Abstract
Because survival with both chemotherapy and allogeneic hematopoietic stem cell transplantation (HSCT) approaches to high risk pediatric acute lymphoblastic leukemia (ALL) generally improves through the years, regular comparisons of outcomes with either approach for a given indication are needed to decide when HSCT is indicated. Improvements in risk classification are allowing clinicians to identify patients at high risk for relapse early in their course of therapy. Whether patients defined as high risk by new methods will benefit from HSCT requires careful testing. Standardization and improvement of transplant approaches has led to equivalent survival outcomes with matched sibling and well-matched unrelated donors, however, survival using mismatched and haploidentical donors is generally worse. Trials comparing chemotherapy and HSCT must obtain sufficient data about therapy and stratify the analysis to assess the outcomes of best-chemotherapy with best-HSCT approaches.
Introduction
Although the large majority of children with ALL are cured with chemotherapy approaches,1–3 HSCT has been used successfully to treat a portion of very high risk patients in first remission or at various stages of relapse.4,5 Through the years, significant improvements have occurred in both chemotherapy and HSCT approaches.6 In addition, sophisticated methods of risk classification based upon clinical and molecular characteristics have allowed the development of approaches targeting intense agents and/or offering HSCT to the highest risk patients.7–9
Because both treatment approaches and diagnostic tools are changing rapidly, clinicians must carefully follow the field to know when HSCT or chemotherapy approaches offer the best chance of cure for their patients. In addition, both treating physicians and clinical researchers need to understand essential study design methods necessary for valid comparison of HSCT and chemotherapy outcomes in order to avoid bias or inappropriate conclusions.10
Our review of this topic will begin with a discussion of current indications for CR1 HSCT from the perspective of a chemotherapist, Dr. Ching-Hon Pui, who leads the St. Jude Total Therapy studies in ALL. While other international study groups vary somewhat in identifying those who should receive HSCT, the general principles outlined by Dr. Pui are useful in considering this question. Dr. Christina Peters, who leads HSCT efforts in Berlin-Frankfurt-Münster (BFM)-based studies, will then review key principles of transplantation learned as the BFM group carefully standardized HSCT approaches over the past decade. Finally, Dr. Michael Pulsipher, a leader in Children’s Oncology Group (COG) ALL HSCT efforts, will discuss data and study design elements that are important in determining which subsets of children with ALL benefit from HSCT approaches.
Indications for Transplantation in First Remission of Childhood ALL: Perspectives from a Chemotherapist
Ching-Hon Pui, MD
Allogeneic hematopoietic stem cell transplantation is considered a treatment modality for patients with ALL who are predicted to respond poorly to intensive chemotherapy. Therefore, the indications for transplant must be periodically reassessed, owing to the continuous improvement in chemotherapy results, transplantation procedures and methods to assess relapse hazard. Small numbers of eligible patients, strong preference for chemotherapy or transplant on the part of the physicians, and a lack of suitable donor are some of the factors that have prevented stratified randomized trials to directly compare the efficacy of transplantation to that of chemotherapy alone in pediatric ALL. Thus, treatment allocation is primarily based on the availability of a suitable donor.
In a study by the BFM group in patients with high-risk T-cell ALL, the 36 patients who received allogeneic transplantation had higher disease-free survival (P=0.01; 67% ± 8% [SE] versus 42% ± 5% at 5 years) and overall survival (P=0.01; 67% ± 8% versus 47% ± 5% at 5 years) than the 120 patients treated with chemotherapy alone.11 In a European multi-institutional study of very high-risk childhood ALL, the 77 transplanted patients also had a superior disease-free survival (P=0.02) than the 280 patients treated with chemotherapy: 56.7% ± 5.7% (SE) versus 40.6% ± 3.1% at 5 years.12 In this study, however, the difference in overall survival rate between the two groups was not statistically significant and there were too few patients to perform meaningful subgroup analyses to identify the specific subtype(s) that benefited from transplantation (the “high-risk” group was defined by many differing factors).12 Based on the outcome of patients treated with chemotherapy alone in these two studies, one could argue that approximately one third of the patients might have been over-treated with transplantation.
Currently, no genetic abnormality per se is an absolute indication for transplantation because even within specific genetic subtypes of ALL there is considerable heterogeneity in terms of drug resistance due to a combination of variables, including secondary cooperating mutations, target cells undergoing malignant transformation, and host pharmacodyamics and pharmacogenetics.13 As shown in Table 1, there is no consensus on the indications for transplantation in childhood ALL in CR1 among major study groups. Discussed here is the perspective of a chemotherapist on this topic.
Table 1.
Indications for allogeneic HSCT in children with ALL during first remission currently being assessed in three ALL study groups
| Study Group | Indications |
|---|---|
| AIEOP/BFM* | Induction failure (≥ 5% blasts in marrow) on day 33 of induction; Hypodiploidy <44 chromosomes, t(4;11) or t(9;22) (BCR-ABL1) plus positive MRD on day 33 of induction or on day 78; T-cell ALL with prednisone- poor response if MRD ≥ 10−3 on day 78 or if MRD data not available; MRD ≥ 10−3 on day 78 of induction |
| Children’s Oncology Group** | Induction failure (≥25% blasts in marrow) on day 29 of induction; Hypodiploidy <44 chromosomes; t(9;22) (BCR-ABL1) [matched-related donor; matched-unrelated donor only if day 29 MRD >1% or week 12 MRD >0.01%] |
| SJCRH | Induction failure (≥5% blasts by MRD in marrow) on day 42 of induction; T-cell precursor ALL; MRD ≥ 10−2 on day 42 of induction; MRD ≥ 10−3 14 weeks after the start of remission induction; re- emergence of leukemia blasts at any level |
Abbreviations: AIEOP, Associazione Italiana Ematologia Oncologia Pediatrica; BFM, Berlin-Frankfurt-Münster; MRD, minimal residual leukemia; SJCRH, St. Jude Children’s Research Hospital
S Hunger (personal communication)
M Schrappe and V Conter (personal communication)
Philadelphia Chromosome-positive ALL
A St. Jude study first recognized that childhood ALL with the t(9;22)(BCR-ABL1) is a heterogeneous disease and that a substantial proportion of patients, i.e., those with low leukocyte count, could be cured with chemotherapy alone.14 This result was confirmed by Aricò and associates15 in a large cooperative group study of 326 pediatric patients treated between 1986 and 1996. Of note, matched-related transplantation was superior to the other types of transplantation and to intensive chemotherapy alone in prolonging disease-free survival and overall survival in this study.15 A subsequent study of 610 patients treated without ABL inhibitors between 1995 and 2006 showed that the overall outcome had improved with advances in chemotherapy and transplantation, that transplantation with matched-related and matched-unrelated transplantation yielded similar results, and that transplantation improved disease-free survival but not overall survival.16 Early treatment response assessed by morphologic examination of blood or bone marrow was the most powerful prognostic indicator in this study. Overall, only 45% of patients were alive 7 years after diagnosis; the study did not identify subsets of patients benefited the most from transplantation.16
In a recent Children’s Oncology Group study, intensive chemotherapy plus continuous imatinib treatment after conventional remission induction therapy yielded a 3-year event-free survival rate of 80%, which was more than twice that of the historical controls and comparable to those of matched-related or matched-unrelated transplant.17 However, the follow-up duration of this study is too short to determine if the treatment with intensive chemotherapy and imatinib truly improved cure rates and not merely prolonged disease-free survival. At the time of the report, positive minimal residual disease (MRD) after conventional remission induction, the most important prognostic factor for childhood ALL, was not significantly associated with poor disease-free survival.17 However, the occurrence of relapse after cessation of therapy in several cases with positive MRD suggested that this factor could become important with additional follow-up. With this uncertainty, it is not surprising that there is no consensus on the indication of transplantation for this group of patients (Table 1). The recent advent of more potent ABL inhibitors and their inclusion into remission induction treatment might make consensus even less likely.
Infant ALL with MLL Rearrangements
The prognosis of MLL-rearranged ALL, including the t(4;11) with MLL-AF4 fusion, is affected by the age of presentation, with infant age predicting the most dismal outcome.18 Although some small studies suggested that transplantation improved outcome of infant cases with MLL rearrangement, results of three large cohort studies failed to show an advantage of transplantation over chemotherapy alone in terms of disease-free survival or survival after adjustment for waiting time to transplantation.18–20 In fact, patients underwent mismatched transplantation had a significantly worse outcome than those treated with chemotherapy alone in one earlier study.18 By contrast, one of the studies (Interfant-99) with a longer follow-up, showed that a very high-risk subgroup of infants with MLL rearrangement (defined by age <6 months and either poor response to glucocorticoid treatment or initial leukocyte count ≥ 300 x 109/L) benefited from transplantation, with a 64% reduction in the risk of relapse or death in remission.21 However, the finding of this subset analysis needs to be confirmed, and studies should also be performed on other subsets characterized by a dismal outcome, such as MLL-rearranged infant cases with persistent MRD after remission induction and consolidation treatment.22 The indications for transplantation should also be evaluated in the context of emerging molecular therapies such as FLT3 inhibitors and DNA methyltransferase inhibitors.23
High-risk T-cell ALL
In the BFM 90 and 95 trials, transplantation improved outcome in patients with T-cell ALL and high-risk features; i.e., poor response to 7 days of prednisone and one dose of intrathecal methotrexate or failure to achieve remission (≥ 5% blast in marrow on day 33 of remission induction).11 However, considering that chemotherapy alone yielded a disease-free survival approaching 50% in this group of patients treated in the BFM 95 trial, one could argue that transplantation was an over-treatment for them. Early T-cell precursor ALL is a recently identified subset of T-cell ALL with immature genetic and immunophenotypic features and a dismal prognosis (event-free survival of 22%), despite the fact that half of the patients received transplantation due to high MRD levels after remission induction.24 Whether transplantation has a therapeutic role in this group of patients remains to be determined by studying a larger number of patients.
Hypodiploid ALL
ALL with hypodiploidy <45 chromosomes occurs in only 1% of childhood ALL. In a recent international collaborative group study (the largest to date), the 80 patients with chromosomal number less than 44 in their leukemic cells fared significantly worse than the 80 patients with 44 chromosomes in terms of event-free survival (P=0.01; 30.1% versus 52.2% at 8 years) and survival (P=0.017; 37.5% versus 69% at 8 years).25 Transplantation was performed in first remission in only 9 patients, 5 of whom subsequently had an adverse event. There was no difference in disease-free survival or overall survival between patients who did or did not undergo transplantation.25 However, the efficacy of transplantation could not be adequately addressed in this study because only a very small number of patients were transplanted and the MRD status after remission induction was unknown in these patients.
Poor Early Responders
Early response to treatment is perhaps the most important prognostic factor because it accounts for the drug sensitivity of leukemic cells, the host pharmacodynamics and pharmacogenetics, the treatment administered, and the patient compliance.26 Among various methods to assess treatment response, MRD determination in bone marrow samples collected during or at the end of induction is the most reliable. Remission induction failure is one of the worst prognostic factors in ALL, with disease-free survival ranging from 21% to 36% in recent studies.12,27–29 It has been universally regarded as an indication for transplantation although there is no uniform definition for “induction failure”, which is generally based on the finding of M2 to M3 marrow between day 29 and day 42 of remission induction. In the study of Oudot et al.,29 patients with Philadelphia chromosome-positive ALL or those with T-cell ALL and no mediastinal mass had a high risk, patients with T-cell ALL and a mediastinal mass had an intermediate-risk, and the other B-cell precursor cases had a low risk of induction failure. Whether these patients have different salvage rates remained to be determined. Matched-related transplantation and chemotherapy yielded a similar outcome in this study.29 However, in the study of Balduzzi et al.12 transplantation prolonged disease-free survival. Recent MRD studies showed that patients with 1% or more leukemic cells at the end of 4 to 6 weeks of remission induction have a prognosis that is almost as poor as that of patients who fail to achieve clinical remission by traditional morphologic standard,26,29 an observation that challenged the current definition of induction failure. In the recently completed St. Jude Total Therapy Study XV, transplantation was performed in 9 patients with 1% or more leukemic cells in bone marrow at day 46 of remission induction, and yielded a 5-year event-free survival of 55.6% ± 26.2% and 5-year survival of 87.5% ± 13.8%.1 Additional studies are needed to determine if transplantation improves outcome in this setting.
Conclusion
There is no absolute indication for transplantation in children with ALL who are in first remission. In view of the dismal outcome of poor early responders with Philadelphia chromosome-positive ALL, early T-cell precursor ALL, or infant ALL with MLL rearrangement, these patients are reasonable candidates for the evaluation of transplantation in first remission.
HSCT Outcomes of BFM Trials: Recent Results, Challenges Moving Forward
Christina Peters, MD
The majority of children and adolescents with ALL can be cured with multimodal chemotherapy.30 However, patients with the very high risk ALL (HR-ALL) or patients who have relapsed have a significantly worse prognosis compared to other patients with ALL.31–34 These patients require additional therapapeutic approaches after achieving remission. Allogeneic HSCT can effectively induce immunological antileukemic control in patients with ALL by means of the graft-versus-leukemia effect (GVL), but treatment related mortality (TRM) remains a serious problem.35,36 In addition, the heterogeneity of available data regarding patient selection, transplantation procedures and study endpoints hampers the interpretation of the value of HSCT.37 To overcome this challenge, in 2003 the Berlin-Frankfurt-Münster (BFM)-Study group initiated a prospective international multicenter trial (ALL-SCT-BFM 2003) for allogeneic HSCT in children with ALL in first, second or subsequent remission who had an indication for HSCT according to the frontline/relapse chemotherapy protocols.38
In addition to developing a standardized approach to HSCT in childhood ALL, the primary objective of this trial was to evaluate whether HSCT from an HLA-identical matched sibling donor (MSD) is equivalent to HSCT from a very well-matched unrelated donor (MD). Secondary objectives were to compare the efficacy between HSCT from HLA-mismatched donors (MMD) and HSCT from MD/MSD, and to determine the incidence of acute and chronic GvHD after HSCT. In 2007 the trial was extended to additional transplant units internationally. This extension of the study has been called the ALL-SCT-BFM-international trial (ALL-SCT-BFMi).
Current Prognostic Factors and Indications for Allogeneic HSCT
As reviewed by Dr. Pui, there are several risk factors for a poor prognosis in childhood ALL that are discernable at diagnosis (cytogenetic characteristics, time and site of relapse, etc.). Additionally, response to induction treatment measured by morphology and/or detection of minimal residual disease has a strong predictive value and defines SCT indications.22,34,39,40 Further details are shown in Table 2 for ALL (CR1) and Table 3 for ALL (>CR1).
Table 2.
Indications for allogeneic stem cell transplantation in ALL in CR1 according to the BFM criteria
| PCR-MRD results | ||||||
|---|---|---|---|---|---|---|
| MRD-SR | MRD- MR | MRD-HR | no MRD result | |||
| MRD- TP2 ≥10−3 | MRD- TP2 ≥10−2 | |||||
| HR criteria (in hierarchica l order) | No CR d33 | MMD | MMD | MMD | MMD | MMD |
| PPR + (9;22) | MMD | MMD | MMD | MMD | MMD | |
| PPR + (4;11) | MD | MD | MD | MMD | MD | |
| PGR + (9;22) | no | MD | MD | MMD | MD | |
| PGR + (4;11) | MSD | MSD | MD | MMD | MSD | |
| PPR + * | no | no | MD | MMD | MD | |
| “Favorable” PPR§ | no | no | MD | MMD | no | |
MSD = matched sibling donor
MD = matched donor (well-matched, unrelated)
MMD = mismatched donor
no = no SCT indicated
PPR + pro-B ALL or T-ALL and/or M3 d15 and/or WBC>100,000/μl
PPR + none of the above criteria
MRD-SR: MRD negativity after 4 and 12 weeks induction treatment, measured with two independent target with a sensitivity of ≤10−4.
MRD-MR: any MRD positivity after 4 and 12 weeks induction treatment, but <10−3 at week 12 (TP2).
MRD-HR: MRD ≥10−3 at week 12 (TP2).
Table 3.
ALL-SCT-BFM 2003/BFMi indications for allogeneic stem cell transplantation in ALL after first relapse
High risk (S3/S4):
|
MMD |
Intermediate risk (S2, MRD* > 10−3):
|
MD |
Intermediate risk (S2, MRD* < 10−3):
|
MSD |
MRD detected after the second induction block; if no MRD is available: MSD-SCT is indicated, MD-SCT indication is dependent on conventional clinical risk factors
MSD = matched sibling donor
MD = matched donor
MMD = mismatched donor
Timepoint of relapse:
very early: <18 months after primary diagnosis
early: ≥ 18 months after primary diagnosis and < 6 months after cessation of front-line therapy
late: ≥ 6 months after cessation of front-line therapy
Donor selection and stem cell source
MSD HSCT has generally led to the best survival rates and is considered the gold standard for all indications.41 Since only 20%–25% of children with an indication for allogeneic HSCT have a MSD, the availability of volunteer HLA matched unrelated donors (MUD) has widened the donor pool over the past decade.42 The chance of finding a suitable donor mainly depends on ethnic group (ranging from 60%–70% for Caucasians to <10% for patients belonging to some ethnic minorities) and the frequency of the HLA phenotype of the patient.43 The primary aim of our trial was to evaluate if a well-matched unrelated donor (MD) is equivalent to a MSD. To assess this accurately, high-resolution (allele level) HLA-typing including the HLA-C locus was required, and an algorithm was developed to choose the best donor if more than one was available. The algorithm was as follows: mismatch at the allele level was considered superior to an antigen mismatch, the subsequent priorities were the matching of the CMV serostatus (CMV antigen positive donor for a positive recipient), then gender (male donor for male recipient) and age (younger donor for children) of donor and recipient. Details for donor definitions are shown in Table 4. Preferably, unmanipulated BM was chosen as the stem cell source from MD in ALL-SCT-BFM 2003, as it was shown that the use of PBSC is associated with a higher TRM and a higher incidence of chronic GvHD.44–46 T-cell depletion was performed only in a MMD situation.
Table 4.
ALL-SCT-BFM 2003/BFMi definition of donor groups by HLA-matching and relationship
| Identity of HLA alleles* | Sibling donor | Family donor | Unrelated donor** |
|---|---|---|---|
| 10/10 | MSD | MD | MD |
| 9/10 | MD | MD | |
| < 9/10 | MMD | MMD | |
high resolution typing of HLA-A, HLA-B, HLA-C, HLA-DRB1, and HLA-DQB1.
If no suitable donor is available cord blood (CB) from a 6/6 matched unrelated donor is an accepted alternative for MD-Indications, and <6/6 matched unrelated CB for MMD-Indications (only valid in trial ALL-SCT-BFMi).
MSD = matched sibling donor
MD = matched donor
MMD = mismatched donor
Conditioning regimen
The choice of the conditioning regimen has a significant impact on survival after HSCT. It was shown retrospectively that conditioning with total body irradiation/etoposide (TBI/VP16) was comparable with TBI/ARA-C/melphalan (MEL) and superior to TBI/cyclophosphamide (Cy). Busulfan/CY/MEL (BU/CY/MEL) as an irradiation-free conditioning was inferior because of higher incidence of relapses as well as treatment-related mortality.47–49 Therefore, the current standard backbone for MSD/MD consists of fractionated TBI (12 Gy) and VP16. Patients who are ineligible for TBI due to young age or previous therapy can substitute TBI with i.v. BU. For MMD, additional TBI or BU, fludarabine (FLU) and CY were given. Alternatively, FLU/thiotepa (THIO)/MEL was an acceptable combination for MMD-transplant for patients at high risk for TRM. In patients with t(4;11), the benefit of allogeneic HSCT could not be clearly demonstrated by retrospective analysis.18 As outcome after treatment by chemotherapy only is also not satisfying, it was decided to choose a conditioning regimen consisting of BU, CY and MEL for these patients, as this regimen was shown to be effective in patients with juvenile myelomonocytic leukemia and AML.50
GVHD prophylaxis and therapy
Heterogeneities in GvHD prophylaxis and therapy are a structural weakness of retrospectively analysed patient cohorts.51,52 Therefore, it was a major goal of our trial to apply a well-standardized and risk-adapted GvHD prophylaxis approach. In MSD, GvHD prophylaxis consisted of CsA only; in MD, additional short MTX and ATG-F were administered. In MMD, CD34-positive selection or CD3/CD19 depletion were performed and no pharmacological immunosuppression was given.53,54
Outcomes
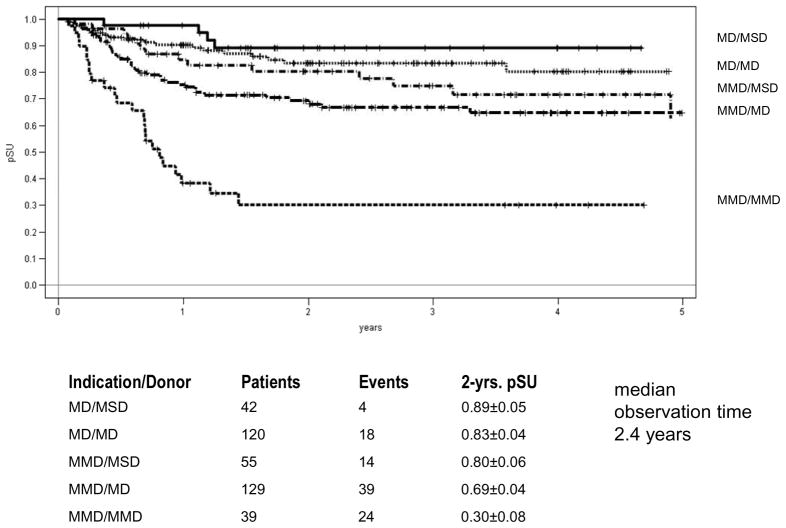
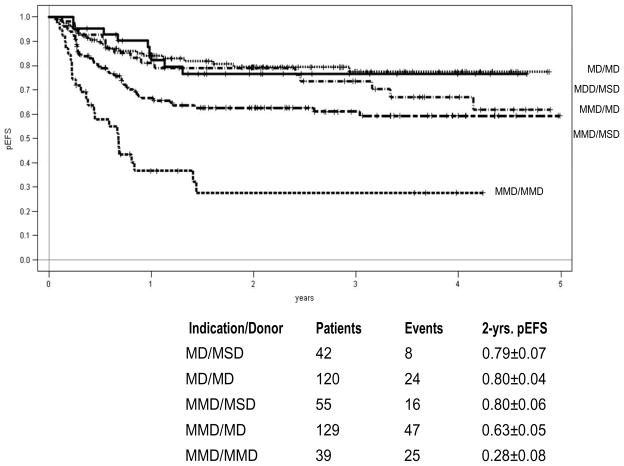
Between September 2003 and September 2009, 624 patients were recruited; 387 patients (188 in CR1, 199 ≥ CR2) were transplanted in 27 participating centers in Austria, Germany and Switzerland. Mean age of the patients at HSCT was 10 years (range 0.5 to 18); 97 patients received a MSD-HSCT, 251 patients a MD-HSCT, and 39 patients a MMD-HSCT. Median follow-up was 2.4 years. Unmanipulated bone marrow was used in 81% of MSD-HSCT and in 65% of MD-HSCT as determined by the protocol.
Acute GvHD (Grade III and IV) occurred in 10% of all patients, the 2-year cumulative incidence of extensive chronic GvHD was 15% after MSD and 12 % after MD HSCT. The 4-year probability of event-free survival (pEFS) after MSD-HSCT was equivalent to MD-HSCT (70% vs. 68%; p=0.37, Figure 1). The cumulative incidence of treatment related mortality (TRM) after 1 year was 5% for MSD and 8% for MD HSCT (n.s.). The 2-years cumulative incidence of relapse was 18% after MSD-HSCT and 20% after MD-HSCT (n.s.). For patients with very high risk of relapse the results for MD/MSD HSCT (n=187) and MMD HSCT (n=39) differed significantly (2-year pEFS 68% vs. 28%; p<0.001). The 2-year incidence of relapse was 23% after MSD/MD HSCT and 37% after MMD-HSCT (n.s.). The 1-year incidence of TRM was 8% after MSD/MD-HSCT and 22% after MMD-SCT (p=0.04). Overall, MMD HSCT showed a significantly worse result with higher TRM and higher relapse rates. For patients beyond CR1 (n=25) transplanted from a MMD, the 2-year pEFS was only 19%.
Figure 1.
Probability of Event Free Survival according to Transplant Indication and Donor Type
The results from this ALL-SCT-BFM 2003 trial, which is the largest prospective, international and multicenter HSCT trial ever performed in childhood ALL, demonstrate the feasibility of a harmonized HSCT approach across multiple international centers. We demonstrate that allogeneic HSCT from well HLA-matched unrelated donors or geno-identical sibling donors are effective treatment options with acceptable toxicity in children with high risk ALL. Precise HLA typing and matching and the inclusion of ATG resulted in a low incidence of extensive chronic GvHD, an important achievement for the quality of life in children and adolescents.
Conclusion
The definition of indications for allogeneic HSCT in children with high-risk (HR) ALL in the first remission or after the first or subsequent relapse depends on biological features, response to treatment, and survival after chemotherapy alone. HSCT indications have to be defined prospectively and must be reevaluated and reconfirmed at intervals dependent on modifications and improvements of the chemotherapeutical approaches of the frontline and relapse protocols. Furthermore, a close cooperation between chemotherapy study groups and HSCT societies enables identification of patients at the highest risk of relapse after HSCT.
There is a strong need for prospective HSCT trials, ensuring a well standardized procedure regarding all relevant components that are potentially responsible for TRM and late effects. Subsequent BFM ALL-HSCT trials will focus on controlled modifications and interventions in patients at highest risk for relapse after HSCT. The most burning questions are:
How to reduce leukemic burden before HSCT?
Whether TBI can be substituted without increasing relapse risk?
Who is the best HLA-mismatched donor: haplo-identical family members, unrelated bone marrow or unrelated cord(s)?
Analysis of Comparative HSCT/Chemotherapy Studies: The Process of Defining when HSCT Should be Considered in Pediatric ALL
Michael A. Pulsipher, MD
Defining when HSCT is appropriate therapy for the ever-multiplying redefinitions of clinical, molecular, and MRD-based risk classifications for children with ALL is a daunting task. In the past decade significant improvements in HSCT have led to less TRM and better survival after unrelated donor transplantation,6 less GVHD in pediatric recipients,55 decreases in TRM and better survival in unrelated cord blood transplantation,56 a possibility of decreased relapse using KIR mismatching or double cord blood approaches,57,58 and the beginning a series of both targeted and non-targeted cellular therapeutic therapies.4,59 As these advances occur, equally compelling advances occur in chemotherapy approaches with the introduction of novel and targeted agents, better definition of high risk groups, and intensification of therapy in order to prevent relapse.23 The pace of advancement in both fields makes it imperative that chemotherapists and HSCT practitioners work together to continually redefine when HSCT should be used for children with ALL.
Approaches to Considering HSCT for High Risk Pediatric ALL
A major challenge in deciding the comparative efficacy of HSCT for pediatric ALL patients is that the modality is not conducive to randomized trials, and past attempts at randomization failed as improvements in HSCT techniques led to widespread non-compliance.60 As highlighted by Dr. Peters above, modern allogeneic transplantation is an effective, well established curative therapy for ALL that combines intensive therapy and an immunological effect (GVL) that can overcome chemotherapy resistance. Our key question is not whether is works, but when it can offer an advantage compared to available therapies.
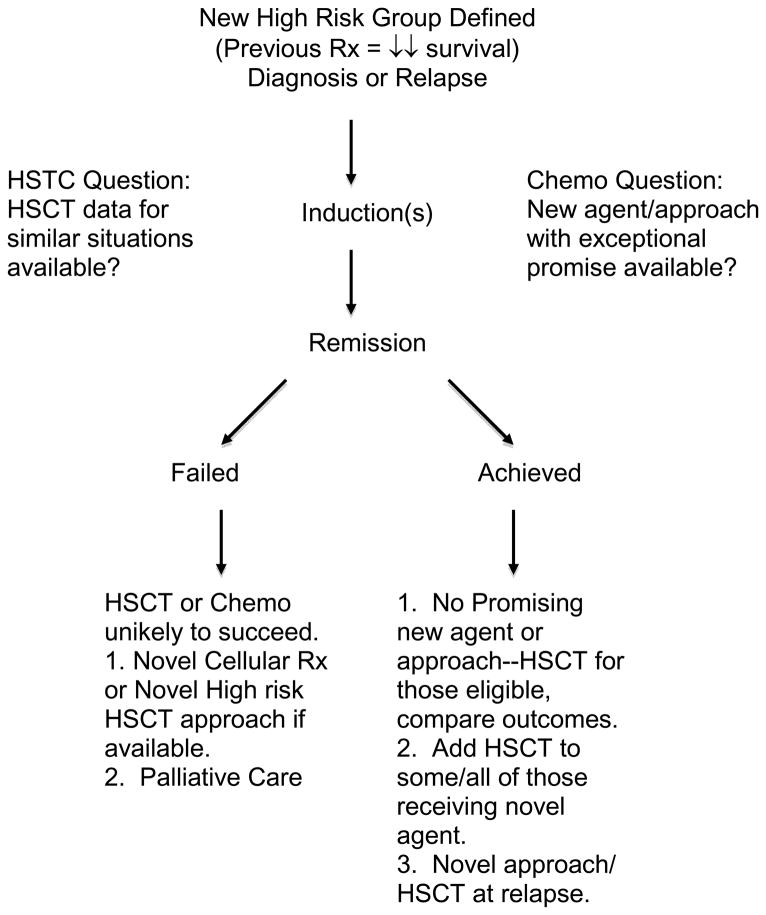
Figure 3 illustrates an approach that could be considered when patients display clinical characteristics that render them to have an unacceptable relapse hazard with established approaches. The first task is to define unacceptable risk which should be considered for each risk group in comparison to a non-HSCT alternative. For example, in CR1 patients, many studies show survival after HSCT with well-matched donors in the 60–70% range.61,62 If a patient has a risk factor resulting mostly in early relapse, the likelihood of achieving a second remission is just under 70%.63 Many of these patients may not be able to get to HSCT due to significant organ toxicity, infections, or insurance issues. Of those who undergo HSCT for early relapse, survival is in the 30–40% range.64 Adding this up, it is likely that survival of patients with this group would increase if an advantage of at least 15–20% over chemotherapy were achieved with HSCT in CR1. If we take the low end of HSCT survival in CR1 (60%), that would mean that a risk factor identifying patients with <40% survival would be a reasonable candidate to consider for HSCT if they achieve remission. Other approaches and logic can be used to decide unacceptable risk, but agreement between transplant physicians and chemotherapists on which approach should be taken is important to protect the integrity of studies.
Figure 3.
Consideration of the Use of HSCT for Newly Defined High Risk Patients
It is vital that patients achieve CR before considering then for comparative study, as both chemotherapy and HSCT for patients who do not achieve remission result in dismal outcomes. Once remission is achieved, key questions that help determine whether to consider HSCT should be answered. The major consideration will be whether a promising alternative therapy exists. Most new chemotherapeutic interventions will not have preliminary data so compelling that a major difference in outcome could be anticipated, therefore clinicians can decide to either perform HSCT on all patients who receive the novel intervention or a high-risk subset. In cases where new approaches are exceptionally promising, investigators may choose to perform HSCT only as a rescue strategy after relapse. Whichever approach is chosen, careful comparison of outcomes with patients who do not undergo HSCT should be performed so that the efficacy of HSCT for that indication vs. chemotherapy can be discerned.
Necessary Elements for Comparative Studies of HSCT and Chemotherapy
Table 5 identifies several areas that are often overlooked when attempts at comparison of HSCT with chemotherapy are performed. It is essential to only include patients who achieve a clinical state where chemotherapy or HSCT has a reasonable chance of cure. Those not attaining remission, therefore, must be excluded, as a disproportionate number of these patients in either arm would skew the results. Well established statistical methods such as adjusting the chemotherapy arm for median time to transplant and intent-to-treat based on a clear marker such as availability of a pre-defined type of donor can then be incorporated if feasible.
Table 5.
Considerations for Studies Comparing HSCT with Chemotherapy
|
A major problem with many past comparative studies is failure to recognize substantial differences in outcome when different HSCT methods are employed and different chemotherapy regimens are pursued. The following characteristics have been shown to result in major differences in survival after HSCT for high risk ALL: 1) allogeneic vs. autologous donor, 2) well-matched related/unrelated donor vs. mismatched or haploidentical donor, 3) myeloablative TBI-based regimen vs. non-TBI containing or reduced intensity regimen,61 4) cord blood HSCT utilizing adequate matching/adequate cell dose vs. inadequate matching/cell dose. Myeloalbative TBI-based regimens using well-matched allogeneic donors (or appropriately matched and dosed cord donors) generally have similar outcomes and can be combined in analyses, but recipients of autologous, haplo-identical or significantly mismatched grafts or non-TBI/reduced intensity approaches must be analyzed separately. In a similar fashion, patients on a chemotherapy comparative arm should be treated with an era and disease stage-appropriate chemotherapy regimen. Patients receiving inadequate chemotherapy approaches should be excluded from the comparison arm or analyzed separately. Sufficient data must be collected to make these distinctions.
Finally, if high-risk cohorts are not defined correctly, comparative analysis of HSCT vs. chemotherapy can be misleading. An example illustrating this is found in the treatment of MLL+ high risk infants with CR1 transplantation. In a retrospective study of children (including infants) with 11q23 abnormalities (MLL rearrangement) treated between 1983 and 1995, investigators showed inferior outcome with HSCT compared to chemotherapy.18 A recent report from COG investigators looking at infants with MLL+ leukemias showed no advantage with CR1 transplant.20 One could conclude that there is no role for HSCT in CR1 for high risk MLL+ infants, however, both studies suffered from a heterogeneous transplant group that mixed high risk and low risk procedures. The major concern with the analysis of these studies, however, is that the defined risk groups contained patients at high, intermediate, and lower risks of relapse with chemotherapy approaches. At the time of the first report, older children with 11q23 had higher rates of survival with chemotherapy approaches (exceeding 60%) compared to the youngest infants, where survival was very poor. The intensive chemotherapy used for the recent COG study resulted in a cure rates for infants greater than 6 months that exceeded 50%,65 while those < 6 months with high WBC had very low rates of survival (<20%). Because chemotherapy cured 50–60%+ of a large amount of the cohort, HSCT, which generally cures about 55% of infants,66 did not improve outcomes. So what about targeting HSCT where it works best, to only the high-risk cohort? The Interfant group recently used this approach by dividing patients into three risk categories; the highest risk group was defined by MLL positivity, age <6 months and either WBC counts ≥ 300 K/μL or prednisone poor response. The group then restricted transplant to the highest risk cohort, although transplant was not mandated. Survival after HSCT of medium and high risk patients was similar (57 and 56%), but chemotherapy outcomes were dramatically different, with disease free survival of 48% of the medium risk and 14% of the high risk children. After appropriate adjustments for waiting time for HSCT, 4-year overall survival was superior for HSCT in the HR cohort (66±12% vs. 19±6%, p=0.001).21 The differing conclusions of these studies regarding the efficacy of transplant in MLL+ infants is a result of standardization of HSCT approaches and better definition of risk groups.
High Risk Areas Where HSCT Approaches Could be Considered
Table 6 lists several risk groups where HSCT is being assessed and/or practiced by some groups. Most of the indications listed for relapsed and multiple relapsed ALL are less controversial, having been verified by several studies.9,61,67 The CR1 indications engender more controversy, although most groups feel that primary induction failure patients who achieve a CR should receive HSCT in CR1.12,62 In addition, most groups feel that patients with persistent MRD define a very high risk group where HSCT is a viable option.9 Ph+ ALL treatment approaches are undergoing significant changes as data using tyrosine kinase inhibitors will dramatically redefine who should and should not receive HSCT over the next few years.17 Although HSCT results for extreme hypodiploidy have not been reported in large numbers, survival with chemotherapy alone is poor,25 and unpublished CIBMTR data on 37 pediatric patients with hypodiploid ALL transplanted between 1990–2005 showed a 2 year OS rate of 68% (Mary Eapen, personal communication), consistent with outcomes of CR1 transplantation for children with other risk factors. As reviewed above, a selected group of very high risk MLL+ infants who achieve CR and have favorable donor options may benefit from HSCT.21
Table 6.
Possible Indications for HSCT in Pediatric ALL
| CR1 Risk Groups Under Study: |
| Primary Induction Failure |
| Persistent MRD after consolidation |
| t(9:22) Philadelphia Chromosome Positive1 |
| Extreme Hypodiploidy (<44 chromosomes) |
| Infants with MLL rearrangements <6m with HR characteristics |
| New High Risk CR1 Groups Not Yet Being Used for Treatment Assignment: |
| Early T-cell precursor ALL |
| T-cell ALL lacking bi-allelic TCR gamma locus deletions |
| IKZF1 deletions (associated with JAK1&2 deletions and deletion of CDKN2A/B) |
| Gene cluster group 8 |
| CR2 Risk Groups Under Study: |
| High Risk |
|
| Intermediate Risk |
|
| CR3+ Risk Groups Under Study: |
| Any second or greater relapse, whether marrow, isolated extramedullary relapse, or combined |
Early COG data shows promising 3 year survival. If verified, Ph+ CR1 transplantation may later be based upon persistent MRD.
There are several risk groups listed in Table 6 that have been recently defined based upon elegant molecular studies.8,24,68–70 Some of these groups have data mature enough to consider testing the role of HSCT, while others require further testing in wider cohorts to verify their predictive power. The approach outlined in Figure 3 should be considered as these and other risk factors begin to be used to define therapeutic approaches in the coming years.
Conclusion
The field of pediatric ALL is rapidly advancing as new agents are being incorporated into up front protocols and molecular diagnostics and sensitive response measures define risk groups more accurately. The field of HSCT is also rapidly advancing as improved approaches decrease TRM and improve GVL, and targeted cellular therapies currently in early development may offer new approaches to very high-risk disease. Careful collaboration between HSCT and chemotherapy practitioners is needed to develop rational approaches and analyze outcomes using appropriate techniques. Such efforts will help optimize ALL therapy, targeting HSCT where it is most effective.
Figure 2.
Probability of Survival according to Transplant Indication and Donor Type
Acknowledgments
This work is supported in part by grant CA21765 from the National Cancer institute and by the American Lebanese Syrian Associated Charities.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pui CH, Campana D, Pei D, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009;360:2730–2741. doi: 10.1056/NEJMoa0900386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moricke A, Zimmermann M, Reiter A, et al. Long-term results of five consecutive trials in childhood acute lymphoblastic leukemia performed by the ALL-BFM study group from 1981 to 2000. Leukemia. 2010;24:265–284. doi: 10.1038/leu.2009.257. [DOI] [PubMed] [Google Scholar]
- 3.Gaynon PS, Angiolillo AL, Carroll WL, et al. Long-term results of the children’s cancer group studies for childhood acute lymphoblastic leukemia 1983–2002: a Children’s Oncology Group Report. Leukemia. 2010;24:285–297. doi: 10.1038/leu.2009.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pulsipher MA, Bader P, Klingebiel T, Cooper LJ. Allogeneic transplantation for pediatric acute lymphoblastic leukemia: the emerging role of peritransplantation minimal residual disease/chimerism monitoring and novel chemotherapeutic, molecular, and immune approaches aimed at preventing relapse. Biol Blood Marrow Transplant. 2008;15:62–71. doi: 10.1016/j.bbmt.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Tallen G, Ratei R, Mann G, et al. Long-term outcome in children with relapsed acute lymphoblastic leukemia after time-point and site-of-relapse stratification and intensified short-course multidrug chemotherapy: results of trial ALL-REZ BFM 90. J Clin Oncol. 2010;28:2339–2347. doi: 10.1200/JCO.2009.25.1983. [DOI] [PubMed] [Google Scholar]
- 6.MacMillan ML, Davies SM, Nelson GO, et al. Twenty years of unrelated donor bone marrow transplantation for pediatric acute leukemia facilitated by the National Marrow Donor Program. Biol Blood Marrow Transplant. 2008;14:16–22. doi: 10.1016/j.bbmt.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 7.Borowitz MJ, Devidas M, Hunger SP, et al. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia and its relationship to other prognostic factors: a Children’s Oncology Group study. Blood. 2008;111:5477– 5485. doi: 10.1182/blood-2008-01-132837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harvey RC, Mullighan CG, Wang X, et al. Identification of novel cluster groups in pediatric high-risk B-precursor acute lymphoblastic leukemia with gene expression profiling: correlation with genome-wide DNA copy number alterations, clinical characteristics, and outcome. Blood. 2010 doi: 10.1182/blood-2009-08-239681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schrauder A, von Stackelberg A, Schrappe M, Cornish J, Peters C. Allogeneic hematopoietic SCT in children with ALL: current concepts of ongoing prospective SCT trials. Bone Marrow Transplant. 2008;41 (Suppl 2):S71–74. doi: 10.1038/bmt.2008.58. [DOI] [PubMed] [Google Scholar]
- 10.Pulsipher MA, Hunger SP, Gamis AS, Wall DA, Grupp SA. Allogeneic marrow transplantation in children with acute leukemia: careful comparison with chemotherapy alternatives required. Leukemia. 2010;24:1212–1216. doi: 10.1038/leu.2010.72. [DOI] [PubMed] [Google Scholar]
- 11.Schrauder A, Reiter A, Gadner H, et al. Superiority of allogeneic hematopoietic stem-cell transplantation compared with chemotherapy alone in high-risk childhood T-cell acute lymphoblastic leukemia: results from ALL-BFM 90 and 95. J Clin Oncol. 2006;24:5742–5749. doi: 10.1200/JCO.2006.06.2679. [DOI] [PubMed] [Google Scholar]
- 12.Balduzzi A, Valsecchi MG, Uderzo C, et al. Chemotherapy versus allogeneic transplantation for very-high-risk childhood acute lymphoblastic leukaemia in first complete remission: comparison by genetic randomisation in an international prospective study. Lancet. 2005;366:635–642. doi: 10.1016/S0140-6736(05)66998-X. [DOI] [PubMed] [Google Scholar]
- 13.Pui CH, Relling MV, Downing JR. Acute lymphoblastic leukemia. N Engl J Med. 2004;350:1535–1548. doi: 10.1056/NEJMra023001. [DOI] [PubMed] [Google Scholar]
- 14.Roberts WM, Rivera GK, Raimondi SC, et al. Intensive chemotherapy for Philadelphia-chromosome-positive acute lymphoblastic leukaemia. Lancet. 1994;343:331–332. doi: 10.1016/s0140-6736(94)91166-5. [DOI] [PubMed] [Google Scholar]
- 15.Arico M, Valsecchi MG, Camitta B, et al. Outcome of treatment in children with Philadelphia chromosome-positive acute lymphoblastic leukemia. N Engl J Med. 2000;342:998–1006. doi: 10.1056/NEJM200004063421402. [DOI] [PubMed] [Google Scholar]
- 16.Arico M, Schrappe M, Hunger SP, et al. Clinical Outcome of Children With Newly Diagnosed Philadelphia Chromosome. Positive Acute Lymphoblastic Leukemia Treated Between 1995 and 2005. J Clin Oncol. 2010 doi: 10.1200/JCO.2010.30.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schultz KR, Bowman WP, Aledo A, et al. Improved early event-free survival with imatinib in Philadelphia chromosome-positive acute lymphoblastic leukemia: a children’s oncology group study. J Clin Oncol. 2009;27:5175–5181. doi: 10.1200/JCO.2008.21.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pui CH, Gaynon PS, Boyett JM, et al. Outcome of treatment in childhood acute lymphoblastic leukaemia with rearrangements of the 11q23 chromosomal region. Lancet. 2002;359:1909–1915. doi: 10.1016/S0140-6736(02)08782-2. [DOI] [PubMed] [Google Scholar]
- 19.Pieters R, Schrappe M, De Lorenzo P, et al. A treatment protocol for infants younger than 1 year with acute lymphoblastic leukaemia (Interfant-99): an observational study and a multicentre randomised trial. Lancet. 2007;370:240–250. doi: 10.1016/S0140-6736(07)61126-X. [DOI] [PubMed] [Google Scholar]
- 20.Dreyer Z, Dinndorf PA, Camitta B, et al. Prospective analysis of the role of hematopoietic stem cell transoplantation in infants with acute lymphoblastic leukemia in first remission and MLL gene rearrangements: a report from the Children’s Oncology Group. J Clin Oncol. 2010 doi: 10.1200/JCO.2009.26.8938. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mann G, Attarbaschi A, Schrappe M, et al. Improved outcome with hematopoietic stem cell transplantation in a poor prognostic subgroup of infants with mixed-lineage-leukemia (MLL)-rearranged acute lymphoblastic leukaemia: results from the Interfant-99 Study. Blood. 2010 doi: 10.1182/blood-2010-03-273532. [DOI] [PubMed] [Google Scholar]
- 22.Van der Velden VH, Corral L, Valsecchi MG, et al. Prognostic significance of minimal residual disease in infants with acute lymphoblastic leukemia treated within the Interfant-99 protocol. Leukemia. 2009;23:1073–1079. doi: 10.1038/leu.2009.17. [DOI] [PubMed] [Google Scholar]
- 23.Pui C, Carroll W, Meshinchi S, Arceci RJ. Biology, risk stratification and therapy of pediatric acute leukemia: an update. J Clin Oncol. 2010 doi: 10.1200/JCO.2010.30.7405. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coustan-Smith E, Mullighan CG, Onciu M, et al. Early T-cell precursor leukaemia: a subtype of very high-risk acute lymphoblastic leukaemia. Lancet Oncol. 2009;10:147–156. doi: 10.1016/S1470-2045(08)70314-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nachman JB, Heerema NA, Sather H, et al. Outcome of treatment in children with hypodiploid acute lymphoblastic leukemia. Blood. 2007;110:1112–1115. doi: 10.1182/blood-2006-07-038299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pui CH, Robison LL, Look AT. Acute lymphoblastic leukaemia. Lancet. 2008;371:1030–1043. doi: 10.1016/S0140-6736(08)60457-2. [DOI] [PubMed] [Google Scholar]
- 27.Arico M, Valsecchi MG, Conter V, et al. Improved outcome in high-risk childhood acute lymphoblastic leukemia defined by prednisone-poor response treated with double Berlin-Frankfurt-Muenster protocol II. Blood. 2002;100:420–426. doi: 10.1182/blood.v100.2.420. [DOI] [PubMed] [Google Scholar]
- 28.Moricke A, Reiter A, Zimmermann M, et al. Risk-adjusted therapy of acute lymphoblastic leukemia can decrease treatment burden and improve survival: treatment results of 2169 unselected pediatric and adolescent patients enrolled in the trial ALL-BFM 95. Blood. 2008;111:4477–4489. doi: 10.1182/blood-2007-09-112920. [DOI] [PubMed] [Google Scholar]
- 29.Oudot C, Auclerc MF, Levy V, et al. Prognostic factors for leukemic induction failure in children with acute lymphoblastic leukemia and outcome after salvage therapy: the FRALLE 93 study. J Clin Oncol. 2008;26:1496–1503. doi: 10.1200/JCO.2007.12.2820. [DOI] [PubMed] [Google Scholar]
- 30.Schrappe M, Reiter A, Zimmermann M, et al. Long-term results of four consecutive trials in childhood ALL performed by the ALL-BFM study group from 1981 to 1995. Berlin-Frankfurt-Munster. Leukemia. 2000;14:2205–2222. doi: 10.1038/sj.leu.2401973. [DOI] [PubMed] [Google Scholar]
- 31.Burkhardt B, Reiter A, Landmann E, et al. Poor outcome for children and adolescents with progressive disease or relapse of lymphoblastic lymphoma: a report from the berlin-frankfurt-muenster group. J Clin Oncol. 2009;27:3363–3369. doi: 10.1200/JCO.2008.19.3367. [DOI] [PubMed] [Google Scholar]
- 32.Einsiedel HG, von Stackelberg A, Hartmann R, et al. Long-term outcome in children with relapsed ALL by risk-stratified salvage therapy: results of trial acute lymphoblastic leukemia-relapse study of the Berlin-Frankfurt-Munster Group 87. J Clin Oncol. 2005;23:7942–7950. doi: 10.1200/JCO.2005.01.1031. [DOI] [PubMed] [Google Scholar]
- 33.Herold R, von Stackelberg A, Hartmann R, Eisenreich B, Henze G. Acute lymphoblastic leukemia-relapse study of the Berlin-Frankfurt-Munster Group (ALL-REZ BFM) experience: early treatment intensity makes the difference. J Clin Oncol. 2004;22:569–570. doi: 10.1200/JCO.2004.99.153. author reply 570–561. [DOI] [PubMed] [Google Scholar]
- 34.von Stackelberg A, Seeger K, Henze G, Eckert C. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia after first relapse. Leukemia. 2004;18:1727–1728. doi: 10.1038/sj.leu.2403475. author reply 1728–1729. [DOI] [PubMed] [Google Scholar]
- 35.Gaynon PS, Harris RE, Altman AJ, et al. Bone Marrow Transplantation Versus Prolonged Intensive Chemotherapy for Children With Acute Lymphoblastic Leukemia and an Initial Bone Marrow Relapse Within 12 Months of the Completion of Primary Therapy: Children’s Oncology Group Study CCG-1941. J Clin Oncol. 2006;24:3150–3156. doi: 10.1200/JCO.2005.04.5856. [DOI] [PubMed] [Google Scholar]
- 36.Rocha V, Cornish J, Sievers EL, et al. Comparison of outcomes of unrelated bone marrow and umbilical cord blood transplants in children with acute leukemia. Blood. 2001;97:2962–2971. doi: 10.1182/blood.v97.10.2962. [DOI] [PubMed] [Google Scholar]
- 37.Eapen M, Rubinstein P, Zhang M-J, et al. Comparable Long-Term Survival After Unrelated and HLA-Matched Sibling Donor Hematopoietic Stem Cell Transplantations for Acute Leukemia in Children Younger Than 18 Months. J Clin Oncol. 2006;24:145–151. doi: 10.1200/JCO.2005.02.4612. [DOI] [PubMed] [Google Scholar]
- 38.Peters C, Schrauder A, Schrappe M, et al. Allogeneic haematopoietic stem cell transplantation in children with acute lymphoblastic leukaemia: the BFM/IBFM/EBMT concepts. Bone Marrow Transplant. 2005;35 (Suppl 1):S9–11. doi: 10.1038/sj.bmt.1704835. [DOI] [PubMed] [Google Scholar]
- 39.Bader P, Kreyenberg H, Henze GH, et al. Prognostic value of minimal residual disease quantification before allogeneic stem-cell transplantation in relapsed childhood acute lymphoblastic leukemia: the ALL-REZ BFM Study Group. J Clin Oncol. 2009;27:377–384. doi: 10.1200/JCO.2008.17.6065. [DOI] [PubMed] [Google Scholar]
- 40.Flohr T, Schrauder A, Cazzaniga G, et al. Minimal residual disease-directed risk stratification using real-time quantitative PCR analysis of immunoglobulin and T-cell receptor gene rearrangements in the international multicenter trial AIEOP-BFM ALL 2000 for childhood acute lymphoblastic leukemia. Leukemia. 2008;22:771–782. doi: 10.1038/leu.2008.5. [DOI] [PubMed] [Google Scholar]
- 41.Miano M, Labopin M, Hartmann O, et al. Haematopoietic stem cell transplantation trends in children over the last three decades: a survey by the paediatric diseases working party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2007;39:89–99. doi: 10.1038/sj.bmt.1705550. [DOI] [PubMed] [Google Scholar]
- 42.Cornish J. Unrelated donor transplant for acute leukaemia in children--the UK experience. Pathol Biol (Paris) 2005;53:167–170. doi: 10.1016/j.patbio.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 43.Rocha V, Locatelli F. Searching for alternative hematopoietic stem cell donors for pediatric patients. Bone Marrow Transplant. 2008;41:207–214. doi: 10.1038/sj.bmt.1705963. [DOI] [PubMed] [Google Scholar]
- 44.Eapen M, Horowitz MM, Klein JP, et al. Higher Mortality After Allogeneic Peripheral-Blood Transplantation Compared With Bone Marrow in Children and Adolescents: The Histocompatibility and Alternate Stem Cell Source Working Committee of the International Bone Marrow Transplant Registry. J Clin Oncol. 2004;22:4872–4880. doi: 10.1200/JCO.2004.02.189. [DOI] [PubMed] [Google Scholar]
- 45.Pulsipher MA, Chitphakdithai P, Miller JP, et al. Adverse events among 2408 unrelated donors of peripheral blood stem cells: results of a prospective trial from the National Marrow Donor Program. Blood. 2009;113:3604–3611. doi: 10.1182/blood-2008-08-175323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pulsipher MA, Levine JE, Hayashi RJ, et al. Safety and efficacy of allogeneic PBSC collection in normal pediatric donors: The Pediatric Blood and Marrow Transplant Consortium Experience (PBMTC) 1996–2003. Bone Marrow Transplant. 2004;35:361–367. doi: 10.1038/sj.bmt.1704743. [DOI] [PubMed] [Google Scholar]
- 47.Bordigoni P, Esperou H, Souillet G, et al. Total body irradiation-high-dose cytosine arabinoside and melphalan followed by allogeneic bone marrow transplantation from HLA-identical siblings in the treatment of children with acute lymphoblastic leukaemia after relapse while receiving chemotherapy: a Societe Francaise de Greffe de Moelle study. Br J Haematol. 1998;102:656–665. doi: 10.1046/j.1365-2141.1998.00825.x. [DOI] [PubMed] [Google Scholar]
- 48.Dopfer R, Henze G, Bender-Gotze C, et al. Allogeneic bone marrow transplantation for childhood acute lymphoblastic leukemia in second remission after intensive primary and relapse therapy according to the BFM- and CoALL-protocols: results of the German Cooperative Study. Blood. 1991;78:2780–2784. [PubMed] [Google Scholar]
- 49.Spitzer TR, Peters C, Ortlieb M, et al. Etoposide in combination with cyclophosphamide and total body irradiation or busulfan as conditioning for marrow transplantation in adults and children. Int J Radiat Oncol Biol Phys. 1994;29:39–44. doi: 10.1016/0360-3016(94)90224-0. [DOI] [PubMed] [Google Scholar]
- 50.Locatelli F, Nollke P, Zecca M, et al. Hematopoietic stem cell transplantation (HSCT) in children with juvenile myelomonocytic leukemia (JMML): results of the EWOG-MDS/EBMT trial. Blood. 2005;105:410–419. doi: 10.1182/blood-2004-05-1944. [DOI] [PubMed] [Google Scholar]
- 51.Miano M, Cancedda R, Hartmann O, et al. Survey on haematopoietic stem cell transplantation for children in Europe. Bone Marrow Transplant. 2005;35 (Suppl 1):S3–8. doi: 10.1038/sj.bmt.1704834. [DOI] [PubMed] [Google Scholar]
- 52.Peters C, Minkov M, Gadner H, et al. Statement of current majority practices in graft-versus-host disease prophylaxis and treatment in children. Bone Marrow Transplant. 2000;26:405–411. doi: 10.1038/sj.bmt.1702524. [DOI] [PubMed] [Google Scholar]
- 53.Handgretinger R, Lang P. The history and future prospective of haplo-identical stem cell transplantation. Cytotherapy. 2008;10:443–451. doi: 10.1080/14653240802251507. [DOI] [PubMed] [Google Scholar]
- 54.Klingebiel T, Cornish J, Labopin M, et al. Results and factors influencing outcome after fully haploidentical hematopoietic stem cell transplant in children with very-high risk acute lymphoblastic leukemia - impact of center size: an analysis on behalf of the Acute Leukemia and Pediatric Disease Working Parties of the European Blood and Marrow Transplant group. Blood. 2009 doi: 10.1182/blood-2009-03-207001. [DOI] [PubMed] [Google Scholar]
- 55.Davies SM, Wang D, Wang T, et al. Recent decrease in acute graft-versus-host disease in children with leukemia receiving unrelated donor bone marrow transplants. Biol Blood Marrow Transplant. 2009;15:360–366. doi: 10.1016/j.bbmt.2008.12.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eapen M, Rubinstein P, Zhang MJ, et al. Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukaemia: a comparison study. Lancet. 2007;369:1947–1954. doi: 10.1016/S0140-6736(07)60915-5. [DOI] [PubMed] [Google Scholar]
- 57.Brunstein CG, Gutman JA, Weisdorf DJ, et al. Allogeneic hematopoietic cell transplantation for hematological malignancy: relative risks and benefits of double umbilical cord blood. Blood. 2010 doi: 10.1182/blood-2010-05-285304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cooley S, Trachtenberg E, Bergemann TL, et al. Donors with group B KIR haplotypes improve relapse-free survival after unrelated hematopoietic cell transplantation for acute myelogenous leukemia. Blood. 2009;113:726–732. doi: 10.1182/blood-2008-07-171926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jena B, Dotti G, Cooper LJ. Redirecting T-cell specificity by introducing a tumor-specific chimeric antigen receptor. Blood. 2010;116:1035–1044. doi: 10.1182/blood-2010-01-043737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lawson SE, Harrison G, Richards S, et al. The UK experience in treating relapsed childhood acute lymphoblastic leukaemia: a report on the medical research council UKALLR1 study. Br J Haematol. 2000;108:531–543. doi: 10.1046/j.1365-2141.2000.01891.x. [DOI] [PubMed] [Google Scholar]
- 61.Eapen M, Raetz E, Zhang MJ, et al. Outcomes after HLA-matched sibling transplantation or chemotherapy in children with B-precursor acute lymphoblastic leukemia in a second remission: a collaborative study of the Children’s Oncology Group and the Center for International Blood and Marrow Transplant Research. Blood. 2006;107:4961–4967. doi: 10.1182/blood-2005-12-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pulsipher MA, Wall DA, Grimley M, et al. A Phase I/II study of the safety and efficacy of the addition of sirolimus to tacrolimus/methotrexate graft versus host disease prophylaxis after allogeneic haematopoietic cell transplantation in paediatric acute lymphoblastic leukaemia (ALL) Br J Haematol. 2009 doi: 10.1111/j.1365-2141.2009.07889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Raetz EA, Borowitz MJ, Devidas M, et al. Reinduction platform for children with first marrow relapse in acute lymphoblastic lymphoma. J Clin Oncol. 2008;26:3971–3978. doi: 10.1200/JCO.2008.16.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gaynon PS, Harris RE, Altman AJ, et al. Bone marrow transplantation versus prolonged intensive chemotherapy for children with acute lymphoblastic leukemia and an initial bone marrow relapse within 12 months of the completion of primary therapy: Children’s Oncology Group study CCG-1941. J Clin Oncol. 2006;24:3150–3156. doi: 10.1200/JCO.2005.04.5856. [DOI] [PubMed] [Google Scholar]
- 65.Hilden JM, Dinndorf PA, Meerbaum SO, et al. Analysis of prognostic factors of acute lymphoblastic leukemia in infants: report on CCG 1953 from the Children’s Oncology Group. Blood. 2006;108:441–451. doi: 10.1182/blood-2005-07-3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eapen M, Rubinstein P, Zhang MJ, et al. Comparable long-term survival after unrelated and HLA-matched sibling donor hematopoietic stem cell transplantations for acute leukemia in children younger than 18 months. J Clin Oncol. 2006;24:145–151. doi: 10.1200/JCO.2005.02.4612. [DOI] [PubMed] [Google Scholar]
- 67.Borgmann A, von Stackelberg A, Hartmann R, et al. Unrelated donor stem cell transplantation compared with chemotherapy for children with acute lymphoblastic leukemia in a second remission: a matched-pair analysis. Blood. 2003;101:3835–3839. doi: 10.1182/blood.V101.10.3835. [DOI] [PubMed] [Google Scholar]
- 68.Gutierrez A, Dahlberg SE, Neuberg DS, et al. Absence of biallelic TCRgamma deletion predicts early treatment failure in pediatric T-cell acute lymphoblastic leukemia. J Clin Oncol. 2010;28:3816–3823. doi: 10.1200/JCO.2010.28.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mullighan CG, Zhang J, Harvey RC, et al. JAK mutations in high-risk childhood acute lymphoblastic leukemia. Proc Natl Acad Sci U S A. 2009;106:9414–9418. doi: 10.1073/pnas.0811761106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mullighan CG, Su X, Zhang J, et al. Deletion of IKZF1 and prognosis in acute lymphoblastic leukemia. N Engl J Med. 2009;360:470–480. doi: 10.1056/NEJMoa0808253. [DOI] [PMC free article] [PubMed] [Google Scholar]