Abstract
The acquisition and transmission of rickettsial pathogens by different tick developmental stages has important epidemiological implications. The purpose of this study was to determine if male Rhipicephalus sanguineus can experimentally acquire and transmit Ehrlichia canis in the absence of female ticks. Two trials were performed where nymphal and male R. sanguineus were simultaneously acquisition fed on the same infected donor hosts, and transstadially or intrastadially exposed male ticks were fed on separate pathogen-free dogs as a test for transmission. A single-step p30-based PCR assay was used to test canine and tick hosts for E. canis infections before and after tick feeding. E. canis was detected after either intrastadial or transstadial passage in male ticks, the organism remained detectable in both tick groups after transmission feeding, and both tick groups transmitted the rickettsia to susceptible dogs. Infection of dogs via tick feeding resulted in milder clinical signs and lower antibody titers than intravenous inoculation of carrier blood, but further investigation is needed to understand the mechanisms responsible for this observation. These results demonstrate that male R. sanguineus can take multiple feedings, and that they can both acquire and transmit E. canis in the absence of female ticks. This tick development stage could be important in transmission of E. canis, and perhaps related pathogens, between vertebrate hosts under natural and experimental conditions.
Keywords: Ehrlichia canis, Canine monocytic ehrlichiosis, Tick transmission, Rhipicephalus sanguineus, Metastriata
1. Introduction
Ehrlichiosis has been a subject of increasing interest from veterinary and public health perspectives over the last few decades (Olano and Walker, 2002; Paddock and Childs, 2003; Rikihisa, 1991; Skotarczak, 2003; Walker and Dumler, 1996). The agents of this disease are members of the genus Ehrlichia, which, like the closely related genus Anaplasma, is classified in the family Anaplasmataceae of the order Rickettsiales (Dumler et al., 2001); all currently defined Ehrlichia species are biologically transmitted by ticks of the family Ixodidae.
Ehrlichia canis is a cosmopolitan parasite of dogs and the primary etiological agent of canine monocytic ehrlichiosis (Rikihisa, 1991; Skotarczak, 2003). Dogs reportedly undergo an acute phase of the disease after an approximately 10-day prepatent period, when pyrexia and thrombocytopenia are often observed (Dawson and Ewing, 1992; de Castro et al., 2004; Harrus et al., 1999; Mathew et al., 1996). Affected hosts then undergo a partial recovery from 20-30 days post-infection, commonly followed by a subclinical to severe chronic phase that is thought to be a carrier state during which tick vectors could still acquire and disseminate the pathogen to other hosts. Rhipicephalus sanguineus is an important experimental and natural biological vector of E. canis (Ewing, 1969; Groves et al., 1975; Harvey et al., 1979). Thus, this relatively well-characterized tick-pathogen interaction could be a suitable experimental model for other Ehrlichia species.
Acquisition and transmission of E. canis by different tick developmental stages may have various implications regarding the epizootiology of canine monocytic ehrlichiosis, and there is evidence that male adult stage ticks of the ixodid subfamily Metastriata could be important vectors of anaplasmal and ehrlichial pathogens. Experimental studies have shown that other species of intrastadially infected male ticks are capable of transmitting the closely related pathogens, A. marginale and Ehrlichia (formerly Cowdria) ruminantium (Andrew and Norval, 1989; Kocan et al., 1992; Potgeiter, 1981; Stiller and Coan, 1995; Stiller et al., 1989). However, transmission of E. canis by male ticks alone has not been reported, and, to our knowledge, only ticks exposed to E. canis during immature stages have been reported to transstadially transmit the pathogen between dogs (Groves et al., 1975; Johnson et al., 1998; Lewis et al., 1977; Mathew et al., 1996). The purpose of this investigation was to determine if male R.sanguineus are capable of experimentally acquiring and transmitting E. canis in the absence of female ticks. Intrastadially and transstadially exposed male ticks were tested for E. canis infection with a PCR assay, and cohorts of these ticks were allowed to feed on specific pathogen-free dogs as a test for transmission. Clinical, serological and molecular methods were used to monitor the canine hosts for evidence of exposure to E. canis.
2. Materials and methods
2.1. E. canis
The Ebony strain of E. canis was used in this study (Mathew et al., 1996). Heparinized carrier blood was inoculated into donor dogs for tick acquisition feeding and to culture E. canis in DH82 cells as described elsewhere (Rikihisa et al., 1994, 1991; Wen et al., 1997).
2.2. Canine hosts
Seven dogs were used in this study and cared for in accordance with a protocol approved by and on file with The Ohio State University Institutional Laboratory Animal Care and Use Committee. All vertebrate hosts were adult (2-3 years old) Beagle dogs and all were female except for dog AHG. These dogs were monitored daily for clinical signs of ehrlichiosis, and semiweekly by PCR and hematology (complete blood and thrombocyte counts). Complete blood counts were performed at the clinical laboratory of The Ohio State University College of Veterinary Medicine with a Cell-Dyne 3500R (Abbot Laboratories, Abbot Park, IL). Dogs were considered parasitemic upon demonstration of E. canis in circulating white blood cells by PCR. Dogs used for tick acquisition feeding were inoculated (i.v.) with 10 ml of heparinized carrier blood from dog A72, an E. canis (Ebony strain) carrier that was previously experimentally infected with transstadially infected ticks (Stich et al., 2002).
2.3. Tick feeding
R. sanguineus were purchased from the Oklahoma State University, Medical Entomology Laboratory. The ticks were allowed to feed on canine hosts as previously described (Stich et al., 2002). For acquisition feeding, 200 nymphal and 100 male ticks were placed into separate orthopedic stockinettes on each donor host at 21 days post-inoculation (dpi) with E. canis, and were allowed to acquisition feed to repletion (nymphs) or for 10 days (adults). Acquisition fed ticks were held in an environmental chamber (rt, 95-100% rh, 12:12 h light:dark photoperiod) and replete nymphs were allowed to molt into adults while the ticks acquisition fed as adults were held for 10 days to ensure that mechanical transmission would not take place. Transstadially and intrastadially exposed male R. sanguineus (50 per dog) were allowed to feed on separate IFA- and PCR-negative dogs for 10 days to test for transmission. Male ticks acquisition fed on donor dog ATK as nymphs or adults were subsequently transmission fed on dogs AHK or AHG, respectively, and male ticks acquisition fed on donor dog BAA as nymphs or adults were transmission fed on dogs AIP or AUF, respectively. Unfed cohorts of these ticks were allowed to feed on a PCR- and IFA-negative dog (AXM) to serve as negative controls and to confirm that these ticks were not previously infected with E. canis.
2.4. PCR assay
A single-step p30-based E. canis PCR assay with primers ECA30-384S (5′-ATAAACACGCTGACTTTACTGTTCC-3′) and ECA30-583A (5′-GTGATGAGATAGAGCGCAGTACC-3′) was adapted to this study (Stich et al., 2002). Canine blood was collected with EDTA, and buffy coats were prepared as previously described (Stich et al., 2002). These cells were incubated in 500 μl of protein digestion buffer (100 mM Tris, pH 8.0; 1 mM EDTA; 0.1% SDS; 0.1 mg/ml proteinase k) overnight at 55 °C. The digests were extracted one time each with equal volumes of buffer-saturated phenol (pH >7.5) (Invitrogen Life Technologies, Carlsbad, CA), phenol/chloroform/isoamyl alcohol (25:24:1), and chloroform/isoamyl alcohol (24:1). DNA templates were precipitated overnight at -20 °C with 1/10 volume of 3 M sodium acetate and 2.5 volumes of absolute ethanol, centrifuged at 14,000 × g for 5 min at 4 °C, dried in a vacuum centrifuge and dissolved in 50 μl of TE; 500 ng of buffy coat DNA was used for each 50 μl PCR.
Tick specimens were assayed for the presence of E. canis with the single step p30-based PCR assay described above. Individual ticks were prepared by incubation for 80 h at 37 °C in 95-100% rh, bisected along the median plane under aseptic conditions, and digested 3 h at 55 °C in 100 μl of protein digestion buffer. These digests were subjected to protein extraction, without the chloroform/isoamyl alcohol step, and DNA precipitation as described for buffy coat samples. These preparations were dissolved in 50 μl of TE and a volume equivalent to 10% of the final reaction volume was assayed from each tick template.
2.5. Immunofluorescence assay (IFA)
E. canis (Ebony isolate)-infected DH82 cells were concentrated to 1.0 × 106 cells/ml in PBS, and 10 μl/well was placed on Teflon-coated slides (Electron Microscopy Sciences, Fort Washington, PA) and allowed to air dry overnight. The slides were placed in ice-cold methanol for 5 min and stored at - 20 °C. For initial screening, plasma was diluted 1:32 in PBS, and 10 μl of the diluted plasma was placed on each well and incubated at 37 °C for 30 min before rinsing 3× with PBS. The slides were then incubated at 37 °C for 30 min with 10 μl per well of Caprine-anti-Canine IgG FITC Conjugate (VMRD, Inc, Pullman, WA) and again rinsed 3× with PBS. The slides were mounted with Prolong Anti-Fade reagent (Molecular Probes, Eugene, OR) according to the manufacturer's instructions and observed with Olympus BX51 fluorescence microscope with filter cube #11001v2 (Chroma Technology, Brattleboro, VT). Titers were determined for samples that tested IFA-positive during the initial screening. The E. canis FA Positive and E. canis FA Negative control dog sera (VMRD) were included on each slide.
2.6. Comparison of clinical parameters
Average values of each dog were calculated for body temperature and hematological parameters observed prior to exposure to E. canis, and these values were compared to peak differences for each group with the two-tailed Student's t-test. Pre-exposure mean values were also used to calculate peak increases and reductions of each parameter, which, along with days to peak values, were compared among the three groups by ANOVA and posthoc analysis with the Tukey procedure when appropriate (Steel and Torrie, 1980).
3. Results
3.1. Dogs used for acquisition feeding
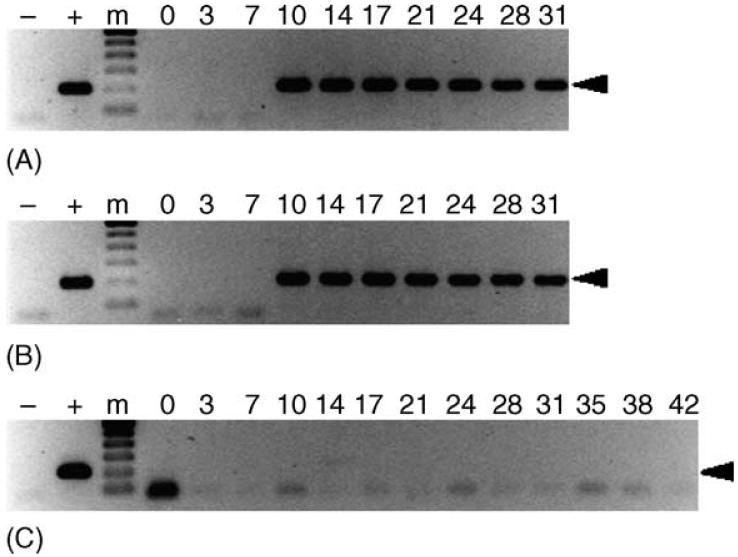
In both trials, dogs inoculated with E. canis carrier blood were PCR-positive (Fig. 1) and seroconverted by day 10. Peak antibody titers of 8192 were reached on days 24 and 21 for dogs ATK and BAA, respectively. Ticks were allowed to begin acquisition feeding on these hosts during the primary stage of infection (21 dpi) to increase the likelihood of tick infection (Johnson et al., 1998; Lewis et al., 1977; Mathew et al., 1996). Dog AXM, the PCR- and IFA-negative host used to feed unexposed cohorts of these ticks, remained PCR-negative (Fig. 1), seronegative and clinically normal throughout the observation period.
Fig. 1.
Detection of E. canis in acquisition hosts. PCR assays were performed on buffy coat samples of dogs used to acquisition feed ticks as described in the text. Lanes containing the 200 bp target amplicon (arrowhead) were considered PCR-positive. Dogs ATK (panel A) and BAA (panel B) were inoculated with whole blood from an E. canis carrier on day 0, and used to acquisition feed ticks on days 21-31. Dog AXM (panel C) was not exposed to E. canis, and was used to feed cohorts of ticks fed on the infected dogs as a negative control. For each panel, template-free reactions served as negative controls (-), template DNA (1 ng) collected from E. canis-infected DH82 cells served as positive control (+) and 0-42 indicate days post-inoculation (panels A and B) or days post-tick attachment (panel C). The molecular size standard (m) is a 100 bp ladder.
3.2. Transmission of E. canis by male ticks exposed as nymphs
E. canis is likely to be transstadially transmitted by R. sanguineus in nature, because (1) all three feeding stages of this tick species readily attach to canine hosts and (2) transstadial transmission has been demonstrated in several experimental studies. Thus, R. sanguineus nymphs were acquisition fed on each donor host to test the vector competence of transstadially exposed male ticks in the absence of infected female cohorts.
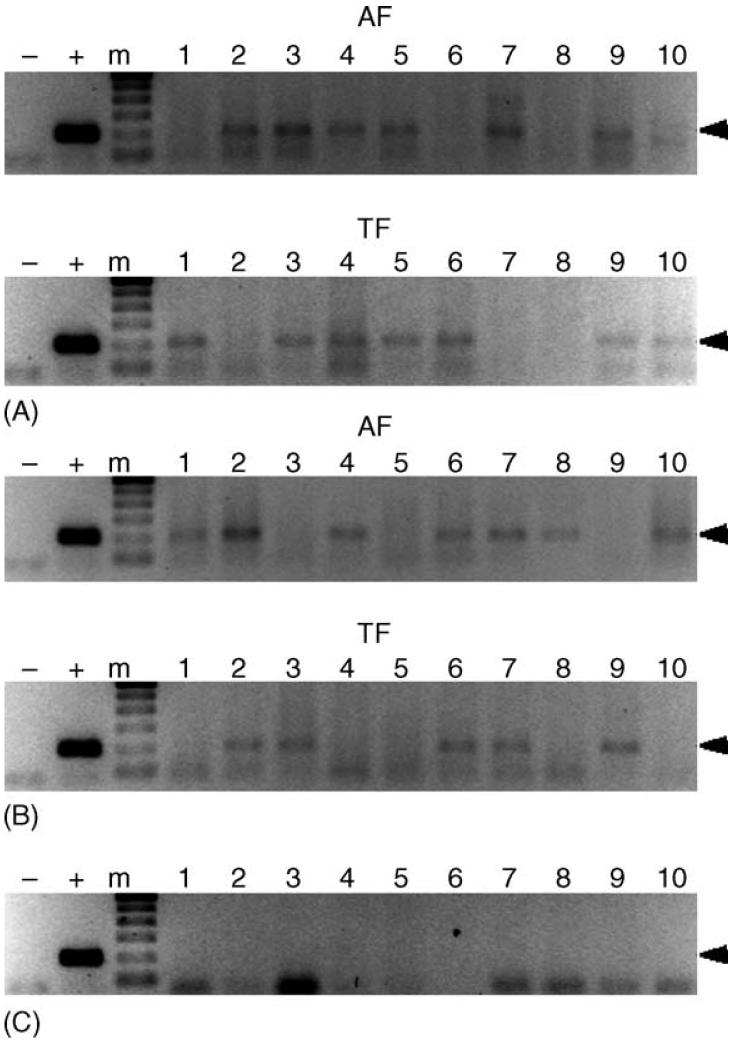
Transstadially exposed R. sanguineus ticks were assayed with PCR to evaluate the frequency of E. canis infection among male ticks exposed as nymphs. Both unfed and transmission-fed cohorts were tested to determine if transmission feeding could affect the frequency of pathogen detection among these ticks. E. canis was detected in transstadially exposed male ticks from both trials (Fig. 2). The pathogen was detected in 6 and 7 of 10 ticks acquisition fed on donors ATK and BAA, respectively, and in 7 and 5 of 10 ticks after transmission feeding on dogs AHK and AIP, respectively. The negative control ticks, male R. sanguineus fed on the negative control host (dog AXM) as nymphs, tested PCR-negative for E. canis.
Fig. 2.
Detection of E. canis in transstadially exposed male R. sanguineus. R. sanguineus nymphs were fed to repletion on E. canis-infected dogs, allowed to molt into adults, and male ticks were assayed before and after transmission feeding. Panels A and B represent ticks acquisition fed on dogs ATK and BAA, respectively, which were assayed after acquisition (AF) and transmission (TF) feeding on dogs AHK and AIP, respectively. Panel C represents male R. sanguineus that molted from nymphs allowed to feed on the negative control, dog AXM. Lanes containing the 200 bp target amplicon were considered PCR-positive. For each panel, template-free reactions served as negative controls (-), template DNA (1 ng) collected from E. canis-infected DH82 cells served as positive control (+) and lanes labeled 1-10 represent individual ticks. The molecular size standard (m) is a 100 bp ladder.
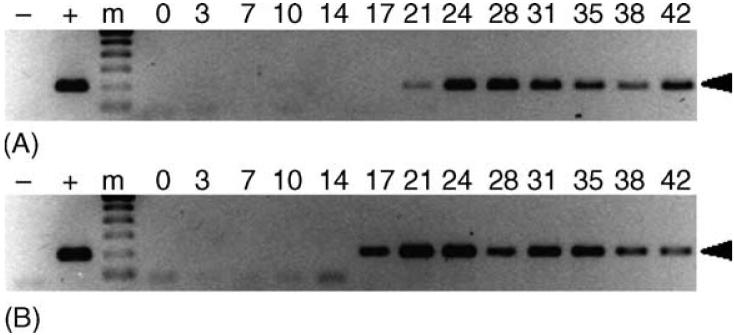
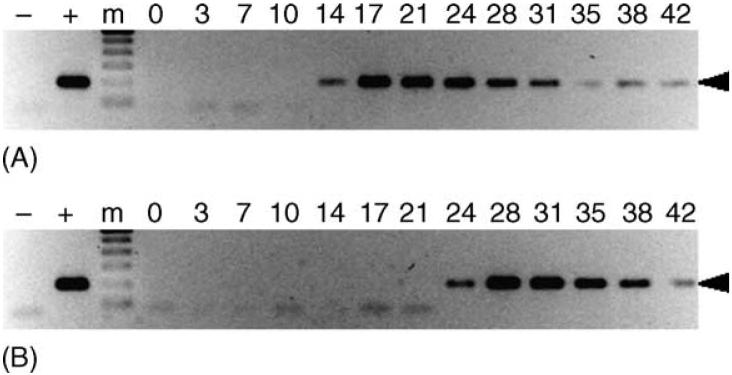
Signs of infection were observed in both dogs fed on by male R. sanguineus exposed to E. canis as nymphs. Dogs AHK and AIP were IFA-positive with a peak antibody titer of 32 by 14 days post tick attachment (dpta) and PCR-positive 21 and 17 dpta, respectively (Fig. 3).
Fig. 3.
Transstadial transmission of E. canis to dogs by male R. sanguineus. PCR assays were performed on peripheral blood from dogs AHK (panel A) and AIP (panel B). Lanes containing the 200 bp target amplicon (arrowhead) were considered PCR-positive. For each panel, template-free reactions served as negative controls (-), template DNA (1 ng) collected from E. canis-infected DH82 cells served as positive control (+) and 0-42 indicate days post-tick attachment. The molecular size standard (m) is a 100 bp ladder.
3.3. Intrastadial transmission of E. canis by male R. sanguineus
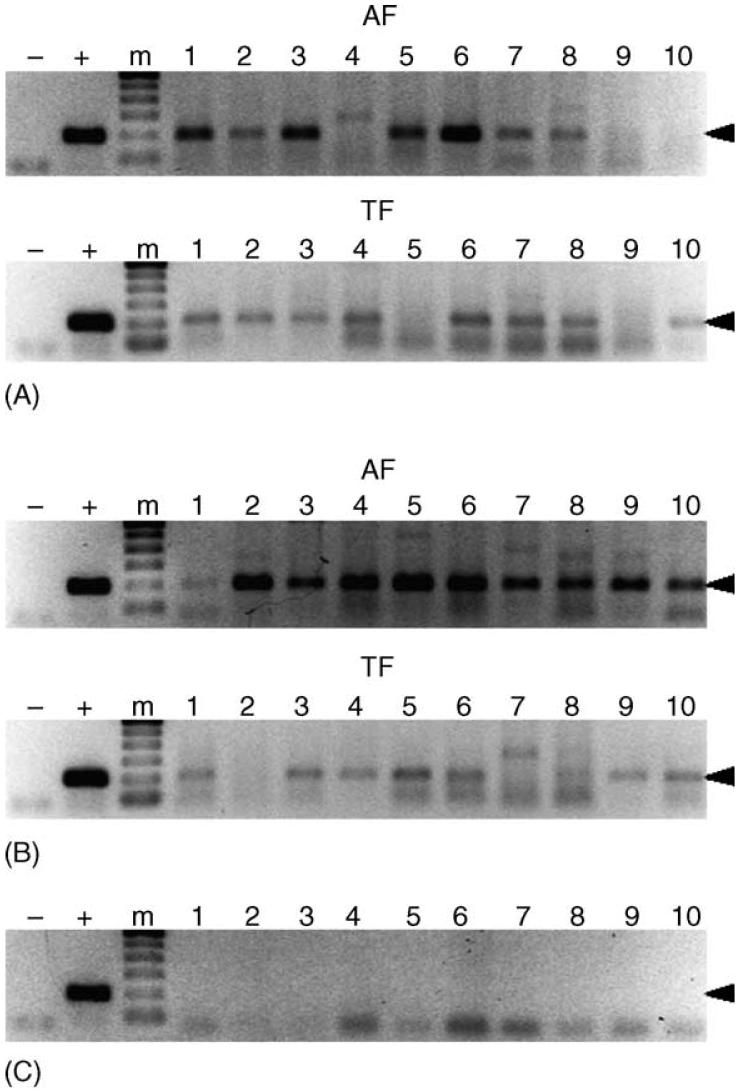
Intrastadial transmission of ehrlichial pathogens by male ticks could have different implications for the transmission of these agents under experimental and natural conditions. Thus, male R. sanguineus were simultaneously fed on the same acquisition hosts as the nymphs described above. Acquisition- and transmission-fed male R. sanguineus exposed to E. canis as adults were also assayed to determine if exposure to the pathogen during the adult stage could affect the frequency of tick infection (Fig. 4). E. canis was detected in 8 and 10 of 10 ticks acquisition fed as adults on dogs ATK and BAA, respectively. Similarly, the pathogen was detected in 8 of 10 ticks from both trials after transmission feeding on either AHG or AUF. No significant difference was observed in the rate of tick infection before or after transmission feeding. Negative controls, male R. sanguineus fed on the uninfected dog (AXM), tested PCR-negative.
Fig. 4.
Detection of E. canis in intrastadially exposed male R. sanguineus. Panels A and B represent male R. sanguineus exposed to E. canis on dogs ATK and BAA, respectively, which were assayed after acquisition (AF) and transmission (TF) feeding on dogs AHG and AUF, respectively. Panel C represents male R. sanguineus allowed feed on the negative control, dog AXM. Samples resulting in a 200 bp amplicon (arrowhead) were considered PCR-positive. For each panel, template-free reactions served as negative controls (-), template DNA (1 ng) collected from E. canis-infected DH82 cells served as positive control (+), and lanes labeled 1-10 represent individual ticks. The molecular size standard (m) is a 100 bp ladder.
Signs of infection were observed in both dogs fed on by male R. sanguineus exposed to E. canis as adults. Dogs AHG and AUF reached peak antibody titers of 128 (31 dpta) and 64 (28 dpta), respectively, and became PCR-positive by 14 and 24 dpta, respectively (Fig. 5).
Fig. 5.
Intrastadial transmission of E. canis to dogs by male R. sanguineus. PCR assays were performed on peripheral blood from dogs AHG (panel A) and AUF (panel B). Lanes containing the 200 bp target amplicon (arrowhead) were considered PCR-positive. For each panel, template-free reactions served as negative controls (-), template DNA (1 ng) collected from E. canis-infected DH82 cells served as positive control (+), and 0-42 indicate days post-tick attachment. The molecular size standard (m) is a 100 bp ladder.
3.4. Clinical ehrlichiosis among dogs exposed to infected male R. sanguineus
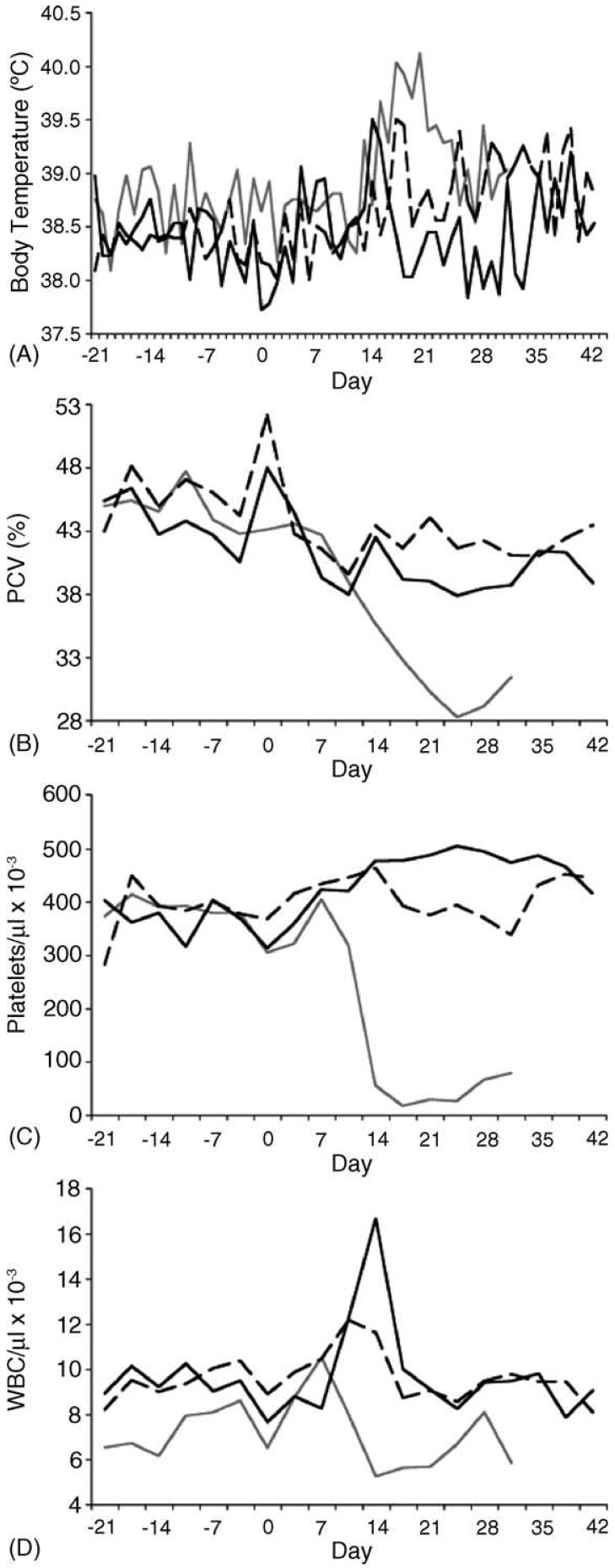
Overt signs of clinical ehrlichiosis were observed among dogs inoculated with carrier blood, including anemia, progressive thrombocytopenia and fever. Interestingly, with the exception of body temperature, relatively mild signs of ehrlichiosis were observed among dogs infected via tick transmission (Fig. 6; Table 1). For all three groups, body temperatures peaked over 39.1 °C at 13-17 days post-exposure, and the blood-inoculated group had the longest period of fever (p < 0.01). There was a gradual decrease of PCV among dogs infected by ticks, but a more pronounced reduction in PCV in the inoculated group; only inoculated dogs (p < 0.05) and those exposed to transstadially infected ticks (p < 0.01) had significant drops in PCV according to the Student's t-test, and the lowest PCV of the inoculated group was lower than those of the tick-infected groups (p < 0.01). Similarly, inoculated hosts had a precipitous drop (p < 0.05) in platelet levels, and a reduction in platelet levels was not observed for dogs exposed to intrastadially infected ticks, where a gradual increase in platelets up to 49% above the pre-exposure levels was observed. Reductions in leukocyte levels were also observed for dogs exposed to carrier blood and transstadially infected ticks (p < 0.05). Interestingly, an initial rise in leukocyte levels was observed for all three groups, followed by a return to pre-exposure levels. Further analysis indicated that this increase was largely due to neutrophil levels, which, for all six dogs, went from 6 ± 1.02 × 103 (mean ± standard deviation) to a post-exposure peak of 10 ± 3.6 × 103neutrophils/μl (p < 0.05). Peak neutrophil levels did not statistically differ among the three groups.
Fig. 6.
Clinical signs of ehrlichiosis among dogs infected with E. canis by different measures. Panels A-D represent body temperature, packed cell volumes, platelets and total leukocyte counts, respectively. For each panel, days -21 through 0 represent values observed prior to exposure to carrier blood or infected ticks. Gray lines represent average values for acquisition hosts inoculated with carrier blood, dark solid lines represent average values for hosts exposed to intrastadially infected male R. sanguineus, and dashed lines represent average values for hosts exposed to transstadially infected male R. sanguineus.
Table 1.
Clinical observations of dogs used in this study
| Exposure to E. canis |
||||||
|---|---|---|---|---|---|---|
| Blood inoculation (i.v.) |
Transstadial tick transmission |
Intrastadial tick transmission |
||||
| ATKa | BAAa | AHKa | AIPa | AHGa | AUFa | |
| Temperature (°C) | ||||||
| Pre-exposureb (n = 21) | 38.5 ± 0.5 | 39.0 ± 0.2 | 38.3 ± 0.2 | 38.5 ± 0.2 | 38.4 ± 0.4 | 38.3 ± 0.3 |
| Minimumc | 38.2 (29) | 37.4 (2) | 37.7 (2) | 38.3 (1) | 37.7 (2) | 37.4 (30) |
| Maximumd | 40.1 (17) | 40.4 (20) | 39.2 (18) | 39.9 (17) | 40.4 (14) | 39.2 (39) |
| PCV (%) | ||||||
| Pre-exposure (n = 6) | 43.7 ± 2.9 | 45.6 ± 1.6 | 46.8 ± 3.1 | 49.2 ± 6.9 | 42.2 ± 2.1 | 46.2 ± 1.6 |
| Minimum | 24.9 (24) | 28.6 (31) | 38.9 (35) | 41.4 (42) | 35.8 (3) | 35.3 (3) |
| Maximum | 42.0 (3) | 45.1 (3) | 50.2 (3) | 48.8 (21) | 46.8 (42) | 48.6 (17) |
| Platelets/μl (× 103) | ||||||
| Pre-exposure (n = 6) | 384 ± 33 | 368 ± 51 | 387 ± 34 | 371 ± 74 | 359 ± 18 | 368 ± 70 |
| Minimum | 32 (17) | 1 (24) | 368 (28) | 265 (31) | 356 (42) | 318 (3) |
| Maximum | 424 (7) | 384 (7) | 488 (14) | 447 (7) | 484 (14) | 559 (21) |
| Neutrophils/μl (× 103) | ||||||
| Pre-exposure (n = 6) | 4.8 ± 0.5 | 4.8 ± 0.6 | 6.1 ± 0.8 | 6.5 ± 0.7 | 7.4 ± 0.6 | 6.4 ± 1.0 |
| Minimum | 3.2 (14) | 4.0 (17) | 5.2 (42) | 4.9 (35) | 4.7 (7) | 5.2 (3) |
| Maximum | 8.0 (3) | 7.1 (28) | 11.9 (10) | 8.3 (14) | 16.9 (14) | 10.8 (14) |
| Lymphocytes/μl (× 103) | ||||||
| Pre-exposure (n = 6) | 2.1 ± 0.9 | 1.8 ± 0.8 | 2.1 ± 0.4 | 2.1 ± 0.5 | 1.8 ± 0.6 | 1.8 ± 0.5 |
| Minimum | 0.4 (17) | 0.4 (17) | 1.1 (24) | 1.0 (10) | 1.2 (7) | 0.5 (7) |
| Maximum | 8.0 (3) | 7.1 (28) | 11.9 (10) | 8.3 (14) | 16.9 (14) | 10.8 (14) |
| Monocytes/μl (× 103) | ||||||
| Pre-exposure (n = 6) | 0.3 ± 0.3 | 0.4 ± 0.4 | 1.0 ± 0.2 | 0.8 ± 0.1 | 0.2 ± 0.0.2 | 0.7 ± 0.4 |
| Minimum | 0.1 (14) | 0.3 (17) | 0.7 (42) | 0.5 (31) | 0.6 (10) | 0.9 (10) |
| Maximum | 1.1 (28) | 1.4 (28) | 1.5 (10) | 1.0 (14) | 1.7 (7) | 2.0 (17) |
Host identification code.
Results reported as mean ± standard deviation for measurements collected over 21 days prior to exposure.
Smallest value recorded after initial exposure to E. canis; days from exposure to reported value are in parenthesis.
Largest value recorded after initial exposure to E. canis; days from exposure to reported value are in parenthesis.
4. Discussion
The results of this investigation demonstrate that male R. sanguineus ticks exposed to E. canis as either nymphs or as adults were capable of transmitting the pathogen under experimental conditions. These male ticks were fed without female counterparts, demonstrating that copulation was not required for acquisition or transmission of E. canis. Intrastadially exposed male ticks readily attached to hosts used for acquisition and transmission feeding, indicating that copulation was not required for multiple feedings by male R. sanguineus.
Detection of E. canis in male ticks after transmission feeding suggests that they could be capable of transmitting the pathogen to additional hosts. Kocan et al. (1992) demonstrated that male Dermacentor andersoni remained capable of transmitting A. marginale, which is closely related to E. canis, to at least five different hosts, and further investigation of this phenomenon with the E. canis model is warranted. E. canis was detected in similar numbers of ticks both before and after transmission feeding.
Comparison of clinical ehrlichiosis parameters was not the original objective of this study, but was prompted by striking differences observed between dogs infected by tick transmission versus inoculation with carrier blood. However, additional work with larger group sizes is necessary before solid conclusions can be drawn regarding these results.
Dogs infected with carrier blood had overt signs including progressive fever, anemia and thrombocytopenia, and significantly higher antibody titers than those infected by tick feeding. Due to the original objective of this investigation, i.e. to determine if male R. sanguineus are capable of acquisition and transmission of E. canis, this group was only monitored until tick acquisition feeding was completed at 31 dpi. Other reports indicated similar signs after dogs were injected with infected cell cultures or host blood (Dawson and Ewing, 1992; de Castro et al., 2004; Gaunt et al., 1996; Rikihisa et al., 1992). At least one study has demonstrated dose-dependent severity of disease associated with subcutaneous inoculation of E. canis in frozen stabilate (Gaunt et al., 1996).
Conversely, relatively mild clinical signs were observed in dogs infected with E. canis after tick feeding compared to those inoculated with carrier blood. Similar mild clinical signs of ehrlichiosis were reported among dogs after transmission feeding of ticks exposed to the closely related zoonotic pathogen, E. chaffeensis (Ewing et al., 1995; Unver et al., 2002). However, other reports associated with experimental tick transmission of E. canis have indicated more severe clinical signs when feeding greater numbers of transstadially infected male and female ticks (Johnson et al., 1998; Mathew et al., 1996). These observations suggest that differences observed in severity of ehrlichiosis among blood inoculated and tick-transmission infected dogs in this study could be due to a dose effect or due to a difference between E. canis strains. Adult female ticks, which were included in other investigations, might also have affected the severity of disease through transmission of larger pathogen numbers or through a more pronounced pharmacological effect on the vertebrate host (Bergman et al., 1995; Stich et al., 1993; Wikel et al., 1994). Regardless of the mechanism(s) underlying the differences in observed clinical signs, these results underscore the relevance of experimental tick transmission in characterization and development of control measures for E. canis and possibly other tick-borne pathogens.
Metastriate male ticks have ample opportunity to acquire and transmit pathogens, because they require a blood meal prior to sexual maturation and because they copulate on the vertebrate host (El Said and Swiderski, 1983; Homsher and Sonenshine, 1972, 1976; Kiszewski et al., 2001; Londt and Van Der Bijl, 1977; Londt and Spickett, 1976; Oliver and Dotson, 1993; Sonenshine, 1991; Valero et al., 1997). The importance of each tick transmission scenario is in part dependent on the preferred host for each tick species and developmental stage, and on the number of individual hosts upon which these stages feed. For R. sanguineus and D. variabilis, the two species experimentally demonstrated capable of transmitting E. canis, the absence of an intermittent molt in the intrastadial transmission cycle would mean a shorter requisite time period between pathogen acquisition and transmission. Intrastadial transmission could be even more important if immature stages are unlikely to feed on hosts that are susceptible to infection with the pathogen. Such is the case for D. variabilis, a tick species with adult stages that readily feed on dogs, but with immature stages with a reported predilection for natural infestation of smaller mammals (Apperson et al., 1993; Clark et al., 2001; Gage et al., 1992; Jackson et al., 1975; Kollars, 1996; Sonenshine et al., 1966). Further work is warranted to determine if male D. variabilis are capable of intrastadial or transstadial transmission of E. canis. Cross sectional surveys of natural canine tick burdens indicated that the majority of adult R. sanguineus collected were male (Horak et al., 1995; L'Hostis et al., 1998), supporting the paradigm that male R. sanguineus, like other metastriates, may persist after their female counterparts have left the host (Sonenshine, 1991). In addition, metastriate male ticks are attracted to feeding sites by aggregation pheromones and to feeding females by sexual pheromones (Andrew and Norval, 1989; Leahy and Booth, 1983; Norval et al., 1989, 1996; Sobbhy et al., 1994; Sonenshine, 1985), suggesting means for inter-host transfer and intermittent feedings on multiple vertebrate hosts. Thus, male R. sanguineus may remain in the environment, potentially transmitting E. canis to multiple canine hosts without further acquisition or molting periods.
In conclusion, male R. sanguineus were capable of both intrastadial and transstadial transmission of E. canis under experimental conditions. The rickettsia was detected in individual ticks from both groups before and after they were allowed to transmission feed on susceptible dogs. Both groups appeared equally capable of transmitting the pathogen to canine hosts. These transmission scenarios took place in the absence of female ticks, indicating that copulation was not required for multiple feedings or pathogen acquisition and transmission by the male ticks. Milder disease associated with transmission of the pathogen by these ticks suggests that tick transmission is an important consideration for experimental characterization of canine monocytic ehrlichiosis. Determining how the presence of female ticks affects acquisition and transmission of E. canis could provide new insights into the mechanisms underlying acquisition and transmission of this pathogen by the tick host. Finally, our results indicate that male R. sanguineus are potentially important vectors of E. canis from both experimental and epizootiological perspectives, and that similar transmission scenarios should be considered for related vector and pathogen species.
Acknowledgements
This work was supported by NIH grant AI47932 (RWS) and The Ohio State University College of Veterinary Medicine Canine Research Fund. The authors thank Kirsten Boughan for excellent technical assistance, Jerry Bowman for R. sanguineus and Dr. R.L. Hamlin for support.
References
- Andrew HR, Norval RA. The role of males of the bont tick (Amblyomma hebraeum) in the transmission of Cowdria ruminantium (heartwater) Vet. Parasitol. 1989;34:15–23. doi: 10.1016/0304-4017(89)90159-3. [DOI] [PubMed] [Google Scholar]
- Apperson CS, Levine JF, Evans TL, Braswell A, Heller J. Relative utilization of reptiles and rodents as hosts by immature Ixodes scapularis (Acari: Ixodidae) in the coastal plain of North Carolina. U.S.A. Exp. Appl. Acarol. 1993;17:719–731. doi: 10.1007/BF00051830. [DOI] [PubMed] [Google Scholar]
- Bergman DK, Ramachandra RN, Wikel SK. Dermacentor andersoni: salivary gland proteins suppressing T-lymphocyte responses to concanavalin A in vitro. Exp. Parasitol. 1995;81:262–271. doi: 10.1006/expr.1995.1117. [DOI] [PubMed] [Google Scholar]
- Clark KL, Oliver JH, Jr., Grego JM, James AM, Durden LA, Banks CW. Host associations of ticks parasitizing rodents at Borrelia burgdorferi enzootic sites in South Carolina. J. Parasitol. 2001;87:1379–1386. doi: 10.1645/0022-3395(2001)087[1379:HAOTPR]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Dawson JE, Ewing SA. Susceptibility of dogs to infection with Ehrlichia chaffeensis, causative agent of human ehrlichiosis. Am. J. Vet. Res. 1992;53:1322–1327. [PubMed] [Google Scholar]
- de Castro MB, Machado RZ, de Aquino LP, Alessi AC, Costa MT. Experimental acute canine monocytic ehrlichiosis: clinicopathological and immunopathological findings. Vet. Parasitol. 2004;119:73–86. doi: 10.1016/j.vetpar.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Dumler JS, Barbet AF, Bekker CP, Dasch GA, Palmer GH, Ray SC, Rikihisa Y, Rurangirwa FR. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and ‘HGE agent’ as subjective synonyms of Ehrlichia phagocytophila. Int. J. Syst. Evol. Microbiol. 2001;51:2145–2165. doi: 10.1099/00207713-51-6-2145. [DOI] [PubMed] [Google Scholar]
- El Said AM, Swiderski Z. Copulation and spermatophore formation in Hyalomma dromedarii. J. Egypt Soc. Parasitol. 1983;13:59–62. [PubMed] [Google Scholar]
- Ewing SA. Canine ehrlichiosis. Adv. Vet. Sci. Comp. Med. 1969;13:331–353. [PubMed] [Google Scholar]
- Ewing SA, Dawson JE, Kocan AA, Barker RW, Warner CK, Panciera RJ, Fox JC, Kocan KM, Blouin EF. Experimental transmission of Ehrlichia chaffeensis (Rickettsiales: Ehrlichieae) among white-tailed deer by Amblyomma americanum (Acari: Ixodidae) J. Med. Entomol. 1995;32:368–374. doi: 10.1093/jmedent/32.3.368. [DOI] [PubMed] [Google Scholar]
- Gage KL, Hopla CE, Schwan TG. Cotton rats and other small mammals as hosts for immature Dermacentor variabilis (Acari: Ixodidae) in central Oklahoma. J. Med. Entomol. 1992;29:832–842. doi: 10.1093/jmedent/29.5.832. [DOI] [PubMed] [Google Scholar]
- Gaunt SD, Corstvet RE, Berry CM, Brennan B. Isolation of Ehrlichia canis from dogs following subcutaneous inoculation. J. Clin. Microbiol. 1996;34:1429–1432. doi: 10.1128/jcm.34.6.1429-1432.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves MG, Dennis GL, Amyx HL, Huxsoll DL. Transmission of Ehrlichia canis to dogs by ticks (Rhipicephalus sanguineus) Am. J. Vet. Res. 1975;36:937–940. [PubMed] [Google Scholar]
- Harrus S, Waner T, Bark H, Jongejan F, Cornelissen AW. Recent advances in determining the pathogenesis of canine monocytic ehrlichiosis. J. Clin. Microbiol. 1999;37:2745–2749. doi: 10.1128/jcm.37.9.2745-2749.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey JW, Simpson CF, Gaskin JM, Sameck JH. Ehrlichiosis in wolves, dogs, and wolf-dog crosses. J. Am. Vet. Med. Assoc. 1979;175:901–905. [PubMed] [Google Scholar]
- Homsher PJ, Sonenshine DE. Spermatogenesis in Dermacentor variabilis (Say) in relation to duration of attachment and the presence of chromosomal aberrations in stocks injected with radioactive glucose (Acarina: Ixodidae) J. Med. Entomol. 1972;9:171–177. doi: 10.1093/jmedent/9.2.171. [DOI] [PubMed] [Google Scholar]
- Homsher PJ, Sonenshine DE. The effect of presence of females on spermatogenesis and early mate seeking behavior in two species of Dermacentor ticks (Acari: Ixodidae) Acarologia. 1976;18:226–233. [PubMed] [Google Scholar]
- Horak IG, Boomker J, Flamand JR. Parasites of domestic and wild animals in South Africa. XXXIV. Arthropod parasites of nyalas in north-eastern KwaZulu-Natal. Onderstepoort. J. Vet. Res. 1995;62:171–179. [PubMed] [Google Scholar]
- Jackson JO, DeFoliart GR, Sonenshine DE, Stout J. Relationships of immature Dermacentor variabilis (Say) (Acari: Ixodidae) with the white-footed mouse, Peromyscus leucopus, in southwestern Wisconsin. J. Med. Entomol. 1975;12:409–412. doi: 10.1093/jmedent/12.4.409. [DOI] [PubMed] [Google Scholar]
- Johnson EM, Ewing SA, Barker RW, Fox JC, Crow DW, Kocan KM. Experimental transmission of Ehrlichia canis (Rickettsiales: Ehrlichieae) by Dermacentor variabilis (Acari: Ixodidae) Vet. Parasitol. 1998;74:277–288. doi: 10.1016/s0304-4017(97)00073-3. [DOI] [PubMed] [Google Scholar]
- Kiszewski AE, Matuschka FR, Spielman A. Mating strategies and spermiogenesis in ixodid ticks. Annu. Rev. Entomol. 2001;46:167–182. doi: 10.1146/annurev.ento.46.1.167. [DOI] [PubMed] [Google Scholar]
- Kocan KM, Goff WL, Stiller D, Claypool PL, Edwards W, Ewing SA, Hair JA, Barron SJ. Persistence of Anaplasma marginale (Rickettsiales: Anaplasmataceae) in male Dermacentor andersoni (Acari: Ixodidae) transferred successively from infected to susceptible calves. J. Med. Entomol. 1992;29:657–668. doi: 10.1093/jmedent/29.4.657. [DOI] [PubMed] [Google Scholar]
- Kollars TM., Jr. Interspecific differences between small mammals as hosts of immature Dermacentor variabilis (Acari: Ixodidae) and a model for detection of high risk areas of Rocky Mountain spotted fever. J. Parasitol. 1996;82:707–710. [PubMed] [Google Scholar]
- L'Hostis M, Rose-Rosette F, Thomas N, Fourgeaud P. Tick infestation of feral dogs in Martinique. Ann. N.Y. Acad. Sci. 1998;849:395–397. doi: 10.1111/j.1749-6632.1998.tb11079.x. [DOI] [PubMed] [Google Scholar]
- Leahy MG, Booth KS. Attraction of metastriate ticks (Acari: Ixodidae) to the sex pheromone 2,6-dichlorophenol and to substituted phenols. J. Med. Entomol. 1983;20:104–105. doi: 10.1093/jmedent/20.1.104. [DOI] [PubMed] [Google Scholar]
- Lewis GE, Jr., Ristic M, Smith RD, Lincoln T, Stephenson EH. The brown dog tick Rhipicephalus sanguineus and the dog as experimental hosts of Ehrlichia canis. Am. J. Vet. Res. 1977;38:1953–1955. [PubMed] [Google Scholar]
- Londt GH, Van Der Bijl EB. The life cycle of the two-host tick Rhipicephalus evertsi evertsi Neumann 1897 under laboratory conditions (Acarina: Ixodidae) Onderstepoort. J. Vet. Res. 1977;44:21–28. [PubMed] [Google Scholar]
- Londt JG, Spickett AM. Gonad development and gametogenesis in Boophilus decoloratus (Koch 1844) (Acarina Metastriata Ixodidae) Onderstepoort. J. Vet. Res. 1976;43:79–96. [PubMed] [Google Scholar]
- Mathew JS, Ewing SA, Barker RW, Fox JC, Dawson JE, Warner CK, Murphy GL, Kocan KM. Attempted transmission of Ehrlichia canis by Rhipicephalus sanguineus after passage in cell culture. Am. J. Vet. Res. 1996;57:1594–1598. [PubMed] [Google Scholar]
- Norval RA, Andrew HR, Yunker CE. Pheromone-mediation of host-selection in bont ticks (Amblyomma hebraeum koch) Science. 1989;243:364–365. doi: 10.1126/science.2911745. [DOI] [PubMed] [Google Scholar]
- Norval RA, Sonenshine DE, Allan SA, Burridge MJ. Efficacy of pheromone-acaricide-impregnated tail-tag decoys for controlling the bont tick, Amblyomma hebraeum (Acari: Ixodidae), on cattle in Zimbabwe. Exp. Appl. Acarol. 1996;20:31–46. doi: 10.1007/BF00051475. [DOI] [PubMed] [Google Scholar]
- Olano JP, Walker DH. Human ehrlichioses. Med. Clin. North Am. 2002;86:375–392. doi: 10.1016/s0025-7125(03)00093-2. [DOI] [PubMed] [Google Scholar]
- Oliver JH, Dotson EM. Hormonal control of molting and reproduction in ticks. Am. Zool. 1993;33:384–396. [Google Scholar]
- Paddock CD, Childs JE. Ehrlichia chaffeensis: a prototypical emerging pathogen. Clin. Microbiol. Rev. 2003;16:37–64. doi: 10.1128/CMR.16.1.37-64.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potgeiter FT. Tick transmission of anaplasmosis in South Africa. Proceedings of the International Conference on Tick Biology and Control; Grahamstown, South Africa. 1981. p. 222. [Google Scholar]
- Rikihisa Y. The tribe Ehrlichieae and ehrlichial diseases. Clin. Microbiol. Rev. 1991;4:286–308. doi: 10.1128/cmr.4.3.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikihisa Y, Ewing SA, Fox JC. Western immunoblot analysis of Ehrlichia chaffeensis, E. canis, or E. ewingii infections in dogs and humans. J. Clin. Microbiol. 1994;32:2107–2112. doi: 10.1128/jcm.32.9.2107-2112.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikihisa Y, Ewing SA, Fox JC, Siregar AG, Pasaribu FH, Malole MB. Analyses of Ehrlichia canis and a canine granulocytic Ehrlichia infection. J. Clin. Microbiol. 1992;30:143–148. doi: 10.1128/jcm.30.1.143-148.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikihisa Y, Stills H, Zimmerman G. Isolation and continuous culture of Neorickettsia helminthoeca in a macrophage cell line. J. Clin. Microbiol. 1991;29:1928–1933. doi: 10.1128/jcm.29.9.1928-1933.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skotarczak B. Canine ehrlichiosis. Ann. Agric. Environ. Med. 2003;10:137–141. [PubMed] [Google Scholar]
- Sobbhy H, Aggour MG, Sonenshine DE, Burridge MJ. Cholesteryl esters on the body surfaces of the camel tick, Hyalomma dromedarii (Koch, 1844) and the brown dog tick, Rhipicephalus sanguineus (Latreille 1806) Exp. Appl. Acarol. 1994;18:265–280. doi: 10.1007/BF00132316. [DOI] [PubMed] [Google Scholar]
- Sonenshine DE. Pheromones and other semiochemicals of the acari. Annu. Rev. Entomol. 1985;30:1–28. doi: 10.1146/annurev.en.30.010185.000245. [DOI] [PubMed] [Google Scholar]
- Sonenshine DE. Biology of Ticks. Oxford University Press; New York: 1991. [Google Scholar]
- Sonenshine DE, Atwood EL, Lamb JT., Jr. The ecology of ticks transmitting Rocky Mountain spotted fever in a study area in Virginia. Ann. Entomol. Soc. Am. 1966;59:1234–1262. doi: 10.1093/aesa/59.6.1234. [DOI] [PubMed] [Google Scholar]
- Steel RGD, Torrie JH. Principles and Procedures of Statistics, A Biometrical Approach. second ed. McGraw-Hill Book Company; New York: 1980. [Google Scholar]
- Stich RW, Rikihisa Y, Ewing SA, Needham GR, Grover DL, Jittapalapong S. Detection of Ehrlichia canis in canine carrier blood and in individual experimentally infected ticks with a p30-based PCR assay. J. Clin. Microbiol. 2002;40:540–546. doi: 10.1128/JCM.40.2.540-546.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stich RW, Sauer JR, Bantle JA, Kocan KM. Detection of Anaplasma marginale (Rickettsiales: Anaplasmataceae) in secretagogue-induced oral secretions of Dermacentor andersoni (Acari: Ixodidae) with the polymerase chain reaction. J. Med. Entomol. 1993;30:789–794. doi: 10.1093/jmedent/30.4.789. [DOI] [PubMed] [Google Scholar]
- Stiller D, Coan ME. Recent developments in elucidating tick vector relationships for anaplasmosis and equine piroplasmosis. Vet. Parasitol. 1995;57:97–108. doi: 10.1016/0304-4017(94)03114-c. [DOI] [PubMed] [Google Scholar]
- Stiller D, Coan ME, Goff WL, Johnson LW, McGuire TC. The importance and putative role of Dermacentor spp. males in anaplasmosis epidemiology: transmission of Anaplasma marginale to cattle by ad libitum interhost transfer of D. andersoni males under semi-natural conditions. Proceedings of the Eighth National Veterinary Hemoparasite Disease Conference; St. Louis, MO. 1989. p. 209. [Google Scholar]
- Unver A, Rikihisa Y, Stich RW, Ohashi N, Felek S. The omp-1 major outer membrane multigene family of Ehrlichia chaffeensis is differentially expressed in canine and tick hosts. Infect. Immun. 2002;70:4701–4704. doi: 10.1128/IAI.70.8.4701-4704.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valero A, Hueli LE, Diaz-Saez V. Spermatogenesis in the Ixodid tick Haemaphysalis (Herpetobia) sulcata (Acarina: Ixodidae) J. Parasitol. 1997;83:212–214. [PubMed] [Google Scholar]
- Walker DH, Dumler JS. Emergence of the ehrlichioses as human health problems. Emerg. Infect. Dis. 1996;2:18–29. doi: 10.3201/eid0201.960102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen B, Rikihisa Y, Mott JM, Greene R, Kim HY, Zhi N, Couto GC, Unver A, Bartsch R. Comparison of nested PCR with immunofluorescent-antibody assay for detection of Ehrlichia canis infection in dogs treated with doxycycline. J. Clin. Microbiol. 1997;35:1852–1855. doi: 10.1128/jcm.35.7.1852-1855.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikel SK, Ramachandra RN, Bergman DK. Tick-induced modulation of the host immune response. Int. J. Parasitol. 1994;24:59–66. doi: 10.1016/0020-7519(94)90059-0. [DOI] [PubMed] [Google Scholar]