Abstract
Objective
The respiratory-related evoked potential (RREP) has become an established technique for studying the neural processing of respiratory signals. However, the increasing availability of high-density EEG systems necessitates new criteria for obtaining acceptable RREPs with these systems.
Methods
The present study examined the minimum criteria for the number of inspiratory occlusions that need to be averaged in order to obtain a signal-to-noise ratio of 2:1 (3dB) for the RREP components Nf, P1, N1, P2 and P3 with a 129 sensor high-density EEG system in 12 healthy volunteers. RREPs resulting from averaging 8, 16, 32 and 64 inspiratory occlusions were compared.
Results
Analyses of signal-to-noise ratios demonstrated that a minimum of 32 and 16 inspiratory occlusions should be averaged for Nf and P1, respectively. For N1, P2, and P3, an average of at least 8 inspiratory occlusions is required. However, to account for inter-individual variability, 64 averaged occlusions for Nf, 32 averaged occlusions for P1, and 16 averaged occlusions for N1, P2, and P3 are recommended which more reliably exceed the signal-to-noise threshold.
Conclusions
These numbers provide the minimum and the recommended criteria for reliable measurements of the RREP for an adequate number of repeated occlusion epochs to be averaged in order to yield a reliable signal-to-noise ratio using a 129 sensor EEG system.
Significance
The present study provides minimum and recommended criteria for obtaining acceptable RREPs with high-density EEG systems.
Keywords: EEG, perception, respiratory-related evoked potential, signal-to-noise ratio
1. Introduction
The respiratory-related evoked potential (RREP) recorded from the electroencephalogram (EEG) has become an important non-invasive technique for studying the neural processing of respiratory mechanosensation (Davenport et al., 1986). The RREP is a measure of cerebral cortical activity elicited by breathing against inspiratory resistive loads or by short inspiratory occlusion and quantifies the initial arrival and subsequent processing of sensory afferent respiratory signals in the cortex. The RREP is comprised of short latency components Nf, P1 and N1 (< 130 ms post stimulus), which presumably reflect the initial arrival and first-order processing of afferent respiratory signals in sensorimotor regions. The longer latency RREP components P2 and P3 (> 150 ms post stimulus) seem to characterize subsequent higher-order cognitive processing in other cortical areas (Chan and Davenport, 2010).
Besides other factors (e.g., low noise levels of the employed EEG system, correct position and low impedances of the sensors), reliable measurements of mechanically elicited somatosensory evoked potentials such as the RREP require an adequate number of stimulus presentation epochs to be averaged in order to yield a reliable signal-to-noise ratio. In this regard, increasing numbers of averaged stimulus epochs usually improve the signal-to-noise ratio of evoked potentials (Duffy et al., 1989). Most previous RREP studies used EEG systems with relatively few (3 to 29) electrode sensors (e.g., Revelette and Davenport, 1990; Webster and Colrain, 2000). For these EEG systems, an average of at least 32 stimulus presentation epochs has been established as minimum criterion for obtaining acceptable signal-to-noise RREPs (Revelette and Davenport, 1990) with many studies averaging even more epochs (e.g., Davenport et al., 1986; Webster and Colrain, 2000). Moreover, these minimum criteria were only established for cephalic referenced (Cz) P1, N1 and P2 RREP components at only two active (C3 and C4) cortical sites (Revelette and Davenport, 1990).
New high-density EEG systems with as many as 129 sensors and improved signal-to-noise properties have recently become available for RREP measurements (von Leupoldt et al., 2010a; 2010b). However, a systematic investigation of the relationship between signal-to-noise and the minimum number of occlusion presentation epochs with high-density EEG systems has not been made. We hypothesized that a minimal signal-to-noise threshold will require more occlusion epochs for small amplitude, short latency RREP components than larger amplitude, long latency RREP components. These criteria are of fundamental importance for reliable RREP studies that use this new generation of EEG methodology
In the present study we determined the minimum signal-to-noise criteria for the number of inspiratory occlusions that were need to be included as stimulus presentation epochs in order to yield acceptable RREP components Nf, P1, N1, P2 and P3 with a 129 sensor high-density EEG system.
2. Method
After providing informed written consent, twelve healthy, non-smoking volunteers without respiratory disease participated in this study (6 female, mean age 20.3 ± 2.7 years, mean forced expiratory volume in 1 s (FEV1) in % predicted 94.9 ± 8.9 L). The study was approved by the Institutional Review Board of the University of Florida.
RREPs were obtained during 4 blocks of occlusion presentations of 7 min each, separated by variable inter-block intervals. Each block began with a 1 min epoch of adaptation to the mouthpiece breathing during which no inspiratory occlusions were presented. This was followed by a 6 min epoch of presenting inspiratory occlusions while participants viewed pictures presented on a monitor. In each block, participants breathed via a mouthpiece through a non-rebreathing valve (Hans Rudolph Inc., Kansas City, USA). The inspiratory port of the valve was connected to a pressure activated occluder. Inspiration was interrupted every 2–6 breaths for 500 ms by manual triggering of the occluder after the onset of inspiration with a parallel marker signal being sent to the EEG recorder. Inspiratory onset was indicated by the continuously displayed mouth pressure signal recorded from the center port of the non-rebreathing valve by a differential-pressure transducer (model MP-45, Validyne Engineering, Northridge, USA), connected to a PowerLab biosignal recording unit (ADInstruments, Bella Vista, Australia).
EEG data were recorded from the scalp using a 129-channel system (Electrical Geodesics Inc., Eugene, USA) with scalp impedance < 70 kΩ, sampling rate = 250 Hz, vertex sensor as reference electrode and on-line bandpass filter (0.1 – 100 Hz). All further processing was performed offline, using functions built into BESA 5.1. After digital low-pass filtering (30 Hz) and artifact corrections, occlusion epochs were extracted (200 ms pre- and 1000 ms post- stimulus) and averaged using a maximum of 200 µV as cutoff amplitude.
For each participant, 4 different averages were calculated that included: a) 8 occlusions (first 8 occlusions from block 1), b) 16 occlusions (first 16 occlusions from block 1), c) 32 occlusions (first 16 occlusions of block 1 and 2) and d) 64 occlusions (first 16 occlusions of all 4 blocks) which were finally averaged across the whole study sample. Based on previous reports (e.g., Davenport et al., 1986; Harver et al., 1995; Redolfi et al., 2005; Webster and Colrain, 2000), the RREP components were identified as follows: Nf = negative peak in the frontal region (latency: 25–50 ms), P1 = positive peak in the centro-parietal region (latency: 45–65 ms), N1 = negative peak in the centro-lateral region (latency: 85–125 ms), P2 = positive peak in the central region (latency: 160–230 ms), and P3 = positive peak in the centro-parietal region (latency: 250–350 ms).
For each RREP component, the individual signal-to-noise ratios were calculated as root mean square of the global signal minus the root mean square of the global signal during the baseline (in µV) separately for each of the 4 averages (e.g., 8, 16, 32 and 64 occlusions). These ratios were compared with repeated measures analyses of variance (ANOVA) including contrast analyses testing for linear increases in the ratios with increasing number of averaged occlusions. A signal-to-noise ratio of 2:1 (3dB) was chosen as the criterion for minimum acceptable signal presence of the RREP in this study. The 3dB point is a commonly used criteria for the presence versus absence of a signal in signal processing (Yariv, 1990), e.g. in filter construction (Boston and Ainslie, 1980).
3. Results
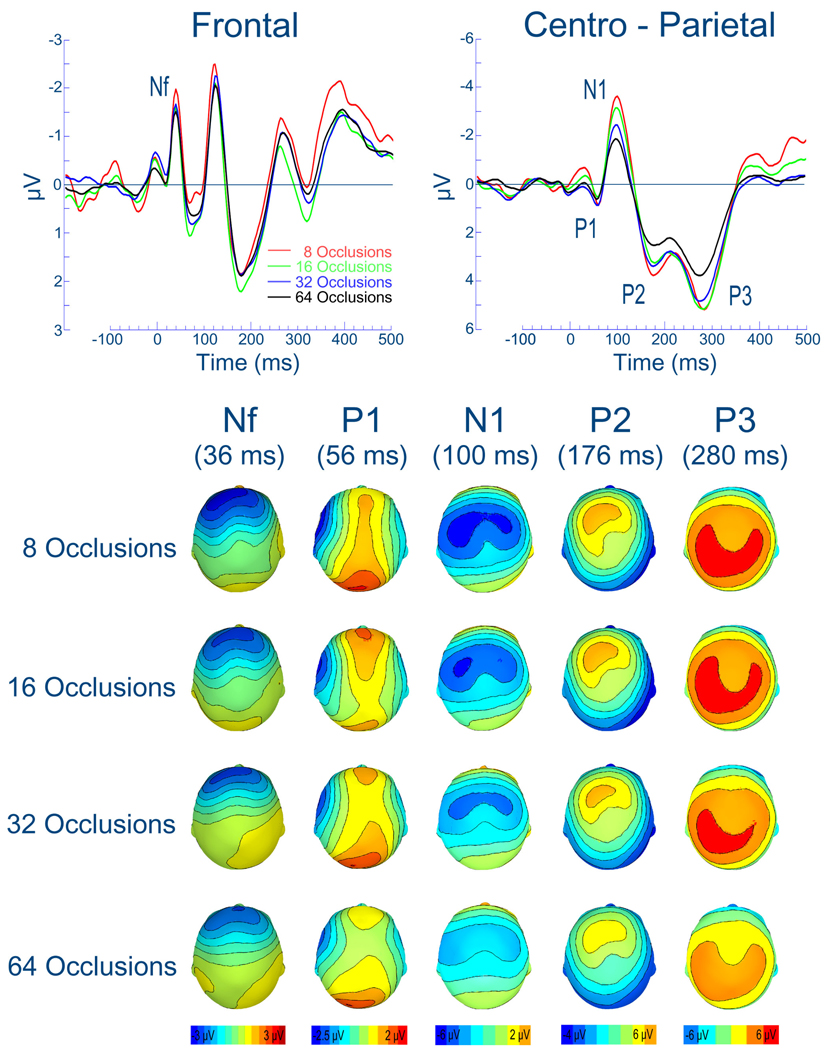
As demonstrated in Figure 1, the RREP components Nf, P1, N1, P2 and P3 could be clearly observed over the frontal and centro-parietal region, respectively, regardless of the number of averaged inspiratory occlusions. Similarly, the scalp topography maps showed comparable localization of the EEG voltage patterns for all RREP components when inspiratory occlusions were averaged across 8, 16, 32 and 64 inspiratory occlusions. Visual inspection of the RREPs indicates that for most RREP components an average of 8 occlusions resulted in the highest amplitudes, but also in the highest baseline noise levels, whereas 64 occlusions resulted in the smallest amplitudes and smallest baseline noise levels.
Figure 1.
Group mean respiratory-related evoked potential averaged across 8, 16, 32 and 64 inspiratory occlusions over the frontal and centro-parietal region (upper panel). Group mean scalp topography maps of the EEG voltage for the RREP components Nf, P1, N1, P2 and P3 (mapped at their peak latencies) averaged across 8, 16, 32 and 64 inspiratory occlusions (lower panel).
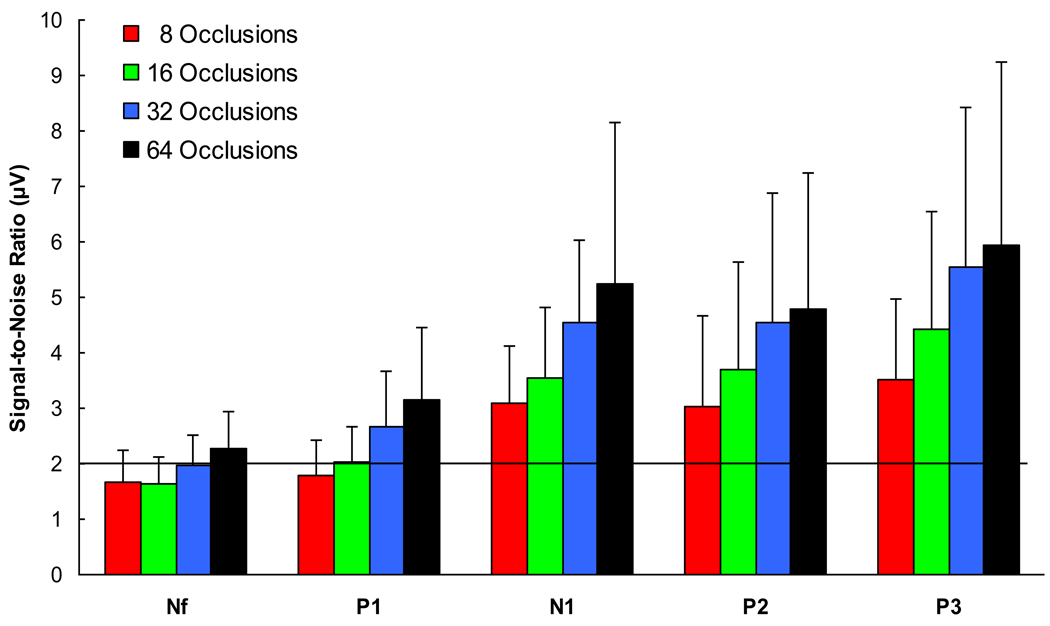
As demonstrated in Figure 2, the signal-to-noise ratios for each RREP component demonstrated a significant linear increase with increasing numbers of averaged occlusions (all p’s < 0.01). In order to reach a threshold of 2:1 in the signal-to-noise-ratio, at least 32 occlusions were necessary for Nf and at least 16 occlusions for P1. However, because these ratios were close to the threshold, 64 averaged occlusions for Nf and at least 32 averaged occlusions for P1 more reliably exceed the signal-to-noise threshold. For the subsequent components N1, P2, and P3, the threshold of 2:1 was reached with 8 averaged occlusions. However, taking the observed inter-individual variability into account, an improved and more reliable signal-to-noise ratio can be reached with 16 averaged occlusions.
Figure 2.
Means (± SD) of the signal-to-noise ratios (root mean square of the global signal minus the baseline) for the RREP components Nf, P1, N1, P2 and P3 averaged across 8, 16, 32 and 64 inspiratory occlusions. The black line denotes the employed signal-to-noise ratio of 2:1 (3dB).
4. Discussion
The present study examined the minimum signal-to-noise criteria for the number of short inspiratory occlusions that need to be averaged as stimulus presentation epochs in order to yield acceptable RREP components with a 129 sensor high-density EEG system. The results recommend that for the short latency RREP components Nf and P1, 64 and 32 inspiratory occlusions, respectively, should be averaged in order to reliably exceed the minimum threshold of 2:1 for the signal-to-noise ratio. For the subsequent components N1, P2, and P3, an average of 16 inspiratory occlusions is recommended in order to reliably meet this criterion. Although the minimum threshold of 2:1 for the signal-to-noise ratio can already be obtained with fewer occlusions for each RREP component, these higher criteria are recommended in order to reduce error variance due to inter-individual variability. These recommended numbers are less than the previous reported minimum criteria of 32 for cephalic referenced RREP measurements with fewer electrode EEG systems (Revelette and Davenport, 1990), which suggests that improved signal-to-noise properties are obtained using high-density EEG systems. In addition, the present findings firstly provide minimum and recommended criteria for the RREP components Nf and P3.
Given the increasing availability of high-density EEG systems, the present criteria are of considerable importance for designing RREP studies using these systems. For example, if attentional or emotional influences on the RREP are to be examined, which have been shown to affect the RREP components N1, P2, and P3, but not Nf and P1, (Davenport et al., 2007; von Leupoldt et al., 2010b; Webster and Colrain, 2000), shorter experimental paradigms with fewer inspiratory occlusions can be used. This reduces the burden for the test participants, which may lead to increased study motivation and compliance and may also prevent habituation effects, i.e. reduced responses due to large numbers of stimulus repetitions. Furthermore, artifacts due to changing impedance of sensors and decreasing attention of participants over the course of long experiments might be prevented.
However, when applying the present criteria it should be kept in mind that these are the numbers of artefact-free occlusions to be averaged. Additional occlusions should be presented because a variable number of occlusions will not survive artefact correction procedures (manual or computerized algorhythms), for example voltage transients due to movements or eye blink. Moreover, each EEG system shows an individual baseline noise level which is dependent on various internal and external sources such as length of electrode cables, amplifier noise, room and shielding characteristics or basic power line artefacts (Duffy et al., 1989). This necessitates careful pilot tests with each EEG system in order to estimate the optimal number of presented occlusions in addition to the reported minimum criteria. In addition, disease state or age might increase neural response variability and thus the presented criteria obtained in healthy younger adults might not apply to older adults, children or patients with neurological or respiratory diseases.
In summary, the present study established for RREP measurements with a 129 sensor high-density EEG system the minimum and the recommended criteria for the number of inspiratory occlusions to be averaged in order to obtain an acceptable 2:1 signal-to-noise ratio for the RREP components Nf, P1, N1, P2 and P3.
Acknowledgement
The authors wish to thank Peter J. Lang and Margaret M. Bradley for their valuable support of this work. The study was supported by a stipend (Heisenberg-Stipendium, DFG LE 1843/9-1) from the German Research Society (Deutsche Forschungsgemeinschaft, DFG) to Andreas von Leupoldt and by a grant from the National Institute of Mental Health (P50 MH 72850) to Peter J. Lang. The funding sources had no impact on the study design, data collection/interpretation or preparation of the present manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All authors report no conflict of interests.
References
- Boston JR, Ainslie PJ. Effects of analog and digital filtering on brain stem auditory evoked potentials. Electroencephalogr Clin Neurophysiol. 1980;48:361–364. doi: 10.1016/0013-4694(80)90273-4. [DOI] [PubMed] [Google Scholar]
- Chan PS, Davenport PW. Respiratory Related Evoked Potential Measures of Cerebral Cortical Respiratory Information Processing. Biol Psych. 2010;84:4–12. doi: 10.1016/j.biopsycho.2010.02.009. [DOI] [PubMed] [Google Scholar]
- Davenport PW, Friedman WA, Thompson FJ, Franzen O. Respiratory-related cortical potentials evoked by inspiratory occlusion in humans. J Appl Physiol. 1986;60:1843–1848. doi: 10.1152/jappl.1986.60.6.1843. [DOI] [PubMed] [Google Scholar]
- Davenport PW, Chan PY, Zhang W, Chou YL. Detection threshold for inspiratory resistive loads and respiratory-related evoked potentials. J Appl Physiol. 2007;102:276–285. doi: 10.1152/japplphysiol.01436.2005. [DOI] [PubMed] [Google Scholar]
- Duffy FH, Iyer VG, Surwillo WW. Clinical Electroencephalography and Topographic Brain Mapping. New York: Springer; 1989. [Google Scholar]
- Harver A, Squires N, Bloch-Salisbury E, Katkin E. Event-related potentials to airway occlusion in young and old subjects. Psychophysiology. 1995;32:121–129. doi: 10.1111/j.1469-8986.1995.tb03303.x. [DOI] [PubMed] [Google Scholar]
- Redolfi S, Raux M, Donzel-Raynaud C, Morelot-Panzini C, Zelter M, Derenne JP, Similowski T, Straus C. Effects of upper airway anaesthesia on respiratory-related evoked potentials in humans. Eur Respir J. 2005;26:1097–1103. doi: 10.1183/09031936.05.00139804. [DOI] [PubMed] [Google Scholar]
- Revelette WR, Davenport PW. Effects of timing of inspiratory occlusion on cerebral evoked potentials in humans. J Appl Physiol. 1990;68:282–288. doi: 10.1152/jappl.1990.68.1.282. [DOI] [PubMed] [Google Scholar]
- von Leupoldt A, Vovk A, Bradley MM, Keil A, Lang PJ, Davenport PW. The impact of emotion on respiratory-related evoked potentials. Psychophysiology. 2010a;47:579–586. doi: 10.1111/j.1469-8986.2009.00956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Leupoldt A, Keil A, Chan PY, Bradley MM, Lang PJ, Davenport PW. Cortical sources of the respiratory-related evoked potential. Respir Physiol Neurobiol. 2010b;170:198–201. doi: 10.1016/j.resp.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster KE, Colrain IM. The respiratory-related evoked potential: effects of attention and occlusion duration. Psychophysiology. 2000;37:310–318. [PubMed] [Google Scholar]
- Yariv A. Signal-to-noise considerations in fiber links with periodic or distributed optical amplification. Opt Lett. 1990;15:1064–1066. doi: 10.1364/ol.15.001064. [DOI] [PubMed] [Google Scholar]