Abstract
The pontine parabrachial nucleus (PBN) and medullary reticular formation (RF) are hindbrain regions that, respectively, process sensory input and coordinate motor output related to ingestive behavior. Neural processing in each hindbrain site is subject to modulation originating from several forebrain structures including the insular gustatory cortex (IC), bed nucleus of the stria terminalis (BNST), central nucleus of the amygdala (CeA), and lateral hypothalamus (LH). The present study combined electrophysiology and retrograde tracing techniques to determine the extent of overlap between neurons within the IC, BNST, CeA and LH that target both the PBN and RF. One fluorescent retrograde tracer, red (RFB) or green (GFB) latex microbeads, was injected into the gustatory PBN under electrophysiological guidance and a different retrograde tracer, GFB or fluorogold (FG), into the ipsilateral RF using the location of gustatory NST as a point of reference. Brain tissue containing each forebrain region was sectioned, scanned using a confocal microscope, and scored for the number of single and double labeled neurons. Neurons innervating the RF only, the PBN only, or both the medullary RF and PBN were observed, largely intermingled, in each forebrain region. The CeA contained the largest number of cells retrogradely labeled after tracer injection into either hindbrain region. For each forebrain area except the IC, the origin of descending input to the RF and PBN was almost entirely ipsilateral. Axons from a small percentage of hindbrain projecting forebrain neurons targeted both the PBN and RF. Target specific and non specific inputs from a variety of forebrain nuclei to the hindbrain likely reflect functional specialization in the control of ingestive behaviors.
Introduction
Several rostral forebrain areas like the insular gustatory cortex (IC), bed nucleus of stria terminalis (BNST), central nucleus of the amygdala (CeA), and lateral hypothalamus (LH) have widespread neural connections with hindbrain sites that process gustatory information and coordinate the control of oromotor nuclei (Veening et al., 1984; van der et al., 1984; Moga et al., 1990). One view is that these diverse centrifugal connections play a significant role in the establishment and elaboration of taste preference that can promote or discourage consumption.
As the second-order relay in the rat central gustatory system, the parabrachial nucleus (PBN) of the pons transmits afferent taste information monosynaptically to the BNST, CeA and LH, and disynaptically via the thalamus to the IC, and, in turn, receives extensive projections from these same forebrain areas (Norgren, 1976; Saper and Loewy, 1980; Li and Cho, 2006; Nishijo et al., 2000; Yasoshima et al., 1995). Similar to decerebration, bilateral lesions of the classically defined caudomedial gustatory PBN eliminate learning and nutritional state dependent alteration of taste preference in rats (Reilly et al., 1993; Grigson et al., 1998; Scalera et al., 1995). In contrast, lesions of the gustatory area in the thalamus that only disrupts taste information along the disynaptic thalamocortical pathway have no obvious effects on these behavioral measures (Scalera et al., 1997). Together these findings support the notion that the neural mechanisms governing gustatory plasticity, at least in part, involve direct reciprocal communication between the PBN and ventral forebrain.
The medullary reticular formation (RF) is another hindbrain area critical for ingestive behavior, because it contains neurons that directly influence nuclei controlling muscles for licking and mastication (Travers et al., 1997; Travers and Norgren, 1983). Infusions of the GABAA agonist muscimol or glutamate antagonists into RF suppresses ingestion and rejection responses to sapid stimulation of the oral cavity in awake-behaving rats (Chen et al., 2001; Chen and Travers, 2003). Similar to the PBN, the medullary RF is targeted by descending axons of IC, BNST, CeA and LH origin (Notsu et al., 2008; Hopkins and Holstege, 1978; Yasui et al., 2004; Berk and Finkelstein, 1982; Notsu et al., 2008). The divergence of forebrain inputs to the PBN and RF provide an anatomical substrate for simultaneous control of sensory input and motor output related to ingestive behavior. However, clear evidence for the existence of individual forebrain neurons with divergent input to both hindbrain sites is lacking.
Thus, the present study investigated the extent to which individual neurons in the IC, BNST, CeA, and LH give rise to efferent axons that project both to the medullary RF and gustatory PBN. A different fluorescent retrograde tracer was injected into each hindbrain site and the number of single- and double-labeled neurons in each forebrain region was quantified.
Results
Injection sites
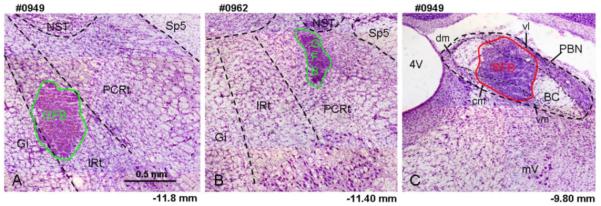
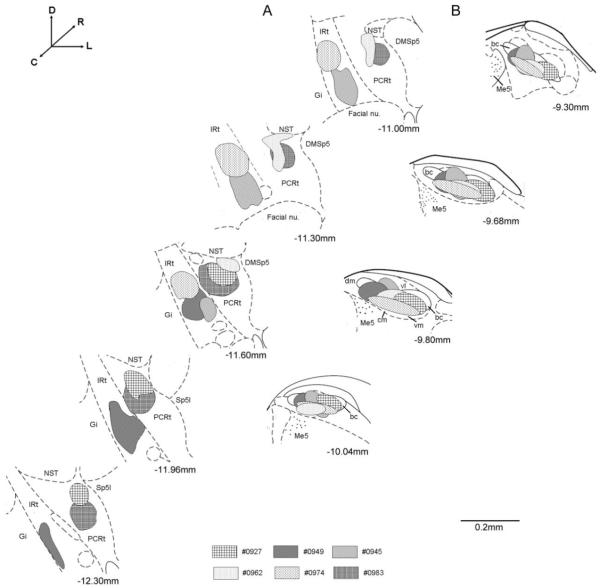
In three animals, retrograde tracer was concentrated in the intermediate zone of the medullary RF (IRt), while in the other three animals tracer material was mostly confined to the more lateral parvocellular region of RF (PCRt). In all six animals, the classically defined caudomedial taste responsive subnuclei of PBN were targeted including the central medial (cm), ventral medial (vm), and waist area between the cm and ventral lateral (vl) subdivisions. These electrophysiologically guided injections resulted in minimal spread into more rostral non gustatory responsive regions. Representative photomicrographs of cresyl violet stained sections revealing the location of tracer material are shown in Figure 1A (IRt), 1B (PCRt), and 1C (PBN). Figure 2 shows a summary of the six tracer injections in the RF and PBN.
Fig. 1.
Light photomicrograph examples of retrograde tracer injections into the RF and PBN counterstained with cresyl violet (5x). A) Injection of GFB into the IRt. B) Injection of GFB into the PCRt. C) Injection of RFB into the PBN of the same animal depicted in A. The extent of the tracer material is outlined with a green (GFB in A & B) or red line (RFB in C). Central medial (cm), dorsal medial (dm), ventral medial (vm) and ventral lateral (vl) subdivision of the PBN are indicated in this figure. The approximate levels relative to bregma are indicated below each photomicrograph (Paxinos and Watson, 1982). 4V, 4th ventricle; BC, brachium conjunctiva; Gi, gigantocellular reticular formation; IRt, intermediate zone of medullary RF; mV, motor trigeminal nucleus; NST, nucleus of the solitary tract; PCRt, parvocellular zone of medullary RF; Sp5, spinal trigeminal tract.
Fig. 2.
Schematic representation of retrograde tracer injections in each animal (n=6). A) Tracer injections concentrated in the IRt and PCRt. B) Tracer injections concentrated in the central medial (cm), dorsal medial (dm), ventral medial (vm) and ventral lateral (vl) subdivision of the PBN. Sections are arranged from rostral (top) to caudal (bottom) and lateral is to the right. The approximate levels relative to bregma are indicated below each photomicrograph (Paxinos and Watson, 1982). bc, brachium conjunctiva; DMSp5, dorsal medial spinal trigeminal nucleus; Gi, gigantocellular reticular formation; IRt, intermediate zone of medullary RF; NST, nucleus of the solitary tract; PCRt, parvocellular zone of medullary RF; Sp5I, interpolar part of spinal trigeminal nucleus; Me5, mesencephalic trigeminal nucleus.
Retrograde labeling
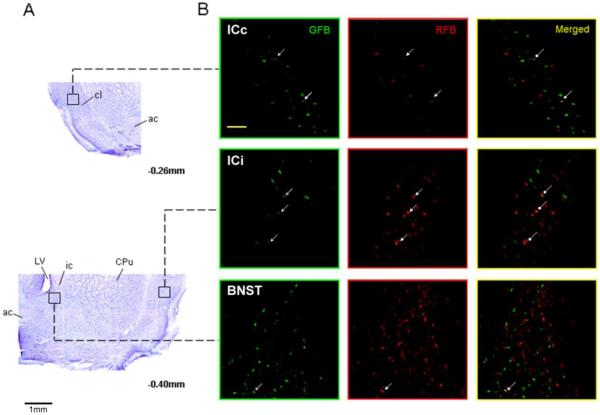
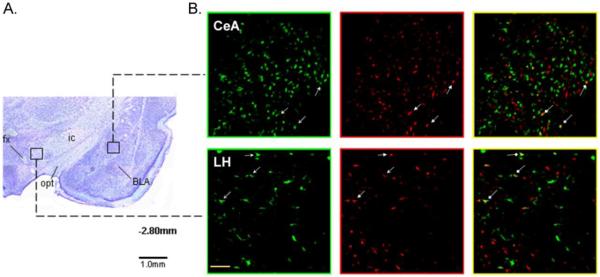
Representative photomicrographs of single- and double-labeled neurons along with alternate cresyl violet stained sections showing the general location within the IC and BNST (Fig. 3), and CeA and LH (Fig. 4). Neurons labeled in three different ways can be seen, green only cells project to the RF, red only cells project to the PBN, and green plus red cells project both to the RF and PBN. Relative to bregma, the approximate rostrocaudal extent of tissue examined for retrogradely labeled neurons was 1.8 to −0.4 for IC, 0.0 to −0.7 for BNST, −1.8 to −3.0 for CeA, and −2.4 to −4.0 for LH (Paxinos and Watson, 1982). All tracer labeled neurons in the LH area were counted without distinguishing whether they were nearer to the fornix (e.g. more medial) or internal capsule (e.g. more lateral). On the basis of prior tract tracing and anatomical studies, the portion of IC examined included the gustatory responsive region as well as a more posterior region that receives convergent inputs from baroreceptor, chemoreceptor, gustatory, and nociceptive organs (Cechetto and Saper, 1987; Kosar et al., 1986; Hanamori et al., 1998). The same is likely true for the other 3 forebrain areas and, thus, the present data cannot distinguish between the type(s) of sensory information processed by retrogradely labeled neurons. We can only say whether they project: 1) to an area that processes taste information (i.e. caudomedial PBN), 2) to one that directly influences oromotor neurons (i.e. RF), or 3) to both areas. Figure 5 shows higher magnification images of single- and double-labeled neurons in each forebrain region.
Fig. 3.
A) Images of cresyl violet-stained brain sections showing the IC and BNST areas in which retrogradely labeled cells were counted (5x). The IC, both contralateral (ICc) and ipsilateral (ICi) to the hindbrain injection, was identified as the area directly lateral to the claustrum (cl). The BNST was identified as the area medial to the internal capsule (ic) and below to the lateral ventricle (LV). The approximate levels relative to bregma are indicated below each photomicrograph (Paxinos and Watson, 1982). B) Corresponding photomicrographs (20x) of alternate sections showing GFB-labeled (green panels), RFB-labeled (red panels), and double-labeled (yellow panels) neurons in each forebrain area. Arrows show examples of cells in each forebrain area that project both to RF and PBN. The scale bar in left top panel of ICc equals 100 um. ac, anterior commissure; CPu, caudate putamen.
Fig. 4.
Images of cresyl violet-stained brain sections showing the CeA and LH areas in which retrogradely labeled cells were counted (5x). The CeA was identified as the area medial to the basolateral nucleus of the amygdala (BLA) and lateral to the optic tract (opt). The LH was identified as the area sandwiched between the ic laterally and the fornix (fx) medially. The approximate level relative to bregma is indicated below the photomicrograph (Paxinos and Watson, 1982). B) Corresponding photomicrographs (20x) of alternate sections showing GFB-labeled (green panels), RFB-labeled (red panels), and double-labeled (yellow panels) neurons in each forebrain area. Arrows show examples of cells in each forebrain area that project both to RF and PBN. The scale bar in left bottom panel of LH equals 100 um.
Fig. 5.
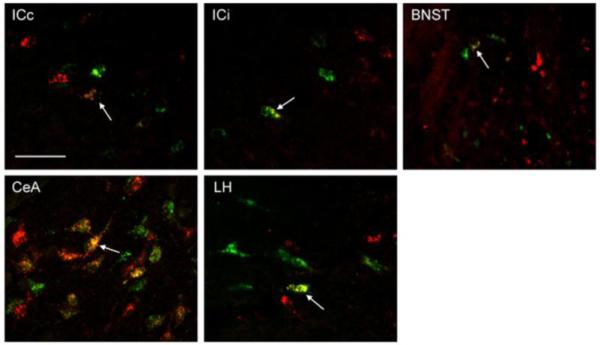
High power (60x) photomicrographs of RF (GFB injection) and PBN (RFB injection) projection neurons in the contralateral insular cortex (ICc), ipsilateral insular cortex (ICi), bed nucleus of the stria terminalis (BNST), central nucleus of the amygdala (CeA), and lateral hypothalamus (LH). Arrows show an example of a cell in each forebrain area that projected both to RF and PBN. The scale bar in left top bar equals 50 um.
Unlike the descending projections from other forebrain areas, which were almost entirely ipsilateral, the RF and PBN receive considerable input from both ipsilateral and contralateral IC. Given that in half of the animals’ tracer material was placed within different subdivisions of the medullary RF, we first used analysis of variance to determine any differences in retrograde labeling between IRt and PCRt injections. Significant differences were not observed in terms of the number of single- and double-labeled neurons (Fs4,30<1.9, ps>0.10; Table 1). Thus, the data from IRt and PCRt injections were combined for further analyses.
Table 1.
Forebrain neurons projecting to different regions of the reticular formation
| Single Labeled | Double Labeled | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ICip | ICcon | CeA | BNST | LH | ICip | ICcon | CeA | BNST | LH | |
| PCRt | 15.0 (2.6) |
32.5 (4.1) |
100.7 (8.1) |
18.6 (2.7) |
25.8 (5.5) |
5.3 (0.9) |
4.3 (1.0) |
20.9 (4.7) |
3.3 (0.8) |
6.4 (2.0) |
| IRt | 17.7 (3.4) |
26.7 (3.9) |
100.9 (13.0) |
36.4 (10.3) |
55.8 (12.3) |
5.6 (1.3) |
3.3 (1.1) |
26.7 (6.3) |
7.3 (2.6) |
11.9 (3.0) |
Show is the mean number of cells per section ± (s.e.).
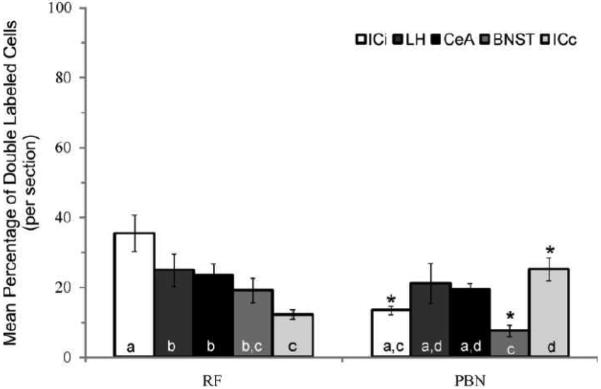
For each forebrain area and animal, the average number of neurons per section resulting from retrograde tracer injection into the RF and PBN was calculated and is graphically shown in Fig. 6. A two-way ANOVA varying injection and forebrain site revealed a significant interaction between factors (F4,60=5.05, p<0.01). Irrespective of injection site, the major source of descending input originated in the CeA. Significantly fewer neurons in the ipsilateral IC projected to the RF, while the contralateral IC contained the fewest number of cells projecting to the PBN. A greater number of neurons in the CeA, BNST, and ipsilateral IC projected to the PBN compared to RF (all ps≤0.03). Moreover, the distribution of cells within the BNST varied as a function of hindbrain target, (F1,24=11.3, p<0.01). While a comparable number of cells in the caudal (60.8 ± 7.6) and rostral (72.9 ± 8.4) half of the BNST projected to the PBN (P = 0.26), almost 5 fold more caudal BNST cells (46.5 ± 8.4) targeted the RF compared to the rostral BNST (8.4 ± 4.7). The photomicrographs of Fig. 7 show an example of the rostrocaudal distribution of RF and PBN projecting neurons in BNST.
Fig. 6.
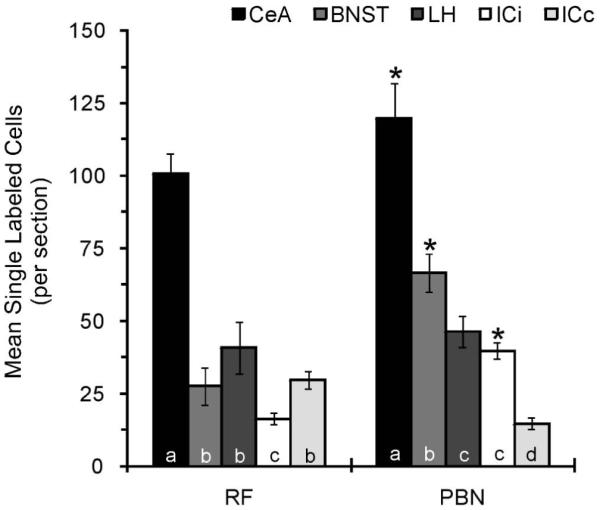
The mean number of single retrogradely labeled neurons per section through the CeA, BNST, LH, ICi, and ICc resulting from tracer injection into the RF and PBN. For each hindbrain injection site, statistical analyses are represented by letters where only those bars with the same letter are not statistically different at alpha < 0.05. *, statistical difference between RF and PBN.
Fig. 7.
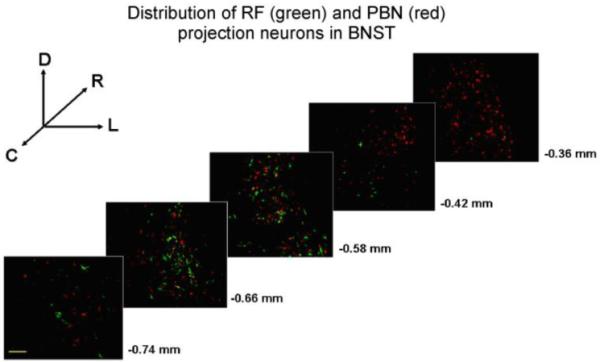
Distribution of BNST neurons projecting to the RF and PBN. Sections are arranged from caudal (bottom) to rostral (top) and lateral is to the right. The approximate levels relative to bregma are indicated below each photomicrograph (Paxinos and Watson, 1982). The scale bar in lower left panel equals 100 um.
Although the actual numbers of labeled neurons per section varied depending on injection size in the RF (ICi, 10.0-21.9; ICc, 19.6-38.0; BNST, 15.1-55.6; CeA, 76.4-120.9; LH, 15.0-76.4) and PBN (ICi, 28.9-45.9; ICc, 10.3-23.1; BNST, 43.7-85.9; CeA, 85.0-150.1; LH, 33.9-64.7), a consistently small number of forebrain neurons were double labeled when expressed as a percentage of the total number of retrogradely labeled cells within a given forebrain site (RF and PBN projecting cells combined). Post hoc analysis of the main effect for forebrain site (F4,29=3.9, p=0.01) revealed that the incidence of tracer co-localization was lowest in BNST (5.5±1.0%) compared to ipsilateral IC (10.5±0.9%), CeA (11.8±1.3%), and LH (11.6±1.9%), but not contralateral IC (8.9±1.0%). When expressed as a function of injection site (RF or PBN projection neurons only), a statistically significant interaction was observed (F4,60=6.5, p<0.01). In the ipsilateral IC and BNST, a greater proportion of the neuronal population projecting to the RF was double labeled (Fig. 8). For instance, approximately 3-fold more ipsilateral IC and BNST neurons retrogradely labeled after RF injections also projected to the PBN compared to the percentage of neurons retrogradely labeled following tracer injection into the PBN giving rise to collaterals that targeted the RF. The opposite pattern was observed for contralateral IC. These findings might be due to the fact that significantly more ipsilateral IC and BNST neurons were labeled following PBN tracer injection compared to RF, while more contralateral IC cells were labeled by RF injections compared to PBN (see Fig. 6). Statistical differences were not observed for CeA and LH. In general, there seems to be a small group of forebrain neurons projecting axons both to the medullary RF and PBN, and two larger populations that target only one of the two hindbrain sites.
Fig. 8.
The mean incidence of tracer co-expression per section in ICi (n=49, 7.8 ± 0.2), LH (n=41, 7.0 ± 0.5), CeA (n=46, 7.7 ± 0.3), BNST (n=44, 7.3 ± 0.3), and ICc (n=43, 7.2 ± 0.7). The total number of sections as well as the mean number of sections per animal ± s.e. are indicated in parentheses. For each hindbrain injection site, statistical analyses are represented by letters where only those bars with the same letter are not statistically different at alpha < 0.05. *, statistical difference between RF and PBN.
Discussion
Based on our results, largely separate populations of cells in the IC, BNST, CeA and LH provide descending input to the PBN and medullary RF. For each forebrain site except the LH and contralateral IC, more neurons projected to the PBN compared to RF. Corticofugal input to the hindbrain was bilateral with a contralateral predominance to the RF and an ipsilateral one to the PBN, while descending input originating in the other forebrain sites was almost entirely ipsilateral. Within the sections examined, descending input from both cortical hemispheres was confined to somatosensory regions bounded dorsal by granular insular cortex and ventral by agranular insular cortex. In all areas except rostral BNST, retrogradely labeled neurons targeting the RF and PBN intermingle anatomically. Nevertheless, forebrain neurons with terminal fields in both hindbrain regions account for a smaller proportion of the population. The present findings mirror results from a previous study showing that a similarly small subset of forebrain neurons give rise to efferent axon collaterals that target gustatory responsive regions both in the NST and PBN (Kang and Lundy, 2009). The existence of at least three populations of forebrain cells with target specific or divergent projections to hindbrain regions processing gustatory sensory information and oromotor signals implies some level of functional specialization in the central control of ingestive behavior.
Medullary Reticular Formation
The medullary reticular formation including IRt and PCRt subdivisions play a critical role in rhythmic jaw and tongue movements due to their innervation of motor nuclei responsible for controlling muscles of mastication and licking (Travers and Norgren, 1983; Fay and Norgren, 1997c; Fay and Norgren, 1997a; Fay and Norgren, 1997b). In fact, the isolated brainstem contains not only the sensory and motor apparatus necessary to produce feeding behavior, but also the integrative capacity to organize normal acceptance and rejection responses to appropriate gustatory stimuli (Grill and Norgren, 1978). Hindbrain circuitry alone (i.e. decerebrate preparation), however, is not sufficient to support learned and some forms of unlearned control of taste-guided behavior indicating a dependence upon neural connections with higher brain centers (Grill and Norgren, 1978a; Grill et al., 1986).
Electrical stimulation of IC, CeA, and LH have been shown to produce rhythmic jaw movements (Sasamoto and Ohta, 1982; Brody et al., 1969; Sasamoto et al., 1990). Transient inactivation of intracellular signaling or immediate early gene transcription in the CeA and IC or neural activity by tetrodotoxin in the LH impairs conditioned taste aversion learning (Lamprecht and Dudai, 1996; Lamprecht et al., 1997; Yasoshima et al., 2006; Caulliez et al., 1996; Berman et al., 1998), while BNST and CeA lesions impair sodium appetite (Zardetto-Smith et al., 1994). Conditioned taste aversion and sodium appetite, respectively, are forms of learned and unlearned modulation of taste preference/aversion behavior. Thus, increases and decreases in the activity of target specific populations of forebrain neurons likely modulate mastication and licking in response to changing physiological conditions, at least in part, through direct control of premotor neurons in IRt and PCRt. Axon terminals of CeA and LH origin are known to synapse directly with RF neurons that project to the motor trigeminal nucleus (Notsu et al., 2008; Yasui et al., 2004). Importantly, a recent study showed that consummatory behavior initiated by 3rd ventricle infusion of the orexigenic peptide neuropeptide Y 3-36 was disrupted by simultaneous infusion of muscimol into the IRt/PCRt (Travers et al., 2010); the implication being that IRt/PCRt provides excitatory drive on oromotor nuclei in response to forebrain appetitive signals.
The variation in the distribution of cells within the BNST as a function of hindbrain target is of potential import and consistent with previous anterograde tracing studies. Specifically, small injections of PHAL (Phaseolus vulgaris leucoagglutinin) into the BNST demonstrated that only neurons in the more caudally located rhomboid division heavily innervate both PBN and medullary RF (Dong and Swanson, 2003). More anterolateral BNST divisions (i.e. oval and fusiform divisions) provide moderate to dense innervation of PBN, but not medullary RF (Dong et al., 2001; Dong and Swanson, 2004). Although the outputs of these BNST divisions as a whole indicate a role at least in modulating the expression of ingestive behaviors, the divergent output of the rhomboid division to hindbrain areas processing sensory input and motor output signals related to feeding suggest some degree of functional specialization.
Although previous research provides convincing evidence that IRt/PCRt plays a critical role in coordinating oromotor behavior through control of motor neurons supplying lingual, masticatory, and facial muscles, this lateral medullary field is also implicated in other functions. For instance, chemical stimulation of regions within IRt/PCRt have been shown to alter cardiovascular and respiratory function (Marchenko and Sapru, 2003; Phillips et al., 2005). Neurons responsive to stimulation of the superior laryngeal nerve and fictive swallowing (Ezure et al., 1993), as well as preganglionic parasympathetic cell bodies controlling parotid and submandibular gland secretion (Toth et al., 1999; Rezek et al., 2008) are also located here. Thus, some of the forebrain neurons projecting to the lateral RF field examined in the present study might also play a role in modulating autonomic output and/or coordinating muscle participation between competing functions.
Gustatory parabrachial nucleus
Neural processing in the PBN is a critical step for the control of preference/aversion behaviors that involve taste-visceral associations, like conditioned taste aversion and sodium appetite (Spector et al., 1992; Scalera et al., 1995). Specifically, the axons necessary for altering taste preference appear to relay through direct projections from the PBN to the ventral forebrain (Hajnal and Norgren, 2005; Norgren et al., 2006; Scalera et al., 1997; Reilly and Pritchard, 1996). Prior investigations demonstrate that the IC, BNST, CeA, and LH modulate taste responsive neurons in the PBN, and taste responsive regions of the PBN project to the RF (Lundy, Jr. and Norgren, 2004; Li et al., 2005; Li and Cho, 2006; Karimnamazi and Travers, 1998). Thus, independent of direct forebrain input to the RF, descending projections from IC, BNST, CeA, and LH might also adjust intake through modulation of PBN taste-elicited drive on specific pools of premotor neurons in RF. The NST similarly might serve as an alternate pathway for centrifugal activity to influence RF neuronal input to oromotor nuclei because cells in the gustatory regions of NST also project to RF (Halsell et al., 1996; Beckman and Whitehead, 1991). Moreover, we and others have shown that the forebrain nuclei examined here project to the gustatory NST, and influence taste-evoked neural activity (Kang and Lundy, 2009; Kang and Lundy, 2010; Smith and Li, 2000; Smith et al., 2005; Cho et al., 2003; Dilorenzo and Monroe, 1995). These descending projections might serve as feed-forward pathways by which cognitive input and behavioral state information modulate direct controls of ingestive behavior (e.g. NST and PBN taste information conveyed to RF).
Together with a prior study from our lab (Kang and Lundy, 2009), a complex circuitry is being defined that involves at least three separate populations of cells within each forebrain region that project to the gustatory NST, gustatory PBN, and RF. Nevertheless, the pools of forebrain neurons projecting to the hindbrain are not completely separate. Small subsets of cells give rise to efferent axons that target both the RF and gustatory PBN or gustatory PBN and NST (Kang and Lundy, 2009). It is likely that individual neurons also project both to the RF and NST as well as some giving rise to collaterals that target all 3 hindbrain sites. In any event, forebrain appetitive signals clearly have the potential to simultaneously influence both sensory and motor output limbs. Future research should be directed at determining the details of the circuitry linking hindbrain taste processing with these distinct pools of forebrain neurons, and how these descending pathways are engaged by conditions that alter taste preference/aversion behavior.
Experimental procedures
Subjects
Nine male Sprague–Dawley rats (Charles River), weighing 350–450 g, were housed in a temperature-controlled colony room on a 12-h light-dark cycle and maintained on ad lib access to normal rat pellets (Teklad 8604) and distilled water. All procedures complied with National Institutes of Health guidelines and were approved by the University of Louisville Institutional Animal Care and Use Committee.
Surgery
The rats were anesthetized with a 50 mg/kg injection (intraperitoneal, ip) of pentobarbital sodium (Nembutal). Atropine was administered to reduce bronchial secretions. Additional doses of Nembutal (10 mg/kg) were administered as necessary to continue a deep level of anesthesia. The animals were placed on a feedback-controlled heating pad and body temperature maintained at 37± 1 °C. Animals were secured in a stereotaxic instrument and the skull was exposed with a midline incision then leveled with reference to bregma and lambda cranial sutures. A small hole was drilled through the bone overlying the cerebellum to allow access to the RF and the PBN. The analgesic buprenex (0.06 mg/kg) was administered for at least two days post surgery.
Electrophysiological recording
Neurons in the NST and PBN responsive to sapid stimulation of the tongue with 0.1 M NaCl were identified by recording multiunit activity through a glass-coated tungsten microelectrode (resistance: 1.5–3 MΩ). Only the anterior 2/3 of the tongue was stimulated because previous studies have shown that forebrain activation has a profound influence on brainstem taste cells that receive input via the chorda tympani nerve and 0.1 M NaCl produces a significant neural response in each “best-stimulus” class of NST and PBN neurons (Cho et al., 2003; Lundy, Jr. and Norgren, 2004; Lundy, Jr. and Norgren, 2001). Cambridge Electronic Design’s Spike2 hardware and software was used to monitor neural responsiveness to NaCl visually relative to baseline discharge during pre-stimulus water flow. For the PBN, the electrode was oriented 20° off the vertical with the tip pointed rostral to avoid the transverse sinus. The coordinates for PBN recordings on the right side of the brainstem were 11.8-12.4 mm posterior to bregma, 1.6-2.2 mm lateral to the midline, and 5.0-6.0 mm below the surface of the cerebellum (Paxinos and Watson, 1982). For access to the NST, the electrode was lowered vertically at coordinates ranging from 12.0-12.8 mm posterior to bregma and 1.4-2.2 mm lateral to the midline. Typically, taste-evoked neural activity was encountered 6.4–7.0 mm ventral to the surface of the cerebellum. Tracer injections into the ipsilateral RF were placed 0.8-1.0 mm ventral to the NST taste responsive area. The cerebellar surface was kept moist throughout surgery with physiological saline.
4.4. Tracer injections
Once the injection region was identified, the tungsten electrode was replaced by a micropipette (tip ID 25–30 μm) filled with a retrograde tracer. For each injection site (i.e. RF or PBN), a single penetration with the micropipette was used to deliver the tracer material. Two of the 9 animals were excluded because histological examination revealed that tracer injection into the RF spread dorsal into the NST. For 4 of the remaining 6 animals, green (GFB) and red (RFB) fluorescent latex microbeads (Lumafluor, Inc.) were pressure injected into the RF and PBN (20 ms at 20 psi). The other 2 animals had 4% fluorogold mixed in saline (FG, Biotium, Inc.) injected iontophoretically (+2 μA for 20 min; 2 min on and 1 min off) into the RF, while GFB or RFB was pressure injected into the PBN (20 ms at 20 psi). The volume of each pressure injection was estimated to be approximately 200 nl. Specifically, we made 2 marks around the outside of our injection pipette; from the tip one mark was made at 9 mm and the other at 10 mm. The latex microbead tracer was loaded into the pipette up to the top mark, which we measured to require 4 μl (e.g. 400 nl/mm). With the aid of a surgical microscope, we ejected tracer until the meniscus was half way between the lines, a movement of approximately 0.5 mm ejecting approximately 200 nl.
4.5. Perfusion and histology
After a 7 day recovery period, the rats were euthanized with a lethal dose of Nembutal (150 mg/kg ip) and perfused through the ascending aorta, initially with 250 ml of 0.9% saline containing 5 ml of 100 units/ml heparin then 300 ml of 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) followed by 150 ml of 20% sucrose. The brains were removed, blocked, and post fixed overnight at 4 °C in 30% sucrose. Coronal sections (40 μm) were cut using a freezing microtome and two series of sections were collected. One series was mounted on slides and cover slipped using Fluor mount G, while the other series was mounted on slides and counter stained with cresyl violet.
4.6. Immunohistochemistry
For the animals that received injections of FG, tissue sections were collected in a small jar and incubated in 5% normal horse serum (NHS; Jackson Labs) mixed in 0.05% triton-x/phosphate buffer saline for 1 h. After overnight incubation (4 °C) in a 1:2,500 dilution of rabbit FG antibody (Chemicon, AB153), sections were rinsed several times in PBS followed by 2 h incubation in Alexa-Fluor 594 anti-rabbit antibody (Invitrogen) diluted 1:200 in phosphate buffer saline.
4.7. Analysis
Cell bodies positive for GFB (excitation filter: 490 nm; barrier filter: 550 nm), RFB, and FG (excitation filter: 520–554 nm; barrier filter: 580 nm) immunoreactivity in the IC, CeA, BNST and LH were identified using an Olympus confocal microscope (sequential scanning). Images of alternate cresyl violet stained sections were acquired using a Leica DM 2000 microscope equipped with UV filter cubes, Hamamatsu monochrome digital camera, and Image-Pro Plus software. The number of single- and double-labeled cells per section (sum of cells divided by the number of sections) was calculated for each forebrain site and used for statistical analyses. The color segmentation function in Image-Pro Plus software was used to separate and count single- and double-labeled neurons. Briefly, confocal images were opened in Image-Pro Plus and invert contrast applied, which changed the black background to white, the green color of retrogradely labeled cells to pink, the red color of RFB or Alex-Fluor 594 to turquoise, and double-labeled cells to dark blue/purple. The threshold for counting a cell as singly or double labeled was set to >10 adjacent pixels exhibiting the same color (Panguluri et al., 2009). The data sets were analyzed with 1 or 2-way ANOVAs when appropriate. The results are presented as mean ± SE and a value of P < 0.05 was considered statistically significant.
ACKNOWLEDGMENTS
The authors wish to thank Dr. Joseph Travers (Ohio State University) for valuable comments on the manuscript. This research was supported by the National Institute on Deafness and Other Communication Disorders Grant DC-006698.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Beckman ME, Whitehead MC. Intramedullary connections of the rostral nucleus of the solitary tract in the hamster. Brain Res. 1991;557:265–279. doi: 10.1016/0006-8993(91)90143-j. [DOI] [PubMed] [Google Scholar]
- Berk ML, Finkelstein JA. Efferent connections of the lateral hypothalamic area of the rat: an autoradiographic investigation. Brain Res. Bull. 1982;8:511–526. doi: 10.1016/0361-9230(82)90009-0. [DOI] [PubMed] [Google Scholar]
- Berman DE, Hazvi S, Rosenblum K, Seger R, Dudai Y. Specific and differential activation of mitogen-activated protein kinase cascades by unfamiliar taste in the insular cortex of the behaving rat. J. Neurosci. 1998;18:10037–10044. doi: 10.1523/JNEUROSCI.18-23-10037.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody JF, DeFeudis PA, DeFeudis FV. Effects of micro-injections of L-glutamate into the hypothalamus on attack and flight behaviour in cats. Nature. 1969;224:1330. doi: 10.1038/2241330a0. [DOI] [PubMed] [Google Scholar]
- Caulliez R, Meile MJ, Nicolaidis S. A lateral hypothalamic D1 dopaminergic mechanism in conditioned taste aversion. Brain Res. 1996;729:234–245. [PubMed] [Google Scholar]
- Cechetto DF, Saper CB. Evidence for a viscerotopic sensory representation in the cortex and thalamus in the rat. J. Comp Neurol. 1987;262:27–45. doi: 10.1002/cne.902620104. [DOI] [PubMed] [Google Scholar]
- Chen Z, Travers JB. Inactivation of amino acid receptors in medullary reticular formation modulates and suppresses ingestion and rejection responses in the awake rat. Am. J. Physiol Regul. Integr. Comp Physiol. 2003;285:R68–R83. doi: 10.1152/ajpregu.00054.2003. [DOI] [PubMed] [Google Scholar]
- Chen Z, Travers SP, Travers JB. Muscimol infusions in the brain stem reticular formation reversibly block ingestion in the awake rat. Am. J. Physiol Regul. Integr. Comp Physiol. 2001;280:R1085–R1094. doi: 10.1152/ajpregu.2001.280.4.R1085. [DOI] [PubMed] [Google Scholar]
- Cho YK, Li CS, Smith DV. Descending influences from the lateral hypothalamus and amygdala converge onto medullary taste neurons. Chem. Senses. 2003;28:155–171. doi: 10.1093/chemse/28.2.155. [DOI] [PubMed] [Google Scholar]
- Dilorenzo PM, Monroe S. Corticofugal Influence on Taste Responses in the Nucleus of the Solitary Tract in the Rat. Journal of Neurophysiology. 1995;74:258–272. doi: 10.1152/jn.1995.74.1.258. [DOI] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Watts AG, Swanson LW. Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain. J. Comp Neurol. 2001;436:430–455. doi: 10.1002/cne.1079. [DOI] [PubMed] [Google Scholar]
- Dong HW, Swanson LW. Projections from the rhomboid nucleus of the bed nuclei of the stria terminalis: implications for cerebral hemisphere regulation of ingestive behaviors. J. Comp Neurol. 2003;463:434–472. doi: 10.1002/cne.10758. [DOI] [PubMed] [Google Scholar]
- Dong HW, Swanson LW. Organization of axonal projections from the anterolateral area of the bed nuclei of the stria terminalis. J. Comp Neurol. 2004;468:277–298. doi: 10.1002/cne.10949. [DOI] [PubMed] [Google Scholar]
- Ezure K, Oku Y, Tanaka I. Location and axonal projection of one type of swallowing interneurons in cat medulla. Brain Res. 1993;632:216–224. doi: 10.1016/0006-8993(93)91156-m. [DOI] [PubMed] [Google Scholar]
- Fay RA, Norgren R. Identification of rat brainstem multisynaptic connections to the oral motor nuclei in the rat using pseudorabies virus. II. Facial muscle motor systems. Brain Res. Brain Res. Rev. 1997a;25:276–290. doi: 10.1016/s0165-0173(97)00027-1. [DOI] [PubMed] [Google Scholar]
- Fay RA, Norgren R. Identification of rat brainstem multisynaptic connections to the oral motor nuclei using pseudorabies virus. I. Masticatory muscle motor systems. Brain Res. Brain Res. Rev. 1997b;25:255–275. doi: 10.1016/s0165-0173(97)00026-x. [DOI] [PubMed] [Google Scholar]
- Fay RA, Norgren R. Identification of rat brainstem multisynaptic connections to the oral motor nuclei using pseudorabies virus. III. Lingual muscle motor systems. Brain Res. Brain Res. Rev. 1997c;25:291–311. doi: 10.1016/s0165-0173(97)00028-3. [DOI] [PubMed] [Google Scholar]
- Grigson PS, Reilly S, Shimura T, Norgren R. Ibotenic acid lesions of the parabrachial nucleus and conditioned taste aversion: further evidence for an associative deficit in rats. Behav. Neurosci. 1998;112:160–171. [PubMed] [Google Scholar]
- Grill HJ, Norgren R. The taste reactivity test. II. Mimetic responses to gustatory stimuli in chronic thalamic and chronic decerebrate rats. Brain Res. 1978;143:281–297. doi: 10.1016/0006-8993(78)90569-3. [DOI] [PubMed] [Google Scholar]
- Hajnal A, Norgren R. Taste pathways that mediate accumbens dopamine release by sapid sucrose. Physiol Behav. 2005;84:363–369. doi: 10.1016/j.physbeh.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Halsell CB, Travers SP, Travers JB. Ascending and descending projections from the rostral nucleus of the solitary tract originate from separate neuronal populations. Neuroscience. 1996;72:185–197. doi: 10.1016/0306-4522(95)00528-5. [DOI] [PubMed] [Google Scholar]
- Hanamori T, Kunitake T, Kato K, Kannan H. Responses of neurons in the insular cortex to gustatory, visceral, and nociceptive stimuli in rats. J. Neurophysiol. 1998;79:2535–2545. doi: 10.1152/jn.1998.79.5.2535. [DOI] [PubMed] [Google Scholar]
- Hopkins DA, Holstege G. Amygdaloid projections to the mesencephalon, pons and medulla oblongata in the cat. Exp. Brain Res. 1978;32:529–547. doi: 10.1007/BF00239551. [DOI] [PubMed] [Google Scholar]
- Kang Y, Lundy RF. Terminal field specificity of forebrain efferent axons to brainstem gustatory nuclei. Brain Res. 2009;1248:76–85. doi: 10.1016/j.brainres.2008.10.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y, Lundy RF. Amygdalofugal influence on processing of taste information in the nucleus of the solitary tract of the rat. J. Neurophysiol. 2010;104:726–741. doi: 10.1152/jn.00341.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimnamazi H, Travers JB. Differential projections from gustatory responsive regions of the parabrachial nucleus to the medulla and forebrain. Brain Res. 1998;813:283–302. doi: 10.1016/s0006-8993(98)00951-2. [DOI] [PubMed] [Google Scholar]
- Kosar E, Grill HJ, Norgren R. Gustatory cortex in the rat. I. Physiological properties and cytoarchitecture. Brain Res. 1986;379:329–341. doi: 10.1016/0006-8993(86)90787-0. [DOI] [PubMed] [Google Scholar]
- Lamprecht R, Dudai Y. Transient expression of c-Fos in rat amygdala during training is required for encoding conditioned taste aversion memory. Learn. Mem. 1996;3:31–41. doi: 10.1101/lm.3.1.31. [DOI] [PubMed] [Google Scholar]
- Lamprecht R, Hazvi S, Dudai Y. cAMP response element-binding protein in the amygdala is required for long- but not short-term conditioned taste aversion memory. J. Neurosci. 1997;17:8443–8450. doi: 10.1523/JNEUROSCI.17-21-08443.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Cho YK. Efferent projection from the bed nucleus of the stria terminalis suppresses activity of taste-responsive neurons in the hamster parabrachial nuclei. Am. J. Physiol Regul. Integr. Comp Physiol. 2006;291:R914–R926. doi: 10.1152/ajpregu.00750.2005. [DOI] [PubMed] [Google Scholar]
- Li CS, Cho YK, Smith DV. Modulation of parabrachial taste neurons by electrical and chemical stimulation of the lateral hypothalamus and amygdala. J. Neurophysiol. 2005;93:1183–1196. doi: 10.1152/jn.00828.2004. [DOI] [PubMed] [Google Scholar]
- Lundy RF, Jr., Norgren R. Pontine gustatory activity is altered by electrical stimulation in the central nucleus of the amygdala. J. Neurophysiol. 2001;85:770–783. doi: 10.1152/jn.2001.85.2.770. [DOI] [PubMed] [Google Scholar]
- Lundy RF, Jr., Norgren R. Activity in the hypothalamus, amygdala, and cortex generates bilateral and convergent modulation of pontine gustatory neurons. J. Neurophysiol. 2004;91:1143–1157. doi: 10.1152/jn.00840.2003. [DOI] [PubMed] [Google Scholar]
- Marchenko V, Sapru HN. Cardiovascular responses to chemical stimulation of the lateral tegmental field and adjacent medullary reticular formation in the rat. Brain Res. 2003;977:247–260. doi: 10.1016/s0006-8993(03)02719-7. [DOI] [PubMed] [Google Scholar]
- Moga MM, Herbert H, Hurley KM, Yasui Y, Gray TS, Saper CB. Organization of cortical, basal forebrain, and hypothalamic afferents to the parabrachial nucleus in the rat. J. Comp Neurol. 1990;295:624–661. doi: 10.1002/cne.902950408. [DOI] [PubMed] [Google Scholar]
- Nishijo H, Ono T, Uwano T, Kondoh T, Torii K. Hypothalamic and amygdalar neuronal responses to various tastant solutions during ingestive behavior in rats. J. Nutr. 2000;130:954S–959S. doi: 10.1093/jn/130.4.954S. [DOI] [PubMed] [Google Scholar]
- Norgren R. Taste pathways to hypothalamus and amygdala. J. Comp Neurol. 1976;166:17–30. doi: 10.1002/cne.901660103. [DOI] [PubMed] [Google Scholar]
- Norgren R, Hajnal A, Mungarndee SS. Gustatory reward and the nucleus accumbens. Physiol Behav. 2006;89:531–535. doi: 10.1016/j.physbeh.2006.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notsu K, Tsumori T, Yokota S, Sekine J, Yasui Y. Posterior lateral hypothalamic axon terminals are in contact with trigeminal premotor neurons in the parvicellular reticular formation of the rat medulla oblongata. Brain Res. 2008;1244:71–81. doi: 10.1016/j.brainres.2008.09.076. [DOI] [PubMed] [Google Scholar]
- Panguluri S, Saggu S, Lundy R. Comparison of somatostatin and corticotrophin-releasing hormone immunoreactivity in forebrain neurons projecting to taste-responsive and non-responsive regions of the parabrachial nucleus in rat. Brain Res. 2009;1298:57–69. doi: 10.1016/j.brainres.2009.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; Sydney: 1982. [DOI] [PubMed] [Google Scholar]
- Phillips SW, Gebber GL, Barman SM. Medullary lateral tegmental field: control of respiratory rate and vagal lung inflation afferent influences on sympathetic nerve discharge. Am. J. Physiol Regul. Integr. Comp Physiol. 2005;288:R1396–R1410. doi: 10.1152/ajpregu.00632.2004. [DOI] [PubMed] [Google Scholar]
- Reilly S, Grigson PS, Norgren R. Parabrachial nucleus lesions and conditioned taste aversion: evidence supporting an associative deficit. Behav. Neurosci. 1993;107:1005–1017. doi: 10.1037//0735-7044.107.6.1005. [DOI] [PubMed] [Google Scholar]
- Reilly S, Pritchard TC. Gustatory thalamus lesions in the rat: II. Aversive and appetitive taste conditioning. Behav. Neurosci. 1996;110:746–759. [PubMed] [Google Scholar]
- Rezek O, Boldogkoi Z, Tombacz D, Kovago C, Gerendai I, Palkovits M, Toth IE. Location of parotid preganglionic neurons in the inferior salivatory nucleus and their relation to the superior salivatory nucleus of rat. Neurosci. Lett. 2008;440:265–269. doi: 10.1016/j.neulet.2008.05.099. [DOI] [PubMed] [Google Scholar]
- Saper CB, Loewy AD. Efferent connections of the parabrachial nucleus in the rat. Brain Res. 1980;197:291–317. doi: 10.1016/0006-8993(80)91117-8. [DOI] [PubMed] [Google Scholar]
- Sasamoto K, Ohta M. Amygdaloid-induced jaw opening and facilitation or inhibition of the trigeminal motoneurons in the rat. Comp Biochem. Physiol A Comp Physiol. 1982;73:349–354. doi: 10.1016/0300-9629(82)90166-9. [DOI] [PubMed] [Google Scholar]
- Sasamoto K, Zhang G, Iwasaki M. Two types of rhythmical jaw movements evoked by stimulation of the rat cortex. Shika. Kiso. Igakkai Zasshi. 1990;32:57–68. doi: 10.2330/joralbiosci1965.32.57. [DOI] [PubMed] [Google Scholar]
- Scalera G, Grigson PS, Norgren R. Gustatory functions, sodium appetite, and conditioned taste aversion survive excitotoxic lesions of the thalamic taste area. Behav. Neurosci. 1997;111:633–645. [PubMed] [Google Scholar]
- Scalera G, Spector AC, Norgren R. Excitotoxic lesions of the parabrachial nuclei prevent conditioned taste aversions and sodium appetite in rats. Behav. Neurosci. 1995;109:997–1008. [PubMed] [Google Scholar]
- Smith DV, Li CS. GABA-mediated corticofugal inhibition of taste-responsive neurons in the nucleus of the solitary tract. Brain Res. 2000;858:408–415. doi: 10.1016/s0006-8993(99)02484-1. [DOI] [PubMed] [Google Scholar]
- Smith DV, Ye MK, Li CS. Medullary taste responses are modulated by the bed nucleus of the stria terminalis. Chem. Senses. 2005;30:421–434. doi: 10.1093/chemse/bji037. [DOI] [PubMed] [Google Scholar]
- Spector AC, Norgren R, Grill HJ. Parabrachial gustatory lesions impair taste aversion learning in rats. Behav. Neurosci. 1992;106:147–161. doi: 10.1037//0735-7044.106.1.147. [DOI] [PubMed] [Google Scholar]
- Toth IE, Boldogkoi Z, Medveczky I, Palkovits M. Lacrimal preganglionic neurons form a subdivision of the superior salivatory nucleus of rat: transneuronal labelling by pseudorabies virus. J. Auton. Nerv. Syst. 1999;77:45–54. doi: 10.1016/s0165-1838(99)00032-6. [DOI] [PubMed] [Google Scholar]
- Travers JB, DiNardo LA, Karimnamazi H. Motor and premotor mechanisms of licking. Neurosci. Biobehav. Rev. 1997;21:631–647. doi: 10.1016/s0149-7634(96)00045-0. [DOI] [PubMed] [Google Scholar]
- Travers JB, Herman K, Travers SP. Suppression of third ventricular NPY-elicited feeding following medullary reticular formation infusions of muscimol. Behav. Neurosci. 2010;124:225–233. doi: 10.1037/a0018928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers JB, Norgren R. Afferent projections to the oral motor nuclei in the rat. J. Comp Neurol. 1983;220:280–298. doi: 10.1002/cne.902200303. [DOI] [PubMed] [Google Scholar]
- van der KD, Koda LY, McGinty JF, Gerfen CR, Bloom FE. The organization of projections from the cortex, amygdala, and hypothalamus to the nucleus of the solitary tract in rat. J. Comp Neurol. 1984;224:1–24. doi: 10.1002/cne.902240102. [DOI] [PubMed] [Google Scholar]
- Veening JG, Swanson LW, Sawchenko PE. The organization of projections from the central nucleus of the amygdala to brainstem sites involved in central autonomic regulation: a combined retrograde transport-immunohistochemical study. Brain Res. 1984;303:337–357. doi: 10.1016/0006-8993(84)91220-4. [DOI] [PubMed] [Google Scholar]
- Yasoshima Y, Sako N, Senba E, Yamamoto T. Acute suppression, but not chronic genetic deficiency, of c-fos gene expression impairs long-term memory in aversive taste learning. Proc. Natl. Acad. Sci. U. S. A. 2006;103:7106–7111. doi: 10.1073/pnas.0600869103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasoshima Y, Shimura T, Yamamoto T. Single unit responses of the amygdala after conditioned taste aversion in conscious rats. Neuroreport. 1995;6:2424–2428. doi: 10.1097/00001756-199511270-00034. [DOI] [PubMed] [Google Scholar]
- Yasui Y, Tsumori T, Oka T, Yokota S. Amygdaloid axon terminals are in contact with trigeminal premotor neurons in the parvicellular reticular formation of the rat medulla oblongata. Brain Res. 2004;1016:129–134. doi: 10.1016/j.brainres.2004.04.080. [DOI] [PubMed] [Google Scholar]
- Zardetto-Smith AM, Beltz TG, Johnson AK. Role of the central nucleus of the amygdala and bed nucleus of the stria terminalis in experimentally-induced salt appetite. Brain Res. 1994;645:123–134. doi: 10.1016/0006-8993(94)91645-4. [DOI] [PubMed] [Google Scholar]