Abstract
Ehrlichia canis is the etiologic agent of canine monocytic ehrlichiosis (CME) and is a useful model for tick-borne zoonotic pathogens, many of which infect dogs. The purpose of this study was to evaluate rifampin and doxycycline regimens for clearance of E. canis infections in addition to alleviation of CME. Beagles were infected with E. canis by intravenous inoculation with carrier blood and treated with either rifampin or doxycycline after the acute phase of CME. Improved hematological values demonstrated that both treatments effectively relieved signs of the disease. Peripheral blood from all dogs became PCR-negative after antibiotic treatment, suggesting that these infections were eliminated and that rifampin is an effective alternative chemotherapeutic agent for treatment of CME.
Keywords: Ehrlichia canis, Rhipicephalus sanguineus, ehrlichiosis, doxycycline, rifampin
INTRODUCTION
Ehrlichia canis is the etiologic agent of canine monocytic ehrlichiosis (CME), a tick-borne disease that is divided into acute, subclinical and chronic phases.1 Acute CME consists of pyrexia and pancytopenia as the most commonly observed clinical signs. Acute illness is followed by long-term subclinical and chronic phases with recurrent clinical and hematologic signs that can include pancytopenia, hemorrhage and weight loss. This malady also serves as a useful infection, transmission and disease model for known tick-borne zoonotic pathogens, the majority of which infect dogs.
Oral doxycycline has long been the drug of choice for treatment of CME. Although doxycycline effectively ameliorates clinical signs of CME, we recently observed persistence of E. canis infection in dogs following a 14-day doxycycline regimen (~10 mg/kg, p.o., q.d.), including pathogen acquisition by Rhipicephalus sanguineus ticks experimentally fed as nymphs on these dogs, suggesting that infected dogs treated with a 14-day doxycycline regimen could remain infective for tick vectors.2 Failure to clear infections from hosts that respond to treatments for ehrlichial diseases can result in asymptomatic carriers that remain sources of infection for vectors and naïve vertebrate hosts, and alternative treatment regimens should be evaluated. The purpose of this study was to test rifampin and an extended doxycycline regimen for clearance of E. canis infections.
METHODS
Four purpose-bred female Beagles (2 – 3 years old) were cared for in accordance with a protocol on file with The Ohio State University Institutional Laboratory Animal Care and Use Committee. Rectal temperatures and overall health were monitored daily, and blood was collected semiweekly for PCR and complete blood counts (CBC) with a Cell-Dyne 3500R 45 (Abbot Laboratories, IL). Experimental acute CME is efficiently induced by inoculation of naïve dogs with E. canis-infected host cells, so blood was collected with heparin from an E. canis (Ebony isolate) carrier (dog A72) and inoculated (iv.) through the cephalic vein into four naïve Beagle dogs (5 ml per dog). This isolate was repeatedly demonstrated to be tick transmissible under experimental conditions.3 Blood was collected and assayed with a p30-based PCR assay was used to detect E. canis in 500 ng of buffy coat DNA as described elsewhere.2, 3
Each regimen was to begin 64 days post-infection (dpi) to ensure the dogs were past acute phase CME. However, doxycycline administration began at 32 dpi for dog AKE (~10 mg/kg, p.o., q.d., ×28d) owing to deteriorating clinical condition (depression and anorexia). The same 28-day doxycycline regimen began as scheduled (64 dpi) for dog AHL. Dogs ABH and ACJ received rifampin at a dose of 150 mg (~15 mg/kg, p.o., q12h) at 64–71 dpi, as recommended for dogs with other conditions4 and approximating the dose used for treatment of human anaplasmosis.5 R. sanguineus larvae were hatched from egg clusters (each egg cluster from a single female tick) purchased from the Oklahoma State University Medical Entomology Laboratory and handled as described elsewhere.3 Larvae (from one egg cluster per dog) were placed on each dog, with the exception of AKE, prior to antibiotic treatment (53 dpi) and acquisition fed on dogs AHL, ABH and ACJ before transmission feeding as nymphs on dogs AFI, ALW and BAH, respectively.
RESULTS AND DISCUSSION
All four hosts inoculated with blood from dog A72 developed severe acute CME, which was demonstrated by hematologic and clinical signs. Pyrexia (i.e., rectal temperature >39 °C) was observed in all four inoculated dogs by 10 – 14 dpi. Body temperatures reached maximum values of 39.8 – 41.0 °C at 14 – 18 dpi, before gradually decreasing back to the normal range prior to administration of treatment regimens. Platelet counts indicated severe thrombocytopenia in all dogs, typically reaching a nadir 14 – 25 dpi. E. canis infections were demonstrated by 200 bp amplicons consistent with the PCR assay. These dogs all tested PCR-negative prior to inoculation with carrier blood, and they all tested PCR-positive for E. canis by 18 dpi.
Doxycycline regimen
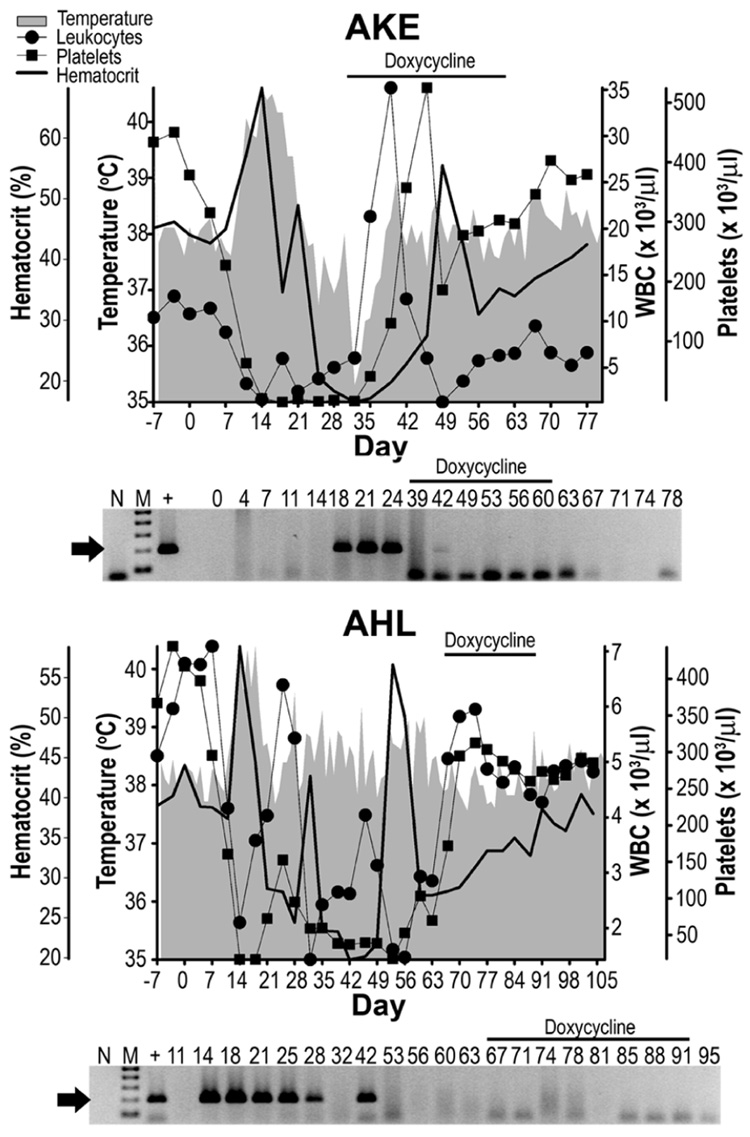
Results for dogs treated with doxycycline are represented in Figure 1. For dog AKE, pyrexia was observed 10 – 20 dpi with a peak of 40.6 °C at 14 dpi. Precipitous drops in peripheral leukocytes and platelets were observed 7–14 dpi, from average baseline values of 10.1 × 103 WBC/µl and 444.2 × 103 platelets/µl, reaching nadirs of 1.93 × 103 platelets/µl at 18 dpi and 1.29 × 103 WBC/µl at 49 dpi. Hematocrit values dropped from the baseline average of 44.9% to 16.9% by 32 dpi. Although signs of CME were observed within 10 dpi, peripheral blood samples did not test PCR-positive for E. canis until 18 – 24 dpi. Rectal temperatures for this dog returned to the normal range by 21 dpi, and remained so through the remainder of the study; however, treatment was required for this dog at 32 dpi. Hematological values rose rapidly after treatment began, and were close to pre-infection levels at the end of the study. CBC values rose from 6.03 to 6.29 × 103 WBC/µl, 3.54 to 304 × 103 platelets/µl and hemotocrits of 16.9 to 34% during the 28 day course of doxycline treatment. These parameters eventually reached maximum values of 9.49 × 103 WBC/µl, 403 × 103 platelets/µl and 42.6% after completion of therapy. With the exception of a single sample from 42 dpi, all of the buffy coats tested PCR-negative after treatment began.
Figure 1. Dogs treated with doxycycline.
Each dog was inoculated with carrier blood on Day 0. Overhead bars indicate samples collected during doxycycline therapy. Shaded areas, lines with circles, squares or solid lines represent body temperature, peripheral leukocytes, platelets or hematocrit values, respectively. An E. canis-specific PCR assay was used to test buffy coat samples and lanes containing the 200 bp amplicon (arrow) were considered PCR-positive. No-template-reactions (N) served as contamination controls, DNA (100 pg) from E. canis-infected DH82 cells served as positive control (+) and 0 – 105 indicate dpi with E. canis carrier blood. The molecular size standard (M) is a 100 bp ladder.
Dog AHL was treated with the same doxycycline regimen at 64 dpi. Pyrexia was first observed 14 – 19 dpi with a peak of 40.4 °C (18 dpi), and spikes > 39.0 °C at 28, 35, 45, 52, 57, 59 and 61 dpi. Precipitous drops in peripheral leukocytes and platelets were observed 7 to 14 dpi, dropping from average baseline values of 371.3 × 103 platelets/µl and 6.15 × 103 WBC/µl to nadirs of 17.8 × 103 platelets/µl and 1.43 × 103 WBC/µl at 14 dpi and 32 dpi, respectively. Hematocrit values dropped a baseline average of 41.9% to a nadir of 19.9% at 42 dpi. PCR-positive buffy coats were observed 14 – 28 dpi and again at 42 dpi, but E. canis was not detected again even though CBC values remained low through 63 dpi, just prior to treatment, and mild fevers repeatedly rose >39 °C. However, rectal temperature remained below 39 °C from initiation of treatment at 64 dpi through the remainder of the study, and CBC values rebounded shortly after treatment began from 2.85 × 103 to 4.27 × 103 WBC/µl, 71 × 103 to 273 × 103 platelets/µl and hematocrits of 27.9 to 38.6% during the 28 day course of doxycline treatment. These values eventually reached 5.01 × 103 WBC/µl, 291 × 103 platelets/µl and a hematocrit of 40.4% after therapy. There was no further PCR evidence of infection after 42 dpi.
Rifampin treatment
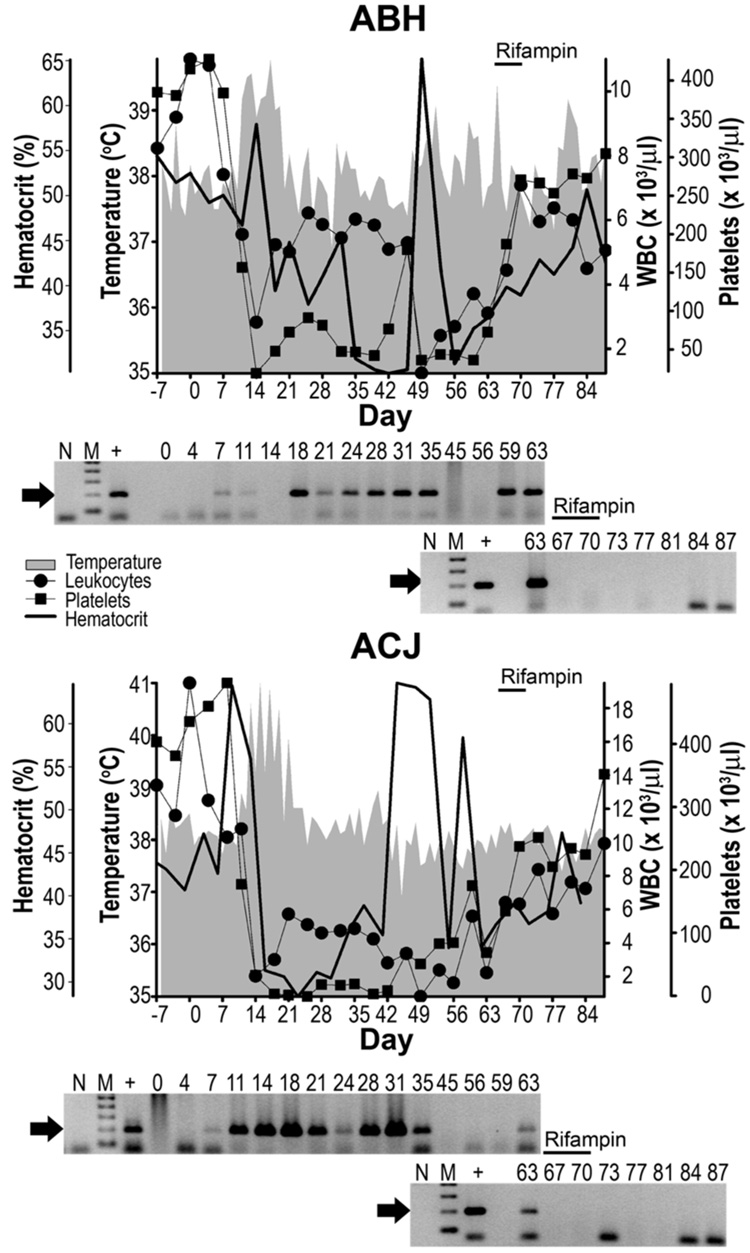
Results for dogs subjected to the rifampin regimen are represented in Figure 2. Both dogs in this group received treatment 64 – 71 dpi. Pyrexia was first observed in ABH at 11 – 19 dpi with a peak of 39.8 °C at 17 dpi, and a single day post-acute phase spike 104 above 39.0 °C at 65 dpi. Precipitous drops in peripheral leukocytes and platelets were observed 7 – 14 dpi, from mean baseline values of 8.2 × 103 WBC/µl and 363.5 × 103 platelets/µl to nadirs of 1.24 × 103 WBC/µl and 21.7 × 103 platelets/µl (each at 14 dpi). Hematocrit values dropped from the baseline average of 52.4% to a nadir of 30.5% at 12 dpi. ABH buffy coats initially tested PCR-positive 7 –10 dpi, and negative 14 dpi, followed by robust positive results 18 – 35 dpi and again 59 – 63 dpi. CBC’s rebounded shortly after initiation of therapy from 3.1 × 103 to 7.08 × 103 WBC/µl, 74.4 × 103 to 305 × 103 platelets/µl and hemotocrits of 36.6 to 39.1%, all during the 7 day course of rifampin treatment. Although leukocytes again dropped to 4.5 × 103 112 WBC/µl after therapy, the remaining CBC values eventually reached up to 305 × 103 platelets/µl and 50.7% hemotocrit by the end of the study. Body temperature again rose above 39 °C at 80 dpi, but there was no further PCR evidence of infection after 63 dpi.
Figure 2. Dogs treated with rifampin.
Labels are as described for Figure 1; except overhead bars are indicative of rifampin therapy.
For dog ACJ, pyrexia was first observed 13 – 19 dpi with a peak of 41 °C at 15 dpi, followed by a single spike >39.0 °C (21 dpi). CBC’s dropped from average baseline values of 8.2 × 103 WBC/µl and 416.8 × 103 platelets/µl to nadirs of 0.81 × 103 WBC/µl (49 dpi) and platelets/µl below detectable levels (25 dpi). Hematocrit values dropped from the baseline average of 42.5% to a nadir of 28.4% (28 dpi). ACJ buffy coats were PCR-positive 7 – 35 dpi and again at 63 dpi. CBC values rebounded shortly after treatment began from 2.2 × 103 to 6.32 × 103 WBC/µl, 69.4 × 103 to 237 × 103 platelets/µl and 34.1 to 36.7% hematocrits during the 7 day course of rifampin treatment, eventually reaching values up to 9.9 × 103 WBC/µl, 351 × 103 123 platelets/µl and 47.3% hematocrit after therapy. Body temperature remained below 39 °C after 21 dpi, with no further PCR evidence of infection after 63 dpi.
Xenodiagnosis
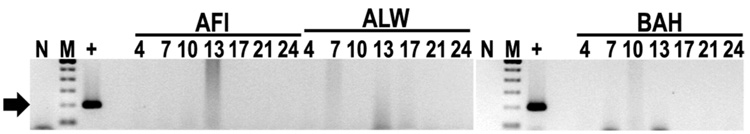
Previous work indicated that a 14-day doxycycline regimen did not prevent acquisition of E. canis by R. sanguineus nymphs fed on dogs that were infected through tick transmission.2 However, only R. sanguineus larvae were commercially available for xenodiagnosis in the current study, so these larvae were placed on dogs AHL, ABH and ACJ prior to antibiotic treatment at 53 dpi, and post-treatment tick feeding began 78 dpi for ABH and ACJ, and 67 or 108 dpi for AKE and AHL, respectively. Cohorts of the ticks used to feed on infected dogs were fed on an uninfected dog (BCI) as a negative control. Nymphs fed as larvae on these dogs at 53 dpi were allowed to transmission feed to repletion on naïve dogs. Buffy coat samples from dogs used for transmission feeding of nymphs acquisition fed as larvae prior to antibiotic treatment did not test PCR-positive (Figure 3), and these dogs did not display clinical signs of CME. Therefore ticks that fed on dogs after antibiotic treatment were not tested for E. canis transmission.
Figure 3. Attempted transmission of E. canis to dogs by R. sanguineus nymphs fed as larvae during post-acute phase CME.
R. sanguineus larvae placed on dogs ACJ, ABH and AHL at 53 dpi (prior to antibiotic treatment) were allowed to feed as nymphs on dogs AFI, ALW and BAH, respectively, as a test of transmission. The E. canis-specific p30-based PCR assay was used to test semiweekly buffy coat samples collected from each dog up to 24 days post-tick attachment (indicated by numerals). Labels are as described for Figure 1.
Resolution of both disease and infection with either doxycycline or rifampin suggested that rifampin might be used as an alternative treatment for CME. These results confirmed other studies where hosts were infected with E. canis by needle-inoculation, but not investigations where dogs were infected via tick feeding.2 Although the same PCR assay was used in the previous investigation, continued efforts to enhance PCR assay sensitivity is warranted to help address this issue. Discrepancies between experimental designs and results of this and other studies together suggested that transmission mode or disease phase might influence efficacy of antibiotics to clear of E. canis infections. Timing of antibiotic administration was among these discrepancies, but disease status might be more relevant with regard to treatment efficacy. For example, severe pancytopenia was still present in every case of the current study while only mild clinical signs were observed among dogs in an earlier report.2, 3 Route of host infection was another difference between the present and the former study; the present study involved needle-inoculation (iv.) with E. canis carrier blood while the earlier study utilized male R. sanguineus ticks to infect dogs. Interestingly, there is at least one report of persistence of natural E. canis infections, most likely acquired through tick transmission, despite doxycycline therapy.6 Further work is needed to compare similar treatments of hosts during different phases of CME as well as hosts infected through tick feeding versus needle-inoculation.
ACKNOWLEDGEMENTS
The authors thank Kirsten Boughan for technical assistance and Dr. R. Hamlin for support. JK was partially supported by The Ohio State University, College of Veterinary Medicine Summer Research Program. This work was supported by NIH grant AI47932.
REFERENCES
- 1.Stich RW, et al. Host surveys, ixodid tick biology and transmission scenarios as related to the tick-borne pathogen, Ehrlichia canis. Vet Parasitol. 2007 doi: 10.1016/j.vetpar.2008.09.013. Manuscript accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schaefer JJ, et al. Tick Acquisition of Ehrlichia canis from Dogs Treated with Doxycycline Hyclate. Antimicrob Agents Chemother. 2007 doi: 10.1128/AAC.00358-07. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bremer WG, et al. Transstadial and intrastadial experimental transmission of Ehrlichia canis by male Rhipicephalus sanguineus. Vet Parasitol. 2005;131:95–105. doi: 10.1016/j.vetpar.2005.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Plumb DC. Veterinary Drug Handbook. Ames, IA: Iowa State University Press; 1999. [Google Scholar]
- 5.Krause PJ, Corrow CL, Bakken JS. Successful treatment of human granulocytic ehrlichiosis in children using rifampin. Pediatrics. 2003;112:e252–e253. doi: 10.1542/peds.112.3.e252. [DOI] [PubMed] [Google Scholar]
- 6.Wen B, et al. Comparison of nested PCR with immunofluorescent-antibody assay for detection of Ehrlichia canis infection in dogs treated with doxycycline. J Clin Microbiol. 1997;35:1852–1855. doi: 10.1128/jcm.35.7.1852-1855.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]