Abstract
Purpose
To determine the relation of high-contrast visual acuity (VA) and low-contrast letter acuity with retinal nerve fiber layer (RNFL) thickness in children with optic pathway gliomas.
Design
Cross-sectional convenience sample, with prospective data collection, from a tertiary care children’s hospital of patients with optic pathway gliomas associated with Neurofibromatosis type 1, sporadic OPG and Neurofibromatosis type 1 without OPG.
Methods
Patients performed best corrected VA testing using surrounded HOTV optotypes and low-contrast letter acuity (5%, 2.5% and 1.25% low contrast Sloan letter charts). Mean RNFL thickness (microns) was measured by a Stratus optical coherence tomography (Carl Zeiss Meditec, Dublin, CA) using the fast RNFL thickness protocol. Eyes were classified as having abnormal vision if they had high-contrast VA > 0.1 logMAR or visual field loss. The association of subject age, glioma location and RNFL thickness with both VA and low-contrast letter acuity scores was evaluated by one-way analysis of variance and linear regression, using the generalized estimating equation approach to account for within-patient intereye correlations.
Results
Eighty-nine eyes of patients with optic pathway gliomas were included and 41 were classified as having abnormal VA or visual field loss. Reduced RNFL thickness was significantly associated with higher logMAR scores for both VA (P < 0.001) and all low-contrast letter acuity charts (P < 0.001) when accounting for age and glioma location.
Conclusions
Eyes of most children with optic pathway gliomas and decreased RNFL thickness had abnormal visual acuity or visual field loss.
Low grade gliomas (World Health Organization grade 1 juvenile pilocytic astrocytomas and grade 2 diffuse fibrillary astrocytomas) are the most common central nervous tumor in children.1 When low grade gliomas involve structures of the afferent visual pathway (i.e., optic nerve, optic chiasm, optic tract, or optic radiations), they are commonly referred to as optic pathway gliomas. Nearly 20% of children with Neurofibromatosis type 1 (NF1) develop optic pathway gliomas, and up to half can cause vision loss.2–4 Optic pathway gliomas in children without NF1 are termed sporadic gliomas and are believed to be clinically more aggressive than NF1 associated optic pathway gliomas.5,6
Standard measures of high contrast visual acuity (VA)—highly dependent upon the child’s cooperation—typically are used to screen and monitor vision outcomes in children with optic pathway gliomas.3 Vision loss from NF1 associated optic pathway gliomas occurs at a median age of 4.9 years7 and infrequently occurs after eight years of age.3 Visual acuity and or visual field loss from optic pathway gliomas can occur despite improvement, stability or progression of radiographic findings. Once vision loss has occurred, it can remain stable, improve or continue to worsen regardless of radiographic changes or therapeutic intervention. Since not all optic pathway gliomas cause vision loss, treatment for an optic pathway glioma is usually initiated only after vision loss has been detected, in the hopes of preventing further vision loss, or after significant radiographic progression with associated symptoms. The management of a child with an optic pathway glioma is more challenging in the absence of knowing the child’s visual acuity. Therefore, a reliable quantitative ophthalmologic tool that does not rely on patient cooperation is needed to evaluate optic pathway integrity in children with an optic pathway glioma, particularly in those who are unable to cooperate with visual acuity and visual field examinations.
The retinal nerve fiber layer (RNFL), the most proximal region of the afferent visual pathway, has been examined as a structural marker of visual integrity in patients with compressive optic neuropathy due to sellar masses8,9 and demyelinating optic neuropathy from multiple sclerosis.10–13 RNFL thickness, as measured by optical coherence tomography (OCT), is closely correlated with low-contrast letter acuity in patients with multiple sclerosis.10,11 Studies of optic neuritis show RNFL thinning over time by OCT, yet eyes of patients with multiple sclerosis and no history of optic neuritis also have RNFL thinning.10,14 To the best of our knowledge, no studies have examined whether RNFL thickness could serve as a structural marker of vision loss in children with optic pathway gliomas.
The primary aim of this study is to determine the relation of VA and low-contrast letter acuity with RNFL thickness, as measured by OCT, in children with optic pathway gliomas.
Methods
Patients
A cross-sectional convenience sample with prospective data collection identified candidate subjects between 6 and 21 years of age during their routine clinical visits to the neuro-ophthalmology or neuro-oncology clinics at Children’s Hospital of Philadelphia from July 2009 and January 2010. Children between 6 and 17 years of age required parental/guardian informed consent, and when appropriate child assent, prior to study enrollment. Children 18 to 21 years of age provided their own consent. Children in the NF1 associated optic pathway gliomas group were required to have both a diagnosis of NF1 based on established National Institute of Health criteria1 and magnetic resonance imaging (MRI) of the brain demonstrating the presence of optic pathway gliomas. Children in the sporadic optic pathway glioma group required documented MRI findings characteristic of a low grade optic pathway gliomas or diagnostic biopsy results. For purposes of descriptive comparison and a secondary analysis, children with a diagnosis of NF1 but without optic pathway gliomas were also enrolled. Patients were excluded if they had a history of ophthalmologic or neurologic disease, other than an optic pathway gliomas, that could have affected their visual acuity or their optic nerve function (e.g., amblyopia, cataracts, glaucoma, retinopathy of prematurity, elevated intracranial pressure requiring ventriculoperitoneal shunting). Healthy control subjects with no significant history of ophthalmologic or neurologic disease were recruited. Institutional review board approval of the protocol and consent form was obtained prior to study initiation.
Using a standardized form, demographic and clinical data were collected from each patient’s clinical chart including: presence of NF1, history of optic pathway gliomas treatment (i.e., chemotherapy or radiation) and visual field (VF) testing results. Findings for the most recent MRI were abstracted from the formal reading and classified as follows: no evidence of optic pathway glioma (tortuous optic nerves allowed), optic pathway glioma involving only the optic nerve, and optic pathway glioma involving the optic chiasm and posterior structures including the hypothalamus.
Neuro-Ophthalmologic Examination
Best corrected visual acuity (VA) was determined using the electronic visual acuity (EVA) system for pediatric patients.15 The subject was seated three meters from the computer monitor in a windowless room. The examination chair was elevated to a level where the subject’s eyes were parallel to the center of the computer monitor. Each eye was tested separately, beginning with the right eye. First, the need for refraction was assessed. Optotypes equivalent to 20/16 were displayed. Children not able to accurately read the 20/16 line were refracted with trial lenses according to a standard protocol.16 If a refraction has been performed within the past 6 months, this was the subject’s starting refraction. High contrast VA was tested according to the Amblyopia Treatment Study visual acuity testing protocol with the EVA monitor placed at 3 meters.15 The EVA computer monitor was calibrated for appropriate luminance prior to each testing session. Single letter (H, O, T, or V) crowded optotypes were presented in four phases: screening, phase 1 (first threshold determination), reinforcement, and phase 2 (second threshold determination). Final visual acuity was the smallest logMAR level passed in phase 1 or phase 2.
Low-contrast letter acuity testing of each eye was performed in the same windowless exam room with the lights turned off using a retroilluminated cabinet. Sloanlow-contrast letter acuity charts at 5%, 2.5% and 1.25% contrast level (Precision Vision, LaSalle, IL) were presented sequentially at 2 meters. Low-contrast letter acuity score (logMAR) for each eye was determined to be the lowest line for which the subject was able to correctly identify at least three of the five letters.
Definition of Clinical Outcomes
High contrast VA testing was used to classify eyes as having normal VA (logMAR ≤ 0.1, 20/25 or better) or abnormal VA (logMAR > 0.1). VF loss, determined by one co-investigator (GTL), was defined as any non-central scotoma (i.e., quadrantanopia, hemianopia, visual field constriction, arcuate defect or altitudinal defect) that was reliably detected with automated perimetry or confrontation testing. Our younger subjects, especially those with NF1, are not able to reliably complete automated perimetry, therefore the VF results were not quantified, but rather categorized as either present or absent. Type of vision loss was classified into categories: normal VA/normal VF, abnormal VA/normal VF, normal VA/abnormal VF and abnormal VA/abnormal VF. Patients with unilateral glioma of the optic nerve contributed that eye as an optic pathway glioma eye, whereas their fellow eye was classified as not having an optic pathway glioma. Both eyes were classified as having optic pathway glioma, when the optic pathway glioma was present in the optic chiasm and or posterior structures.
OCT Imaging
RNFL thickness (microns) of each eye was measured using the “Fast RNFL” thickness protocol with a Stratus OCT (Zeiss Meditec, Dublin CA). Scan quality was optimized before imaging each eye by adjusting the polarization. Scanning commenced when the 3.4 mm diameter circle was centered over the optic nerve head as the subject fixated on an external or internal fixation light. Scans with a signal strength less than 6 were discarded. Three scans were averaged to produce anatomic quadrant and average RNFL thickness in microns. If the pupil diameter was not sufficient to obtain OCT images successfully, mydriatic eye drops (1% tropicamide and 2.5% phenylephrine hydrochloride) were used.
Statistical Analysis
Demographic and clinical characteristics were summarized by standard descriptive summaries (e.g. means and standard deviations for continuous variables such as age and percentages for categorical variables such as gender). RNFL thickness was categorized into quartiles from the optic pathway glioma patients due to the non-linear association between RNFL thickness and the measures of vision. RNFL measurements from healthy controls and NF1 patients without an optic pathway glioma were not used to calculate the quartiles and were presented for descriptive comparison.
One-way analysis of variance (ANOVA) was used to determine if high contrast VA (logMAR) and low-contrast letter acuity (logMAR) was associated with RNFL quartiles in patients with optic pathway gliomas. The generalized estimating equation (GEE) approach to variance estimation was used to account for the correlation between eyes of patients and to provide a robust estimator to accommodate for unequal variance in vision measures among RNFL groups.17 Similarly, the GEE approach was also used in multiple regression analyses to examine the influence of RNFL thickness quartiles, patient age, and tumor location on both high contrast VA and low-contrast letter acuity in patients with optic pathway gliomas.
Because we were not aware of any previously published studies of RNFL thickness in children with optic pathway gliomas, sample size was calculated using RNFL data from studies of optic neuritis in multiple sclerosis.10 RNFL differs by roughly 20% between patients with optic neuritis (80±20 microns) and healthy controls (103±12 microns). Setting the alpha to 0.01 (based on multiple comparisons) to achieve a power of 0.9 necessitates recruitment of a total of 32 eyes (16 eyes with vision loss and 16 eyes without vision loss). The multivariable model contained 4 variables and thus required a minimum of 80 study eyes (20 eyes per variable) to achieve sufficient precision.
Results
Sixty-two patients (124 study eyes) were enrolled and attempted to complete the study procedures. OCT imaging was unsuccessful for both eyes in three patients and for one eye in seven patients due to patient cooperation, immobile eye or facial plexiform fibroma impeding the OCT. During the study, one patient originally diagnosed to have NF1 was found to have a yet unidentified genetic mutation and this child’s data were not included in the analysis. Therefore, 58 patients contributed 109 eyes to the study. Forty-eight patients (89 study eyes) had optic pathway gliomas (both eyes, N=41; one eye, N=7) and were included in the primary analysis. Fourteen NF1 subjects without an optic pathway glioma (20 study eyes; mean age 11.3 years; 65% female) and fourteen healthy control (28 study eyes, mean age 11.5 years; 64% female) subjects were enrolled. Table 1 lists the demographic and MRI findings for the NF1 associated optic pathway gliomas and sporadic optic pathway glioma patients.
Table 1.
Demographic and Clinical Characteristics of Study Eyes with Optic Pathway Gliomas.
| Study Eyes (N = 89) | |
|---|---|
| Agea | 11.6/11.0 |
| Range | (6.4–20.8) |
| Female sex – no. (%) | 40 (45) |
| Race – no. (%) | |
| White/Caucasian | 80 (89.9) |
| Black/African American | 3 (3.4) |
| Asian | 0 (0) |
| Multiple races | 6 (6.7) |
| Ethnicity – no. (%) | |
| Non-Hispanic | 89 (100) |
| Hispanic | 0 (0) |
| Diagnosis – no. (%) | |
| NF1 with optic pathway glioma | 61 (68.5) |
| Sporadic optic pathway glioma | 28 (31.5) |
| Location of optic pathway glioma – no. (%) | |
| Optic nerve only | 20 (22.5) |
| Optic chiasm and posterior | 69 (77.5) |
| Category of vision loss – no. (%) | |
| Normal VA/normal VF | 48 (53.9) |
| Abnormal VA/normal VF | 12 (13.5) |
| Normal VA/abnormal VF | 12 (13.5) |
| Abnormal VA/abnormal VF | 17 (19.1) |
| Treatment for optic pathway gliomab – no. (%) | 70 (78.7) |
| Age at onsetc | 3.6 |
In years mean/median and (range);
Includes chemotherapy, radiation or surgical debulking alone or in combination with other treatments;
Mean age at which first treatment was initiated for study eyes.
Of those with NF1 associated optic pathway gliomas and sporadic optic pathway gliomas, 48 study eyes were found to have normal high contrast VA and normal VF, 12 had decreased VA (> 0.1 logMAR) with a normal VF, 12 had normal VA but an abnormal VF, and 17 had both abnormal VA and abnormal visual field (Table 1). Table 2 lists the high-contrast VA, low-contrast letter acuity and RNFL thickness measures for those with normal VA and VF compared with those with abnormal VA and or VF, and healthy controls.
Table 2.
Visual Acuity and Retinal Nerve Fiber Layer Thickness Outcomes in Eyes
| Optic Pathway Glioma Patients, Normal Visiona (N=48) | Optic Pathway Glioma Patients, Abnormal Visionb (N=41) | Healthy Control (N=28) | |
|---|---|---|---|
| High Contrastc | −0.0/0.0 | 0.6/0.3 | −0.1/−0.1 |
| Visual Acuity | (−0.1–0.1) | (−0.1–1.5) | (−0.1–0) |
| Low Contrastc | 0.3/0.3 | 0.8/0.7 | 0.2/0.2 |
| Letter Acuity (5.0%) | (0.1–0.5) | (0.1–1.1) | (0.0–0.4) |
| Low Contrastc | 0.4/0.4 | 0.9/0.9 | 0.3/0.3 |
| Letter Acuity (2.5%) | (0.2–0.6) | (0.3–1.1) | (0.1–0.4) |
| Low Contrastc | 0.6/0.6 | 1.0/1.1 | 0.5/0.5 |
| Letter Acuity (1.25%) | (0.4–1.0) | (0.1–1.1) | (0.3–0.8) |
| Mean RNFLd (microns) | 101/101 (45–159) | 60/56 (32–120) | 113/110 (85–155) |
Patient eyes with a normal high contrast visual acuity (logMAR ≤ 0.1) and normal visual fields.
Includes patients with abnormal VA/normal VF, normal VA/abnormal VF or abnormal VA/abnormal VF.
Mean/median logMAR and range in parenthesis.
Mean/median RNFL thickness and range in parenthesis.
Average RNFL thickness in microns was divided into quartiles based on the 89 study eyes from patients with optic pathway gliomas: Top 25% (mean = 121.4, s.d. = 14.3), 51–75% (mean = 93.5, s.d. = 6.1), 26–50% (mean = 67.5, s.d. = 7.5), and 0–25% (mean = 44.7, s.d. = 6.8). One-way ANOVA of RNFL quartiles demonstrated significant between-group differences in mean values for high-contrast VA (F = 27.45, P < 0.001), 5% low-contrast letter acuity (F = 36.45, P < 0.001), 2.5% low-contrast letter acuity (F = 36.28, P < 0.001) and 1.25% low-contrast letter acuity (F = 25.53, P < 0.001).
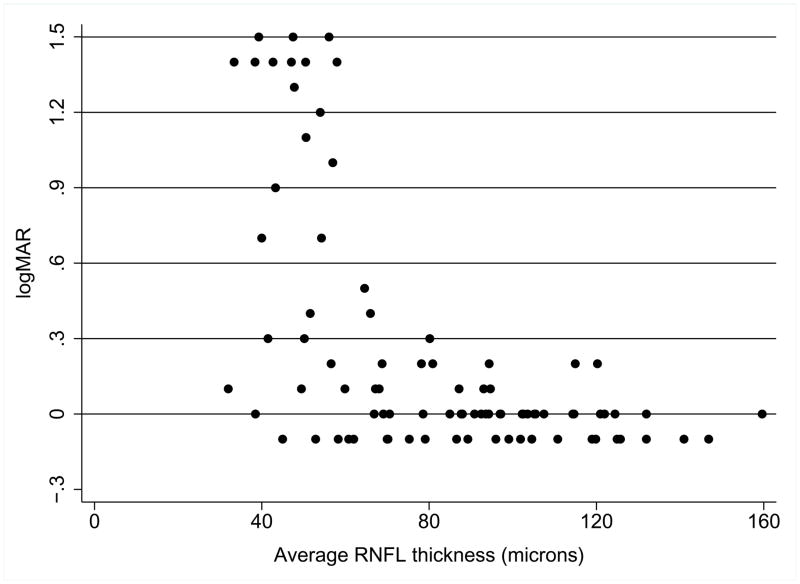
Table 3 lists the unadjusted and adjusted multiple regression analysis of the effect of RNFL quartile, age and optic pathway glioma location on high-contrast VA in optic pathway glioma patients. In unadjusted and adjusted analysis, the bottom two RNFL quartiles were associated with worse VA scores. Figure 1 is a scatterplot of VA and average RNFL thickness. Higher RNFL thickness (>80 microns approximately) was associated with uniformly normal or near normal VA, whereas lower RNFL thickness was associated with worse VA scores, but with wide variability in VA at particular RNFL thickness values.
Table 3.
Factors Associated with High Contrast Visual Acuity in Univariable and Multivariable Linear Regression for Patients with Optic Pathway Gliomas.
| Variable | Unadjusted Coefficient | Adjusted Coefficient | 95% Confidence Interval | Pa |
|---|---|---|---|---|
| RNFL Quartile | ||||
| 76–100% | Reference | Reference | … | … |
| 51–75% | 0.034 | 0.034 | −0.06, 0.13 | 0.489 |
| 26–50% | 0.192b | 0.240 | 0.05, 0.43 | 0.014 |
| 0–25% | 0.857c | 0.882 | 0.64, 1.13 | <0.001 |
| Age | −0.012 | −0.014 | −.03, 0.01 | 0.242 |
| Optic pathway glioma location | ||||
| Optic Nerve | Reference | Reference | … | … |
| Optic Chiasm and Posterior | 0.104 | 0.044 | −0.17, 0.09 | 0.504 |
P-value in adjusted analysis.
Denotes P-value <0.05 in unadjusted analysis.
Denotes P-value <0.01 in unadjusted analysis.
Figure 1.
Relation between high-contrast visual acuity and average RNFL thickness in optic pathway glioma patients. Lower logMAR represents better visual acuity. Abnormal visual acuity (i.e., > 0.1 logMAR) occurs more frequently when average RNFL thickness is below 80 microns.
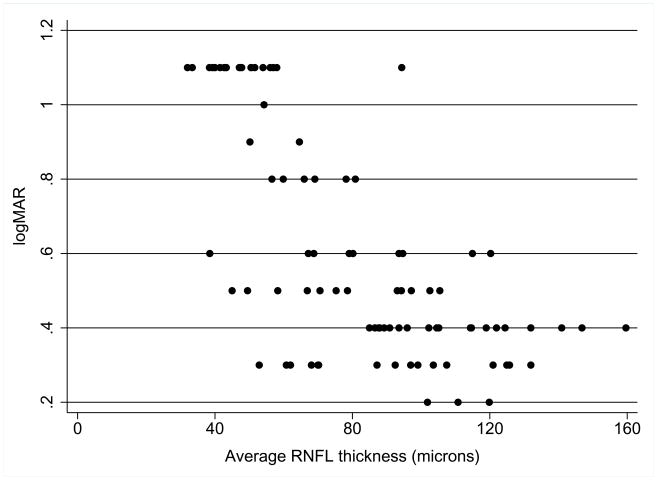
A multiple regression analysis was repeated using 5%, 2.5%, and 1.25% low-contrast letter acuity as the dependent variable. Table 4 lists the multiple regression results for the 2.5% low-contrast letter acuity. The bottom two RNFL quartiles, as with the high-contrast VA analysis, and optic pathway glioma location in the optic chiasm significantly were associated with VA in the unadjusted analysis. In adjusted analysis, the bottom two RNFL quartiles maintained an association with worse scores for the 2.5% low-contrast letter acuity. Multiple regression analysis for the 5% and 1.25% contrast levels generated similar results (data not shown). Figure 2 is a scatter plot of 2.5% low-contrast letter acuity and average RNFL thickness as a continuous variable. A pattern similar to the one in Figure 1 was observed, having higher RNFL values associated with normal or near normal levels of low-contrast letter acuity whereas lower RNFL thickness was associated with worse scores, but with wide variability in low-contrast letter acuity at particular RNFL thickness values.
Table 4.
Factors Associated with 2.5% Low Contrast Letter Acuity in Univariable and Multivariable Linear Regression for Patients with Optic Pathway Gliomas.
| Variable | Unadjusted Coefficient | Adjusted Coefficient | 95% Confidence Interval | Pa |
|---|---|---|---|---|
| RNFL Quartile | ||||
| 76–100% | Reference | Reference | … | … |
| 51–75% | 0.029 | 0.026 | −0.05, 0.10 | 0.513 |
| 26–50% | 0.25c | 0.26 | 0.15, 0.37 | <0.001 |
| 0–25% | 0.595c | 0.597 | 0.49, 0.70 | <0.001 |
| Age | −0.001 | −0.007 | −0.02, 0.01 | 0.365 |
| Optic pathway glioma location | ||||
| Optic Nerve | Reference | Reference | … | … |
| Optic Chiasm and posterior | 0.182b | 0.001 | −0.08, 0.08 | 0.994 |
P-value in adjusted analysis.
Denotes P-value <0.05 in unadjusted analysis.
Denotes P-value <0.01 in unadjusted analysis.
Figure 2.
Relation between 2.5% low contrast letter acuity and average RNFL thickness in optic pathway glioma patients. Lower logMAR represents better low-contrast letter acuity. Ability to identify low-contrast letters decreases when average RNFL thickness is below 80 microns.
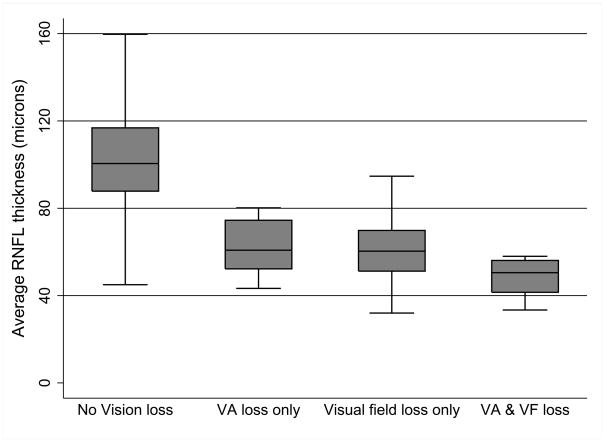
The relation between high-contrast VA and RNFL thickness and between low-contrast letter acuity and RNFL thickness, displayed in Figures 1 and 2, respectively, do not conform to a linear relationship. Because some patients with RNFL thickness less than 80 microns were able to achieve a normal high contrast VA, we examined the visual function of the eyes more closely. Figure 3 displays box plots for groups of eyes with normal VA/normal VF, abnormal VA/normal VF, normal VA/abnormal VF and abnormal VA/abnormal visual field. Post-hoc analysis using a one-way ANOVA of RNFL thickness and category of vision loss demonstrated between-group differences (F = 28.28, P < 0.001).
Figure 3.
Relation between average RNFL thickness and category of vision loss in optic pathway glioma patients. One-way analysis of variance for RNFL thickness and category of vision loss demonstrated between-group differences (F = 28.28, P < 0.001). Abnormal visual acuity or visual field defects primarily occur when the average RNFL thickness was below 80 microns.
RNFL thickness measures were comparable between NF1 patients without optic pathway gliomas (mean = 112.2, s.d. = 16.6) and healthy controls (mean = 113.3, s.d. = 15.3). A secondary analysis of the relation between high-contrast VA, low-contrast letter acuity and RNFL thickness included NF1 patients without optic pathway gliomas (N=20 eyes), thereby resulting in a total of 109 study eyes. Similar to the primary analysis, multiple regression analysis demonstrated that the bottom two RNFL quartiles were associated with worse high-contrast VA (P < 0.001) and low-contrast letter acuity (P < 0.001) in both unadjusted and adjusted analysis.
Discussion
This study demonstrates that most children with optic pathway gliomas and decreased RNFL thickness have abnormal VA, visual field loss or both. Some children with decreased RNFL thickness had normal high-contrast VA, but abnormal visual fields. Interestingly, other children were found to have normal VA and normal visual fields despite a significantly decreased RNFL—possibly suggesting that visual acuity or visual field loss may not have been detected with current techniques or the child has yet to manifest symptoms. On the other hand, if our vision testing was accurate, a normal RNFL thickness shows a strong relation to normal VA and visual fields. Therefore, in children who are not cooperative enough to provide a reliable sensory vision examination, a normal RFNL thickness might be reassuring to the clinician that the patient likely has a normal VA and visual field. In contrast, the finding of a reduced RNFL thickness may or may not have a strong relation to clinical visual sensory function and thus should not be taken as absolute evidence of clinically significant visual sensory dysfunction.
Our findings are similar to other studies that use OCT to correlate RNFL axonal loss with visual function.8–12 Studies of patients with optic neuritis and multiple sclerosis have demonstrated a strong association between reduced RNFL thickness and reduction of high and low contrast visual acuity.10,12 VA loss and VF loss associated with RNFL thinning in multiple sclerosis are indicators of axonal loss in the anterior (pregeniculate) visual pathway (i.e., optic nerve, optic chiasm or optic tract).11,12 Danesh-Meyer and colleagues8 have demonstrated a correlation between RNFL thickness and visual field deficits in adults with tumors compressing the optic chiasm.
Comparable to studies of optic neuritis in multiple sclerosis,10,12,14 we found that low-contrast letter acuity measures had a strong relation with RNFL using the identical OCT acquisition protocol. Although not formally analyzed, we frequently noticed subtle inter-eye differences (i.e., 0.1 logMAR) in high-contrast VA testing, but discovered more robust differences using low-contrast letter acuity. One possible explanation is that our high-contrast VA protocol using the EVA can overestimate visual acuity, especially in eyes with decreased visual acuity, as compared to ETDRS letters in the clinic.18 Chang and colleagues have found that children with optic pathway gliomas and normal visual acuity had normal grating acuity but impaired contrast sensitivity using sweep visual evoked potentials.19 These findings suggest low contrast acuity measures, as in multiple sclerosis, may be more sensitive to visual pathway damage as compared to high contrast VA measures. Comparison of the discrimination ability between high-contrast VA and low-contrast letter acuity requires further study.
Visual evoked potentials (VEP), proposed as an objective measure of visual pathway integrity, have been used in an attempt to detect vision loss20,21 or to correlate VEP with visual acuity22 in children with optic pathway gliomas. The accuracy and utility of VEPs are limited since most optic pathway gliomas involve the optic chiasm, eliminating the ability to make meaningful inter-eye comparisons as the visual pathway of both eyes are affected. Also, very subtle changes (i.e., < 5%) in VEP results can profoundly alter the sensitivity of the test and suggests that VEP is an inadequate management tool in this population.23 An expert review has determined that insufficient evidence exists to use VEP for the detection or progression of visual loss in children with optic pathway gliomas.3
Our cross-sectional study design contains some degree of selection bias because we obtained a convenience sample and this may limit our interpretation of the relation between RNFL thickness and vision in optic pathway gliomas. However, one author (RAA) followed a pre-established study protocol for VA and low-contrast letter acuity testing to minimize the possibility that the examiner’s knowledge of clinical diagnosis could influence the patient’s performance on the tests of visual function.15 Also, without knowing the within subject change in RNFL over time, we cannot exclude other factors contributing to our findings, such as the effect of chemotherapy. Longitudinal within subject measures of RNFL before and after vision loss are needed to better establish this relation.
Our secondary analysis that included data from NF1 patients without optic pathway gliomas revealed nearly identical results as the primary analysis. This secondary analysis was performed since children with NF1 frequently have cognitive delays and attention-deficit hyperactivity disorder that could influence their ability to accurately complete the high contrast VA and low-contrast letter acuity tests. Also, children with NF1 demonstrate brain MRI findings such as tortuous optic nerves that are not formally considered an optic pathway glioma, but could conceivably influence both VA and RNFL. If RNFL is to be used as a structural marker or screening tool for NF1 associated optic pathway glioma related vision loss in the future, NF1 children without optic pathway gliomas will undoubtedly be part of the screening population.
The interpretation of the RNFL values is problematic since normative data for children have not been established and our study relied on our own control subjects. We observed a wide range of RNFL, high-contrast VA and low-contrast letter acuity values in children with and without abnormal visual acuity and visual fields. This may be a result of performance variability, especially in children with NF1 and developmental delay. It is also possible that some subjects with decreased visual acuity but normal RNFL may have unrecognized amblyopia. We performed an undilated refraction to determine best corrected visual acuity rather than a cycloplegic refraction. However, if our refraction was incorrect, thus causing the patient to have worse VA, this error would only weaken, rather than strengthen the observed association between VA and RNFL. However, this is unlikely since nearly all children in this cohort benefit from a thorough neuro-ophthalmologic exam on a regular basis by one investigator (GTL), reducing the chance of overlooking a new and clinically meaningful refractive error.
Six patient eyes with normal VA and normal visual fields were found to have decreased RNFL, all of which had fellow eyes with vision loss from an optic pathway glioma affecting the optic chiasm. Two of these patient eyes were myopic, which is known to be associated with a lower RNFL.24 Also, it is possible that some of these patients had an abnormal visual field that was not detected because of poor patient cooperation.
Our interpretation of the relation between RNFL and high-contrast VA is further complicated by the finding of normal high-contrast VA in children with abnormal visual fields. In most cases, this can be explained by attributing the visual field loss to chiasmal or optic tract damage. Interestingly, all but one subject with normal VA and abnormal visual field had an RNFL below 80 microns (see Figure 3). This suggests that VA might not be the most sensitive measure in children with optic pathway gliomas, especially in younger children where VA and VF testing is difficult due to patient cooperation.
This study demonstrates that most children with an optic pathway glioma and decreased RNFL thickness have abnormal VA or visual fields from their tumors. This study suggests RNFL thickness is a rapid, non-invasive, objective quantitative measure of visual pathway integrity in children with optic pathway gliomas. If our results can be replicated in younger children, who are at the greatest risk for vision loss from their optic pathway gliomas (i.e., less than six years old), then RNFL thickness may be useful as a marker of VA in patients who are unable to cooperate with standard VA testing. OCT performed while the child is sedated, could possibly be used in this young population. Future longitudinal studies comparing serial measures of RNFL thickness need to examine whether decreasing RNFL might precede VA loss and to determine if OCT could possibly augment the clinical management of children with optic pathway gliomas.
Acknowledgments
Funding/Support: This article was supported by National Institutes of Health/National Eye Institute Pediatric Research Loan repayment program (Dr. Avery), grant 1T32NS061779-01 for the Neurologic Clinical Epidemiology Training Program (PI: Laura Balcer, MD MSCE) from the National Institutes of Health (Dr. Avery), the Lynn Saligman League of the Children’s Hospital of Philadelphia (Dr. Avery), and grant K24 EY018136 from the National Eye Institute (Dr. Balcer and Dr. Maguire).
This study was approved by the Children’s Hospital of Philadelphia IRB and was in accordance with HIPAA regulations. Written consent for the study was obtained from the patients and or their legal guardian prior study participation.
We are thankful to Jean Bennett MD, PhD for the use of her OCT, Kathleen Marshall COT for technical advice with OCT acquisition and Angie Miller RN for help in subject recruitment.
Biographies
 Dr. Robert A. Avery recently completed his neuro-ophthalmology fellowship at the Children’s Hospital of Philadelphia/University of Pennsylvania and a Master’s degree in Clinical Epidemiology at the University of Pennsylvania. In July 2010, Dr. Avery joined the faculty of the Department of Neurology and the Gilbert Family Neurofibromatosis Institute at Children’s National Medical Center in Washington DC where he has a dedicated pediatric neuro-ophthalmology practice and clinical research program.
Dr. Robert A. Avery recently completed his neuro-ophthalmology fellowship at the Children’s Hospital of Philadelphia/University of Pennsylvania and a Master’s degree in Clinical Epidemiology at the University of Pennsylvania. In July 2010, Dr. Avery joined the faculty of the Department of Neurology and the Gilbert Family Neurofibromatosis Institute at Children’s National Medical Center in Washington DC where he has a dedicated pediatric neuro-ophthalmology practice and clinical research program.
 Laura J. Balcer, M.D., M.S.C.E., is a Professor of Neurology, Ophthalmology and Epidemiology at the University Pennsylvania School of Medicine. She is the director of the Multiple Sclerosis Division in the Department of Neurology. Dr. Balcer’s primary research focus is on the identification and development of visual and ocular imaging outcome measures for Multiple Sclerosis clinical trials.
Laura J. Balcer, M.D., M.S.C.E., is a Professor of Neurology, Ophthalmology and Epidemiology at the University Pennsylvania School of Medicine. She is the director of the Multiple Sclerosis Division in the Department of Neurology. Dr. Balcer’s primary research focus is on the identification and development of visual and ocular imaging outcome measures for Multiple Sclerosis clinical trials.
Footnotes
Financial Disclosures: none
Author Contributions: Design of the study (RAA, GTL, MJF, GEQ, MGM, LJB), Conduct of the study (RAA, GTL); Collection, management, analysis and interpretation of the data (RAA, GTL, MJF, GEQ, JBB, PCP, MGM, LJB); preparation, approval, and review of the manuscript (RAA, GTL, MJF, GEQ, JBB, PCP, MGM, LJB).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lynch TM, Gutmann DH. Neurofibromatosis 1. Neurologic Clin N Am. 2002;20(3):841–865. doi: 10.1016/s0733-8619(01)00019-6. [DOI] [PubMed] [Google Scholar]
- 2.Listernick R, Darling C, Greenwald M, Strauss L, Charrow J. Optic pathway tumors in children: the effect of neurofibromatosis type 1 on clinical manifestations and natural history. Pediatrics. 1995;127(5):718–722. doi: 10.1016/s0022-3476(95)70159-1. [DOI] [PubMed] [Google Scholar]
- 3.Listernick R, Ferner RE, Liu GT, Gutmann DH. Optic pathway gliomas in Neurofibromatosis-1: Controversies and recommendations. Ann Neurol. 2007;61(3):189–198. doi: 10.1002/ana.21107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balcer LJ, Liu GT, Heller G, et al. Visual loss in children with Neurofibromatosis type 1 and optic pathway gliomas: Relation to tumor location by magnetic resonance imaging. Am J Ophthalmol. 2001;131(4):442–5. doi: 10.1016/s0002-9394(00)00852-7. [DOI] [PubMed] [Google Scholar]
- 5.Kornreich L, Blaser S, Schwarz M, et al. Optic pathway glioma: correlation of imaging findings with the presence of neurofibromatosis. Am J Neuroradiol. 2001;22(10):1963–9. [PMC free article] [PubMed] [Google Scholar]
- 6.Opocher E, Kremer LC, Da Dalt L, et al. Prognostic factors for progression of childhood optic pathway glioma: a systematic review. Eur J Cancer. 2006;42(12):1807–1816. doi: 10.1016/j.ejca.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 7.Listernick R, Charrow J, Greenwald M, Mets M. Natural history of optic pathway tumors in children with neurofibromatosis type 1: a longitudinal study. J Pediatr. 1994;125(1):63–6. doi: 10.1016/s0022-3476(94)70122-9. [DOI] [PubMed] [Google Scholar]
- 8.Danesh-Meyer HV, Carroll SC, Foroozan R, et al. Relationship between retinal nerve fiber layer and visual field sensitivity as measured by optical coherence tomography in chiasmal compression. Invest Ophthalmol Vis Sci. 2006;47(11):4827–4835. doi: 10.1167/iovs.06-0327. [DOI] [PubMed] [Google Scholar]
- 9.Danesh-Meyer HV, Papchenko T, Savino PJ, Law A, Evans J, Gamble GD. In vivo retinal nerve fiber layer thickness measured by optical coherence tomography predicts visual recover after surgery for postchiasmal tumors. Invest Ophthalmol Vis Sci. 2008;49(5):1879–1885. doi: 10.1167/iovs.07-1127. [DOI] [PubMed] [Google Scholar]
- 10.Fisher JB, Jacobs DA, Markowitz CE, et al. Relation of visual function to retinal nerve fiber layer thickness in multiple sclerosis. Ophthalmology. 2006;113(2):324–332. doi: 10.1016/j.ophtha.2005.10.040. [DOI] [PubMed] [Google Scholar]
- 11.Frohman E, Costello F, Zivadinov R, et al. Optical coherence tomography in multiple sclerosis. Lancet Neurol. 2006;5(10):853–863. doi: 10.1016/S1474-4422(06)70573-7. [DOI] [PubMed] [Google Scholar]
- 12.Trip SA, Schlotmann PG, Jones SJ, et al. Retinal nerve fiber layer axonal loss and visual dysfunction in optic neuritis. Ann Neurol. 2005;58(3):383–391. doi: 10.1002/ana.20575. [DOI] [PubMed] [Google Scholar]
- 13.Costello F, Coupland S, Hodge W, et al. Quantifying axonal loss after optic neuritis with optical coherence tomography. Ann Neurol. 2006;59(6):963–9. doi: 10.1002/ana.20851. [DOI] [PubMed] [Google Scholar]
- 14.Talman LS, Bisker ER, Sackel DJ, et al. Longitudinal study of vision and retinal nerve fiber layer thickness in multiple sclerosis. Ann Neurol. 2010;67(6):749–760. doi: 10.1002/ana.22005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moke PS, Turpin AH, Beck RW, et al. Computerized method of visual acuity testing: Adaptation of the amblyopia treatment study visual acuity protocol. Am J Ophthalmol. 2001;132(6):903–9. doi: 10.1016/s0002-9394(01)01256-9. [DOI] [PubMed] [Google Scholar]
- 16.Modified Early Treatment Diabetic Retinopathy Study (ETDRS) Refraction Protocol. Web-based methods resource. Center Center for Eye Disease Trials, Jaeb Center for Health Research; Tampa FL: [Accessed April 21, 2010]. Available at http://drcrnet.jaeb.org. [Google Scholar]
- 17.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrics. 1986;73:13–22. [Google Scholar]
- 18.Birch EE, Strauber SF, Beck RW, Holmes JM. Comparison of the amblyopia treatment study HOTV and electronic-early treatment diabetic retinopathy study visual acuity protocols in amblyopic children aged 5 to 11 years. J AAPOS. 2009;13(1):75–8. doi: 10.1016/j.jaapos.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang BCM, Mirabella G, Yagev R, et al. Screening and diagnosis of optic pathway gliomas in children with Neurofibromatosis type 1 by using sweep visual evoked potentials. Invest Ophthalmol Vis Sci. 2007;48(6):2895–2902. doi: 10.1167/iovs.06-0429. [DOI] [PubMed] [Google Scholar]
- 20.Kelly JP, Weiss AH. Comparison of pattern visual-evoked potentials to perimetry in the detection of visual loss in children with optic pathway gliomas. J AAPOS. 2006;10(4):298–306. doi: 10.1016/j.jaapos.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Wolsey DH, Larson SA, Creel D, Hoffman R. Can screening for optic nerve gliomas in patients with Neurofibromatosis type 1 be performed with visual-evoked potential testing? JAAPOS. 2006;10(4):307–311. doi: 10.1016/j.jaapos.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 22.Triscuizzi MTS, Riccardi R, Piccardi M, et al. A fast visual evoked potential method for functional assessment and follow-up of optic pathway gliomas. Clin Neurophysiol. 2004;115(1):217–226. doi: 10.1016/s1388-2457(03)00282-7. [DOI] [PubMed] [Google Scholar]
- 23.Siatkowski RM. VEP testing and visual pathway gliomas: not quite ready for prime time. J AAPOS. 2006;10(4):293–295. doi: 10.1016/j.jaapos.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 24.Salchow DJ, Oleynikov YS, Chiang MF, et al. Retinal nerve fiber layer thickness in normal children measured with optical coherence tomography. Ophthalmology. 2006;113(5):786–791. doi: 10.1016/j.ophtha.2006.01.036. [DOI] [PubMed] [Google Scholar]