Abstract
Impaired function of pancreatic β-cells is one of the hallmarks of type 2 diabetes. β-cell function is regulated by the activity of many hormones and neurotransmitters which bind to specific cell surface receptors. The M3 muscarinic acetylcholine receptor (M3R) belongs to the superfamily of G protein-coupled receptors and, following ligand-dependent activation, selectively activates G proteins of the Gq/11 family. Recent studies with M3R mutant mice strongly suggest that β-cell M3Rs play a central role in promoting insulin release and maintaining proper glucose homeostasis. In this review, we highlight recent studies indicating that β-cell M3Rs and components of downstream signaling pathways may represent promising new targets for the treatment of type 2 diabetes.
Modulation of β-cell function by G protein-coupled receptors
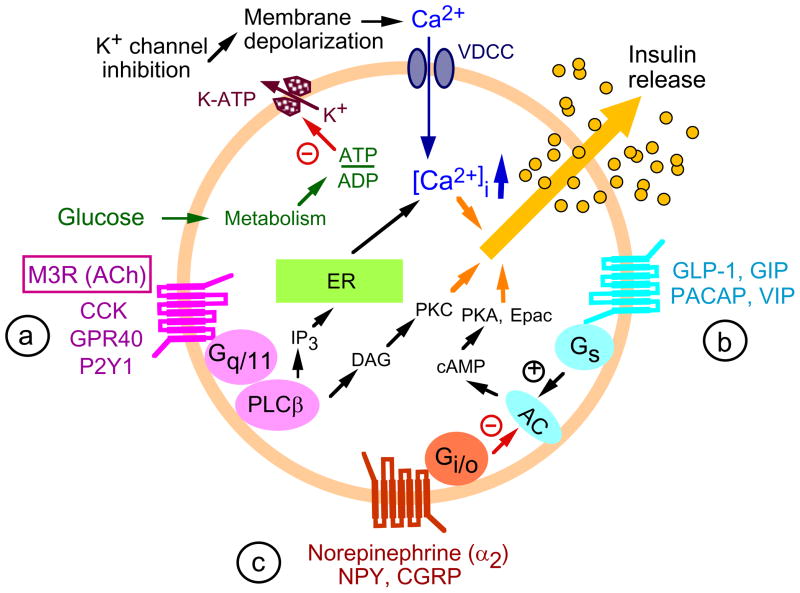
A key feature of type 2 diabetes (T2D) is the failure of pancreatic β-cells to secrete sufficient amounts of insulin to maintain normal blood glucose levels [1]. Glucose-stimulated insulin secretion (GSIS) is modulated by various neurotransmitters and hormones that bind to specific receptors present on the surface of pancreatic β-cells. The majority of these receptors are members of the superfamily of G protein-coupled receptors (GPCRs) [2]. Based on their G protein coupling properties, GPCRs can subdivided into different functional classes, primarily Gq/11-, Gi-, or Gs-coupled receptors (Figure 1). G proteins are linked to specific signaling pathways which have multiple effects on β-cell function inducing the regulation of insulin release (Figure 1). Whereas Gi-coupled receptors exert an inhibitory effect on insulin release, Gs- and Gq/11- coupled receptors promote insulin release and may have several other beneficial effects on β-cell function [2]. For example, during the past decade, the glucagon-like peptide 1 (GLP-1) receptor, a Gs-coupled GPCR that is preferentially expressed by pancreatic β-cells, has attracted attention as a novel drug target. GLP-1 receptor agonists (e.g. exenatide) or dipeptidyl peptidase 4 (DPP-4) inhibitors (e.g. sitagliptin), which interfere with the breakdown of endogenous GLP-1, can facilitate GSIS and improve whole body glucose homeostasis [3,4]. As a result, several agents that act by promoting signaling through GLP-1 receptors have been approved recently by the FDA for the treatment of T2D [2].
Figure 1.
GPCR-dependent signaling pathways involved in regulating insulin release from pancreatic β-cells. Like essentially all other cell types, pancreatic β-cells express many different GPCRs [2,5] which are linked to different functional classes of heteromeric G proteins, primarily (a) Gq/11-, (b) Gs-, and (c) Gi -type G proteins. The different classes of G proteins activate or inhibit specific signaling pathways or networks that play important roles in modulating glucose-dependent insulin release. Please note that only the major effectors known to be critical for G protein-regulated insulin secretion are shown in this figure. In general, activation of Gs- and Gq/11- coupled receptors enhances insulin release, whereas activation of Gi-coupled receptors usually leads to inhibition of insulin secretion [2]. This figure shows only a few representative GPCRs found on the surface of pancreatic β-cells. The M3 muscarinic receptor (M3R), the main focus of this review, is shown boxed. AC, adenylyl cyclase; ACh, acetylcholine; GLP-1, glucagon-like peptide 1; CCK, cholecystokinin; NPY, neuropeptide Y; CGRP, calcitonin gene-related peptide; GIP, glucose-dependent insulinotropic peptide i; M3R, M3 muscarinic receptor; PACAP, pituitary adenylate cyclase-activating polypeptide; VIP, vasoactive intestinal polypeptide; VDCC, voltage-dependent Ca2+ channel; DAG, diacylglycerol; IP3, inositol 1,4,5-trisphosphate; PKA, protein kinase A; PKC, protein kinase C; ER, endoplasmic reticulum; Epac, exchange protein activated by cAMP; K-ATP, ATP-sensitive K+ channel.
Pancreatic β-cells also express several Gq/11-coupled receptors [2, 5] including the M3 muscarinic acetylcholine (ACh) receptor (M3R), a prototypic class I (rhodopsin-like) GPCR. ACh, the major neurotransmitter of peripheral parasympathetic nerves, exerts a pronounced stimulatory effect on pancreatic insulin release [6,7]. It is well known that this effect is of particular importance during the preabsorptive phase of feeding which is accompanied by an increase in the activity of efferent vagal nerves [6,7]. Studies with isolated islets derived from M3R knockout (KO) mice clearly demonstrated that the stimulatory effect of ACh on insulin release is mediated by the M3R subtype [8,9], consistent with the outcome of classical pharmacological studies [10]. Following ACh binding, the M3R is able to preferentially activate G proteins of the Gq/11 family [11].
Recent studies with M3R mutant mice have clearly established a role for β-cell M3Rs in maintaining proper blood glucose homeostasis [12]. These studies also suggested that strategies aimed at enhancing signaling through β-cell M3Rs may prove useful for the treatment of T2D. Herein, we summarize the major findings from the recent phenotypic analysis of M3R mutant mice and other mouse models, focusing on proteins critical for β-cell M3R function.
M3R-mediated signaling pathways in pancreatic β-cells
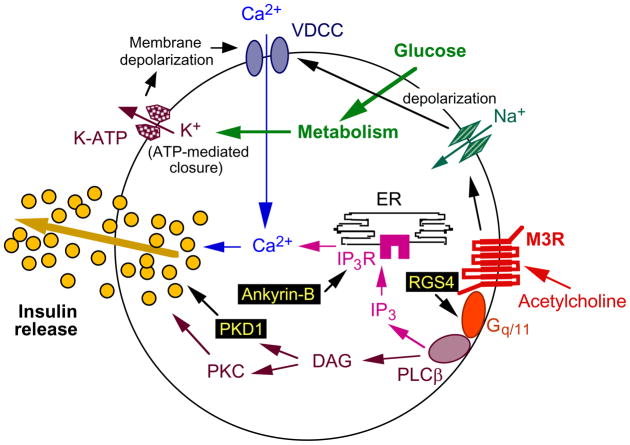
ACh binding to β-cell M3Rs triggers a sequence of biochemical events that are typically seen after activation of Gq/11-type G proteins (for a comprehensive review, see [7]). One key event is the Gαq/11-induced stimulation of distinct isoforms of phospholipase Cβ (PLCβ), which catalyze the hydrolytic breakdown of phosphatidylinositol (PI) 4,5-bisphosphate, resulting in the generation of two second messengers, diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3). Whereas DAG plays a key role in the activation of various protein kinase C (PKC) isoforms, IP3 promotes the release of Ca2+ from endoplasmic reticulum (ER) stores via binding to specific IP3 receptors (Figure 2). The M3R-receptor mediated increase in intracellular calcium levels and the ability of activated PKC to increase the efficiency of calcium on insulin exocytosis are of key importance for M3R-dependent augmentation of GSIS [7]. In addition, β-cell muscarinic receptors also modulate other cellular activities, including the activation of an inward Na+ current ([13, 14]; Figure 2), which is predicted to facilitate insulin release by augmenting membrane depolarization and subsequent Ca2+ influx.
Figure 2.
Scheme highlighting several key components involved in M3R-mediated facilitation of insulin release from pancreatic β-cells. Recent studies suggest that RGS4 is a potent negative regulator of M3R signaling in pancreatic β-cells [21] and that ankyrin-B and protein kinase D1 (PKD1) are required for M3R-mediated augmentation of insulin release (note that RGS4, like ankyrin-B and PKD1, is located in the cytoplasm). Whereas ankyrin-B plays a role in stabilizing IP3 receptors present in the ER (IP3R; [44]), PKD1 is thought to promote membrane fission events necessary for insulin exocytosis [45].
β-cell M3Rs play a critical role in maintaining blood glucose homeostasis
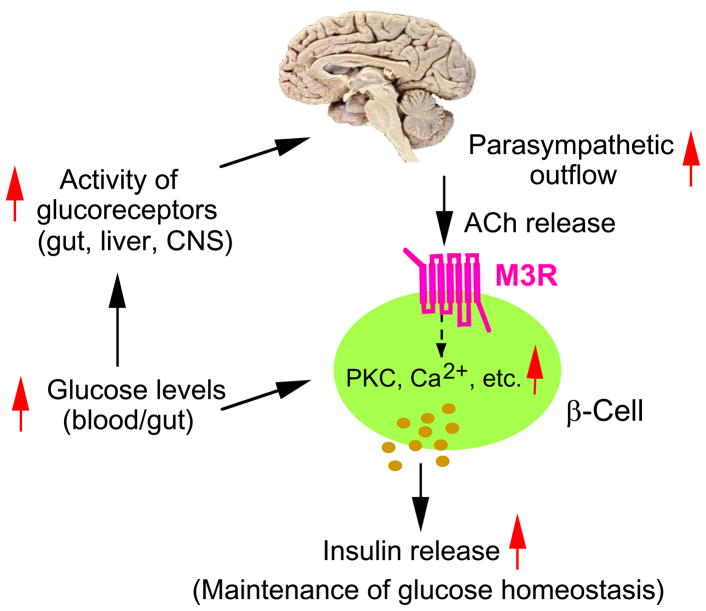
To assess the physiological relevance of β-cell M3Rs, Gautam et al. [12] generated mutant mice that lack M3Rs in pancreatic β-cells only (β-M3R KO mice) as well as transgenic mice that selectively overexpress M3Rs in pancreatic β-cells (β-M3R Tg mice). β-M3R KO mice displayed impaired glucose tolerance with significantly reduced insulin release. In contrast, β-M3R Tg mice showed greatly improved glucose tolerance, increased insulin release, and were resistant to diet-induced glucose intolerance and hyperglycemia [12]. Taken together, these findings clearly indicated that β-cell M3Rs play a central role in maintaining normal glucose homeostasis and insulin release in vivo (Figure 3). Moreover, the beneficial metabolic changes displayed by β-M3R Tg mice suggested that strategies aimed at enhancing signaling through β-cell M3Rs might prove useful for the treatment of T2D.
Figure 3.
Central role of β-cell M3Rs in maintaining proper blood glucose homeostasis. Food intake results in the activation of glucoreceptors in the gut, liver, and brain, leading to enhanced central parasympathetic outflow [7]. Acetylcholine (ACh) released from pancreatic parasympathetic nerve terminals promotes insulin release via stimulation of β-cell M3Rs, due to an increase in intracellular calcium levels and the enhanced formation of several other second messengers [7]. Disruption of this pathway in mice lacking β-cell M3Rs leads to reduced insulin release and impaired glucose tolerance, whereas overexpression of M3Rs in β-cells of transgenic mice results in enhanced insulin secretion and greatly improved glucose tolerance [12].
Interestingly, the opposing changes in glucose tolerance observed with the two M3R mutant mouse strains discussed above were observed independent of the route of glucose administration (intragastric or i.p.) [12]. This finding strongly suggests that glucose-induced activation of pancreatic efferent parasympathetic nerves is not only a transient phenomenon associated with the vagus-mediated preabsorptive phase of feeding [6,7], but also persists throughout the absorptive phase of feeding (Figure 3).
Beneficial metabolic effects of persistent activation of β-cell M3Rs
At present, muscarinic agonists that can selectively activate the M3R subtype are not available (note that the muscarinic receptor family consists of five molecularly distinct subtypes, M1-M5; [11]). Thus, to mimic the effects of a drug that chronically stimulates β-cell M3Rs, Gautam et al. [15] generated a transgenic mouse line that expressed a mutant version of the M3R in pancreatic β-cells only. This mutant receptor contained the Gln490Leu point mutation which renders the M3R constitutively active (i.e. this mutant receptor can activate Gq/11 even in the absence of agonist ligands; [16]).
In vitro insulin release experiments carried out with perifused islets from wild-type (WT) and β-M3R-Q490L Tg mice confirmed that the Q490L mutant M3R was indeed constitutively active in mouse β-cells [15]. Strikingly, in vivo studies demonstrated that the β-M3R-Q490L Tg mice showed elevated serum insulin levels (despite unchanged peripheral insulin sensitivity), significantly decreased blood glucose levels (under both fed and fasting conditions), and greatly improved glucose tolerance. Moreover, β-M3R-Q490L Tg mice were protected against diet-induced hyperglycemia and glucose intolerance, most likely due to enhanced insulin release [15]. Persistent muscarinic stimulation of β-cells may sensitize β-cells to the stimulatory effects of glucose (see, for example, ref. [17]) or other nutrients such as fatty acids or amino acids, providing a possible explanation for the observation that β-M3R-Q490L Tg mice displayed reduced blood glucose levels even under fasting conditions.
To confirm that the changes in blood glucose and insulin levels displayed by the β-M3R-Q490L Tg mice were indeed due to persistent signaling through the mutant M3R, rather than a consequence of secondary changes in β-cell metabolism, Gautam et al. [15] injected freely fed β-M3R-Q490L Tg mice and WT littermates with an acute dose of the inverse agonist, atropine (10 mg/kg i.p.). In WT mice, atropine treatment had little or no effect on blood glucose and insulin levels. In contrast, atropine administration restored WT-like blood glucose and insulin levels in the mutant animals (these mice exhibited reduced blood glucose and increased serum insulin levels prior to atropine injection). This observation strongly supported the concept that sustained signaling through β-cell Q490L mutant M3Rs was responsible for the metabolic changes displayed by the β-M3R-Q490L Tg mice.
Importantly, these findings strongly suggest that chronic, continuous activation of β-cell M3Rs does not lead to a loss or significant reduction in M3R activity caused by receptor desensitization or counter-regulatory cellular events. Clearly, this observation is of considerable therapeutic relevance.
RGS4 selectively impairs signaling through β-cell M3Rs
Given the major breakthroughs that have been achieved in the development of muscarinic receptor subtype-selective ligands recently [18], it is likely that M3R-selective agonists will become available soon. However, the potential clinical use of such agents for the treatment of T2D may be complicated by unwanted side effects, since M3Rs also play an important role in mediating several other peripheral actions of ACh including stimulation of smooth muscle activity [19,20].
To overcome this potential obstacle, Ruiz de Azua et al. [21] recently initiated work in MIN6 insulinoma cells to identify proteins that modulate signaling through β-cell M3Rs and, hopefully, are endowed with a more favorable pattern of expression. MIN6 cells almost exclusively express the M3R subtype, and treatment of these cells with muscarinic agonists leads to robust increases in intracellular calcium levels and GSIS [21].
As is the case with other GPCRs, agonist activation of the M3R triggers a number of cellular events, such as phosphorylation of the M3R by various kinases, which dampens M3R signaling [22]. In addition, the lifetime of the receptor-activated G proteins is greatly reduced by the action of RGS proteins (Regulators of G protein Signaling), which catalyze the hydrolysis of GTP that is bound to activated Gα subunits [23].
RGS proteins represent a rather large protein family consisting of more than 30 different members in mammals [23]. Real-time qRT-PCR studies demonstrated that RGS4 mRNA was the most abundant RGS transcript detectable in MIN6 cells [21]. RGS4 mRNA was also found to be highly expressed in mouse pancreatic islets [21]. Interestingly, siRNA-mediated knockdown of RGS4 expression in MIN6 cells led to significant increases in muscarinic agonist-induced elevations in intracellular calcium levels and GSIS [21]. In agreement with these observations, in vitro studies with pancreatic islets prepared from RGS4-deficient mice and control littermates showed that muscarinic agonist-induced enhancement of GSIS was significantly enhanced in islets lacking RGS4 [21]. Taken together, these findings clearly indicated that RGS4 represents a potent negative regulator of M3R function in pancreatic β-cells.
Interestingly, RGS4 deficiency had little or no effect on the ability of ADP, arginine vasopressin (AVP), and GLP-1 to enhance GSIS in MIN6 cells or mouse pancreatic islets [21]. ADP and AVP exert their stimulatory effects on insulin release via activation of β-cell Gq/11-coupled receptors (P2Y1/6 and V1b vasopressin receptors, respectively), whereas GLP-1 acts on the Gs-coupled GLP-1 receptor [2]. These findings suggest that RGS4 selectively interferes with M3R function in insulin-containing cells. Consistent with this observation, several studies have shown that certain RGS proteins can exert selective inhibitory actions on specific receptor/G protein combinations (for recent reviews, see refs. [24–26]). Indeed, several laboratories have demonstrated that RGS proteins can directly interact with specific GPCRs, most likely within the context of GPCR/RGS signaling complexes containing additional signaling or scaffolding proteins [24–26]. It is therefore likely that the ability of RGS4 to selectivity regulate M3R signaling in pancreatic β-cells may be due to the existence of M3R/RGS4 signaling complexes. In agreement with this notion, M3Rs can be co-immunoprecipitated in a complex with RGS4 in co-transfected mammalian cells [21].
Finally, to examine the potential physiological role of RGS4 in regulating β-cell function in vivo, Ruiz de Azua et al. [21] generated mutant mice that lacked RGS4 in pancreatic β-cells only (β-RGS4 KO mice). Under basal conditions, the β-RGS4 KO mutant mice did not show any obvious metabolic phenotype. However, WT and β-RGS4 KO mutant showed significantly different responses when challenged with bethanechol (2 μg/g, s.c.), a peripherally acting muscarinic agonist [21]. In WT control mice, bethanechol induced a transient increase in serum insulin levels, which was associated with a moderate decrease in blood glucose levels. These effects were absent in β-M3R KO mice, indicating that they were mediated by β-cell M3Rs. Strikingly, in β-RGS4 KO mice, bethanechol-induced insulin secretion remained very high throughout the entire 1 hr observation period, associated with more robust decreases in blood glucose levels (compared to bethanechol-injected WT littermates; [21]). It remains to be determined whether β-RGS4 KO mice also show enhanced insulin and glucose responses after oral administration of glucose.
Taken together, these observations indicate that RGS4 acts as a potent negative regulator of M3R-mediated insulin secretion in vitro and in vivo. Recent studies have shown that RGS4 also modulates other peripheral functions including heart rate [27], renal blood flow [28], secretion of catecholamines from adrenal glands [29], and breast cancer migration [30]. Most importantly, RGS4 is abundantly expressed in the brain [31], and various reports have linked changes in RGS4 expression to several major CNS disorders [32–34]. In order to reduce the incidence of unwanted central side effects, efforts to develop RGS4 inhibitors for the treatment of T2D should therefore focus on agents that are unable to penetrate the blood-brain barrier.
A novel chemical-genetic strategy to study G protein regulation of β-cell function in vivo
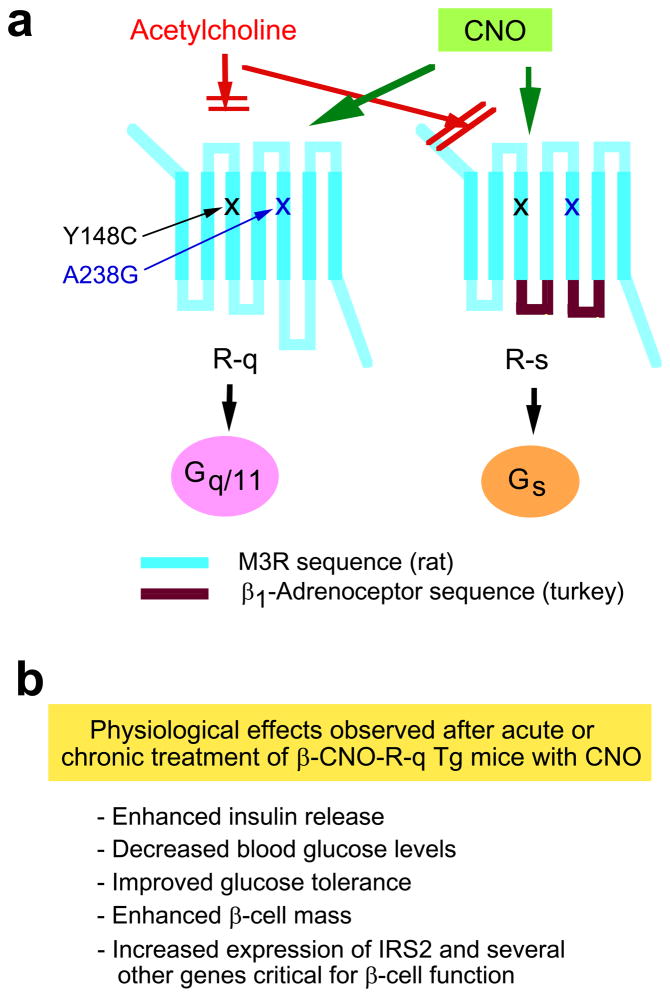
Several years ago, Armbruster et al. [35] reported that a mutant version of the human M3R containing the Y149C and A239G point mutations (referred to from now on as R-q [36]) lost the ability to bind the endogenous ligand, ACh. However, when incubated with clozapine-N-oxide (CNO), a pharmacologically inert metabolite of the antipsychotic drug, clozapine, this mutant receptor was able to efficiently and selectively activate G proteins of the Gq/11 family ([35]; Figure 4a).
Figure 4.
Studies with clozapine-N-oxide (CNO)-responsive mutant M3Rs endowed with distinct G protein-coupling properties. (a) Structure of the M3R-based R-q and R-s designer receptors that can be selectively activated by CNO. The Y148C and A238G point mutations (rat M3R sequence) prevent acetylcholine binding to the M3R [35, 36]. Note that the Y148C and A238G point mutations (rat M3R sequence) correspond to the Y149C and A239G substitutions in the human M3R [35]. Both designer receptors can be efficiently activated by CNO, a pharmacologically inert compound. CNO binding to R-q leads to the selective activation of G proteins of the Gq/11 family [35, 36]. On the other hand, binding of CNO to R-s results in the selective activation of Gs [36]. (b) Phenotypic features of transgenic mice that express the R-q designer receptor selectively in pancreatic β-cells (β-CNO-R-q Tg mice). This short summary lists the key phenotypes that were observed after acute or chronic treatment of β-CNO-R-q Tg mice with CNO [36].
On the basis of these findings, Guettier et al. [36] generated transgenic mice that selectively expressed the R-q receptor in pancreatic β-cells (β-CNO-R-q Tg mice). In these mutant mice, β-cell Gq/11 signaling can be initiated in vivo in a conditional, drug-dependent fashion by simply treating the β-CNO-R-q mutant animals with CNO. Acute CNO injection in freely fed β-CNO-R-q Tg mice led to dose-dependent increases in serum insulin levels, accompanied by dose-dependent decreases in blood glucose concentrations. Guettier et al. [36] also demonstrated that selective activation of β-cell Gq/11 signaling in β-CNO-R-q Tg mice caused a significant increase in both first- and second-phase insulin release. Since a reduction in first-phase insulin is usually indicative of β-cell dysfunction in the early stages of T2D[37, 38], the observed enhancement of first-phase insulin release following activation of β-cell Gq/11 signaling is of considerable clinical interest.
To examine the effect of prolonged activation of β-cell Gq/11 signaling on β-cell mass, Guettier et al. [36] treated β-CNO-R-q Tg mice and WT littermates for two weeks with CNO (1 mg/kg/day i.p.) or saline (controls) and then subjected islets prepared from these animals to morphological studies. This analysis demonstrated that prolonged activation of β-cell Gq/11 signaling resulted in a significant elevation in β-cell mass, associated with an increase in mean islet size and β-cell hypertrophy. Since T2D is characterized by a progressive reduction in β-cell mass[39], strategies aimed at promoting signaling through β-cell Gq/11 may prove beneficial in treating T2D by increasing β-cell mass.
Interestingly, CNO treatment of islets derived from β-CNO-R-q Tg mice led to enhanced expression of several genes critical for β-cell function including a robust increase in IRS-2 mRNA expression[36], probably due to Gq/11-mediated increases in intracellular Ca2+ levels [40]. Since IRS-2 plays a key role in maintaining β-cell function and mass[41, 42], it is likely that elevated β-cell IRS-2 expression is intimately involved in the observed Gq/11-dependent increases in β-cell mass.
To compare the in vivo effects of activating β-cell Gq/11 versus β-cell Gs signaling pathways, Guettier et al. [36] generated a CNO-sensitive mutant M3R which was no longer able to couple to Gq/11 but gained the ability to efficiently activate Gs (R-s; Figure 4a). The R-s receptor was then selectively expressed in β-cells of transgenic mice (β-CNO-R-s Tg mice) at levels similar to those of R-q in β-CNO-R-q Tg mice. Phenotypic analysis of the β-CNO-β-R-s Tg mice yielded results that were largely consistent with findings of previous studies, suggesting that activation of β-cell Gs improves β-cell function, including an increase in β-cell mass[3, 4]. Interestingly, β-CNO-R-q Tg mice showed generally more robust metabolic phenotypes than β-CNO-R-s Tg mice[36]. However, the proper interpretation of this finding was complicated by the fact that the R-s receptor showed some degree of agonist-independent signaling which may have triggered counter-regulatory responses in the β-CNO-R-s Tg mice.
Clearly, the novel chemical-genetic approach employed by Guettier et al. [36] yielded important new insights into the roles of Gq/11-dependent signaling pathways in regulating β-cell function in vivo (Figure 4b). Importantly, these findings strongly support the concept that agents that promote signaling through β-cell Gq/11 proteins have considerable therapeutic potential.
New mutant mouse models provide novel insights into β-cell M3R function
Studies with mutant mice selectively lacking Gαq and Gα11 in pancreatic β-cells
Recently, Sassmann et al. [43] generated mutant mice that lacked both Gαq and Gα11, the two key members of the Gq protein family, selectively in pancreatic β-cells (β-Gαq/Gα11 double KO mice). Muscarinic agonist-induced stimulation of PI hydrolysis and intracellular calcium mobilization was absent in isolated islets prepared from these mutant mice. Moreover, muscarinic agonist-mediated augmentation of GSIS was essentially abolished in Gαq/Gα11-deficient islets. These observations are in agreement with the notion that M3R-mediated biochemical and physiological effects on β-cell function require the activation of Gq/11-type G proteins (Figure 2).
Somewhat surprisingly, Sassmann et al. [43] also found that GSIS was reduced in Gαq/Gα11 double KO mice. Consistent with this observation, glucose-induced membrane depolarization was also impaired in β-cells lacking both Gαq and Gα11. Evidence suggested that these deficits were due to loss of β-cell–autonomous potentiation of insulin secretion through factors co-secreted with insulin, such as uridine diphosphate (UDP) and calcium ions. Specifically, UDP, via acting on β-cell P2Y6 receptors, and calcium, by stimulating β-cell calcium-sensing receptors, require the presence of Gq/11 to promote GSIS in an autocrine fashion. These findings further highlight the key role of Gq/11-mediated signaling pathways in maintaining proper β-cell function.
Critical role of ankyrin-B in modulating M3R activity in pancreatic β-cells
The magnitude of M3R-mediated enhancement of GSIS is critically dependent on proper β-cell IP3 receptor levels [44]. Ankyrin-B, an IP3 receptor-associated adaptor protein that is enriched in β-cells, plays a role in stabilizing β-cell IP3 receptors (Figure 2). Strikingly, muscarinic agonist-induced GSIS was significantly reduced in pancreatic islets prepared from heterozygous ankyrin-B mutant mice (ankB+/− mice) or in rat islets following acute knockdown of ankyrin-B expression [44]. Finally, an ankyrin-B point mutation (R1788W) failed to rescue muscarinic-agonist-induced augmentation of GSIS in ankyrin-B-deficient islets. This point mutation was found to be associated with T2D [44]. These findings indicate that β-cell IP3 receptor expression levels represent a critical factor determining the efficiency of M3R signaling in pancreatic β-cells.
PKD1 as a novel regulator of glucose- and M3R-induced insulin release
Carbachol, a muscarinic agonist, strongly stimulates the activity of protein kinase D1 (PKD1), a novel serine/threonine protein kinase, in pancreatic β-cells [45]. PKD1 activation occurs via binding to the membrane-bound second messenger, DAG [46]. Interestingly, deletion of PKD1 in INS1 insulinoma cells completely abolished glucose- and carbachol-induced insulin release, indicating that PKD1 is essential for insulin secretion from β-cells. Activated PKD1 is likely to promote membrane fission events required at different steps during insulin exocytosis [45] (Figure 2). Studies with mutant mice and cultured insulinoma cells also showed that the p38δ mitogen-activated protein kinase (MAPK) suppresses PKD1-mediated insulin secretion by an inhibitory phosphorylation of PKD1 [45]. Additional in vivo studies clearly support the novel concept that the p38δ-PKD1 pathway plays a critical role in modulating β-cell function including β-cell survival. Taken together, these new findings highlight that M3R-mediated facilitation of GSIS requires the coordinated activity and proper regulation of many different β-cell proteins (Figure 2).
Role of β-cell M3Rs in humans
Studies with human subjects have shown that the early phase of insulin release, occuring during the first few minutes after food ingestion – the so-called preabsorptive phase of insulin secretion, is triggered by increased activity of parasympathetic nerves innervating the endocrine pancreas, resulting in M3R-mediated insulin release [6, 7]. This early phase of insulin release is thought to play an important role in maintaining optimum glucose homeostasis (reviewed in [7]). Emerging evidence also suggests that the parasympathetic stimulation of pancreatic β-cells persists during the absorptive phase of insulin release [7], consistent with the metabolic phenotypes displayed by the β-cell-selective M3R mutant mice. So far, polymorphisms in the M3R gene that are associated with T2D or impaired glucose tolerance in humans have not been identified. However, Johnson et al. [47] recently demonstrated that olanzapine and clozapine, two atypical antipsychotic drugs, impair M3R-mediated augmentation of GSIS by blocking M3Rs with high affinity, suggesting the possibility that this effect contributes to the increased risk of hyperglycemia and T2D associated with the clinical use of these agents. Since the physiological role of β-cell M3Rs appears to be conserved among species, β-cell M3Rs and/or downstream signaling components represent attractive targets for human drug therapy.
Future research and concluding remarks
This review discusses the role of β-cell M3Rs and β-cell Gq/11-dependent signaling pathways in modulating β-cell function, including the facilitation of insulin secretion. Clearly, however, more detailed studies are needed to obtain a complete picture of the factors regulating the signaling cascades that mediate the beneficial effects of M3R (Gq/11) activation on β-cell function. Such knowledge may pave the way towards the development of new anti-diabetic drugs, with reduced side effects. An interesting new finding that emerged from the analysis of β-CNO-R-q Tg mice was that chronic activation of β-cell Gq/11 signaling leads to an increase in β-cell mass [36]. Thus, elucidation of the biochemical pathways involved in mediating this response also represents an important avenue for future research.
Recently, several GPCR-mediated physiological effects have been described that do not seem to require G protein activation but are mediated by different isoforms of β-arrestin (arrestin 2 and 3) which bind with high affinity to phosphorylated GPCRs [48]. Members of the arrestin protein family were originally identified as mediators of GPCR desensitization. However, arrestin 2 and 3 are now recognized as adaptor proteins that can initiate various intracellular signaling cascades independent of G protein activation [48]. In fact, a recent study using M3R knockin mice expressing a phosphorylation-deficient version of the M3R strongly suggests that M3R-mediated learning and memory depends, at least partially, on M3R phosphorylation/arrestin signaling [49]. It remains to be explored whether β-cell M3Rs or other β-cell GPCRs also use arrestin-dependent signaling networks to modulate β-cell function.
From a therapeutic point of view, the most important goal is the development of novel pharmacological agents that can target β-cell M3Rs or downstream signaling proteins with high potency and selectivity. The most direct approach would involve the development of peripherally acting, selective M3R agonists (note that such agents are not available at present). However, since M3Rs are also involved in mediating several other functions of the peripheral parasympathetic nervous system [19,20], the clinical use of selective M3R agonists may be limited by peripheral side effects. One may speculate that such side effects could be avoided or ameliorated by the judicious use of so-called ‘allosteric enhancers’ of M3R function which increase the affinity and/or efficacy of ACh at M3Rs without directly activating these receptors (note that such compounds remain to be identified). It should be noted in this context that positive allosteric enhancers have been developed recently for other (non-M3R) muscarinic receptor subtypes and that the potential clinical usefulness of such agents has been demonstrated [18].
Besides M3Rs, pancreatic β-cells express many other Gq/11-coupled receptors – several of which are known to stimulate insulin secretion [2]. These receptors include, for example, receptors for fatty acids (e.g. GPR40), cholecystokinin (CCKA), arginine vasopressin (V1b), or extracellular nucleotides (P2Y1, P2Y6). In most cases, it remains unclear to which extent activation of these receptors contributes to the maintenance of proper insulin release and glucose homeostasis in vivo. The development of new mutant mouse models will be necessary to shed light on this question. Given the beneficial metabolic effects mediated by activation of β-cell M3Rs and β-cell Gq/11-dependent signaling pathways, it is likely that other Gq/11-coupled GPCRs will emerge as important new targets for the treatment of T2D.
Acknowledgments
The authors’ own research covered in this review was supported by the Intramural Research Program of the NIH, NIDDK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kahn SE. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of Type 2 diabetes. Diabetologia. 2003;46:3–19. doi: 10.1007/s00125-002-1009-0. [DOI] [PubMed] [Google Scholar]
- 2.Ahrén B. Islet G protein-coupled receptors as potential targets for treatment of type 2 diabetes. Nat Rev Drug Discov. 2009;8:369–385. doi: 10.1038/nrd2782. [DOI] [PubMed] [Google Scholar]
- 3.Doyle ME, Egan JM. Mechanisms of action of glucagon-like peptide 1 in the pancreas. Pharmacol Ther. 2007;113:546–593. doi: 10.1016/j.pharmthera.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 5.Regard JB, et al. Probing cell type-specific functions of Gi in vivo identifies GPCR regulators of insulin secretion. J Clin Invest. 2007;117:4034–4043. doi: 10.1172/JCI32994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahrén B. Autonomic regulation of islet hormone secretion--implications for health and disease. Diabetologia. 2000;43:393–410. doi: 10.1007/s001250051322. [DOI] [PubMed] [Google Scholar]
- 7.Gilon P, Henquin JC. Mechanisms and physiological significance of the cholinergic control of pancreatic β-cell function. Endocr Rev. 2001;22:565–604. doi: 10.1210/edrv.22.5.0440. [DOI] [PubMed] [Google Scholar]
- 8.Duttaroy A, et al. Muscarinic stimulation of pancreatic insulin and glucagon release is abolished in M3 muscarinic acetylcholine receptor-deficient mice. Diabetes. 2004;53:1714–1720. doi: 10.2337/diabetes.53.7.1714. [DOI] [PubMed] [Google Scholar]
- 9.Zawalich WS, et al. Effects of muscarinic receptor type 3 knockout on mouse islet secretory responses. Biochem Biophys Res Commun. 2004;315:872–876. doi: 10.1016/j.bbrc.2004.01.139. [DOI] [PubMed] [Google Scholar]
- 10.Henquin JC, Nenquin M. The muscarinic receptor subtype in mouse pancreatic B-cells. FEBS Lett. 1988;236:89–92. doi: 10.1016/0014-5793(88)80290-4. [DOI] [PubMed] [Google Scholar]
- 11.Wess J. Molecular biology of muscarinic acetylcholine receptors. Crit Rev Neurobiol. 1996;10:69–99. doi: 10.1615/critrevneurobiol.v10.i1.40. [DOI] [PubMed] [Google Scholar]
- 12.Gautam D, et al. A critical role for β cell M3 muscarinic acetylcholine receptors in regulating insulin release and blood glucose homeostasis in vivo. Cell Metab. 2006;3:449–461. doi: 10.1016/j.cmet.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 13.Rolland JF, et al. G protein-independent activation of an inward Na+ current by muscarinic receptors in mouse pancreatic β-cells. J Biol Chem. 2002;277:38373–38380. doi: 10.1074/jbc.M203888200. [DOI] [PubMed] [Google Scholar]
- 14.Swayne LA, et al. The NALCN ion channel is activated by M3 muscarinic receptors in a pancreatic β-cell line. EMBO Rep. 2009;10:873–880. doi: 10.1038/embor.2009.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gautam D, et al. Beneficial metabolic effects caused by persistent activation of β-cell M3 muscarinic acetylcholine receptors in transgenic mice. Endocrinology. 2010 doi: 10.1210/en.2010-0519. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmidt C, et al. Random mutagenesis of the M3 muscarinic acetylcholine receptor expressed in yeast. Identification of point mutations that “silence” a constitutively active mutant M3 receptor and greatly impair receptor/G protein coupling. J Biol Chem. 2003;278:30248–30260. doi: 10.1074/jbc.M304991200. [DOI] [PubMed] [Google Scholar]
- 17.Zawalich WS, Zawalich KC. Species differences in the induction of time-dependent potentiation of insulin secretion. Endocrinology. 1996;137:1664–1669. doi: 10.1210/endo.137.5.8612499. [DOI] [PubMed] [Google Scholar]
- 18.Conn PJ, et al. Subtype-selective allosteric modulators of muscarinic receptors for the treatment of CNS disorders. Trends Pharmacol Sci. 2009;30:148–155. doi: 10.1016/j.tips.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caulfield MP, Birdsall NJM. International Union of Pharmacology. XVII Classification of muscarinic acetylcholine receptors. Pharmacol Rev. 1998;50:279–290. [PubMed] [Google Scholar]
- 20.Wess J, et al. Muscarinic acetylcholine receptors: mutant mice provide new insights for drug development. Nat Rev Drug Discov. 2007;6:721–733. doi: 10.1038/nrd2379. [DOI] [PubMed] [Google Scholar]
- 21.Ruiz de Azua I, et al. RGS4 is a negative regulator of insulin release from pancreatic β-cells in vitro and in vivo. Proc Natl Acad Sci USA. 2010;107:7999–8004. doi: 10.1073/pnas.1003655107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Koppen CJ, Kaiser B. Regulation of muscarinic acetylcholine receptor signaling. Pharmacol Ther. 2003;98:197–220. doi: 10.1016/s0163-7258(03)00032-9. [DOI] [PubMed] [Google Scholar]
- 23.Ross EM, Wilkie TM. GTPase-activating proteins for heterotrimeric G proteins: regulators of G protein signaling (RGS) and RGS-like proteins. Annu Rev Biochem. 2000;69:795–827. doi: 10.1146/annurev.biochem.69.1.795. [DOI] [PubMed] [Google Scholar]
- 24.Abramow-Newerly M, et al. RGS proteins have a signalling complex: interactions between RGS proteins and GPCRs, effectors, and auxiliary proteins. Cell Signal. 2006;18:579–591. doi: 10.1016/j.cellsig.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 25.Neitzel KL, Hepler JR. Cellular mechanisms that determine selective RGS protein regulation of G protein-coupled receptor signaling. Semin Cell Dev Biol. 2006;17:383–389. doi: 10.1016/j.semcdb.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 26.Bansal G, et al. R4 RGS proteins: regulation of G-protein signaling and beyond. Pharmacol Ther. 2007;116:473–495. doi: 10.1016/j.pharmthera.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cifelli C, et al. RGS4 regulates parasympathetic signaling and heart rate control in the sinoatrial node. Circ Res. 2008;103:527–535. doi: 10.1161/CIRCRESAHA.108.180984. [DOI] [PubMed] [Google Scholar]
- 28.Siedlecki A, et al. RGS4 controls renal blood flow and inhibits cyclosporine-mediated nephrotoxicity. Am J Transplant. 2010;10:231–241. doi: 10.1111/j.1600-6143.2009.02930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iankova I, et al. Regulator of G protein signaling-4 controls fatty acid and glucose homeostasis. Endocrinology. 2008;149:5706–5712. doi: 10.1210/en.2008-0717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xie Y, et al. Breast cancer migration and invasion depend on proteasome degradation of regulator of G-protein signaling 4. Cancer Res. 2009;69:5743–5751. doi: 10.1158/0008-5472.CAN-08-3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Erdely HA, et al. Regional expression of RGS4 mRNA in human brain. Eur J Neurosci. 2004;19:3125–3128. doi: 10.1111/j.0953-816X.2004.03364.x. [DOI] [PubMed] [Google Scholar]
- 32.Ding J, et al. RGS4-dependent attenuation of M4 autoreceptor function in striatal cholinergic interneurons following dopamine depletion. Nat Neurosci. 2006;9:832–842. doi: 10.1038/nn1700. [DOI] [PubMed] [Google Scholar]
- 33.Levitt P, et al. Making the case for a candidate vulnerability gene in schizophrenia: Convergent evidence for regulator of G-protein signaling 4 (RGS4) Biol Psychiat. 2006;60:534–537. doi: 10.1016/j.biopsych.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 34.Hooks SB, et al. A role of RGS proteins in drug addiction. Biochem Pharmacol. 2008;75:76–84. doi: 10.1016/j.bcp.2007.07.045. [DOI] [PubMed] [Google Scholar]
- 35.Armbruster BN, et al. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci USA. 2007;104:5163–5168. doi: 10.1073/pnas.0700293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guettier JM, et al. A chemical-genetic approach to study G protein regulation of β cell function in vivo. Proc Natl Acad Sci USA. 2009;106:19197–19202. doi: 10.1073/pnas.0906593106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Del Prato S, et al. Phasic insulin release and metabolic regulation in type 2 diabetes. Diabetes. 2002;51 (Suppl 1):S109–S116. doi: 10.2337/diabetes.51.2007.s109. [DOI] [PubMed] [Google Scholar]
- 38.Nesher R, Cerasi E. Modeling phasic insulin release: immediate and time-dependent effects of glucose. Diabetes. 2002;51 (Suppl 1):S53–S59. doi: 10.2337/diabetes.51.2007.s53. [DOI] [PubMed] [Google Scholar]
- 39.De Koning EJ, et al. Preservation of β-cell function by targeting β-cell mass. Trends Pharmacol Sci. 2008;29:218–227. doi: 10.1016/j.tips.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 40.Lingohr MK, et al. Specific regulation of IRS-2 expression by glucose in rat primary pancreatic islet β-cells. J Biol Chem. 2006;281:15884–15892. doi: 10.1074/jbc.M600356200. [DOI] [PubMed] [Google Scholar]
- 41.Niessen M. On the role of IRS2 in the regulation of functional β-cell mass. Arch Physiol Biochem. 2006;112:65–73. doi: 10.1080/13813450600711409. [DOI] [PubMed] [Google Scholar]
- 42.White MF. Regulating insulin signaling and β-cell function through IRS proteins. Can J Physiol Pharmacol. 2006;84:725–737. doi: 10.1139/y06-008. [DOI] [PubMed] [Google Scholar]
- 43.Sassmann A, et al. The Gq/G11-mediated signaling pathway is critical for autocrine potentiation of insulin secretion in mice. J Clin Invest. 2010;120:2184–2193. doi: 10.1172/JCI41541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Healy JA, et al. Cholinergic augmentation of insulin release requires ankyrin-B. Sci Signal. 2010;3:ra19. doi: 10.1126/scisignal.2000771. [DOI] [PubMed] [Google Scholar]
- 45.Sumara G, et al. Regulation of PKD by the MAPK p38δ in insulin secretion and glucose homeostasis. Cell. 2009;136:235–248. doi: 10.1016/j.cell.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oancea E, et al. Mechanism of persistent protein kinase D1 translocation and activation. Dev Cell. 2003;4:561–574. doi: 10.1016/s1534-5807(03)00087-x. [DOI] [PubMed] [Google Scholar]
- 47.Johnson DE, et al. Inhibitory effects of antipsychotics on carbachol-enhanced insulin secretion from perifused rat islets: role of muscarinic antagonism in antipsychotic-induced diabetes and hyperglycemia. Diabetes. 2005;54:1552–1558. doi: 10.2337/diabetes.54.5.1552. [DOI] [PubMed] [Google Scholar]
- 48.Rajagopal S, et al. Teaching old receptors new tricks: biasing seven-transmembrane receptors. Nat Rev Drug Discov. 2010;9:373–386. doi: 10.1038/nrd3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Poulin B, et al. The M3-muscarinic receptor regulates learning and memory in a receptor phosphorylation/arrestin-dependent manner. Proc Natl Acad Sci USA. 2010;107:9440–9445. doi: 10.1073/pnas.0914801107. [DOI] [PMC free article] [PubMed] [Google Scholar]