Abstract
Objective
To estimate the risk of large for gestational age (LGA) across categories of glucose tolerance.
STUDY DESIGN
In a cohort of 89,141, women without GDM were categorized by their screening and diagnostic test results, those with GDM were categorized as meeting the NDDG or only the ADA criteria. Multivariable logistic regression models estimated the risk of LGA; screening values 5.5–6.0 mmol/L comprised the referent.
RESULTS
In women without GDM, the OR for LGA was 1.89 (95% CI 1.45–2.45) for fasting, 1.57 (95% CI 1.31–1.89) for 1-hr, 1.60 (95% CI 1.33–1.93) for 2-hr and 1.62 (95% CI 1.23–2.14) for 3-hr values meeting the ADA time-point specific thresholds.
CONCLUSIONS
For GDM identified in a two-step procedure, our findings support the use of isolated abnormal fasting values according to the ADA threshold in identifying women who could benefit from treatment.
Keywords: glycemia, large for gestational age, pregnancy
INTRODUCTION
Gestational diabetes mellitus (GDM), defined as carbohydrate intolerance with onset or first recognition during pregnancy1, is associated with an increased risk of adverse perinatal outcomes.2;3 In the United States, it is currently recommended that GDM be diagnosed in women with at least two plasma glucose values on a diagnostic 100-g, 3-hour OGTT that meet or exceed the thresholds recommended by the American Diabetes Association (ADA) in 20004; the 100-g, 3-hour OGTT is only performed in women with abnormal values (≥ 7.8 mmol/L) on a 50-g, 1-hour GCT screening.
The Hyperglycemia and Adverse Pregnancy Outcome Study (HAPO)5, a large multicenter study designed to identify potential glucose thresholds on the 75-g 2-hour OGTT associated with increased risk of adverse infant outcomes, found that the risk of delivering a large for gestational age (LGA) infant or an infant with hyperinsulinemia increased continuously across increasing categories of maternal glycemia at all time points. Providers were blinded to glucose tolerance status except for among women with overt GDM, defined as fasting plasma glucose > 5.8 mmol/L and/or 2 hour > 11.1 mmol/L, who were excluded from the primary analyses. Diagnostic criteria based on the presence of one abnormal value on the 75-g 2-hour OGTT have been recently proposed from these data.6 The International Association of Diabetes and Pregnancy Study Groups Consensus Panel (IADPSG) used ORs ≥ 1.75 relative to the cohort mean value for each time point in arriving at the following diagnostic criteria: fasting value ≥ 5.1 mmol/L, 1 hour ≥ 10.0 mmol/L, or 2 hour ≥ 8.5 mmol/L.
To provide further information on potential thresholds for the diagnosis of GDM, we estimated the risk of LGA across increasing categories of pregnancy glycemia assessed via 100-g, 3-hour OGTT in women who did not have GDM and did not receive treatment. We also estimated the risk of LGA among women with GDM by the ADA criteria4 only, who did not receive treatment during the study period, and women with GDM by the National Diabetes Data Group (NDDG) criteria7, who were treated. Finally, we estimated the prevalence of GDM if only 1 abnormal glucose value according to the ADA thresholds4, or lower glucose cut points previously found to be associated with LGA risk5;6, were used for diagnosis.
MATERIALS AND METHODS
The study setting is Kaiser Permanente Northern California (KPNC), a large group-practice prepaid health plan that provides comprehensive medical services to members residing in a 14 county region. Approximately 30% of the population that resides in the area served by the KPNC is enrolled in the health plan, which is representative of the underlying population.8;9
To estimate the association between pregnancy glycemia and LGA, we identified 191,095 pregnancies to 158,073 women who delivered a live singleton infant between 1995 and 2004, were 15–45 years of age at delivery, had a maternal weight measurement available between 9–24 weeks gestation and no history of recognized diabetes. We then excluded 4,862 pregnancies missing maternal glucose values on the 50-g, 1-hr GCT, 742 pregnancies missing one or more values on the 100-g, 3-hr OGTT and 1,862 pregnancies with screening glucose values ≥ 7.8 mmol/L but no 100-g, 3-hr OGTT on record. From among the remaining 183,629 pregnancies, we selected the first pregnancy to each woman during the study period, leaving 152,671 pregnancies. We then restricted the cohort to those with height available in the electronic records for the calculation of body mass index (BMI; n= 90,966) and those who delivered between 35 and 42 weeks gestation, resulting in a final analytic cohort of 89,141 mother-infant pairs. To assess whether restriction of the cohort to those with height data biased our results, a sensitivity analysis was conducted among the larger cohort of women with weight data available (n= 149,699). Maternal BMI and maternal weight were highly correlated in those women with height data (R2 = 0.86).
In this setting, during the entire study period, the diagnosis of GDM was based exclusively on the NDDG criteria7 [2 or more plasma glucose values on the 100-g, 3-hour OGTT at or higher than the NDDG thresholds: fasting 5.8 mmol/L; 1-hour 10.5 mmol/L; 2-hour 9.1 mmol/L; 3-hour 8.0 mmol/L]. Among women who did not meet the NDDG criteria7, GDM by the ADA criteria4 only was defined as the presence of 2 plasma glucose values meeting or exceeding the following thresholds: fasting 5.3 mmol/L; 1-hour 10.0 mmol/L; 2-hour 8.6 mmol/L; 3-hour 7.8 mmol/L. Women who met the ADA criteria4 only for GDM did not receive treatment during the study period.
Infant birth weight and gestational age at birth were obtained from the KPNC electronic medical records. A previous study in this setting during the same period10 found 98.5% agreement between gestational age in the electronic medical records and estimates from ultrasonographic data collected prior to 24 weeks. LGA was defined as birth weight greater than the 90th percentile for the study population’s race-ethnicity and gestational-age specific birth weight distribution. The following maternal racial-ethnic groups, obtained by linkage with the State of California’s Birth Certificate data (99% successful linkage), were used to define these distributions: Non-Hispanic white, African American, Asian, Hispanic, and Other/Unknown. Maternal age at delivery, height (meters), weight (kilograms) and gestational age (weeks) at the weight measurement were obtained from the KPNC electronic medical records. Maternal BMI was calculated as weight, in kilograms, at the second trimester divided by height, in meters, squared. Plasma glucose values on the screening 50-g, 1-hr glucose challenge test (hereafter referred to as the screening test) and the diagnostic 100 g, 3-h OGTT (hereafter referred to as the diagnostic test) were obtained from the KPNC clinical laboratory database.
Logistic regression models were used to obtain odds ratios that estimated the relative risk of LGA associated with increasing levels of maternal glycemia. A separate model was constructed for each time point of the 100-g, 3-hour OGTT. Women with normal screening values were categorized as follows: < 5.5 mmol/L, 5.5–6.0 mmol/L, 6.1–6.6 mmol/L, 6.7–7.1 mmol/L, and 7.2–7.7 mmol/L. Women with abnormal screening values (plasma glucose ≥ 7.8 mmol/L) that did not have 2 or more values on the 100-g, 3-hour OGTT meeting or exceeding the ADA glucose thresholds4 for the diagnosis of GDM were further categorized by their plasma glucose values on the diagnostic test. There were six groups for each time point of the diagnostic test; fasting, 1-hour, 2-hour, and 3-hour plasma glucose value classifications are displayed in Figures 1 and 2. For each time point, the sixth group included women with only one abnormal value on the 100-g, 3-hour OGTT according to the ADA thresholds.4 Each model included the screening categories listed above, the plasma glucose categories specific to that time point on the diagnostic test (see Figures 1 and 2), and two indicator terms for GDM (by the ADA criteria only4 and by the NDDG criteria7). Among women with normal screening results (plasma glucose values < 7.8 mmol/L), the mean 1 hour value was 5.83 mmol/L. Similar to the referent selection method of the IADPSG6, the screening category that contained this mean value, 5.5–6.0 mmol/L, served as the reference for all models; the prevalence of LGA in this group was 9.2%.
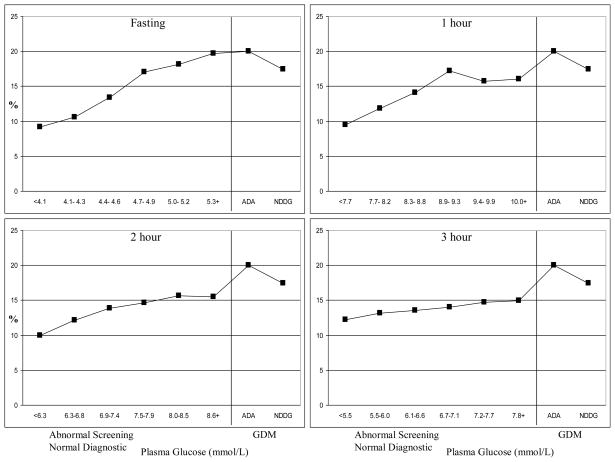
Figure 1. Prevalence of large for gestational age across categories of maternal plasma glucose among women delivering at Kaiser Permanente Northern California, 1995–2004.
ADA = American Diabetes Association
NDDG = National Diabetes Data Group
GDM = gestational diabetes mellitus
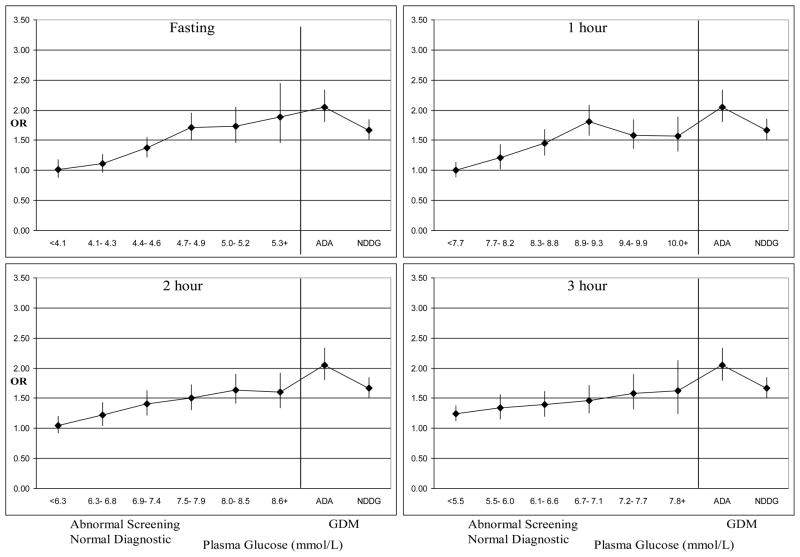
Figure 2. Adjusted odds ratios* for large for gestational age across categories of maternal plasma glucose among women delivering at Kaiser Permanente Northern California, 1995–2004.
* Adjusted for maternal age, race-ethnicity, parity, education, maternal BMI and gestational age at the weight measurement, screening glucose 5.5–6.0 mmol/l is the reference group for all ORs
ADA = American Diabetes Association
NDDG = National Diabetes Data Group
GDM = gestational diabetes mellitus
The models were adjusted for maternal age (15 to 24 years as the reference, 25 to 29 years, 30 to 34 years and 35 to 45 years), race-ethnicity (Non-Hispanic white as the reference, African American, Asian, Hispanic and Other/Unknown), parity (0 as the reference, 1, 2, 3+ and unknown), education (high school graduate, some college, completed college as the reference, beyond college, and unknown), maternal BMI in the second trimester (< 25.0 as the reference, 25.0–29.9, and > 30) and gestational age at the second trimester weight measurement (continuous).
To assess the impact of lower glucose thresholds on the prevalence of GDM, we identified 33,921 women who delivered a live or stillborn infant at KPNC in 2007. We then estimated the prevalence of GDM if 1 versus 2 abnormal glucose values according to the ADA thresholds4 for the 100-g, 3-hour OGTT were used for diagnosis. A hierarchical, stepwise approach was utilized. To estimate the prevalence of GDM if only 1 abnormal value were used for diagnosis, we first identified women with fasting glucose ≥ 5.3 mmol/L. From among the remaining women, we identified those with 1-hour glucose ≥ 10.0 mmol/L, followed by those with 2-hour glucose ≥ 8.6 mmol/L and lastly, those with 3-hour glucose ≥ 7.8 mmol/L. To estimate the prevalence of GDM by the current ADA criteria4 (2 abnormal values), women with 2 abnormal values who had a fasting glucose ≥ 5.3 mmol/L were first identified. Next, we identified women with 2 abnormal values that had a 1-hour glucose ≥ 10.0 mmol/L and had not been captured in the previous step. Women with 2 abnormal values that had a 2-hour glucose ≥ 8.6 mmol/L and were not identified in the first two steps were captured last.
SAS 9.1 (SAS Institute Inc., Cary, NC)11 was used for all analyses. This study was approved by the human subjects committees of the Kaiser Foundation Research Institute and the State of California.
RESULTS
The characteristics of the cohort are displayed in Table 1. Over half of the women (57.7%) were from minority racial-ethnic groups and 53.9% were pregnant with their first child. Seventeen percent of the cohort had abnormal screening values and underwent the diagnostic test, four percent of the cohort met the NDDG criteria7 for GDM and an additional 2% meeting the ADA criteria4 only. The prevalence of LGA increased with increasing categories of screening plasma glucose: 7.0% in the < 5.5 mmol/L, 9.2% in the 5.5–6.0 mmol/L, 10.4% in the 6.1–6.6 mmol/L, 11.4% in the 6.7–7.1 mmol/L, and 12.6% in the 7.2–7.7 mmol/L categories. The prevalence also increased with increasing plasma glucose levels on the diagnostic test (Figure 1). The prevalence of LGA was 20% in women with GDM by the ADA criteria only, who did not receive treatment during the study period, as well as among women with fasting levels ≥ 5.3 mmol/L on the diagnostic test.
Table 1.
Characteristics of 89,141 women delivering between January 1995 and December 2004 at Kaiser Permanente Northern California.
| Characteristics | % |
|---|---|
| Age at delivery (years) | |
| 15–24 | 24.8 |
| 25–29 | 32.2 |
| 30–34 | 32.2 |
| 35–45 | 10.8 |
| Race/Ethnicity | |
| Non-Hispanic White | 39.6 |
| African American | 9.5 |
| Asian | 20.5 |
| Hispanic | 27.7 |
| Other or unknown | 2.7 |
| Education | |
| Less than high school | 9.9 |
| High school graduate | 29.2 |
| Some college | 29.0 |
| College graduate and beyond | 29.8 |
| Unknown | 2.1 |
| Parity | |
| 0 | 53.9 |
| 1 | 28.3 |
| 2 | 12.5 |
| 3+ | 5.3 |
| Unknown | 0.03 |
| Maternal Weight (kg) | |
| Mean (SD) | 69.8 (16.5) |
| < 50 | 5.8 |
| 50–59 | 24.2 |
| 60–69 | 29.9 |
| 70–79 | 19.0 |
| 80+ | 21.1 |
| Maternal BMI (kg/m2) | |
| Mean (SD) | 26.4 (5.6) |
| < 25 | 49.0 |
| 25–29.9 | 30.1 |
| 30+ | 20.8 |
| Gestational Age at Weight Measurement (weeks) | |
| Mean (SD) | 16.8 (1.3) |
| 9–14 | 1.1 |
| 15–17 | 69.7 |
| 18–20 | 28.9 |
| 21–24 | 0.3 |
Figure 2 displays ORs (adjusted for maternal age, race-ethnicity, parity, education, maternal BMI and gestational age at the weight measurement) for delivering an LGA infant across maternal plasma glucose categories on the diagnostic test. In women without GDM, the risk of LGA steadily increased with increasing categories of plasma glucose at the fasting, 2-hr and 3-hr time points of the diagnostic test. Women without GDM comprising the two highest glucose categories (5th and 6th) at all time points of the diagnostic test had ≥ 1.5 times the risk of delivering an LGA infant when compared to women with screening values 5.5–6.0 mmol/L (Figure 2). The largest risk estimate was obtained for the 6th category of fasting plasma glucose: the OR for fasting plasma glucose values ≥ 5.3 mmol/L was 1.89 (95% CI 1.45–2.45).
Among women with GDM, the risk of LGA was higher in women who met the ADA criteria only4 when compared to women who met the NDDG criteria7 for GDM and received treatment [OR= 2.06 (95% CI 1.80–2.35) and OR= 1.67 (95% CI 1.50–1.86), respectively, with the 5.5–6.0 mmol/L screening category comprising the reference group]. However, this difference was not statistically significant.
The models also contained estimates of the ORs for LGA associated with maternal pregnancy BMI (Table 2). In models for each time point of the diagnostic test, maternal overweight and obesity were significantly associated with an increased risk of LGA. When compared to women with BMI < 25 kg/m2, those with BMI 25–29.9 kg/m2 had ORs for LGA ranging from 1.61 to 1.62, depending on the time point (all p values < 0.001); for women with pregnancy BMI ≥ 30.0 kg/m2, the ORs for LGA ranged from 2.19 to 2.23, depending on the time point (all p values < 0.001).
Table 2.
Odds Ratios* for large for gestational age associated with maternal pregnancy body mass index in multiple-adjusted models* specific to each time point of the diagnostic test among 89,141 women delivering at Kaiser Permanente Northern California, 1995–2004.
| Model with Fasting values | Model with 1-hr values | Model with 2-hr values | Model with 3-hr values | |
|---|---|---|---|---|
| Maternal Pregnancy BMI | ||||
| <25.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| - | - | - | - | |
| 25–29.9 | 1.61 (1.52–1.70) | 1.61 (1.52–1.70) | 1.61 (1.53–1.70) | 1.62 (1.53–1.71) |
| >30.0 | 2.19 (2.07–2.32) | 2.21 (2.08–2.34) | 2.22 (2.09–2.35) | 2.23 (2.10–2.36) |
Adjusted for maternal age, race-ethnicity, parity, education, plasma glucose level, and gestational age at the weight measurement
When the analytic cohort was expanded to include those who were missing height data in the electronic records (n = 149,699), similar results were obtained for the association between increasing levels of maternal glycemia and the risk of delivering an LGA infant in models adjusting for maternal weight instead of BMI (data not shown).
Table 3 displays the prevalence of GDM diagnosed by 1 versus 2 abnormal plasma glucose values according to the ADA thresholds4, calculated among 33,921 women who delivered at KPNC in 2007. If the presence of only 1 abnormal plasma glucose value were used for diagnosis, the prevalence of GDM would increase from 7.1% to 11.4%, resulting in a 60.6% increase in the prevalence of GDM. If the presence of fasting plasma glucose ≥ 5.1 mmol/L were the threshold, as recently recommended for the 75-g 2-hour OGTT6, the prevalence of GDM would increase to 11.6%.
Table 3.
Prevalence of GDM diagnosed by 2 versus 1 abnormal glucose values according to the ADA thresholds among 33,921 women who delivered at Kaiser Permanente Northern California in 2007.
| Subjects above ADA Thresholds | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| ADA Thresholds | 2 abnormal values | 1 abnormal values | |||||||
| mmol/L | Total % | Add % | N | Add subjects | Total % | Add % | N | Add subjects | |
| FPG | 5.3 | 2.8 | ---- | 937 | ---- | 3.4 | ---- | 1139 | ---- |
| 1-hr | 10.0 | 6.6 | 3.8 | 2243 | 1306 | 8.8 | 5.4 | 3000 | 1861 |
| 2-hr | 8.6 | 7.1 | 0.5 | 2417 | 174 | 11.0 | 2.2 | 3716 | 716 |
| 3-hr | 7.8 | 7.1 | 0 | 2417 | 0 | 11.4 | 0.4 | 3877 | 161 |
| 7.1 | --- | 2417 | --- | 11.4 | --- | 3877 | 2738 | ||
COMMENT
In this cohort, among women without GDM, the risk of LGA steadily increased with increasing plasma glucose values for three of the four time points on the diagnostic test. Fasting plasma glucose demonstrated the most pronounced, linear increase in the risk of LGA across categories of plasma glucose. The risk of LGA in women with fasting plasma glucose values ≥ 5.3 mmol/L, or isolated abnormal fasting glucose according to the ADA threshold4, exceeded the predefined IADPSG risk threshold6 (OR ≥ 1.75) and was similar to that in untreated women with GDM by the ADA criteria4 only. This finding suggests that isolated abnormal fasting glucose may be clinically useful in identifying women at risk of LGA. Women with GDM by the ADA criteria4 only, who did not receive treatment during the study period, had a higher risk of LGA than treated women whose glucose levels met the stricter NDDG criteria.7 It is also worth noting that our estimate of the association between LGA and milder GDM, defined as meeting the ADA criteria4 only, was as strong as the associations between LGA and pregnancy obesity, defined as BMI ≥ 30.
Our study has several limitations. Height data were not universally available in the electronic records, but results obtained for the expanded cohort (the main analytic cohort plus those missing data on height) adjusted for pregnancy weight instead of BMI were very similar to those presented. We also lacked data on several potential confounding variables, including early pregnancy weight gain, hypertension, renal disease, and smoking. In this cohort, only women with screening values ≥7.8 mmol/L received the diagnostic test, so we can not rule out the possibility of misclassification of maternal plasma glucose category, particularly for women with higher screening values that were still < 7.8 mmol/L. Thus some women with GDM were likely included among those with normal screening values, suggesting that our results provide conservative estimates of the effects of maternal glycemia on the risk LGA.
Although data for the diagnostic test were restricted to those women with abnormal screening values and providers were not blinded in this observational study, our findings are similar to the results of the HAPO study5, which used the 75-g, 2-hour OGTT to assess maternal hyperglycemia in all participants. The prevalence of LGA was approximately 17% at the 5th highest fasting plasma glucose category in the HAPO study (fasting plasma glucose 5.0–5.2 mmol/L) and then continued to increase with increasing fasting plasma glucose levels. In the present study, the prevalence of LGA among women with fasting plasma glucose in this same category was very similar (18.1%).
Our results are also consistent with previous studies conducted among women without GDM by the NDDG criteria7 who did not receive treatment for hyperglycemia.13–15 The Toronto Tri-Hospital Gestational Diabetes Project13 found a continuous relationship between the pregnancy glucose quartiles at each time point of the 100-g, 3-hour OGTT and fetal macrosomia. Several large case-control studies15 reported that the risk of macrosomia, hypoglycemia, and hyperbilirubinemia significantly increased with an increasing number of glucose values meeting the ADA thresholds4. Scholl et al.14 reported a significantly increased risk of LGA with increasing maternal plasma glucose on the screening test among women without GDM.
In conclusion, this study provides additional data on the association between only 1 abnormal value and LGA that could be considered prior to the adoption of such criteria for the 100-g, 3-hour OGTT. These data show that the risk of LGA in women with isolated fasting hyperglycemia was the only estimate to exceed the IADPSG6 threshold. Except for the fasting time point, our results do not support the use of only 1 abnormal OGTT value to diagnose GDM, as the risk was only modestly elevated in women with isolated abnormal values at the 1 hour, 2 hour and 3 hour time points. In addition, since the prevalence of the condition would increase by approximately 60% if such criteria were used, we recommend that the costs and benefits be carefully evaluated and the health system adequately prepared prior to any change in the criteria for diagnosing GDM. Finally, it should also be noted that although several randomized trials have demonstrated the benefit of treatment for mild GDM16;17, the benefit of treatment for one abnormal value has yet to be thoroughly examined.
Acknowledgments
This research was supported by a R01 DK 54834 from the National Institute of Diabetes and Digestive and Kidney Diseases and a grant from the American Diabetes Association.
Financial Support: This research, conducted at the Kaiser Permanente Division of Research, was supported by a R01 DK 54834 from the National Institute of Diabetes and Digestive and Kidney Diseases and a grant from the American Diabetes Association.
Footnotes
The authors do not have any conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Metzger BE. Summary and recommendations of the Third International Workshop- Conference on Gestational Diabetes Mellitus. Diabetes. 1991;40 (Suppl 2):197–201. doi: 10.2337/diab.40.2.s197. [DOI] [PubMed] [Google Scholar]
- 2.Kjos SL, Buchanan TA. Gestational diabetes mellitus. N Engl J Med. 1999;341(23):1749–1756. doi: 10.1056/NEJM199912023412307. [DOI] [PubMed] [Google Scholar]
- 3.Jovanovic L, Pettitt DJ. Gestational diabetes mellitus. JAMA. 2001;286(20):2516–2518. doi: 10.1001/jama.286.20.2516. [DOI] [PubMed] [Google Scholar]
- 4.Gestational Diabetes Mellitus. Diabetes Care. 2000;23(Suppl 1):S77–S79. [PubMed] [Google Scholar]
- 5.Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358(19):1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- 6.International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33(3):676–682. doi: 10.2337/dc09-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. National Diabetes Data Group. Diabetes. 1979;28:1039–1057. doi: 10.2337/diab.28.12.1039. [DOI] [PubMed] [Google Scholar]
- 8.Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health. 1992;82(5):703–710. doi: 10.2105/ajph.82.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285(18):2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 10.Hedderson MM, Ferrara A, Sacks DA. Gestational diabetes mellitus and lesser degrees of pregnancy hyperglycemia: association with increased risk of spontaneous preterm birth. Obstet Gynecol. 2003;102(4):850–856. doi: 10.1016/s0029-7844(03)00661-6. [DOI] [PubMed] [Google Scholar]
- 11.SAS 9.1.3 Help and Documentation. Cary, NC: SAS Institute Inc; 2002. [Google Scholar]
- 12.O’Sullivan JB, Mahan CM, Charles D, Dandrow RV. Screening criteria for high-risk gestational diabetic patients. Am J Obstet Gynecol. 1973;116(7):895–900. doi: 10.1016/s0002-9378(16)33833-9. [DOI] [PubMed] [Google Scholar]
- 13.Sermer M, Naylor CD, Gare DJ, Kenshole AB, Ritchie JW, Farine D, et al. Impact of increasing carbohydrate intolerance on maternal-fetal outcomes in 3637 women without gestational diabetes. The Toronto Tri-Hospital Gestational Diabetes Project. Am J Obstet Gynecol. 1995;173:146–156. doi: 10.1016/0002-9378(95)90183-3. [DOI] [PubMed] [Google Scholar]
- 14.Scholl TO, Sowers M, Chen X, Lenders C. Maternal glucose concentration influences fetal growth, gestation, and pregnancy complications. Am J Epidemiol. 2001;154(6):514–520. doi: 10.1093/aje/154.6.514. [DOI] [PubMed] [Google Scholar]
- 15.Ferrara A, Weiss NS, Hedderson MM, Quesenberry CP, Jr, Selby JV, Ergas IJ, et al. Pregnancy plasma glucose levels exceeding the American Diabetes Association thresholds, but below the National Diabetes Data Group thresholds for gestational diabetes mellitus, are related to the risk of neonatal macrosomia, hypoglycaemia and hyperbilirubinaemia. Diabetologia. 2007;50:298–306. doi: 10.1007/s00125-006-0517-8. [DOI] [PubMed] [Google Scholar]
- 16.Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005;352(24):2477–2486. doi: 10.1056/NEJMoa042973. [DOI] [PubMed] [Google Scholar]
- 17.Landon MB. A prospective multicenter randomized treatment trial of mild gestational diabetes (GDM) American Journal of Obstetrics and Gynecology. 2008;199(6 SuppA):S2. [Google Scholar]