Abstract
Triterpene derivatives were analyzed for anti-HIV-1 activity and for cellular toxicity. Betulinic aldehyde, betulinic nitrile, and morolic acid derivatives were identified to have anti-HIV-1 activity. These derivatives inhibit a late step in virus replication, likely virus maturation.
Keywords: retrovirus, antiviral, antiretroviral, proteolysis, protease
Human immunodeficiency virus type 1 (HIV-1), the etiologic agent of AIDS, remains a serious global public health problem. Highly active antiretroviral therapy (HAART) prevents viral replication and the development of AIDS. Though HIV-1 infection can be inhibited, antiretroviral drug resistance and off-target effects require the continual development of novel antiretroviral agents to combat HIV-1 infection for inclusion into HAART regimens.
Fifteen of the 25 anti-HIV-1 drugs approved by the United States Food and Drug Administration (FDA) inhibit early steps in the HIV-1 life cycle. Specifically, these drugs target either viral entry, co-receptor recognition, fusion, reverse transcription, or integration.1 The 10 approved anti-HIV-1 drugs that inhibit late steps in the HIV-1 life cycle all target the viral protease.1 There are numerous late steps in the HIV-1 life cycle that could be potential drug targets. Examples of such targets include viral RNA transcription, protein translation, virus particle assembly, release, or maturation. Maturation of HIV-1 particles occurs primarily after virus release from infected cells. Maturation is a process that is targeted by the triterpene bevirimat (3-O-(3′,3′-dimethylsuccinyl)betulinic acid, PA-457, DSB).2–11 Bevirimat (Figure 1) inhibits HIV-1 maturation by preventing the cleavage of CA-SP1 (p25) Gag into Capsid (p24) and SP1 (p2).2–11 Bevirimat, in the absence of the viral protease, has also been shown to inhibit virus assembly.12, 13 Drug resistance studies have revealed many resistance-bearing mutations at the carboxy-terminus of capsid and in the SP1 spacer peptide.3, 6, 11, 14, 15 Bevirimat has been shown to inhibit HIV-1 replication in cell culture2–4, 6–9, 11, 16 and has shown promise in clinical trials,17, 18 but has not been approved as a drug for the treatment of HIV-1 infection. The goal of this study was to identify other triterpenes that could inhibit HIV-1 replication and would be useful for better understanding the mechanism of action and the structure-activity relationship. Such information would clearly be useful in the design of new derivatives that have potential for clinical translation.
Figure 1.
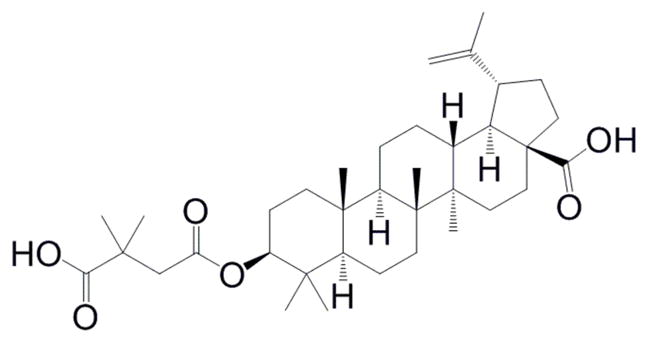
Structure of bevirimat.
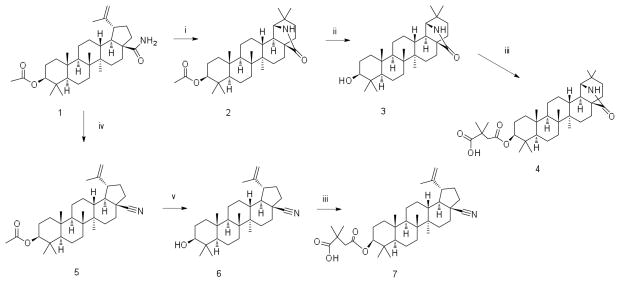
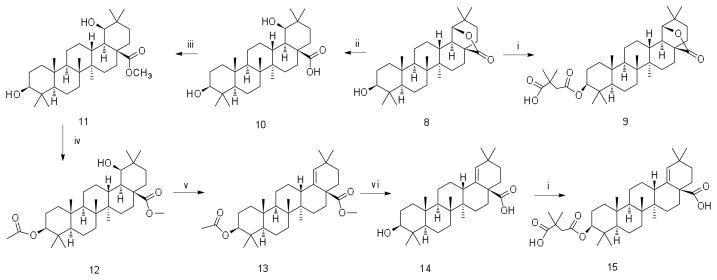
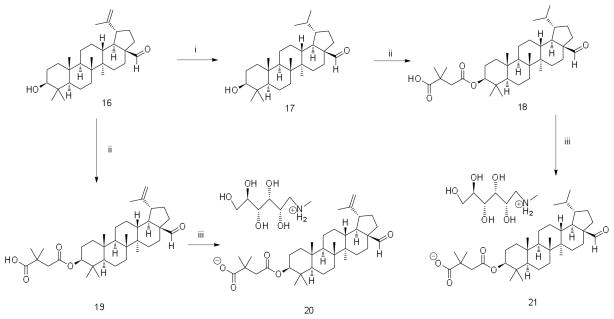
Triterpenes have great potential for drug development.19 Triterpenes can be extracted from natural sources and are abundant in Betula papyrifera (North American birch tree).19 Betulin was extracted and purified from birch bark of Betula papyrifera in accordance with a previously developed procedure.20 Other precursors - betulinic aldehyde and betulinic acid - were synthesized from betulin by the method of electrochemical oxidation.21 Further triterpene modifications were provided in accordance with newly developed Schemes 1, 2 and 3. All synthesized derivatives were characterized by 1H and 13C NMR and HRMS (Supplementary Material).
Scheme 1.
(i) CF3COOH, CHCl3; (ii) KOH, EtOH; (iii) DMSA, DMAP, Py; (iv) (CF3CO)2O, CHCl3; (v) KOH, MeOH
Scheme 2.
(i) DMSA, DMAP, Py; (ii) KOH, t-BuOH; (iii) (CH3O)2SO2, K2CO3, DMF; (iv) (CH3CO)2O, Py; (v) POCl3, Py; (vi) 1) KOH, t-BuOH; 2) HCl, H2O
Scheme 3.
(i) H2, Pd/C, THF/MeOH (ii) DMSA, DMAP, Py; (iii) N-methyl-D-glucamine, MeOH
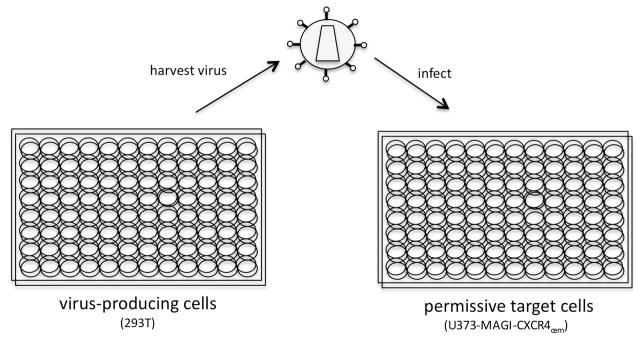
The triterpene derivatives were screened for anti-HIV-1 activity using a single-cycle HIV-1 replication assay. This assay is particularly useful because it can differentiate whether a derivative targets the late or early steps in the viral life cycle (Figure 2). In the assay, 293T cells were transfected in parallel22, 23 with the HIV-1 vector pHIG,23 which encodes for the green fluorescence protein (GFP), and a HIV-1 envelope vector, pIIINL4 Env.24 The cell culture supernatant from the virus-producing cells was harvested, cellular debris removed, and used to infect HIV-1 permissive U373-MAGI- CXCR4cem target cells.25 A minimum of three independent replicates of the assay were performed. Permissive cells were all treated with the equivalent volume of compound treated virus containing supernatant as the no drug treatment control. The no drug treatment control virus had a multiplicity of infection (MOI) of 0.05. The low MOI reduces the possibility of multiple infections in individual target cells. Infected cells were monitored for GFP expression 48 h post infection by flow cytometry. GFP expression allowed for the monitoring of HIV-1 infection, and a reduction in the percentage of GFP-expressing target cells was indicative of a reduction in virus infectivity due to the antiretroviral activity of a triterpene derivative. To determine whether the triterpene derivatives could inhibit HIV-1 replication, virus-producing or permissive target cells were exposed to triterpene derivatives in a concentration range from 5 nM to 50 μM. The EC50 values were determined using Graph Pad Prism 5 statistical software.
Figure 2.
Assay for antiretroviral activity of triterpene derivatives. HIV-1 vector was cotransfected with a HIV-1 envelope expression plasmid into 293T cells in a 96-well tissue culture plate. Forty-eight hours post-trasfection, cell culture supernatants were harvested from the virus-producing cells and used to infect permissive target cells (i.e., U373-MAGI-CXCR4cem cells). Infected cells were harvested 48 hours postinfection and analyzed by flow cytometry. To test triterpene derivatives for antiretroviral activity, virus-producing cells (prior to harvesting virus) or permissive target cells after virus infection were exposed individually with each derivative at a range of concentrations (i.e., 5 nM to 50 μM).
When permissive target cells were treated with the triterpene derivatives, none of the derivatives inhibited HIV-1 replication – testing up to concentrations of 50 μM. When treating virus-producing cells, bevirimat, 3-O-(3′,3′-dimethylsuccinyl)betulinic acid inhibited HIV-1 replication with an EC50 value of 0.29 μM. The betulinic aldehyde derivatives (18–21) had EC50 values of 1.8 μM, 1.2 μM, 1.5 μM, and 1.4 μM, respectively. The betulinic nitrile derivative (7) had a similar EC50 value of 1.5 μM. Derivatives (4) and (9) had EC50 values of 15 and 14 μM, respectively, which was ~10-fold less potent than the betulinic aldehyde or betulinic nitrile derivatives. Interestingly, derivative 15 was about 130 times less active than bevirimat, with an EC50 of 39 μM.
Potential cell toxicity was assayed using a commercially available kit (CellTiter Non-Radioactive Cell Proliferation Assay, Promega) following the manufacturer’s instructions (Table 1). Briefly, cells were treated with serial dilutions of each individual triterpene derivative and IC50 values were determined. If cell proliferation was not inhibited by at least 50% at the highest concentration tested, the estimated IC50 was reported as > the highest concentration tested in the assay. Bevirimat, the betulinic nitrile derivative (7), the betulinc aldehyde derivatives (18, 19, and 21), and the morolic acid derivative 15 had the lowest cellular toxicity values: IC50 >300 μM. The betulinic aldehyde derivative had an IC50 value of 29 μM. Derivatives 4 and 9 were less soluble than the other derivatives analyzed in the study, and had minimal toxicity. The triterpene derivatives 4 and 9 were found to have IC50 values of >130 μM and >150 μM, respectively.
Table 1.
Antiretroviral activity, cell toxicity, and therapeutic indices for triterpene derivatives.
| Triterpene derivative | Antiretroviral activity (treatment of virus-producing cells) | Antiretroviral activity (treatment of permissive target cells) | Cell toxicity | Therapeutic indexc |
|---|---|---|---|---|
| EC50, μMa | EC50, μMb | IC50, μMb | ||
| Bevirimat | 0.28 (±0.09) | >50 | >300 | >1000 |
| 4 | 15 (±2.5) | >50 | >150 | >10 |
| 7 | 1.5 (±0.4) | >50 | >300 | >200 |
| 9 | 14 (±2.7) | >50 | >130 | >9.2 |
| 15 | 39 (±10) | >50 | >300 | >7.6 |
| 18 | 1.8 (±0.5) | >50 | >300 | >160 |
| 19 | 1.2 (±0.3) | >50 | >300 | >250 |
| 20 | 1.5 (±0.5) | >50 | 29 | 19 |
| 21 | 1.4 (±0.4) | >50 | >300 | >210 |
Mean of four experiments, standard deviation is given in parentheses.
Mean of three experiments.
Therapeutic index = IC50 cytotoxicity/EC50 antiretroviral activity.
A therapeutic index (TI) value (i.e., TI = IC50/EC50) was determined for each of the triterpene derivatives. Bevirimat had a TI of >1000. The betulinic aldehydes (18, 19, and 21) and the betulinic nitrile derivative (7) had TI values of >160, >250, >210 and >200 respectively. The betulinic aldehyde derivative 20 had a TI of 19. Derivatives 4 and 9, which were less soluble, had TI values of >10 and > 9.2, respectively. Finally, the dimethyl succinate derivative of morolic acid, 15, had a TI value of >7.6.
The 3,3-dimethylsuccinate functional group was common to all nine triterpene derivatives listed in Table 1. The difference in anti-HIV-1 activity between 15 and bevirimat suggests that the morolic acid scaffold is less active at inhibiting HIV-1 replication than the betulinic acid scaffold. In addition, the dimethylsuccinate group in the C-3 position of the derivatives may be important for antiretroviral activity. Furthermore, the C-28 position or the triterpene can be variable, having either a carboxylic (bevirimat), aldehyde (18, 19, 20, and 21) or nitrile (7) group and still maintaining antiretroviral activity. Finally, dimethylsuccinate derivatives of 3β-hydroxyoleananes (4, 9) or morolic acid (15) have antiretroviral activity. These observations provide useful information for further investigation of the structure-activity relationship.
In this study, several triterpene derivatives have been characterized to have anti-HIV-1 activity, using a novel single-cycle replication assay. These derivatives should be useful for better understanding the mechanism of action of bevirimat and the general structure-activity relationship. Though it is possible the compounds could inhibit virus release or the viral protease, it is most likely, due to structural similarity with bevirimat, that the compounds in Table 1 inhibit HIV-1 replication by blocking CA-SP1 processing. It should be noted that the derivatives of 3-O-(3′,3′-dimethylsuccinyl)betulinic aldehyde (18, 19, 20, and 21) or 3-O-(3′,3′-dimethylsuccinyl)betulinic nitrile (7) may be metabolized by aldolases or nitrolases and therefore represent prodrugs for bevirimat. In general, the derivatives analyzed in this study have the desirable and practical feature that they can be produced in high quantities and at low cost from birch bark. In summary, the information from this study should be useful in the design of new derivatives that have potential for clinical translation.
Supplementary Material
Acknowledgments
Supported by the Continuing Umbrella of Research Experiences Supplement to NIH T32 CA09138 (Cancer Biology Training Grant) (to CDR), NIH Supplement to NIH GM56615 to Promote Diversity in Health-Related Research (to CDR), and a Natural Resources Research Institute funding agreement.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Notes
- 1.Mehellou Y, De Clercq E. J Med Chem. 2009;53:521. doi: 10.1021/jm900492g. [DOI] [PubMed] [Google Scholar]
- 2.Li F, Goila-Gaur R, Salzwedel K, Kilgore NR, Reddick M, Matallana C, Castillo A, Zoumplis D, Martin DE, Orenstein JM, Allaway GP, Freed EO, Wild CT. Proc Natl Acad Sci U S A. 2003;100:13555. doi: 10.1073/pnas.2234683100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou J, Chen CH, Aiken C. Retrovirology. 2004;1:15. doi: 10.1186/1742-4690-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou J, Yuan X, Dismuke D, Forshey BM, Lundquist C, Lee KH, Aiken C, Chen CH. J Virol. 2004;78:922. doi: 10.1128/JVI.78.2.922-929.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou J, Huang L, Hachey DL, Chen CH, Aiken C. J Biol Chem. 2005;280:42149. doi: 10.1074/jbc.M508951200. [DOI] [PubMed] [Google Scholar]
- 6.Adamson CS, Ablan SD, Boeras I, Goila-Gaur R, Soheilian F, Nagashima K, Li F, Salzwedel K, Sakalian M, Wild CT, Freed EO. J Virol. 2006;80:10957. doi: 10.1128/JVI.01369-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li F, Zoumplis D, Matallana C, Kilgore NR, Reddick M, Yunus AS, Adamson CS, Salzwedel K, Martin DE, Allaway GP, Freed EO, Wild CT. Virology. 2006;356:217. doi: 10.1016/j.virol.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 8.Sakalian M, McMurtrey CP, Deeg FJ, Maloy CW, Li F, Wild CT, Salzwedel K. J Virol. 2006;80:5716. doi: 10.1128/JVI.02743-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou J, Chen CH, Aiken C. J Virol. 2006;80:12095. doi: 10.1128/JVI.01626-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adamson CS, Waki K, Ablan SD, Salzwedel K, Freed EO. J Virol. 2009;83:4884. doi: 10.1128/JVI.02659-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adamson CS, Sakalian M, Salzwedel K, Freed EO. Retrovirology. 7:36. doi: 10.1186/1742-4690-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DaFonseca S, Blommaert A, Coric P, Hong SS, Bouaziz S, Boulanger P. Antivir Ther. 2007;12:1185. [PubMed] [Google Scholar]
- 13.Dafonseca S, Coric P, Gay B, Hong SS, Bouaziz S, Boulanger P. Virol J. 2008;5:162. doi: 10.1186/1743-422X-5-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heider D, Verheyen J, Hoffmann D. BMC Bioinformatics. 11:37. doi: 10.1186/1471-2105-11-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verheyen J, Verhofstede C, Knops E, Vandekerckhove L, Fun A, Brunen D, Dauwe K, Wensing AM, Pfister H, Kaiser R, Nijhuis M. AIDS. 24:669. doi: 10.1097/QAD.0b013e32833160fa. [DOI] [PubMed] [Google Scholar]
- 16.Hashimoto F, Kashiwada Y, Cosentino LM, Chen CH, Garrett PE, Lee KH. Bioorg Med Chem. 1997;5:2133. doi: 10.1016/s0968-0896(97)00158-2. [DOI] [PubMed] [Google Scholar]
- 17.Yebra G, Holguin A. Antivir Ther. 2008;13:1083. [PubMed] [Google Scholar]
- 18.Smith PF, Ogundele A, Forrest A, Wilton J, Salzwedel K, Doto J, Allaway GP, Martin DE. Antimicrob Agents Chemother. 2007;51:3574. doi: 10.1128/AAC.00152-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krasutsky PA. Nat Prod Rep. 2006;23:919. doi: 10.1039/b606816b. [DOI] [PubMed] [Google Scholar]
- 20.Krasutsky PA, Kolomitsyn IV, Krastuskyy DA. 2007/121482. WIPO IP Services; WO. 2007
- 21.Krasutsky PA, Khotkevych AB, Pushechnikov A, Rudnitskaya A. 2006/105357. WIPO IP Services; WO. 2006
- 22.Dorweiler IJ, Ruone SJ, Wang H, Burry RW, Mansky LM. J Virol. 2006;80:3634. doi: 10.1128/JVI.80.7.3634-3643.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dapp MJ, Clouser CL, Patterson S, Mansky LM. J Virol. 2009;83:11950. doi: 10.1128/JVI.01406-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murakami T, Freed EO. Proc Natl Acad Sci U S A. 2000;97:343. doi: 10.1073/pnas.97.1.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vodicka MA, Goh WC, Wu LI, Rogel ME, Bartz SR, Schweickart VL, Raport CJ, Emerman M. Virology. 1997;233:193. doi: 10.1006/viro.1997.8606. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.