Abstract
Biotinidase deficiency is the primary enzymatic defect in biotin-responsive, late-onset multiple carboxylase deficiency. Untreated children with profound biotinidase deficiency usually exhibit neurological symptoms including lethargy, hypotonia, seizures, developmental delay, sensorineural hearing loss and optic atrophy; and cutaneous symptoms including skin rash, conjunctivitis and alopecia. Although the clinical features of the disorder markedly improve or are prevented with biotin supplementation, some symptoms, once they occur, such as developmental delay, hearing loss and optic atrophy, are usually irreversible. To prevent development of symptoms, the disorder is screened for in the newborn period in essentially all states and in many countries. In order to better understand many aspects of the pathophysiology of the disorder, we have developed a transgenic biotinidase-deficient mouse. The mouse has a null mutation that results in no detectable serum biotinidase activity or cross-reacting material to antibody prepared against biotinidase. When fed a biotin-deficient diet these mice develop neurological and cutaneous symptoms, carboxylase deficiency, mild hyperammonemia, and exhibit increased urinary excretion of 3-hydroxyisovaleric acid and biotin and biotin metabolites. The clinical features are reversed with biotin supplementation. This biotinidase-deficient animal can be used to study systematically many aspects of the disorder and the role of biotinidase, biotin and biocytin in normal and in enzyme-deficient states.
Keywords: Biotinidase deficiency, biotin, biotin-responsive, transgenic, knockout mouse, animal model, carboxylase deficiency
Introduction
Biotin is an essential vitamin required for the activation of four biotin-dependent carboxylases. In humans, holocarboxylase synthetase (HCS) converts inactive apocarboxylases into functionally active holocarboxylases by covalently attaching biotin to ε-amino groups of specific lysyl-residues of each of the four biotin-dependent carboxylases (propionyl CoA carboxylase (PCC), β-methylcrotonyl CoA carboxylase (MCC), pyruvate carboxylase (PC), and acetyl CoA carboxylase (ACC)) (1). These holocarboxylases have important roles in gluconeogenesis, fatty acid synthesis and the catabolism of several branch-chain amino acids and odd-carbon fatty acids. The holocarboxylases are proteolytically degraded to biocytin (biotinyl-ε-N-lysine) and biotinylated-peptides. Biocytin is the product of the proteolytic degradation of biotin-dependent carboxylases (1). Similarly proteolysis of dietary proteins generates biotinylated-peptides and biocytin. Biotinidase (EC 3.5.1.12) is an amido-hydrolase that hydrolyzes biocytin (biotinyl–ε-lysine) and small biotinyl-peptides thereby recycling biotin (2,3). In addition, biotinidase has biotinyl-transferase activity capable of transferring biotin to nucleophilic acceptor molecules, such as histones (4).
Biotinidase deficiency (OMIM 253260) is a biotin-responsive, autosomal recessively inherited metabolic disorder (5). If untreated, children with profound biotinidase deficiency (less than 10 % of mean normal serum activity) can exhibit neurological features such as hypotonia, seizures, developmental delay, sensorineural hearing loss and optic atrophy; and cutaneous abnormalities, such as skin rash and alopecia (6). Biochemically, these individuals may exhibit metabolic acidosis, mild hyperammonemia and/or organic acidemia/uria. The symptoms improve or can be prevented if affected children are treated with pharmacological doses of biotin (7). However, the developmental delay, hearing loss, and visual abnormalities are usually irreversible if they occur prior to initiation of biotin therapy (8). Currently, most states in the United States and many countries screen their newborns for biotinidase deficiency (9).
With the success of newborn screening for biotinidase deficiency, we are losing the window-of-opportunity to learn about the natural history and the pathophysiology of symptomatic, untreated biotinidase-deficient individuals. Therefore, we developed a transgenic mouse with biotinidase deficiency by knocking-out the biotinidase gene (BTD). Biotinidase-deficient (BTD−/−) mice have no detectable biotinidase activity and develop neurocutaneous symptoms when placed on a biotin-deficient diet. In addition, symptoms can be reversed in BTD−/− mice treated with pharmacological doses of biotin. This biotinidase-deficient mouse model will allow us to gain a better understanding of the natural history of biotinidase deficiency, the pathophysiology of the disorder, and the role biotinidase and biotin deficiency play in normal and biotinidase-deficient states.
Material and Methods
Generation of vector constructs
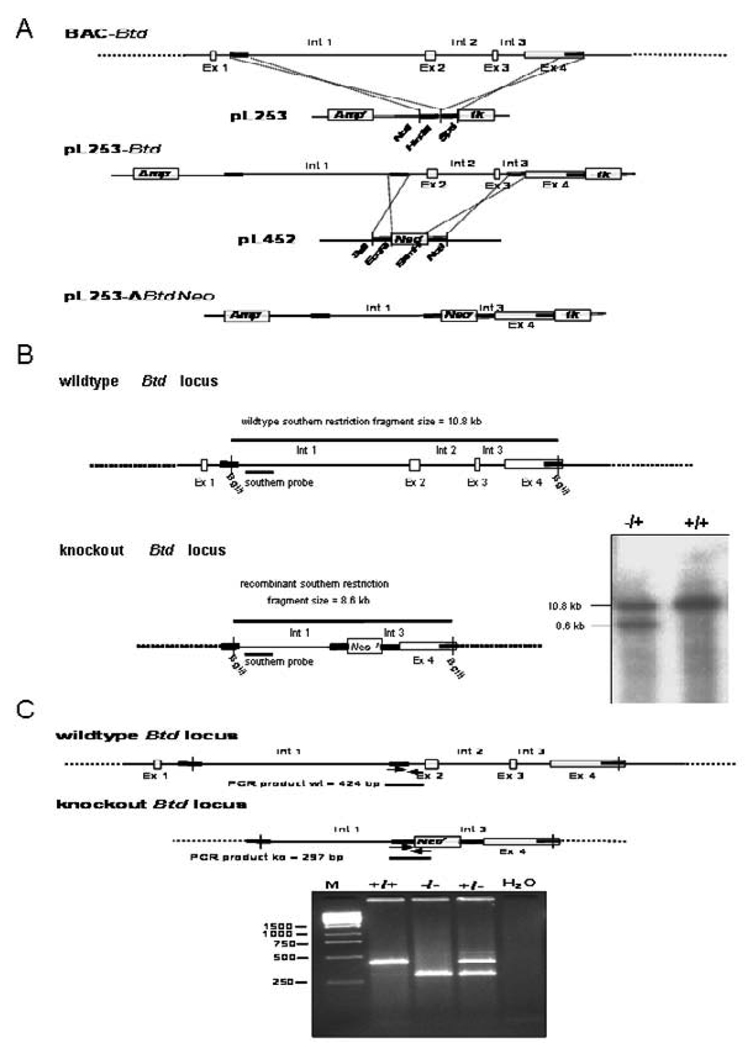
Construction of the target vector pL253-ΔBtdNeo with the disrupted BTD gene sequence required multi-step recombinant engineering to generate multiple vectors. BTD homologous sequence (from BAC-Btd) was cloned into pL253 in a series of recombination steps involving PCR amplifications, restriction digestions, gel purification of correct fragments, ligation, colony screening, and plasmid preparation. We adopted a highly efficient recombination based method as described (10). This method takes advantage of homologous recombination mediated by the λ phage Red proteins. In general, the procedure involves retrieving a gene fragment from BAC clones into a high-copy plasmid for relatively easy subsequent modification of the gene before creating the target vector; in this case deleting the gene sequence.
BAC-Btd (RP23-16M15: AC109509; 216410 bp) clone was obtained from the Children’s Hospital Oakland Research Institute containing a 14.6 kb mouse genome segment spanning the BTD gene sequence after identifying it through a Blast search at http://genome.ucsc.edu. The bacterial strain EL350 (expressing the Red genes of bacteriophage λ upon induction at 42 °C for homologous recombination via gap repair), vectors pL253 (5878 bp; containing thymidine kinase (tk) and ampicillin resistance (Ampr) genes), and pL452 (5755 bp; containing Neo cassette harboring Neor gene flanked by loxP sites for conditional recombination at Neor site) were used in this work for targeted disruption of germline DNA. This was a generous gift from Dr. Copeland of National Cancer Institute, Maryland.
Construction of the retrieval vector
The procedure for constructing the retrieval vector pL253-Btd is described here. As shown in Figure 2A, initially, the two small segments flanking the BTD gene from BAC-Btd using sets of primers (primer set A and B to produce a 465 bp segment AB corresponding to Intron 1 sequence and primer set Y and Z to produce a 370 bp segment YZ corresponding to Exon 4 sequence) with incorporated restriction sites needed to directionally clone the fragments into vector pL253 were generated. The two primer sets with the following sequences were designed with their corresponding restriction sites (underlined nucleotides):
A: 5’-AATA GCGGCCGC TCTGTCGTCGTTGTTGCTGT-3’ (with NotI)
B: 5’-GTCA AAGCTT CTTGCTATGGGCACTTCAG-3’ (with HindIII)
Y: 5’-GTCA AAGCTT GTGAGATGGACCCGTCCCAT-3’ (with HindIII)
Z: 5’-TCT ACTAGT TCTCCTGCCCGCAGGTGTCGAA-3’ (with SpeI)
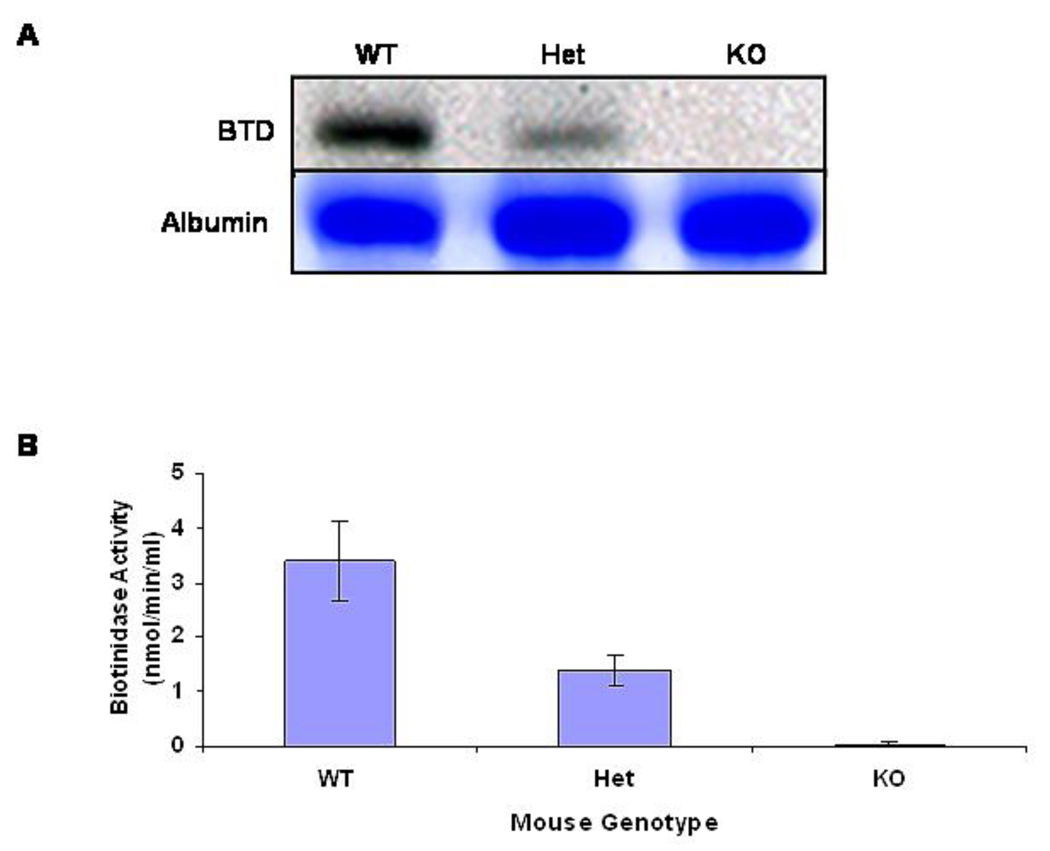
Fig. 2. Serum biotinidase expression and enzyme activity.
A. Biotinidase expression in the serum samples were accessed for wildtype (WT; BTD+/+), heterozygous (Het; BTD+/−), and biotinidase-deficient knockout (KO; BTD−/−). Upper panel shows detection of biotinidase with the antibody prepared against biotinidase peptide and the lower panel shows coomassie-stained serum albumin for respective lanes as a protein loading control. B. The mean serum biotinidase activity with standard deviation for the three groups of animals with wildtype (WT; BTD+/+; n = 5), heterozygous (Het; BTD+/−; n = 7), and biotinidase-deficient knockout (KO; BTD−/−; n = 18) genotypes are shown.
The PCR products were digested with corresponding sets of restriction enzymes and were introduced into NotI and SpeI digested pL253. The correct clone of pL253 (with segments AB and YZ) was then linearized with HindIII followed by dephosphorylation before electroporation into EL350 bacterial strain (post Red gene induction) containing BAC-Btd to homologously retrieve the BTD gene fragment spanning Intron1 to Exon 4 (13,766 bp) and to generate pL253-Btd (19,644 bp).
Construction of the target vector
Second, the majority of the BTD sequence (Exon 2, Intron 2, Exon 3, Intron 3, and a portion of Exon 4) from pL253-Btd with Neo cassette was replaced to construct the target vector pL253-ΔBtdNeo (Figure 2A). Two additional sets of primers with the following sequences were designed below with corresponding restriction sites (underlined):
C: 5’- GTC GTCGAC TGACACAGCAGAAGCTAGGA-3’ (with SalI)
D: 5’- GTCGAATTC GCAGGAGAGAAGAGCTGAAT-3’ (with EcoRI)
E: 5’- TCTGGGATCCAAACCACCGA-3’ (with BamHI)
F: 5’- ATAA GCGGCCGC ACCTGGACCAGTAGACTCAA-3’ (with NotI)
Fragment CD (corresponding to 493 bp of Intron 1) and EF (corresponding to 430 bp of Intron 3) were PCR amplified using pL253-Btd as a template and were introduced into pL452 on either side of the Neo cassette to construct pL253-ΔBtdNeo using the similar recombinant strategy described above to generate pL253-Btd (Figure 1A). The resultant targeting vector pL253-ΔBtdNeo (12457 bp) was amplified in DH10B and purified using Qiagen endotoxin-free plasmid preparation kit (Qiagen Inc. Valencia, CA).
Fig. 1. Generation of biotinidase-deficient mice.
A. Generation of the target vector, pL253-ΔBTDNeo. A BAC clone contains the wildtype biotinidase (BTD) sequence. All four exons (Ex 1 to Ex 4) are shown as unfilled boxes with intervening introns (Int 1 to Int 3). In the first step, part of the BTD sequence (excluding Ex 1) was cloned into pL253 in a series of recombination steps for the ease of subsequent gene modification. In the second step, most of the BTD sequence (including partial Int 1, Ex 2, Int 2, Ex 3, and partial Int 3) was replaced by a Neo cassette from pL452. The resultant target vector pL253-ΔBTDNeo has disrupted nonfunctional BTD (partial Int 1, partial Int 3, and Ex 4) in addition to Ampr, Neor, and tk. B. Wildtype and knockout BTD locus is shown with the restriction fragment when subjected to restriction endonuclease digestion with BglII. In addition, the location of the southern probe used for discriminating two different alleles (wildtype and knockout) is shown. In the inset, southern analysis of restriction fragments generated from genomic DNA (DNA was isolated from ES cells with both alleles being wildtype and the ES cells with one wildtype and one knockout allele) is clearly discriminatory for two different alleles on the basis of size; the wildtype allele is 10.8 kb and the disrupted knockout allele is 8.6 kb. C. The two allele three-primer PCR strategy to discriminate animals with three different genotypes (i.e., wildtype, knockout, and heterozygous) is shown. The wildtype locus produces a PCR product of 424 bp, whereas the knockout locus produces a PCR product of 297 bp. Gel electrophoresis of the PCR fragments of a representative experiment are shown in the inset: lane marked M was loaded with a DNA marker; lanes with BTD+/+, BTD−/−, and BTD+/− were loaded with PCR products generated from DNA of wildtype, knockout, and heterozygous animals, respectively. The last lane, a negative control, has the PCR reaction product using water instead of template DNA.
Development of BTD-deficient mice
After expanding the selected clone of pL253-ΔBtdNeo by preparing endotoxin-free plasmid preparation, the linearized vector clone was electroporated into D2 ES cell line to generate the biotinidase knockout locus.
Multiple ES clones were selected that were resistant to G418 and ganciclovir to identify ES clones with truncated endogenous BTD allele. Nested PCR was used to screen for the targeted clones with intended disruption. Southern blot analysis of genomic DNA digested with Bgl II with a specific probe was used to confirm truncation of one biotinidase allele (Figure 1B). One of the ES clones with intended modification at the biotinidase gene locus was expanded to produce chimera following fusion with CD-1 host embryo. The resulting F1 mice were backcrossed to C57BL/6 background for ten generations to produce a congenic animal line.
Animal care, housing and diet
Mice were maintained under standard housing conditions in a 12-hour light-dark cycle with free access to food and water. Approval was obtained from the Institutional Animal Care and Use Committee (IACUC) for maintaining breeding colonies and all procedures performed on mice in this project. Standard breeder diet (Harlan Teklad, Madison, WI) was used to feed the mice used for breeding colonies until the litters were weaned and/or used for specific experiments.
The pups used in specific experiments were nursed by either wildtype or heterozygous dams until they were three weeks old. At this time, they were genotyped and the mice with the appropriate genotype were utilized for specific experiments as needed.
Genotyping
We genotyped mice by two-allele three-primer PCR of genomic DNA obtained from either tail clips or blood. We employed BTD gene specific sense primer common to wildtype and knockout alleles (5’–AACACAGCGAGCCGTTTGAA-3’), the antisense primer specific for the wildtype allele (5’–GTCGAGGTTCTGCTTCATGA-3’), and the knockout allele (5’–GCTGGACGTAAACTCCTCTT-3’). We carried out 35 cycles of PCR with annealing at 62 °C for 45 seconds and extension for 45 seconds, generating different size fragments corresponding to the wildtype allele (424 bp) and knockout allele (297 bp) in multiplex fashion. The PCR product was electrophoresed on a 2 % agarose gel (Figure 1C).
Dietary Manipulation
Two types of diets, biotin-depleted and biotin-deficient (custom formulated by Harlan Teklad, Madison, WI) were used. The biotin-depleted diet contains 30 % egg-white, whereas the biotin-deficient diet is specially formulated from the vitamin-free, alcohol-extracted casein as the sole source of protein. Biotin content of this specially formulated diet is negligible (0.007 mg/kg); far below the estimated daily requirement for a normal rodent diet (0.2 mg/kg). The first dietary regiment consisted solely of the biotin-deficient diet. In order to reduce the time necessary to develop biotin deficiency and symptoms, a second dietary regiment was used in some experiments as described. For this, the animals were fed the biotin-depleted diet for five days followed by the biotin-deficient diet for an additional eight days.
Blood collection and serum procurement
Retro-orbital bleeding was used to collect approximately 0.1 to 0.2 ml of blood from each mouse. Mice were anesthetized using ketamine/xylazine (60 mg/kg and 8 mg/kg, respectively). Serum was separated from the blood after centrifugation (1500 × g for 5 min in a temperature-controlled table top centrifuge). Specimens were stored in airtight sealed tubes in a deep freezer at −80°C until they were analyzed.
Biotinidase activity in serum
Serum biotinidase enzyme activity was determined in six-week-old mice maintained on a normal, biotin-supplemented diet. Serum biotinidase activity is determined by colorimetric quantitation of p-aminobenzoic acid cleaved from the artificial substrate, biotinyl-4-aminobenzoic acid (11).
Western blot analysis
Western blot analysis was performed following a standard procedure described previously (12). Two µl of serum samples were subjected to SDS-polyacrylamide electrophoresis. Serum samples were boiled in 10 µl of sample buffer (20 % glycerol, 4 % SDS, 0.2 % Bromophenol Blue, 125 mM Tris–HCl (pH 7.5) and 640 mM 2-mercaptoethanol) for five minutes and electrophoresed on (12 %) SDS-polyacrylamide (37.5:1) gels at 120V for 140 min. Proteins on the gels were transferred to PVDF membranes. Membranes were blocked with 2 % Tropix I-Block (Applied Biosystems) in 1x PBS (pH 7.4), with 0.05 % Tween 20 (TPBS) and probed with rabbit polyclonal antibody raised against human biotinidase peptide (Santa Cruz Biotechnology) and Horse Radish Peroxidase-conjugated secondary antibody (Pierce, ThermoScientific). Immune complexes or cross-reacting material (CRM) were visualized with enhanced chemiluminescence (ECL) detection system (GE Healthcare).
Hepatic biotin concentration
Gravimetrically measured, small aliquots of the frozen liver were used to determine biotin content. The aliquots were homogenized in water using a glass homogenizer. The homogenates were acid hydrolyzed with 1.5 M HC1, which releases >95 % of protein-bound biotin. Hydrolyzed liver lysates were then subjected to HPLC to separate biotin from its metabolites. The HPLC fractions containing biotin were analyzed using an avidin-binding assay described previously (13). Biotin concentrations were normalized to a unit protein concentration (14). Protein concentrations were determined by a bicinchoninic acid assay (Pierce, Rockford, IL).
Urinary excretion of biotin and biotin metabolites
Biotin and biotinylated metabolites (BBM) were determined in urinary specimens using a methodology described for total avidin-bindable substances (TABS) (14). Urinary excretions of BBM were normalized to the urinary excretion of creatinine. The urinary creatinine concentrations were measured using the picric acid method (15). The normalized urinary BBM concentrations are presented as µmoles/mole of creatinine. Urinary specimens were collected from mice of each genotype before the mice were started on the diet regiment (“Day 0”) and between days three and five of being on the diet (“Days 3–5”). The mean urinary BBM excretion of the mice of the various genotypes at “Days 0” and “Days 3–5” were statistically compared using one-tail T test analysis.
Hepatic propionyl-CoA carboxylase (PCC) activity
Small portions of frozen liver samples were cut and homogenized in buffer (25 mM sucrose, 50 mM Tris, pH 7.9, 5mM glutathione and 1 mM EDTA). The ratios of homogenization buffer to the samples were maintained at 5 ml of buffer to 1 gram of sample. Homogenized samples were sonicated (three cycles of 30 sec) on ice to release membrane-bound enzymes followed by centrifugation at 105,000 × g to remove insoluble cellular debris. PCC activity was assayed using the incorporation of C14-bicarbonate in the soluble supernatant as described previously (16). PCC activity was expressed per milligram of protein. Enzyme activity was linear for at least 30 min with a coefficient of variation that was typically <5 %.
Blood chemistries
Serum electrolytes (sodium, potassium, chloride and bicarbonate) were analyzed in frozen serum samples that were sealed in airtight tubes and shipped on dry ice to Comparative Pathology Laboratory, School of Veterinary Medicine University of California, Davis. Samples were analyzed using COBAS INTEGRA 400 Plus instrument from Roche Diagnostics.
Plasma ammonia concentrations were determined using an ammonia assay kit from BioVision Research Products (Mountain View, CA). A standard curve was generated with each assay run using an ammonium chloride standard solution in triplicate according to the manufacturer’s instructions.
Urinary organic acid analysis
Urine samples were collected individually following the procedure described earlier (17). The urinary specimens were sealed and shipped on dry ice to the Pediatric Biochemical Genetics Laboratory at University of Maryland School of Medicine. The samples were stored at −80 °C until they were analyzed using capillary gas chromatography-mass spectrometry (GC-MS) based Selected Ion Monitoring (SIM) with an Agilent ChemStation platform. Urinary concentrations of 3-hydroxypropionic acid (3HPA), 3-hydroxyisovaleric acid (3HIVA), 3-methylcrotonylglycine (3MCG), and 2-methylcitric acid (2MCA) were determined. Relative concentration of each organic acid metabolite was normalized to urinary creatinine. The urinary creatinine concentrations were measured using the picric acid method (15). Urinary organic acid metabolite concentrations are presented as nmoles/10 mg of creatinine.
Weight measurements
Three-week-old mice were placed on the biotin-deficient diet immediately after being weaned. All mice were weighed regularly after the specific diet regiment was initiated, until the termination of the experiments. For most of the experiments, this was 24 days after the initiation of the diet regimen, unless specified otherwise.
Motor-neuronal assessments
We adopted a general staging method to assess the motor-neuron deficiency based on gross observation of motor neuron function or deficit as described (18). Mice were assigned to one of six stages of physical impairment (stage 0 = normal; stage 1 = loss of tail tone; stage 2 = hindlimb weakness; stage 3 = hindlimb paralysis; stage 4 = hind and forelimb paralysis; and stage 5 = moribund). Euthanasia was performed on mice when they reached the moribund stage or in some cases earlier, depending on the objectives of the specific experiments.
Computerized tomography
High-resolution computerized tomography images (120 kVP, 250 mAs) of anesthetized mice were performed using a clinical scanner (Philips Brilliance Big Bore). Slice thickness was 1 mm and the matrix size was 512 pixels × 512 pixels. We used the Eclipse Treatment Planning System by Varian to reconstruct and analyze the images. Bone volumes were measured following image processing to enhance bone structures.
Results
Resultant transgenic knockout mice
The resulting F1 mice were back-crossed to C57BL/6 background for ten generations to produce a congenic animal line. Currently, we have bred the genetically engineered mice for 20 generations without any breeding complications.
Mice were genotyped using the three primer two allele PCR methodology. They were assigned to one of three genotypic groups: BTD+/+ or wildtype, BTD+/− or heterozygous, and BTD−/− or deficient. A representative genotyping agarose gel is shown in Figure 1C. The ratio of BTD+/+ toBTD−/− to BTD+/− pups born to BTD+/− by BTD+/−matings was 59:105:59 (expected ratio is 1:2:1); thus demonstrating autosomal recessive inheritance for the BTD- knockout genotype.
The body weights for the pups of all three genotypes were comparable at birth. All newborn pups, regardless of their genotype, develop normally as long as they are nursed by lactating wildtype or heterozygous dams. Animals of all three genotypes thrived and gained appropriate and comparable body weight when weaned and fed a normal biotin-supplemented diet. Deficient mice prior to being fed the biotin-depleted or deficient diet are asymptomatic and will be designated as such. However, when these animals were fed a biotin-deficient or biotin-depleted diet for 10 to 14 days they exhibited behavioral and neurological symptoms (symptomatic), whereas animals of the other two genotypes remained asymptomatic.
Serum biotinidase activity and expression
As shown in a western blot in Figure 2A, serum from a representative wildtype mouse had the greatest quantity of biotinidase protein with the heterozygous mouse having about half the quantity of biotinidase protein in serum. There was essentially no detectable biotinidase protein in serum of the biotinidase-deficient animal.
Wildtype mice had a mean serum biotinidase activity of 3.4 nmol/min/ml (n = 5), heterozygous mice had a mean activity of 1.4 nmol/min/ml (n = 7), and biotinidase-deficient mice had a mean activity of 0.022 nmol/min/ml (n = 18); the latter is essentially indistinguishable from background (Figure 2B).
Hepatic biotin content and PCC activity
Six-week-old mice (biotinidase deficient, n=3; heterozygous, n=2; and wildtype, n=l) weighing 15 to 20 grams were fed a biotin-depleted diet for five days followed by a biotin-deficient diet for an additional eight days. The mice were then sacrificed and liver specimens were collected for hepatic biotin content and PCC activity determination.
The mean concentration of biotin was 1,780 pmol/gram of liver for wildtype mice, 1,477 pmol/gram of liver for heterozygous mice and 519 pmol/gram of liver for deficient mice.
Because PCC activity is an early and sensitive indicator of biotin deficiency (16), hepatic PCC activity was determined in hepatic extracts of the mice. Mean PCC activity was similar in the wildtype and heterozygous animals, with activities of 71 and 89 nmol of bicarbonate incorporated per minute per gram of liver extract, respectively; whereas deficient animals had an activity of 22 nmol of bicarbonate incorporated per minute per gram of liver extract.
Urinary excretion of biotin and biotin metabolites
Six-week-old mice (wildtype, n = 4; heterozygous, n = 4; and biotinidase-deficient, n = 3) weighing between 15 to 20 grams were fed a biotin-depleted diet for five days followed by a biotin-deficient diet for an additional eight days to expedite symptom development. Once the mice become symptomatic, it was difficult to collect the urine specimen consistently. Because of this difficulty, urine specimens from days three through five were combined and then analyzed (designated “Days 3-to-5).” The mean urinary BBM excretions in the “Day 0” groups of wildtype, heterozygous, and biotinidase-deficient mice were 336.9 ± 164.3, 461.8 ± 106.6, and 510.9 ± 131.3 µmoles/mole of creatinine, respectively. The mean urinary BBM excretions of the “Days 3–5” group of wildtype, heterozygous, and deficient mice were 34.0 ± 5.7, 47.5 ± 15.2, and 120.3 ± 39.6 µmoles/mole of creatinine, respectively. Using a one-tail T test analysis, the mean urinary excretions of the various genotypes at “Day 0” were not significantly different from each other. However, the mean urinary BBM excretion of the biotinidase-deficient mice at “Days 3–5” was statistically different from that of both wildtype and heterozygous mice; p = 0.03 and 0.04, respectively.
Blood chemistries
Three-week-old mice were placed on the biotin-deficient diet for three weeks. Sera were collected prior to the start of the diet and again at the end of three weeks. Serum electrolytes, including sodium, potassium, chloride and bicarbonate, and anion gap were determined for heterozygous, asymptomatic deficient and symptomatic deficient animals. Mean serum electrolytes values for sodium, potassium, chloride and bicarbonate concentrations in heterozygous mice were 135 ± 5 mmol/L (± 2 S.D.), 15.02 ±1.88 mmol/L, 116.6 ± 3.6 mmol/L, 15.7 ± 3 mmol/L, respectively. Similarly, in asymptomatic knockout mice corresponding concentrations were 141 ± 9 mmol/L, 14.72 ± 2.39 mmol/L, 115.3 ± 8.1 mmol/L, 15.2 ± 2.5 mmol/L, respectively, and in symptomatic knockout mice they were 149 ± 12 mmol/L; 17.81 ± 5.42 mmol/L, 122.6 ± 11.9 mmol/L, 15.3 ±4.1 mmol/L, respectively. The electrolyte concentrations were not statistically different between any of the animal groups. Most importantly, there was no evidence of metabolic acidosis (lower bicarbonate concentration or increased anion gap).
The wildtype mice had a mean plasma ammonia concentration of 74 µmoles/ml (range: 31 to 104 µmoles/ml; n = 3) before being fed the biotin-deficient diet and 59 µmoles/ml (33 to 106 µmoles/ml; n = 3) after being fed this diet. The mean plasma ammonia concentration of asymptomatic deficient mice was 146 µmoles/ml (75 to 243 µmoles/ml; n = 9), whereas the mean concentration of symptomatic mice was 207 µLmoles/ml (133 to 309 µmoles/ml; n = 9).
Urinary organic acid analysis
Urinary organic acid concentrations of 3-hydroxypropionic acid (3HPA), 3-hydroxyisovaleric acid (3HIVA), 3-methylcrotonylglycine (3MCG), and the standard 2-methylcitric acid (2MCA) were analyzed. Three-week-old mice were placed on the biotin-deficient diet for a period of three weeks. Two sample t-test (assuming equal variance) was performed to establish statistical significance. As shown in Table 1, both the asymptomatic and symptomatic biotinidase-deficient mice had statistically increased mean 3MCG concentrations (p<0.01 and p<0.004, respectively) relative to the that of the normal or heterozygous animals, but the concentrations were not statistically different from each other. However, the mean concentration of 3HIVA of the symptomatic deficient mice was markedly statistically increased (p<0.009), approximately twenty-fold, compared to that of the normal/heterozygous and to the asymptomatic deficient mice. The mean concentrations of the 3HPA and 2MCA were not significantly different among the groups.
Table 1.
Urinary Organic Acid Metabolites (mmol/mol of creatinine) of Mice of Various Gentoypes and of Asymptomatic and Symptomatic Mice
| Genotype | 3HPA | 3HIVA | 3MCG | 2MCA |
|---|---|---|---|---|
| Btd+/+ | 6.66 ± 1.25 (n = 7) | l.16 ± 0.78 (n = 9) | 14.20 ± 12.96 (n = 9) | 15.12 ± 9.80 (n = 9) |
| Btd−/+ | 8.52 ± 3.74 (n = 15) | 1.38 ± l.57 (n = 14) | 18.55 ± 17.48 (n = 13) | 9.44 ± 6.18 (n=16) |
| Btd−/− Asymptomatic | 9.02 ± 4.18 (n = 5) | 0.54 ± 0.26 (n = 5) | 62.84 ± 36.25 (n = 5)† | 23.42 ± 5.44 (n = 5) |
| Btd−/− Symptomatic | 4.43 ± 0.92 (n = 3) | 23.5 ± 25.79 (n = 4)* | 84.75 ± 72.76 (n = 2)† | 4.32 ± 2.79 (n = 4) |
Physical features, weight changes, and behavioral and neurological characterization of mice on the biotin-deficient diet
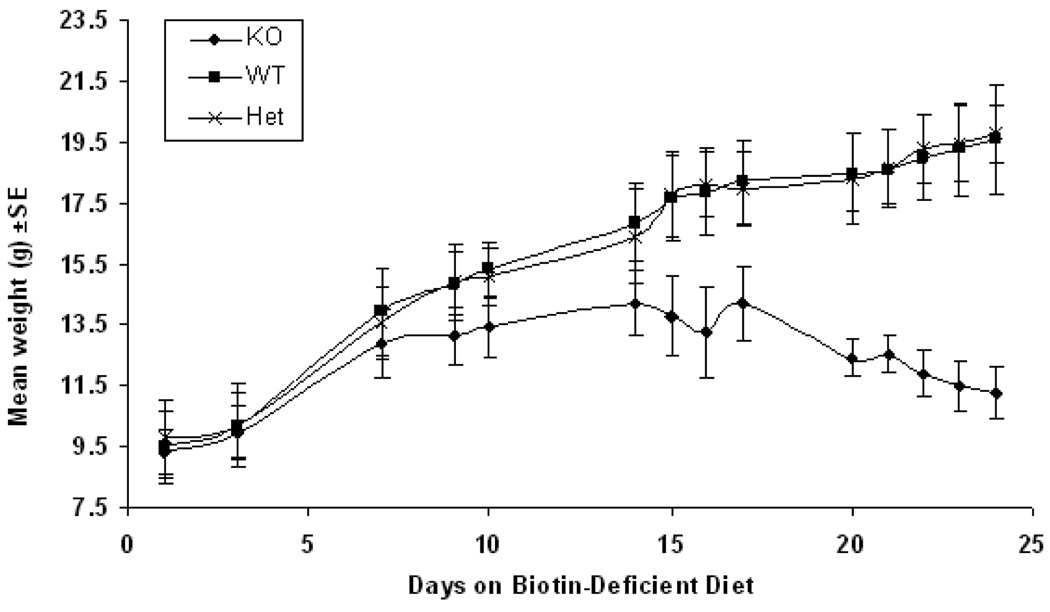
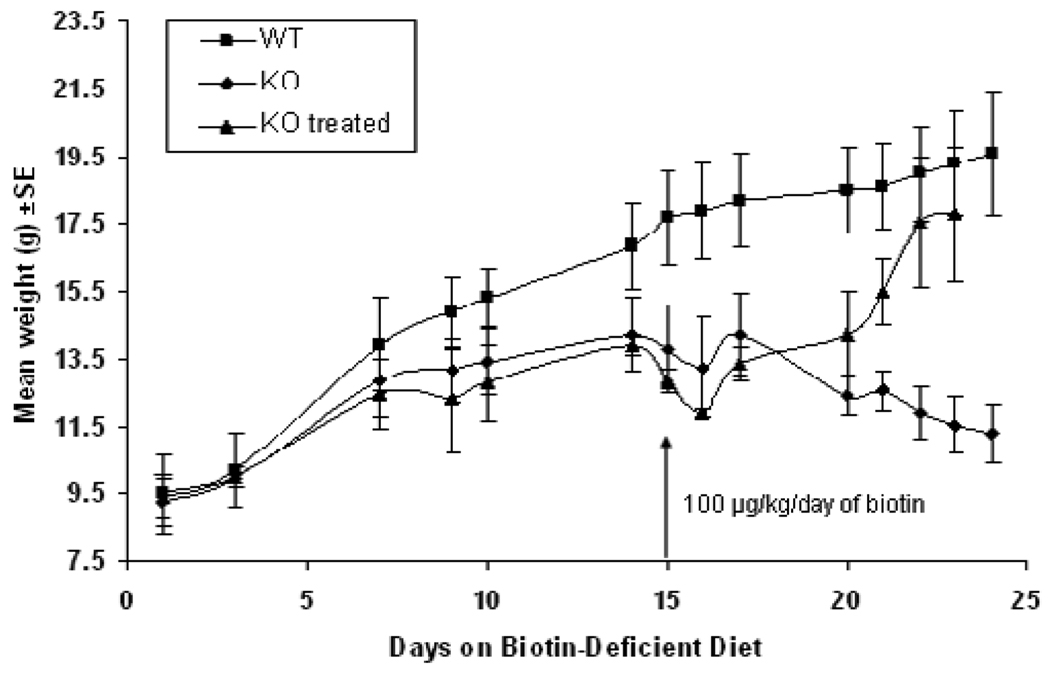
Deficient mice (n=8), heterozygous mice (n=5), and wildtype mice (n=5) were fed the biotin-deficient diet immediately after weaning; three weeks of age. Weight measurements for the mice were obtained throughout that period of the experiment as shown in Figure 3. Weight decreased rapidly in the deficient mice compared to the normal weight gain of wildtype and heterozygous mice. On day 14, the deficient mice exhibited whitening of the fur around the eyes. Subsequently, graying of the fur gradually extended over the entire body and eventually the biotinidase-deficient mice began to lose their fur (Figure 5A).
Fig. 3. Weight changes of wildtype, heterozygous and biotinidase-deficient mice on a biotin-deficient diet.
Three groups of mice, WT (BTD+/+), Het (BTD+/−) and KO (BTD−/−), were placed on biotin-deficient diet immediately after being weaned. Until weaning, the mice were nursed by dams on a normal, biotin-replete diet. At this time, the mice weighed between 9 and 11 grams. Weights were measured until the KO mice became symptomatic at approximately 17 days. The rate of mean weight gain slowed at about day 5 of the diet and the KO mice eventually lost weight at about day 15, whereas the WT and Het animals remained healthy and continued to gain weight throughout.
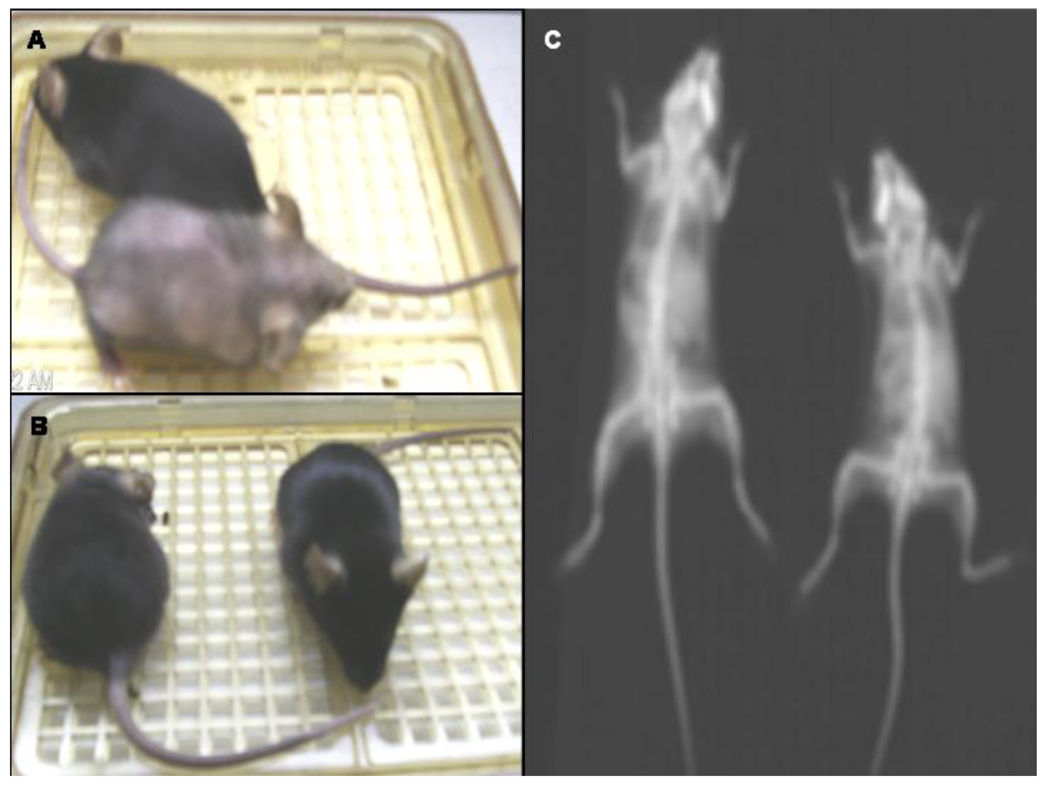
Fig. 5. Computer tomography symptomatic and non-symptomatic mice.
A. A symptomatic KO mouse is shown with loss of fur next to a non-symptomatic WT mouse. B. Following biotin treatment, a symptomatic KO mouse regrew all of its lost fur and regained most of its weight. C. Computerized tomography scan image of a symptomatic mouse (left) exhibiting 33 % bone volume loss compared to a non-symptomatic mouse (right).
After 14 days on the diet, all of the deficient mice appeared lethargic, displayed tremors and walked abnormally with a hunched posture. In addition, they were markedly hypotonic and had difficulty keeping their eyelids open; whereas the wildtype and heterozygous mice did not show any abnormal behaviors or neurological symptoms. At this time we also assessed motor-neuron function of these animals. On day 14 of the diet, heterozygous and wildtype mice were normal (stage 0), whereas all of the deficient mice showed signs of tail and limb weakness (stage 2). On day 16, the deficient mice exhibited symptoms ranging from hindlimb and forelimb weakness to paralysis (between stage 3 and 4).
A symptomatic deficient mouse exhibited bone volume loss (33 % less) compared to a heterozygous mouse by computerized tomography scan analysis performed on day 15 of the diet (Figure 5C). All other organs appeared normal and there were no detectable bone deformations at this stage.
Rescue by biotin supplementation
After confirming that the deficient mice exhibited clinical symptoms and biochemical features of biotinidase deficiency when fed a biotin-deficient diet, we attempted a pharmacological rescue with biotin supplementation. Once the deficient mice (n=8) began exhibiting symptoms (two weeks on the diet), we initiated biotin therapy for two of the mice while maintaining the animals on the biotin-deficient diet. Each of the biotin-treated mice received doses of biotin dissolved in 100 µl of normal saline based on their weight; one hundred µg of biotin/kg/day (based on the pharmacological dose given to children with biotinidase deficiency disorder) was administered once a day by intraperitoneal injection. The six other symptomatic deficient mice and four wildtype mice each received 100 µl of normal saline injection once a day. All untreated deficient mice continued to lose large patches of fur; however, within several days of treatment there was marked improvement in the biotin-treated deficient mice. The treated mice became more energetic and active and began to gain weight (Figure 4). The untreated deficient mice continued to deteriorate and lose weight. By day 8 of biotin treatment thec mice began to grow back their body fur and continued to gain weight (Figure 5B). They also did not exhibit tremors and were considered normal by motor neuron criteria (stage 0).
Fig. 4. Pharmacological rescue of symptomatic KO mice.
Eight KO mice and four WT mice were placed on biotin-deficient diet after weaning. KO mice became symptomatic and started losing weight at about day 15. At this time, two of the symptomatic mice were treated with intra-peritoneal (IP) injections of biotin (100 µg/kg/day in 100 µl of normal saline), whereas the other six symptomatic mice and four WT mice received IP injections of l00µl of normal saline. Mice were weighed regularly until day 23 when they were sacrificed. While they were biotin treated, symptomatic KO mice began recovering as seen by their mean weight gain, whereas the untreated symptomatic mice continued to deteriorate and eventually became moribund. In comparison, the WT mice did well on the biotin-deficient diet the entire time.
Discussion
Our laboratory first demonstrated that late-onset multiple carboxylase deficiency was due to a deficiency of biotinidase activity (5), characterized the variability in the clinical expression of the biotinidase deficiency (19–21), developed a method to screen newborns for biotinidase deficiency using blood-soaked filter paper spots (22), piloted the first newborn screening program for the disorder (23). We subsequently isolated and cloned the human biotinidase gene (24) characterized the genomic organization of the gene (25), and identified over one hundred different mutations that cause profound biotinidase deficiency (26). In the past many investigators, including our laboratory, have attempted to infer information about biotinidase deficiency by studying rats made biotin-deficient by feeding them biotin-depleted diets. However, this approach is obviously flawed because these animals still have normal biotinidase activity and retain the ability to recycle biotin. Our biotinidase-deficient mice now eliminate this obstacle.
We observed the ratio of BTD+/+, BTD+/− and BTD−/− genotypes for newborn pups of about 1:2:1 in animals bred from pairs of heterozygous mice. This is the expected ratio for an autosomal recessive trait. The observed ratio and normal gestational development also indicates that biotinidase deficiency does not adversely affect fertilization, implantation or embryonic development.
Individuals heterozygous for one biotinidase-deficient mutation allele and one normal allele have half the serum biotinidase activity compared to that of the normal individuals. We confirmed that wildtype mice had about twice the serum biotinidase activity and CRM to antibody prepared against human biotinidase compared to heterozygous mice and that deficient mice have essentially undetectable biotinidase activity. The deficient mice have no detectable CRM present in serum consistent with what is observed for individuals with profound biotinidase deficiency due to a null mutation and individuals who are heterozygous for such a mutation.
Furthermore, biotin content and PCC activities in liver extracts of wildtype and heterozygous mice were similar, whereas both the hepatic biotin content and PCC activity of deficient mice were markedly reduced. These results support that only one functional copy of the BTD gene is sufficient to maintain biotin content and PCC activity and is consistent with what is observed in individuals who are heterozygous for profound biotinidase deficiency.
Interestingly, urinary excretion of biotin and biotinylated metabolites (BBM) was significantly greater in biotinidase-deficient mice compared to that of both the wildtype and heterozygous mice after 3–5 days of being fed the biotin-depleted diet. This is similar to what is observed in humans with biotinidase deficiency when withdrawn from biotin supplementation (27,28). On the contrary, the mice with normal biotinidase activity when fed a biotin-depleted diet have decreased urinary biotin excretion (29). The results indicate that the biotinidase, known to play a central role in intestinal absorption of biotin at the brush-border, may also have a role in renal reabsorption of the vitamin.
Neither wildtype nor heterozygous mice exhibited symptoms when placed on a biotin-deficient diet for the length of time necessary to elicit symptoms in deficient mice. This is consistent with what is observed in humans; heterozygous carrier individuals who have one defective and one functional copy of the BTD gene do not exhibit symptoms of the disorder.
Biotinidase-deficient mice exhibited growth delay and dramatic weight loss after being fed a biotin-deficient diet. They also exhibited hypotonia, deterioration of motor-neuron function, both hindlimb, forelimb and tail weakness, graying of fur with eventual loss of fur after about two weeks on the diet. All of the deficient mice became lethargic and began to limp with a hunched-up posture and stunted body frame, whereas heterozygous mice did not show any of these abnormalities and continued to grow normally.
In addition, bone volume of symptomatic deficient mice was decreased by 33 % compared to that of asymptomatic mice. Skeletal malformation and shortening of the long bones has been reported in fetuses of biotin-deficient dams (30). We did not observe any bone malformations or defects nor limbs deformations in the heterozygous animal.
Biotin deficiency due to less than adequate dietary biotin in individuals with biotinidase deficiency usually leads to multiple carboxylase deficiency and subsequently to the accumulation of abnormal organic acids. In particular, decreased activity of 3MCG shunts the 3-methylcrotonyl-CoA to alternate metabolic pathways resulting in the accumulation of 3HIVA, 3MCG, and isovalerylglycine (IVG). In humans, urinary excretion of 3HIVA is considered the earliest, most sensitive indicator of biotin deficiency (31,32), whereas 3-HPA and 2MCA are not (33). Although it was experimentally challenging to obtain sufficient urine samples for analysis from symptomatic deficient mice, we found a 20-fold increase in the urinary excretion of 3HIVA in these mice compared to that of asymptomatic biotin-replete deficient mice, whereas the other organic acids were not elevated.
Many symptomatic children with biotinidase deficiency exhibit metabolic acidosis; however, this is not always present. In fact, we have found that symptomatic individuals often have elevated lactate and organic acid accumulation in the cerebrospinal fluid or by MRI spectroscopy, whereas there is no lactic acidemia/uria (34–36). We did not find reduced serum bicarbonate or an increased anion gap in symptomatic deficient mice compared to deficient asymptomatic mice. We did observe mild hyperammonemia in symptomatic deficient mice, whereas the ammonia concentrations in asymptomatic deficient and normal mice were not elevated.
We attempted a pharmacological biotin rescue of symptomatic deficient mice by maintaining them on a biotin-deficient diet and supplementing them with pharmacological doses of biotin. The symptomatic deficient mice were treated with biotin injections, whereas symptomatic deficient mice and one heterozygous mouse received normal saline injections. Within several days of administering supplemental biotin, the symptomatic deficient mice showed marked improvement, whereas the symptomatic animals administered normal saline in place of biotin continued to deteriorate and lose weight. The biotin-treated deficient mice became more energetic and active and began to gain weight. In addition, these animals eventually grew back their body fur. This is similar to the reversibility of symptoms observed when symptomatic biotinidase-deficient children are diagnosed and treated with biotin.
We have clearly demonstrated that the transgenic, profoundly biotinidase-deficient mouse exhibits many of the clinical and biochemical features of untreated biotinidase deficiency in humans. In addition, biotin supplementation is able to reverse the clinical features of the disorder. Although many other enzyme-deficient mouse models have not met all the expectation for their usefulness in studying the pathophysiology or clinical features of the disorder seen in humans, our initial characterization of the biotinidase-deficient mouse is promising. Following further characterization, this animal model is likely to be useful in answering questions about biotinidase deficiency in a systematic and controlled environment. We will use this animal to study and compare the effect of biotin-restriction on the cutaneous, neurological (specifically auditory and ophthalmological) and immunological systems because these organ systems are affected in untreated children with biotinidase deficiency. It is important for us to determine if these mice develop sensorineural hearing loss and myelination defects, such as optic atrophy. We will also be able to study the potential toxicity of biocytin in the untreated and treated state.
In summary, the observations presented in this study indicate that our transgenic, knock-out mouse with biotinidase deficiency exposed to a biotin-deficient diet exhibits many of the clinical and biochemical characteristics of untreated children with biotinidase deficiency. Moreover, symptomatic, biotinidase-deficient mice markedly improve following treatment with pharmacological doses of biotin. This enzyme-deficient animal should provide important information about the disorder that cannot be obtained by studying individuals with the enzyme deficiency, especially now that fewer children with biotinidase deficiency will be available because of universal newborn screening.
Acknowledgment
This work was funded by the Safra Research Foundation (B.W.) and National Institutes of Health grants R37 DK36823 (DMM) and R37 DK36823-26S1 (DMM). We thank Dr. Kenneth Barton from the Department of Radiation Oncology at Henry Ford Hospital for helping us with the Computer Tomography studies and Dr. Jieli Chen from the Department of Neurology at Henry Ford Hospital for helping us with Motor-neuronal assessment studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moss J, Lane MD. The biotin-dependent enzymes. Adv.Enzymol. 1971;35:321–442. doi: 10.1002/9780470122808.ch7. [DOI] [PubMed] [Google Scholar]
- 2.Thoma RW, Peterson WH. The enzymatic degradation of soluble bound biotin. J.Biol.Chem. 1954;210:569–579. [PubMed] [Google Scholar]
- 3.Wright LD, Driscoll CA, Boger WP. Biocytinase, an enzyme concerned with hydrolytic cleavage of biocytin. Proc.Soc.Exp.Biol.Med. 1954;86:335–337. doi: 10.3181/00379727-86-21090. [DOI] [PubMed] [Google Scholar]
- 4.Wolf B, Heard GS, McVoy JS, Raetz HM. Biotinidase deficiency: The possible role of biotinidase in the processing of dietary protein-bound biotin. J.lnherited.Metab.Dis. 1984;7 Suppl 2:121. doi: 10.1007/978-94-009-5612-4_35. [DOI] [PubMed] [Google Scholar]
- 5.Wolf B, Grier RE, Allen RJ, Goodman SL, Kien CL. Biotinidase deficiency: The enzymatic defect in late-onset multiple carboxylase deficiency. Clin.Chim.Acta. 1983;131:273–281. doi: 10.1016/0009-8981(83)90096-7. [DOI] [PubMed] [Google Scholar]
- 6.Wolf B. Disorders of Biotin Metabolism. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease. New York: McGraw-Hill; 2001. pp. 3935–3962. [Google Scholar]
- 7.Wolf B, Grier RE, Secor McVoy JR, Heard GS. Biotinidase deficiency: A novel vitamin recycling defect. J.Inherited.Metab.Dis. 1985;8 Suppl 1:53–58. doi: 10.1007/BF01800660. [DOI] [PubMed] [Google Scholar]
- 8.Wolf B. Disorders of biotin metabolism: Treatable neurological syndromes. In: Rosenberg R, Prusiner SB, Di Mauro S, Barchi RL, Kunkel LM, editors. The Molecular and Genetic Basis of Neurological Disease. Stoneham, Mass: Butterworth Publishers; 1992. pp. 569–581. [Google Scholar]
- 9.Wolf B. Worldwide survey of neonatal screening for biotinidase deficiency. J.Inherited.Metab.Dis. 1991;14:923–927. doi: 10.1007/BF01800475. [DOI] [PubMed] [Google Scholar]
- 10.Liu P, Jenkins NA, Copeland NG. A highly efficient recombineering-based method for generating conditional knockout mutations. Genome Res. 2003;13:476–484. doi: 10.1101/gr.749203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hymes J, Fleischhauer K, Wolf B. Biotinidase in serum and tissues. Methods Enzymol. 1997;279:422–432. doi: 10.1016/s0076-6879(97)79046-3. [DOI] [PubMed] [Google Scholar]
- 12.Hart PS, Hymes J, Wolf B. Isoforms of human serum biotinidase. Clin.Chim.Acta. 1991;197:257–264. doi: 10.1016/0009-8981(91)90146-4. [DOI] [PubMed] [Google Scholar]
- 13.Mock DM, Mock NL, Stewart CW, LaBorde JB, Hansen DK. Marginal biotin deficiency is teratogenic in ICR mice. J.Nutr. 2004;133:2519–2525. doi: 10.1093/jn/133.8.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mock DM. Sequential soldi-phase assay for biotin based on 125I-labeled avidin. Methods Enzymol. 1990;184:224–233. doi: 10.1016/0076-6879(90)84278-o. [DOI] [PubMed] [Google Scholar]
- 15.Determination of creatinine by a direct colorimetric method. Clin.Chim.Acta. 1973;43:305–310. doi: 10.1016/0009-8981(73)90466-x. [DOI] [PubMed] [Google Scholar]
- 16.Mock DM, Mock N. Lymphocyte propionly-CoA carboxylase is an early and sensitive indicator of biotin deficiency in rats, but urinary excretion of 3-hydroxypropionic acid is not. J.Nutr. 2002;132:1945–1950. doi: 10.1093/jn/132.7.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sweetman L. Qualitative and quantitative analysis of organic acids in physioplogic fluids for diagnosis of the organic acidurias. In: Nyhan WL, editor. Abnormalities in amino acid metabolism in clinical medicine. Norwalk: Appelton-Century-Crofts; 1984. pp. 149–156. [Google Scholar]
- 18.Human bone marrow stromal cell treatment improves neurological functional recovery in EAE mice. Exp.Neurol. 2005;195:16–25. doi: 10.1016/j.expneurol.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 19.Wolf B, Heard GS, Weissbecker KA, Secor McVoy JR, Grier RE, Leshner RT. Biotinidase deficiency: Initial clinical features and rapid diagnosis. Ann.Neurol. 1985;18:614–617. doi: 10.1002/ana.410180517. [DOI] [PubMed] [Google Scholar]
- 20.Wolf B, Grier RE, Allen RJ, Goodman SL, Kien CL, Parker WD, Howell DM, Hurst DL. Phenotypic variation in biotinidase deficiency. J.Pediatr. 1983;103:233–237. doi: 10.1016/s0022-3476(83)80351-5. [DOI] [PubMed] [Google Scholar]
- 21.Wolf B, Pomponio RJ, Norrgard KJ, Lott IT, Baumgartner ER, Suormala T, Raemaekers VTh, Coskun T, Tokatli A, Ozalp L, Hymes J. Delayed-onset profound biotinidase deficiency. J.Pediatr. 1998;132:362–365. doi: 10.1016/s0022-3476(98)70464-0. [DOI] [PubMed] [Google Scholar]
- 22.Heard GS, Secor McVoy JR, Wolf B. A screening method for biotinidase deficiency in newborns. Clin.Chem. 1984;30:125–127. [PubMed] [Google Scholar]
- 23.Heard GS, Wolf B, Jefferson LG, Weissbecker KA, Nance WE, Secor McVoy JR, Napolitano A, Mitchell PL, Lambert FW, Linyear AS. Neonatal screening for biotinidase deficiency: Results of a 1-year pilot study. J.Pediatr. 1986;108:40–46. doi: 10.1016/s0022-3476(86)80766-1. [DOI] [PubMed] [Google Scholar]
- 24.Wolf B, Heard GS, Jefferson LG, Proud VK, Nance WE, Weissbecker KA. Clinical findings in four children with biotinidase deficiency detected through a statewide neonatal screening program. N.Engl. J.Med. 1985;313:16–19. doi: 10.1056/NEJM198507043130104. [DOI] [PubMed] [Google Scholar]
- 25.Knight HC, Reynolds TR, Meyers GA, Pomponio RJ, Buck GA, Wolf B. Structure of the human biotinidase gene. Mammal. Genome. 1998;9:327–330. doi: 10.1007/s003359900760. [DOI] [PubMed] [Google Scholar]
- 26.Hymes J, Stanley CM, Wolf B. Mutations in BTD causing biotinidase deficiency. Hum.Mutat. 2001;200:375–381. doi: 10.1002/humu.1208. [DOI] [PubMed] [Google Scholar]
- 27.Baumgartner ER, Suormala T, Wick H, Bausch J, Bonjour J-P. Biotinidase deficiency: Factors responsible for the increased biotin requirement. J.Inherit.Metab.Dis. 1985;8 Suppl. 1:59–64. doi: 10.1007/BF01800661. [DOI] [PubMed] [Google Scholar]
- 28.Suormala T, Wick H, Bonjour JP, Baumgartner ER. Intestinal absorption and renal excretion of biotin in patients with biotinidase deficiency. Eur.J.Pediatr. 1985;144:21–26. doi: 10.1007/BF00491919. [DOI] [PubMed] [Google Scholar]
- 29.Mock NL, Malik ML, Stumbo PJ, Bishop WP, Mock DM. Increased urinary excretion of 3-hydroxyisovaleric acid and decreased urinary excretion of biotin are sensitive early indicators of decreased biotin status in experimental biotin deficiency. Am.J.Clin.Nutr. 1997;65:951–958. doi: 10.1093/ajcn/65.4.951. [DOI] [PubMed] [Google Scholar]
- 30.Mock DM. Marginal biotin deficiency is common in normal human pregnancy and is highly teratogenic in mice. J.Nutr. 2009;139:154–157. doi: 10.3945/jn.108.095273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stevenson DE, Feng R, Dumas F, Groleau D, Mihoc A, Storer AC. Mechanistic and structural studies on Rhodococcus ATCC 39484 nitrilase. Biotechnol Appl Biochem. 1992;15:283–302. [PubMed] [Google Scholar]
- 32.Mock DM, Heinrich CL, Carnell N, Mock NI. Indicators of marginal biotin deficiency and repletion in humans: validaion of 3-hydroxyisovaleric acid excretion and a leucine challenge. Am.J.Clin.Nutr. 2002;76:1061–1068. doi: 10.1093/ajcn/76.5.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mock DM, Henrich-Shell CL, Carnell N, Stumbo P, Mock NI. 3-Hydroxypropionic acid and methylcitric acid are not reliable indicators of marginal biotin deficiency in humans. J.Nutr. 2004;134:317–320. doi: 10.1093/jn/134.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diamantopoulos N, Painter MJ, Wolf B, Heard GS, Roe C. Biotinidase deficiency: Accumulation of lactate in the brain and response to physiologic doses of biotin. Neurology. 1986;36:1107–1109. doi: 10.1212/wnl.36.8.1107. [DOI] [PubMed] [Google Scholar]
- 35.Duran M, Baumgartner ER, Suormala T, Bruinvis L, Dorlland L, Smeitnk JAM, Poll-The BT. Cerebrospinal fluid organic acids in biotinidase deficiency. J.Inherit.Metab.Dis. 1993;16:513–516. doi: 10.1007/BF00711668. [DOI] [PubMed] [Google Scholar]
- 36.Desai S, Ganesan K, Hegde A. Biotinidase deficiency: a reversible metabolic encephalopathy. Neuroimaging and MR spectroscopic findings in a series of four patients. Pediatr.Radiol. 2008;38:848–856. doi: 10.1007/s00247-008-0904-z. [DOI] [PubMed] [Google Scholar]