Summary
Allergic asthma is a debilitating disease of the airways characterized by airway hyperresponsiveness, eosinophilic inflammation, goblet cell metaplasia with associated mucus hypersecretion, and airway wall remodelling events, particularly subepithelial fibrosis and smooth muscle cell hyperplasia. Animal models that accurately mimic these hallmarks of allergic airways disease are critical for studying mechanisms associated with the cellular and structural changes that lead to disease pathogenesis. Aspergillus fumigatus, is a common aeroallergen of human asthmatics. The intratracheal (IT) delivery of A. fumigatus conidia into the airways of sensitized mice has been described as a model of allergic disease. Here, we compared the IT model with a newly developed inhalation (IH) challenge model. The IH model allowed multiple fungal exposures, which resulted in an exacerbation to the allergic asthma phenotype. Increased recruitment of eosinophils and lymphocytes, the hallmark leukocytes of asthma, were noted with the IH model as compared to the IT model in which macrophages and neutrophils were more prominent. Immunoglobulin E (IgE) production was significantly greater after IH challenge, while that of IgG2a was higher after IT challenge. Airway wall remodelling was pronounced in IH-treated mice, particularly after multiple allergen challenges. Although the IT model may be appropriate for the examination of the played by innate cells in the acute response to fungus, it fails to consistently reproduce the chronic remodelling hallmarks of allergic asthma. The ability of the IH challenge to mimic these characteristics recommends it as a model suited to study these important events.
Keywords: Aspergillus fumigatus, Allergic asthma, Inhalation challenge model
Introduction
Asthma is a debilitating disease of the airways that affects over 300 million people globally (World Health Organization, 2007). Asthmatic airways sensitized to a particular allergen respond violently to subsequent exposures, resulting in asthma attacks which can be fatal. Extrinsic asthma, characterized by eosinophilic airway inflammation (eosinophilia), airway hyperresponsiveness (AHR), goblet cell metaplasia with associated mucus hypersecretion, subepithelial fibrosis, and smooth muscle cell hyperplasia, may be triggered by environmental allergens which include those associated with fungi.
The genus Aspergillus consists of over 182 species of which A. fumigatus is routinely associated with human allergies (Wang et al., 2009) due to inhalation of conidia into the alveoli (Latge, 2001). The nature of the fungal conidium, its distribution, and incidence of causing human disease has inevitably made A. fumigatus a target for research within the context of host-fungal interaction in allergy. Animal models that use A. fumigatus have evolved over the past 22 years initially from the utilization of antigenic extracts from A. fumigatus cultures to induce allergic airways disease (Kurup et al., 1994a; Kurup et al., 1994b). However, since conidia are the most commonly encountered fungal component for human disease, models that utilize conidia have also been developed.
The prominent models of conidia-induced airways disease use a specified number of conidia in suspension that is administered intratracheally into animals previously sensitized with A. fumigatus fungal extracts (Hogaboam et al., 2000; Land et al., 1989). While these models provide a closer representation of human airways disease, the conidia administration method does not take full advantage of the dispersal strategies that allow them to be inhaled deeply into the lung. Our laboratory has created a model system of fungal allergic asthma that fully exploits the nature of the A. fumigatus conidia by exposing sensitized mice to un-manipulated airborne fungal conidia (Hoselton et al., 2010). While each protocol provides a means to study fungal allergic airways disease, we hypothesized that the ability to expose animals multiple times via inhalation of conidia would make the inhalation model more useful for the understanding of the mechanisms involved in chronic characteristics of airway wall remodelling, such as subepithelial fibrosis and smooth muscle cell hyperplasia. In this study, we compared the outcomes of the intratracheal (IT) and inhalation (IH) challenge protocols in C57BL/6 mice to determine the extent to which IH challenge results in an enhanced allergy phenotype.
Materials and Methodology
Animals
C57BL/6 mice were purchased from Jackson Laboratories, Bar Harbor, ME. Animals were housed on Alpha-dri paper bedding (Shepherd Specialty Papers, Watertown, TN) in microfilter-topped cages (Ancare, Bellmore, NY) in a specific pathogen-free facility with ad libitum access to food and water. Age-matched mice were used in groups of five for each experiment. The study described was performed in accordance with IACUC guidelines (Research) of North Dakota State University.
Animal sensitization
Animals were sensitized and challenged as previously described (Hoselton et al., 2010; Samarasinghe et al., 2010). Sensitization procedure followed Hogaboam’s published protocol (Hogaboam et al., 2000) with a slight modification in the choice of adjuvant. A. fumigatus extract purchased from Greer Laboratories (Lenoir, NC) consisted of antigens from whole cultures including mycelia and spores. The extract was resuspended to 1 mg protein/ml, and aliquots were stored at −20°C. Mice were globally sensitized with subcutaneous and intraperitoneal injections of ten micrograms of A. fumigatus extract in 0.1 ml normal saline mixed with 0.1 ml of Imject® Alum (Pierce, Rockford, IL). Two weeks after the global sensitization, the animals were held upright and administered with 20 μg of A. fumigatus extract in 20 μl of normal saline intranasally with a pipet. This local sensitization procedure was performed once a week for three weeks (Hogaboam et al., 1999; Hogaboam et al., 2000). All experimental procedures utilizing A. fumigatus were conducted with prior approval of the Institutional Biological Safety Office of North Dakota State University.
Fungal culture
A lyophilized A. fumigatus fungal culture stock (strain NIH 5233) was purchased from American Type Culture Collection (Manassas, VA), reconstituted in 5 ml phosphate buffered saline (PBS) and stored in 60μl aliquots at 4°C. A single aliquot was used to inoculate each Petri dish or 25-cm2 flask with Sabouraud Dextrose Agar which were then incubated at 37°C for eight days to obtain mature fungal cultures. Conidia were harvested from Petri dish in 20 ml of PBS with 0.1% TweenTM 80. The suspension was passed through several layers of gauze and washed to remove mycelia and conidia counts were obtained with a hemacytometer. The conidial suspension was immediately used to challenge the mice via IT delivery as detailed below. The flasks with fungal cultures were used for the IH challenge as described below.
Intratracheal animal challenge protocol
Mice were anesthetized with a cocktail of ketamine (75mg/kg) and xylazine (25mg/kg) for challenge. One week after the last intranasal sensitization, tracheas were surgically exposed in anesthetized mice, and 5 × 106 conidia in 30μl of PBS with 0.1% T-80 delivered into the trachea (Hogaboam et al., 2000) constituting the IT challenge.
Inhalation animal challenge protocol
The airborne conidia challenge protocol was carried out in a Class II-Type A2 biological safety cabinet. A flask containing an 8-day-old fungal culture was attached to the inhalation chamber with the ports plugged and air was passed over the culture at 2 psi for 10 mins to liberate the conidia to coat the inner chamber of the inoculation system (Hoselton et al., 2010).
For IH challenge, three anesthetized mice were placed supine with noses in the inhalation chamber and exposed to airborne conidia liberated from a mature culture for 10 mins (Hoselton et al., 2010). The fungal culture was changed with each new set of mice. Additional groups of mice were exposed to airborne conidia two weeks after the first IH challenge (2x) (Samarasinghe et al., 2010), of which one group was challenged a third time, four weeks after the second challenge (3x).
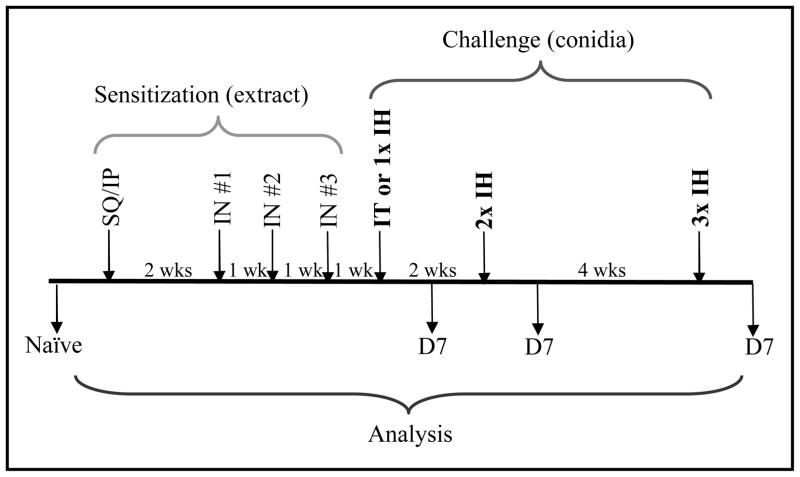
The invasive nature of the IT delivery causes scarring at the surgical site surrounding the trachea, making intubation and subsequent surgeries impractical. Therefore, the study described herein compares a single IT challenge with single or multiple IH challenges. All animals were sacrificed 7 days after their respective challenge protocols. Naïve animals were neither sensitized nor challenged. Figure 1 provides a schematic representation of the sensitization and challenge protocol.
Figure 1.
Animal sensitization and challenge protocol scheme. Mice were sensitized by subcutaneous (SQ) and intraperitoneal (IP) injections, and intranasal (IN) administration of commercially available extract from Aspergillus fumigatus. Sensitized mice were challenged with conidia via intratracheal (IT) or inhalation (IH) delivery methods. Samples were obtained at day 7 (D7) after each challenge. Mice that were neither sensitized, nor challenged served as naïve controls.
Measurement of airway hyperresponsiveness (AHR) and sample collection
Restrained plethysmography (Buxco, Troy, NY) was used to assess airway response (Hogaboam et al., 2000). Mice were anesthetized with sodium pentobarbital (Butler, Columbus OH; 0.1 mg/10 g of mouse body weight), intubated, and ventilated with a Harvard pump ventilator (Harvard Apparatus, Reno, NV). Buxco plethysmography (Buxco, Troy, NY) was used to assess airway responses. Baseline airway resistance was measured for each animal and averaged for the group. The dosage of a non-specific spasmogen, acetyl-β-methacholine, that doubled the baseline airway resistance in naïve mice was selected to analyze AHR. Airway resistance was measured in each animal following an intravenous injection of acetyl-β-methacholine (420 μg/kg) to determine AHR at day 7 after A. fumigatus challenge (Hoselton et al.).
Approximately 500 μl of blood was collected by ocular bleed, and a bronchoalveolar lavage (BAL) was performed on each mouse with 1 ml of sterile saline. Left lungs were harvested, inflated with 10% neutral buffered formalin, fixed in formalin for 16 h, and paraffin-embedded for histological and immunohistochemical analyses.
Determination of allergic airways disease
Individual BAL samples were centrifuged at 3000 × g for 10 min to separate the cells from the fluid. BAL fluid samples were stored at −20 °C until use, while BAL cells were re-suspended and cytospun (Shandon Scientific, Runcorn) onto microscope slides for differential staining (Quick-Dip stain, Mercedes Medical, Sarasota, FL) and counting. Macrophages, eosinophils, neutrophils, and lymphocytes from five randomly chosen high-power fields (HPFs) in each sample, identified based on morphology and eosin dye uptake, were counted to determine the mean number of each cell type in the airway lumen of each group. A large influx of inflammatory leukocytes (such as eosinophils) into the BAL compartment compared to naïve controls constituted allergic responses against fungal challenge. Blood harvested from each mouse was centrifuged at 13,000 × g for 10 min to obtain sera. IgE and IgG2a concentrations in the sera and BAL fluid were quantified by ELISA per manufacturer’s guidelines (BD OptEIA, San Diego, CA). Briefly, plates were coated with anti-IgE, or anti-IgG2a capture antibodies and incubated overnight at 4°C. Washed plates were blocked for one hour at room temperature in PBS with 10% fetal bovine serum. Washed plates were incubated in sera (1:100) or BAL fluid (1:40 for IgG2a and undiluted for IgE) samples at room temperature for one hour, washed, then incubated in detection antibody, enzyme conjugate, and developed in substrate prior to absorbance measurements.
Histological and immunohistochemical analyses
Formalin-fixed, paraffin-embedded lungs were cut longitudinally across the coronal plane in 5-μm sections and stained with hematoxylin and eosin (H&E) stain (Richard-Allan Scientific, Kalamazoo, MI) to assess inflammation, with periodic acid Schiff’s (PAS) stain (Richard-Allan Scientific) to assess goblet cell metaplasia, or Gomori’s trichrome stain (Richard-Allan Scientific) to assess subepithelial collagen deposition.
Immunohistochemistry (IHC) was used to determine the localization of a prominent marker of smooth muscle, αSMA. Sections affixed to glass slides were submerged in 10-mM citric acid at pH 6.0 and microwaved in a pressure cooker for 10 min for antigen retrieval. Staining was performed using the HRP-AEC cell and tissue staining kits by R&D Systems (Minneapolis, MN) following the manufacturer’s recommended protocol as follows. Endogenous peroxidase, serum, avidin, and biotin blocking steps were carried out at room temperature using the appropriate blocking reagents provided in the staining kits. Sections were incubated in rabbit monoclonal antibody against αSMA (Abcam, Cambridge, MA) at 1:500, overnight at 4°C. Control sections were not incubated in primary antibody. Incubation in secondary antibody was carried out at room temperature for one hour. A 30-min incubation in horseradish peroxidase conjugate followed by a 10-min incubation in 3-amino-9-ethylcarbazole resulted in the formation of a red precipitate in areas of antigen-antibody localization. Sections were counterstained in Gill’s III hematoxylin (Surgipath, Richmand, IL) for 10 sec.
Statistical analysis
All groups were analyzed against one another with Prism GraphPad software (San Diego, CA) using the one-way ANOVA with Newman-Keuls post test to determine statistical significance. Results are expressed as mean ± standard error of the mean (SEM). p <0.05 was considered statistically significant. Letters (a, b, c, and d) placed on bars of graphs denote their comparison to other groups. Bars with no letters in common are significantly different, while samples with the same letters are indicative of data sets that are not statistically significant.
Results
While each protocol yielded an allergic response in mice that was marked by airway inflammation, hyperresponsiveness, goblet cell metaplasia, and elevated serum IgE production, our findings indicate that particular characteristics of fungal allergic asthma can be manipulated depending upon the route of delivery employed. The most dramatically different features were the enhanced recruitment of eosinophils and lymphocytes to the airways and the exacerbation of airway wall remodelling. Multiple IH challenges, 2x and 3x, yielded similar results in all parameters analyzed; this is indicative that the immune response remains unaltered after the initial exposure to conidial antigens irrespective of the time lapse between exposures.
Airway hyperresponsiveness
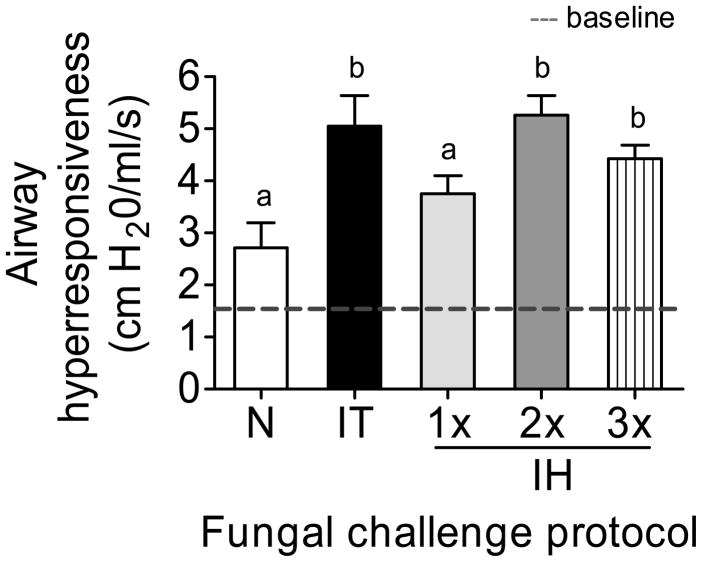
Airways of allergic animals challenged via intratracheal or multiple inhalations of A. fumigatus conidia were hyperresponsive to methacholine provocation. Airway reactivity of each animal was measured prior to methacholine administration and averaged 1.51 ±0.10 cm H2O/ml/s (Fig 2, dotted line). The single exposure to inhaled conidia was insufficient to induce a significant increase in airway reactivity. However, IT challenge and multiple IH challenges resulted in significant airway hyperresponsiveness compared to naïve controls. However, there were no differences in AHR between IT and multiple IH challenges.
Figure 2.
Airway hyperresponsiveness measured in naïve and allergic animals 7 days after each challenge protocol. The mean baseline measurement of 1.51 ±0.10 cm H2O/ml/s is represented by the dotted line. n=4–5 mice per group. Data analyzed using one way ANOVA with the Newman-Keuls post test. Bars having no letters in common are significantly different (p<0.05).
Leukocyte recruitment into the airways following conidia challenge
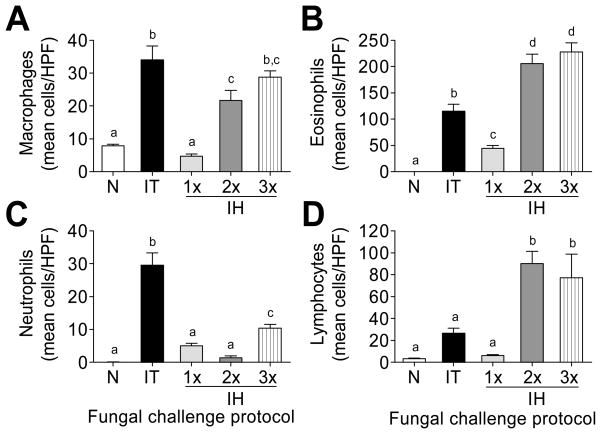
Inflammatory cells in the BAL contents 7 days after each challenge protocol were differentially stained and counted based on morphology and eosin dye uptake in comparison to naïve mice. Macrophages, neutrophils, eosinophils, and lymphocytes in the BAL compartment were counted and reported as the mean/HPF for each group analyzed (Fig 3). Although a single IH challenge led to the recruitment of all the cell types analyzed, cell recruitment was more pronounced after multiple exposures (Fig 3). The IT challenge protocol resulted in more innate-type cells, macrophages (Fig 3A) and neutrophils (Fig 3C), into the allergic airways at day 7 compared to the IH challenge protocol. While macrophage recruitment increased gradually with each IH challenge (Fig 3A), neutrophil recruitment decreased upon re-challenge (2x IH), and increased slightly after the third (3x) IH challenge (Fig 3C). Multiple IH challenges resulted in the recruitment of approximately twice as many eosinophils compared to the IT challenge at this time point (Fig 3B). Although the peak in eosinophil recruitment after IT challenge occurred at day 7, it occurred at day 3 after IH challenge. The mean number of eosinophils/HPF was 433.8 ±36.1 at day 3 after 2x IH (data not shown) emphasizing that multiple inhalations of conidia polarize the immune response in favour of allergy. Similarly, lymphocyte recruitment was elevated after additional IH challenges with approximately a 3-fold increase compared to the IT challenge protocol (Fig 3D).
Figure 3.
Morphological analysis of inflammatory leukocytes in the bronchoalveolar lavage contents of naïve and allergic mice. n=5 mice per group. Data analyzed using one-way ANOVA with the Newman-Keuls post test. Bars having no letters in common are significantly different (p<0.05). HPF = high power fields; N = naïve; IT = intratracheal challenge; 1× 2× 3× IH = single, double, and triple inhalation challenge.
Systemic and local antibody production
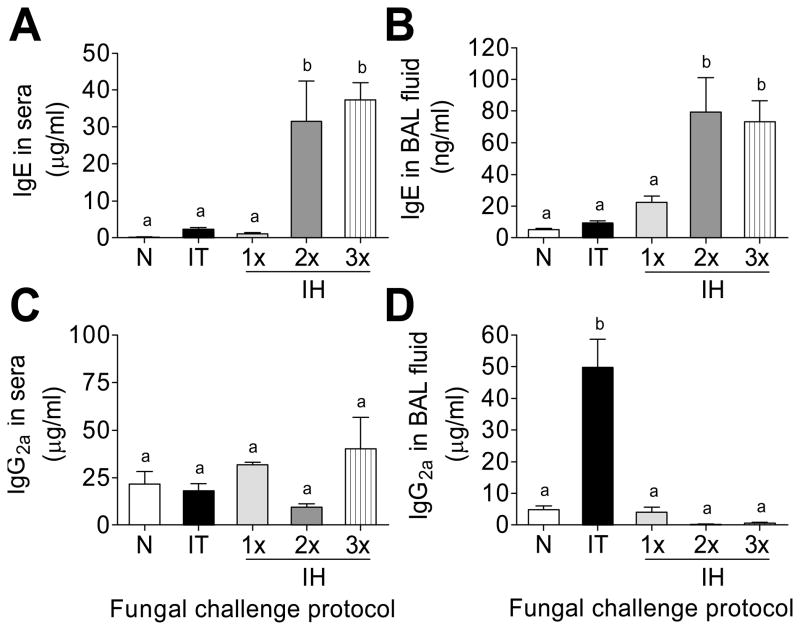
Elevations in serum IgE, an important hallmark of allergic asthma, was observed in A. fumigatus sensitized and challenged animals. The production of serum IgE was strikingly enhanced after multiple inhalations compared to IT challenge and single IH challenge protocols (Fig 4A). IT and 1x IH resulted in similar levels of BAL IgE, while additional IH challenge produced a significant increase in BAL IgE when compared to either IT or 1x IH (Fig 4B).
Figure 4.
Immunoglobulin (Ig) quantification 7 days after each challenge protocol with ELISA compared to naïve mice. n=4–5 mice per group. Data analyzed using one-way ANOVA with the Newman-Keuls post test. Bars having no letters in common are significantly different (p<0.05). BAL = bronchoalveolar lavage; N = naïve; IT = intratracheal challenge; 1× 2× 3× IH = single, double, and triple inhalation challenge.
IgG2a is the major immunoglobulin G sub-class produced by TH1-activated B cells. Although not crucial for pathogen elimination via complement fixation, its ability to function as an opsonin to mediate phagocytosis of invading fungi (Mukherjee et al., 1995) indicates its importance in host protection against fungal growth. Since asthma is a TH2-mediated disease, the production of IgG2a was expected to decrease with sensitization and fungal challenge. Systemic IgG2a did not change after conidia challenge (Fig 4C). While a massive increase in IgG2a was noted in the BAL fluid after the IT challenge, IH exposures resulted in a marked reduction in local IgG2a (Fig 4D). The increase in systemic and local IgE with a decrease in IgG2a in the BAL (as compared to IT) shows that IH challenge reinforces the allergic phenotype particularly at the level of local tissue.
Pathological changes in the lungs after intratracheal and inhalation challenge protocols compared to naïve controls
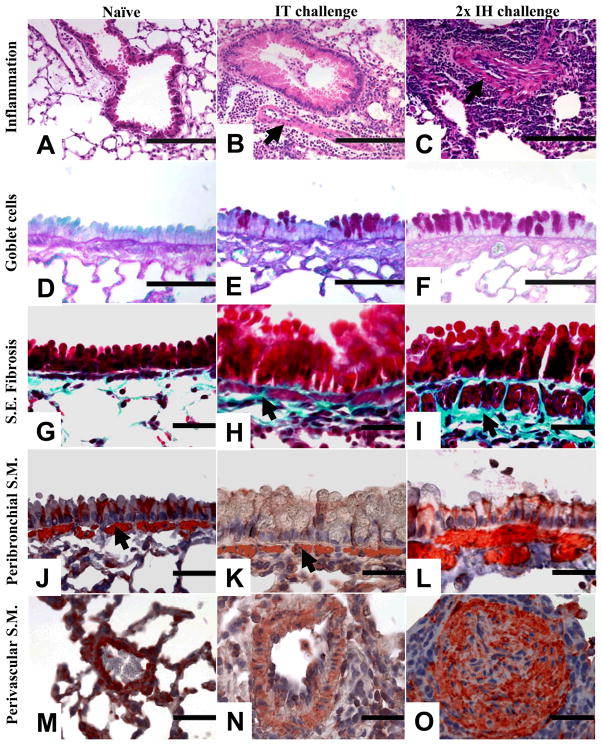
A single IH challenge with A. fumigatus conidia induced eosinophilic airway inflammation and goblet cell metaplasia that were equivalent to the IT challenge method. Airway wall remodelling events such as subepithelial fibrosis and smooth muscle cell hyperplasia were visually more apparent after multiple IH challenges as compared to the IT challenge. The third IH challenge (3x) resulted in the further enhancement of airway wall remodelling events observed after the second IH (2x). Photomicrograph representations are of IT challenge and two IH challenges (Fig 5).
Figure 5.
Representative photomicrographs showing inflammation, goblet cell metaplasia, subepithelial fibrosis, and peribronchovascular smooth muscle cell changes in naïve controls or at 7 days after either a single intratracheal (IT) or two inhalation (IH) challenges. H&E-stained histological sections from naïve (A), IT-treated (B), and IH-treated (C) lungs were assessed for inflammation (arrows indicate perivascular smooth muscle cell increases). Goblet cells were examined in periodic acid Schiff’s stained sections in naïve (D), IT-challenged (E), or IH-challenged (F) lungs. Gomorri’s trichrome stain was used to visualize subepithelial (S.E.) collagen deposition (blue) and to visualize smooth muscle (S.M., pink) in histological sections. Naïve (G), IT-challenged (arrow, H), and IH-challenged (arrow, I) lungs were assessed for peribronchial collagen. Peribronchovascular smooth muscle was assessed in naïve lungs (J, M), after IT challenge (arrow, K), and after IH challenge (arrow, L). Smooth muscle changes in the distal blood vessels were noted as a prominent and differentiating feature after IH challenge (O) as compared to naïve (M) or IT-challenged (N) lungs. Scale bars; A–C = 200μm, D–F = 100μm, G–O = 50μm.
Naïve animals did not exhibit pulmonary inflammation (Fig 5A) while allergic mice challenged with single IT (Fig 5B) or IH conidia had prominent peribronchovascular inflammation. Peribronchovascular inflammation increased after a second IH challenge (Fig 5C). Smooth muscle cell hyperplasia around the blood vessels was apparent after multiple IH challenges (arrow, Fig 5C) but minimal after IT challenge (arrow, Fig 5B). The number of goblet cells that interspersed the columnar epithelia was minimal in naïve lungs (Fig 5D), while allergen challenge led to an increase in the goblet cell numbers shown by magenta staining after periodic acid Schiff’s stain in all groups. However, fewer goblet cells were observed after IT challenge (Fig 5E) compared to IH challenge (Fig 5F).
Airway remodelling events are marked by subepithelial collagen deposition and smooth muscle cell hyplerplasia. Subepithelial collagen deposition was scarce in naïve lungs (Fig 5G) while fungal challenge caused a marked increase in the amount of collagen observed in these areas (aqua staining) after Gomori’s trichrome staining procedure. The layer of subepithelial collagen was thinner in the IT challenged lungs (arrow, Fig 5H) while multiple IH challenges led to a marked increase in the thickness of this layer (arrow, Fig 5I). Naïve (arrow, Fig 5J) and IT-challenged (arrow, Fig 5K) lungs showed no signs of smooth muscle hyperplasia around the airways. The peribronchial smooth muscle cell layer was markedly thicker after IH challenge (arrow, Fig 5L).
Perivascular smooth muscle cell hyperplasia was a prominent and unique feature of the inhalation protocols. Naïve lungs had no hyperplasia in this cell layer (Fig 5M), while some rare distal blood vessels were noted to be slightly thickened after IT challenge (Fig 5N). In contrast, massive hyperplasia of vascular smooth muscle was a major feature after 2x IH challenges (Fig 5O).
Prominent features of the intratracheal and inhalation challenge protocols
The utilization of the single IH challenge, while sufficient to induce the allergic phenotype in C57BL/6 mice, was not sufficient to attain maximum eosinophilia or IgE. Multiple IH challenges mimicked the hallmarks of human patients as demonstrated in this study. Therefore, the major differences between the IT and 2x IH challenge protocols are summarized in Table 1. The 2x IH challenge is more suited for the induction of eosinophilia and smooth muscle cell hyperplasia compared to the IT protocol. However, the IT challenge resulted in the recruitment of more acute inflammatory cells (neutrophils and macrophages) compared to IH challenges, and may reflect events in the early sensitization of the allergic lung.
Table 1.
Summary of parameters analyzed after intratracheal (IT) and two inhalation (2x IH) challenges with Aspergillus fumigatus in antigen-sensitized C57BL/6 mice.
| Analyzed parameter | IT | 2x IH |
|---|---|---|
| Macrophage recruitment | +(+) | + |
| Neutrophil recruitment | +++ | + |
| Eosinophil recruitment | + | ++ |
| Lymphocyte recruitment | + | +++ |
| Serum IgE | + | +++ |
| BAL fluid IgE | + | +++ |
| Serum IgG2a | + | (+) |
| BAL fluid IgG2a | +++ | + |
| Peribronchovascular inflammation | ++ | +++ |
| Goblet cell metaplasia | ++ | +++ |
| Subepithelial fibrosis | + | +++ |
| Smooth muscle cell hyperplasia | (+) | +++ |
Relative increases in the modulation of these parameters are represented with + symbols as compared to naïve controls. Parentheses are indicative of incremental changes.
Discussion
Animal model systems of fungal allergic asthma are limited, but crucial to our understanding of host-pathogen interactions involved in the initiation and development of the disease which results in structural changes that compromise the normal physiology of the lungs. A. fumigatus produces massive numbers of conidia commonly inhaled by humans and other animals. In models that employ A. fumigatus, modes of challenge include the delivery of conidia into the respiratory system via involuntary aspiration, intranasal, intratracheal, nebulization, and dry-inhalation methods. Since variations in challenge protocols alter the outcomes induced in mice (Zhang et al., 1997), we hypothesized that the mode and type of fungal exposure may alter the allergic phenotype drastically. To this aim, we compared the outcomes of intratracheal and inhalation challenge protocols in sensitized mice.
The antigenic exposure of the conidia may differ between these challenge protocols due to the manipulation involved with the IT delivery and the lack thereof for the IH delivery. The IT delivery method requires the suspension of conidia in buffer with detergent to reduce surface hydrophobicity which prevents conidial clumping. TweenTM is a non-ionic surfactant commonly used to enhance the delivery of particles by increasing cell permeability, but may interfere with the host-pathogen interaction (Henderson et al., 1995) and can also be toxic to lung epithelial cells (Nakate et al., 2003). Repeated intratracheal delivery of conidia in TweenTM may thus lead to pathology that is enhanced by the presence of the detergent. Suspension in TweenTM enhances the solubility of conidia (by limiting agglomeration), the number of conidia that enters the sol layer of the mucus may increase abnormally thereby inducing more neutrophil recruitment for fungal clearance. The increase in neutrophil recruitment corresponds to the increase in IgG2a in the BAL fluid after IT challenge. Since neutrophils express Fc receptors for IgG2a (Lopez et al., 1985) and IgG2a functions as an opsonin to mediate pathogen clearance (Mukherjee et al., 1995), the influx of neutrophils at the time of increased IgG2a in BAL fluid may further illustrate that this mode of fungal challenge mediates a prominent inflammatory response compared to conidia inhalation.
Conidial interaction with host defences dictates the outcome of the immune response. The conidial wall contains external (melanin and rodlets) and internal layers (α-, β-glucans, chitins, galactomannans). The prior, which provides immune protection (Chai et al., 2009), is gradually lost during swelling and germination exposing the latter which mediates aggregation (Fontaine et al., 2010). A. fumigatus conidial mitosis occurs within hours in favourable conditions (Momany and Taylor, 2000). The necessity for macrophages and neutrophils in the effective elimination of A. fumigatus conidia and germinating spores has been demonstrated extensively (Bonnett et al., 2006; Philippe et al., 1997; Schaffner et al., 1982), and their phagocytic functions are enhanced by IgG2a opsonisation (Mukherjee et al., 1995). Whether the differences in leukocyte influx after IT versus IH fungal challenge is a result of variations in the conidial surface expression of antigens or conidial impact on the epithelia which alters cellular recruitment and transmigration needs to be elucidated. Nonetheless, the enhanced recruitment of macrophages and neutrophils along with increased availability of IgG2a in the BAL fluid after IT challenge advocates this protocol for the investigation of innate cellular defences against A. fumigatus conidia.
While the recruitment of eosinophils into the lungs is a common hallmark of allergic airways disease, the role played by these cells is still ambiguous. Exposure to A. fumigatus extracts leads to eosinopoiesis and the subsequent increase in peripheral blood eosinophils (Murali et al., 1992) reiterating that fungal antigens are responsible for the recruitment of eosinophils into the affected tissues. The association between eosinophils and airway remodelling demonstrated in various model systems classify eosinophils as regulators of this chronic feature of asthma (Bousquet et al., 2000; Cho et al., 2004; Humbles et al., 2004; Pegorier et al., 2006). In this study, subepithelial fibrosis and smooth muscle cell hyperplasia corresponded with massive eosinophilia in the lungs after multiple inhalation exposures to conidia demonstrating that this model may provide a means to determine the extent to which eosinophils mediate airway remodelling events in genetically unaltered mice.
Asthma is considered to be a TH2-mediated disease based on increased TH2-type cytokines and the role played by TH2 cells in the activation of IgE producing B cells. Exposure to A. fumigatus stimulates the induction of cytokines, chemokines, and antibodies (Hogaboam et al., 2000; Knutsen et al., 1994; Knutsen et al., 2004; Skov Olsen et al., 1986) that are mediated by TH2 lymphocytes. The recruitment of lymphocytes correlating with the induction of local IgE following conidia challenge in this study underscores the allergenicity A. fumigatus conidia. Since murine eosinophils do not express Fc receptors for IgE (de Andres et al., 1997; Kita and Gleich, 1997), and mast cells are limited in mouse lungs (Gersch et al., 2002), the exact role of IgE in mouse models of allergic asthma is still unclear. Nonetheless, the significant enhancement of IgE after IH challenge highlights the relationship between the mode of fungal delivery and the strength of the allergic output.
Airway wall remodelling events are believed to be extensive repair processes initiated in response to repeated injuries induced by allergen exposure. The decrease in diameter of the airway lumen due to increased deposition of collagen and smooth muscle hyperplasia makes breathing difficult. Airway wall thickening is observed in asthmatics irrespective of disease severity, although subepithelial fibrosis and smooth muscle cell hyperplasia are more evident in severe asthmatics and have been correlated with AHR (Elias, 2000). However, these characteristics are seldom demonstrated in models utilizing wild-type mice, thereby placing genetically modified animals at the forefront of airway wall remodelling research (Elias, 2000). We have demonstrated the ability to produce subepithelial fibrosis in wild-type mice by exposure to conidia (Hoselton et al., 2010), a feature that is more pronounced than those published previously (Hogaboam et al., 2000). We conclude that the differences between our demonstration of this feature than that of Hogaboam et al. (2000) are due to variations in conidial delivery methods, choice of adjuvant and mouse strains. However, the changes to the lung architecture were more apparent after multiple IH challenges compared to the IT challenge which did not generate smooth muscle cell hyperplasia. This may indicate that cellular responses leading to architectural changes in the lung differ with the mode of allergen delivery.
The advantages and disadvantages of using intratracheal instillation technique have been reviewed extensively (Driscoll et al., 1976; Henderson et al., 19955; Leong et al., 1998). The advantages of using the IT model include the ability to administer a predetermined number of conidia, lack of animal ingestion of conidia, and the ability to use a single fungal culture to treat a large number of animals. The most important benefit of using the IH model is that it mimics a natural allergen as well as the natural route of exposure in humans thereby eliminating the need for surgery. In contrast, the major disadvantages of the IT model are that the challenge protocol requires invasive surgery and may limit the allergen to the upper airways. A major concern with the IT model is that liquid suspension alters the antigens expressed by conidia resulting in an immune response that is more inflammatory in nature compared to the IH model which does not manipulate the conidia. The inability to expose individual animals to an exact number of conidia each time is a major, the possibility of conidial ingestion, and the high number of fungal cultures required to treat animals are the major drawbacks of the IH model. As described in this study, the IH model would provide a platform for the study of eosinophils and airway remodelling in fungal allergic asthma, while the IT model may be more useful in elucidating the roles played by macrophages and neutrophils in this disease. While each method has its unique advantages, the utilization of the method to produce a mouse model must be informed by the desired outputs and time allotted for experiments and will vary depending upon the goals of the investigation.
Acknowledgments
Funding for this project was supported by NIH/NCRR 2P20 RR015566 (including the use of Core Biology Facilities), NIH/NIAID 1R15AI69061, and through a faculty development grant from the NDSU Advance FORWARD program (NSF HRD-0811239).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Amali E. Samarasinghe, Email: a.samarasinghe@ndsu.edu.
Scott A. Hoselton, Email: Scott.Hoselton@ndsu.edu.
Jane M. Schuh, Email: Jane.Schuh@ndsu.edu.
References
- Bonnett CR, Cornish EJ, Harmsen AG, Burritt JB. Early neutrophil recruitment and aggregation in the murine lung inhibit germination of Aspergillus fumigatus conidia. Infect Immun. 2006;74:6528–6539. doi: 10.1128/IAI.00909-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. Asthma. From bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med. 2000;161:1720–1745. doi: 10.1164/ajrccm.161.5.9903102. [DOI] [PubMed] [Google Scholar]
- Chai LY, Netea MG, Sugui J, Vonk AG, van de Sande WW, Warris A, Kwon-Chung KJ, Jan Kullberg B. Aspergillus fumigatus conidial melanin modulates host cytokine response. Immunobiology. 2009 doi: 10.1016/j.imbio.2009.10.002. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JY, Miller M, Baek KJ, Han JW, Nayar J, Lee SY, McElwain K, McElwain S, Friedman S, Broide DH. Inhibition of airway remodeling in IL-5-deficient mice. J Clin Invest. 2004;113:551–560. doi: 10.1172/JCI19133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Andres B, Rakasz E, Hagen M, McCormik ML, Mueller AL, Elliot D, Metwali A, Sandor M, Britigan BE, Weinstock JV, Lynch RG. Lack of Fc-epsilon receptors on murine eosinophils: implications for the functional significance of elevated IgE and eosinophils in parasitic infections. Blood. 1997;89:3826–3836. [PubMed] [Google Scholar]
- Driscoll KE, Costa DL, Hatch G, Henderson R, Oberdorster G, Salem H, Schlesinger RB. Intratracheal instillation as an exposure technique for the evaluation of respiratory tract toxicity: uses and limitations. Toxicol Sci. 2000;55:24–35. doi: 10.1093/toxsci/55.1.24. [DOI] [PubMed] [Google Scholar]
- Elias JA. Airway Remodeling in Asthma. Am J Respir Crit Care Med. 2000;161:S168–S171. doi: 10.1164/ajrccm.161.supplement_2.a1q4-4. [DOI] [PubMed] [Google Scholar]
- Fontaine T, Beauvais A, Loussert C, Thevenard B, Fulgsang CC, Ohno N, Clavaud C, Prevost MC, Latge JP. Cell wall alpha1-3glucans induce the aggregation of germinating conidia of Aspergillus fumigatus. Fungal Genet Biol. 2010;47:707–712. doi: 10.1016/j.fgb.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Gersch C, Dewald O, Zoerlein M, Michael LH, Entman ML, Frangogiannis NG. Mast cells and macrophages in normal C57/BL/6 mice. Histochem Cell Biol. 2002;118:41–49. doi: 10.1007/s00418-002-0425-z. [DOI] [PubMed] [Google Scholar]
- Henderson RF, Driscoll KE, Harkema JR, Lindenschmidt RC, Chang IY, Maples KR, Barr EB. A comparison of the inflammatory response of the lung to inhaled versus instilled particles in F344 rats. Fundam Appl Toxicol. 1995;24:183–197. doi: 10.1006/faat.1995.1022. [DOI] [PubMed] [Google Scholar]
- Hogaboam CM, Blease K, Mehrad B, Steinhauser ML, Standiford TJ, Kunkel SL, Lukacs NW. Chronic Airway Hyperreactivity, Goblet Cell Hyperplasia, and Peribronchial Fibrosis during Allergic Airway Disease Induced by Aspergillus fumigatus. American Journal of Pathology. 2000;156:723–732. doi: 10.1016/S0002-9440(10)64775-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogaboam CM, Gallinat CS, Taub DD, Strieter RM, Kunkel SL, Lukacs NW. Immunomodulatory role of C10 chemokine in a murine model of allergic bronchopulmonary aspergillosis. J Immunol. 1999;162:6071–6079. [PubMed] [Google Scholar]
- Hoselton SA, Samarasinghe AE, Seydel JM, Schuh JM. An inhalation model of airway allergic response to inhalation of environmental Aspergillus fumigatus conidia in sensitized BALB/c mice. Med Mycol. 2010 doi: 10.3109/13693786.2010.485582. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humbles AA, Lloyd CM, McMillan SJ, Friend DS, Xanthou G, McKenna EE, Ghiran S, Gerard NP, Yu C, Orkin SH, Gerard C. A critical role for eosinophils in allergic airways remodeling. Science. 2004;305:1776–1779. doi: 10.1126/science.1100283. [DOI] [PubMed] [Google Scholar]
- Kita H, Gleich GJ. Eosinophils and IgE receptors: a continuing controversy. Blood. 1997;89:3497–3501. [PubMed] [Google Scholar]
- Knutsen AP, Hutcheson PS, Slavin RG, Kurup VP. IgE antibody to Aspergillus fumigatus recombinant allergens in cystic fibrosis patients with allergic bronchopulmonary aspergillosis. Allergy. 2004;59:198–203. doi: 10.1046/j.1398-9995.2003.00310.x. [DOI] [PubMed] [Google Scholar]
- Knutsen AP, Mueller KR, Levine AD, Chouhan B, Hutcheson PS, Slavin RG. Asp f I CD4+ TH2-like T-cell lines in allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol. 1994;94:215–221. doi: 10.1016/0091-6749(94)90043-4. [DOI] [PubMed] [Google Scholar]
- Kurup VP, Choi H, Murali PS, Coffman RL. IgE and eosinophil regulation in a murine model of allergic aspergillosis. J Leukoc Biol. 1994a;56:593–598. doi: 10.1002/jlb.56.5.593. [DOI] [PubMed] [Google Scholar]
- Kurup VP, Seymour BW, Choi H, Coffman RL. Particulate Aspergillus fumigatus antigens elicit a TH2 response in BALB/c mice. J Allergy Clin Immunol. 1994b;93:1013–1020. doi: 10.1016/s0091-6749(94)70050-8. [DOI] [PubMed] [Google Scholar]
- Land CJ, Sostaric B, Fuchs R, Lundstrom H, Hult K. Intratracheal exposure of rats to Aspergillus fumigatus spores isolated from sawmills in Sweden. Appl Environ Microbiol. 1989;55:2856–2860. doi: 10.1128/aem.55.11.2856-2860.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latge JP. The pathobiology of Aspergillus fumigatus. Trends Microbiol. 2001;9:382–389. doi: 10.1016/s0966-842x(01)02104-7. [DOI] [PubMed] [Google Scholar]
- Leong BK, Coombs JK, Sabaitis CP, Rop DA, Aaron CS. Quantitative morphometric analysis of pulmonary deposition of aerosol particles inhaled via intratracheal nebulization, intratracheal instillation or nose-only inhalation in rats. J Appl Toxicol. 1998;18:149–160. doi: 10.1002/(sici)1099-1263(199803/04)18:2<149::aid-jat490>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Lopez AF, Battye FL, Vadas MA. Fc receptors on mouse neutrophils and eosinophils: antigenic characteristics, isotype specificity and relative cell membrane density measured by flow cytometry. Immunology. 1985;55:125–133. [PMC free article] [PubMed] [Google Scholar]
- Momany M, Taylor I. Landmarks in the early duplication cycles of Aspergillus fumigatus and Aspergillus nidulans: polarity, germ tube emergence and septation. Microbiology. 2000;146(Pt 12):3279–3284. doi: 10.1099/00221287-146-12-3279. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Lee SC, Casadevall A. Antibodies to Cryptococcus neoformans glucuronoxylomannan enhance antifungal activity of murine macrophages. Infect Immun. 1995;63:573–579. doi: 10.1128/iai.63.2.573-579.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murali PS, Dai G, Kumar A, Fink JN, Kurup VP. Aspergillus antigen-induced eosinophil differentiation in a murine model. Infect Immun. 1992;60:1952–1956. doi: 10.1128/iai.60.5.1952-1956.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakate T, Yoshida H, Ohike A, Tokunaga Y, Ibuki R, Kawashima Y. Improvement of pulmonary absorption of cyclopeptide FK224 in rats by co-formulating with beta-cyclodextrin. Eur J Pharm Biopharm. 2003;55:147–154. doi: 10.1016/s0939-6411(02)00158-3. [DOI] [PubMed] [Google Scholar]
- Pegorier S, Wagner LA, Gleich GJ, Pretolani M. Eosinophil-derived cationic proteins activate the synthesis of remodeling factors by airway epithelial cells. J Immunol. 2006;177:4861–4869. doi: 10.4049/jimmunol.177.7.4861. [DOI] [PubMed] [Google Scholar]
- Philippe B, Ibrahim-Granet O, Prevost MC, Gougerot-Pocidalo MA, Sanchez Perez M, Van der Meeren A, Latge JP. Killing of Aspergillus fumigatus by alveolar macrophages is mediated by reactive oxidant intermediates. Infect Immun. 2003;71:3034–3042. doi: 10.1128/IAI.71.6.3034-3042.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Research IfLA. Guide for the Care and Use of Laboratory Animals. National Academy Press; Washington, D.C: 1996. [Google Scholar]
- Samarasinghe AE, Hoselton SA, Schuh JM. The absence of the VPAC(2) receptor does not protect mice from Aspergillus induced allergic asthma. Peptides. 2010;31:1068–1075. doi: 10.1016/j.peptides.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner A, Douglas H, Braude A. Selective protection against conidia by mononuclear and against mycelia by polymorphonuclear phagocytes in resistance to Aspergillus. Observations on these two lines of defense in vivo and in vitro with human and mouse phagocytes. J Clin Invest. 1982;69:617–631. doi: 10.1172/JCI110489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skov Olsen P, Kirkegaard P, Poulsen SS, Nexo E. Vasoactive intestinal polypeptide and acetylcholine stimulate exocrine secretion of epidermal growth factor from the rat submandibular gland. Regul Pept. 1986;15:37–46. doi: 10.1016/0167-0115(86)90073-x. [DOI] [PubMed] [Google Scholar]
- Wang K, Ussery DW, Brunak S. Analysis and prediction of gene splice sites in four Aspergillus genomes. Fungal Genet Biol. 2009;46(Suppl 1):S14–18. doi: 10.1016/j.fgb.2008.09.010. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Lamm WJ, Albert RK, Chi EY, Henderson WR, Jr, Lewis DB. Influence of the route of allergen administration and genetic background on the murine allergic pulmonary response. Am J Respir Crit Care Med. 1997;155:661–669. doi: 10.1164/ajrccm.155.2.9032210. [DOI] [PubMed] [Google Scholar]