Abstract
The factors that influence Leydig cell activity currently include peptides such as neuropeptide Y (NPY). In this work we investigated the ability of this compound, injected directly into the testes of adult male rats, to alter testosterone (T) release into the general circulation. At a 5 μg/kg dose administered 1 h prior to challenge with human chorionic gonadotropin (hCG, 1.0 U/kg, iv), NPY significantly (P<0.01) blunted the T response to this gonadotropin. The inhibitory effect of NPY was observed in animals pretreated with an antagonist to gonadotropin-releasing hormone or not, indicating that the decrease in plasma T found was most likely independent of pituitary luteinizing hormone. However, testicular levels of steroidogenic acute regulatory (STAR) protein or translocator protein (TSPO) in the Leydig cells did not exhibit consistent changes, which suggested that other mechanisms mediated the blunted T response to hCG. We therefore used autoradiography and immunohistochemistry methodologies to identify NPY receptors in the testes, and found them primarily located on blood vessels. Competition studies further identified these receptors as being Y1, a subtype previously reported to modulate the vasoconstrictor effect of NPY. The absence of significant changes in STAR and TSPO levels, as well as the absence of Y1 receptors on Leydig cells, suggest that NPY-induced decreases in T release is unlikely to represent a direct effect of NPY on these cells. Rather, the very high expression levels of Y1 found in testicular vessels supports the concept that NPY may alter gonadal activity, at least in part, through local vascular impairment of gonadotropin delivery to, and/or blunted T secretion from, Leydig cells.
Keywords: Neuropeptide Y, Y1 receptor subtype, Leydig cells, rat, testosterone
INTRODUCTION
The production of testosterone (T) by mammalian testes is under the regulation of complex factors that can be initiated both in the brain or within the male gonads themselves. In the brain, the hypothalamic peptide gonadotropin-releasing hormone (GnRH) stimulates luteinizing hormone (LH) production by the pituitary, which then acts on Leydig cells (Breen and Karsch, 2006; Kalra and Kalra, 1983; McCann et al., 1993). Within the gonads, an array of factors that include amines, prostaglandins, opiates and peptides {ref. in (Rivier, 2008; Saez, 1994)} act locally to regulate the activity of the compounds essential for androgen synthesis, such as the steroidogenic acute regulatory (STAR) protein, the translocator protein (TSPO) previously known as the peripheral-type benzodiazepine receptor and the cytochrome P450 side-chain cleavage enzyme (P450scc) (Bose et al., 2002; Papadopoulos, 1993; Payne and Hales, 2004; Stocco and Clark, 1996).
Recently, neuropeptide Y (NPY) has been added to the list of secretagogues that influence reproductive functions (Pedrazzini et al., 2003). NPY was originally identified in the mammalian brain (Tatemoto et al., 1982) where it is one of the most abundant peptides, and exerts many influences upon endocrine functions (Pedrazzini et al., 2003). For example, it is involved in the seasonal regulation of GnRH and subsequent LH release in male sheep, and does so in a T-dependent manner during a long daylight photoperiod (Dobbins et al., 2004). The ability of this peptide to lower plasma LH levels (Pierroz et al., 1999) was recently corroborated by the finding that it also inhibits the neuronal activity of explanted GnRH neurons (Klenke et al., 2010). In addition, the intracerebroventricular (icv) injection of NPY in mice inhibits the gonadotropic axis by decreasing T secretion (Wahlestedt and Reis, 1993). This is due to either the occurrence of nonfunctional single LH peaks or the absence of LH peak “clusters” (Pierroz et al., 1999). More recently NPY was also reported to inhibit the neuronal activity of explanted GnRH neurons (Klenke et al., 2010). In order to understand how NPY influences steroid hormone release, experiments have focused on the location and direct action sites of this peptide in the testes. Both NPY mRNA levels (Kanzaki et al., 1996) and immunoreactivity (Wang et al., 2004) have been reported in rat Leydig cells, as well as in nerve fibers around the testicular tubules and vessels. Interestingly, NPY gene expression increases with testicular development (Terado et al., 2006). While collectively these results suggested a potential influence of NPY on T production, to our knowledge this has not been reported.
NPY binds to several receptors characterized as Y1, Y2, Y4 and Y5 (Berglund et al., 2003; Larhammar, 1996; Lin et al., 2004; Pedrazzini, 2004; Pedrazzini et al., 2003). A study that examined the regulation of NPY Y1 receptors by testosterone found high levels of their immunoreactivity (ir) and mRNA in the smooth muscle cells of the rat testis (Kopp et al., 1997). As these receptors promote vasoconstriction (Collin et al., 1998; Kopp et al., 2008), it is possible that the potential influence exerted by NPY on Leydig cell activity might be due at least in part to decreased delivery of blood-borne secretagogues to these cells, and/or altered T release into the general circulation. The questions we therefore aimed to ask were: (a) does NPY act within the testes to alter T secretion; and (b) if so, does this involve the inhibition of the proteins that control cholesterol delivery into mitochondria, a rate-limiting step in steroidogenesis. Answering these two questions was accomplished by first examining the direct effects of intra-testicular (itt) injection of NPY on the T response to human chorionic gonadotropin (hCG) administration, a model that we have extensively validated for the investigation of testicular steroidogenic function (Ogilvie and Rivier, 1998; Selvage and Rivier, 2003; Turnbull and Rivier, 1997b). We then measured testicular levels of STAR and TSPO following itt NPY administration according to protocols that we had used in other models (Herman et al., 2006; Herman and Rivier, 2006; Ogilvie et al., 1999). These two proteins were chosen because they modulate essential steps in steroidogenesis (Bauer et al., 2000; Lin et al., 1995; Papadopoulos, 1993; Payne and Hales, 2004). Finally, using a morphological binding assay, in vitro NPY receptor autoradiography, we located the different NPY receptors within the testis to determine where NPY might bind following itt injection.
Animals
Sixty-five to 70 day old adult male Sprague-Dawley or Wistar rats were housed under controlled lighting conditions (lights on at 0600 h and lights off at 1800 h), with water and rat chow provided ad libitum. All protocols were approved by the Salk Institute and Bern University Institutional Animal Care and Use Committees (IACUCs).
Protocols and Surgeries
• For the experiments illustrated in Fig. 1, the animals were implanted with permanent intravenous (iv) cannulae under isoflurane anesthesia (Selvage and Rivier, 2003). After surgery, the animals were individually housed and allowed to recover for 2 d before experimentation. On the experimental day, the animals were placed individually in opaque buckets in a soundproof room no later than 0700 h. At this time, the iv cannulae were connected to polyethylene tubing and a syringe filled with heparinized saline. The freely-moving animals remained in the buckets for 3 h until treatment administration in both experiments. In a first series of experiments, NPY (5 μg/kg) or its vehicle (1% BSA and 0.1% ascorbic acid in sterile PBS) was administered itt under isoflurane anesthesia 1 h before an hCG challenge (1 IU/kg, iv) in order to investigate the ability of this peptide to alter the T response to hCG. Blood (jugular) samples were collected from all animals immediately before as well as 15, 30, 60 and 90 min after gonadotropin administration to measure plasma T concentrations. The second series of experiments consisted of four groups of animals, and was designed to determine whether the decreased T response to hCG was modulated by altered LH release. To block LH secretion and therefore eliminate its influence, the GnRH antagonist Azaline B (40 g/kg) was administered iv to 2 groups of rats (A and B), while the other 2 groups (C and D) received the vehicle. We have extensively validated this model in our laboratory {see (Rivier, 2008; Selvage et al., 2004; Turnbull and Rivier, 1997a)}. One h later, under isoflurane anesthesia NPY was administered itt to groups B and D, and its vehicle was given itt to groups A and C. The hCG challenge (1 IU/kg, iv) was administered to all animals one h later (i.e., 2 h after Azaline B). Blood samples were collected from all four groups immediately before as well as 15, 30, 60 and 90 min after hCG injection to measure plasma T levels.
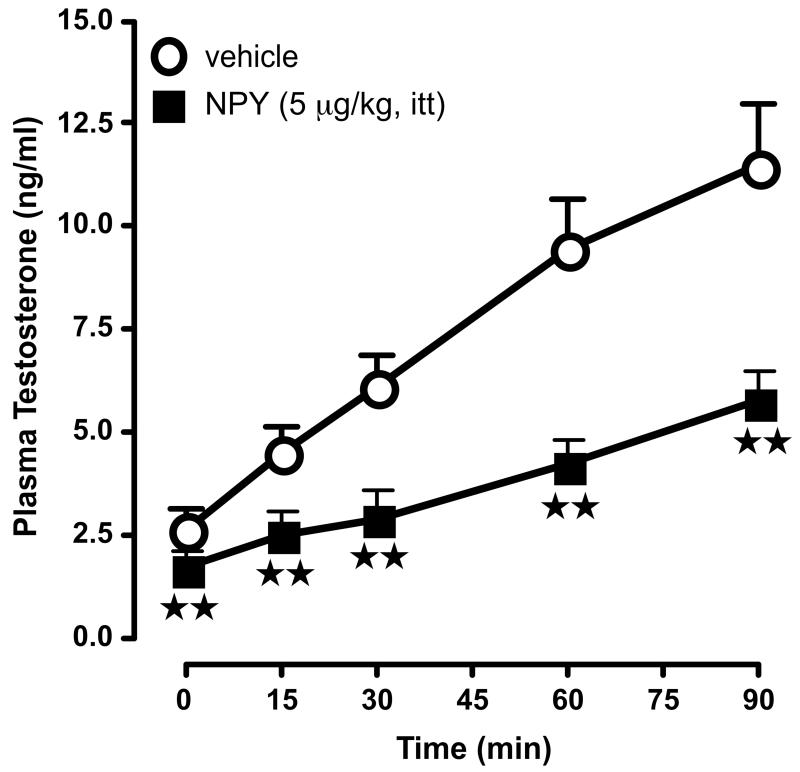
Figure 1.
Compared to vehicle administration, the intratesticular (itt) injection of NPY (5 μg/kg) lowers basal T levels and interferes with Leydig cells response to hCG administration (1.0 U/kg, iv, - 1 h), as measured by changes in plasma T values. N = 5-7/point.  , P<0.01 vs. vehicle.
, P<0.01 vs. vehicle.
• For the experiment focused on measurement of testicular STAR and TSPO by Western analysis, two groups of animals were used. After 1 to 3 h in opaque buckets in a soundproof room, all animals were anesthetized with isoflurane prior to the itt injection of 5 μg/kg NPY (n = 8) or its vehicle (n = 8). Using a Hamilton syringe, 2 injections (25 μl each) were made in 2 different sites of each testis (Rivier, 2008) for a total of 100 μL per rat. Preliminary studies conducted with Evans Blue had indicated that this protocol was consistent with wide spread disbursement of the injection volumes. The animals recovered from anesthesia, were placed back into their buckets and decapitated under minimal stress 1 h later for testes collection and subsequent Leydig cell isolation.
Reagents
Dr. Jean Rivier at the Salk Institute (La Jolla, CA) generously provided the GnRH antagonist, Azaline B, and NPY peptide that were synthesized by solid-phase methodology (Kornreich et al., 1992; Rivier et al., 1988). Azaline B and NPY were reconstituted in sterile 0.04 M phosphate-buffered saline (PBS, pH 7.4) that contained 0.1% bovine serum albumin (BSA; Sigma-Aldrich, St. Louis, MO) and 0.01% ascorbic acid. HCG (Sigma-Aldrich, St. Louis, MO) was prepared in PBS. The rat anti-STAR and rat anti-TSPO antiserum used for Western Blot analysis were generous gifts from Dr. D. B. Hales (Southern Illinois University School of Medicine at Carbondale, IL) and Dr. V. Papadopoulos (McGill University Health Center, Montréal, Quebec), respectively. The antiserum were generated as previously described (Hales and Payne, 1989; Papadopoulos, 1993) and used according to the parameters suggested by the related investigators. For Leydig cell isolation, Medium 199 with Hanks Salts was purchased through Invitrogen (San Diego, CA), collagenase was obtained from Worthington Biochemical Corp. (Type 4, Lakewood, NJ), and Percoll was purchased from GE Healthcare (Piscataway, NJ).
Leydig Cell Isolation
The Leydig cell isolation process has been previously described by Herman and Rivier (2006). Briefly, the testes from rapidly decapitated animals were quickly removed, decapsulated and cleared of large blood vessels. The samples were dispersed in M199 that contained 0.1% w/v collagenase and 1.25% w/v BSA (81003, Fraction V, MP Biomedicals, Solon, OH). They were then washed in Medium 199 with BSA and penicillin/streptomycin (GIBCO, Invitrogen, San Diego, CA), suspended in an isotonic Percoll gradient and centrifuged at 4°C for 45 min at 6,500 x g. Isolated Leydig cells were then removed from the gradient, washed in heparin dissociation buffer, pelleted and re-suspended in freezing media (Medium 199, BSA, penicillin/streptomycin and glycerol) and frozen until Western blot analysis.
Western Blot Analysis
Frozen Leydig cell samples were defrosted on ice, pelleted, washed with HDB and then repelleted. Pelleted Leydig cells were suspended in 150 μL of RIPA lysis and extraction buffer (Pierce, Rockford, IL) with Halt protease inhibitor cocktail (Pierce, Rockford, IL). The samples were then mixed, briefly sonicated and centrifuged at 3,000 x g for 10 min. The Bradford assay (Bio-Rad, Hercules, CA) was used to determine total protein concentrations for each sample. Equal amounts (25 μg) of protein per sample were loaded onto 10% Bis-Tris Midi Gels (Invitrogen, San Diego, CA) followed by transfer onto a nitrocellulose membrane with a 0.2 μm pore size (Bio-Rad, Hercules, CA). The blot was probed separately with 1:1,500 rabbit anti-STAR and 1:1,000 rabbit anti-TSPO. Mouse anti-β-Actin (Sigma, St. Louis, MO) was used to control for total protein and showed linear increases over a 10 to 75 μg range (R = 0.93). Horseradish peroxidase conjugated secondary antibodies, goat anti-rabbit and goat anti-mouse IgG, were used at 1:30,000 and 1:20,000 for STAR and TSPO, respectively, and at 1:5,000 for β-Actin. Supersignal West Pico enhanced chemiluminescent (Pierce, Rockford, IL) was used for Western blot detection for all antibodies, but 5% Femto was added for STAR and TSPO development and ImageJ (NIH, Bethesda, MD) was used to quantify the signal. The results were expressed as a percentage of control using β-Actin as an internal standard. As STAR is rapidly processed in the mitochondria, the active 37-kDA form of STAR cannot be measured using the current procedures used for Leydig cell isolation and, subsequently, the “mature” 30-kDa form of STAR can be reliably measured and used as an index of changes in the synthesis of STAR (Granot et al., 2003; Granot et al., 2002).
Testosterone Assay
After collection, the blood samples were centrifuged at 4°C and 3000 x g for 10 min and plasma was frozen (−20°C) and stored until assay. T levels were measured with a commercial kit from Diagnostics Products Corporation (Catalog Number TKTT1, Los Angeles, CA). The detection limit of the assay ranged from 0 to 16 ng/mL with the lowest detection level being 0.2 ng/mL. The intra- and inter-assay coefficients of variation were 7.3 and 13.2%, respectively.
NPY receptors localization
• Autoradiography and competition studies
NPY receptor expression was assessed with in vitro NPY receptor autoradiography, as previously described (Reubi et al., 2001). Briefly, cryostat sections were incubated with 10,000 cpm/100 μl 125I-PYY (2,000 Ci/mmol; Anawa, Wangen, Switzerland) either alone or in competition with 25 nM cold PYY to assess non-specific binding. To differentiate the NPY receptor subtypes, 125I-PYY was incubated in competition with increasing concentrations of NPY receptor subtype-selective analogs, including the Y1-selective agonist [Leu31,Pro34]-PYY (Bachem, Bubendorf, Switzerland), the Y1-selective antagonist Bis(31/31′)[Pro30,Cys31,Tyr32,Leu34]NPY(28-36)-NH2 (Balasubramaniam et al., 2001), the Y2-selective antagonist BIIE 0246 (Boehringer Ingelheim, Biberach, Germany), the Y4 preferring pancreatic polypeptide (PP; Bachem), and the Y5-selective ligand [cPP(1-17),NPY(19-23),Ala31, Aib32, Pro34]hPP (Cabrele et al., 2002). In all experiments, rat brain was included as positive control. After incubation, the slides were exposed to radiosensitive films. The signals on the films were analyzed in correlation with morphology using corresponding tissue sections stained either with H&E or immunohistochemically with an anti-Factor VIII antibody highlighting the blood vessels or with an anti-STAR antibody staining Leydig cells. The signal densities were measured with a computer-assisted imaging system (Interfocus, Mering, Germany).
• Immunohistochemistry
Immunohistochemistry for Factor VIII and STAR was carried out as follows: Ten micrometer thick cryostat sections were fixed in acetone and postfixed in 4% formalin. The primary antibody was either a polyclonal anti-Factor VIII antibody (1:600; DAKO, Glostrup, Denmark) or a polyclonal anti-STAR antibody (1:1000). The secondary antibody was a biotynilated goat anti-rabbit immunoglobulin. Antibody binding was visualized using the Vectastain ABC Kit (Vector Laboratories, Burlingame, CA). Staining was carried out with 3,3′-diaminobenzidine and counterstaining with hemalum.
Statistical analysis
Data were expressed as the mean ± SEM and were analyzed using one- or two-way ANOVA followed by the least square means post hoc test, and/or by repeated measures design. Differences were considered significant from P ≤ 0.05.
Effect of itt-injected NPY on T release
Preliminary experiments were carried out to determine the dose of NPY that would be used for all subsequent studies, as well as the optimum timing of its injection. Cumulative T release measured 15, 30, 60 and 90 min after hCG injection were: control (no NPY), 31.5 ± 4.68 ng/ml; 1 μg NPY/kg, 22.69 ± 3.15 ng/ml; 2 μg NPY/kg, 15.3 ± 2.3 ng/ml; 5 μg NPY/kg = 8.8 ± 1.8 ng/ml; 10 μg NPY/kg = 8.5 ± 1.7 ng/ml. On the basis of the results, a 5 μg/kg total dose administered 1 h prior to hCG was chosen as inducing a consistent inhibition of T release. As illustrated in Fig. 1, under this protocol, NPY significantly (P<0.01) decreased basal plasma T levels, compared to vehicle-treated rats. It also markedly (P<0.01) blunted the T response to hCG at all times studied. As NPY has been reported to alter plasma LH levels (Pierroz et al., 1999), and because itt-administered compounds can reach the general circulation {discussion in (Rivier, 2008)}, it was important to assess the potential role of NPY in mediating the changes in T release found. However, measuring LH levels does not represent a valid approach because these levels are very low in adult male rats and fall in an unreliable part of the RIA curve, thereby rendering interpretation of the data difficult. We therefore used a different approach, i.e., we used rats pretreated with the GnRH antagonist Azaline B {40 mg/kg, iv; (Rivier, 1999; Rivier, 2008; Turnbull and Rivier, 1997b)} to ensure that the influence exerted by itt-injected NPY was not due to its effect on the pituitary. As indicated in Table 1, this effect was also observed under these conditions, which supports the hypothesis that it is independent of altered LH levels.
Table 1.
The intratesticular (itt) administration of NPY (5 μg/ kg) inhibited plasma testosterone secretion induced by a hCG challenge (1 U/ kg, iv) following pre-treatment with or without a GnRH antagonist (Azaline B, 40 mg/ kg) in comparison with animals that were administered the vehicle for NPY
| Pre-treatment | itt treatment | ng T/ ml* |
|---|---|---|
| vehicle | vehicle | 22.20 ± 3.25 |
| vehicle | NPY | 10.60 ± 0.98Ra |
| GnRH antagonist | vehicle | 23.15 ± 3.11b |
| GnRH antagonist | NPY | 11.59 ± 1.70a,b |
Results are presented as means ± SEM, N = 5-7, and represent cumulative T values measured 15, 30, 60 and 90 min after hCG injection.
P<0.01 vs. itt vehicle
P>0.05 vs. corresponding vehicle pre-treatment.
Effect of itt-injected NPY on testicular STAR and TSPO levels
We ran four different experiments in which the vehicle or NPY (5 μg/kg) was injected itt 1 h prior to rapid decapitation of the animals, followed by Leydig cell collection and processing for Western analysis. This approach was chosen for two reasons. First, measurement of mRNA levels is not warranted because we wanted to focus on changes in testicular STAR and TSPO levels that corresponded to the time frame of the altered T response to hCG. Second, detection of changes in STAR and TSPO by immunohistochemistry would not have been feasible because in order to be quantitative, data would have to be collected in every Leydig cell (i.e., through the entire gonad), as the ability of all cells to display comparable changes in protein levels has not been established. Results obtained with Western blot analysis were both variable and inconsistent, and while STAR and TSPO levels decreased in some studies, they did not in others (Mean range ± SEM: STAR/Actin, % control = 74.1 ± 13.2 - 110.0 ± 7.5; TSPO/Actin, % control = 81.7 ± 13.8 - 122.4 ± 15.1; all P>0.05).
Localization of testicular NPY receptors
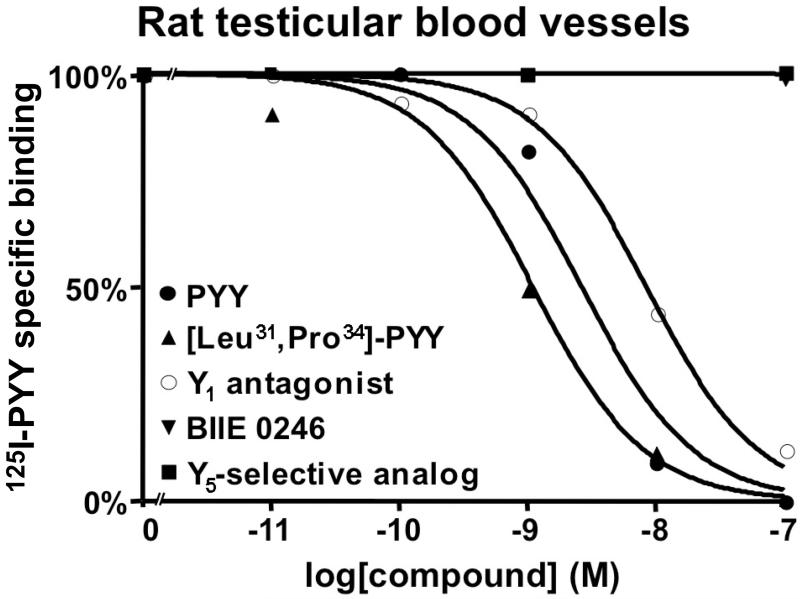
NPY receptors were expressed extensively and at very high density in Factor VIII positive testicular blood vessels (Table 2, Fig. 2). These vascular NPY receptors were localized mainly in the smooth muscle layer, and no NPY receptor expression was discernable in STAR-positive testicular Leydig cells or in the seminiferous tubules. Competition binding experiments demonstrated that the vascular NPY receptors corresponded mainly to the Y1 subtype, as 125I-PYY was displaced by the Y1-selective ligands with high affinity, but with only low affinity or not at all by Y2-, Y4- and Y5-preferring ligands (Fig. 3).
Table 2.
Expression of the NPY receptor subtype Y1, but not all other subtypes, is found at high density in the testicular blood vessels of the rat testis but not in the Leydig cells or seminiferous tubules
| Case | Blood vessels | Leydig cells |
Seminiferous tubules |
|
|---|---|---|---|---|
| NPY receptors subtype |
NPY receptor density | |||
| 1 | Y1 | 4717 dpm/ mg tissue | - | - |
| 2 | Y1 | 3654 dpm/ mg tissue | - | - |
| 3 | Y1 | 3737 dpm/ mg tissue | - | - |
| 4 | Y1 | 4265 dpm/ mg tissue | - | - |
| 5 | Y1 | 4438 dpm/ mg tissue | - | - |
no NPY receptors detected
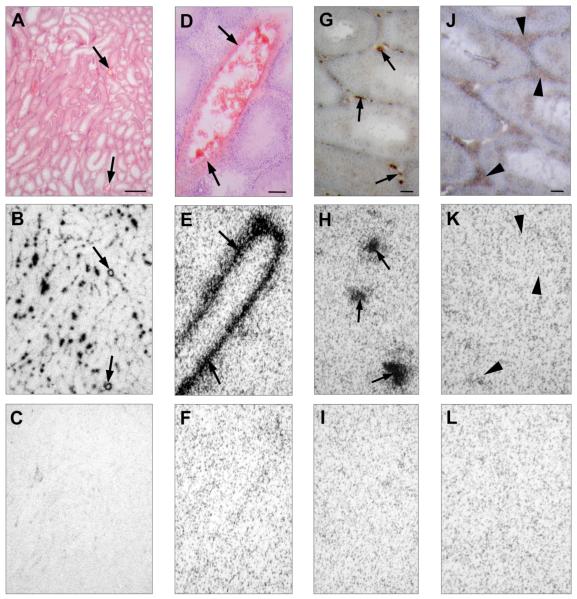
Figure 2.
In vitro NPY receptor autoradiography on serial tissue sections of rat testis using 10,000 cpm/100microliter 125I-PYY (specific activity 2,000 Ci/mmol) corresponding to a peptide concentration of 22.5 pM. The data indicate that a high density of Y1 receptors is found in testicular vessels, but there was no evidence for the presence of any NPY receptors on Leydig cells that expressed STAR-ir. A, D: H&E-stained tissue sections showing rat testis (A) at low magnification with blood vessels ( ) between the seminiferous tubules (bar = 1 mm) and (D) at high magnification with a large blood vessel in the center surrounded by seminiferous tubules (
) between the seminiferous tubules (bar = 1 mm) and (D) at high magnification with a large blood vessel in the center surrounded by seminiferous tubules ( indicate the vascular smooth muscle layer; bar = 0.1 mm). G: Immunohistochemical staining for Factor VIII highlighting the small blood vessels between the seminiferous tubules (
indicate the vascular smooth muscle layer; bar = 0.1 mm). G: Immunohistochemical staining for Factor VIII highlighting the small blood vessels between the seminiferous tubules ( ; bar = 0.1 mm). J: Immunohistochemical staining for STAR demonstrating the interstitial Leydig cells (
; bar = 0.1 mm). J: Immunohistochemical staining for STAR demonstrating the interstitial Leydig cells ( ; bar = 0.1 mm). B, E, H, K: Autoradiograms showing total binding of 125I-PYY to rat testis. B: At low magnification, widespread and strong 125I-PYY binding between the seminiferous tubules and particularly in larger blood vessels (
; bar = 0.1 mm). B, E, H, K: Autoradiograms showing total binding of 125I-PYY to rat testis. B: At low magnification, widespread and strong 125I-PYY binding between the seminiferous tubules and particularly in larger blood vessels ( ) can be appreciated. E: High magnification shows 125I-PYY binding to the smooth muscle layer of a larger blood vessel (
) can be appreciated. E: High magnification shows 125I-PYY binding to the smooth muscle layer of a larger blood vessel ( ); no binding to surrounding seminiferous tubules. H: Factor VIII-staining demonstrates that the autoradiography signals also correspond to many small blood vessels (
); no binding to surrounding seminiferous tubules. H: Factor VIII-staining demonstrates that the autoradiography signals also correspond to many small blood vessels ( ) between seminiferous tubules. K: STAR-expressing Leydig cells show no 125I-PYY binding. C, F, I, L: Autoradiograms showing non-specific 125I-PYY binding in the presence of 25 nM cold PYY. Complete displacement of 125I-PYY binding to testicular blood vessels by cold PYY provides evidence of specific 125I-PYY binding.
) between seminiferous tubules. K: STAR-expressing Leydig cells show no 125I-PYY binding. C, F, I, L: Autoradiograms showing non-specific 125I-PYY binding in the presence of 25 nM cold PYY. Complete displacement of 125I-PYY binding to testicular blood vessels by cold PYY provides evidence of specific 125I-PYY binding.
Figure 3.
Competition binding experiments demonstrated that the vascular NPY receptors corresponded mainly to the Y1 subtype, as 125I-PYY is displaced with high affinity by cold PYY (●), the Y1-selective agonist [Leu31,Pro34]-PYY (▲) and the Y1-selective antagonist Bis(31/31′)[Pro30,Cys31,Tyr32,Leu34]NPY(28-36)-NH2 (○), but not by the Y2-selective antagonist BIIE 0246 (▼) and the Y5-selective ligand [cPP(1-17),NPY(19-23),Ala31,Aib32,Pro34]hPP (■). The Y4-preferring pancreatic polypeptide showed an IC50 of 10.7 nM (data not shown).
The work presented here had three goals: to determine whether NPY was able to alter T release independently of LH secretion; to examine the potential role of decreased STAR and TSPO levels as mediators of the influence of NPY on T secretion; and to further support a role of this peptide in the rat testes, by localizing receptors in this organ. We report here that the injection of NPY directly into the testes blunted the T response to hCG. As these results were obtained regardless of whether the rats were or were not pretreated with a GnRH antagonist, the findings suggest that NPY acted independently of LH secretion. This conclusion is further supported by the fact that altered LH levels are not believed to acutely influence testicular responsiveness to this hormone (Saez, 1994), which further negates an important role of this hormone in our model. It should be mentioned, however, that these data do not influence the potential importance of a central (i.e., GnRH-mediated) influence of NPY on gonadal activity (see Introduction), but rather point to an additional site of action within the testes themselves. The next question was, what are the mechanisms responsible for this inhibitory effect? As mentioned in the Introduction, NPY has been reported to cause vasoconstriction in the testes (Collin et al., 1998). These findings indicate that in rats receiving an itt injection of NPY, changes in the T response to hCG might be due at least in part to decreased delivery of this gonadotropin to Leydig cells, and/or interference with T release in the general circulation. However, interpreting data from direct measurement of testicular blood flow would be difficult, as sorting out the various influences of decreased hCG delivery to Leydig cells, impaired T release into the general circulation, and altered levels of other blood-borne factors believed to influence testicular activity, is not easily feasible. Rather, we chose to measure the testicular proteins STAR and TSPO, as these parameters are not believed to be influenced by blood flow when NPY is injected directly into the testes. The overall finding from these experiments is that changes in steroidogenic proteins levels were inconsistent and did not reach statistical significance. The adult male rat displays large animal-to-animal variation in basal T levels (Desjardins, 1981), which undoubtedly result from the variance that we measured in terms of STAR and TSPO values. However, as we observed significantly decreased STAR and TSPO concentrations in other models, including systemic alcohol administration (Herman et al., 2006), it is unlikely that this phenomenon represents the sole explanation for the present findings. Moreover, since no NPY receptors were identified by immunohistochemistry or receptor autoradiography in rat Leydig cells {(Kopp et al., 1997) and present work}, it is unlikely that this peptide would act directly on these cells to influence T responses.
In order to further investigate the mechanisms through which NPY alters Leydig cell activity, we then used binding methodology to examine the location of receptors for this peptide. While we found a high density of Y1 receptors in testicular vessels, there was no evidence for the presence of any NPY receptors on Leydig cells that expressed STAR-ir, which corresponds to a previous report based on immunohistochemistry (Kopp et al., 1997). However, as Leydig cells only represent ca. 3% of total cell count in the rat testis (Desjardins, 1981), it remains possible that the methodology we used might have missed sparse receptors within these cells. Nevertheless, the apparent absence of NPY receptors on Leydig cells, coupled with the absence of significant changes in steroidogenic protein levels observed in rat testes injected with NPY, further supports the importance of mechanisms other than those directly involved in steroid-producing cells. Future experiments conducted in cultured Leydig cells exposed to NPY, a model that bypasses confounding factors such as Y1 receptors on blood cells and changes in blood flow, and/or direct measurement of testicular blood flow, will be needed in order to improve our ability to differentiate between the influence of this peptide on STAR and TSPO, and testicular vasoconstriction.
In conclusion, we showed that while the itt injection of NPY significantly blunted both basal and hCG-induced T release, there were no consistent changes in STAR and TSPO levels. On the other hand, using binding studies as well as immunohistochemistry, we observed expression of NPY receptor subtype Y1 at high density restricted to testicular blood vessels. As activation of Y1 is reported to cause vasoconstriction (Collin et al., 1998; Kopp et al., 2008), it seems reasonable to suggest that the high number of these receptors may represent one of the molecular mechanisms through which NPY influences T secretion, either by impairing gonadotropin delivery to Leydig cells, and/or by interfering with T secretion into the general circulation. Collectively, our results suggest that NPY needs to be added to the increasing number of factors that influence testicular activity through a local site of action.
Acknowledgments
Grant Support: The project described was supported by Award Number 5 R01 AA012810 from the National Institute of Alcohol Abuse and Alcoholism. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Alcohol Abuse and Alcoholism or the National Institutes of Health.
We would like to thank the following institutions and individuals for their generous gifts of reagents: Boehringer Ingelheim, Biberach, Germany (BIIE0246), as well as Drs. Jean Rivier, The Salk Institute, La Jolla (NPY and GnRH antagonist), D.B. Hales, Southern Illinois University School of Medicine at Carbondale, Chicago, IL (STAR antibody) and V. Papadopoulos, McGill University Health Centre, Montreal, Quebec, Canada (TSPO antibody). We are also grateful for the excellent technical help of Cristin Roach, Brian Baridon, Yaira Haas and Calvin Lau.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
C.D.A., B.W., M.K., J.C.R., S.L., and C.R. have nothing to disclose.
References
- Balasubramaniam A, Dhawan VC, Mullins DE, Chance WT, Sheriff S, Guzzi M, Prabhakaran M, Parker EM. Highly selective and potent neuropeptide Y (NPY) Y1 receptor antagonists based on [Pro(30), Tyr(32), Leu(34)]NPY(28-36)-NH2 (BW1911U90) J. Med. Chem. 2001;44:1479–1482. doi: 10.1021/jm010031k. [DOI] [PubMed] [Google Scholar]
- Bauer M, Bridgham J, langenau D, Johnson A, Goetz F. Conservation of steroidogenic acute regulatory (StAR) protein structure and expression in vertebrates. Mol. Cell. Endocrinol. 2000;168:119–125. doi: 10.1016/s0303-7207(00)00316-6. [DOI] [PubMed] [Google Scholar]
- Berglund MM, Hipskind PA, Gehlert DR. Recent developments in our understanding of the physiological role of PP-fold peptide receptor subtypes. Exp. Biol. Med. (Maywood) 2003;228:217–244. doi: 10.1177/153537020322800301. [DOI] [PubMed] [Google Scholar]
- Bose H, Lingappa V, Miller W. Rapid regulation of steroidogenesis by mitochondrial protein import. Nature. 2002;417:87–91. doi: 10.1038/417087a. [DOI] [PubMed] [Google Scholar]
- Breen KM, Karsch FJ. New insights regarding glucocorticoids, stress and gonadotropin suppression. Front. Neuroendocrinol. 2006;27:233–245. doi: 10.1016/j.yfrne.2006.03.335. [DOI] [PubMed] [Google Scholar]
- Cabrele C, Wieland HA, Koglin N, Stidsen C, Beck-Sickinger AG. Ala31-Aib32: identification of the key motif for high affinity and selectivity of neuropeptide Y at the Y5-receptor. Biochemistry. 2002;41:8043–8049. doi: 10.1021/bi0201421. [DOI] [PubMed] [Google Scholar]
- Collin O, Enfalt E, Astrom M, Lissbrant E, Bergh A. Unilateral injection of neuropeptide Y decreases blood flow in the injected testis but may also increase blood flow in the contralateral testis. J. Androl. 1998;19:580–584. [PubMed] [Google Scholar]
- Desjardins C. Endocrine signaling and male reproduction. Biol. Reprod. 1981;24:1–21. doi: 10.1095/biolreprod24.1.1. [DOI] [PubMed] [Google Scholar]
- Dobbins A, Lubbers LS, Jackson GL, Kuehl DE, Hileman SM. Neuropeptide Y gene expression in male sheep: influence of photoperiod and testosterone. Neuroendocrinology. 2004;79:82–89. doi: 10.1159/000076631. [DOI] [PubMed] [Google Scholar]
- Granot Z, Geiss-Friendlander R, Melamed-Book N, Eimerl S, Timberg R, Weiss A, Hales K, Hales D, Stocco D, Orly J. Proteolysis of normal and mutated steroidogenic acute regulatory proteins in the mitochondria: the fate of unwanted proteins. Mol. Endocrinol. 2003;17:2461–2476. doi: 10.1210/me.2003-0074. [DOI] [PubMed] [Google Scholar]
- Granot Z, Silverman E, Friedlander R, Melamed-Book N, Eimeri S, Timberg R, Hales K, Hales D, Stocco D, Orly J. The life cycle of the steroidogenic acute regulatory (StAR) protein: From transcription through proteolysis. Endocr. Rev. 2002;28:375–386. doi: 10.1081/erc-120016812. [DOI] [PubMed] [Google Scholar]
- Hales D, Payne A. Glucocorticoid-mediated repression of P450scc mRNA and de novo synthesis in cultured Leydig cells. Endocrinology. 1989;124:2099–2104. doi: 10.1210/endo-124-5-2099. [DOI] [PubMed] [Google Scholar]
- Herman M, Kang S, Lee S, James P, Rivier C. Systemic administration of alcohol to adult rats inhibits Leydig cell activity: Time course of effect and role of nitric oxide. Alcoholism: Clin. Exp. Res. 2006;30:1479–1491. doi: 10.1111/j.1530-0277.2006.00179.x. [DOI] [PubMed] [Google Scholar]
- Herman M, Rivier C. Activation of a neural brain-testicular pathways lowers Leydic cell levels of the steroidogenic acute regulatory protein and the peripheral-type benzodiazepine receptor while increasing levels of neural nitric oxide synthase. Endocrinology. 2006;147:624–633. doi: 10.1210/en.2005-0879. [DOI] [PubMed] [Google Scholar]
- Kalra SP, Kalra PS. Neural regulation of luteinizing hormone secretion in the rat. Endocr. Rev. 1983;4:311–351. doi: 10.1210/edrv-4-4-311. [DOI] [PubMed] [Google Scholar]
- Kanzaki M, Fujisawa M, Okuda Y, Okada H, Arakawa S, Kamidono S. Expression and regulation of neuropeptide Y messenger ribonucleic acid in cultured immature rat Leydig and Sertoli cells. Endocrinology. 1996;137:1249–1257. doi: 10.1210/endo.137.4.8625896. [DOI] [PubMed] [Google Scholar]
- Klenke U, Constantin S, Wray S. Neuropeptide Y directly inhibits neuronal activity in a subpopulation of gonadotropin-releasing hormone-1 neurons via Y1 receptors. Endocrinology. 2010;151:2736–2746. doi: 10.1210/en.2009-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp J, Collin O, Villar M, Mullins D, Bergh A, Hokfelt T. Regulation of neuropeptide Y Y1 receptors by testosterone in vascular smooth muscle cells in rat testis. Neuroendocrinology. 2008;88:216–226. doi: 10.1159/000138250. [DOI] [PubMed] [Google Scholar]
- Kopp J, Zhang X, Hokfelt T. Neuropeptide Y1 receptors in the rat genital tract. Regul. Pept. 1997;70:149–160. doi: 10.1016/s0167-0115(97)00028-1. [DOI] [PubMed] [Google Scholar]
- Kornreich WD, Galyean R, Hernandez J-F, Craig AG, Donaldson CJ, Yamamoto G, Rivier C, Vale W, Rivier J. Alanine series of ovine corticotropin releasing factor (oCRF): A structure-activity relationship study. J. Med. Chem. 1992;35:1870–1876. doi: 10.1021/jm00088a024. [DOI] [PubMed] [Google Scholar]
- Larhammar D. Structural diversity of receptors for neuropeptide Y, peptide YY and pancreatic polypeptide. Regul. Pept. 1996;65:165–174. doi: 10.1016/0167-0115(96)00110-3. [DOI] [PubMed] [Google Scholar]
- Lin D, Sugawara T, 3rd, S JF, Clark BJ, Stocco DM, Saenger P, Rogol A, Miller WL. Role of steroidogenesis acute regulatory protein in adrenal and gonadal steroidogenesis. Science. 1995;267:1828–1831. doi: 10.1126/science.7892608. [DOI] [PubMed] [Google Scholar]
- Lin S, Boey D, Herzog H. NPY and Y receptors: lessons from transgenic and knockout models. Neuropeptides. 2004;38:189–200. doi: 10.1016/j.npep.2004.05.005. [DOI] [PubMed] [Google Scholar]
- McCann SM, Marubayashi U, Sun H-Q, Yu WH. Control of follicle-stimulating hormone and luteinizing hormone release by hypothalamic peptides. Ann. N. Y. Acad. Sci. 1993;687:55–58. doi: 10.1111/j.1749-6632.1993.tb43853.x. [DOI] [PubMed] [Google Scholar]
- Ogilvie K, Hales K, Roberts M, Hales D, Rivier C. The inhibitory effect of intracerebroventricularly injected interleukin 1b on testosterone secretion in the rat: Role of steroidogenic acute regulatory protein. Biol. Reprod. 1999;60:527–533. doi: 10.1095/biolreprod60.2.527. [DOI] [PubMed] [Google Scholar]
- Ogilvie K, Rivier C. The intracerebroventricular injection of interleukin-1b blunts the testosterone response to human chorionic gonadotropin: Role of prostaglandin- and adrenergic-dependent pathways. Endocrinology. 1998;139:3088–3095. doi: 10.1210/endo.139.7.6090. [DOI] [PubMed] [Google Scholar]
- Papadopoulos V. Peripheral-type benzodiazepine/diazepam binding inhibitor receptor: biological role in steroidogenic cell function. Endocr. Rev. 1993;14:222–240. doi: 10.1210/edrv-14-2-222. [DOI] [PubMed] [Google Scholar]
- Payne AH, Hales DB. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr. Rev. 2004;25:947–970. doi: 10.1210/er.2003-0030. [DOI] [PubMed] [Google Scholar]
- Pedrazzini T. Importance of NPY Y1 receptor-mediated pathways: assessment using NPY Y1 receptor knockouts. Neuropeptides. 2004;38:267–275. doi: 10.1016/j.npep.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Pedrazzini T, Pralong F, Grouzmann E. Neuropeptide Y: the universal soldier. Cell. Mol. Life Sci. 2003;60:350–377. doi: 10.1007/s000180300029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierroz DD, Aebi AC, Huhtaniemi IT, Aubert ML. Many LH peaks are needed to physiologically stimulate testosterone secretion: modulation by fasting and NPY. Am. J. Physiol. 1999;276:E603–E610. doi: 10.1152/ajpendo.1999.276.4.E603. [DOI] [PubMed] [Google Scholar]
- Reubi JC, Gugger M, Waser B, Schaer JC. Y(1)-mediated effect of neuropeptide Y in cancer: breast carcinomas as targets. Cancer Res. 2001;61:4636–4641. [PubMed] [Google Scholar]
- Rivier C. Alcohol rapidly lowers plasma testosterone levels in the rat: Evidence that a neural brain-gonadal pathway may be important for decreased testicular responsiveness to gonadotropin. Alcoholism: Clin. Exp. Res. 1999;23:38–45. doi: 10.1111/j.1530-0277.1999.tb04021.x. [DOI] [PubMed] [Google Scholar]
- Rivier CL. Urocortin 1 inhibits rat Leydig cell function. Endocrinology. 2008;149:6425–6432. doi: 10.1210/en.2008-0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivier J, Kupryszewski G, Varga J, Porter J, Rivier C, Perrin M, Hagler A, Struthers S, Corrigan A, Vale W. Design of potent cyclic gonadotropin releasing hormone (GnRH) antagonists. J. Med. Chem. 1988;31:677–682. doi: 10.1021/jm00398a030. [DOI] [PubMed] [Google Scholar]
- Saez JM. Leydig cells: endocrine, paracrine, and autocrine regulation. Endocr. Rev. 1994;15:574–626. doi: 10.1210/edrv-15-5-574. [DOI] [PubMed] [Google Scholar]
- Selvage D, Rivier C. Importance of the paraventricular nucleus of the hypothalamus as a component of a neural pathway between the brain and the testes that modulates testosterone secretion independently of the pituitary. Endocrinology. 2003;144:594–598. doi: 10.1210/en.2002-220781. [DOI] [PubMed] [Google Scholar]
- Selvage DJ, Hales DB, Rivier CL. Comparison between the influence of the systemic and central injection of alcohol on Leydig cell activity. Alcoholism: Clin. Exp. Res. 2004;28:480–488. doi: 10.1097/01.alc.0000117839.69352.b3. [DOI] [PubMed] [Google Scholar]
- Stocco DM, Clark BJ. Regulation of the acute production of steroids in steroidogenic cells. Endocr. Rev. 1996;17:221–244. doi: 10.1210/edrv-17-3-221. [DOI] [PubMed] [Google Scholar]
- Tatemoto K, Carlquist M, Mutt V. Neuropeptide Y - a novel brain peptide with structural similarities to peptide YY and pancreatic polypeptide. Nature. 1982;296:659–660. doi: 10.1038/296659a0. [DOI] [PubMed] [Google Scholar]
- Terado M, Nomura M, Mineta K, Fujimoto N, Matsumoto T. Expression of Neuropeptide Y gene in mouse testes during testicular development. Asian J. Androl. 2006;8:443–449. doi: 10.1111/j.1745-7262.2006.00166.x. [DOI] [PubMed] [Google Scholar]
- Turnbull AV, Rivier C. Inhibition of gonadotropin-induced testosterone secretion by the intracerebroventricular injection of interleukin-1 beta in the male rat. Endocrinology. 1997a;138:1008–1013. doi: 10.1210/endo.138.3.5019. [DOI] [PubMed] [Google Scholar]
- Turnbull AV, Rivier C. Inhibition of gonadotropin-induced testosterone secretion by the intracerebroventricular injection of interleukin-1b in the male rat. Endocrinology. 1997b;138:1008–1013. doi: 10.1210/endo.138.3.5019. [DOI] [PubMed] [Google Scholar]
- Wahlestedt C, Reis DJ. Neuropeptide Y-related peptides and their receptors--are the receptors potential therapeutic drug targets? Annu. Rev. Pharmacol. Toxicol. 1993;33:309–352. doi: 10.1146/annurev.pa.33.040193.001521. [DOI] [PubMed] [Google Scholar]
- Wang X, Cui Y, Chen Z, Pan Q. Expression of neuropeptide Y in rat testes and its significance. Zhonghua Nan Ke Xue. 2004;10:334–336. 339. [PubMed] [Google Scholar]