Abstract
Vaccinia virus infection can confer immunity to smallpox by inducing potent T cell and antibody responses. While the CD8 T cell response to vaccinia virus has been well characterized, less is known about factors required for priming and memory for the CD4 T cells. Focusing on two recently described epitopes, we show that after intranasal infection, both I1L and L4R epitopes are co-dominant during the acute response, but the I1L epitope dominates during memory. CD4 T cell priming was intact in the absence of CD80/86, however secondary responses were reduced. This contrasts with our previous data showing CD80/86-CD28 interaction is required for optimal primary and memory CD8 T cell responses. The absence of CD80/86 also changed the immunodominance hierarchy during memory, with the I1L and L4R responses becoming co-dominant in knockout mice. These data highlight different costimulatory requirements for primary CD4 and CD8 T cell responses to vaccinia virus.
Keywords: Vaccinia virus, T cell memory, CD4 T cell, B7, CD80, CD86, CD28, Secondary response, Recall response, Smallpox
1. Introduction
Vaccinia virus (VAVC) is a double-stranded DNA virus belonging to the Poxviridae family and shares high similarities to other orthopoxviruses such as variola virus, the causative agent of smallpox. Vaccinia virus infection induces potent cellular and humoral responses [1–3], and intradermal inoculation with this virus provides protective immunity against smallpox infection. While the CD8 T cell response to this virus has been studied extensively, less is know regarding the CD4 T cell response. CD4 T cells are crucial for protection from primary vaccinia virus infection, largely by providing help for the antibody response [4]. Recently several CD4 T cell epitopes on the virus were mapped, mostly on structural proteins, and these are highly correlated with antigens recognized by the antibody response [5]. The pattern of responses to these epitopes during infection has not been reported in detail, nor have the requirements for effective primary and secondary responses to these epitopes. Understanding the secondary response is particularly critical for vaccine responsiveness, as rapid T cell expansion promotes containment of virus replication in a timely manner.
CD80 and CD86, the most well-studied costimulatory molecules, are expressed on antigen-presenting cells (APCs), upregulated upon cell activation, and bind to CD28 on the T cell, delivering a crucial signal for T-cell activation together with the T cell receptor [6]. CD28 signaling is mediated through the phosphatidylinositol 3-kinase-protein kinase B (Akt) and growth factor-receptor-bound protein 2 (Grb2) pathways, enhancing the production of interleukin-2 and other cytokines, upregulating antiapoptotic molecules (such as Bcl-xL), promoting energy metabolism (glucose uptake and rate of glycolysis), and facilitating cell-cycle progression [7–9]. Once T cells are activated, cytotoxic T-lymphocyte antigen 4 (CTLA-4), another receptor for CD80/CD86, is upregulated. CTLA-4 negatively regulates T cell responses by several mechanisms: sequestering CD80/CD86 because of the higher affinity for CTLA-4 compared with CD28, recruiting phosphatases such as Src homology region 2 domain-containing phosphatase 1 (SHP-1), to dephosphorylate downstream signals, and transducing signals into the APCs to induce indoleamine 2.3-dioxygenase and the catabolism of tryptophan, resulting an inhibitory environment for the T cell [10–12].
The effects of CD80/CD86 costimulation on T cell responses depends on the strength of the TCR signal, and whether the cell is a naïve, activated or memory T cell [13]. T cell responses to some viruses, such as LCMV, do not require costimulation through this pathway [14, 15], whereas with other viruses, such as influenza or VSV, the response is impaired in the absence of CD28 signaling [16, 17]. The dogma has been that CD80/86-CD28 interactions are needed to initiate a response from naïve T cells, however this is not necessary for activating memory T cells. Work by our lab and others in recent years, focusing on the CD8 T cell response, has shown that, while CD80/86 is not necessary for a secondary T cell response, the magnitude of the response is significantly reduced without this costimulatory signal [18–21]. However CD80/86 is always necessary for a high-avidity neutralizing antibody response, including in the vaccinia virus system (EJU, unpublished data). Given the virus-specific nature of costimulatory requirements for the T cell response, and the importance of vaccinia virus as a vaccine, we wished to determine whether CD80/86 was necessary for efficient primary and secondary CD4 T cell responses to vaccinia virus.
Here we show that after intranasal infection with VACV, responses to the two immunodominant epitopes for CD4 T cells were initially of similar magnitude. One month after infection the I1L-specific response was dominant over the L4R-specific response. The magnitude of neither response was affected by the absence of CD80/86, although the response to secondary infection was sub-optimal. These data provide a kinetic profile of the CD4 T cell response to VACV, and highlight the key role for CD80/86-CD28 interactions in memory but not primary CD4 T cell immunity in this infection.
2. Materials and Methods
2.1 Mice and virus
The Western Reserve strain of vaccinia virus (VV-WR) was originally obtained from Dr. William R. Green (Dartmouth Medical School, Lebanon, NH). C57BL/6 mice were purchased from The National Cancer Institute (Bethesda, MD). CD80/CD86−/− mice on the C57BL/6 background were bred in the Dartmouth-Hitchcock Medical Center mouse facility. Mice were infected with 103 PFU of VV-WR intranasally under anesthesia with 2,2,2-tribromoethanol. At day 35 after infection, mice were rechallenged intranasally with 6×105 PFU of VV-WR. All animal experiments were approved by the Animal Care and Use Program of Dartmouth College.
2.2 Tissue preparation
For the primary response, lungs and spleen were taken at day 10, 14, 22 and 29 after infection. For the recall responses, lungs and spleens were taken at day 8 post re-challenge. Single-cell suspensions of spleen and lung lymphocytes were prepared as described previously [22]. Briefly, spleens were prepared by passing through cell strainers. Lungs were injected with 2 ml of minimal essential medium containing 417.5 µg/ml Liberase CI and 200 µg/ml DNase I (both obtained from Roche, Indianapolis, IN), minced with scissors, and then incubated for 30 min at 37°C and passed through cell strainers. Suspensions were resuspended in 80% isotonic Percoll and subsequently overlaid with 40% isotonic Percoll. Samples were then centrifuged at 400g for 25 min at 4°C, and the cells at the 80%/40% interface were collected, washed, and counted.
2.3 Antibody staining and flow cytometric analysis
Cells were stained with APC-conjugated anti-CD4 (RM4-5; Biolegend). Stained samples were analyzed using a FACS Calibur flow cytometer and CellQuest software (BD Immunocytometry Systems).
2.4 IFN-γ ELISPOT assay
The number of IFN-γ secreting cells was determined after stimulation with peptides in an ELISPOT assay. Epitopes derived from I1L (7–21, QLVFNSISARALKAY) and L4R (176–190, ISKYAGINILNVYSP) proteins were synthesized as peptides and used in this study. Peptides were purchased from New England peptide. In brief, 96-well Multiscreen HTS nitrocellulose plates (Millipore) were coated overnight at 4°C with 100 µl per well of rat anti-mouse IFN-γ antibody (R4-6A2; BD Pharmingen), at a concentration of 2 µg/ml. The plates were then washed and blocked before the addition of irradiated (3000RAD) normal C57BL/6 spleen cells (5×105 cells/well), a graded number of responder spleen cells, 2 µg/ml of each peptide and 10 U/ml recombinant human IL-2 (Tecin, National Cancer Institute). Plates were then incubated for 24 h at 37°C and developed for 2 h with a biotinylated rat anti-mouse IFN-γ antibody (XMG1.2; BD Pharmingen) at a concentration of 2 µg/ml, followed by streptavidin-alkaline phosphatase (Biolegend) at a 1/500 dilution for 1 h at room temperature. Following addition of the chromogenic substrate 5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium (BCIP/NBT; Sigma-Aldrich), visible spots were enumerated using a dissecting microscope. The frequency of CD4 T cells producing IFN-γ was calculated, together with the total number per spleen or set of lungs.
2.5 Statistical analysis
Student’s t-test was used to compare experimental groups. A P value of <0.05 was considered significant.
3. Results
3.1 Quantification of lymphocyte and CD4 T cell populations in spleen and lung
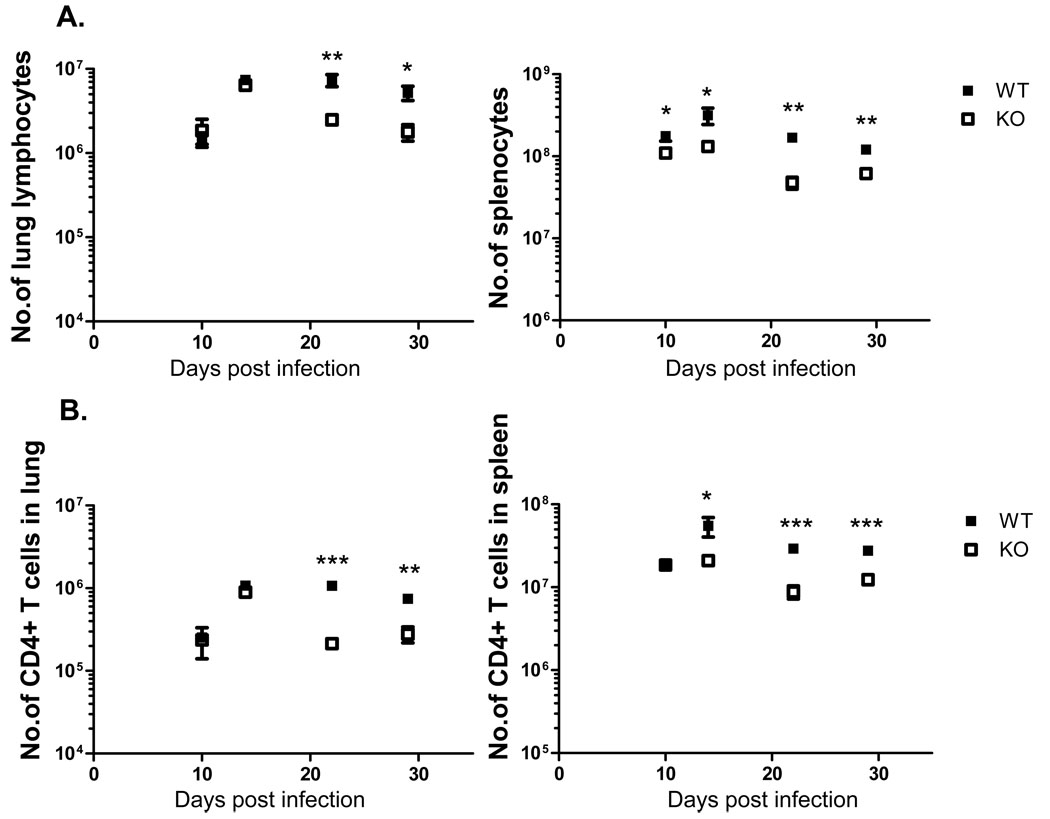
Previous studies identified 14 VAVC-specific CD4 T cell epitopes in C57BL/6 mice. Two epitopes that induced the strongest responses were derived from the I1L and L4R proteins, which are both structural proteins with core-DNA binding activity [5, 23]. We wished to profile the kinetics of responses to these two epitopes following intranasal infection with VAVC, and measure the effect of CD80/CD86-CD28 costimulation on the response using CD80/86-deficient mice. The intranasal route was chosen as it approximates the natural route of infection for smallpox virus. Our previous studies in mice lacking the CD28/CD80/86 pathway infected by VACV by the i.n. route show this pathway does not affect the clearance of the virus [20]. In the lungs at early times post-infection there was no difference in either the number of lymphocytes in the lung (Fig. 1A) or the number of CD4 T cells (Fig. 1B), however at later times there were larger numbers of cells in the wild-type animals. A similar pattern was observed in the spleen, with significantly larger cell numbers present in wild-type animals at later timepoints, although in this organ there was an earlier divergence in the size of the response between the two mouse strains (Fig. 1A and B).
Figure 1.
Lymphocyte and CD4 T cell populations in lungs and spleen in B6 and CD80/86−/− knockout (KO) mice following VACV infection. (A) Total numbers of lymphocytes in the lungs (left panel) or spleen (right panel). (B) Numbers of CD4 T cells in the lung (left panel) and spleen (right panel). * P<0.05, ** P<0.01, *** P<0.05. Data shown is representative of two experiments with 3–4 mice per group.
3.2. Primary CD4 T cell responses to VACV epitopes
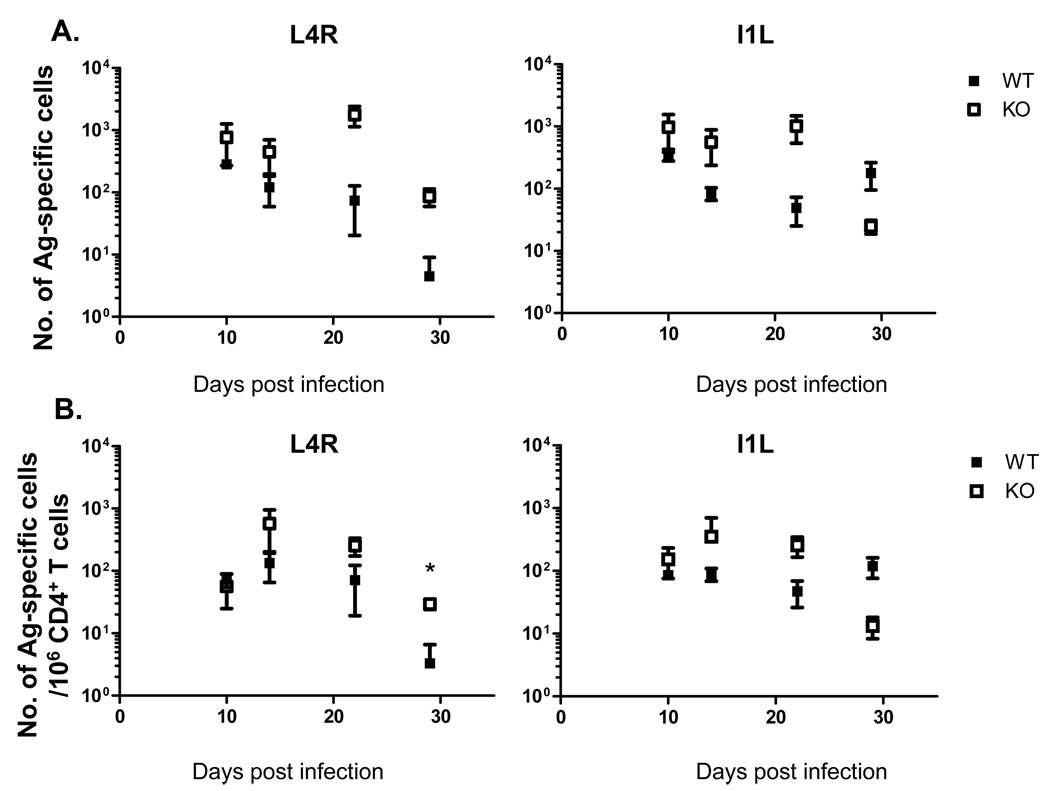
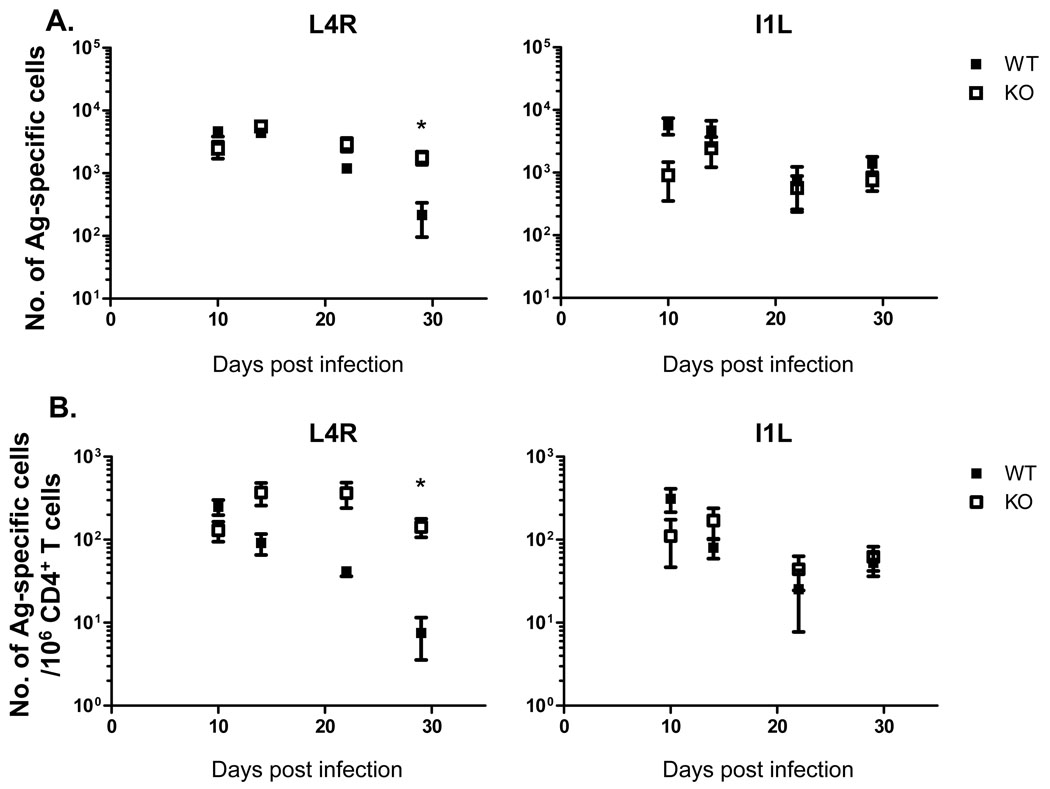
Next we analyzed the epitope-specific CD4 T cell response to VACV using IFN-γ ELISPOT analysis. We used the L4R and I1L epitopes in these studies, as these were shown to elicit the strongest responses among the MHC class II-restricted epitopes identified [5, 23]. A strong CD4 T cell response was detectable in the lungs (Fig. 2) and spleen (Fig. 3) at 10 days post-infection, which slowly declined to a lower, but readily detectable level by day 29 days post-infection. The absence of CD80/86 did not reduce the magnitude of the response, in fact in some cases the response was higher in the knockout animals (Fig. 2A–B, Fig. 3 A–B). Interestingly, the absence of CD80/86 altered the relative strength of the response to the two epitopes during memory. In B6 mice the I1L-specific response was larger than the L4R response at day 29, however the L4R response was elevated to the level of the I1L response in CD80/86-deficient mice in both the lungs and spleen. This was only seen at this late timepoint, as during the acute response both I1L and L4R-specific response were of similar magnitude in both mouse strains. We therefore concluded that CD80/86 is not required for the induction of a potent CD4 T cell response against VACV after i.n. infection, but it does affect the relative sizes of individual epitope-specific responses at late times after infection.
Figure 2.
Lung CD4 T cell responses to VACV epitopes in B6 and CD80/86−/− knockout (KO) mice. Frequencies (A) and total numbers (B) of CD4 T cells recognizing the L4R or I1L epitopes from in the lungs. Error bars show one standard deviation. Representative data from two experiments are shown. * P<0.05. Data shown is representative of two experiments with 3–4 mice per group.
Figure 3.
Responses to VACV CD4 epitopes in the spleen in B6 and CD80/86 knockout (KO) mice. Data shows the frequencies (A) and total numbers (B) of CD4 T cells recognizing the L4R or I1L epitopes in the spleen. Error bars show one standard deviation. Representative data from two experiments are shown. * P<0.05. Data shown is representative of two experiments with 3–4 mice per group.
3.3 Secondary response of antigen-specific CD4 T cells
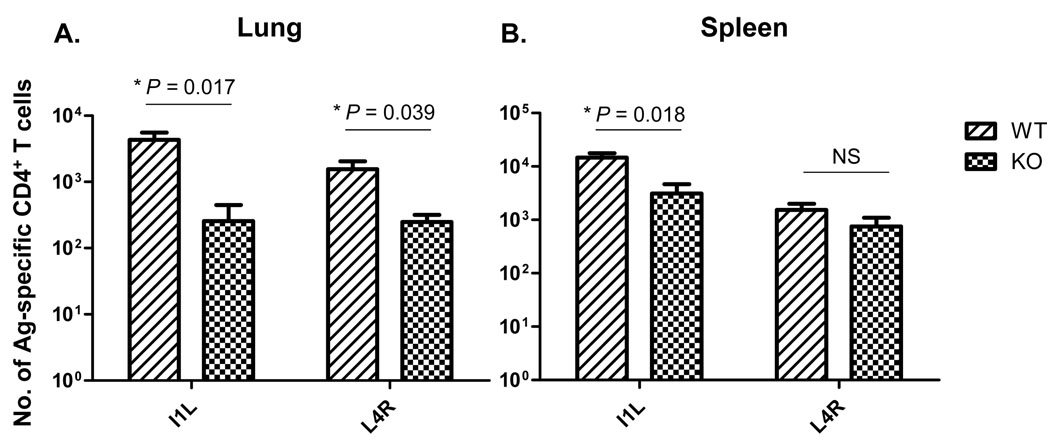
As memory responses were established in both wild-type and CD80/86-deficient mice, we next tested whether there was a difference in the ability of these cells to undergo a recall response upon secondary exposure to virus. At day 35 after infection, mice were challenged with a high dose of vaccinia virus (6×105 PFU) i.n. to overcome antibody responses induced by the prior infection. Eight days later mice were sacrificed, and the expansion of virus-specific CD4 T cells measured using ELISPOT analysis. It should be noted that the IFN-γ ELISPOT assay used included naive B6 splenocytes in all cases, providing a source of CD80/CD86-positive antigen presenting cells. In wild type mice there were robust recall responses to both epitopes in lungs (Fig 4A) and spleen (Fig. 4B), however responses were much weaker in CD80/86−/− mice. The differences shown in Fig. 4 actually represent an underestimate of the expansion deficit, as in several cases the epitope-specific responses during memory were larger in the knockout strain (Figs 2 and 3), but nevertheless the magnitude of the secondary response was smaller in CD80/86-deficient mice. We concluded that although CD80/86 is dispensable for primary CD4 T cell responses to VACV, it is required for an optimal recall response to this virus.
Figure 4.
Expansion of antigen-specific CD4 T cell responses after VAVC re-challenge in wild-type and CD80/86−/− mice. Data shows the number of antigen-specific T cells in wild-type (WT) and CD80/86−/− (KO) mice in the lung (A) and spleen (B) following secondary i.n. infection with vaccinia virus. Data is representative of two experiments. Data shown is representative of two experiments with 3–4 mice per group.
4. Discussion
In this study we show very clearly that priming of the CD4 T cell response to vaccinia virus occurs independently of CD80/86, but there is a suboptimal recall response in the absence of these costimulatory molecules. We previously reported that CD8 T cell priming after i.n. infection with VACV in the absence of the CD80/86-CD28 pathway results in a robust T cell response, although peak responses are significantly reduced relative to responses seen in wild-type mice [20]. This is consistent with other studies reporting reductions in T cell priming in the absence of the CD80/86-CD28 pathway, where mice were infected by the intraperitoneal route [24, 25]. Thus in our current studies it was surprising that that the magnitude of the antiviral CD4 T cell response was unaffected by CD80/86 deficiency. The absence of CD80/86 impacted upon overall CD4 T cell recruitment to the lung and spleen, particularly during later times after infection. However the antigen-specific response was similar to wild-type mice, indicating that there were no deficits in the ability to generate the requisite number of virus-specific CD4 T cells. Nevertheless, in both cases the memory population has a functional deficit and expands suboptimally in the absence of CD80/86 upon re-exposure to virus.
The role of CD80/CD86-CD28 costimulation in CD8 T cell responses varies with the identity of the pathogen. During acute LCMV (Armstrong) infection, the number and function of antigen-specific memory CD8 T cell were maintained in the absence of CD28 costimulation [14, 15]. In VSV or influenza virus infection, the primary CD8 T cell response was reduced in the absence of this costimulatory signal [21, 26, 27]. Recall responses were impaired in mice challenged with influenza virus [16, 18, 21], L. monocytogenes [28] or vesicular stomatitis virus (VSV) [17] in the absence of CD80/86 signals. Without either CD80/CD86 or CD28, the primary virus-specific CD8+ T-cell and antibody responses were significantly impaired in the murine gammaherpesvirus-68 (MHV-68) model [22]. Furthermore, antigen-specific memory CD8+ T cells were impaired in their phenotype, their ability to produce IFN-γ, and to proliferate upon secondary antigenic challenge, indicating impaired memory CD8 T cell differentiation [22]. It has been suggested that LCMV, being a non-cytolytic virus, prolongs the presentation of viral epitopes on infected antigen presenting cells, providing a very robust signal to the T cell receptor of the CD8 T cell during direct antigen presentation, therefore costimulation is not required [27, 29]. Possibly this signal is not as prolonged in most other infections, either due to death of the cell or through other mechanisms, so the response depends more upon costimulation.
A number of studies have reported the primary CD4 T cell response is dependent upon CD80/86-CD28 costimulation in several virus infections such as influenza, HSV-1 and LCMV [26, 27, 30]. Costimulation dependence of the memory CD4 response is less well-studied. One report used the influenza virus system, where CD80/86 was blocked with recombinant CTLA-4-Ig, and measured the effect on the recall response mediated by memory CD4 T cells. CTLA-4-Ig treatment resulted in less cell proliferation, and diminished IL-2 and IFN-γ production from influenza-specific memory CD4 T cells [31]. In addition, CD80/86 interactions were found to be critical for the secondary response of CD4 T cells adoptively transferred into mice chronically infected with LCMV [32]. As there is a clear role for CD80/86-CD28 costimulation in these models, it will be interesting to determine whether costimulation dependence of memory CD4 T cell responses is a general phenomenon, or if it is infection-dependent as for the CD8 T cell response. Responses that are independent of CD80/86 may instead obtain sufficient costimulation from other costimulatory molecules such as 4-1BB, CD27, LIGHT or OX40. There is a degree redundancy between the different costimulatory molecules, however it is also emerging that each has subtly different effects on the character of the ensuing immune response. It will therefore be interesting in future studies to determine which other costimulatory molecules may be substituting for CD80/86 in vaccinia virus infection.
The effect of CD80/86 in this system could be acting at multiple different levels. Costimulation during priming may program cells that can differentiate into memory cells but lack the capacity to mount an optimal recall response. Alternatively, CD80/86 may act during the recall response itself, leading to the response being truncated in the absence of this signal. It is also possible costimulation may be important at other stages of the response. Our previous studies showed that CD80/86-CD28 costimulation is required both for the programming or maintenance of memory CD8 T cells, and during the recall response for an optimal secondary response to vaccinia virus [20]. It will therefore be very interesting in future studies to determine whether costimulation during priming, during the recall response, or both, is critical for the recall CD4 T cell response.
Acknowledgements
We thank Dr. W.R. Green for providing the Western Reserve strain of vaccinia virus. This work was funded by NIH grants AI069943 and CA103642.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Belyakov IM, Earl P, Dzutsev A, Kuznetsov VA, Lemon M, Wyatt LS, Snyder JT, Ahlers JD, Franchini G, Moss B, Berzofsky JA. Proc Natl Acad Sci U S A. 2003;100(16):9458–9463. doi: 10.1073/pnas.1233578100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Demkowicz WE, Jr, Littaua RA, Wang J, Ennis FA. J Virol. 1996;70(4):2627–2631. doi: 10.1128/jvi.70.4.2627-2631.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hammarlund E, Lewis MW, Hansen SG, Strelow LI, Nelson JA, Sexton GJ, Hanifin JM, Slifka MK. Nat Med. 2003;9(9):1131–1137. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- 4.Xu R, Johnson AJ, Liggitt D, Bevan MJ. J Immunol. 2004;172(10):6265–6271. doi: 10.4049/jimmunol.172.10.6265. [DOI] [PubMed] [Google Scholar]
- 5.Sette A, Moutaftsi M, Moyron-Quiroz J, McCausland MM, Davies DH, Johnston RJ, Peters B, Rafii-El-Idrissi Benhnia M, Hoffmann J, Su HP, Singh K, Garboczi DN, Head S, Grey H, Felgner PL, Crotty S. Immunity. 2008;28(6):847–858. doi: 10.1016/j.immuni.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carreno BM, Collins M. Annu Rev Immunol. 2002;20:29–53. doi: 10.1146/annurev.immunol.20.091101.091806. [DOI] [PubMed] [Google Scholar]
- 7.Alegre ML, Frauwirth KA, Thompson CB. Nat Rev Immunol. 2001;1(3):220–228. doi: 10.1038/35105024. [DOI] [PubMed] [Google Scholar]
- 8.Sharpe AH, Freeman GJ. Nat Rev Immunol. 2002;2(2):116–126. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- 9.Wang S, Chen L. Microbes Infect. 2004;6(8):759–766. doi: 10.1016/j.micinf.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Egen JG, Kuhns MS, Allison JP. Nat Immunol. 2002;3(7):611–618. doi: 10.1038/ni0702-611. [DOI] [PubMed] [Google Scholar]
- 11.Grohmann U, Orabona C, Fallarino F, Vacca C, Calcinaro F, Falorni A, Candeloro P, Belladonna ML, Bianchi R, Fioretti MC, Puccetti P. Nat Immunol. 2002;3(11):1097–1101. doi: 10.1038/ni846. [DOI] [PubMed] [Google Scholar]
- 12.Orabona C, Grohmann U, Belladonna ML, Fallarino F, Vacca C, Bianchi R, Bozza S, Volpi C, Salomon BL, Fioretti MC, Romani L, Puccetti P. Nat Immunol. 2004;5(11):1134–1142. doi: 10.1038/ni1124. [DOI] [PubMed] [Google Scholar]
- 13.McAdam AJ, Schweitzer AN, Sharpe AH. Immunol Rev. 1998;165:231–247. doi: 10.1111/j.1600-065x.1998.tb01242.x. [DOI] [PubMed] [Google Scholar]
- 14.Andreasen SO, Christensen JE, Marker O, Thomsen AR. J Immunol. 2000;164(7):3689–3697. doi: 10.4049/jimmunol.164.7.3689. [DOI] [PubMed] [Google Scholar]
- 15.Suresh M, Whitmire JK, Harrington LE, Larsen CP, Pearson TC, Altman JD, Ahmed R. J Immunol. 2001;167(10):5565–5573. doi: 10.4049/jimmunol.167.10.5565. [DOI] [PubMed] [Google Scholar]
- 16.Bertram EM, Lau P, Watts TH. J Immunol. 2002;168(8):3777–3785. doi: 10.4049/jimmunol.168.8.3777. [DOI] [PubMed] [Google Scholar]
- 17.McAdam AJ, Farkash EA, Gewurz BE, Sharpe AH. J Virol. 2000;74(1):203–208. doi: 10.1128/jvi.74.1.203-208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boesteanu AC, Katsikis PD. Semin Immunol. 2009;21(2):69–77. doi: 10.1016/j.smim.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fang M, Sigal LJ. J Immunol. 2006;177(11):8027–8036. doi: 10.4049/jimmunol.177.11.8027. [DOI] [PubMed] [Google Scholar]
- 20.Fuse S, Zhang W, Usherwood EJ. J Immunol. 2008;180(2):1148–1157. doi: 10.4049/jimmunol.180.2.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borowski AB, Boesteanu AC, Mueller YM, Carafides C, Topham DJ, Altman JD, Jennings SR, Katsikis PD. J Immunol. 2007;179(10):6494–6503. doi: 10.4049/jimmunol.179.10.6494. [DOI] [PubMed] [Google Scholar]
- 22.Fuse S, Obar JJ, Bellfy S, Leung EK, Zhang W, Usherwood EJ. J Virol. 2006;80(18):9159–9170. doi: 10.1128/JVI.00422-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moutaftsi M, Bui HH, Peters B, Sidney J, Salek-Ardakani S, Oseroff C, Pasquetto V, Crotty S, Croft M, Lefkowitz EJ, Grey H, Sette A. J Immunol. 2007;178(11):6814–6820. doi: 10.4049/jimmunol.178.11.6814. [DOI] [PubMed] [Google Scholar]
- 24.Salek-Ardakani S, Arens R, Flynn R, Sette A, Schoenberger SP, Croft M. J Immunol. 2009;182(5):2909–2918. doi: 10.4049/jimmunol.0803545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sigal LJ, Reiser H, Rock KL. J Immunol. 1998;161(6):2740–2745. [PubMed] [Google Scholar]
- 26.Bertram EM, Tafuri A, Shahinian A, Chan VS, Hunziker L, Recher M, Ohashi PS, Mak TW, Watts TH. Eur J Immunol. 2002;32(12):3376–3385. doi: 10.1002/1521-4141(200212)32:12<3376::AID-IMMU3376>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 27.Christensen JE, Christensen JP, Kristensen NN, Hansen NJ, Stryhn A, Thomsen AR. Int Immunol. 2002;14(7):701–711. doi: 10.1093/intimm/dxf037. [DOI] [PubMed] [Google Scholar]
- 28.Mittrucker HW, Kursar M, Kohler A, Hurwitz R, Kaufmann SH. J Immunol. 2001;167(10):5620–5627. doi: 10.4049/jimmunol.167.10.5620. [DOI] [PubMed] [Google Scholar]
- 29.nChristensen JE, Christensen JP, Kristensen NN, Hansen NJ, Stryhn A, Thomsen AR. Int Immunol. 2002;14(7):701–711. doi: 10.1093/intimm/dxf037. [DOI] [PubMed] [Google Scholar]
- 30.Edelmann KH, Wilson CB. J Virol. 2001;75(2):612–621. doi: 10.1128/JVI.75.2.612-621.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ndejembi MP, Teijaro JR, Patke DS, Bingaman AW, Chandok MR, Azimzadeh A, Nadler SG, Farber DL. J Immunol. 2006;177(11):7698–7706. doi: 10.4049/jimmunol.177.11.7698. [DOI] [PubMed] [Google Scholar]
- 32.Garidou L, Heydari S, Truong P, Brooks DG, McGavern DB. J Virol. 2009;83(17):8905–8915. doi: 10.1128/JVI.00027-09. [DOI] [PMC free article] [PubMed] [Google Scholar]