Abstract
The clinical evaluation of microbicide formulations presents variable and interacting challenges. Specific domains of acceptability, pharmacokinetics, and toxicity interact with each other to potentially inhibit or enhance a microbicide's efficacy. Each of these is further influenced by application and use parameters in the relative presence (or absence) of vaginal and/or seminal fluids, ultimately impacting effectiveness. Historically, acceptability of formulation and use parameters, and their concomitant behavioral influences, have been considered separately from pharmacokinetics and toxicity. While independent evaluation of these elements is necessary in some respects, we must acknowledge that this approach is not sufficient for the successful development of microbicides. Each needs to be considered in an integrated clinical evaluation strategy. This article presents the rationale for such an approach. This article forms part of a special supplement covering two presentations on clinical evaluation of microbicides from the symposium on “Recent Trends in Microbicide Formulations” held on 25 and 26 January 2010, Arlington, VA.
Keywords: Microbicide Development, HIV Prevention, Pharmacokinetics, Toxicity, Acceptability, Clinical Evaluation
1. Introduction
Clinical evaluation of microbicide formulations involves a variety of challenges uncommonly seen in most areas of drug development when one considers the variable and interacting evaluation domains of acceptability, pharmacokinetics (PK), and toxicity. Each of these domains interacts with the others, potentially inhibiting or enhancing the ability of a microbicide candidate to prevent sexual HIV infection (Figure 1). The benefit of direct application of a drug to its site of action is balanced by the potential for direct toxic effects at that site, as seemingly innocuous toxicities for most other clinical indications may result in enhanced HIV infection. Acceptability, with important linkages to adherence and drug exposure, may be very sensitive to local effects at the site of drug application in ways avoided by oral drug administration. Assessment of distribution and clearance of drug applied to the vagina or rectum are more challenging than traditional PK assessments in blood, and they may only be interpretable in light of simultaneous HIV distribution and clearance – a complicated moving target following sex, quite unlike most other drug targets. Novel methods of development in each of these domains are being pursued to meet these formulation development challenges.
Figure 1.
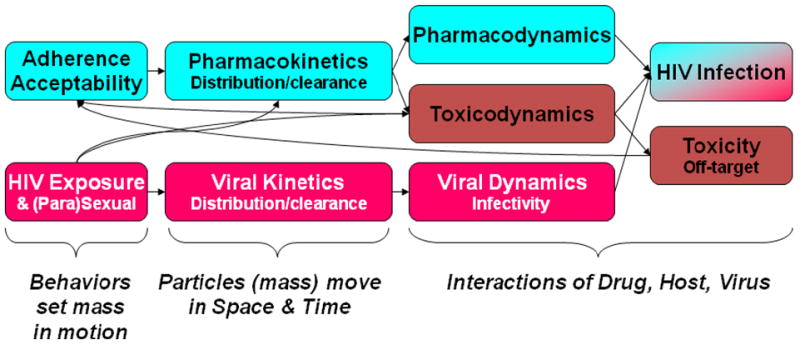
Conceptual model of the interactions of variables modifying the success of HIV prevention with topical microbicides. From left to right, upper blue boxes indicate drug-related variables which influence drug concentration (acceptability, adherence, and pharmacokinetics) and the resultant drug effects, which are separated into pharmacodynamics of drug-HIV interactions and toxicodynamics of off-target, non-HIV effects. In parallel, the lower red boxes indicate HIV “dose”-related variables (type of sexual exposure, sexual practices influencing HIV load, distribution and clearance of HIV anatomically) which influence HIV infectivity (viral dynamics). At far right, the outcomes are seroconversion and toxicity resulting from drug-virus and drug-host interactions, respectively. Some of the interrelationships among these various effects are indicated by arrows to indicate the complex nature of these interacting variables.
This paper forms part of a group of seven reviews covering presentations from the Trends in Microbicide Formulations Workshop that was held on 25-26 January, 2010 in Arlington, Virginia, USA. The other articles discuss preclinical evaluation of microbicides (Doncel and Clark, 2010), gel, film, and tablet formulations (Garg et al., 2010), the prevention of mucosal transmission (Hladik and Doncel, 2010), intravaginal rings (Malcolm et al. 2010), dual protection (Friend and Doncel, 2010) and novel approaches to microbicide delivery and safety assessment (Whaley et al. 2010).
2. Acceptability
The microbicide development field has understood from its early history that product acceptability will be critical to overall effectiveness in decreasing HIV infection rates. Indeed, the microbicide field has historically integrated basic acceptability evaluations into clinical safety and efficacy studies since candidates began advancing beyond preclinical development (Bentley et al., 2004; Bentley et al., 2000; Carballo-Dieguez et al., 2007; Dutta et al., 2008; El-Sadr et al., 2006; Greene et al., 2010; Morrow et al., 2003; Rosen et al., 2008). However, the acceptability data derived from those studies has had limited impact on decisions to move any given candidate forward in the development pipeline: the weight of “go/no-go” pipeline decisions in Phase 1 trials has rested on safety profiles and efficacy studies. Only in instances where a formulation was so fraught with problems as to be insurmountable has a poorly accepted candidate undergone reformulation. As specific candidates have advanced to Phase III trials, it has become increasingly evident that acceptability will play a critical role in microbicide use (Coly and Gorbach, 2008; Mantell et al., 2005; Montgomery et al., 2010). Indeed, it is likely that the omnibus construct known as “acceptability” has already played a crucial role in clinical trials thus far (Greene et al., 2010; Tolley et al., 2009; Turner et al., 2009; Vandebosch et al., 2004).
In the absence of an approved product with which to study “acceptability” as a phenomenon in its own right, the field has morphed the constructs of “acceptability” and “adherence” (i.e., the degree to which a product is consistently and correctly used, as per instructions), using the latter as a surrogate by which to presume the former: if a product were “acceptable,” women would use it (and/or recommend it to others). Thus “acceptability” has been presumed to have a near-perfect positive correlation to reported use, or to potential users' intentions to use a given candidate. This presumption, however, is limited by our understanding of both what makes a product acceptable (Barnhart et al., 2004; Lai et al., 2008) and what elicits consistent and correct use (Koo et al., 2005; Montgomery et al., 2008; Morrow et al., 2007a; Morrow et al., 2007b; Severy et al., 2005; Tolley et al., 2006). In clinical trials, this process is further obscured by the reality of adherence within a clinical trial protocol: that is, even if products are adhered to within the context of a clinical trial, we cannot assume that this behavioral phenomenon is anything more than protocol adherence, as opposed to product adherence indicative of acceptability. One can easily conceptualize conditions in which the correlation between adherence and acceptability is far from positive or perfect. Some may be likely to use an efficacious microbicide even if it leaks or reduces sexual pleasure (high use, low acceptability). Others may be less likely to use an efficacious microbicide even if it increases sexual pleasure (low use, high acceptability). There is likely more to a user's decision to use a microbicide than basic acceptability: in the above examples, risk perception may be playing a key role, as well as numerous other factors.
Microbicide developers historically have used two approaches to better understand the factors that impact “acceptability.” In one approach, surrogate products (those with overall formulation and application characteristics similar to candidate microbicides under development) are evaluated for indications of acceptability via a user's intention to use ‘a product like this one’ in the future (Mason et al., 2003; Tanner et al., 2009; Weeks et al., 2004; Zubowicz et al., 2006). In the second approach, candidate microbicides are evaluated for acceptability in the context of clinical trials, via adherence rates and with respect to intentions to use the candidate in the future should it become available (Bax et al., 2002; Bentley et al., 2004; El-Sadr et al., 2006; Jones et al., 2009; Kamali et al., 2010; Mauck et al., 2001; Mauck et al., 2004b; Morrow et al., 2003; Ramjee et al., 2007; Rosen et al., 2008; Schwartz et al., 2006; Trottier et al., 2007). As a result of these evaluations, much is now known about various factors that might impact potential users' decisions to use – or not use – a microbicide. These range from user-related characteristics, to contextual factors, to product-related factors. User-related factors that have been shown to impact willingness to use a given product include demographics reflecting socioeconomic status (Darroch and Frost, 1999; Hammett et al., 2000b; Morrow et al., 2007a), and a potential microbicide user's history using other types of prevention products (Hardy et al., 2003; Morrow et al., 2007a; Weeks et al., 2004): inherent within these are potential knowledge, attitude and motivation variables, as well as past behavior (Mantell et al., 2005; Morrow et al., 2007a; Reiff et al., 2008; Rupp and Rosenthal, 2003; Severy and Newcomer, 2005). Contextual factors include dynamics inherent in the sexual partnership, such as the type of partner a woman considers using a microbicide with (main/committed/monogamous partner versus non-main/other-than-committed/nonmonogamous partners) (Greene et al., 2010; Hammett et al., 2000a; Koo et al., 2005; Mason et al., 2003; Morrow and Ruiz, 2008; Wang et al., 2008), as well as gender-power dynamics (Montgomery et al., 2008; Tanner, 2008; Weeks et al., 2004), sociocultural influences (Montgomery et al., 2010; Morrow et al., 2003), and decisional balance regarding risk (Bentley et al., 2004; Greene et al., 2010; Hammett et al., 2000a; Montgomery et al., 2010; Morrow and Ruiz, 2008; Severy and Newcomer, 2005; Woodsong, 2004). Product-related factors shown to impact willingness to use a given product include both broad-based vehicle characteristics (product color and scent) (Bentley et al., 2004; Bentley et al., 2000; El-Sadr et al., 2006; Hardy et al., 1998; Mason et al., 2003; Morrow et al., 2007b; Morrow et al., 2003; Morrow and Ruiz, 2008; Ramjee et al., 1999; Ramjee et al., 2007; Rosen et al., 2008; Rupp and Rosenthal, 2003) and use parameters such as dose frequency or potential for leakage (Bentley et al., 2000; Coggins et al., 2000; El-Sadr et al., 2006; Elias and Coggins, 2001; Hammett et al., 2000a; Mason et al., 2003; Mauck et al., 2004a; Morrow et al., 2007b; Morrow et al., 2003; Morrow and Ruiz, 2008; Ramjee et al., 1999; Rosen et al., 2008; Wang et al., 2008).
Given the microbicide field's understandable primacy afforded to proof-of-concept, what can we do to minimize the unintentional entanglement of acceptability and adherence, and make advances to optimize both as we continue to develop novel and more efficacious products? Once proof-of-concept is achieved and one or more microbicides have been approved for marketing, it will be the responsibility of policy makers, health care providers, and behavioral scientists to develop effective strategies for introducing microbicides to potential users, educating potential users about their benefits, and developing interventions to teach potential users how to use the products correctly and consistently. Until then, we propose that we expand research efforts in the context of preclinical frameworks to rationally design microbicides to have the most optimal impact on potential users' willingness to use a specific dosage form. While these efforts may not impact products currently in clinical trials, they can influence the development of later generations of microbicides with respect to dosage form or device, and increase the impact of future microbicides.
Morrow and colleagues have been pioneering an emerging science designed to link user sensory perceptions and drug delivery parameters in novel formulations and devices (Morrow, 2008; Morrow, et al., 2008; Morrow, et al., 2009; Morrow, 2009; Morrow et al., 2010a; Morrow et al., 2010b). The rationale for this approach derives from multidisciplinary efforts in microbicide development. On the one hand, the sociobehavioral acceptability field has provided a wealth of both hypothetical and surrogate product acceptability data that clearly illustrate the experiences of female users of topical vaginal gels as varied, each contributing to the interpretations users derive from those product experiences and their ultimate opinions of those products. This is also true in clinical trials of microbicide candidates. In most instances, characterizations of product properties and behaviors have been limited to broad descriptions. These descriptions aggregate around global (and often dichotomous) conceptions of acceptability, such as leakage of product from the vagina, opinions of whether the product could be used covertly, and what impact, if any, the product would have on sexual pleasure. Specific and replicable measures are not provided, and often are at odds across data collection methods. Quantitative survey data, for example, often overestimate acceptability or obscure more specific evaluations of the products.
On the other hand, formulation science has also provided a wealth of methods to characterize topical vaginal gels as a function of such variables as coating time, spread, sheer, yield stress and viscosity, among others. These have been utilized to build mathematical models with both drug delivery and acceptability outcomes as dependent variables (Geonnotti et al., 2005; Lai et al., 2008; Szeri et al., 2008). This approach provides the means to iteratively optimize formulations based on physicochemical modifications to achieve desired acceptability and biofunctionality outcomes.
The results of experiments by Morrow et al (in preparation) provide increasing evidence that users have highly specific experiences with gel formulation properties, as well as mechanical properties of delivery devices such as intravaginal rings (Morrow, 2010; Morrow et al., 2010a; Morrow et al., 2010b). These experiences, in the form of sensory perceptions, can be quantified in rating scales, and disparate experiences can be articulated by the user via these scales. These sensory experiences are interpreted by users, who apply those interpretations in the formation of opinions of the product. If these perceptions can be correlated with users' willingness to use a microbicide, and if these same perceptions can be attributed to specific formulation properties or device mechanics, parameters for optimizing both drug delivery and users' product opinions and willingness to use them can be articulated (Figure 2). Additionally, because these parameters are defined by sensory perception scales and linked to user opinions, cultural values and differences in experience preferences can be calibrated differently for different users in different geographic or cultural environments. If such parameters could be articulated and evaluated within preclinical frameworks as novel microbicide formulations and devices are being developed, the user experience could assume greater weight in preclinical drug development pipeline decisions, and preserve valuable resources by increasing the likelihood of “acceptable” formulations and devices being used in microbicides advanced to clinical trials.
Figure 2.
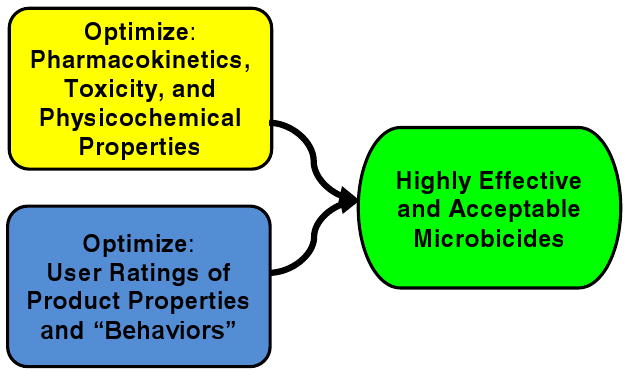
Linking User Sensory Perceptions and Drug Delivery in Novel Formulations and Devices. By optimizing physicochemical properties, toxicity and pharmacokinetics for drug distribution and clearance, and optimizing physicochemical properties for user perceptions most conducive to acceptability and adherence (e.g., coating/spreading, “feel,” retention, leakage) this interdisciplinary work can facilitate development of the most highly effective and acceptable microbicides.
At the 2010 Trends in Microbicide Formulations Workshop sponsored by CONRAD, Morrow presented results from recent studies (Morrow et al., in preparation) designed to gather formative data on user characterizations of product properties and “behavior” in situ. The studies are showing promising results. User perception rating scales have been developed that can be understood as related to traditional acceptability conceptualizations, as well as related to the formulation properties that elicit the experiences that are then translated by the user into perceptions and opinions. Similar approaches are now being used to objectively define important properties of other dosage forms, for example, intravaginal rings and quick-dissolving films. Morrow, Katz, and Kiser (Morrow et al., 2010a) have postulated that, by better understanding the correspondence between user experience and drug delivery efficiency, the correspondence between the two can be used to co-optimize user experience and drug delivery, resulting in a microbicide that is both effective and acceptable.
3. Pharmacokinetics
As the articles in this issue illustrate, formulation and device developers are providing increasing evidence that an actual drug delivery vehicle or device will require specific physicochemical properties, rheological performance properties, and/or mechanical properties to be maximally effective: that is, optimized drug delivery is dependent on formulation or device properties, just as optimized acceptability is dependent on formulation or device properties. Without attention given to these requirements, products will be less than optimally successful in delivering drugs to target areas, ultimately undermining the public health impact microbicides could achieve.
Case in point is the critical domain in early phase microbicide development that involves the assessment of candidate product pharmacokinetics (PK), the distribution and clearance of active ingredient and vehicle within the vaginal and rectal lumen and their surrounding mucosal tissue. The goal of rational microbicide development is to select a vehicle that delivers the active pharmaceutical ingredient (API) to locations within the vagina, rectum, and surrounding mucosal tissue in a concentration, location, and duration sufficient to prevent viral transmission. This requires a quantitative understanding of the distribution and clearance (PK) of both virus and microbicide candidate in the same spatio-temporal context. Tissue PK is essential for tissue-active drugs, like antiretrovirals (ARVs). Understanding luminal drug and vehicle distribution is critical for understanding luminally active drug action (e.g., surfactants, polyanions). Luminal distribution is also imperative for understanding tissue-active drugs because luminal distribution likely exerts greater influence on mucosal tissue concentration through lumen-to-tissue drug movement when compared to local spread within tissue to distant mucosal sites not reached by luminal drug.
The effects of vaginal and anal intercourse are also of potential importance on microbicide PK as sexual forces may greatly influence both distribution and clearance of microbicide and virus. This is more clearly plausible for luminally-active drugs, especially those with a relatively short luminal half-life with daily microbicide dosing, or for any luminal half-life when using coitally-dependent dosing. Coital forces may cause significant dislocation of API from the sexual lumen before ejaculation, but these same forces may cause favorable mixing of virus and API following ejaculation which may be beneficial for luminally-active drug action. Post-coital luminal PK may also be highly relevant for some tissue-active microbicides in specific settings. For example, coital forces which displace drug from the sexual lumen prior to complete absorption of the dose into tissue may effectively reduce drug exposure to ineffective tissue concentrations when the drug has a short tissue half-life or in persons who have sex frequently enough that most doses are influenced by coital forces. This dose reduction effect is magnified in persons who are also poorly adherent or on intermittent dosing regimens less resistant to missed doses.
The eventual goal of a richly described pharmacokinetic (PK) picture of drug distribution and clearance from various body compartments – lumen, tissue, blood - is the relationship of drug concentrations among these locations and the correlation of these concentrations with relevant clinical pharmacodynamic (PD) outcomes, like prevention of HIV seroconversion (Figure 3). The goals of PK/PD linkage is, first, defining the critical concentration target of the active drug in the site of action associated with desired efficacy and second, to use that concentration target to inform future study design and clinical drug monitoring to maximize efficacy.
Figure 3.
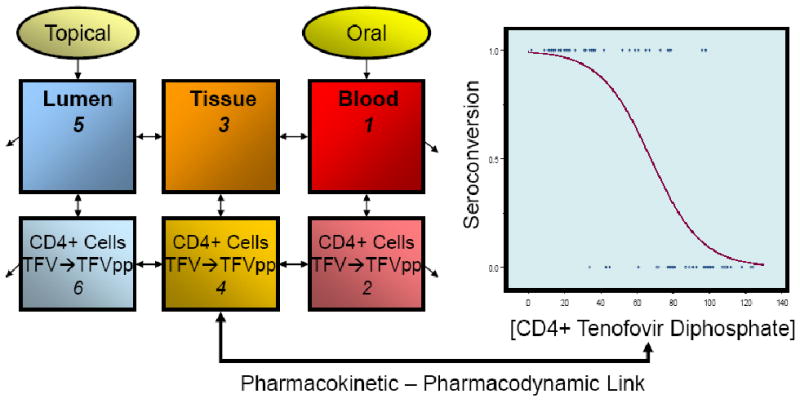
Pharmacokinetic – Pharmacodynamic Link. Tenofovir (TFV) and its active intracellular form, tenofovir diphosphate (TFV-DP), are illustrated in this conceptual model of a 6-compartment PK model linked to PD (seroconversion) outcomes. The PK model shows TFV being dosed either topically into the lumen of the rectum or vagina, or orally by mouth. The drug then circulates among various body compartments as shown: lumen (colonic or vaginal), tissue, and blood. Each of these is further divided into fluid and cellular components. Each compartment is indicated as discrete boxes on the left side of the figure. Within the cellular compartment, conversion of TFV to TFV-DP is shown. Movement of drug between compartments is a time dependent phenomenon indicated by the bidirectional arrows between compartments; the unidirectional arrows pointing away from the luminal and blood compartments indicate removal from the body by several clearance mechanisms. The graph on the right indicates a theoretical PD outcome (e.g., seroconversion as in a phase 3 efficacy study like CAPRISA 004 or VOICE) on the y-axis. Drug exposure (shown here as CD4+ cell intracellular active TFV-DP concentrations) is on the x-axis. The PK and PD models are linked via active site drug concentration.
Unlike acceptability studies that were commonly employed in early phases of microbicide testing in humans, PK studies have not been a frequent feature in early microbicide development and almost no data exists on viral distribution in the intact human lumen or tissue following sex (Louissaint et al., 2010; Nimmagadda et al., 2007). The first generation of luminally active drugs were uncommonly evaluated for luminal PK, but methods to assess PK in the vaginal and rectal compartments were not well developed when those drugs were moving into clinical development. Subsequently, considerable PK method development has been undertaken to provide these tools, primarily by several active groups (Barnhart et al., 2009; Barnhart et al., 2006; Braun et al., 2006; Dumond et al., 2009; Henderson et al., 2007; Hendrix et al., 2009; Hendrix et al., 2008; Mauck et al., 2008; Talameh et al., 2010). These groups have pioneered novel methods for direct luminal and tissue sampling or indirect imaging methods using MR and gamma imaging agents. Some of these methods allow assessment of concentration within the lumen and tissue, over distance and time, of both drug and virus, simultaneously, providing a complete PK picture with which to make go/no go assessments and enable iterative optimization of vaginal or rectal specific microbicide vehicles suitable for specific APIs (Hendrix et al., 2009). Unlike the early generation of microbicide candidates, PK studies of ARVs are being completed in parallel with and, increasingly, prior to, clinical outcome studies in a manner much more akin to traditional drug development.
As with acceptability studies, vehicle optimization studies are currently underway with a variety of formulations (small volume gels and liquids or larger volume enemas, films, vaginal rings) to correlate specific physicochemical characteristics (e.g., viscosity, yield stress, sheer rate) with PK parameters. Eventually, it is hoped that a more complete understanding of viral distribution will inform which PK characteristics of a microbicide are desirable. Given that understanding, these desirable PK characteristics can be rationally built into microbicide candidates based on physicochemical properties of formulation ingredients from the very earliest stages of development.
4. Discussion
PK and acceptability interact in several critical respects. First, insofar as acceptability impacts adherence, acceptability can have a major impact on effective drug dose. Perhaps more important than any PK parameter, poor adherence secondary to poor acceptability will result in relatively lower concentrations of the microbicide where it is needed, possibly dropping below effective concentrations. As with treatment, this is a double-insult for prevention as low drug concentrations may both fail to prevent HIV infection and select for resistant HIV in the process, thus reducing potential future treatment options in such cases. A second linkage between PK, acceptability, and adherence lies in the potential use of PK methods with observed drug concentration to improve our ability to quantitatively assess adherence, which remains a limitation to the understanding of the acceptability–adherence linkage identified earlier in this paper. Several studies are underway to evaluate the use of drug concentration in blood or hair as an adherence measure, either alone, or in combination with, other adherence measures, like MEMS devices (Hendrix, 2010; Liu et al., 2010).
More than acceptability or PK, assessment of toxicity in clinical microbicide development is critical to go/no go decisions early in development to avoid further development of vehicles and APIs that will either increase the risk of HIV transmission or reduce the acceptability of the product. Initially, these evaluations included little more than clinical inspection for mucosal inflammation, but this area of evaluation has evolved rapidly and broadly to include histology, cytokine expression, ex vivo challenge with HIV in tissue explants, permeability, impedance, impact on vaginal flora, and effects on natural immune peptides within the vaginal environment as evaluations for toxicity. Even though toxicity assessments have been studied more thoroughly, especially in the more recent generation of ARV microbicide candidates, the interpretation of these varied laboratory tests remains unclear. What degree of alteration from normal for any of these parameters is sufficient to cause the elimination of an API or vehicle from further clinical development remains a matter of active discussion while, at the same time, go/no go decisions are being made based upon these tests. Optimally, these assessments would be performed in a pre-clinical setting to rule out further development of vehicles and APIs before they reach clinical development, applying similar methods to those described above wherein correlations are established between physicochemical characteristics and changes in mucosal tissue perceived to enhance susceptibility to HIV infection. Ultimately, as with acceptability and PK evaluations, the best validation of any of these tests as surrogates for clinically-relevant toxicity will depend on demonstration of a close association between these biomarker results and seroconversion outcomes in clinical trials. Until such evidence is available, well-intentioned decisions based on biologic plausibility, but without clinical validation, could lead to needless rejection of viable microbicide candidates.
There remains substantial uncertainty about the predictive value for preventive efficacy of any of these tests in each of the three domains discussed – acceptability, PK, toxicity – such that making go/no go decisions based on these tests in early clinical studies is far from a science. In addition to the uncertainty within any of these domains, even more difficult is the ability to synthesize data from each domain, especially if it is derived from separate studies, each focusing on a specific domain of testing in the absence of the others. To go beyond this one dimensional approach, investigators are increasingly combining acceptability, PK, and toxicity assessments in the same early clinical, if not first in human, studies of microbicide vehicles in order to simultaneously evaluate a panel of variables to understand relationships among these variables and the physicochemical properties of the vehicles. Given the complex interaction of these variables across domains, some would argue this comprehensive approach, especially in early phase clinical studies, will be highly informative for either candidate selection among several options, or for informing further vehicle optimization, based on physicochemical correlations from simultaneously observed outcomes of acceptability, PK, and toxicity. The risk of prematurely rejecting a candidate through the use of a poorly predictive test outcome remains, but a fuller picture of the spectrum of relevant variables and their interrelationships may prove to be an improvement over the multiple studies designed to ask a more limited number of questions. The evidence of the success of this approach also lies in the future as clinical trials provide the definitive test of microbicide candidates.
Acknowledgments
Dr. Morrow would like to acknowledge the contributions of Dr. David F Katz (Duke University) and Dr. Patrick Kiser (University of Utah), as co-investigators in the Microbicide Innovation Program award that provided the intellectual seeds to combine knowledge of behavioral science and engineering in microbicide development. Similarly, our colleagues engaged in other projects with us, Dr. Robert Buckheit, Dr. Lisa Rohan, and Dr. Tony Ham also deserve thanks for their support and intellectual discussions. We would also like to acknowledge Drs. James Turpin, Roberta Black, Andrew Forsyth and our other colleagues at NIAID/NIMH/NIH for their support in these innovations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Kathleen M. Morrow, The Miriam Hospital and The Warren Alpert Medical School of Brown University.
Craig Hendrix, John Hopkins School of Medicine.
References
- Barnhart K, Kulp JL, Rosen M, Shera DM. A randomized trial to determine the distribution of four topical gel formulations in the human vagina. Contraception. 2009;79:297–303. doi: 10.1016/j.contraception.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Barnhart KT, Pretorius ES, Shera DM, Shabbout M, Shaunik A. The optimal analysis of MRI data to quantify the distribution of a microbicide. Contraception. 2006;73:82–7. doi: 10.1016/j.contraception.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Barnhart KT, Pretorius ES, Timbers K, Shera D, Shabbout M, Malamud D. In vivo distribution of a vaginal gel: MRI evaluation of the effects of gel volume, time and simulated intercourse. Contraception. 2004;70:498–505. doi: 10.1016/j.contraception.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Bax R, Douville K, McCormick D, Rosenberg M, Higgins J, Bowden M. Microbicides--Evaluating multiple formulations of C31G. Contraception. 2002;66:365–368. doi: 10.1016/s0010-7824(02)00389-x. [DOI] [PubMed] [Google Scholar]
- Bentley ME, Fullem AM, Tolley EE, Kelly CW, Jogelkar N, Srirak N, Mwafulirwa L, Khumalo-Sakutukwa G, Celentano DD. Acceptability of a microbicide among women and their partners in a 4-country phase I trial. Am J Public Health. 2004;94:1159–1164. doi: 10.2105/ajph.94.7.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley ME, Morrow K, Fullem A, Chesney MA, Horton SD, Rosenberg Z, Mayer KH. Acceptability of a novel vaginal microbicide during a safety trial among low-risk women. Fam Plann Perspect. 2000;32:184–8. [PubMed] [Google Scholar]
- Braun KE, Boyer JD, Henderson MH, Katz DF, Wax A. Label-free measurement of microbicidal gel thickness using low-coherence interferometry. J Biomed Opt. 2006;11:20504. doi: 10.1117/1.2192767. [DOI] [PubMed] [Google Scholar]
- Carballo-Dieguez A, Balan IC, Morrow K, Rosen R, Mantell JE, Gai F, Hoffman S, Maslankowski L, El-Sadr W, Mayer K. Acceptability of tenofovir gel as a vaginal microbicide by US male participants in a phase I clinical trial (HPTN 050) AIDS Care. 2007;19:1026–31. doi: 10.1080/09540120701294237. [DOI] [PubMed] [Google Scholar]
- Coggins C, Blanchard K, Alvarez F, Brache V, Weisberg E, Kilmarx PH, Lacarra M, Massai R, Mishell D, Jr, Salvatierra A, Witwatwongwana P, Elias C, Ellertson C. Preliminary safety and acceptability of a carrageenan gel for possible use as a vaginal microbicide. Sex Transm Infect. 2000;76:480–483. doi: 10.1136/sti.76.6.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coly A, Gorbach PM. Microbicide acceptability research: recent findings and evolution across phases of product development. Curr Opin HIV AIDS. 2008;3:581–6. doi: 10.1097/COH.0b013e32830aba00. [DOI] [PubMed] [Google Scholar]
- Darroch JE, Frost JJ. Women's interest in vaginal microbicides. Fam Plann Perspect. 1999;31:16–23. [PubMed] [Google Scholar]
- Doncel G, Clark M. Preclinical evaluation of anti-HIV microbicide products: New models and biomarkers. Antiviral Res. 2010 doi: 10.1016/j.antiviral.2010.09.018. in press. [DOI] [PubMed] [Google Scholar]
- Dumond JB, Patterson KB, Pecha AL, Werner RE, Andrews E, Damle B, Tressler R, Worsley J, Kashuba AD. Maraviroc concentrates in the cervicovaginal fluid and vaginal tissue of HIV-negative women. J Acquir Immune Defic Syndr. 2009;51:546–53. doi: 10.1097/QAI.0b013e3181ae69c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta S, Joglekar N, Joshi S. Experience of conducting a phase-I safety & acceptability clinical trial of a candidate vaginal microbicide & lessons learned. Indian J Med Res. 2008;128:212–213. [PubMed] [Google Scholar]
- El-Sadr WM, Mayer KH, Maslankowski L, Hoesley C, Justman J, Gai F, Mauck C, Absalon J, Morrow K, Masse B, Soto-Torres L, Kwiecien A. Safety and acceptability of cellulose sulfate as a vaginal microbicide in HIV-infected women. AIDS. 2006;20:1109–1116. doi: 10.1097/01.aids.0000226950.72223.5f. [DOI] [PubMed] [Google Scholar]
- Elias C, Coggins C. Acceptability research on female-controlled barrier methods to prevent heterosexual transmission of HIV: Where have we been? Where are we going? J Women Health Gen-B. 2001;10:163–173. doi: 10.1089/152460901300039502. [DOI] [PubMed] [Google Scholar]
- Friend DR, Doncel GF. Combining prevention of HIV-1, other sexually transmitted infections and unintended pregnancies: Development of dual-protection technologies. Antiviral Res. 2010 doi: 10.1016/j.antiviral.2010.09.005. in press. [DOI] [PubMed] [Google Scholar]
- Garg S, Goldman D, Krumme M, Rohan LC, Smoot S, Friend DR. Advances in development, scale-up and manufacturing of microbicide gels, films, and tablets. Antiviral Res. 2010 doi: 10.1016/j.antiviral.2010.09.010. in press. [DOI] [PubMed] [Google Scholar]
- Geonnotti AR, Peters JJ, Katz DF. Erosion of microbicide formulation coating layers: Effects of contact and shearing with vaginal fluid or semen. J Pharm Sci. 2005;94:1705–1712. doi: 10.1002/jps.20386. [DOI] [PubMed] [Google Scholar]
- Greene E, Batona G, Hallad J, Johnson S, Neema S, Tolley EE. Acceptability and adherence of a candidate microbicide gel among high-risk women in Africa and India. Cult Health Sex. 2010 doi: 10.1080/13691051003728599. Published First Online 14 April 2010. [DOI] [PubMed] [Google Scholar]
- Hammett TM, Mason TH, Joanis CL, Foster SE, Harmon P, Robles RR, Finlinson HA, Feudo R, Vining-Bethea S, Jeter G, Mayer KH, Doherty-Iddings P, Seage GR., 3rd Acceptability of formulations and application methods for vaginal microbicides among drug-involved women: results of product trials in three cities. Sex Transm Dis. 2000a;27:119–126. doi: 10.1097/00007435-200002000-00011. [DOI] [PubMed] [Google Scholar]
- Hammett TM, Norton GD, Mason TH, Langenbahn S, Mayer KH, Robles RR, Feudo R, Seage GR., 3rd Drug-involved women as potential users of vaginal microbicides for HIV and STD prevention: A three-city survey. J Women Health Gen-B. 2000b;9:1071–1080. doi: 10.1089/152460900445983. [DOI] [PubMed] [Google Scholar]
- Hardy E, de Padua KS, Hebling EM, Osis MJ, Zaneveld LJ. Women's preferences for vaginal antimicrobial contraceptives. V: Attitudes of Brazilian women to the insertion of vaginal products. Contraception. 2003;67:391–395. doi: 10.1016/s0010-7824(03)00026-x. [DOI] [PubMed] [Google Scholar]
- Hardy E, Jimenez AL, de Padua KS, Zaneveld LJ. Women's preferences for vaginal antimicrobial contraceptives. III. Choice of a formulation, applicator, and packaging. Contraception. 1998;58:245–249. doi: 10.1016/s0010-7824(98)00104-8. [DOI] [PubMed] [Google Scholar]
- Henderson MH, Couchman GM, Walmer DK, Peters JJ, Owen DH, Brown MA, Lavine ML, Katz DF. Optical imaging and analysis of human vaginal coating by drug delivery gels. Contraception. 2007;75:142–51. doi: 10.1016/j.contraception.2006.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrix CW. Pharmacokinetic assessment of adherence. Microbicides 2010 Conference; May 22-25; Pittsburgh, PA. 2010. Abstract 12. [Google Scholar]
- Hendrix CW, Cao YJ, Fuchs EJ. Topical microbicides to prevent HIV: clinical drug development challenges. Annu Rev Pharmacol. 2009;49:349–75. doi: 10.1146/annurev.pharmtox.48.113006.094906. [DOI] [PubMed] [Google Scholar]
- Hendrix CW, Fuchs EJ, Macura KJ, Lee LA, Parsons TL, Bakshi RP, Khan WA, Guidos A, Leal JP, Wahl R. Quantitative imaging and sigmoidoscopy to assess distribution of rectal microbicide surrogates. Clin Pharmacol Ther. 2008;83:97–105. doi: 10.1038/sj.clpt.6100236. [DOI] [PubMed] [Google Scholar]
- Hladik F, Doncel GF. Preventing mucosal HIV transmission with topical microbicides – challenges and opportunities. Antiviral Res. 2010 doi: 10.1016/j.antiviral.2010.09.011. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones HE, Chaikummao S, van de Wijgert JH, Friedland BA, Manopaiboon C, Witwatwongwana P, Wankrairot M, Chantharojwong N, Kilmarx PH. Acceptability of a Carrageenan-based candidate vaginal microbicide and matching placebo: Findings from a phase II safety trial among women in Chiang Rai, Thailand. J Womens Health. 2009;18:1003–10. doi: 10.1089/jwh.2008.0862. [DOI] [PubMed] [Google Scholar]
- Kamali A, Byomire H, Muwonge C, Bakobaki J, Rutterford C, Okong P, Profy A, Byaruhanga R, Namukwaya S, McCormack S, Grosskurth H, Nunn AJ, Lacey CJ. A randomised placebo-controlled safety and acceptability trial of PRO 2000 vaginal microbicide gel in sexually active women in Uganda. Sex Transm Infect. 2010;86:222–6. doi: 10.1136/sti.2009.038372. [DOI] [PubMed] [Google Scholar]
- Koo HP, Woodsong C, Dalberth BT, Viswanathan M, Simons-Rudolph A. Context of acceptability of topical microbicides: Sexual relationships. J Soc Issues. 2005;61:67–93. doi: 10.1111/j.0022-4537.2005.00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai BE, Xie YQ, Lavine ML, Szeri AJ, Owen DH, Katz DF. Dilution of microbicide gels with vaginal fluid and semen simulants: effect on rheological properties and coating flow. J Pharm Sci. 2008;97:1030–8. doi: 10.1002/jps.21132. [DOI] [PubMed] [Google Scholar]
- Liu A, Vittinghoff E, Gandhi M, Huang Y, Chillag K, Wiegand R, Anderson P, Grant R, Greenblatt R, Buchbinder S. Validating measures of Tenofovir drug exposure in US pre-exposure prophylaxis trial. 17th CROI; February 16-19; San Francisco, CA. 2010. Abstract 86. [Google Scholar]
- Louissaint N, Nimmagadda S, Bakshi RP, Anderson J, Fuchs EJ, King K, Macura K, Hendrix CW. Vaginal distribution of cell-free and cell-associated HIV surrogates following simulated intercourse. Microbicides; 2010, May 22-25; Pittsburgh, PA. 2010. Abstract 211. [Google Scholar]
- Malcolm RK, Edwards KL, Kiser P, Romano J, Smith T. Advances in microbicide vaginal rings. Antiviral Res. 2010 doi: 10.1016/j.antiviral.2010.09.003. in press. [DOI] [PubMed] [Google Scholar]
- Mantell JE, Myer L, Carballo-Dieguez A, Stein Z, Ramjee G, Morar NS, Harrison PF. Microbicide acceptability research: Current approaches and future directions. Soc Sci Med. 2005;60:319–330. doi: 10.1016/j.socscimed.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Mason TH, Foster SE, Finlinson HA, Morrow K, Rosen R, Vining S, Joanis CL, Hammett TM, Seage GR., 3rd Perspectives related to the potential use of vaginal microbicides among drug-involved women: Focus groups in three cities in the United States and Puerto Rico. AIDS Behav. 2003;7:339–51. doi: 10.1023/b:aibe.0000004726.61630.96. [DOI] [PubMed] [Google Scholar]
- Mauck CK, Katz D, Sandefer EP, Nasution MD, Henderson M, Digenis GA, Su I, Page R, Barnhart K. Vaginal distribution of Replens and K-Y Jelly using three imaging techniques. Contraception. 2008;77:195–204. doi: 10.1016/j.contraception.2007.11.016. [DOI] [PubMed] [Google Scholar]
- Mauck CK, Weiner DH, Ballagh S, Creinin M, Archer DF, Schwartz J, Pymar H, Lai JJ, Callahan M. Single and multiple exposure tolerance study of cellulose sulfate gel: A phase I safety and colposcopy study. Contraception. 2001;64:383–391. doi: 10.1016/s0010-7824(01)00271-2. [DOI] [PubMed] [Google Scholar]
- Mauck CK, Weiner DH, Ballagh SA, Creinin MD, Archer DF, Schwartz JL, Pymar HC, Lai JJ, Rencher WF, Callahan MM. Single and multiple exposure tolerance study of polystyrene sulfonate gel: A phase I safety and colposcopy study. Contraception. 2004a;70:77–83. doi: 10.1016/j.contraception.2004.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauck CK, Weiner DH, Creinin MD, Barnhart KT, Callahan MM, Bax R. A randomized phase I vaginal safety study of three concentrations of C31G vs. Extra Strength Gynol II. Contraception. 2004b;70:233–240. doi: 10.1016/j.contraception.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Montgomery CM, Gafos M, Lees S, Morar NS, Mweemba O, Ssali A, Stadler J, Pool R. Re-framing microbicide acceptability: findings from the MDP301 trial. Cult Health Sex. 2010 doi: 10.1080/13691051003736261. 1. Published First Online 14 April 2010. [DOI] [PubMed] [Google Scholar]
- Montgomery CM, Lees S, Stadler J, Morar NS, Ssali A, Mwanza B, Mntambo M, Phillip J, Watts C, Pool R. The role of partnership dynamics in determining the acceptability of condoms and microbicides. AIDS Care. 2008;20:733–40. doi: 10.1080/09540120701693974. [DOI] [PubMed] [Google Scholar]
- Morrow KM. Microbicide formulations and delivery systems: behavioral perspectives. Microbicides 2008 Conference; February 24-27; New Delhi, India. 2008. Invited keynote address at: [Google Scholar]
- Morrow KM. Engineering an anti-HIV gel for user acceptability. Food Sciences Department, Pennsylvania State University; October; State College, PA. 2009. Invited oral presentation at: [Google Scholar]
- Morrow KM. Acceptability of different dosage forms. CONRAD Trends in Microbicide Formulations Workshop; January 25; Arlington, VA. 2010. Invited oral presentation at: [Google Scholar]
- Morrow KM, Fava JL, Rosen RK, Kiser P, Katz D. Linking biophysical functions to user perceptions and acceptability in preclinical product development. 1st International Symposium on Advancing Prevention Technologies for Sexual and Reproductive Health: A Strategy Symposium; March 24-25; Berkeley, CA. 2009. Poster presented at: [Google Scholar]
- Morrow KM, Fava J, Kiser P, Rosen R, Katz D. The LINK between gel properties and user perceptions: Implications for rational design of microbicides. Microbicides 2010 Conference; May 22-25; Pittsburgh, PA. 2010a. Oral presentation at: [Google Scholar]
- Morrow KM, Kiser P, Fava J, Katz D. Linking physicochemical properties of vaginal gel formulations with user perceptions and willingness to use in preparation. [Google Scholar]
- Morrow KM, Rosen RK, Henderson M, Vargas S, Barroso C, Katz D. Co-optimizing vaginal microbicide gel acceptability and vaginal deployment. Microbicides 2008 Conference; February 24-27; New Delhi, India. 2008. Poster presented at: [Google Scholar]
- Morrow KM, Rosen R, Vargas S, Barroso C, Kiser P, Katz D. User-identified vaginal gel characteristics: A qualitative exploration of perceived product efficacy. Microbicides 2010 Conference; May 22-25; Pittsburgh, PA. 2010b. Oral presentation at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow KM, Fava JL, Rosen RK, Christensen AL, Vargas S, Barroso C. Willingness to use microbicides varies by race/ethnicity, experience with prevention products, and partner type. Health Psychol. 2007a;26:777–86. doi: 10.1037/0278-6133.26.6.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow KM, Fava JL, Rosen RK, Vargas S, Barroso C, Christensen AL, Woodsong C, Severy L. Willingness to use microbicides is affected by the importance of product characteristics, use parameters, and protective properties. J Acquir Immune Defic Syndr. 2007b;45:93–101. doi: 10.1097/QAI.0b013e3180415ded. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow KM, Rosen R, Richter L, Emans A, Forbes A, Day J, Morar N, Maslankowski L, Profy AT, Kelly C, Abdool Karim SS, Mayer KH. The acceptability of an investigational vaginal microbicide, PRO 2000 Gel, among women in a phase I clinical trial. J Womens Health. 2003;12:655–666. doi: 10.1089/154099903322404302. [DOI] [PubMed] [Google Scholar]
- Morrow KM, Ruiz MS. Assessing microbicide acceptability: a comprehensive and integrated approach. AIDS Behav. 2008;12:272–83. doi: 10.1007/s10461-007-9266-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmagadda S, Fuchs EF, Cao Y, Louissaint N, Bakshi RP, Guidos A, Menendez F, Purdy E, Lee L, King K, Hendrix CW. Autologous lymphocytes and HIV-sized particles in autologous seminal plasma penetrate colonic tissue following simulated receptive anal intercourse in healthy men. 4th IAS Conference on HIV Pathogenesis, Treatment, and Prevention; July 22-25; Sydney, Australia. 2007. Abstract WEPEA112. [Google Scholar]
- Ramjee G, Abdool Karim SS, Morar N, Gwamanda Z, Xulu G, Ximba T, Gouws E. Acceptability of a vaginal microbicide among female sex workers. S Afr Med J. 1999;89:673–676. [Google Scholar]
- Ramjee G, Morar NS, Braunstein S, Friedland B, Jones H, van de Wijgert J. Acceptability of Carraguard, a candidate microbicide and methyl cellulose placebo vaginal gels among HIV-positive women and men in Durban, South Africa. AIDS Res Ther. 2007;4:20. doi: 10.1186/1742-6405-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiff M, Wade C, Chao MT, Kronenberg F, Cushman LF. Health practices and vaginal microbicide acceptability among urban black women. J Womens Health. 2008;17:1345–51. doi: 10.1089/jwh.2008.0886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen RK, Morrow KM, Carballo-Dieguez A, Mantell JE, Hoffman S, Gai F, Maslankowski L, El-Sadr WM, Mayer KH. Acceptability of tenofovir gel as a vaginal microbicide among women in a phase I trial: a mixed-methods study. J Womens Health. 2008;17:383–92. doi: 10.1089/jwh.2006.0325. [DOI] [PubMed] [Google Scholar]
- Rupp RE, Rosenthal SL. Vaginal microbicides and teenagers. Curr Opin Obstet Gyn. 2003;15:371–375. doi: 10.1097/00001703-200310000-00004. [DOI] [PubMed] [Google Scholar]
- Schwartz JL, Mauck C, Lai JJ, Creinin MD, Brache V, Ballagh SA, Weiner DH, Hillier SL, Fichorova RN, Callahan M. Fourteen-day safety and acceptability study of 6% cellulose sulfate gel: a randomized double-blind phase I safety study. Contraception. 2006;74:133–40. doi: 10.1016/j.contraception.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Severy LJ, Newcomer S. Critical issues in contraceptive and STI acceptability research. J Soc Issues. 2005;61:45–65. [Google Scholar]
- Severy LJ, Tolley E, Woodsong C, Guest G. A framework for examining the sustained acceptability of microbicides. AIDS Behav. 2005;9:121–131. doi: 10.1007/s10461-005-1687-y. [DOI] [PubMed] [Google Scholar]
- Szeri AJ, Park SC, Verguet S, Weiss A, Katz DF. A model of transluminal flow of an anti-HIV microbicide vehicle: Combined elastic squeezing and gravitational sliding. Phys Fluids. 2008;20:83101–10. doi: 10.1063/1.2973188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talameh JA, Rezk NL, Kashuba AD. Quantifying the HIV-1 integrase inhibitor raltegravir in female genital tract secretions using high-performance liquid chromatography with ultraviolet detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:92–6. doi: 10.1016/j.jchromb.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner A, Zimet G, Fortenberry JD, Reece M, Graham C, Murray M. Young women's use of a vaginal microbicide surrogate: The role of individual and contextual factors in acceptability and sexual pleasure. J Sex Res. 2009;46:15–23. doi: 10.1080/00224490802398407. [DOI] [PubMed] [Google Scholar]
- Tanner AE. Perceptions of acceptability and utility of microbicides in Ghana, West Africa: a qualitative, exploratory study. Sahara J. 2008;5:11–8. doi: 10.1080/17290376.2008.9724897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolley EE, Eng E, Kohli R, Bentley ME, Mehendale S, Bunce A, Severy LJ. Examining the context of microbicide acceptability among married women and men in India. Cult Health Sex. 2006;8:351–369. doi: 10.1080/13691050600793071. [DOI] [PubMed] [Google Scholar]
- Tolley EE, Harrison PF, Goetghebeur E, Morrow K, Pool R, Taylor D, Tillman SN, van der Straten A. Adherence and its measurement in phase 2/3 microbicide trials. AIDS and Behav. 2009 doi: 10.1007/s10461-009-9635-x. Published first Online 19 November 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trottier S, Omar RF, Desormeaux A, Drouin J, Gagnon MT, Vezina F, Guilbert E, Masse B, Bergeron MG. Safety, tolerance and acceptability of the Invisible Condom and its vaginal applicator in healthy women and their male sexual partners. Contraception. 2007;76:117–25. doi: 10.1016/j.contraception.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Turner AN, Van Damme K, Jamieson DJ, Khan MR, Pettifor AE, Swezey TA, Bell AJ, Newman DR, Penman-Aguilar A, Raharinivo MS, Randrianasolo B, Ramiandrisoa FN, Behets FM. Predictors of adherent use of diaphragms and microbicide gel in a four-arm, randomized pilot study among female sex workers in Madagascar. Sex Transm Dis. 2009;36:249–57. doi: 10.1097/OLQ.0b013e3181901aec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandebosch A, Goetghebeur E, Ramjee G, Alary M, Ettiegne-Traore V, Chandeying V, Van Damme L. Acceptability of COL-1492, a vaginal gel, among sex workers in one Asian and three African cities. Sex Trans Infect. 2004;80:241–243. doi: 10.1136/sti.2003.005934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Liao SS, Weeks MR, Jiang JM, Abbott M, Zhou YJ, He B, Liu W, Mosack KE. Acceptability of hypothetical microbicides among women in sex establishments in rural areas in Southern China. Sex Transm Dis. 2008;35:102–10. doi: 10.1097/OLQ.0b013e31814b8546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks MR, Mosack KE, Abbott M, Sylla LN, Valdes B, Prince M. Microbicide acceptability among high-risk urban U.S. women: Experiences and perceptions of sexually transmitted HIV prevention. Sex Transm Dis. 2004;31:682–690. doi: 10.1097/01.olq.0000143113.04524.a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whaley KJ, Hanes J, Shattock R, Cone RA, Friend DR. Novel approaches to vaginal delivery and safety of microbicides: Biopharmaceuticals, nanoparticles and vaccines. Antiviral Res. 2010 doi: 10.1016/j.antiviral.2010.09.006. in press. [DOI] [PubMed] [Google Scholar]
- Woodsong C. Covert use of topical microbicides: Implications for acceptability and use. Int Fam Plan Perspec. 2004;30:94–98. doi: 10.1363/3009404. [DOI] [PubMed] [Google Scholar]
- Zubowicz EA, Oakes JK, Short MB, Perfect MM, Succop PS, Rosenthal SL. Adolescents' descriptions of the physical characteristics of microbicide surrogates and experiences of use. J Womens Health. 2006;15:952–61. doi: 10.1089/jwh.2006.15.952. [DOI] [PubMed] [Google Scholar]


