease; the contrast enhancement provided can be equivalent to millions of molecular counterparts; and they can be integrated with a combination of different functions for multi-modal imaging. Here, we review recent advances in the development of contrast agents based on inorganic nanoparticles for molecular imaging, with a touch on contrast enhancement, surface modification, tissue targeting, clearance, and toxicity. As research efforts intensify, contrast agents based on inorganic nanoparticles that are highly sensitive, target-specific, and safe to use are expected to enter clinical applications in the near future.
Nanoparticles as molecular imaging agents
Molecular imaging is a new frontier of biomedical research for visualizing, characterizing, and monitoring biological processes in cells, tissues, and organisms using sensitive instrumentation and contrast mechanisms [1]. Molecular imaging interrogates biological processes to report on and reveal the molecular abnormalities that form the basis of diseases. As a result, molecular imaging provides a powerful tool for the diagnosis of diseases including cancer, cardiovascular syndrome, and neurological disorders. It can also assist treatment planning by providing information on the physiological state of a tissue or stage of a disease. Molecular imaging differs from traditional imaging in that contrast agents are typically utilized to help identify particular biomarkers or pathways with high sensitivity and selectivity [2]. Ideally, the contrast agents would selectively accumulate at the site of interest; the accumulated agents then interact with the target physically, chemically, and/or biochemically, and thereby alter the imaging contrast according to the ensuing changes. Recent advances in both the development of new imaging techniques and the synthesis of novel contrast agents offer a broad range of exciting opportunities, including early diagnosis and effective treatment of disease.
Although small molecules such as organic dyes and radioisotopes conjugated to targeting ligands have been widely used as contrast agents in both research and clinical settings [2, 3], inorganic nanoparticles (NPs) are receiving increasing attention as future contrast agents because of their superb properties [4–8]. For example, semiconductor quantum dots (QDs) exhibit not only optical emission wavelengths similar to organic dyes (with peaks tunable in the visible and near-infrared regions) but also unique features such as superior brightness, extraordinary photostability, and multi-color capability under single source excitation [4]. Inorganic NPs can also be readily designed and prepared to include an array of properties (e.g., magnetic and optical scattering, absorption, or luminescence) for use with multiple imaging modalities [9–16]. In addition, the surfaces of inorganic NPs can be easily conjugated with different functional groups without changing their physical properties, making it feasible to selectively target the site of interest (e.g., cancerous tissue) for maximum contrast enhancement [17, 18].
Nanoparticles made of organic materials (e.g., liposome, micelles, and polymeric particles) have also been explored as contrast agents for molecular imaging [19, 20]. However, most of them are simply used as carriers to encapsulate functional components such as inorganic NPs, coordination compounds, and organic dyes. Part of the reason can be attributed to the fact that most organic materials that can be easily and conveniently processed as NPs do not exhibit relevant magnetic and optical properties. Some organic materials such as conjugated polymers do exhibit interesting and tunable optical (fluorescence) properties, but they can be difficult to process and degrade in the body. As a result, inorganic NPs are beginning to receive more attention than their organic counterparts as contrast agents for molecular imaging.
Here, we highlight recent progress in the development of new contrast agents based on inorganic NPs for molecular imaging. Specifically, we first introduce imaging modalities that can benefit from contrasts agents based on inorganic NPs, and we concentrate on some of the most extensively explored systems that are in various stages of preclinical and clinical development. We then discuss the mechanisms of targeting for inorganic NPs by engineering the surface properties. We also touch upon the clearance and toxicity issues associated with inorganic NPs. Finally, we elaborate on a set of recent applications enabled by contrast agents based on inorganic NPs.
Molecular imaging modalities
Inorganic NPs is an actively explored technology for the development of contrast agents for molecular imaging. Figure 1 and Table 1 list the types of inorganic NPs being developed as imaging agents, and Figure 1 also shows some typical examples of inorganic NP-based molecular imaging [6, 9, 13–15, 21–33]. Before discussing the performance of these contrast agents, it is helpful to give a brief introduction to the imaging modalities whose success will greatly benefit from these contrast agents. We divide these modalities into two major groups, depending on the penetration depth.
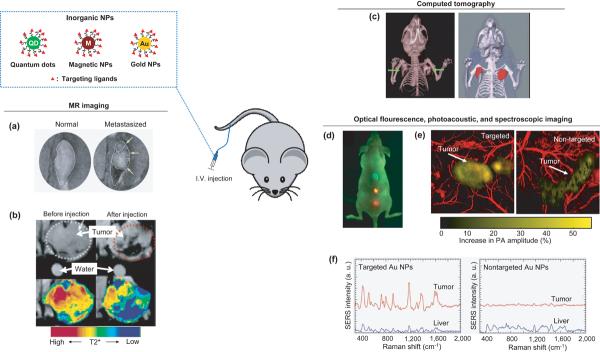
Figure 1.
Typical examples of in vivo molecular imaging with inorganic NPs as contrast agents that are often modified with ligands to target tumors or other diseased lesions. (a) MR imaging of lymph nodes with iron oxide NPs for detecting the metastasis of prostate cancer. (Reproduced with the permission of [24].) (b) MR imaging with antibody-conjugated superparamagnetic iron oxide NPs for tumor targeting. (Reproduced with the permission of [25].) (c) X-ray CT of a mouse bearing tumors (indicated by arrows) as enhanced by PEGylated gold nanorods. (Reproduced with the permission of [31].) (d) Optical fluorescence imaging for detecting human prostate cancer with targeted QDs. (Reproduced with the permission of [21].) (e) Photoacoustic imaging with targeted gold nanocages for detecting human melanoma in a nude mouse. Reproduced with the permission of [27]. (f) SERS spectra with targeted gold NPs for detecting a human squamous cell carcinoma. (Reproduced with the permission of [15].)
Table 1.
Inorganic NPs that have been explored as contrast agents for molecular imaging.
| Nanoparticle type | Imaging modality | Stage of development1 | Ref. |
|---|---|---|---|
| Semiconductor quantum dots | Fluorescence imaging | in vivo | [21–23] |
| Magnetic NPs | Magnetic resonance (MR) imaging | Clinical | [6,9,24,25] |
| Gold colloids | Photoacoustic tomography (PAT) | in vivo | [16,26,27] |
| Surface-enhanced Raman microscopy | in vivo | [15,28] | |
| Multi-photon luminescence imaging | in vivo | [29,30] | |
| X-ray computed tomography (CT) | in vivo | [13,14,31] | |
| Rare earth-doped NPs | Luminescence imaging | in vivo | [32] |
| MRI and near-infrared imaging | in vivo | [33] |
The stage of development is based on the data we could find in literature when this review article was prepared. For examples, the magnetic NPs (especially, iron oxide NPs) have been approved for clinical use so that we denoted this agent as “Clinical”. The references shown in the table are based on in vivo imaging with mice.
Deep-tissue modalities
Magnetic resonance (MR) imaging [34]
MR imaging is used clinically to visualize the structure and function throughout the body. MR imaging detects the different proton relaxation times (T) of water in response to a strong field in the radio frequency (RF) range. It offers great contrast between different soft tissues of the body. There are two types of MR imaging mechanisms: T1-weighted and T2-weighted.
T1, the “longitudinal” relaxation time required for a substance to regain longitudinal magnetization following an RF pulse, represents the correlation between the frequency of molecular motions and the Larmor frequency. The frequencies of small molecules (e.g., water) and large molecules (e.g., proteins) are significantly different from the Larmor frequency and thus have long T1. By contrast, cholesterol, a medium-sized molecule, has natural frequencies close to those used for MR imaging, hence it has a short T1. Thus, cholesterol-rich tissues appear bright while water and proteins looks dark on T1-weighted images. T1 can be decreased from an interaction between the unpaired electrons in the paramagnetic iron (or gadolinium ions) and the protons in water.
T2 is the “transverse” relaxation time, which is a measure of how long the resonating protons of a substance can be coherent or rotated by a following 90° RF pulse. Depending on whether the protons can align with/against the external magnetic field, the local regions of increased/decreased magnetic fields can be created by the fluctuating magnetic fields of the substance. Following the 90° RF pulse, the protons might lose their coherence and transverse magnetization can be lost. This results in T2 relaxation. The existence of a magnetic contrast agent in the tissue results in short T2.
Positive contrast agents (i.e., those containing gadolinium, manganese, or iron ions) appear brighter on MR images owing to a reduction in T1. By contrast, negative contrast agents (appearing darker on MR images) result in shorter T1 and T2. They are usually small particles and aggregates such as superparamagnetic iron oxide NPs.
Computed tomography (CT) imaging [35]
CT is one of the most heavily utilized clinical imaging modalities. CT uses radiation-rays to create a three-dimensional image by employing tomographic imaging techniques. Because of this, it can quickly image various diseases throughout the entire human body. Contrast in the image between different tissues arises from differential attenuation of X-rays in the body. X-ray attenuation is very efficient in bone, but less so in soft tissues. Therefore, for imaging soft tissues, CT might require a contrast agent to help attenuate X-ray in the vicinity of soft tissues. Although there has been a debate regarding the risk of cancer due to frequent exposure to X-rays, this convenient modality has provided valuable information to help cure disease.
Positron emission tomography (PET) [36]
PET is a clinically used, nuclear imaging technique, and it produces a three-dimensional image of functional processes in the body. The modality detects gamma rays indirectly emitted by a positron-emitting radiotracer (contrast agent). Images of the tracer in the body are reconstructed by computer analysis. Depending on the purpose of imaging, the tracers can be either a biologically active molecule (e.g., fluorodeoxyglucose) or metal isotopes chelate compounds, such as 64Cu-1,4,7,10-tetraazacyclododecane-N,N',N”,N'”-tetraacetic acid (DOTA) chelate [37–40]. Because the metal chelates are generally not considered to be inorganic NPs, we do not give a detailed account on this class of contrast agents. Instead, we will touch on these contrast agents when we discuss the inorganic hybrid NPs for dual imaging modalities.
Shallow-tissue modalities
Optical coherence tomography (OCT) [41]
OCT captures three-dimensional images from optical scattering media (e.g., biological tissue), and it sometimes can provide sub-micrometer resolution. However, the imaging depth in OCT is limited by optical scattering rather than absorption because scattering tends to attenuate and randomize the light. Depending on the wavelength of light, this technique can achieve imaging depths of up to 2 mm in most tissues. This technique has been used clinically for some applications such as eye examination and has been tested in vivo and ex vivo for cancer diagnosis. In order to generate sufficient contrast, the imaging agents for this modality need to have large scattering cross sections.
Photoacoustic tomography (PAT) [26]
PAT is a hybrid imaging modality that provides strong optical absorption contrast and high ultrasonic resolution. Because the spatial resolution beyond one optical transport mean free path (~1 mm) is determined by ultrasonic parameters, the maximum imaging depth and resolution of PAT are scalable when diffusive photons are available. One can greatly increase the penetration depth of PAT with near-infrared light because the optical absorption of hemoglobin and scattering of tissues are weak in this regime. Therefore, a proper combination of PAT with the right contrast agent can accurately detect and diagnose tumors. As in the case of OCT, the imaging depth depends on the wavelength of light, but the imaging depth (~30 mm) is higher than OCT. This modality is currently being evaluated in vivo. Additionally, contrast agents for PAT need to have large absorption cross sections.
Two-photon microscopy [42]
Two-photon microscopy is a fluorescence-based technique that offers images of living tissue up to ~1 mm in depth. It usually uses red-shifted light to minimize scattering in the tissue, and the background signal is strongly suppressed owing to multiphoton absorption. It is being tested in vivo. Recently, Nguyen and colleagues reported that surgery with molecular fluorescence imaging can be efficient for complete removal of tumors [43]. The results might suggest a breakthrough in the application of two-photon microscopy for molecular imaging to overcome the disadvantage of imaging depth.
Surface-enhanced Raman spectroscopy (SERS) imaging [44]
SERS utilizes the enhancement of Raman scattering by molecules adsorbed on surfaces of metal NPs. The increment can be as much as 1014–1015, hence Raman-active dyes placed on the surface of gold and silver colloids will exhibit greatly amplified Raman signals. The ability of gold colloids to easily conjugate with targeting ligands enables the detection of tumors in vivo using this technique [15, 28]. Contrast agents for this modality additionally require Raman active molecules (i.e., SERS probes) on the surface of gold or silver colloids.
Major inorganic nanoparticles for molecular imaging
Among the inorganic NPs displayed in Figure 1a, iron oxide NPs have been used clinically as contrast agents for MR imaging, whereas the other two have been mainly evaluated in the preclinical setting. In addition, recent advances in synthesis have produced many novel NPs with potential applications in molecular imaging and Table 1 shows a partial list of these inorganic NPs. These and additional examples can also be found in The Molecular Imaging and Contrast Agent Database (MICAD, http://www.ncbi.nlm.nih.gov/). In principle, one can easily design a contrast agent based on an inorganic NP with desired properties for a specific imaging modality because the electronic, optical, and magnetic properties of NPs can be tailored and fine tuned by controlling the composition (e.g., metals, semiconductors, and metal oxides), geometry (e.g., size and shape), and structure (e.g., solid, hollow, and core-shell). In this section, we briefly discuss a few examples, including semiconductor QDs and magnetic, gold and rare-earth doped NPs. Specifically, we want to illustrate why these NPs are attractive as contrast agents for a specific imaging modality.
Semiconductor quantum dots
Semiconductor QDs are nanocrystals with typical sizes in the range of 1-10 nm. Unlike the bulk semiconductors, these minuscule structures exhibit discrete energy levels and tunable optical absorption/emission properties that are dependent on their size, shape, and chemical composition [45, 46]. Compared with organic dyes and fluorescent proteins, QDs offer a broader range of emission spectra that cover both the visible and near-infrared (NIR) wavelengths, exhibit larger absorption coefficients, and have much higher photostability [4]. Therefore, QDs have been extensively explored and are commercially available from several companies as contrast agents for fluorescence imaging modalities including both single- and multi-photon microscopy [21–23, 47]. Some QDs can also be applied as contrast agents for photoacoustic imaging [10].
Magnetic nanoparticles
Magnetism is highly sensitive to size and temperature because this property arises from the collective interaction of atomic magnetic dipoles. When a ferro- or ferrimagnetic magnet is reduced to a critical size (rc), it will change from a state that has multiple magnetic domains to one with only one single domain. Below this critical size, the thermal energy becomes comparable to what is needed for spins to flip, leading to randomization of the magnetic dipoles in a short period of time. Such nanoparticles are referred to as superparamagnetic as they do not have permanent magnetic moments in the absence of an external field but can quickly respond to an external magnetic field [48]. Hard (high coercivity) and soft (low coercivity) ferromagnetic materials are characterized by different values of rc: for spherical NPs, the rc is typically 3–4 nm and over 20 nm for very hard and soft magnetic materials, respectively [48].
More than ten types of superparamagnetic NPs are now commercially marketed as contrast agents for MR imaging. The most commonly utilized superparamagnetic NPs are those based on iron oxides, generally smaller than 20–30 nm in size. Iron oxide NPs enhance proton relaxation of specific tissues and can serve as MR contrast agents for clinical diagnosis [49, 50]. Both γ-Fe2O3 (maghemite) and Fe3O4 (magnetite) NPs have been investigated as MR contrast agents for more than two decades and are already approved for clinical use in the Europe [6, 9, 51]. Other types of magnetic NPs have also been prepared and actively explored for MR imaging, including those doped with alternative metals to achieve higher relaxivities than the commercially available varieties (Table 2). Notable examples include doped ferrites such as MnFe2O4, CoFe2O4, NiFe2O4, as well Gd2O3 and gold-coated cobalt NPs [53, 59, 60].
Table 2.
T2 relaxivities of magnetic nanoparticles.1
| Magnetic NPs | Core size (nm) | dh(nm) | T2 | Ref. |
|---|---|---|---|---|
| USPIO, transferrin-coated | 5 | 36 | 52.1 | [52] |
| CLIO | -2 | - | 62 | [53] |
| CLIO, cyclic RGD-modified | 28 | - | 111 | [54] |
| CLIO, scrambled RGD-modified | 36 | - | 118 | |
| Carboxydextran-coated SPIO SHU-555 | - | 62 | [55] | |
| Dextran-coated SPIO AMI-25 | - | 58 | [56] | |
| Dextran-coated SPIO AMI-227 | - | 17–20 | ||
| 2–4 | 33 | 72 | ||
| 6–8 | 46 | 95 | ||
| Dextran-coated iron oxide | 10–15 | 59 | 185 | [57] |
| 15–20 | 75 | 242 | ||
| 20–25 | 91 | 320 | ||
| Chlorotoxin-PEG-iron oxide | 10–15 | - | 5.20 | [58] |
| 9 | - | 265 | ||
| Fe3O4 | 6 | - | 208 | [53] |
| 12 | - | 218 | ||
| MnFe2O4 | 12 | - | 358 | [53] |
| 9 | - | 134 | ||
| CoFe2O4 | 6 | - | 109 | [53] |
| 12 | - | 172 | ||
| NiFe2O4 | 12 | - | 152 | [53] |
| Gold-coated Co NPs | - | - | 107 | [59] |
The unit for T2 is (mM×s)−1
The dimensions were not specified
Symbol and abbreviations: dh, hydrodynamic diameter of the NPs; USPIO, ultra small paramagnetic iron oxide; CLIO, cross-linked iron oxide; SPIO, superparamagnetic iron oxide.
Gold nanoparticles
Gold NPs can show strong extinction peaks in the visible and NIR regions. Such strong extinction peaks originate from the collective oscillations of their conduction electrons in the presence of an incident light, a phenomenon commonly known as localized surface plasmon resonance (LSPR) [7, 8]. Because of LSPR, gold NPs can strongly scatter and/or absorb light, and the magnitude of light being scattered or absorbed is largely determined by their sizes and internal structures (solid vs. hollow) [61]. It is relatively straightforward to control these parameters to tune LSPR properties of gold NPs for a specific imaging modality. For example, gold NPs with relatively large scattering cross sections are ideal for enhancing the contrast in OCT [62] whereas PA imaging requires the use of gold NPs with large absorption cross sections [16, 26, 27]. In addition, gold NPs can have large absorption coefficients against the X-ray source used for CT imaging [13, 14, 31]; exhibit a photoluminescence capability suitable for multi-photon microscopy [29, 30]; and even enhance the intensity of Raman-active molecules for the SERS technique [15, 28]. To date, four different types of gold NPs have been tested in a preclinical setting as contrast agents for molecular imaging: nanospheres [8], nanocages [7], nanorods [8, 15], and nanoshells [63].
Rare-earth doped nanoparticles
Owing to their luminescence properties, rare-earth ions are often used for optoelectronic applications, with notable examples including the Nd:YAG (neodymium-doped yttrium aluminum garnet) laser and Erbium-doped fiber amplifiers used in optical-fiber communication systems. Similar to QDs, rare-earth doped NPs exhibit sharp emission peaks (sharper than QDs), long fluorescence lifetimes, high quantum yields, and excellent photostability. For these reasons, they possess great potential for noninvasive and real-time in vivo diagnosis of disease [32, 33]. The synthesis and optical properties of rare-earth doped NPs have been actively studied and their application in biomedicine is being explored and expanded [64, 65].
Surface modification of inorganic nanoparticles
The surfaces of inorganic NPs often need to be modified: i) to allow transfer from a nonhydrolytic solvent into an aqueous medium; ii) to increase the circulation time in the blood stream by adding poly(ethylene glycol) (PEG) coating, or PEGylation; iii) to enhance the targeting efficiency via conjugation of binding ligands; and iv) to increase the functionality via addition of other components including drugs. Three important issues must be taken into consideration when modifying the surfaces of inorganic NPs. First, the physicochemical properties of the NPs required for a specific imaging modality should not be significantly altered during the modification. If there is extensive aggregation between the NPs or substantial changes to their sizes, shapes, and morphologies, their optical or magnetic properties could be shifted or completely destroyed. Secondly, the molecular structure and conformation of the targeting ligand should be preserved. This could be a major problem for macromolecules such as proteins and antibodies because they can be easily denatured during conjugation [66–68], and yet the interaction between a ligand and the receptor on the surface of a cell is extremely sensitive to their chemical and molecular structures [69, 70]. Finally, the surface-modified NPs should be nontoxic for use in biological applications. An organic coating can improve the biocompatibility of inorganic NPs and facilitate a rapid clearance of the NPs from the body, but an unwise choice of an organic layer could also cause the NPs to be cleared from the blood prior to reaching the site of interest. The biocompatibility and toxicity issues surrounding inorganic NPs will be discussed later. In general, every step of surface modification requires careful monitoring to avoid possible aggregation of the NPs and potential denaturation of the targeting ligands, as well as to ensure biocompatibility.
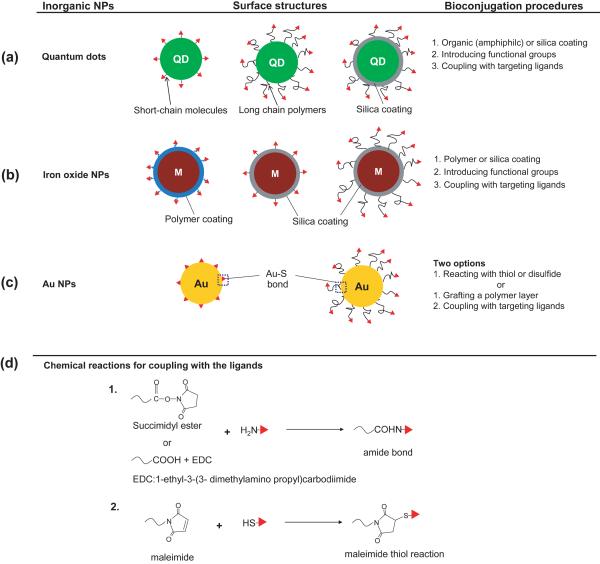
Figure 2 shows examples of QDs, iron oxide NPs, and gold colloids whose surfaces have been conjugated with targeting ligands for molecular imaging. Most modifications require more than two steps to attach targeting ligands; some also need pretreatment with an organic or inorganic coating to ensure a good stability for the NPs in a buffer solution and to facilitate the conjugation of targeting ligands [4, 71]. Amphiphilic lipids (e.g., fatty acids) and polymers are useful in stabilizing QDs and iron oxide NPs as they can form self-assembled structures on the surfaces of NPs through hydrophobic interactions [21, 51, 72, 73]. Such treatments can often supply chemical groups for further coupling with the targeting ligands. Coating the surfaces of NPs with silica shells is another way to enhance the stability of these NPs in aqueous media [74–76]. In this case, organic molecules with various functional groups can be readily attached to the silica surface using the siloxane monolayer chemistry [77, 78]. Among various types of inorganic NPs, the surfaces of gold colloids can be most easily and reproducibly modified with organic molecules through the well-established, controllable, and robust monolayer chemistry based on gold-thiolate bonding [79]. When the targeting ligand contains a thiol or disulfide group, it can be covalently conjugated to the surface of a gold NP at room temperature [80]. Prior to bioconjugation, it is beneficial to introduce a stabilizing layer such as PEG chains of various lengths to help maintain the original conformations of the targeting ligands, enhance the stability of gold colloids, and to prolong the circulation times of the resultant conjugates in the blood stream [7, 8, 16, 63].
Figure 2.
Strategies for the bioconjugation of targeting ligands to the surfaces of inorganic NPs. (a) QDs and (b) magnetic NPs are typically hydrophobic or easy to aggregate owing to their magnetic properties, but surface coating is an essential step to ensure their stability and facilitate their conjugation with targeting ligands. Surface coating can be generally achieved using amphiphilic molecules or inorganic materials (e.g. silica), and the targeting ligands can then be added through coupling reactions. (c) The bioconjugation of gold NPs with targeting ligands is easier than the other two types of NPs owing to their ability to form gold–thiolate bonding. As such, the target ligands can be directly coupled to the surfaces of Au NPs or conjugated to the surface of an organic layer (e.g. PEG) that is chemically grafted to the Au surface. (d) Typical chemical reactions involved in coupling the targeting ligands to the NPs. In general, one can use the primary amine in a protein or peptide to react with succinimidyl esters or carboxylic acid with the aid of EDC. Alternatively, if the targeting ligands have thiol groups they can be attached to the NPs via the maleimide–thiol reaction.
Figure 2d shows schematics of three typical reactions between the targeting ligands and the organic groups anchored to the surface of NPs. Because the most commonly used targeting ligands are based on antibodies and peptides, amine groups at the amino termini or thiol groups in moieties such as cysteine can be directly employed for bioconjugation through the following coupling reactions: succimidylester-amine, carboxylic acid-amine by means of 1-ethyl-3-(3-dimethylamino propyl)carbodiimide (EDC), and maleimide-thiol.
Delivery and targeting of inorganic nanoparticles
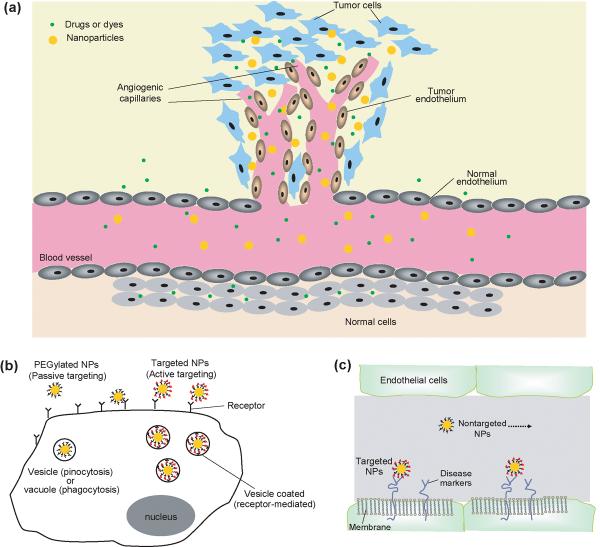
Nanoparticles provide a range of advantages over molecular contrast agents traditionally used for molecular imaging. For example, the differences in vasculatures between normal and cancerous tissues enhance the accumulation of NPs in solid tumors (Figure 3a). Endothelial cells in normal tissues are well aligned and closely packed, whereas those in solid tumors are relatively leaky [81–83]. Because of their large sizes, inorganic NPs circulating in the bloodstream cannot easily enter normal tissues yet they can leak into tumor tissues through the dilated blood vessels. This is in contrast to small molecules that enter both normal and cancerous tissues, attenuating their utility for imaging contrast enhancement. The selective accumulation of inorganic NPs in cancerous tissues is commonly known as the enhanced permeation and retention (EPR) effect or passive targeting, and this phenomenon has been extensively explored for the systemic delivery of NPs and macromolecular drugs [17, 18, 84]. Strictly speaking, passive targeting should not be considered as a mechanism of molecular imaging as no specific molecular interaction is involved in the targeting process.
Figure 3.
The delivery of inorganic NPs to the site of interest. (a) A schematic showing the enhanced permeation and retention effect in selectively delivering NPs to a tumor site. In contrast to normal tissue, the vasculature in a tumor is leaky and the cancer cells are less densely packed owing to their tendencies to grow fast. This allows NPs to enter the tumor tissue more easily than they can the normal tissue. This effect is also known as “passive targeting.” (b) The comparison of delivery with targeted NPs for active targeting with nontargeted (typically PEGylated) NPs for passive targeting. When the surfaces of NPs are modified with PEG, the NPs are hardly taken up by cells owing to the antifouling property of PEG. By comparison, NPs modified with target ligands can bind to the receptors on the surfaces of cells and thereby facilitate the internalization of NPs by cells. (c) When NPs are used to detect a disease other than cancer, NPs bearing target ligands can preferentially bind to the diseased site relative to the nontargeted NPs, and thereby the targeted NPs will provide better contrast enhancement.
Without targeting ligands, as in the case of PEGylated NPs, the NPs accumulated in the tumor can leach out with time. To maintain the NPs in the tumor and to further increase the efficiency of delivery, it is necessary for them to adhere to or enter the cancer cells once they have reached the tumor site (Figure 3b). To this end, targeting ligands that can selectively bind to the receptors typically overexpressed on the surfaces of cancer cells can be used. Because the interactions between the targeting ligands and the receptors can be highly selective, NPs bearing the targeting ligands should be retained preferentially by the cancer cells. This phenomenon is commonly referred to as active targeting, which can be combined with passive targeting to achieve maximum localization of inorganic NPs in malignant tissues for the maximal enhancement of contrast. Similar mechanism can also be applied for molecular imaging of other diseases (Figure 3c) [85].
It is important to choose a proper targeting ligand to maximize the performance of a NP-based contrast agent for molecular imaging. A summary of the targeting ligands that have been examined for cancer detection is presented in Table 3 [21, 27, 52–54, 86–104]. Ideally, the targeting ligand should bind exclusively to a receptor on a specific type of cancer cell. Notable examples include both mono- or polyclonal antibodies, antibody of the prostate specific membrane antigen (PSMA) for prostate cancer, and α-melanocyte stimulating hormone (α-MSH) for melanoma. Some targeting ligands, such as RGD (arginine-glycine-aspartate) peptides and folic acid, can bind to many types of cancer cells. Therefore, the identification of a targeting ligand is probably the most important step in the design and development of contrast agents for molecular imaging.
Table 3.
Targeting ligands used for cancer diagnosis.
| Type of cancer | Deaths1 | Receptor | Targeting ligands | Ref. |
|---|---|---|---|---|
| Brain | 12,920 | VEGF | VEGF protein | [86] |
| αvβ3 integrin | RGD peptide | [87,88] | ||
| Nucleolin | F3 peptide | [89] | ||
| MMP-2 | Chlorotoxin | [90] | ||
| Breast | 40,610 | HER2 | Monoclonal antibody | [25,53,91] |
| αvβ3 integrin | RGD peptide | [54] | ||
| Transferrin | Transferrin protein | [52] | ||
| Tumor vasculature | CREKA peptide | [92,93] | ||
| Colon | 49,920 | Mucin-1 | EPPT peptide | [94] |
| Fibrosarcoma | 1,470 | Tumor vasculature | CREKA peptide | [92] |
| Ovarian | 14,600 | αvβ3 integrin | RGD peptide | [95] |
| Pancreatic | 35,240 | Mucin-1 | EPPT peptide | [96] |
| Prostate | 27,360 | PSMA | Monoclonal antibody | [21] |
| Hepsin | Peptide | [97] | ||
| Squamous cell carcinoma | 11,590 | αvβ3 integrin | RGD peptide | [98] |
| Melanoma | 8,650 | αvβ3 integrin | RGD peptide | [99,100] |
| α-MSH receptor | α-MSH | [27,101] | ||
| All cancers | Folic acid | [102–104] |
Annual estimated death in the US (2009).
Abbreviation: VEGF, vascular endothelial growth factor; MMP-2, matrix metalloproteinase 2; HER2, human epidermal growth factor receptor 2; PSMA, prostate-specific membrane antigen; α-MSH, α-melanocyte stimulating hormone.
Although most of these targeting ligands have been proven to be effective in vitro, the binding capability and efficiency of ligands bound to NPs in vivo is often a different story. This is because the targeted NPs will encounter the reticuloendothelial system (RES) and interact with many types of cells during circulation in the body. During these interactions, the NPs can be cleared by or accumulated in tissues and organs before reaching the site of interest. Therefore, it is of great importance to evaluate and report in vivo targeting efficiency in addition to collecting in vitro data for a targeted NP. Table 4 summarizes the in vivo targeting efficacy of ligands frequently used with inorganic NPs. Although these data are qualitative, they might provide some guidance for choosing the optimum ligand to target various types of cancers.
Table 4.
In vivo targeting efficiency of target-specific, ligand-conjugated inorganic NPs.
| NPs | Targeting ligands | Cell line | Analysis | Summary of results | Ref. |
|---|---|---|---|---|---|
| QD | RGD | U87MG | Fluorescence | 4.4-times higher in signal than the nonspecific QD | [87] |
| Cu64-QD | VEGF | U87MG | PET imaging | 3.8-times higher in accumulation than the nonspecific NPs. | [86] |
| RGD | U87MG | 3.6-times higher in intensity than the nonspecific NPs | [88] | ||
| SPIO | HER2 | NIH3T6.7 | MRI | 80% reduction in T2 compared with the nonspecific SPIO | [25] |
| Transferrin | SMT/2A | 30% reduction in T2 than the nonspecific SPIO | [52] | ||
| RGD | HatCat-ras-A-5RT3 | 25% reduction in T2 than nonspecific SPIO | [98] | ||
| A431 | 10% reduction in T2 than nonspecific SPIO | [98] | |||
| Gold nanorods | EGFR | Ca127 | PAT | 2.8-times higher in PA signal than the nonspecific nanorods | [16] |
| HER2 | OECM1 | 2-times higher in PA signal than the nonspecific nanorods | [16] | ||
| Gold nanocages | α-MSH receptor | B16 | PAT | 3.6-times higher in PA signal than the nonspecific nanocages | [27] |
Abbreviation: SPIO, superparamagnetic iron oxide.
Biodistribution, clearance, and toxicity of inorganic nanoparticles
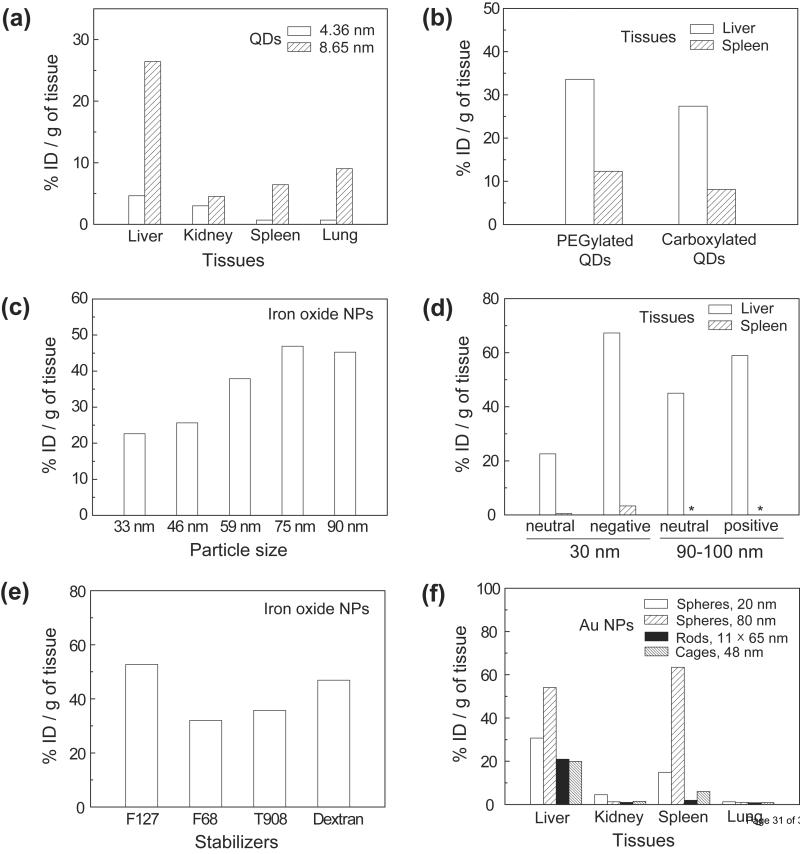
In reality, it is impossible to have all injected NPs accumulated at the site of disease. As such, the biodistribution of inorganic NPs has to be considered and optimized from the very beginning. Typically, inorganic NPs administered intravenously are mostly taken up by liver and spleen, with the amount of accumulated particles depending on the particle size, shape, and surface chemistry. There are several reports on the biodistribution of QDs, iron oxide NPs, and gold colloids (Figure 4) [57, 105–109]. The amount of QDs that accumulate in different organs depends on the size when spherical particles were used (Figure 4a) [106] and the amount of gold colloids accumulated in different organs differs substantially depending on the geometries of the particles (Figure 4f) [106–108]. In addition, it has been reported that the biodistributions of QDs and iron oxide NPs might differ depending on the charges and functional groups on their surfaces [57, 109].
Figure 4.
The biodistributions of inorganic NPs after intravenous injections into mice. (a) The biodistributions of QDs with two different sizes. (The figure was replotted with the permission of [105].) (b) The biodistribution of QDs covered with different functional groups. (The figure was replotted with the permission of [109].) (c) The amount of dextran-coated iron oxide NPs accumulated in the liver as a function of their sizes. (d) The biodistribution of iron oxide NPs with different surface charges. (e) The amount of iron oxide NPs accumulated in the liver when their surfaces were coated with different functional groups. (The figures in (c–e) were replotted with the permission of [57].) (f) The biodistributions of Au colloids with different geometries. The dimensions of the Au nanospheres, nanorods and nanocages are labeled as diameter, width × length and outer edge length, respectively. (The figure was replotted with the permission of [106–108].)
For all types of inorganic NPs, clearance and toxicity need to be completely evaluated before they can move to clinical development. Ideally, the contrast agents would be nontoxic while in the body and then completely cleared. Clearance usually proceeds through the kidney and liver, depending on the particle size [105, 110]. Clearance of inorganic NPs via the kidney is preferred because it rapidly removes NPs from vascular compartments in their original forms. In addition, this process involves minimal intracellular catabolism, which might cause cytotoxicity. The slit diaphragms between the “foot processes” of the podocytes in the kidney are roughly 43 nm in size [108], but the functional pore size of these diaphragms is much smaller, typically less than 5 nm [109]. These diaphragms limit the size of NPs that can be excreted through the kidney.
Gold colloids used as contrast agents for X-ray and CT imaging can be as small as 2 nm in size; hence, they can be cleared mostly by renal excretion [14]. Although the core sizes of QDs are usually less than 10 nm, their hydrodynamic diameters could be much larger than the functional pore size of the diaphragms in a kidney [21–23, 105]. Most gold colloids being evaluated as optical contrast agents are larger than 10 nm in hydrodynamic diameter [7, 8, 63]. The sizes of iron oxide NPs used for MR imaging are usually larger than the pore size of diaphragms, but these NPs can be cleared from the body via other routes such as RES [71] or by degradation [113, 114]. If inorganic NPs are not degraded into ionic forms or small clusters (subnanometer to a few nanometers in size), they might remain in the body for long times. In this case, the spleen and liver are the primary homes for the accumulated NPs. The mechanism of liver clearance is more complicated than that of renal excretion, and most inorganic NPs cannot be degraded and cleared through this mechanism.
The toxicity of inorganic NPs is another critical issue that might hinder their application in biomedical imaging. A recent review by Nel and colleagues suggested that nanomaterials can influence a living organism through different biological pathways [115]. Although QDs affect both cells and tissues, there are some reports claiming that QDs are not significantly toxic [116, 117]. This discrepancy largely results from the diversity of materials being used; hence, toxicity should be studied with QDs having well-defined physicochemical properties [116, 118]. Some QDs might become cytotoxic only after oxidative and/or photolytic destruction of their surface coatings [116]. Iron oxide NPs and gold colloids seem to be less of a concern in terms of toxicity. Iron oxide can be cleared from the body via various routes with minimal toxicity [71, 113, 114], and gold is bio-inert. For these reasons, several products based on iron oxide and gold NPs are in clinical use or have been approved by the US Food and Drug Administration for phase I clinical trials (see, for example, www.clinicaltrials.gov). To date the toxicity of inorganic NPs has mostly been assessed by nanotechnology researchers; hence a systematic study involving biologists is necessary to reach conclusions regarding the toxicity of inorganic NPs.
Taken together, the clearance and toxicity issues can be a major bottleneck for the clinical use of inorganic NPs as contrast agents. However, it is a trade-off between toxicity and overall health benefit. It remains an urgent and important task to explore new contrast agents based on inorganic NPs for early and accurate detection of diseases even though there is uncertainty about their potential clearance and toxicity.
To avoid the long-term accumulation and possible toxicity of inorganic NPs, it is important to use a minimal dose of NPs for imaging and/or treatment applications. There are several strategies to achieve this goal, with notable examples including: i) development of inorganic NPs with optimized optical, fluorescence, and magnetic properties or having a combination of at least two functions for multi-modal imaging; and ii) improvement of the selectivity and sensitivity of contrast agents by both optimizing their physical properties and by introducing highly selective targeting ligands. Chemists are currently working on the first approach, and inorganic NPs are engineered with state-of-the-art technologies for maximum signal enhancement for each respective imaging modality. As a notable example of an MR imaging contrast agent, as shown in Table 2, Bouchard and colleagues recently developed gold-coated cobalt NPs with T2 relaxivities over 105 times higher than the commercially available iron oxide NPs [59]. Lee and colleagues also tried to combine various types of magnetic elements with iron oxide NPs to improve the relaxivity of established contrast agents [53]. For the optical contrast agents, increasing the scattering and/or absorption cross sections by engineering the internal structures of gold colloids has been a fruitful approach [61]. However, it is important for chemists, biologists, and biomedical scientists to work together in an effort to solve the problem of selective targeting.
Multimodal imaging with inorganic nanoparticles
In order to diagnose malignancy accurately and in an early stage, it is often necessary to use a combination of different imaging modalities simultaneously. As such, there has been a strong interest in developing new imaging techniques based on optical and spectroscopic mechanisms. Notable examples include OCT, PAT, SERS, and multi-photon microscopy. To keep pace with these emerging techniques, inorganic NPs need to be engineered with a variety of properties, and in some cases, they also need to enhance the signals coming from other contrast agents (e.g., organic dyes used for SERS) included in the NPs. In addition, it is often required that the inorganic NPs have at least two different properties for multimodal imaging. Some NPs, such as QD and gold NPs, can exhibit more than two different properties, making them well-suited for multimodal imaging. In addition, inorganic hybrid NPs have been designed for in vivo multimodal imaging (Figure 5) [119, 120].
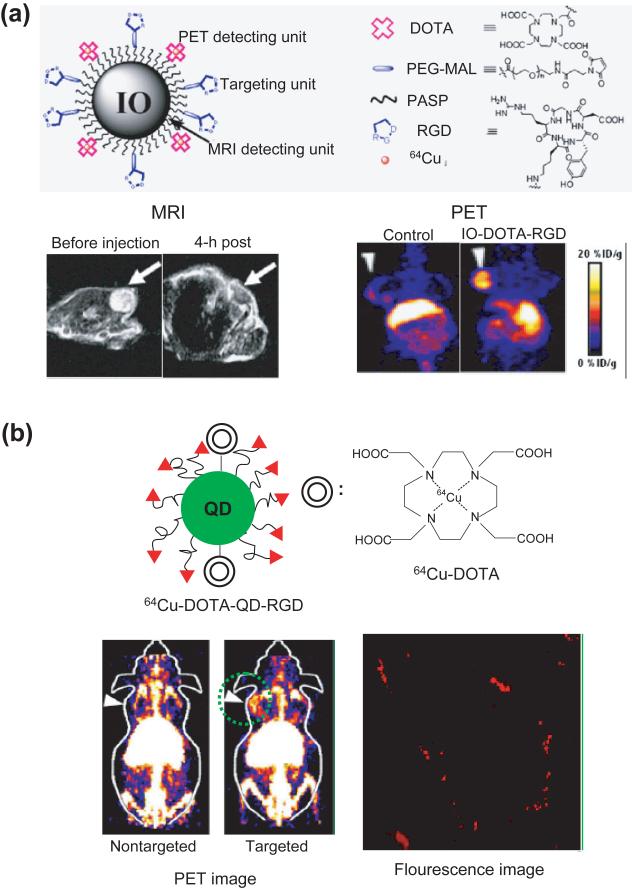
Figure 5.
Inorganic NPs as contrast agents for multimodal imaging. (a) Top: schematic showing iron oxide (IO) NPs conjugated with DOTA and RGD for MR imaging and PET. Bottom: MR (left) and PET images (right) after the intravenous injection of the IO-DOTA-RGD. Arrows indicate the tumors in nude mice. (Reproduced with the permission of [119].) (b) Top: schematic showing a QD conjugated with DOTA and RGD for PET and fluorescence imaging. Bottom: PET and fluorescence images after the intravenous injection of the DOTA-QD-RGD NPs. Arrows indicate the tumors in nude mice and the fluorescence image was taken from the circled region in the PET image. (Reproduced with the permission of [120].)
The combination of more than two imaging modalities would be beneficial if the two (or more) imaging modalities provide different information about the diseased site. For example, PET can provide a functional data for the tumor while CT and MR imaging offer anatomic information of the body. Therefore, a combination of PET and CT/MR can provide accurate information about the tumor including its location and boundary. For PET and MR imaging, the contrast agents are made of metal (or metal oxide) and radiometal-chelate compounds (Figure 5a) [119, 121]. QDs can also be combined with the radiometal chelates as a dual contrast agent for both fluorescence and PET imaging, as shown in Figure 5b [120]. Several other multifunctional NPs have also been developed as contrast agents for multimodal imaging. These include gold-coated QDs for optical plus fluorescence imaging [122], gold-coated iron oxide NPs for optical imaging and MRI [123], and gold NPs conjugated with Gd-chelate compounds for X-ray imaging and MRI (in vivo) [124], and QDs conjugated with Gd-chelate compounds for fluorescence imaging and MRI (in vivo) [125]. Silica particles loaded with QDs and iron oxide NPs have also been constructed for both MRI and fluorescence imaging [76].
Concluding remarks
Many types of inorganic NPs have been proposed and evaluated as potential contrast agents for molecular imaging, but most of them are still being tested in vitro or in vivo with small animals. Although a few of them (e.g., iron oxide NPs for MR imaging) have been approved for clinical use, most are still in development and will need to solve the potential toxicity issue. It is expected that some of the inorganic NPs (e.g., gold colloids) will overcome these hurdles and eventually move into the clinical phase. In addition, recent demonstrations applying similar NPs in new therapeutic settings (e.g., magnetic hyperthermia treatment [126], photothermal therapy [127], and controlled release of drugs [128]) will likely push the field of inorganic NPs into new directions, including their use as theranostic agents for both diagnosis and treatment.
Acknowledgements
Our research in this field has been supported in part by an National Institutes of Health (NIH) Director's Pioneer Award (DP1 OD000798), an NIH research grant (1R01 CA138527) from the NCI, and startup funds from Washington University in St. Louis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weissleder R, Mahmood U. Molecular imaging. Radiology. 2001;219:316–333. doi: 10.1148/radiology.219.2.r01ma19316. [DOI] [PubMed] [Google Scholar]
- 2.Achilefu S. Introduction to concept and strategies for molecular imaging. Chem. Rev. 2010;110:2575–2578. doi: 10.1021/cr1001113. [DOI] [PubMed] [Google Scholar]
- 3.Licha K. Contrast agents for optical imaging. Topics Curr. Chem. 2002;222:1–29. [Google Scholar]
- 4.Gao X, et al. In vivo molecular and cellular imaging with quantum dots. Curr. Opin. Biotechnol. 2005;16:63–72. doi: 10.1016/j.copbio.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Erdi YM. The use of PET for radiotherapy. Curr. Medical Imaging Rev. 2007;3:3–16. [Google Scholar]
- 6.Lanza GM, et al. Magnetic resonance molecular imaging with nanoparticles. Journal of Nuclear Cardiology. 2004;11:733–743. doi: 10.1016/j.nuclcard.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Skrabalak SE, et al. Gold nanocages: synthesis, properties, and applications. Acc. Chem. Res. 2008;41:1587–1595. doi: 10.1021/ar800018v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murphy CJ, et al. Gold nanoparticles in biology: beyond toxicity to cellular imaging. Acc. Chem. Res. 2008;41:1721–1730. doi: 10.1021/ar800035u. [DOI] [PubMed] [Google Scholar]
- 9.Thorek DL, et al. Superparamagnetic iron oxide nanoparticle probes for molecular imaging. Annal. Biomed. Eng. 2006;34:23–38. doi: 10.1007/s10439-005-9002-7. [DOI] [PubMed] [Google Scholar]
- 10.Shashkov EV, et al. Quantum dots as multimodal photoacoustic and photothermal contrast agents. Nano Lett. 2008;8:3953–3958. doi: 10.1021/nl802442x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farrer RA, et al. Highly efficient multiphoton-absorption-induced luminescence from gold nanoparticles. Nano Lett. 2005;5:1139–1142. doi: 10.1021/nl050687r. [DOI] [PubMed] [Google Scholar]
- 12.Dulkeith E, et al. Plasmon emission in photoexcited gold nanoparticles. Phys. Rev. B. 2004;70:205424. [Google Scholar]
- 13.Popovtzer R, et al. Targeted gold nanoparticles enable molecular CT imaging of cancer. Nano Lett. 2008;8:4593–4596. doi: 10.1021/nl8029114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hainfeld JF, et al. Gold nanoparticles: a new X-ray contrast agent. British J. Radiol. 2006;79:248–253. doi: 10.1259/bjr/13169882. [DOI] [PubMed] [Google Scholar]
- 15.Qian X, et al. In vivo tumor targeting and spectroscopic detection with surface-enhanced raman nanoparticle tags. Nature Biotechnol. 2008;26:83–90. doi: 10.1038/nbt1377. [DOI] [PubMed] [Google Scholar]
- 16.Li PC, et al. In vivo photoacoustic molecular imaging with simultaneous multiple selective targeting using antibody-conjugated gold nanorods. Opt. Express. 2008;16:18605–18615. doi: 10.1364/oe.16.018605. [DOI] [PubMed] [Google Scholar]
- 17.Iyer AK, et al. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov. Today. 2006;11:812–818. doi: 10.1016/j.drudis.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Ghosh P, et al. Gold nanoparticles in delivery applications. Adv. Drug Deliv. Rev. 2008;60:1307–1315. doi: 10.1016/j.addr.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 19.van Tilborg GAF, et al. Annexin A5-functionalized bimodal lipid-based contrast agents for the detection of apoptosis. Bioconjugate Chem. 2006;17:741–749. doi: 10.1021/bc0600259. [DOI] [PubMed] [Google Scholar]
- 20.Karathanasis E, et al. Imaging nanoprobe for prediction of outcome of nanoparticle chemotherapy by using mammography. Radiology. 2009;250:398–406. doi: 10.1148/radiol.2502080801. [DOI] [PubMed] [Google Scholar]
- 21.Gao X, et al. In vivo cancer targeting and imaging with semiconductor quantum dots. Nature Biotechnol. 2004;22:969–976. doi: 10.1038/nbt994. [DOI] [PubMed] [Google Scholar]
- 22.Allen PM, et al. InAs(ZnCdS) quantum dots optimized for biological imaging in the near-Infrared. J. Am. Chem. Soc. 2009;132:470–471. doi: 10.1021/ja908250r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao J, et al. Ultrasmall near-infrared non-cadmium quantum dots for in vivo tumor imaging. Small. 2010;6:256–261. doi: 10.1002/smll.200901672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heesakkers RA, et al. MRI with a lymph-node-specific contrast agent as an alternative to CT scan and lymph-node dissection in patients with prostate cancer: a prospective multicohort study. Lancet Oncol. 2008;9:850–856. doi: 10.1016/S1470-2045(08)70203-1. [DOI] [PubMed] [Google Scholar]
- 25.Huh Y-M, et al. In Vivo magnetic resonance detection of cancer by using multifunctional magnetic nanocrystals. J. Am. Chem. Soc. 2005;127:12387–12391. doi: 10.1021/ja052337c. [DOI] [PubMed] [Google Scholar]
- 26.Yang X, et al. Nanoparticles for photoacoustic imaging. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology. 2009;1:360–368. doi: 10.1002/wnan.42. [DOI] [PubMed] [Google Scholar]
- 27.Kim C, et al. In vivo molecular photoacoustic tomography of melanomas targeted by bio-conjugated gold nanocages. ACS Nano. 2010;4:4559–4564. doi: 10.1021/nn100736c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zavaleta CL, et al. Multiplexed imaging of surface enhanced raman scattering nanotags in living mice using noninvasive raman spectroscopy. Proc. Natl. Acad. Sci. 2009;106:13511–13516. doi: 10.1073/pnas.0813327106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tong L, et al. Bright three-photon luminescence from gold/silver alloyed nanostructures for bioimaging with negligible photothermal toxicity. Angew. Chem. Int. Ed. 2010;49:3485–3488. doi: 10.1002/anie.201000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park J, et al. Two-photon-induced photoluminescence imaging of tumors using near-infrared excited gold nanoshells. Opt. Exp. 2008;16:1590–1599. doi: 10.1364/oe.16.001590. [DOI] [PubMed] [Google Scholar]
- 31.Maltzahn GV, et al. Computationally guided photothermal tumor therapy using long-circulating gold nanorod antennas. Cancer Res. 2009;69:3892–3900. doi: 10.1158/0008-5472.CAN-08-4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nyk M, et al. High contrast in vitro and in vivo photoluminescence bioimaging using near infrared to near infrared up-conversion in Tm3+ and Yb3+ doped fluoride nanophosphors. Nano Lett. 2008;8:3834–3838. doi: 10.1021/nl802223f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou J, et al. Dual-modality in vivo imaging using rare-earth nanocrystals with near-infrared to near-infrared (NIR-to-NIR) upconversion luminescence and magnetic resonance properties. Biomaterials. 2010;31:3287–3295. doi: 10.1016/j.biomaterials.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 34.Hashemi RH, et al. MRI: The basics. 3rd Ed Lipincott Williams and Wilkins; 2010. [Google Scholar]
- 35.Herman GT. Fundamentals of computerized tomography: Image reconstruction from projection. 2nd Ed. Springer; 2009. [Google Scholar]
- 36.Phelps ME. PET: physics, instrumentation, and scanners. Springer; 2006. [Google Scholar]
- 37.Rodriguez-Porcel M, et al. Imaging of VEGF receptor in a rat myocardial infarction model using PET. J. Nucl. Med. 2008;49:667–673. doi: 10.2967/jnumed.107.040576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olafsen T, et al. Optimizing radiolabeled engineered anti-p185HER2 antibody fragments for in vivo imaging. Cancer Res. 2005;65:5907–5916. doi: 10.1158/0008-5472.CAN-04-4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li WP, et al. Receptor-binding, biodistribution, and metabolism studies of 64Cu-DOTA-cetuximab, a PET-imaging agent for epidermal growth-factor receptor-positive tumors. Cancer Biotherapy Radiopharmaceuticals. 2008;23:158–171. doi: 10.1089/cbr.2007.0444. [DOI] [PubMed] [Google Scholar]
- 40.Niu G, et al. Monitoring therapeutic response of human ovarian cancer to 17-DMAG by noninvasive PET imaging with 64Cu-DOTA-trastuzumab. Eur. J. Nucl. Med. Mol. Imaging. 2009;36:1510–1519. doi: 10.1007/s00259-009-1158-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fujimoto JG, et al. Optical biopsy and imaging using optical coherence tomography. Nature Med. 1995;1:970–972. doi: 10.1038/nm0995-970. [DOI] [PubMed] [Google Scholar]
- 42.Denk W, et al. Two-photon laser scanning fluorescence microscopy. Science. 1990;248:73–76. doi: 10.1126/science.2321027. [DOI] [PubMed] [Google Scholar]
- 43.Nguyen QT, et al. Surgery with molecular fluorescence imaging using activatable cell penetrating peptides decreases residual cancer and improves survival. Proc. Natl. Acad. Sci. 2010;107:4317–4322. doi: 10.1073/pnas.0910261107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nie S, Emory SR. Probing single molecules and single nanoparticles by surface-enhanced Raman scattering. Science. 1997;275:1102–1106. doi: 10.1126/science.275.5303.1102. [DOI] [PubMed] [Google Scholar]
- 45.Smith AM, Nie S. Semiconductor nanocrystals: structure, properties, and band gap engineering. Acc. Chem. Res. 2010;43:190–200. doi: 10.1021/ar9001069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alivisatos AP. Perspectives on the physical chemistry of semiconductor nanocrystals. J. Phys. Chem. 1996;100:13226–13239. [Google Scholar]
- 47.Chan WCW, et al. Luminescent quantum dots for multiplexed biological detection and imaging. Curr. Opin. Biotechnol. 2002;13:40–46. doi: 10.1016/s0958-1669(02)00282-3. [DOI] [PubMed] [Google Scholar]
- 48.Jeong U, et al. Superparamagnetic colloids: controlled synthesis and niche application. Adv. Mater. 2007;19:33–60. [Google Scholar]
- 49.Lin W, et al. Magnetic nanoparticles for early detection of cancer by magnetic resonance imaging. MRS Bulletin. 2009;34:441–448. doi: 10.1557/mrs2009.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun C, et al. Magnetic nanoparticles in MR imaging and drug delivery. Adv. Drug Deliv. Rev. 2008;60:1252–1265. doi: 10.1016/j.addr.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weissleder R, et al. Long-circulating iron oxides for MR imaging. Adv.Drug Deliv. Rev. 1995;16:321–334. [Google Scholar]
- 52.Kresse M, et al. Targeting of ultrasmall superparamagnetic iron oxide (USPIO) particles to tumor cells in vivo by using transferrin receptor pathways. Mag. Resonance Med. 1998;40:236–242. doi: 10.1002/mrm.1910400209. [DOI] [PubMed] [Google Scholar]
- 53.Lee J-H, et al. Artificially engineered magnetic nanoparticles for ultra-sensitive molecular imaging. Nature Med. 2007;13:95–99. doi: 10.1038/nm1467. [DOI] [PubMed] [Google Scholar]
- 54.Montet X, et al. Nanoparticle Imaging of Integrins on Tumor Cells. Neoplasia. 2006;8:214–222. doi: 10.1593/neo.05769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reimer P, Balzer T. Ferucarbotran (Resovist): a new clinically approved RES-specific contrast agent for contrast-enhanced MRI of the liver: properties, clinical development, and applications. Eur. Radiol. 2003;13:1266–1276. doi: 10.1007/s00330-002-1721-7. [DOI] [PubMed] [Google Scholar]
- 56.Jung CW, Jacobs P. Physical and chemical properties of superparamagnetic iron oxide MR contrast agents: ferumoxides, ferumoxtran, ferumoxsil. Magn. Reson. Imaging. 1995;13:661–674. doi: 10.1016/0730-725x(95)00024-b. [DOI] [PubMed] [Google Scholar]
- 57.Choulyt D, et al. Development of superparamagnetic nanoparticles for MRI: effect of particle size, charge and surface nature on biodistribution. J. Microencapsulation. 1996;13:245–255. doi: 10.3109/02652049609026013. [DOI] [PubMed] [Google Scholar]
- 58.Sun C, et al. In vivo MRI detection of gliomas by chlorotoxin-conjugated superparamagnetic nanoprobes. Small. 2008;4:372–379. doi: 10.1002/smll.200700784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bouchard L-S, et al. Picomolar sensitivity MRI and photoacoustic imaging of cobalt nanoparticles. Proc. Natl. Acad. Sci. 2009;106:4085–4089. doi: 10.1073/pnas.0813019106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bridot J-L, et al. Hybrid gadolinium oxide nanoparticles: multimodal contrast agents for in vivo imaging. J. Am. Chem. Soc. 2007;129:5076–5084. doi: 10.1021/ja068356j. [DOI] [PubMed] [Google Scholar]
- 61.Cho EC, et al. Measuring the optical absorption cross sections of Au-Ag nanocages and Au nanorods by photoacoustic Imaging. J. Phy. Chem. C. 2009;113:9023–9028. doi: 10.1021/jp903343p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen J, et al. Gold nanocages: bioconjugation and their potential use as optical contrast agents. Nano Lett. 2005;5:473–477. doi: 10.1021/nl047950t. [DOI] [PubMed] [Google Scholar]
- 63.Lal S, et al. Nanoshell-enabled photothermal cancer therapy: impending clinical impact. Acc. Chem. Res. 2008;41:1842–1851. doi: 10.1021/ar800150g. [DOI] [PubMed] [Google Scholar]
- 64.Beaurepaire E, et al. Functionalized fluorescent oxide nanoparticles: artificial toxins for sodium channel targeting and imaging at the single-molecule level. Nano Lett. 2004;4:2079–2084. [Google Scholar]
- 65.Bunzli J-CG. Lanthanide luminescence for biomedical analyses and imaging. Chem. Rev. 2010;110:2729–2755. doi: 10.1021/cr900362e. [DOI] [PubMed] [Google Scholar]
- 66.Haynes CA, Norde W. Globular proteins at solid/liquid interfaces. Colloid Sur. B. 1994;2:517–566. [Google Scholar]
- 67.Gray JJ. The interaction of proteins with solid surfaces. Curr. Opin. Struct. Biol. 2004;14:110–115. doi: 10.1016/j.sbi.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 68.Sigal GB, et al. Effect of surface wettability on the adsorption of proteins and Detergents. J. Am. Chem. Soc. 1998;120:3464–3473. [Google Scholar]
- 69.Vajda S, et al. Effect of conformational flexibility and solvation on receptor-ligand binding free energies. Biochemistry. 1994;33:13977–13988. doi: 10.1021/bi00251a004. [DOI] [PubMed] [Google Scholar]
- 70.Hruby VJ, et al. Emerging approaches in the molectilar design of receptor-selective peptide ligands: conformational, topographical and dynamic considerations. Biochem. J. 1990;268:249–262. doi: 10.1042/bj2680249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Corot C, et al. Recent advances in iron oxide nanocrystal technology for medical imaging. Adv. Drug Deliv. Rev. 2006;58:1471–1504. doi: 10.1016/j.addr.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 72.Dubertret B, et al. In vivo imaging of quantum dots encapsulated in phospholipid micelles. Science. 2002;298:1759–1762. doi: 10.1126/science.1077194. [DOI] [PubMed] [Google Scholar]
- 73.Zhang Y, et al. Surface modification of superparamagnetic magnetite nanoparticles and their intracellular uptake. Biomaterials. 2002;23:1553–1561. doi: 10.1016/s0142-9612(01)00267-8. [DOI] [PubMed] [Google Scholar]
- 74.Gerion D, et al. Synthesis and properties of biocompatible water-soluble silica-coated CdSe/ZnS semiconductor quantum Dots. J. Phys. Chem. B. 2001;105:8861–8871. [Google Scholar]
- 75.Lu Y, et al. Modifying the surface properties of superparamagnetic iron oxide nanoparticles through a sol–gel approach. Nano Lett. 2002;2:183–186. [Google Scholar]
- 76.Sathe TR, et al. Mesoporous silica beads embedded with semiconductor quantum dots and iron oxide nanocrystals: dual-function microcarriers for optical encoding and magnetic separation. Anal. Chem. 2006;78:5627–5632. doi: 10.1021/ac0610309. [DOI] [PubMed] [Google Scholar]
- 77.Laurent S, et al. Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 2008;108:2064–2110. doi: 10.1021/cr068445e. [DOI] [PubMed] [Google Scholar]
- 78.Minakata S, Komatsu M. Organic reaction on silica in water. Chem. Rev. 2009;109:711–724. doi: 10.1021/cr8003955. [DOI] [PubMed] [Google Scholar]
- 79.Love JC, et al. Self-assembled monolayers of thiolates on metals as a form of nanotechnology. Chem. Rev. 2005;105:1103–1169. doi: 10.1021/cr0300789. [DOI] [PubMed] [Google Scholar]
- 80.Jiang W, et al. Nanoparticle-mediated cellular response is size-dependent. Nature Nanotechnol. 2008;3:145–150. doi: 10.1038/nnano.2008.30. [DOI] [PubMed] [Google Scholar]
- 81.Hashizume H. Openings between defective endothelial cells explain tumor vessel leakiness. Am. J. Pathol. 2000;156:1363–1380. doi: 10.1016/S0002-9440(10)65006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Folkman J. Fighting cancer by attacking its blood supply. Scientific Am. 1996;275:150–154. doi: 10.1038/scientificamerican0996-150. [DOI] [PubMed] [Google Scholar]
- 83.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 84.Yuan F, et al. Vascular permeability in a human tumor xenograft: molecular size dependence and cutoff size. Cancer Res. 1995;55:3752–3756. [PubMed] [Google Scholar]
- 85.Villanueva FS, Wagner WR. Ultrasound molecular imaging of cardiovascular disease. Nature Clin. Pract. Cardiovasc. Med. 2008;5:S26–S32. doi: 10.1038/ncpcardio1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen K, et al. Dual-modality optical and positron emission tomography imaging of vascular endothelial growth factor receptor on tumor vasculature using quantum dots. Eur.J. Nucl. Med. Mol.Imaging. 2008;35:2235–2244. doi: 10.1007/s00259-008-0860-8. [DOI] [PubMed] [Google Scholar]
- 87.Cai W, et al. Peptide-labeled near-infrared quantum dots for imaging tumor vasculature in living subjects. Nano Lett. 2006;6:669–676. doi: 10.1021/nl052405t. [DOI] [PubMed] [Google Scholar]
- 88.Cai W, et al. Dual-function probe for PET and near-infrared fluorescence imaging of tumor vasculature. J. Nucl. Med. 2007;48:1862–1870. doi: 10.2967/jnumed.107.043216. [DOI] [PubMed] [Google Scholar]
- 89.Reddy GR, et al. Vascular targeted nanoparticles for imaging and treatment of brain tumors. Clinical Cancer Res. 2006;12:6677–6686. doi: 10.1158/1078-0432.CCR-06-0946. [DOI] [PubMed] [Google Scholar]
- 90.Sun C, et al. In vivo MRI detection of gliomas by chlorotoxin-conjugated superparamagnetic nanoprobes. Small. 2008;4:372–379. doi: 10.1002/smll.200700784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tada H, et al. In vivo real-time tracking of single quantum dots conjugated with monoclonal anti-HER2 antibody in tumors of mice. Cancer Res. 2007;67:1138–1144. doi: 10.1158/0008-5472.CAN-06-1185. [DOI] [PubMed] [Google Scholar]
- 92.Park J-H, et al. Systematic surface engineering of magnetic nanoworms for in vivo tumor targeting. Small. 2009;5:694–700. doi: 10.1002/smll.200801789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Simberg D, et al. Biomimetic amplification of nanoparticle homing to tumors. Proc. Natl. Acad. Sci. 2007;104:932–936. doi: 10.1073/pnas.0610298104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Moore A, et al. In vivo targeting of underglycosylated MUC-1 tumor antigen using a multimodal imaging probe. Cancer Res. 2004;64:1821–1827. doi: 10.1158/0008-5472.can-03-3230. [DOI] [PubMed] [Google Scholar]
- 95.Smith BR, et al. Real-time intravital imaging of RGD-quantum dot binding to luminal endothelium in mouse tumor neovasculature. Nano Lett. 2008;8:2599–2606. doi: 10.1021/nl080141f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Medarova Z, et al. In vivo imaging of tumor response to therapy using a dual-modality imaging strategy. Int. J. Cancer. 2006;118:2796–2802. doi: 10.1002/ijc.21672. [DOI] [PubMed] [Google Scholar]
- 97.Kelly KA, et al. Detection of early prostate cancer using a hepsin-targeted imaging agent. Cancer Res. 2008;68:2286–2291. doi: 10.1158/0008-5472.CAN-07-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang C, et al. Specific targeting of tumor angiogenesis by RGD-conjugated ultrasmall superparamagnetic iron oxide particles using a clinical 1.5-T magnetic resonance scanner. Cancer Res. 2007;67:1555–1562. doi: 10.1158/0008-5472.CAN-06-1668. [DOI] [PubMed] [Google Scholar]
- 99.Albelda SM, et al. Integrin distribution in malignant melanoma: association of the β3 subunit with tumor progression. Cancer Res. 1990;50:6757–6764. [PubMed] [Google Scholar]
- 100.Schmieder AH, et al. Molecular MR imaging of melanoma angiogenesis with αvβ3-targeted paramagnetic nanoparticles. Mag. Reson. Med. 2005;53:621–627. doi: 10.1002/mrm.20391. [DOI] [PubMed] [Google Scholar]
- 101.Miao Y, et al. 111In-Labeled lactam bridge-cyclized α-melanocyte stimulating Hormone peptide analogues for melanoma imaging. Bioconjugate Chem. 2008;19:539–547. doi: 10.1021/bc700317w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang ZJ, et al. MR imaging of ovarian tumors using folate-receptor-targeted contrast agents. Pediatr. Radiol. 2008;38:529–537. doi: 10.1007/s00247-008-0764-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Corot C, et al. Tumor imaging using P866, a high-relaxivity gadolinium chelate designed for folate receptor targeting. Magn. Reson. Med. 2008;60:1337–1346. doi: 10.1002/mrm.21773. [DOI] [PubMed] [Google Scholar]
- 104.Saborowski O, et al. MR imaging of antigen-induced arthritis with a new, folate receptor targeted contrast agent. Contrast Media Mol. Imaging. 2007;2:72–81. doi: 10.1002/cmmi.128. [DOI] [PubMed] [Google Scholar]
- 105.Choi HS, et al. Renal clearance of quantum dots. Nature biotechnol. 2007;25:1165–1170. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang G, et al. Influence of anchoring ligands and particle size on the colloidal stability and in vivo biodistribution of polyethylene glycol-coated gold nanoparticles in tumor-xenografted mice. Biomaterials. 2009;30:1928–1936. doi: 10.1016/j.biomaterials.2008.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Niidome T, et al. PEG-modified gold nanorods with a stealth character for in vivo applications. J. Controlled Release. 2006;114:343–347. doi: 10.1016/j.jconrel.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 108.Chen J, et al. Gold nanocages as photothermal transducers for cancer treatment. Small. 2010;6:811–817. doi: 10.1002/smll.200902216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schipper ML, et al. Particle size, surface coating, and pegylation influence the biodistribution of quantum dots in living mice. Small. 2009;5:126–134. doi: 10.1002/smll.200800003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Longmire M, et al. Clearance properties of nano-sized particles and molecules as imaging agents: considerations and caveats. Nanomedicine. 2008;3:703–717. doi: 10.2217/17435889.3.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Deen WM, et al. Structural determinants of glomerular permeability. Am. J. Physiol. Renal Physiol. 2001;281:F579–F596. doi: 10.1152/ajprenal.2001.281.4.F579. 2001. [DOI] [PubMed] [Google Scholar]
- 112.Ohlson M, et al. A gel-membrane model of glomerular charge and size selectivity in series. Am. J. Physiol. Renal Physiol. 2001;280:F396–F405. doi: 10.1152/ajprenal.2001.280.3.F396. 2001. [DOI] [PubMed] [Google Scholar]
- 113.Jain TK, et al. Biodistribution, clearance, and biocompatibility of Iron oxide magnetic nanoparticles in rats. Mol. Pharm. 2008;5:316–327. doi: 10.1021/mp7001285. [DOI] [PubMed] [Google Scholar]
- 114.Briley-Saebo K, et al. Hepatic cellular distribution and degradation of iron oxide nanoparticles following single intravenous injection in rats: implications for magnetic resonance imaging. Cell Tissue Res. 2004;316:315–323. doi: 10.1007/s00441-004-0884-8. [DOI] [PubMed] [Google Scholar]
- 115.Nel A, et al. Toxic potential of materials at the nanolevel. Science. 2006;311:622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- 116.Hardman RA. Toxicologic review of quantum dots: toxicity depends on physicochemical and environmental factors. Environmental Health Perspectives. 2008;114:165–172. doi: 10.1289/ehp.8284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hauck TS, et al. In vivo quantum-dot toxicity assessment. Small. 2010;6:138–144. doi: 10.1002/smll.200900626. [DOI] [PubMed] [Google Scholar]
- 118.Hoshino A, et al. physicochemical properties and cellular toxicity of nanocrystal quantum dots depend on their surface modification. Nano Lett. 2004;4:2163–2169. [Google Scholar]
- 119.Lee H-Y, et al. PET/MRI dual-modality tumor imaging using arginine-glycine-aspartic (RGD)–conjugated radiolabeled iron oxide nanoparticles. J. Nucl. Med. 2008;49:1371–1379. doi: 10.2967/jnumed.108.051243. [DOI] [PubMed] [Google Scholar]
- 120.Cai W, et al. Dual-function probe for PET and near-infrared fluorescence imaging of tumor vasculature. J. Nuclear Med. 2007;48:1862–1870. doi: 10.2967/jnumed.107.043216. [DOI] [PubMed] [Google Scholar]
- 121.Glaus C, et al. In Vivo Evaluation of 64Cu-Labeled magnetic nanoparticles as a dual-modality PET/MR imaging agent. Bioconjugate Chem. 21:715–722. doi: 10.1021/bc900511j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jin Y, Gao X. Plasmonic fluorescence quantum dots. Nature Nanotech. 2009;4:571–576. doi: 10.1038/nnano.2009.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schevchenco EV, et al. Gold/iron oxide core/hollow-shell nanoparticles. Adv. Mater. 2008;20:4323–4329. [Google Scholar]
- 124.Alric C, et al. Gadolinium chelate coated gold nanoparticles as contrast agents for both X-ray computed tomography and magnetic resonance imaging. J. Am. Chem. Soc. 2008;130:5908–5915. doi: 10.1021/ja078176p. [DOI] [PubMed] [Google Scholar]
- 125.Oostendorp M, et al. Quantitative molecular magnetic resonance imaging of tumor angiogenesis using cNGR-labeled paramagnetic quantum dots. Cancer Res. 2008;68:7676–7683. doi: 10.1158/0008-5472.CAN-08-0689. [DOI] [PubMed] [Google Scholar]
- 126.Prasad NK, et al. Mechanism of cell death induced by magnetic hyperthermia with nanoparticles of g-MnxFe22-xO3 synthesized by a single step process. J. Mater. Chem. 2007;17:5042–5051. [Google Scholar]
- 127.Park JH, et al. Cooperative nanomaterial system to sensitize, target, and treat tumors. Proc. Natl. Acad. Sci. 2010;107:981–986. doi: 10.1073/pnas.0909565107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yavuz MS, et al. Gold nanocages covered by smart polymers for controlled release with near-infrared light. Nat. Mater. 2009;8:935–939. doi: 10.1038/nmat2564. [DOI] [PMC free article] [PubMed] [Google Scholar]