Abstract
The association between alcohol use and internalizing symptoms during adolescence varies across studies, and the causes underlying this association remain unclear. The current study examines the relationship between symptoms of anxiety and depression and intoxication frequency in a sample of Swedish twins assessed longitudinally from ages 13–14 to 19–20. The objectives of the study were to assess the stability of genetic and environmental influences on each trait across adolescence; to investigate whether these traits share genetic and/or environmental liabilities; and to explore quantitative changes in the shared liability over time. We found that the magnitude of genetic influences on internalizing symptoms remained relatively stable across adolescence, while their impact on intoxication frequency was dynamic. Symptoms of anxiety and depression were influenced by unique environmental factors, while both shared and unique environmental factors influenced intoxication frequency. Genetic and environmental innovation and attenuation were observed for both traits. While no significant genetic correlation was observed between traits, unique environmental factors did contribute to a shared liability. This environmental correlation was positive and moderate (rE=0.41) in the early assessment, but decreased and changed direction at later waves (rE=−.04 –−.01). The genetic and environmental factors underlying internalizing symptoms and intoxication frequency appear to be developmentally dynamic. Early environmental factors contribute to the association between these traits, but this shared liability diminishes across adolescence.
Keywords: genetic innovation and attenuation, shared liability, twin modeling, internalizing symptoms, alcohol use
1. Introduction
Internalizing symptoms and alcohol use are often phenotypically correlated. Studies examining the relationship between clinical manifestations of these traits (for example, major depression and alcohol dependence) in adults typically report that individuals suffering from one of the disorders are two to six times more likely to suffer from the other disorder as well (B. F. Grant, et al., 2004; Hasin, Goodwin, Stinson, & Grant, 2005; Kendler, Heath, Neale, Kessler, & Eaves, 1993; Kessler, et al., 1997). Adolescence is a critical developmental period for investigating this association: internalizing symptoms commonly manifest during this time, and the lifetime prevalence of a DSM-IV major depressive episode during adolescence is 14% (Office of Applied Studies, 2005). In addition, initial experimentation with alcohol typically occurs in adolescence (Johnston, O'Malley, Bachman, & Schulenberg, 2006), and these early experiences can be predictive of later alcohol problems (B. F. Grant, Stinson, & Harford, 2001).
A number of epidemiological studies have examined the co-occurrence of internalizing symptoms and alcohol use prior to adulthood. A study of a sample of adolescent Finnish twins found that early-onset (before age 15) depressive disorders were positively associated with frequency of alcohol use and intoxication (Sihvola, et al., 2008). Likewise, Strandheim and colleagues (Strandheim, Holmen, Coombes, & Bentzen, 2009) found that, among female Norwegian teenagers, symptoms of anxiety and depression were positively associated with alcohol intoxication frequency. Another study indicated that depressed mood during childhood is associated with early age of onset of alcohol use, problematic alcohol use during adolescence, and adult alcohol dependence (AD) (Crum, et al., 2008). Some studies have found evidence of a causal relationship between these traits (Fergusson, Boden, & Horwood, 2009; Kuo, Gardner, Kendler, & Prescott, 2006; Lyons, et al., 2006), though these results are based on adult samples and are inconsistent in terms of whether alcohol use influences mood or vice versa. There is also evidence that mood-related drinking motivations impact the association between internalizing symptoms and alcohol use (V. V. Grant, Stewart, & Mohr, 2009; Young-Wolff, Kendler, Sintov, & Prescott, 2009).
Genetically informative samples can be used to determine whether the phenotypic association between these traits is partially attributable to non-causal influences, such as latent genetic and/or environmental factors that influence liability to both traits. Results from genetic epidemiological studies investigating shared liability across internalizing symptoms and alcohol use have been inconsistent. One study of U.S. adult women found that major depression (MD) and AD were genetically correlated (rA=0.4–0.6, Kendler et al., 1993). A study of young adult Norwegian twins reported that, among males, the modest phenotypic correlation between alcohol consumption and symptoms of anxiety and depression could be accounted for by shared genetic factors; for women, the correlation was due to either shared environmental influences or a combination of common genetic and environmental influences (Tambs, Harris, & Magnus, 1997). However, within a sample of adult U.S. male twins, the genetic relationship between AD and MD could be accounted for by the genetic correlation between both of these traits and antisocial personality disorder (Fu, et al., 2002). Despite these inconsistencies, the concept of shared genetic liability is supported by evidence from molecular genetic studies, which have identified genetic variants that are associated with both phenotypes (see (Saraceno, Munafò, Heron, Craddock, & B. M. van den Bree, 2009) for a review). Unfortunately, many such findings have not been consistently replicated, and must be considered tentative in the absence of more compelling evidence.
Despite the fact that the phenotypic association between internalizing symptoms and alcohol use often begins during adolescence, little information regarding genetic correlations between traits during this critical developmental period is available. There is evidence that both traits are genetically dynamic during this period (Bergen, Gardner, & Kendler, 2007; Hicks, et al., 2007; Kendler, Gardner, & Lichtenstein, 2008), with heritability for both typically increasing with age. Shared environmental factors are often influential during adolescence, particularly on alcohol use phenotypes (Pagan, et al., 2006; Rose, Dick, Viken, Pulkkinen, & Kaprio, 2001, 2004), and could also partially account for phenotypic associations between traits.
Here, we present results from a longitudinal study of a population-based sample of Swedish twins who were assessed for internalizing symptoms and frequency of alcohol intoxication across several waves of data collection spanning adolescence. A previous report detailed changes in the heritability of internalizing symptoms for this sample (Kendler, Gardner, et al., 2008); the current analyses differ methodologically in several ways (see Methods). In the current analyses, we sought to assess genetic and environmental on internalizing symptoms and intoxication frequency, as well as their innovation and attenuation across adolescence; to determine whether shared genetic and/or environmental factors contribute to the phenotypic association between phenotypes; and to investigate quantitative changes in shared liability across adolescence.
2. Methods
2.1. Sample
These analyses include participants in the longitudinal Swedish Twin Study of Child and Adolescent Development (TCHAD), which began with twin pairs born in Sweden between May 1985 and December 1986, and where both twins were alive and residing in Sweden in 1994 (Lichtenstein, Tuvblad, Larsson, & Carlstrom, 2007). The first wave involved parental responses to questionnaires when children were aged 8–9 years; the current analyses include only adolescents’ self-reports for wave 2 (age 13–14), wave 3 (age 16–17), and wave 4 (age 19–20). Only same-sex twins were included in the analyses (482 monozygotic (MZ) female twins, 362 female dizygotic (DZ) twins, 474 MZ male twins, and 333 DZ male twins). Zygosity was determined based on well-validated questions to parents and twins, which in turn were based on algorithms derived from analyses of a subset of twin pairs of known zygosity (Lichtenstein, et al., 2007). This sample was 47.2% male. Data were available for 1586, 1663, and 1223 individuals at waves 2, 3, and 4, respectively. The Ethics Committee of the Karolinska Institute, Stockholm, Sweden approved each questionnaire; Swedish rules state that response to the questionnaire constitutes informed consent.
2.2. Measures
2.2.1. Symptoms of anxiety and depression
Self-reports of symptoms of anxiety and depression were obtained using items from the Anxious/Depressed subscale of the Child Behavior Checklist (CBCL; (Achenbach, 1991) for waves 2 and 3; at wave 4, items were from the similar Adult Behavior Checklist (Achenbach & Rescorla, 2003). Only the 12 items that are common across both scales were used for these analyses. Items are scored on a three-point (0, 1, or 2 points) scale: not true, somewhat or sometimes true, and very or often true. Sum-scores were calculated for each individual, with a possible range of 0–24. The sum score was then converted to an ordinal variable from 0 (lowest level of anxiety/depression) to 3 (highest level of anxiety/depression for twin modeling. We will refer to these variables as “symptoms of anxiety and depression,” or “SxAnxDep.” Readers should note that a previous report using these data also used this variable name (Kendler, Gardner, et al., 2008). However, that report employed a continuous factor score from the same measure, which was derived from parental and self-reports, across four waves of data. The variables used for the current analysis are derived from only self-reports across three waves of data, and are used as an ordinal measure in twin modeling.
2.2.2. Alcohol use
Questions regarding alcohol use varied across waves; we focused on frequency of intoxication, a measure that remained relatively consistent across waves. In Wave 2 (the first wave of adolescents’ self-reports), respondents were asked how frequently in the past 12 months they had “drunk beer, wine, liquor, or other alcohol so that you felt intoxicated?” Options ranged in frequency from “never” to “more than 50 times.” In Wave 3, respondents were asked how often they get drunk when drinking alcohol, and response options ranged from “don’t drink” to “always.” Finally, in the fourth wave, participants responded to the question “How often do you get drunk when you drink?” with response options ranging from “don’t drink alcohol” to “daily.” Because the exact response options varied across waves, responses were condensed into ordinal scales ranging from 0 (least frequent intoxication) to 3 (most frequent intoxication). For simplicity, we will refer to these measures of intoxication frequency as “intoxication frequency”, or “IntoxFreq”, at different waves.
2.3. Statistical Analyses
2.3.1. Descriptive Statistics
Descriptive statistics were calculated in using SAS version 9.1 and Mx (Neale, Boker, Xie, & Maes, 2003). In regression analyses, significance level was corrected for the correlational structure of the twin data. Twin correlations were calculated in SAS.
2.3.2. Twin Modeling
Twin modeling was conducted in Mx using the raw ordinal data option. The use of ordinal data assumes that the categories are representative of an underlying normal distribution of liability, with thresholds in liability discriminating between categories. In twin modeling, liability to phenotypes such as depression or alcohol use can be attributed to several latent sources of variance: additive genetic factors (A), shared environment (C), and unique environment (E). The C variance component represents environmental exposures and experiences that are shared by both members of a twin pair and contribute to twins’ increased similarity irrespective of zygosity in a given phenotype. Environmental factors that are unique to one twin are accounted for by the E component; these factors reduce twin similarity for a given phenotype. The E component also includes measurement error. Estimates of each of these variance components are calculated by comparing the phenotypic correlation between monozygotic twins, who share all their genes, to dizygotic twins, who share half of their genes on average identical by descent.
We used a Cholesky decomposition (model) with six variables: SxAnxDep and IntoxFreq at each of the three waves described above. Variables were ordered by wave; within each wave, SxAnxDep preceded intoxication frequency. The full model allows for genetic influences that impact both outcomes (SxAnxDep and intoxication frequency), across all three waves (2–4) (factor A1); genetic influences that don’t impact SxAnxDep at wave 2, but influence all other variables (A2); other genetic influences that only become evident at wave 3 (A3), influence both variables and carry through to wave 4, etc. The C and E pathways follow the same pattern. This is an atheoretical model from which submodels are fit to determine the best-fitting model for the data. Figure 1 depicts the genetic structure of a full model; the structure is identical for shared and unique environmental paths.
Figure 1.
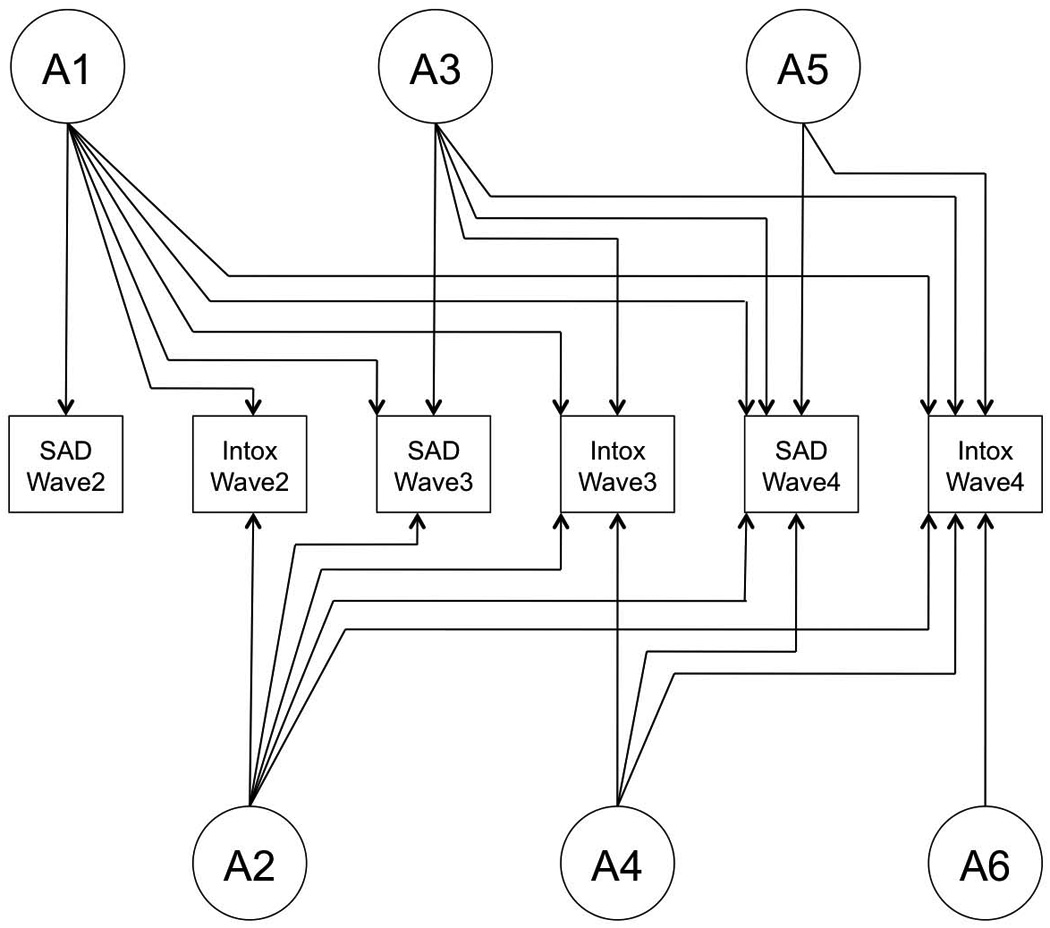
Genetic structure of a full model. Shared and unique environmental structures are identical to that of genetic factors.
Models were estimated using a full information maximum likelihood estimation method. The fit of nested models was assessed using the Akaike Information Criterion (AIC, (Akaike, 1987) to select models. A lower AIC value indicates a better balance between the explanatory power of a model and its parsimony. Our goal is to minimize the AIC value so a submodel is accepted if the change in AIC is negative.
3. Results
3.1. Descriptive Statistics and Regression Analyses
Descriptive statistics for self-reported symptoms of anxiety and depression (SxAnxDep), before being ordinalized for twin modeling, are provided in Table 1. Significant sex differences in SxAnxDep sum scores were observed at each wave, with girls reporting higher levels of symptoms. Self-reported frequency of intoxication at each wave is provided in Table 2; no significant differences were seen between the sexes at any wave.
Table 1.
Descriptive data. Descriptives of sum scores of self-reported symptoms of anxiety and depression, by sex, for Waves 2–4 of data collection. Significance of sex differences is corrected for the correlation between twins.
| Boys | Girls | Sex Differences |
|||
|---|---|---|---|---|---|
| Mean (SE) | Range | Mean (SE) | Range | ||
| Wave 2 SxAnxDep | 2.47 (0.10) | 0–18 | 4.00 (0.14) | 0–21 | Z= −7.42*** |
| Wave 3 SxAnxDep | 2.05 (0.10) | 0–20 | 4.49 (0.15) | 0–19 | Z= −11.94*** |
| Wave 4 SxAnxDep | 2.52 (0.16) | 0–22 | 5.41 (0.18) | 0–22 | Z= −10.71*** |
p<0.001
Table 2.
Distribution of intoxication frequency at each wave of data collection, separately by sex. See Methods for item description.
| Wave 2 | ||
|---|---|---|
| Boys | Girls | |
| Never | 81.6% | 84.5% |
| Occasionally | 12.7% | 9.4% |
| A few times | 3.5% | 3.2% |
| Quite a few times | 1.3% | 1.6% |
| Often | 0.7% | 0.9% |
| Very often | 0.3% | 0.3% |
| Sex differences | Z=0.26 (n.s.) | |
| Wave 3 | ||
|---|---|---|
| Boys | Girls | |
| Don’t drink | 38.1% | 34.2% |
| Never/seldom | 16.6% | 17.2% |
| Sometimes | 18.8% | 19.9% |
| Nearly every time | 18.1% | 21.7% |
| Always | 8.3% | 7.0% |
| Sex differences | Z= −0.93 (n.s.) | |
| Wave 4 | ||
|---|---|---|
| Boys | Girls | |
| Don’t drink | 7.5% | 5.7% |
| Never | 5.8% | 9.3% |
| Once per year | 6.0% | 5.7% |
| 3–4 times per year | 10.7% | 11.2% |
| Once per 2 months | 11.2% | 13.4% |
| Once per month | 18.4% | 16.5% |
| Twice per month | 35.6% | 26.1% |
| Once per week | 11.1% | 10.1% |
| Twice per week | 3.7% | 2.2% |
| Daily | 0% | 0% |
| Sex differences | Z= −0.71 (n.s.) | |
We conducted regressions between SxAnxDep and IntoxFreq where temporally appropriate (i.e., contemporaneous SxAnxDep and IntoxFreq, or where the independent variable temporally preceded the dependent variable); for later waves, preceding waves’ phenotypes were included as covariates. We detected statistically significant associations between traits at Wave 2 for both sexes, wherein higher SxAnxDep scores were associated with more frequent intoxication (boys: beta=0.02, Z=2.06, p=0.04; girls: beta=0.03, Z=2.81, p=0.005). At Wave 3, no contemporaneous association existed, but Wave 2 drinking was significantly associated with Wave 3 IntoxFreq (boys: beta=0.45, Z=5.11, p<0.0001; girls: beta=0.45, Z=7.18, p<0.0001). Associations with Wave 4 IntoxFreq differed by sex. For boys, Wave 3 SxAnxDep was negatively associated with Wave 4 IntoxFreq (beta=−0.12, Z=−2.32, p=0.02); for girls, Wave 2 SxAnxDep was positively associated with Wave 4 IntoxFreq (beta=0.04, Z=2.09, p=0.04). In regressions using SxAnxDep as the dependent variable, there were no significant associations between IntoxFreq variables and concurrent or later SxAnxDep outcome. These findings underscore the complicated association between these traits, and suggest that modest sex differences exist.
3.2. Twin Modeling
Twin correlations were derived in Mx from ordinalized data and are presented in Table 3. The cross-twin, within-trait correlations are consistently higher for MZ twins than for DZ twins, indicating that genetic factors can at least partially account for phenotypic variance. Cross-trait correlations – that is, correlations across SxAnxDep and IntoxFreq -- whether contemporaneous or across waves – were quite low, and were not consistently higher for MZ than for DZ twins.
Table 3.
Twin correlations. Polychoric correlations for ordinalized twin data are presented for monozygotic twins (below diagonal, and listed first on the diagonal) and dizygotic twins (above the diagonal, and listed second on the diagonal).
| Wave 2 SxAnxDep |
Wave 3 SxAnxDep |
Wave 4 SxAnxDep |
Wave 2 Intox |
Wave 3 Intox |
Wave 4 Intox |
|
|---|---|---|---|---|---|---|
| Wave 2 SxAnxDep | 0.50/0.36 | 0.37 | 0.28 | 0.09 | 0.08 | 0.02 |
| Wave 3 SxAnxDep | 0.44 | 0.54/0.46 | 0.34 | 0.17 | 0.05 | 0.02 |
| Wave 4 SxAnxDep | 0.38 | 0.41 | 0.47/0.32 | 0.08 | 0.11 | 0.00 |
| Wave 2 Intox | 0.01 | −0.01 | −0.04 | 0.84/0.57 | 0.39 | 0.09 |
| Wave 3 Intox | 0.13 | 0.08 | 0.09 | 0.59 | 0.85/0.66 | 0.23 |
| Wave 4 Intox | 0.01 | 0.01 | 0.00 | 0.37 | 0.54 | 0.72/0.32 |
Twin modeling statistics are provided in Table 4. We found that sources of variance could be constrained to be equal across the sexes, so subsequent submodels included this constraint. We next tested whether an AE model (which excludes shared environmental variance) or a CE model (which excludes additive genetic variance) fit the data well; both models fit significantly worse than the full ACE model and were rejected. However, subsequent testing of submodels (Models 5 and 6 in Table 4) indicated that shared environmental (C) influences on SxAnxDep could be removed from the model without worsening fit, i.e., shared environmental factors significantly influenced only the IntoxFreq variables.
Table 4.
Model fitting procedure with fit statistics. Submodels were adopted if the AIC was negative.
| # | Model Description | Comparison | Δdf | Δχ2 | p-value | ΔAIC |
|---|---|---|---|---|---|---|
| 1 | Full | (7793) | (15897.445) | n/a | (311.445) | |
| 2 | Constrain variance to be equal across sexes | 2 vs. 1 | 63 | 47.238 | 0.931 | −78.762 |
| 3 | AE Model | 3 vs. 2 | 21 | 40.938 | 0.006 | −1.062 |
| 4 | CE Model | 4 vs. 2 | 21 | 71.151 | <0.001 | 29.151 |
| 5 | Drop all loadings from C1, C3, and C5 | 5 vs. 2 | 12 | 4.972 | 0.959 | −19.028 |
| 6 | Drop loadings onto SxAnxDep from C2 & C4 | 6 vs. 5 | 3 | 4.923 | 0.178 | −1.077 |
| 7 | Drop cross-wave genetic effects | 7 vs. 6 | 12 | 169.430 | <0.001 | 145.430 |
| 8 | Drop genetic correlations between SxAnxDep and IntoxFreq* | 8 vs. 6 | 9 | 4.676 | 0.854 | −13.324 |
| 9 | Drop cross-wave unique environmental effects | 9 vs. 8 | 12 | 71.779 | <0.001 | 47.779 |
| 10 | Drop unique environmental correlations between SxAnxDep and IntoxFreq | 10 vs. 8 | 9 | 28.318 | 0.001 | 10.318 |
This submodel became the Final Model referred to in the text.
Removing cross-wave genetic factor loadings (Model 7) significantly worsened model fit, indicating that genetic influences evident at early waves remain relevant at later waves. Model 8 tested the hypothesis that genetic correlations existed between SxAnxDep and IntoxFreq. Results indicated that genetic correlations across these traits were not significant: removal of these paths did not significantly reduce model fit, and resulted in a negative change in AIC; thus, subsequent models did not include these paths.
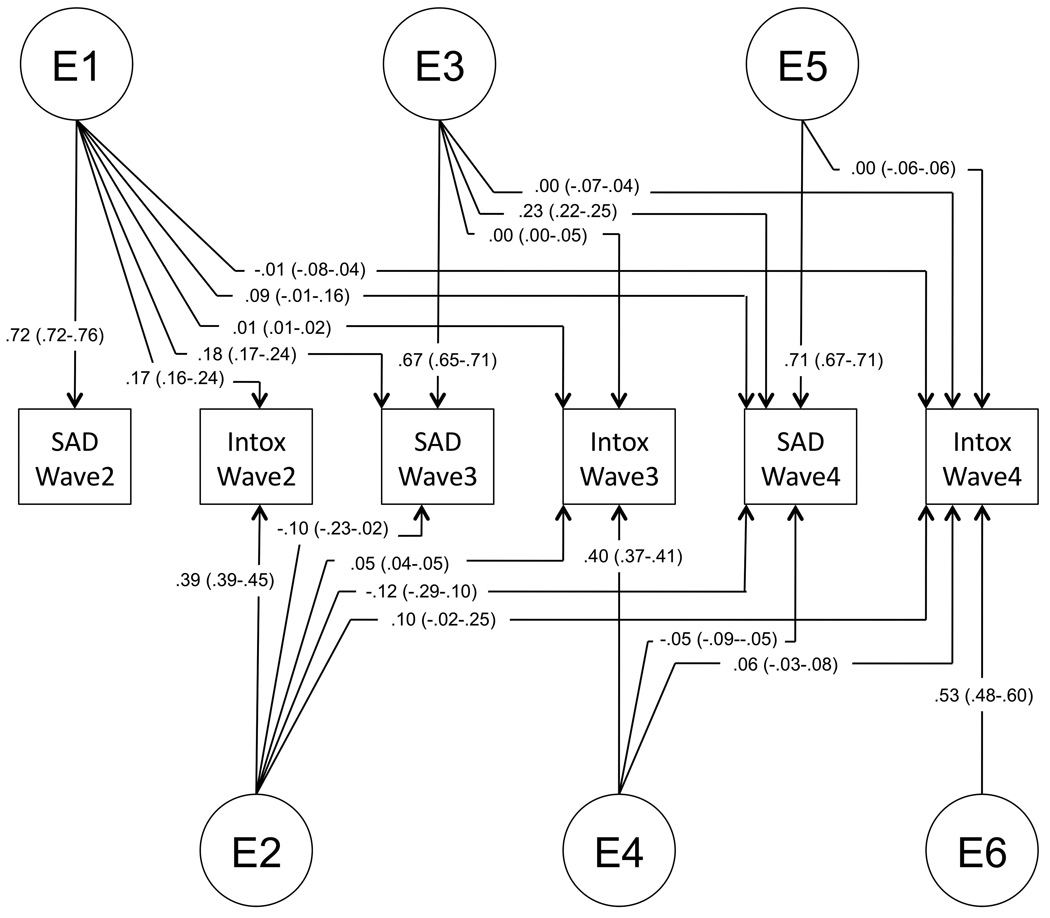
Finally, we tested for the specificity of unique environmental (E) influences, those that are not shared by both twins but might contribute to covariance of traits. Model fitting indicated that unique environmental factors are significantly correlated across SxAnxDep and IntoxFreq. Furthermore, these influences remain relevant across waves, though they are supplemented by novel environmental factors that become evident at later waves. In the interest of avoiding over-fitting the model, which could bias remaining factor loadings, we did not test whether individual paths with low factor loadings could be removed from the model. Factor loadings from the final model are provided in Figure 2A–C.
Figure 2.
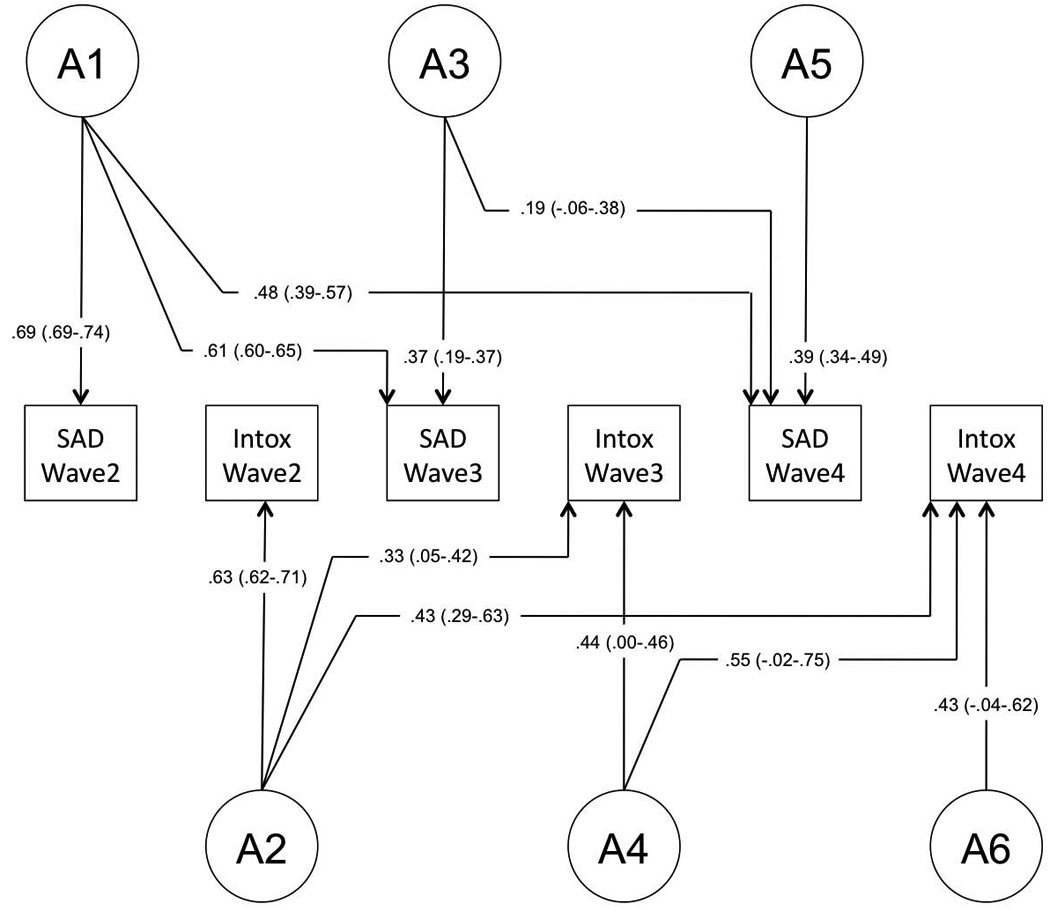
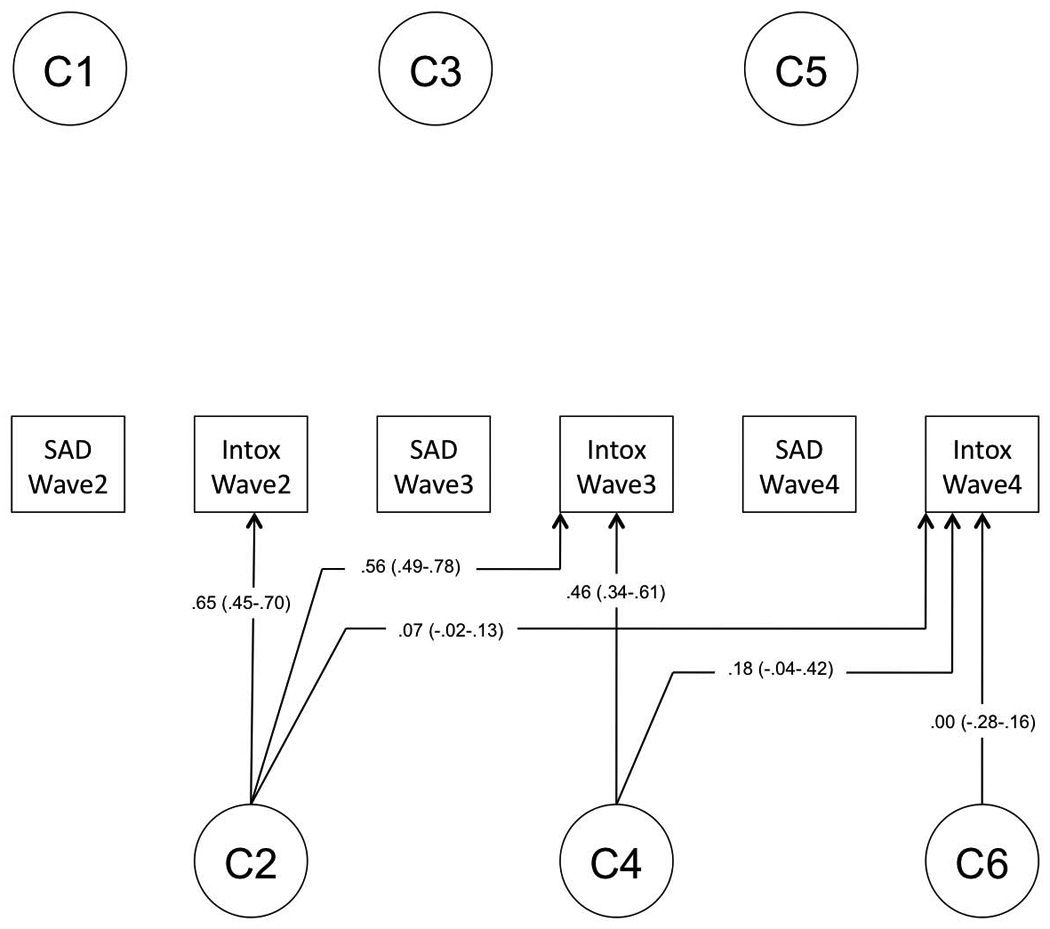
Final model. Final model structure, with path estimates (and 95% confidence intervals) for (A) genetic factor loadings, (B) shared environmental factor loadings, and (C) unique environmental factor loadings.
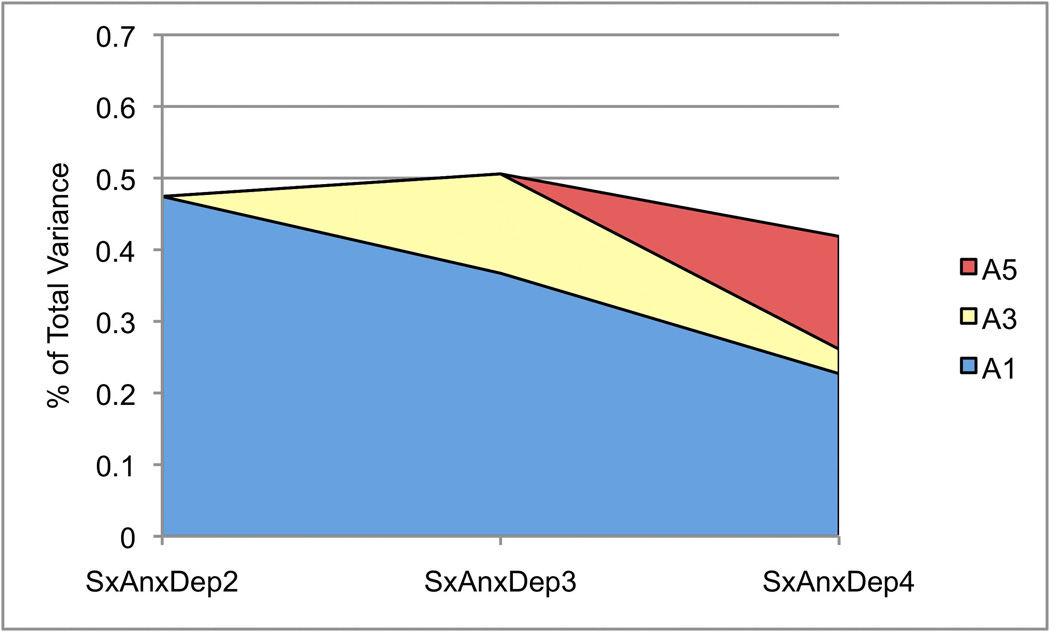
Variance components from the final model are provided in Table 6, and genetic and environmental correlations are provided in Table 7. The heritability of SxAnxDep ranged from 0.42–0.51. The dynamic nature of genetic factors underlying SxAnxDep is illustrated in Figure 3A. Factor A1 remains influential across Waves 3 and 4; Factor A3 continues to contribute to genetic variance in Wave 4, though to a lesser degree than A1. A moderate proportion (37%) of the total genetic variance is novel to Wave 4.
Table 6.
Genetic and environmental correlations from final model. Genetic correlations are given below the diagonal. Above the diagonal, shared environmental correlations are only listed for IntoxFreq variables (see Figure 2A–C); these values are in italicized and grey text. Unique environmental correlations are in black and are not italicized.
| SxAnxDep2 | IntoxFreq2 | SxAnxDep3 | IntoxFreq3 | SxAnxDep4 | IntoxFreq4 | |
|---|---|---|---|---|---|---|
| SxAnxDep2 | 1/1 | 0.41 | 0.25 | 0.03 | 0.11 | −0.01 |
| IntoxFreq2 | 0 | 1/1 | −0.03 | 0.77, 0.12 | −0.10 | 0.35, 0.16 |
| SxAnxDep3 | 0.85 | 0 | 1/1 | −0.01 | 0.34 | −0.03 |
| IntoxFreq3 | 0 | 0.59 | 0 | 1/1 | −0.08 | 0.87, 0.14 |
| SxAnxDep4 | 0.74 | 0 | 0.78 | 0 | 1/1 | −0.04 |
| IntoxFreq4 | 0 | 0.52 | 0 | 0.85 | 0 | 1/1 |
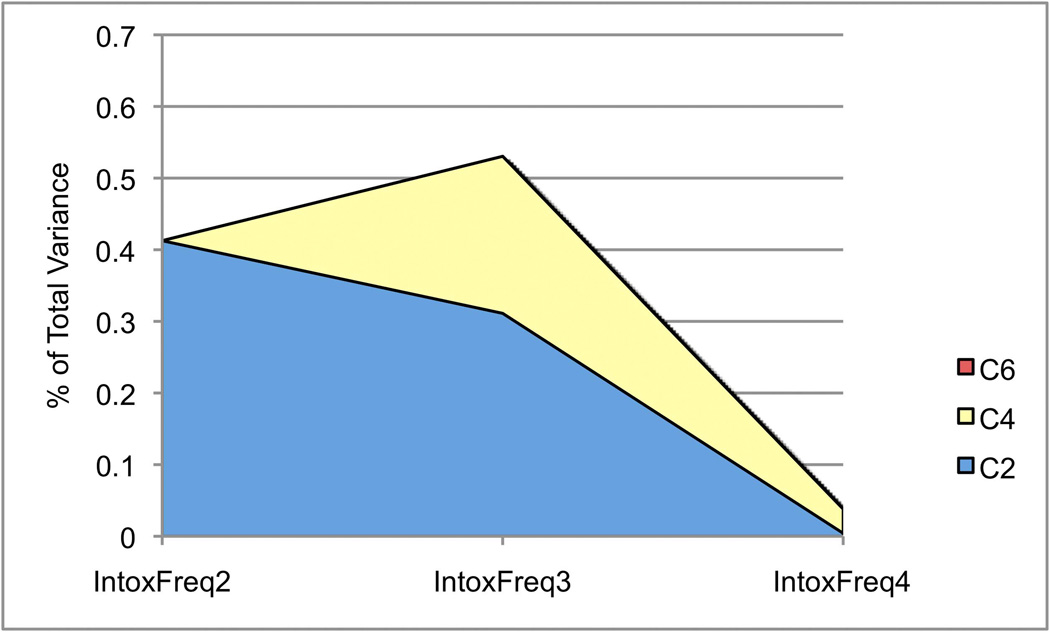
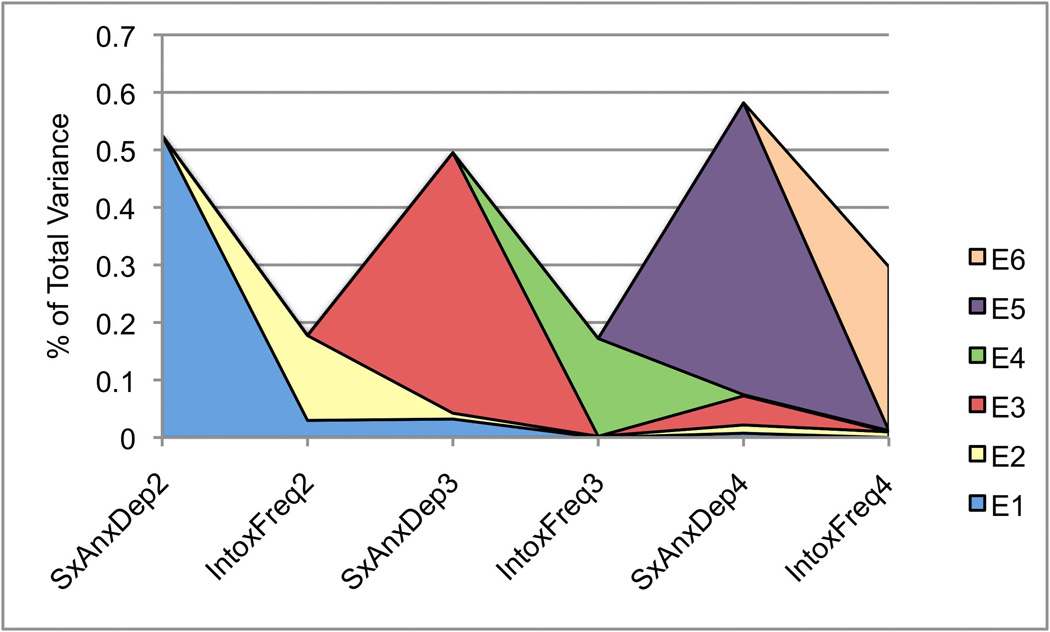
Figure 3.
Contributions of latent factors to variance. The proportion of total variance at each wave is depicted for SxAnxDep and IntoxFreq. A) Genetic components of variance for SxAnxDep; B) genetic components of variance for IntoxFreq; C) shared environmental components of variance for IntoxFreq; and D) unique environmental components of variance for both SxAnxDep and IntoxFreq.
The heritability of IntoxFreq differed considerably at different waves, ranging from 0.30–0.67, with the lowest estimate at Wave 3 when respondents are aged 16–17. Figure 3B depicts the composition of genetic variance for IntoxFreq, which, like that of SxAnxDep, is dynamic in that we observe genetic attenuation and innovation at each wave. The decrease in heritability of IntoxFreq at Wave 3 occurs alongside an increase in shared environmental influences; Figure 3C illustrates changes in these effects across waves. Notably, total shared environmental influences at Wave 4 were quite low (c2=0.04).
Figure 3D depicts the influence of unique environmental factors across traits and waves. These factors were the only significant source of variance shared by SxAnxDep and IntoxFreq in this sample, though each E factor loads predominantly onto SxAnxDep or IntoxFreq phenotypes. In addition, cross-wave factor loadings are generally low. With the exception of E1 – which loads positively on SxAnxDep waves 2 and 3 and IntoxFreq at wave 2, unique environmental factors that load first onto SxAnxDep phenotypes actually have negative factor loadings onto IntoxFreq phenotypes, and vice versa. However, these factor loadings are quite low, with confidence intervals spanning 0.
4. Discussion
The three primary goals of these analyses were as follows: i) assess the sources of variance underlying SxAnxDep and IntoxFreq across adolescence, including innovation and attenuation of genetic and environmental factors; ii) determine whether a shared liability, in the form of genetic and/or environmental correlations, contributes to the phenotypic association between traits; and iii) assess whether there are quantitative changes in any shared liability changes across adolescence. We address each of these questions in turn.
Consistent with the prior examination of genetic influences on symptoms of anxiety and depression (Kendler, Gardner, et al., 2008), we found that heritable factors are strongly influential across development, though the current estimates are lower than were previously reported (see Methods, and below). Though the total heritability of SxAnxDep does not differ dramatically across waves (ranging from a2=0.42–0.51), the underlying factors are dynamic, with novel genetic influences becoming evident at each successive wave (i.e., genetic innovation) and the influence of genetic factors already apparent at earlier waves waning over time (genetic attenuation). In contrast, the total heritability of intoxication frequency varies considerably across waves, with an estimate of 0.40 at Wave 2, decreasing to 0.30 at Wave 3, and then increasing to 0.67 at Wave 4, when participants are aged 19–20. As with SxAnxDep, both genetic attenuation and innovation are observed: factors A2 and A4 remain influential across waves.
A decrease in the heritability of IntoxFreq from Wave 2 to Wave 3 occurs alongside an increase in the relative influence of shared environmental factors. By Wave 4, shared environmental factors have nearly disappeared. These findings are consistent with the notion that, as individuals mature, they “age out” of shared environmental influences and their behavior is more a function of genetic factors and unique environmental experiences, as well as established behavioral practices (e.g., (Bergen, et al., 2007; Kendler, Schmitt, Aggen, & Prescott, 2008)). The absence of significant shared environmental influences on SxAnxDep is also consistent with some previous research, primarily on clinical manifestations of internalizing disorders (Hettema, Neale, & Kendler, 2001; Sullivan, Neale, & Kendler, 2000).
For both SxAnxDep and IntoxFreq, total unique environmental variance remained relatively stable across waves, ranging from 0.49–0.58 for the former and 0.17–0.30 for the latter. Interestingly, the vast majority of environmental variance for either trait, at any wave, is largely specific to one trait and for only one wave, though loadings are higher within SxAnxDep or intoxication frequency than they are across phenotypes. Given that the cross-time and cross-trait unique environment factor loadings are so low (with confidence intervals for many of them spanning 0) it is surprising that Models 9 and 10 indicated that removing these loadings substantially deteriorated model fit. However, the cross-time, within-trait unique environmental correlations suggest that these factors do contribute to stability of each phenotype over time.
Despite low unique environmental factor loadings, the environmental correlations across waves and phenotypes are nontrivial in several cases; for example, the environmental correlation between traits at Wave 2 is rE=0.41. Critical to our research questions, these results indicate that the shared liability between SxAnxDep and IntoxFreq is limited to environmental factors in this sample. More specifically, early environmental influences appear to account for much of the shared liability: factor E1 is the source of the only positive correlations between traits. Later environmental factors (E3–E6) have only very minimal loadings across traits or time. Furthermore, the sign of the unique environmental correlation between SxAnxDep and IntoxFreq changes: after Wave 2, these correlations become negative, albeit weakly. Thus, the nature of the shared liability between SxAnxDep and IntoxFreq changes across adolescence, though in a nuanced manner: the degree to which environmental factors influence covariance between these phenotypes certainly decreases over time, and is limited almost entirely to the effects of E1.
What might these influential environmental factors be? Epidemiological studies have identified a number of risk factors for adolescent internalizing symptoms and alcohol use. Socioeconomic status, ethnicity, adverse home environment, and exposure to maternal depression have all been reproducibly associated with both phenotypes (Saraceno, et al., 2009); these circumstances are presumably experienced by both twins, and would only contribute to unique environmental variance if twin responses to them differed. Other risk factors are more likely to be at least partly twin-specific. These include peer influences (Beitchman, et al., 2005), such as rejection or bullying (Calles, 2007), or exposure to deviant peers (Schulte, Ramo, & Brown, 2009); neglect, or physical or sexual abuse (Bhatia & Bhatia, 2007; Sartor, et al., 2007; Zalsman, Brent, & Weersing, 2006); or life stressors (Garber, 2006; Wills, Vaccaro, & McNamara, 1992).
As noted above, the sign of the environmental correlation across SxAnxDep and IntoxFreq changes from positive at Wave 2 to weakly negative at Waves 3 and 4. Given the age of participants at Wave 2, we might speculate that environmental risk factors influencing SxAnxDep also influence an individual’s likelihood of alcohol use initiation. The change in sign at later waves suggests that, once initiation is established, environmental risk factors for SxAnxDep actually reduce, although weakly, the frequency of alcohol intoxication. These possibilities are consistent with some previous research that indicates that children with symptoms of depression (Kaplow et al. 2001, Crum et al. 2008) or generalized anxiety (Kaplow et al. 2001) were at increased risk for early alcohol use initiation. Pitkanen and colleagues (2008) reported that age 14 anxiety was negatively associated with later alcohol use. Although the strength of these findings differed by sex, alcohol use measure, and age of later alcohol use, they support the notion that the relationship between symptoms of anxiety and alcohol use changes across development. Similar to our own speculation about the potential nature of the environmental factors underlying this relationship, other researchers (e.g., Kaplow et al. 2001, Sher et al. 2005) have suggested that peer influences are a critical factor in adolescent alcohol use. Perhaps at early ages, adolescents with internalizing problems use alcohol to “fit in” with peers; but as they mature, these same individuals might instead seek out non-drinking peers. Although we cannot determine from our analyses which environmental influences are conferring risk, it is clear that these factors can have detrimental consequences on both mood and alcohol intake.
In contrast to some (e.g., males in Tambs and colleagues (Tambs, et al., 1997); (Edwards, et al., submitted; Fu, et al., 2002; Kendler, et al., 1993) but not all (e.g., females in Tambs et al., 1997) previous findings, genetic factors common to both traits do not significantly influence the phenotypic association between them. Such disparate results might be due to true sample differences, but differences in how each phenotype is measured likely also contribute. The use of non-clinical measures of internalizing problems and alcohol use is likely particularly relevant to our findings: the variables used in these analyses capture normative variation in these traits. Perhaps syndromal levels of internalizing symptoms and alcohol use share genetic and/or environmental influences to a greater degree than do phenotypic levels more typical of the general population.
We conducted post hoc cross-sectional bivariate analyses to investigate further the nature of the shared liability between phenotypes at each wave. The results of these analyses essentially confirmed that these relationships are dynamic (results available upon request): the influences of genetic, shared environmental, and unique environmental factors on and between phenotypes changed at each wave. Ultimately, the longitudinal Cholesky model is more powerful and enables us to specifically address our questions regarding cross-time influences, making it the preferred model for hypothesis testing. However, the results of the within-wave bivariate analyses provide some insight as to why different studies might reach inconsistent conclusions regarding the relationship between internalizing symptoms and alcohol use: this relationship is quite dynamic, at least during adolescence.
Because the manner by which model reduction proceeds can influence results, we also conducted post-hoc model fitting to test whether genetic correlations between traits could be detected if these paths were dropped after the environmental components of the model had already been reduced. Our findings were largely in agreement with the final model described here: genetic correlations could be removed without a significant deterioration in model fit, nor did final variance component estimates differ substantially depending on the order of model reduction. Thus, we are confident that the absence of genetic correlations between traits is not a function of the specific model reduction procedure utilized.
5. Conclusions
In summary, our analyses indicate that the heritability of internalizing symptoms and alcohol use is dynamic during adolescence. Although in the case of internalizing symptoms, heritability is superficially stable, genetic attenuation and innovation are observed for both internalizing and alcohol use. Common environmental effects do not significantly influence internalizing symptoms, but are a prominent source of variance for alcohol use until late adolescence. Unique environmental factors underlying both internalizing and alcohol use vary considerably across adolescence, and their effects are generally ephemeral. However, these factors – particularly those evident early in adolescence – establish a shared liability to symptoms of internalizing and alcohol use. Thus, environmental circumstances identified as risk factors for one trait should be investigated as risk factors for the other as well. These findings potentially have implications for prevention, intervention, and treatment of psychopathology and substance use that manifest during adolescence.
The results of these analyses must be considered in light of several limitations. First, the sample is entirely native born Swedish twins and these results may not extrapolate to other ethnicities. Second, the measure used of internalizing symptoms consisted of items related to both anxiety and depression, which, on a clinical level, could be differentially related to alcohol use. However, attempts to identify distinct phenotypic factors within the measure failed (Kendler, Gardner, et al., 2008), suggesting that the measure is largely homogenous for this sample. Finally, though the primary goals of the analyses differ considerably, we note that the current results differ from those reported by Kendler and colleagues (2008) in that the current heritability estimates of SxAnxDep are lower. This could be due in part to differences in how the final variables were constructed; in particular, the previous report used a continuous factor score, which likely has lower error than our ordinal measure. In addition, the previous study corrected for the unreliability of individual raters through the use of multiple reporters. Finally, model fitting proceeded somewhat differently with regards to unique environmental effects. Our analyses are concerned primarily with the nature of a shared liability between SxAnxDep and IntoxFreq rather than more specifically with developmental changes in the (distinct) measure of SxAnxDep used by Kendler et al.; our model fitting approach was designed accordingly.
Table 5.
Final variance components. Variance components (95% CI) for symptoms of anxiety and depression and intoxication frequency at each wave of self-reported data. Figures might not sum to 1 due to rounding.
| SxAnxDep | IntoxFreq | ||||
|---|---|---|---|---|---|
| A | E | A | C | E | |
| Wave 2 | 0.47 (0.39–0.52) | 0.53 (0.46–0.53) | 0.40 (0.18–0.57) | 0.42 (0.24–0.52) | 0.18 (0.14–0.24) |
| Wave 3 | 0.51 (0.50–0.58) | 0.49 (0.49–0.58) | 0.30 (0.19–0.49) | 0.53 (0.41–0.55) | 0.17 (0.17–0.28) |
| Wave 4 | 0.42 (0.35–0.47) | 0.58 (0.49–0.64) | 0.67 (0.52–0.67) | 0.04 (0.01–0.07) | 0.30 (0.27–0.37) |
Abbreviations
- SxAnxDep
symptoms of anxiety and depression
- IntoxFreq
intoxication frequency
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achenbach TM. Manual for the Child Behavior Checklist/4–18 and 1991 Profile. Burlington, VT: University of Vermont; 1991. [Google Scholar]
- Achenbach TM, Rescorla LA. Manual for the ASEBA Adults Forms and Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, and Families; 2003. [Google Scholar]
- Akaike H. Factor analysis and AIC. Psychometrika. 1987;52:317–332. [Google Scholar]
- Beitchman JH, Adlaf EM, Atkinson L, Douglas L, Massak A, Kenaszchuk C. Psychiatric and substance use disorders in late adolescence: the role of risk and perceived social support. American Journal on Addictions. 2005;14:124–138. doi: 10.1080/10550490590924755. [DOI] [PubMed] [Google Scholar]
- Bergen SE, Gardner CO, Kendler KS. Age-related changes in heritability of behavioral phenotypes over adolescence and young adulthood: a meta-analysis. Twin Research and Human Genetics. 2007;10:423–433. doi: 10.1375/twin.10.3.423. [DOI] [PubMed] [Google Scholar]
- Bhatia SK, Bhatia SC. Childhood and adolescent depression. American Family Physician. 2007;75:73–80. [PubMed] [Google Scholar]
- Calles JL., Jr Depression in children and adolescents. Primary Care; Clinics in Office Practice. 2007;34:243–258. doi: 10.1016/j.pop.2007.04.008. abstract vi. [DOI] [PubMed] [Google Scholar]
- Crum RM, Green KM, Storr CL, Chan YF, Ialongo N, Stuart EA, Anthony JC. Depressed mood in childhood and subsequent alcohol use through adolescence and young adulthood. Archives of General Psychiatry. 2008;65:702–712. doi: 10.1001/archpsyc.65.6.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards AC, Sihvola E, Korhonen T, Pulkkinen L, Moilanen I, Kaprio J, Rose RJ, Dick DM. Depressive symptoms and alcohol use are genetically and environmentally correlated across adolescence. doi: 10.1007/s10519-010-9400-y. (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergusson DM, Boden JM, Horwood LJ. Tests of causal links between alcohol abuse or dependence and major depression. Archives of General Psychiatry. 2009;66:260–266. doi: 10.1001/archgenpsychiatry.2008.543. [DOI] [PubMed] [Google Scholar]
- Fu Q, Heath AC, Bucholz KK, Nelson E, Goldberg J, Lyons MJ, True WR, Jacob T, Tsuang MT, Eisen SA. Shared genetic risk of major depression, alcohol dependence, and marijuana dependence: contribution of antisocial personality disorder in men. Archives of General Psychiatry. 2002;59:1125–1132. doi: 10.1001/archpsyc.59.12.1125. [DOI] [PubMed] [Google Scholar]
- Garber J. Depression in children and adolescents: linking risk research and prevention. American Journal of Preventive Medicine. 2006;31:S104–S125. doi: 10.1016/j.amepre.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton W, Pickering RP, Kaplan K. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Archives of General Psychiatry. 2004;61:807–816. doi: 10.1001/archpsyc.61.8.807. [DOI] [PubMed] [Google Scholar]
- Grant BF, Stinson FS, Harford TC. Age at onset of alcohol use and DSM-IV alcohol abuse and dependence: a 12-year follow-up. Journal of Substance Abuse. 2001;13:493–504. doi: 10.1016/s0899-3289(01)00096-7. [DOI] [PubMed] [Google Scholar]
- Grant VV, Stewart SH, Mohr CD. Coping-anxiety and coping-depression motives predict different daily mood-drinking relationships. Psychology of Addictive Behaviors. 2009;23:226–237. doi: 10.1037/a0015006. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Goodwin RD, Stinson FS, Grant BF. Epidemiology of major depressive disorder: results from the National Epidemiologic Survey on Alcoholism and Related Conditions. Archives of General Psychiatry. 2005;62:1097–1106. doi: 10.1001/archpsyc.62.10.1097. [DOI] [PubMed] [Google Scholar]
- Hettema JM, Neale MC, Kendler KS. A review and meta-analysis of the genetic epidemiology of anxiety disorders. American Journal of Psychiatry. 2001;158:1568–1578. doi: 10.1176/appi.ajp.158.10.1568. [DOI] [PubMed] [Google Scholar]
- Hicks BM, Blonigen DM, Kramer MD, Krueger RF, Patrick CJ, Iacono WG, McGue M. Gender differences and developmental change in externalizing disorders from late adolescence to early adulthood: A longitudinal twin study. Journal of Abnormal Psychology. 2007;116:433–447. doi: 10.1037/0021-843X.116.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Teen Drug Use Continues Down in 2006, Particularly Among Older Teens; But Use of Prescription-Type Drugs Remains High. Ann Arbor, MI: 2006. [Google Scholar]
- Kendler KS, Gardner CO, Lichtenstein P. A developmental twin study of symptoms of anxiety and depression: evidence for genetic innovation and attenuation. Psychological Medicine. 2008;38:1567–1575. doi: 10.1017/S003329170800384X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Heath AC, Neale MC, Kessler RC, Eaves LJ. Alcoholism and major depression in women. A twin study of the causes of comorbidity. Archives of General Psychiatry. 1993;50:690–698. doi: 10.1001/archpsyc.1993.01820210024003. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Schmitt E, Aggen SH, Prescott CA. Genetic and environmental influences on alcohol, caffeine, cannabis, and nicotine use from early adolescence to middle adulthood. Archives of General Psychiatry. 2008;65:674–682. doi: 10.1001/archpsyc.65.6.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Crum RM, Warner LA, Nelson CB, Schulenberg J, Anthony JC. Lifetime co-occurrence of DSM-III-R alcohol abuse and dependence with other psychiatric disorders in the National Comorbidity Survey. Archives of General Psychiatry. 1997;54:313–321. doi: 10.1001/archpsyc.1997.01830160031005. [DOI] [PubMed] [Google Scholar]
- Kuo PH, Gardner CO, Kendler KS, Prescott CA. The temporal relationship of the onsets of alcohol dependence and major depression: using a genetically informative study design. Psychological Medicine. 2006;36:1153–1162. doi: 10.1017/S0033291706007860. [DOI] [PubMed] [Google Scholar]
- Lichtenstein P, Tuvblad C, Larsson H, Carlstrom E. The Swedish Twin study of CHild and Adolescent Development: the TCHAD-study. Twin Res Hum Genet. 2007;10:67–73. doi: 10.1375/twin.10.1.67. [DOI] [PubMed] [Google Scholar]
- Lyons MJ, Schultz M, Neale M, Brady K, Eisen S, Toomey R, Rhein A, Faraone S, Tsuang M. Specificity of familial vulnerability for alcoholism versus major depression in men. Journal of Nervous and Mental Disease. 2006;194:809–817. doi: 10.1097/01.nmd.0000244480.78431.49. [DOI] [PubMed] [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical Modeling. 6th ed. Richmond, VA: Virginia Institute for Psychiatric and Behavioral Genetics. Virginia Commonwealth University; 2003. [Google Scholar]
- Office of Applied Studies. Results from the 2004 National Survey on Drug Use and Health: National Findings. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2005. [Google Scholar]
- Pagan JL, Rose RJ, Viken RJ, Pulkkinen L, Kaprio J, Dick DM. Genetic and environmental influences on stages of alcohol use across adolescence and into young adulthood. Behavior Genetics. 2006;36:483–497. doi: 10.1007/s10519-006-9062-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose RJ, Dick DM, Viken RJ, Pulkkinen L, Kaprio J. Drinking or abstaining at age 14? A genetic epidemiological study. Alcoholism, Clinical and Experimental Research. 2001;25:1594–1604. [PubMed] [Google Scholar]
- Rose RJ, Dick DM, Viken RJ, Pulkkinen L, Kaprio J. Genetic and environmental effects on conduct disorder and alcohol dependence symptoms and their covariation at age 14. Alcoholism, Clinical and Experimental Research. 2004;28:1541–1548. doi: 10.1097/01.alc.0000141822.36776.55. [DOI] [PubMed] [Google Scholar]
- Saraceno L, MunafÛ M, Heron J, Craddock N, B. M. van den Bree M. Genetic and non-genetic influences on the development of co-occurring alcohol problem use and internalizing symptomatology in adolescence: a review. Addiction. 2009;104:1100–1121. doi: 10.1111/j.1360-0443.2009.02571.x. [DOI] [PubMed] [Google Scholar]
- Sartor CE, Lynskey MT, Bucholz KK, McCutcheon VV, Nelson EC, Waldron M, Heath AC. Childhood sexual abuse and the course of alcohol dependence development: findings from a female twin sample. Drug and Alcohol Dependence. 2007;89:139–144. doi: 10.1016/j.drugalcdep.2006.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte MT, Ramo D, Brown SA. Gender differences in factors influencing alcohol use and drinking progression among adolescents. Clinical Psychology Review. 2009;29:535–547. doi: 10.1016/j.cpr.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sihvola E, Rose RJ, Dick DM, Pulkkinen L, Marttunen M, Kaprio J. Early-onset depressive disorders predict the use of addictive substances in adolescence: a prospective study of adolescent Finnish twins. Addiction. 2008;103:2045–2053. doi: 10.1111/j.1360-0443.2008.02363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strandheim A, Holmen TL, Coombes L, Bentzen N. Alcohol intoxication and mental health among adolescents - a population review of 8983 young people, 13–19 years in North-Trondelag, Norway: the Young-HUNT Study. Child and Adolescent Psychiatry and Mental Health. 2009;3:18. doi: 10.1186/1753-2000-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. American Journal of Psychiatry. 2000;157:1552–1562. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- Tambs K, Harris JR, Magnus P. Genetic and environmental contributions to the correlation between alcohol consumption and symptoms of anxiety and depression. Results from a bivariate analysis of Norwegian twin data. Behavior Genetics. 1997;27:241–250. doi: 10.1023/a:1025662114352. [DOI] [PubMed] [Google Scholar]
- Wills TA, Vaccaro D, McNamara G. The role of life events, family support, and competence in adolescent substance use: a test of vulnerability and protective factors. American Journal of Community Psychology. 1992;20:349–374. doi: 10.1007/BF00937914. [DOI] [PubMed] [Google Scholar]
- Young-Wolff KC, Kendler KS, Sintov ND, Prescott CA. Mood-related drinking motives mediate the familial association between major depression and alcohol dependence. Alcoholism, Clinical and Experimental Research. 2009;33:1476–1486. doi: 10.1111/j.1530-0277.2009.00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalsman G, Brent DA, Weersing VR. Depressive disorders in childhood and adolescence: an overview: epidemiology, clinical manifestation and risk factors. Child and Adolescent Psychiatric Clinics of North America. 2006;15:827–841. doi: 10.1016/j.chc.2006.05.002. vii. [DOI] [PubMed] [Google Scholar]