Introduction
Uric acid stones constitute a minority of all nephrolithiasis cases, but are significantly more common among stone formers with the metabolic syndrome. An overly acidic urine is recognized as the major abnormality responsible for uric acid nephrolithiasis. This article reviews the epidemiology, pathogenesis, and management of uric acid stone disease, with a focus on its recent association with the metabolic syndrome and the underlying mechanisms.
Epidemiology
Uric acid nephrolithiasis comprises 8–10% of all kidney stones in the United States, 16% of stones in Okinawa, Japan, and 25% of stones in certain regions in Germany [1]. Furthermore, this prevalence is significantly higher in certain subsets of stones formers: Stone formers with type 2 diabetes mellitus (T2DM) have UA stones as the main stone constituent more frequently than non-diabetic stone formers [2–4]. As an example, in one retrospective study, UA stones constituted 34% of the stone burden in patients with T2DM compared with 6% in stone-forming patients without diabetes [3]. A higher prevalence of UA stones has also been described among obese stone formers [5–6]. It also appears that greater body mass index and T2DM are independent risk for factors for UA nephrolithiasis [4]. The clustering of obesity and insulin resistance along with hypertension and dyslipidemia describes the metabolic syndrome. In recent studies, a large proportion of uric acid nephrolithiasis patients were found to be affected by various features of the metabolic syndrome [7–8]. In summary, the metabolic syndrome and its individual features are linked with uric acid nephrolithiasis.
Uric acid metabolism and physical chemistry
Uric acid is the end product of purine metabolism in humans who display serum and urinary UA concentrations significantly higher than other mammals. Since urinary excretion of uric acid in humans generally exceeds 600 mg/day, the limited urinary uric acid solubility of 96 mg/L poses a great risk for UA precipitation.
Uric acid, a weak acid, in urine is in equilibrium with its base urate: Uric Acid ← → H+ + Urate−. The pKa for this equilibrium is 5.35 at 37°C, which lies within the clinical range for urine pH, rendering urine pH an important determinant of urinary UA solubility. An unduly acidic urine (urine pH ≤ 5.5) leads to precipitation of sparingly soluble uric acid (UA), increasing the predisposition to UA nephrolithiasis. Another important physicochemical property is that uric acid and sodium urate crystals in urine increase the propensity toward formation of mixed UA and calcium oxalate stones through the process of heterogeneous nucleation and epitaxial crystal growth.
Pathophysiology of uric acid stone formation
The pathogenetic mechanisms for UA stone formation are diverse, and may be congenital, acquired, or idiopathic [1]. Three significant urinary abnormalities have been described in patients with UA nephrolithiasis: low urinary pH, hyperuricosuria, and low urine volume.
A. Low urine volume
Low urinary volume (<2 liters/day) increases urinary saturation with respect to stone-forming constituents, and contributes the development of all stone types, including uric acid stones.
B. Hyperuricosuria
Conditions associated with excessive uric acid excretion include dietary indiscretion (consumption of purine-rich diets) [9], use of drugs that prevent renal proximal tubule reabsorption of uric acid (losartan, probenecid, and high doses of salicylates), and increased purine catabolism (myeloproliferative disorders, hemolytic diseases and following chemotherapy) [1]. A number of congenital metabolic disorders are also associated with uric acid overproduction and subsequent hyperuricemia and significant hyperuricosuria. The relatively infrequent occurrence of stone disease in inherited and acquired hyperuricosuric states underscores the importance of additional factor(s) in uric acid precipitation. In fact, unless present in massive amounts, hyperuricosuria per se without low urine pH is insufficient to cause uric acid nephrolithiasis.
C. Low urine pH
An overly acidic urine is the overriding pathogenetic mechanism underlying uric acid nephrolithiasis. A low 24-hr urine pH (<5.5) is seen in the vast majority of uric acid stone formers, many of whom exhibit normal urinary uric acid excretion [7, 10]. Conditions that can contribute to acidic urine include chronic diarrhea and increased endogenous acid generation (as occurs with ingestion of an acidogenic diet [11]). However, the vast majority of UA stone formers lack these features, and are classified under Idiopathic UA nephrolithiasis (IUAN), previously termed “gouty diathesis”.
Metabolic syndrome and the genesis of uric acid stones
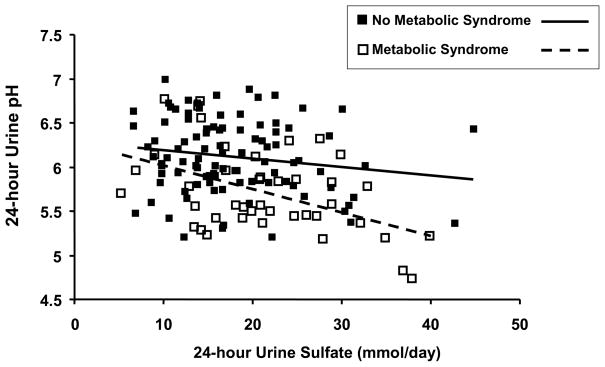
IUAN patients comprise the vast majority of UA stone formers [7]. In this group, the most important and invariant feature is an overly acidic urine that increases the urinary content of undissociated UA and the propensity for UA precipitation. Dietary factors – primarily consumption of animal protein – could underly this more acidic urine. However, dietary factors alone do not account for the overly acidic urine in IUAN, as studies have shown greater acid excretion in IUAN patients vs. controls while these subjects consumed a fixed metabolic diet low in acid ash [7]. The pathophysiologic mechanism(s) accounting for this increased acid excretion appears to be related to obesity/insulin resistance, as T2DM patients without stones as well as IUAN patients appear to have greater acid excretion than lean, non-diabetic, insulin sensitive controls [12]. In addition, obesity itself is associated with low urine pH in patients with nephrolithiasis [13]. Finally, compared with subjects without the metabolic syndrome, those with the metabolic syndrome exhibit low urine pH at any level of dietary acid intake (Figure 1).
Figure 1. Relationship between 24-hr urine pH and 24-hr urine sulfate (a marker of dietary animal protein intake) in subjects with (□) and without (■) the metabolic syndrome.
Data from 148 non-stone forming volunteers. Subjects with the metabolic syndrome exhibited a significantly higher 24-hr urine sulfate, indicating greater consumption of acidogenic diet. The regression line for each group shows that 24-hr urine pH declines with increasing acid intake (24-hr urine sulfate). However, at any given 24-hr urine sulfate, 24-hr urine pH is significantly lower in subjects with the metabolic syndrome, suggesting that factors besides dietary acid intake contribute to the more acidic urine.
Greater acid excretion alone is not sufficient to lower urine pH, as the excess acid can be buffered in urine. However, if excess acid is not appropriately buffered, urine becomes unduly acidic. Ammonium (NH4+) is an important urinary buffer, because of its high pK, and its renal production and excretion are regulated by acid-base status. Reduced NH4+ excretion has been shown in subjects with IUAN and this defect was amplified by administration of an acute acid load [7]. A similar finding was also demonstrated in non-stone forming patients with the metabolic syndrome [14]. These findings are consistent with a defect in renal ammoniagenesis and/or excretion that leads to impaired buffering and amplifies the acidic urine caused by the increased acid excretion in patients with IUAN and the metabolic syndrome.
Management of uric acid stones – Nutritional and pharmacological approaches
The initial management of patients with uric acid nephrolithiasis consists of medical dissolution therapy as this non-invasive approach is successful in the vast majority of cases [15]. Medical management is directed at correcting the underlying metabolic abnormalities that lead to stone formation: Low urine volume, hyperuricosuria and low urine pH. Correction of low urine pH is the cornerstone of therapy in uric acid nephrolithiasis. It results in the dissolution of existing stones and prevents recurrent stone formation. Reduction in dietary intake of animal proteins is helpful, and should be recommended to all patients [16]. However, pharmacological therapy with potassium citrate is most commonly used in this setting [15]. Sodium salts (e.g. sodium bicarbonate) are used in patients who cannot tolerate or are poor candidates for potassium citrate (due to hyperkalemia or renal insufficiency). Correction of low urine volume requires increasing fluid intake to maintain a urinary volume of approximately 2 L/d. Compared with consumption of water, some fruit juices (such as orange juice) may provide an alkali load and raise urine pH in addition to raising urine volume [17]. Correction of hyperuricosuria can be achieved with lifestyle changes (consumption of a balanced diet and avoidance of food sources of purine [16]) and pharmacological therapy (allopurinol).
Dissolution therapy is generally effective, and most patients achieve durable results with lifelong alkali therapy. Urological intervention is infrequently used in the management of uric acid nephrolithiasis and is reserved for patients with prolonged obstruction, progressive renal insufficiency, intractable pain and/or poor response to medical therapy.
Conclusions
Uric acid stone disease is primarily a disorder in which an overly acidic urine results in the precipitation of uric acid in the urinary tract. While it comprises a minority of all kidney stones, it is significantly more common in stone formers with the metabolic syndrome. Treatment is primarily medical, and relies on stone dissolution with alkalinization of urine.
Abbreviations
- UA
Uric acid
- IUAN
Idiopathic Uric Acid Nephrolithasis
- T2DM
Type 2 Diabetes
- MS
Metabolic Syndrome
Footnotes
Dr. Maalouf has no conflicts relevant to this publication.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Maalouf NM, Cameron MA, Moe OW, Sakhaee K. Novel insights into the pathogenesis of uric acid nephrolithiasis. Curr Opin Nephrol Hypertens. 2004;13:181–189. doi: 10.1097/00041552-200403000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Lieske JC, de la Vega LS, Gettman MT, et al. Diabetes mellitus and the risk of urinary tract stones: a population-based case-control study. Am J Kidney Dis. 2006;48:897–904. doi: 10.1053/j.ajkd.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Pak CY, Sakhaee K, Moe O, et al. Biochemical profile of stone-forming patients with diabetes mellitus. Urology. 2003;61:523–527. doi: 10.1016/s0090-4295(02)02421-4. [DOI] [PubMed] [Google Scholar]
- 4.Daudon M, Traxer O, Conort P, Lacour B, Jungers P. Type 2 diabetes increases the risk for uric acid stones. J Am Soc Nephrol. 2006;17:2026–2033. doi: 10.1681/ASN.2006030262. [DOI] [PubMed] [Google Scholar]
- 5.Ekeruo WO, Tan YH, Young MD, et al. Metabolic risk factors and the impact of medical therapy on the management of nephrolithiasis in obese patients. J Urol. 2004;172:159–163. doi: 10.1097/01.ju.0000128574.50588.97. [DOI] [PubMed] [Google Scholar]
- 6.Daudon M, Lacour B, Jungers P. Influence of body size on urinary stone composition in men and women. Urol Res. 2006;34:193–199. doi: 10.1007/s00240-006-0042-8. [DOI] [PubMed] [Google Scholar]
- 7.Sakhaee K, Adams-Huet B, Moe OW, Pak CY. Pathophysiologic basis for normouricosuric uric acid nephrolithiasis. Kidney Int. 2002;62:971–979. doi: 10.1046/j.1523-1755.2002.00508.x. [DOI] [PubMed] [Google Scholar]
- 8.Abate N, Chandalia M, Cabo-Chan AV, Jr, Moe OW, Sakhaee K. The metabolic syndrome and uric acid nephrolithiasis: novel features of renal manifestation of insulin resistance. Kidney Int. 2004;65:386–392. doi: 10.1111/j.1523-1755.2004.00386.x. [DOI] [PubMed] [Google Scholar]
- 9.Fellstrom B, Danielson BG, Karlstrom B, Lithell H, Ljunghall S, Vessby B. The influence of a high dietary intake of purine-rich animal protein on urinary urate excretion and supersaturation in renal stone disease. Clin Sci (Lond) 1983;64:399–405. doi: 10.1042/cs0640399. [DOI] [PubMed] [Google Scholar]
- 10.Pak CY, Sakhaee K, Peterson RD, Poindexter JR, Frawley WH. Biochemical profile of idiopathic uric acid nephrolithiasis. Kidney Int. 2001;60:757–761. doi: 10.1046/j.1523-1755.2001.060002757.x. [DOI] [PubMed] [Google Scholar]
- 11.Reddy ST, Wang CY, Sakhaee K, Brinkley L, Pak CY. Effect of low-carbohydrate high-protein diets on acid-base balance, stone-forming propensity, and calcium metabolism. Am J Kidney Dis. 2002;40:265–274. doi: 10.1053/ajkd.2002.34504. [DOI] [PubMed] [Google Scholar]
- 12.Cameron MA, Maalouf NM, Adams-Huet B, Moe OW, Sakhaee K. Urine composition in type 2 diabetes: predisposition to uric acid nephrolithiasis. J Am Soc Nephrol. 2006;17:1422–1428. doi: 10.1681/ASN.2005121246. [DOI] [PubMed] [Google Scholar]
- 13.Maalouf NM, Sakhaee K, Parks JH, Coe FL, Adams-Huet B, Pak CY. Association of urinary pH with body weight in nephrolithiasis. Kidney Int. 2004;65:1422–1425. doi: 10.1111/j.1523-1755.2004.00522.x. [DOI] [PubMed] [Google Scholar]
- 14.Maalouf NM, Cameron MA, Moe OW, Adams-Huet B, Sakhaee K. Low urine pH: a novel feature of the metabolic syndrome. Clin J Am Soc Nephrol. 2007;2:883–888. doi: 10.2215/CJN.00670207. [DOI] [PubMed] [Google Scholar]
- 15.Preminger GM. Pharmacologic treatment of uric acid calculi. Urol Clin North Am. 1987;14:335–338. [PubMed] [Google Scholar]
- 16.Siener R, Hesse A. The effect of a vegetarian and different omnivorous diets on urinary risk factors for uric acid stone formation. Eur J Nutr. 2003;42:332–337. doi: 10.1007/s00394-003-0428-0. [DOI] [PubMed] [Google Scholar]
- 17.Odvina CV. Comparative value of orange juice versus lemonade in reducing stone-forming risk. Clin J Am Soc Nephrol. 2006;1:1269–1274. doi: 10.2215/CJN.00800306. [DOI] [PubMed] [Google Scholar]